Abstract
The mammalian immune response to infection is mediated by 2 broad arms, the innate and adaptive immune systems. Innate immune cells are a first-line defense against pathogens and are thought to respond consistently to infection, regardless of previous exposure, i.e., they do not exhibit memory of prior activation. By contrast, adaptive immune cells display immunologic memory that has 2 basic characteristics, antigen specificity and an amplified response upon subsequent exposure. Whereas adaptive immune cells have rearranged receptor genes to recognize the universe of antigens, natural killer (NK) cells are innate immune lymphocytes with a limited repertoire of germ-line encoded receptors for target recognition. NK cells also produce cytokines such as IFN-gamma (IFN-γ) to protect the host during the innate response to infection. Herein, we show that cytokine-activated NK cells transferred into naïve hosts can be specifically detected 7–22 days later when they are phenotypically similar to naïve cells and are not constitutively producing IFN-γ. However, they produce significantly more IFN-γ when restimulated. This memory-like property is intrinsic to the NK cell. By contrast, memory-like NK cells do not express granzyme B protein and kill targets similarly to naïve NK cells. Thus, these experiments identify an ability of innate immune cells to retain an intrinsic memory of prior activation, a function until now attributed only to antigen-specific adaptive immune cells.
Keywords: innate immunity, interferon, NK cells
The mammalian immune system has evolved to comprise both innate and adaptive immune responses to efficiently control the vast array of pathogens encountered daily. Although the adaptive system provides long-lasting specific immunity, the first line of defense against pathogens is the innate immune system. Indeed, defects in innate immunity, including natural killer (NK) cells, often lead to uncontrolled, fatal infections (1–3). NK cells are innate lymphocytes capable of recognizing and killing target cells and producing immunoregulatory cytokines, especially IFN-γ (4). In concert with other members of the innate response, NK cells are important for the initial control of many viral and bacterial pathogens (5–7). Unlike adaptive T and B lymphocytes, NK cells do not somatically rearrange their receptor genes, but rely upon a finite number of germ line-encoded inhibitory and activating NK receptors capable of recognizing MHC class I and class I-like molecules (8, 9). Engagement of self-MHC class I by inhibitory NK receptors prevents NK cell killing of normal cells, and NK cell activity is dictated by a complex integration of signals from both inhibitory and activating NK receptors (10). There are a few examples of NK receptors that recognize specific antigens, most notably murine Ly49H, which recognizes the virally-encoded ligand m157 (11). However, the overall NK receptor repertoire is very limited. Thus, under many circumstances NK cells use other activation signals, including dendritic cell-derived cytokines (12, 13).
One classical distinction between innate and adaptive immunity is the limitation of immunologic memory to adaptive T and B lymphocytes. Memory has 2 primary features, antigen specificity and an amplified response upon subsequent antigen exposure. Cellular components of the innate immune system have a small repertoire of recognition receptors and are thought to react in a similar manner upon repeated stimulation. However, a prior study suggests that NK cells may exhibit memory-like properties, because they were shown to mediate a hapten-specific contact hypersensitivity-like reaction in mice lacking T and B lymphocytes (14). The mechanism of NK cell activation is unclear in this model and whether the observed memory-like property is intrinsic to the NK cell is unknown. When considering the possibility of a memory-phenotype among innate immune cells, such as NK cells, it is important to note that current concepts of memory are built upon studies of adaptive immune lymphocytes, which recognize a limitless number of antigens. However, does this definition of memory confine its functional consequence, i.e., the capacity to respond more robustly upon repeated stimulation, only to antigen-specific immune cells?
In the current study, we explored whether NK cells exhibit innate immune memory with more robust responses upon restimulation. NK cells are short-lived in culture (15), and an inability to maintain them long-term in vitro without high-doses of cytokines has made it technically challenging to assess their potential to exhibit memory because they cannot revert to a resting state in vitro. Herein, we used an in vivo adoptive transfer system to study NK cell responses to reactivation. We found that activated NK cells return to what appears to be a quiescent state after transfer into naïve hosts. However, NK cells with a history of prior activation by cytokines display an intrinsic capacity to respond more robustly after reactivation with cytokines or via engagement of activating NK receptors. These studies suggest an ability of NK cells to retain memory of prior activation, indicating that innate immune cells can have memory-like properties.
Results
Cytokine-Activated NK Cells Adoptively Transferred into Naïve Hosts Are Phenotypically Similar to Control NK Cells.
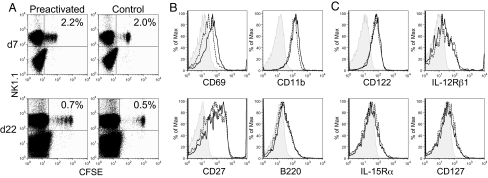
Splenic NK cells were enriched by negative selection (60–95% purity by NK1.1+) from Rag1−/− hosts (which lack T and B cells) and cultured overnight (13–15 h) with a combination of IL-12 (10 ng/mL) and IL-18 (50 ng/mL) plus low-dose IL-15 (10 ng/mL) as a survival factor (15) (activated) or with low-dose IL-15 alone (control). Activation with IL-12 and IL-18 (plus low-dose IL-15) induced >90% of NK cells to produce IFN-γ protein (Fig. S1), indicating that the vast majority of these cells were activated at the time of adoptive transfer. By contrast, overnight culture with IL-15 alone did not stimulate IFN-γ production (<2% positive NK cells, see Fig. S1). Cells were washed at least 4 times to remove cytokines, labeled with CFSE, and transferred i.v. into Rag1−/− hosts. Donor-derived, CFSE+NK1.1+ NK cells were easily detected 1 to 3 weeks later (Fig. 1A). Donor-derived splenic NK cells that were previously-activated (preactivated) or control-transferred NK cells were phenotypically similar with regard to expression of NK cell activation markers CD69, CD11b, CD11c, gp49B, and B220 (Fig. 1B and Fig. S2) and cytokine receptors CD122 (IL2/15 Rβ chain), IL-15Rα, IL-12Rβ1, and CD127 (Fig. 1C). In addition, we did not observe a preferential expansion of CD27hi NK cells (Fig. 1B), a subset of cells known to have an enhanced capacity for cytokine production (16). Similar percentages of preactivated vs. control donor-derived CFSE+ NK cells were found in the spleen and liver, whereas a higher percentage of preactivated NK cells than control NK cells trafficked to the lymph nodes 7 days after adoptive transfer (Fig. S3). Interestingly, NK cells with a history of prior activation proliferated in vivo (Fig. 2A) whereas control-treated cells did not (40.5% v. 6.3% of recovered cells proliferated). We did not observe any additional proliferation between 7 and 22 days, suggesting that previous activation with cytokines is responsible for early NK cell proliferation independent of continued activation. Otherwise, there was no obvious distinguishing phenotype of previously-activated NK cells.
Fig. 1.
Adoptively transferred, preactivated, and control NK cells can be detected after 1–3 weeks and are phenotypically similar. (A) CFSE-labeled preactivated (IL-12 + IL-18 with 10 ng/mL IL-15) or control-treated (10 ng/mL IL-15) NK cells were transferred into Rag1−/− hosts and could be detected 7 (Upper) to 22 (Lower) days later in the spleen, identified as CFSE+NK1.1+. Percentages represent transferred NK cells, gated on live lymphocytes. (B) Preactivated (solid black line) and control (dashed line) NK cells express similar levels of NK activation markers (CD69 and CD11b), CD27, and B220. (C) Cytokine receptor expression 7 days after adoptive transfer (isotype control, shaded). Results are representative of 2–5 independent experiments.
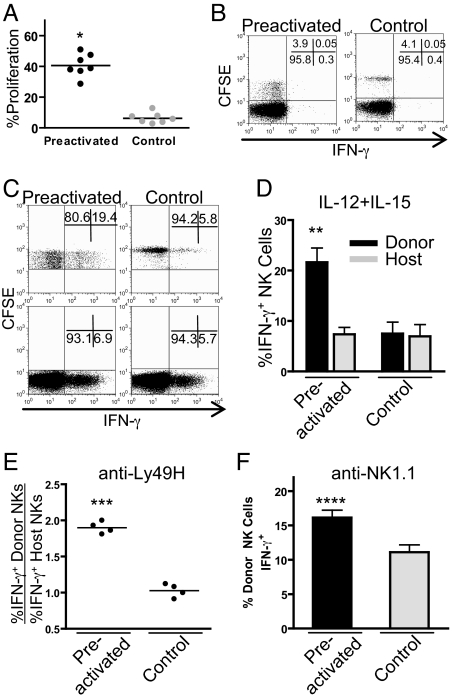
Fig. 2.
Preactivated NK cells proliferate in vivo and do not constitutively make IFN-γ but produce abundant IFN-γ upon restimulation. (A) NK cells preactivated with IL-12 + IL-18 (plus 10 ng/mL IL-15) proliferate in vivo significantly more than control-treated (10 ng/mL IL-15) cells (40.5% v. 6.3%;*, P < 0.0001; n = 7). (B) Preactivated (Left) donor-derived (CFSE+) and host (CFSE−) NK cells do not constitutively produce IFN-γ 7 days after adoptive transfer. (C) Representative FACS plot of CFSE+ donor NK cell (Upper) and CFSE− host NK cell (Lower) IFN-γ production (x axis) after restimulation with IL-12 + IL-15 for 4 h. All plots are gated on NK1.1+ NK cells. The numbers indicate the percentage of CSFE+ (donor) cells (Upper) or CSFE− (host) cells (Lower) in the corresponding gates, demonstrating significantly more preactivated NK cells produce IFN-γ upon restimulation. (D) Percentage of IFN-γ positive CFSE+ (donor) and CFSE− (host) NK cells after stimulation with IL-12 + IL-15 (**, P < 0.01, n = 4). (E and F) Engagement of Ly49H (E) or NK1.1 (F) by culture with plate-bound monoclonal antibody for 8 h (***, P < 0.0001; ****, P = 0.004). IFN-γ production with anti-Ly49H is shown as a ratio of % IFN-γ positive donor NK cells (CFSE+NK1.1+) to % IFN-γ positive host NK cells (CFSE−NK1.1+) to account for well-to-well variability. Error bars indicate SEM.
Previously Activated NK Cells Respond More Robustly to Reactivation 1 to 3 Weeks Later.
NK cells activated with IL-12 + IL-18 produce abundant IFN-γ at the time of adoptive transfer into naïve Rag1−/− mice, but 7 days after transfer these previously-activated donor NK cells do not spontaneously produce detectable IFN-γ protein (Fig. 2B). However, these cells respond more robustly upon restimulation with cytokines (IL-12 + IL-15, Fig. 2 C and D) or via engagement of activating NK cell receptors (Ly49H and NK1.1) with plate-bound antibody (Fig. 2 E and F). By comparison, unactivated control-transferred cells had a response similar to endogenous host NK cells.
This was an NK-intrinsic effect because there was no difference in IFN-γ production by host NK cells from mice that received previously-activated NK cells versus recipients of control NK cells. In addition, transfer of activated NK cells sorted by flow cytometry (>97% purity) rather than enriched NK cells resulted in similar findings (Fig. S4A). An enhanced capacity for IFN-γ production by previously-activated NK cells persisted for at least 3 weeks after transfer (Fig. S4B), suggesting this is a long-lived response considering that the half-life of NK cells is ≈7 days (17). Thus, cytokine activation of NK cells leads to differentiation of cells with the capacity to respond more robustly upon restimulation, a key attribute of immunologic memory, suggesting that these are memory-like NK cells.
Interestingly, the length of time of the initial NK cell activation had an effect on both in vivo proliferation and subsequent capacity for IFN-γ production. NK cells initially activated for only 5 h before adoptive transfer did proliferate in vivo and respond more robustly to restimulation as compared with control-treated NK cells, however, this response was less robust than cells preactivated overnight (Fig. S5). These data suggest a time of activation effect, whereby NK cell memory-like responses are proportional to the length of time of the initial activation. Alternatively, an increased activation time may allow for a higher percentage of NK cells to differentiate into memory-like cells. Because NK cells cannot survive overnight in vitro without IL-15 (15), we were also able to test the requirement for low-dose IL-15 in the differentiation of memory-like NK cells with this 5-hour preactivation. Our results demonstrate that NK cells preactivated with IL-12 + IL-18 with or without low-dose IL-15 for 5 h had an enhanced capacity to produce IFN-γ and proliferated 7 days after adoptive transfer into wild-type C57BL/6 hosts (Fig. S5).
Prior Activation of NK Cells Does Not Result in Enhanced Cytotoxicity.
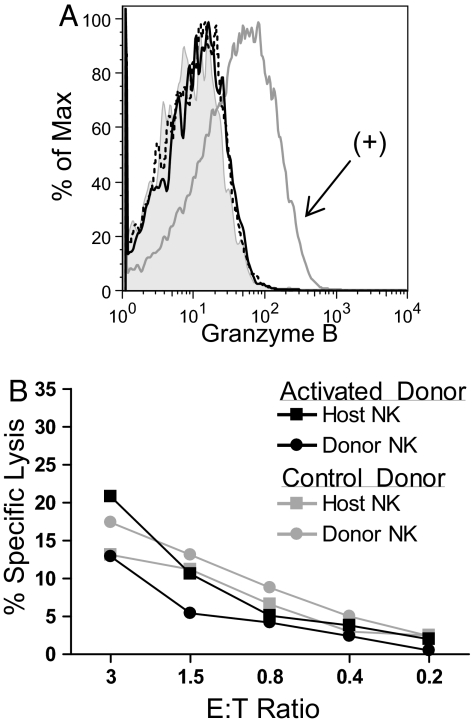
To determine whether memory-like NK cells are also more cytotoxic, we evaluated expression of granzyme B, a protein necessary for NK cell killing, in memory-like and control-transferred NK cells at 7 days. Neither population of NK cells expressed granzyme B protein (Fig. 3A), suggesting that these cells are not especially cytotoxic (18). Indeed, when donor memory-like and control NK cells and host NK cells were sorted by flow cytometry 7 days after adoptive transfer and tested for their ability to kill YAC-1 targets, all 4 groups of NK cells exhibited similar cytotoxicity (Fig. 3B). Thus, memory-like NK cells acquire a selective capacity to produce IFN-γ but do not constitutively up-regulate the machinery to kill and are no more cytotoxic than control NK cells.
Fig. 3.
Memory-like NK cells lack granzyme B protein and kill target cells similar to control NK cells. (A) Preactivated, memory-like NK cells (solid black line) do not express granzyme B protein 7 days after adoptive transfer into Rag1−/− hosts as compared with a positive control, NK cells activated with high-dose (100 ng/mL) IL-15 for 2 days. (Dashed line, control-treated NK cells; shaded, negative control; gray line, positive control). (B) Donor and host NK cells were sorted from recipients that had received activated or control NK cells 7 days prior and were used as effectors in a cytotoxicity assay with Yac-1 targets at the indicated effector to target (E:T) ratios. Data represent the mean of duplicate wells and results are representative of 2 independent experiments.
Capacity for Enhanced NK Cell IFN-γ Production Is Not Related to a History of Proliferation and Is Passed on to Daughter Cells.
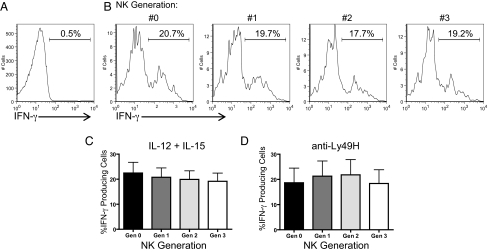
One potential mechanism for enhanced NK cell IFN-γ production by memory-like NK cells is the possibility that cellular division allows for transcriptional or epigenetic changes. This possibility predicts that enhanced cytokine-secretion would only be seen in those cells with a history of proliferation. To test this, we identified parental (generation 0) and daughter generations (generations 1–3) of preactivated NK cells based on CFSE dilution and analyzed each population for IFN-γ production after restimulation (Fig. 4). Similar percentages of IFN-γ-producing cells were seen among previously-activated NK cells that had never proliferated as compared with those that had undergone up to 3 divisions. Thus, the robust IFN-γ response of memory-like NK cells is not dependent on proliferation and is an intrinsic property of this NK cell population, which appears to be passed on to subsequent generations.
Fig. 4.
Similar IFN-γ production by memory-like NK cells regardless of proliferation. (A) Resting NK cells did not produce IFN-γ protein. (B) After restimulation with IL-12 + IL-15, parental (generation 0) and 3 daughter generations of preactivated memory-like NK cells were identified based on CFSE dilution and IFN-γ production in each generation measured 7 days after adoptive transfer. (C and D) There was no significant difference in IFN-γ production in generations 0–3 after restimulation with either IL-12 + IL-15 (C) or anti-Ly49H plate-bound antibody (D). Results represent the mean of 3 independent experiments. Error bars indicate SEM.
Discussion
In this study we demonstrate that based on a prior experience, NK cells can fundamentally change the way they respond to later activation, a memory-like property. NK cells with a history of prior cytokine activation have an NK-intrinsic, enhanced capacity to produce IFN-γ upon restimulation that is not dependent on proliferation. These findings do not rule out the potential for antigen-driven NK cell memory, but strongly suggest it is not required for this memory-like property.
Previously-activated NK cells do not spontaneously produce cytokines 1–3 weeks after adoptive transfer. However, these NK cells produced significantly more IFN-γ protein when restimulated 1–3 weeks later as compared with control-treated NK cells. Memory-like NK cells were induced here by culture with IL-12 and IL-18 in the presence of low-dose IL-15 as a survival factor, a stimulation that results in >90% of cells producing IFN-γ before adoptive transfer. Several groups have recently shown that freshly isolated NK cells are poorly cytotoxic and do not spontaneously produce cytokines without “arming” or “priming” via signals from IL-15 during infection or endogenous IL-18 (18–20). However, our findings suggest that cytokine-activation of NK cells that leads to NK cell IFN-γ production results in long-term changes in the original NK cell and daughter cells that were never exposed to high doses of cytokine in vitro. Thus, based on prior activation, we propose that NK cells can differentiate into a stable memory-like state, whereby the subsequent behavior of an NK cell is changed, a process distinct from short-term arming/priming during an infection.
Cytokine-activated NK cells showed slight differences in trafficking and proliferated in vivo after removal of IL-12 and IL-18 and adoptive transfer into naïve hosts, suggesting that NK cells receiving activation signals during a local infection might traffic to other, noninflamed sites, and subsequently proliferate. It is possible that we were unable to detect some NK cells due to proliferation and CFSE dilution, resulting in the inclusion of these cells among the host NK cell pool. However, this technical issue does not alter the conclusions of this study because identification of these cells would have enhanced the memory-like effect. Moreover, these cells were likely to be few in number as they did not change the response of the host NK cell pool. Regardless, proliferation per se was not required for differentiation of memory-like NK cells as we observed similar percentages of NK cells producing IFN-γ in parental and daughter generations after restimulation. These data suggest that the mechanism of generating NK memory-like properties does not require the NK cell to enter cell cycle and is heritable. This aspect is in contrast to CD4+ T cells, in which proliferation was associated with an enhanced capacity to produce IFN-γ protein mediated by epigenetic modifications (21).
NK cell production of IFN-γ appears to be regulated at the posttranscriptional level. Using an IFN-γ reporter mouse, Stetson et al. (22) demonstrated that NK cells express IFN-γ transcript, but not protein, during early development in vivo. The mechanism of NK cell posttranscriptional regulation of IFN-γ is unclear (23), but may provide a means for NK cells to rapidly produce protein when needed. Indeed, NK cell activation with high doses of IL-15 was shown to release a translational block of perforin and granzyme B mRNAs allowing translation of preformed mRNAs and subsequent protein production (18), suggesting that posttranscriptional regulation may be a common mechanism of controlling NK cell function. Consistent with this possibility, we did not detect any differences in the amount of IFN-γ transcript in memory-like NK cells compared with naïve host NK cells (Fig. S6), indicating that excess IFN-γ transcript is not responsible for memory-like NK cell function. Thus, further elucidation of the mechanisms of NK cell IFN-γ regulation may also shed light on the mechanism underlying the phenotype of memory-like NK cells.
The innate immune system has increasingly been recognized to be more intricate and sophisticated than simply a primitive response blindly firing during infection. For example, innate immune cells can orchestrate specific immune responses to infection by recognition of pathogens through germ line encoded receptors such as toll-like receptors (TLRs) (24). In addition, studies by Kurtz and Franz have revealed specific memory by the innate immune system of invertebrates (25, 26). Here, we have discovered another layer of complexity to the innate immune response with the finding that NK cells can develop memory-like properties based on prior activation. These amplified NK cell responses are likely important during the early response to pathogens and it may be possible to boost the NK cell response to subsequent infection by stimuli that result in the memory-like NK cell phenotype.
Our data have a number of broader implications. By inducing a permanent and heritable change, the process of memory-like NK cell differentiation might result in a population of experienced NK cells with enhanced function not dependent on constant stimulation. A related possibility is that the potential pathogens encountered by the host on a regular basis may serve to differentiate and continually renew a pool of memory-like NK cells that have enhanced responses to infectious challenge. Inasmuch as NK cells are innate immune cells, perhaps other immune cells similarly respond more robustly after initial exposure to stimuli. For example, cytokine production by dendritic cells may be more efficient in those cells that had been previously stimulated. For adaptive immunity, our studies also suggest that part of the amplified response by T memory cells could be due to processes that may resemble those that regulate the memory-like NK cell response. In other words, robust T memory responses may be due to T cell receptor-independent factors that may be distinguishable from antigen-specificity per se. Interestingly, memory CD8 T cells were protective against Listeria monocytogenes infection without regard to antigen but rather in response to early, innate, IL-12 and IL-18 production (27), suggesting that antigen-specificity is not always required by memory lymphocytes. Finally, our findings may lead to development of new therapeutics that can harness the potential of inducing more robust responses to augment pathogen defenses or throttling autoimmune phenomena. Consequently, our finding of memory-like NK cell function may be more broadly applicable to understanding of adaptive and innate immunity.
Materials and Methods
Mice.
B6.129 Rag1 deficient (Rag1−/−) mice were purchased from The Jackson Laboratory and bred at our institution. Mice were housed in specific pathogen free conditions and were used in accordance with institutional guidelines for animal experimentation. All mice were used between 6 and 12 weeks of age.
Antibodies and Flow Cytometry.
Antibodies to the following antigens were obtained from BD PharMingen: anti-NK1.1 (PK136); anti-IFN-γ (XMG1.2); anti-CD69 (H1.2F3); CD11b (M1/70); anti-CD11c (HL3); anti-CD122 (TM-β1), IL-12Rβ1 (clone 114), anti-CD27 (LG.3A10). Antibodies to IFN-γ (XMG1.2), CD127 (A7R34), CD62L (MEL-14), CD117 (2B8), and NKG2D (CX5) were purchased from eBioscience. Anti-IL-15Rα (BAF551) was from R&D Systems. Anti-B220 (RA3–62) was purchased from Caltag. Anti-gp49B (H1.1) was produced in our laboratory as described in ref. 28. Nonspecific antibody binding was blocked with 2.4G2 (anti-FcγRII/III, American Type Culture Collection). All flow cytometry data were collected on FACSCanto and FACSCalibur machines (BD Biosciences).
NK Cell Isolation and Adoptive Transfer.
NK cells were enriched from Rag1−/− splenocytes by negative selection using a mixture of antibodies including anti-CD19, anti-CD4, anti-CD8a, anti-CD5, anti-Gr1, and anti-Ter-119 (Miltenyi Biotec). NK cells were 60–95% pure by NK1.1 staining. Alternatively, NK cells from Rag1−/− mice were sorted by flow cytometry to >98% purity based on NK1.1 positivity. NK cells were activated with IL-12 (10 ng/mL, PeproTech), and IL-18 (50 ng/mL, Medical and Biological Laboratories Co.) in the presence of low-dose IL-15 (10 ng/mL, PeproTech), or control-treated with IL-15 alone overnight (13–15 h). In separate experiments, NK cells were cultured for 5 h with cytokines before adoptive transfer (SI Materials and Methods). The next day cells were harvested, washed a total of 4 times in PBS (PBS), and labeled with 1 μM CFSE (Invitrogen) or for 22 day experiments NK cells were labeled with 2.5 μM CFSE. Equal numbers of CFSE-labeled NK cells (≈1.5–3 × 106) were adoptively transferred into Rag1−/− mice by tail vein injection.
Cytokine Stimulation Assays and Intracellular Flow Cytometry.
Splenocytes from adoptive transfer hosts were cultured with IL-12 (10 ng/mL) + IL-15 (100 ng/mL) for 4 h or plate-bound antibody, anti-Ly49H (clone 3D10, 5 μg/mL) or anti-NK1.1 (clone PK136, 5 μg/mL), for 8 h. Brefeldin-A was added after the first hour, and at the end of the culture IFN-γ protein was measured by intracellular flow cytometry gating on total donor (CFSE+NK1.1+) or host (CFSE−NK1.1+) NK cells as described in ref. 29.
Granzyme B Intracellular Flow Cytometry.
Splenocytes from adoptive transfer hosts were isolated and immediately surface stained for NK1.1 followed by fixation and permeabilization (BD PharMingen) and intracellular staining for granzyme B with anti-granzyme B (clone GB12, Caltag). NK cells from Rag1−/− mice activated in vitro with high-dose IL-15 (100 ng/mL) for 48 h served as a positive control for granzyme B staining (18).
Cytotoxicity Assays.
Donor-derived and host NK cells were purified by flow cytometric sorting (Siteman Cancer Center High Speed Cell Sorter Core) as NK1.1+CFSE+ or NK1.1+ respectively and used as effectors in killing assays as described in ref. 30. Briefly, 5 × 103 YAC-1 targets were labeled with 51Cr (as Na2CrO4) and incubated for 4 h with the sorted effectors at the indicated effector to target (E:T) ratios.
Statistical Analysis and Flow Cytometric Analysis.
Student's t test was used for statistical analyses between 2 groups and a 1-way ANOVA test was used to compare 3 or more groups with P < 0.05 considered significant. All flow cytometric analysis, including proliferation analysis, was performed with FlowJo software (Tree Star).
Supplementary Material
Acknowledgments.
We thank Emil Unanue for critical review of this manuscript. Work in the W.M.Y. laboratory is supported by the Howard Hughes Medical Institute and National Institute of Health Grants AI34385, AI33903, and AI51345. M.A.C. is supported by the National Institutes of Health under National Institute of Child Health and Human Development Ruth L. Kirschstein National Research Service Award T32 HD043010.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/cgi/content/full/0813192106/DCSupplemental.
References
- 1.Biron CA, Byron KS, Sullivan JL. Severe herpesvirus infections in an adolescent without natural killer cells. N Engl J Med. 1989;320:1731–1735. doi: 10.1056/NEJM198906293202605. [DOI] [PubMed] [Google Scholar]
- 2.Orange JS. Human natural killer cell deficiencies. Curr Opin Allergy Clin Immunol. 2006;6:399–409. doi: 10.1097/ACI.0b013e3280106b65. [DOI] [PubMed] [Google Scholar]
- 3.Bustamante J, et al. Novel primary immunodeficiencies revealed by the investigation of paediatric infectious diseases. Curr Opin Immunol. 2008;20:39–48. doi: 10.1016/j.coi.2007.10.005. [DOI] [PubMed] [Google Scholar]
- 4.Yokoyama WM. Mistaken notions about natural killer cells. Nat Immunol. 2008;9:481–485. doi: 10.1038/ni1583. [DOI] [PubMed] [Google Scholar]
- 5.Biron CA, Brossay L. NK cells and NKT cells in innate defense against viral infections. Curr Opin Immunol. 2001;13:458–464. doi: 10.1016/s0952-7915(00)00241-7. [DOI] [PubMed] [Google Scholar]
- 6.French AR, Yokoyama WM. Natural killer cells and viral infections. Curr Opin Immunol. 2003;15:45–51. doi: 10.1016/s095279150200002x. [DOI] [PubMed] [Google Scholar]
- 7.Lodoen MB, Lanier LL. Natural killer cells as an initial defense against pathogens. Curr Opin Immunol. 2006;18:391–398. doi: 10.1016/j.coi.2006.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yokoyama WM, Plougastel BF. Immune functions encoded by the natural killer gene complex. Nat Rev Immunol. 2003;3:304–316. doi: 10.1038/nri1055. [DOI] [PubMed] [Google Scholar]
- 9.Lanier LL. NK cell recognition. Annu Rev Immunol. 2005;23:225–274. doi: 10.1146/annurev.immunol.23.021704.115526. [DOI] [PubMed] [Google Scholar]
- 10.Bryceson YT, Long EO. Line of attack: NK cell specificity and integration of signals. Curr Opin Immunol. 2008;20:344–352. doi: 10.1016/j.coi.2008.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Scalzo AA, Yokoyama WM. Cmv1 and natural killer cell responses to murine cytomegalovirus infection. Curr Top Microbiol Immunol. 2008;321:101–122. doi: 10.1007/978-3-540-75203-5_5. [DOI] [PubMed] [Google Scholar]
- 12.Andrews DM, Scalzo AA, Yokoyama WM, Smyth MJ, Degli-Esposti MA. Functional interactions between dendritic cells and NK cells during viral infection. Nat Immunol. 2003;4:175–181. doi: 10.1038/ni880. [DOI] [PubMed] [Google Scholar]
- 13.Cooper MA, Fehniger TA, Fuchs A, Colonna M, Caligiuri MA. NK cell and DC interactions. Trends Immunol. 2004;25:47–52. doi: 10.1016/j.it.2003.10.012. [DOI] [PubMed] [Google Scholar]
- 14.O'Leary JG, Goodarzi M, Drayton DL, von Andrian UH. T cell- and B cell-independent adaptive immunity mediated by natural killer cells. Nat Immunol. 2006;7:507–516. doi: 10.1038/ni1332. [DOI] [PubMed] [Google Scholar]
- 15.Cooper MA, et al. In vivo evidence for a dependence on interleukin 15 for survival of natural killer cells. Blood. 2002;100:3633–3638. doi: 10.1182/blood-2001-12-0293. [DOI] [PubMed] [Google Scholar]
- 16.Hayakawa Y, Smyth MJ. CD27 dissects mature NK cells into two subsets with distinct responsiveness and migratory capacity. J Immunol. 2006;176:1517–1524. doi: 10.4049/jimmunol.176.3.1517. [DOI] [PubMed] [Google Scholar]
- 17.Koka R, et al. Interleukin (IL)-15Rα-deficient natural killer cells survive in normal but not IL-15Rα-deficient mice. J Exp Med. 2003;197:977–984. doi: 10.1084/jem.20021836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fehniger TA, et al. Acquisition of murine NK cell cytotoxicity requires the translation of a pre-existing pool of granzyme B and perforin mRNAs. Immunity. 2007;26:798–811. doi: 10.1016/j.immuni.2007.04.010. [DOI] [PubMed] [Google Scholar]
- 19.Lucas M, Schachterle W, Oberle K, Aichele P, Diefenbach A. Dendritic cells prime natural killer cells by trans-presenting interleukin 15. Immunity. 2007;26:503–517. doi: 10.1016/j.immuni.2007.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chaix J, et al. Cutting edge: Priming of NK cells by IL-18. J Immunol. 2008;181:1627–1631. doi: 10.4049/jimmunol.181.3.1627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bird JJ, et al. Helper T cell differentiation is controlled by the cell cycle. Immunity. 1998;9:229–237. doi: 10.1016/s1074-7613(00)80605-6. [DOI] [PubMed] [Google Scholar]
- 22.Stetson DB, et al. Constitutive cytokine mRNAs mark natural killer (NK) and NK T cells poised for rapid effector function. J Exp Med. 2003;198:1069–1076. doi: 10.1084/jem.20030630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Young HA, Bream JH. IFN-gamma: Recent advances in understanding regulation of expression, biological functions, and clinical applications. Curr Top Microbiol Immunol. 2007;316:97–117. doi: 10.1007/978-3-540-71329-6_6. [DOI] [PubMed] [Google Scholar]
- 24.Janeway CA, Jr, Medzhitov R. Innate immune recognition. Annu Rev Immunol. 2002;20:197–216. doi: 10.1146/annurev.immunol.20.083001.084359. [DOI] [PubMed] [Google Scholar]
- 25.Kurtz J, Franz K. Innate defence: Evidence for memory in invertebrate immunity. Nature. 2003;425:37–38. doi: 10.1038/425037a. [DOI] [PubMed] [Google Scholar]
- 26.Kurtz J. Specific memory within innate immune systems. Trends Immunol. 2005;26:186–192. doi: 10.1016/j.it.2005.02.001. [DOI] [PubMed] [Google Scholar]
- 27.Berg RE, Crossley E, Murray S, Forman J. Memory CD8+ T cells provide innate immune protection against Listeria monocytogenes in the absence of cognate antigen. J Exp Med. 2003;198:1583–1593. doi: 10.1084/jem.20031051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang LL, Chu DT, Dokun AO, Yokoyama WM. Inducible expression of the gp49B inhibitory receptor on NK cells. J Immunol. 2000;164:5215–5220. doi: 10.4049/jimmunol.164.10.5215. [DOI] [PubMed] [Google Scholar]
- 29.Kim S, et al. In vivo developmental stages in murine natural killer cell maturation. Nat Immunol. 2002;3:523–528. doi: 10.1038/ni796. Epub 2002 May 2013. [DOI] [PubMed] [Google Scholar]
- 30.Smith HR, et al. Nonstochastic coexpression of activation receptors on murine natural killer cells. J Exp Med. 2000;191:1341–1354. doi: 10.1084/jem.191.8.1341. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.