Abstract
Mincle (also called as Clec4e and Clecsf9) is a C-type lectin receptor expressed in activated phagocytes. Recently, we have demonstrated that Mincle is an FcRγ-associated activating receptor that senses damaged cells. To search an exogenous ligand(s), we screened pathogenic fungi using cell line expressing Mincle, FcRγ, and NFAT-GFP reporter. We found that Mincle specifically recognizes the Malassezia species among 50 different fungal species tested. Malassezia is a pathogenic fungus that causes skin diseases, such as tinea versicolor and atopic dermatitis, and fatal sepsis. However, the specific receptor on host cells has not been identified. Mutation of the putative mannose-binding motif within C-type lectin domain of Mincle abrogated Malassezia recognition. Analyses of glycoconjugate microarray revealed that Mincle selectively binds to α-mannose but not mannan. Thus, Mincle may recognize specific geometry of α-mannosyl residues on Malassezia species and use this to distinguish them from other fungi. Malassezia activated macrophages to produce inflammatory cytokines/chemokines. To elucidate the physiological function of Mincle, Mincle-deficient mice were established. Malassezia-induced cytokine/chemokine production by macrophages from Mincle−/− mice was significantly impaired. In vivo inflammatory responses against Malassezia was also impaired in Mincle−/− mice. These results indicate that Mincle is the first specific receptor for Malassezia species to be reported and plays a crucial role in immune responses to this fungus.
Keywords: ITAM, macrophages, signal transduction
Mincle (also called as Clec4e and Clecsf9) is a C-type lectin receptor expressed in activated macrophages (1). We have recently demonstrated that Mincle is associated with FcRγ chain, an immunoreceptor tyrosine-based activation motif (ITAM)-containing adaptor, and acts as an activating receptor that senses damaged cells (2). Mincle is genetically mapped to a cluster on mouse chromosome 6F2 and human chromosome 12p31 (1, 3). Among these clusters, Dectin-1 and Dectin-2 are ITAM-coupled C-type lectin receptors that directly recognize specific fungi (4, 5). Given that Mincle possesses a similar structure to these receptors, it is possible that Mincle also recognizes nonself ligand such as specific fungi (6).
Malassezia species are ubiquitous residents of human skin but are associated with several diseases, such as tinea versicolor, folliculitis, and atopic dermatitis (7, 8). In neonate, invasive infection by Malassezia often causes lethal sepsis (9). Despite the important role played by Malassezia in multiple diseases, little is known about the molecular mechanism involved in host cell recognition.
In this study, we show that Mincle is a specific receptor for Malassezia species and plays a crucial role in immune responses to this fungus.
Results and Discussion
Specific Recognition of Malassezia Species by Mincle.
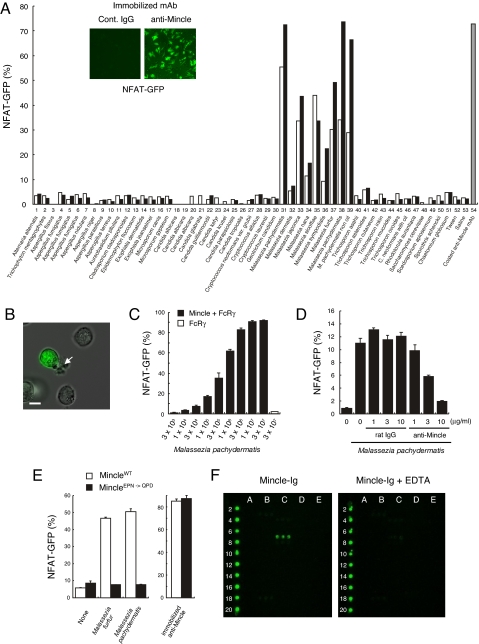
We first examined whether Mincle act as a receptor for living fungi by establishing cell-based indicator system. To discriminate putative Mincle ligands from possible contaminating TLR ligands in fungi, we used nonmyeloid T cell hybridomas as host cells and used an NFAT-GFP reporter as a specific detector of ITAM-mediated signals (10). Crosslinking of Mincle with immobilized anti-Mincle mAb induced strong GFP expression in an indicator cell line expressing NFAT-GFP reporter (10), Mincle and FcRγ (Fig. 1A, Inset) (2). We therefore used this reporter cell line to screen reactive species from more than 50 species of pathogenic fungi (Table 1). Intriguingly, only Malassezia fungi induced profound NFAT activation (Fig. 1A, lane 31–39). Although structurally similar Dectin-1, Dectin-2, and DC-SIGN are reported to recognize Candida albicans or Aspergillus species (5, 11–13), at least three strains of C. albicans (serotype A) or Aspergillus species tested did not activate Mincle-expressing cells (Fig. 1A, lane 18–20). Some species, which were negative for GFP but partially affected the viability of the reporter line, were also confirmed at a nontoxic concentration (data not shown). Microscopic analysis revealed that only Malassezia-bound cells were GFP positive (Fig. 1B). The activation was dose-dependent of Malassezia (Fig. 1C). Reporter cells expressing only FcRγ did not respond to Malassezia (Fig. 1C), demonstrating that introduced Mincle, but not other endogenous receptors associated with FcRγ, is responsible for NFAT activation. Furthermore, soluble anti-Mincle mAb blocked Malassezia-induced NFAT activation in a dose-dependent manner (Fig. 1D). These results indicate that Mincle directly recognizes Malassezia species.
Fig. 1.
Mincle recognizes Malassezia species and delivers activation signal. (A) Screening of pathogenic fungus for Mincle-ligand activity. Reporter cell line expressing Mincle, FcRγ, and NFAT-GFP was established and left unstimulated or stimulated with plate-coated anti-Mincle mAb (10 μg/ml) for 18 h (inset). Mincle-expressing NFAT-GFP reporter cells were cocultured with indicated pathogenic fungi (open, 1%; closed, 10%) for 18 h, or stimulated with plate-coated anti-Mincle as a positive control (gray). NFAT-GFP induction was analyzed by flow cytometry. Data are representative of 3 independent experiments. (B) Microscopic analysis. Mincle-expressing reporter cells were cocultured with M. pachydermatis (arrowhead) and analyzed with fluorescence microscopy (IX-81; Olympus). (Scale bars: 5 μm.) (C) Dose-dependent activation by M. pachydermatis. Reporter cells expressing Mincle and FcRγ (closed) and only FcRγ (open) were cocultured with indicated amount (cells/ml) of M. Pachydermatis for 18 h. (D) Anti-Mincle mAb blocked Malassezia-induced NFAT activation. Reporter cells were cultured with M. pachydermatis in the presence of rat IgG or anti-Mincle mAbs and GFP expression was determined by flow cytometry. (E) Critical role of Mannose-binding motif of Mincle for Malassezia recognition. MincleWT (WT), MincleE169Q/N171D (EPN -> QPD) were expressed together with FcRγ in NFAT-GFP reporter cells. Cells were treated and analyzed as in (D). (F) Mincle specifically recognizes α-mannose. Mincle-Ig (1 μg/ml) precomplexed with Cy3-anti-human IgG (0.5 μg/ml) in TBS containing 1 mM CaCl2 or 10 mM EDTA was applied on the glycoconjugated microarray. After incubation at 20 °C for 3 h, binding was detected by an evanescent-field fluorescence-assisted scanner. Data were analyzed with the Array Pro analyzer ver. 4.5.
Table 1.
IFM number of fungi used in this study
| Lane no. | Strain | IFM No. |
|---|---|---|
| 1 | Alternaria alternata | 53969 |
| 2 | Trichophyton mentagrophytes | 5218 |
| 3 | Aspergillus flavus | 54306 |
| 4 | Aspergillus fumigatus | 47439 |
| 5 | Aspergillus fumigatus | 47450 |
| 6 | Aspergillus fumigatus | 49824 |
| 7 | Aspergillus nidulans | 54308 |
| 8 | Aspergillus niger | 54309 |
| 9 | Aspergillus parasiticus | 42197 |
| 10 | Aspergillus terreus | 54310 |
| 11 | Aureobasidium pllulans | 4802 |
| 12 | Cladosporium cladosporioides | 41450 |
| 13 | Epidermophyton floccosum | 46991 |
| 14 | Exophiala dermatitidis | 4826 |
| 15 | Exophiala jeanselmei | 54222 |
| 16 | Microsporum canis | 41134 |
| 17 | Microsporum gypseum | 53930 |
| 18 | Candida albicans | 54349 |
| 19 | Candida albicans | 40009 |
| 20 | Candida albicans | 54366 |
| 21 | Candida glabrata | 54350 |
| 22 | Candida guilliermondii | 5787 |
| 23 | Candida kefyr | 5800 |
| 24 | Candida krusei | 46839 |
| 25 | Candida parapsiolosis | 51754 |
| 26 | Candida tropicalis | 55047 |
| 27 | Cryptococcus neoformans var. grubii | 5807 |
| 28 | Cryptococcus albidus | 5763 |
| 29 | Cryptococcus laurentii | 50261 |
| 30 | Geotrichum candidum | 45995 |
| 31 | Malassezia pachydermatis | 48586 |
| 32 | Malassezia dermatis | 51970 |
| 33 | Malassezia japonica | 52993 |
| 34 | Malassezia nana | 53376 |
| 35 | Malassezia slooffiae | 48587 |
| 36 | Malassezia sympodialis | 48588 |
| 37 | Malassezia furfur | 52635 |
| 38 | Malassezia pachydermatis | 48586 |
| 39 | M. pachydermatis non oil | 48586 |
| 40 | Trichosporon asahii | 48429 |
| 41 | Trichosporon asteroides | 51965 |
| 42 | Trichosporon cutaneum | 40066 |
| 43 | Trichosporon inkin | 48551 |
| 44 | Trichosporon mucoides | 48611 |
| 45 | Trichosporon ovoides | 49887 |
| 46 | C. neoformans with oil | 5807 |
| 47 | Rhodotorula aurantiaca | 40059 |
| 48 | Saccharomyces cerevisiae | 40022 |
| 49 | Scedosporium apiosermum | 51940 |
| 50 | Sporothrix schenckii | 55052 |
| 51 | Chaetomium globosum | 40872 |
Mincle Recognizes Mannosyl Residue.
Next, we addressed the structure of Malassezia species recognized by Mincle. Although several Malassezia proteins are known as major antigens for IgE in atopic dermatitis patient (8), recombinant Mal f2 (14) and Mal f6 (15) did not induce NFAT activation in the Mincle reporter cells (data not shown). Heat-killed Malassezia retained the stimulatory activity, suggesting that Mincle recognizes the nonprotein determinant of Malassezia (data not shown). Since Mincle possesses a typical carbohydrate recognition domain (CRD), it is possible that Mincle may recognize any specific carbohydrate structure of Malassezia species.
Mincle CRD contains an EPN (Glu-Pro-Asn) motif (1), which is a putative mannose-binding motif (16). To test whether Malassezia recognition by Mincle involves mannose or a related carbohydrate determinant, we mutated the EPN motif of Mincle into the QPD (Gln-Pro-Asp) motif that shows preference for galactose (16). Mutant Mincle bearing E169Q/N171D (MincleEPN->QPD) failed to respond to Malassezia furfur and Malassezia pachydermatis (Fig. 1E, Left), while it did not affect anti-Mincle-induced activation (Fig. 1E, Right). Thus, mannose recognition seems to be crucial for Mincle to sense Malassezia species.
We further examined glycan-binding specificity of Mincle by glycoconjugate microarray (Fig. S1) (17). Intriguingly, soluble Mincle protein, Mincle-Ig, showed specific binding on the spots of α-mannose-polyacrylaminde conjugates which are a highly multivalent form of α-mannose (Fig. 1F, Left, position 7C). Since EDTA completely blocked α-mannose binding to Mincle (Fig. 1F, Right, position 7C), the binding required Ca2+, suggesting that CRD of Mincle is involved in this recognition. However, Mincle-Ig showed no binding on the spots of mannan (18) (Fig. 1F, position 5E-6E). Consistently, soluble mannan did not block Mincle-mediated NFAT-activation induced by Malassezia (data not shown).
These results suggest that Mincle may recognize specific geometry of α-mannosyl residues or any related carbohydrate on Malassezia species to distinguish them from other fungi. To identify such specific determinant, anti-Malassezia monoclonal antibodies which block Mincle—Malassezia interaction are now under development.
Establishment of Mincle-Deficient Mice.
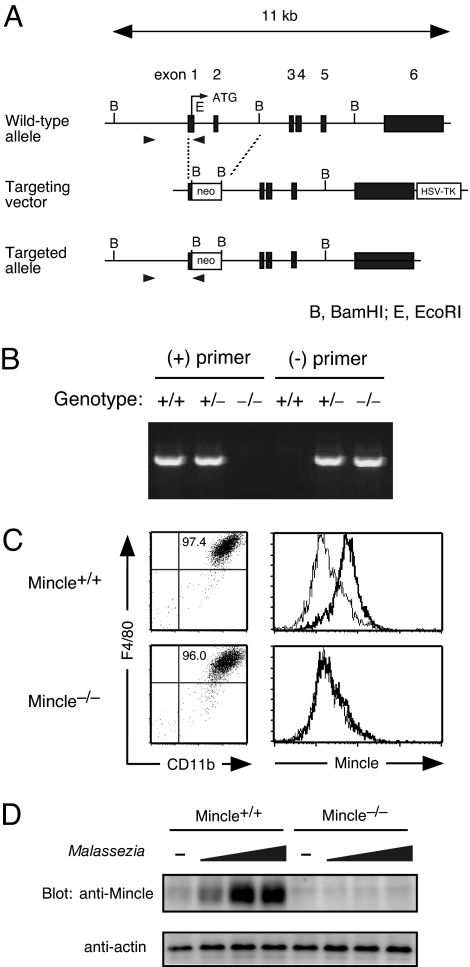
Finally, to investigate the role of Mincle in immune responses to Malassezia, we established Mincle-deficient mice (Fig. 2A). Mincle−/− mice were born following Mendelian law and showed no obvious abnormalities, and their cellularity and subpopulation of thymus, spleen, lymph node, and peritoneal cells were not altered (data not shown). BMMφ showed normal development in the absence of Mincle (Fig. 2B). However, surface Mincle expression was completely absent in Mincle−/− mice even after LPS stimulation (Fig. 2C). Malassezia stimulation markedly up-regulated Mincle protein expression in WT macrophages, suggesting that macrophages up-regulate a receptor for Malassezia after sensing the fungal bodies, possibly for the purpose of initiating immune response against this fungus (Fig. 2D). Importantly, the corresponding bands detected by anti-Mincle were completely lost in Mincle−/− mice (Fig. 2D).
Fig. 2.
Generation of Mincle-deficient mice. (A) Genomic Mincle structure and targeting constructs with neomycin resistance (Neo) insertion. The Mincle exons are shown as black box. (B) Genomic PCR analysis of Mincle-targeted allele. Genomic DNA isolated from +/+, +/− and −/− mice were amplified with primer pairs for wild-type allele (+) or targeted allele (−) as shown in arrowheads in (A). (C) Surface expression of Mincle. BMMφ from WT (Mincle+/+) and Mincle-deficient (Mincle−/−) mice were stimulated with 1 ng/ml LPS for 18 h and stained with anti-rIgG1-biotin (thin line) or anti-Mincle-biotin (thick line) and Streptavidine-APC. (D) Western blot analysis. Thioglycolate-elicited peritoneal macrophages from Mincle+/+ and Mincle−/− mice were left unstimulated (−) or stimulated with 1, 3, 10 × 106 of M. pachydermatis for 18 h. Cells were lysed and blotted with anti-Mincle and anti-actin mAbs as a control.
These results confirmed that Mincle protein expression was successfully disrupted in this mutant mouse, and also demonstrated that Mincle is dispensable for the development of macrophages as well as the hematopoietic lineage.
Crucial Role of Mincle in Malassezia-Mediated Immune Responses.
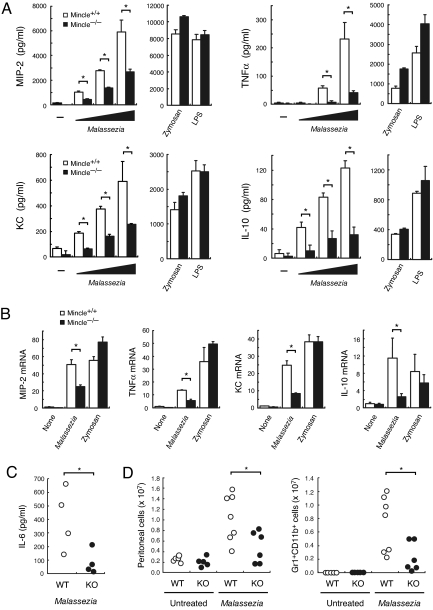
We also examined the immunostimulatory effect of Malassezia species on macrophages. Coculture of increasing amount of M. pachydermatis activated BMMφ to produce MIP-2, TNFα, KC, and IL-10 in a dose-dependent manner (Fig. 3A). The cytokine production was significantly impaired in Mincle−/− macrophages, whereas TLR-mediated stimuli such as zymosan and LPS induced comparable responses (Fig. 3A). We confirmed that the induction of inflammatory cytokine/chemokine mRNA expression was also significantly suppressed in Mincle−/− mice (Fig. 3B). These results indicate that Mincle is critical for macrophages to evoke inflammatory responses to Malassezia.
Fig. 3.
Mincle deficiency failed to respond to Malassezia. (A) Malassezia-induced cytokine production. Mincle+/+ and Mincle−/− BMMφ were stimulated with 1, 3, 10 × 106 of M. pachydermatis, 100 μg/ml zymosan or 1 ng/ml LPS for 18 h. *, P < 0.05. (B) Malassezia-induced cytokine transcription. BMMφ was stimulated with 10 × 106 M. pachydermatis or 100 μg/ml zymosan for 4 h, and mRNA expression was determined by real time PCR. Relative mRNA levels were expressed as fold induction. *, P < 0.05. (C) Mincle+/− (WT) and Mincle−/− (KO) mice were injected with 4 × 107 M. pachydermatis i.p. After 18 h, the peritoneal cavity was washed out with 5 ml of saline and cytokine concentration was determined by ELISA. Each symbol represents an individual mouse. Data are representative of two independent experiments. *, P < 0.05. (D) Mincle+/+ or Mincle+/− (WT) and Mincle−/− (KO) mice were injected with 2.5 × 107 M. pachydermatis i.p. At 20 h after injection, peritoneal cells were stained with CD11b and Gr1 and analyzed by flowcytometry. Total number of peritoneal cells (Left) and CD11b+Gr1+ cells (Right) were indicated. Data are representative of two independent experiments. *, P < 0.05.
Since Malassezia species possess multiple cell-wall components (19), they might also stimulate cytokine production through TLR engagement (20). Marginal induction of cytokines by Malassezia observed in Mincle−/− macrophages (Fig. 3 A-B) may reflect cooperative contribution of Mincle and TLR to Malassezia response, as is shown between Dectin-1 and TLR2 for the recognition of yeast (21). The analysis of mice lacking both Mincle and MyD88 (22) may clarify this issue. In addition, inducible expression of Mincle may suggest that Mincle plays a crucial role during late/chronic phase of the infection.
To demonstrate a role of Mincle in host defense against Malassezia in detail, additional experiment of in vivo Malassezia infection should be conducted. However, no such in vivo models with pathogenic invasion are available in mice so far, partly because Malassezia species are residents with weak pathogenicity in healthy animals. Instead, we injected WT and Mincle-deficient mice with M. pachydermatis i.p. and cytokine production in the peritoneal cavitiy was examined. Malassezia induced detectable amount of inflammatory cytokines, such as IL-6 and TNF (data not shown), in WT mice, whereas it was impaired in Mincle-deficient mice (Fig. 3C). Malassezia-induced neutrophil infiltration into peritoneal cavity was also significantly suppressed in Mincle-deficient mice (Fig. 3D).
In normal skin, Malassezia species can live as commensal. However, in atopic/eczema dermatitis syndrome and psoriasis, these fungi elicit an inflammatory response in the skin lesions (7). Mincle expression is up-regulated by several stresses (1), and indeed we observed that it was slightly induced on dermal dendritic cells upon stimulation with chemokines or LPS (data not shown). Therefore, it is likely that Mincle-induced inflammatory cytokine may contribute to the maintenance of the lesions.
Multiple diseases, such as tinea versicolor, atopic dermatitis, and lethal sepsis, have been shown to be caused by invasive infection of Malassezia fungus. The identification of Mincle as a specific receptor for Malassezia will provide valuable information for the development of therapy and effective drugs against Malassezia-related diseases.
Very recently, it was reported that Mincle is involved in response to C. albicans (23). Three strains of C. albicans were not recognized by Mincle in our reporter systems, although these strains differ from that used by Wells, et al. (23). This implies that Mincle may distinguish structural difference of the substrain of C. albicans. Alternatively, Mincle alone may not be sufficient to recognize C. albicans to induce cell activation and some other receptor(s) expressed in macrophages may cooperate with Mincle to recognize C. albicans.
Materials and Methods
Mice.
Mincle-deficient mice were established using R1 embryonic stem cells following a general procedure as described elsewhere (24), and used as C57BL/6 and 129 mixed genetic background. All mice were maintained in a filter-air laminar flow enclosure and provided standard laboratory food and water ad libitum. All animal experiments were performed in compliance with our institutional guidelines.
Cells.
Thioglycolate-elicited peritoneal macrophages and bone marrow-derived macrophages (BMMφ) were prepared as described elsewhere (1). Cytokine production was determined by ELISA or Meso Scale Discovery assay kit. Western blotting was performed as described previously (25).
Antibodies.
Anti-Mincle mAbs were established by immunizing Wistar rats with rat basophilic leukemia (RBL-2H3) cells expressing murine Mincle as described previously (2). Clone 1B6 (IgG1, κ) was used in this study.
Fungi.
The fungal strains and Institute of Food Microbiology (IFM) number used in this study (Table 1) were inoculated on potato dextrose agar (PDA; Difco Laboratories) slants and incubated for 3 to 14 days at 25 °C. Fungal spores or mycelia were collected and suspended in 0.85% NaCl or 0.1% Tween80 solution. Some Malassezia species were cultured on PDA in the presence of olive oil or on CHROMagar Malassezia Candida medium (CHROMagar).
Glycoarray.
Preparation of glycoarray, hybridization of Ig-fusion protein, and data analysis were performed as described (17).
RT-PCR.
Gene-specific primer sequences were as follows: MIP-2, 5′-GCTTCCTCGGGCACTCCAGAC-3′ (forward) 5′-TTAGCCTTGCCTTTGTTCAGTAT-3′ (reverse); TNFα, 5′-GCGACGTGGAACTGGCAGAAG-3′ (forward) 5′-GGTACAACCCATCGGCTGGCA-3′ (reverse); KC 5′-GCCAATGAGCTGCGCTGTCAATGC-3′ 5′-CTTGGGGACACCTTTTAGCATCTT-3′ (reverse); IL-10, 5′-TAGAGCTGCGGACTGCCTTCA-3′ (forward) 5′-TCATGGCCTTGTAGACACCTTG-3′ (reverse); β-actin, 5′-TGGAATCCTGTGGCATCCATGAAAC-3′ (forward) 5′-TAAAACGCAGCTCAGTAACAGTCCG-3′ (reverse).
Reagent.
LPS (L4516) and Zymosan (Z4250) were purchased from SIGMA. Candida albicans Cell wall mannan (MG001), Mannan from Yeast (21338–34) and D-Galacto-D-mannan from Ceratonia siliqua (48230) were from takara Bio Inc. and SIGMA and nacalai tesque, respectively. Recombinant M. furfur peroxisomal membrane protein (Mal f2) and Cyclophilin (Mal f6) were from takara Bio Inc.
Construct.
cDNAs for FcRγ and Mincle were cloned by PCR and inserted into pMX-IRES-rCD2 and pMX-IRES-hCD8 vectors (25), respectively.
Ig Fusion Protein.
The extracellular domain of Mincle (a.a. 46–214) was fused to the N terminus of hIgG Fc region by PCR and inserted into the XhoI fragment of pME18S-SLAMsig-hIgG Fc. The preparation of Ig fusion protein was described elsewhere (2). 293T cells were transiently transfected with pME18S-SLAMsig-hIgG Fc (Ig) or pME18S-SLAMsig-hIgG Fc-Mincle (Ig-Mincle). Cells were cultured in protein-free medium (PFHM-II). Filtered supernatant was applied to Protein A-Sepharose column and bound fraction was eluted with 50 mM diethylamine and immediately neutralized with Tris-HCl (pH 7.5). The major fraction was dialyzed against PBS and used as purified Ig fusion solution.
Supplementary Material
Acknowledgments.
We thank Drs. R. Tanaka, N. Shibata, and H. Hara for discussion; Drs. A. Mori, S. Seki, and Mr. T. Ishikawa for technical assistance; and Ms. H. Yamaguchi for secretarial help.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0805177106/DCSupplemental.
References
- 1.Matsumoto M, et al. A novel LPS-inducible C-type lectin is a transcriptional target of NF-IL6 in macrophages. J Immunol. 1999;163:5039–5048. [PubMed] [Google Scholar]
- 2.Yamasaki S, et al. Mincle is an ITAM-coupled activating receptor that senses damaged cells. Nat Immunol. 2008;9:1179–1188. doi: 10.1038/ni.1651. [DOI] [PubMed] [Google Scholar]
- 3.Flornes LM, et al. Identification of lectin-like receptors expressed by antigen presenting cells and neutrophils and their mapping to a novel gene complex. Immunogenetics. 2004;56:506–517. doi: 10.1007/s00251-004-0714-x. [DOI] [PubMed] [Google Scholar]
- 4.Saijo S, et al. Dectin-1 is required for host defense against Pneumocystis carinii but not against Candida albicans. Nat Immunol. 2007;8:39–46. doi: 10.1038/ni1425. [DOI] [PubMed] [Google Scholar]
- 5.Sato K, et al. Dectin-2 is a pattern recognition receptor for fungi that couples with the Fc receptor gamma chain to induce innate immune responses. J Biol Chem. 2006;281:38854–38866. doi: 10.1074/jbc.M606542200. [DOI] [PubMed] [Google Scholar]
- 6.Willment JA, Brown GD. C-type lectin receptors in antifungal immunity. Trends Microbiol. 2008;16:27–32. doi: 10.1016/j.tim.2007.10.012. [DOI] [PubMed] [Google Scholar]
- 7.Ashbee HR. Recent developments in the immunology and biology of Malassezia species. FEMS Immunol Med Microbiol. 2006;47:14–23. doi: 10.1111/j.1574-695X.2006.00057.x. [DOI] [PubMed] [Google Scholar]
- 8.Scheynius A, Johansson C, Buentke E, Zargari A, Linder MT. Atopic eczema/dermatitis syndrome and Malassezia. Int Arch Allergy Immunol. 2002;127:161–169. doi: 10.1159/000053860. [DOI] [PubMed] [Google Scholar]
- 9.Devlin RK. Invasive fungal infections caused by Candida and Malassezia species in the neonatal intensive care unit. Adv Neonatal Care. 2006;6:68–77. doi: 10.1016/j.adnc.2006.01.005. quiz 78–9. [DOI] [PubMed] [Google Scholar]
- 10.Ohtsuka M, et al. NFAM1, an immunoreceptor tyrosine-based activation motif-bearing molecule that regulates B cell development and signaling. Proc Natl Acad Sci USA. 2004;101:8126–8131. doi: 10.1073/pnas.0401119101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cambi A, et al. The C-type lectin DC-SIGN (CD209) is an antigen-uptake receptor for Candida albicans on dendritic cells. Eur J Immunol. 2003;33:532–538. doi: 10.1002/immu.200310029. [DOI] [PubMed] [Google Scholar]
- 12.Taylor PR, et al. Dectin-1 is required for beta-glucan recognition and control of fungal infection. Nat Immunol. 2007;8:31–38. doi: 10.1038/ni1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Steele C, et al. The beta-glucan receptor dectin-1 recognizes specific morphologies of Aspergillus fumigatus. PLoS Pathog. 2005;1:e42. doi: 10.1371/journal.ppat.0010042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yasueda H, et al. Identification and cloning of two novel allergens from the lipophilic yeast, Malassezia furfur. Biochem Biophys Res Commun. 1998;248:240–244. doi: 10.1006/bbrc.1998.8944. [DOI] [PubMed] [Google Scholar]
- 15.Lindborg M, et al. Selective cloning of allergens from the skin colonizing yeast Malassezia furfur by phage surface display technology. J Invest Dermatol. 1999;113:156–161. doi: 10.1046/j.1523-1747.1999.00661.x. [DOI] [PubMed] [Google Scholar]
- 16.Drickamer K. Engineering galactose-binding activity into a C-type mannose-binding protein. Nature. 1992;360:183–186. doi: 10.1038/360183a0. [DOI] [PubMed] [Google Scholar]
- 17.Tateno H, et al. Glycoconjugate microarray based on an evanescent-field fluorescence-assisted detection principle for investigation of glycan-binding proteins. Glycobiology. 2008;18:789–798. doi: 10.1093/glycob/cwn068. [DOI] [PubMed] [Google Scholar]
- 18.Cambi A, et al. Dendritic cell interaction with Candida albicans critically depends on N-linked mannan. J Biol Chem. 2008;283:20590–20599. doi: 10.1074/jbc.M709334200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mittag H. Fine structural investigation of Malassezia furfur. II. The envelope of the yeast cells. Mycoses. 1995;38:13–21. doi: 10.1111/j.1439-0507.1995.tb00003.x. [DOI] [PubMed] [Google Scholar]
- 20.Baroni A, et al. Toll-like receptor 2 (TLR2) mediates intracellular signalling in human keratinocytes in response to Malassezia furfur. Arch Dermatol Res. 2006;297:280–288. doi: 10.1007/s00403-005-0594-4. [DOI] [PubMed] [Google Scholar]
- 21.Brown GD. Dectin-1: A signalling non-TLR pattern-recognition receptor. Nat Rev Immunol. 2006;6:33–43. doi: 10.1038/nri1745. [DOI] [PubMed] [Google Scholar]
- 22.Kawai T, Adachi O, Ogawa T, Takeda K, Akira S. Unresponsiveness of MyD88-deficient mice to endotoxin. Immunity. 1999;11:115–122. doi: 10.1016/s1074-7613(00)80086-2. [DOI] [PubMed] [Google Scholar]
- 23.Wells CA, et al. The macrophage-inducible C-type lectin, mincle, is an essential component of the innate immune response to Candida albicans. J Immunol. 2008;180:7404–7413. doi: 10.4049/jimmunol.180.11.7404. [DOI] [PubMed] [Google Scholar]
- 24.Hemmi H, et al. The roles of two IkappaB kinase-related kinases in lipopolysaccharide and double stranded RNA signaling and viral infection. J Exp Med. 2004;199:1641–1650. doi: 10.1084/jem.20040520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yamasaki S, et al. Mechanistic basis of pre-T cell receptor-mediated autonomous signaling critical for thymocyte development. Nat Immunol. 2006;7:67–75. doi: 10.1038/ni1290. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.