Abstract
Hematopoiesis is a tightly controlled process maintained by a small pool of hematopoietic stem cells (HSCs). Here, we demonstrate that the LT-HSC, MPP, premegakaryocytic/erythroid, Pre CFU-E, Pre GM, MkP, and granulocyte-macrophage compartments were all significantly reduced in E2A-deficient bone marrow. Despite a severe depletion of erythroid progenitors, the erythrocyte and megakaryocyte compartments were equivalent in E2A-deficient bone marrow as compared with wild-type mice. E2A-deficient HSCs also failed to efficiently maintain the HSC pool on serial transplantation, and we demonstrate that the E2A proteins regulate cell cycle progression of HSCs by regulating the expression of p21Cip1, p27Kip1, and the thrombopoietin receptor, known regulators of HSC self-renewal activity. Based on these observations, we propose that the E2A proteins promote the developmental progression of the entire spectrum of early hematopoietic progenitors and to suppress an erythroid specific program of gene expression in alternative cell lineages. Last, the data mechanistically link E2A, cell cycle regulators, and the maintenance of the HSC pool in a common pathway.
Keywords: E47, lymphoid/myeloid versus erythroid/megakaryocyte development, self-renewal
Hematopoiesis is a tightly regulated process maintained by a small pool of hematopoietic stem cells (HSC) uniquely capable of undergoing self-renewal and generating mature progeny of all of the hematopoietic cell lineages. To sustain the proper levels of blood cells, HSCs must continuously monitor and regulate the balance between self-renewal and lineage differentiation. Following the decision to differentiate, hematopoiesis proceeds in a step-wise manner from the primordial long-term (LT)-HSCs. LT-HSCs possess the ability to self-renew and the capacity for long-term reconstitution of lethally irradiated hosts (1). Upon differentiation, LT-HSCs lose their capacity for self-renewal and give rise to a population of short-term (ST)-HSCs. The ST-HSCs, limited to a transient ability to self-renew and reconstitute lethally irradiated hosts, differentiate into a multipotent progenitor (MPP) population. The MPPs lack the capacity to undergo self-renewal, but retain multipotentcy. From the MPP population develops a series of intermediate progenitors that give rise to the assorted hematopoietic lineages. In the classical pathway of hematopoiesis, these intermediates include the common lymphoid progenitors (CLPs) that differentiate into lymphoid, but not myeloid, progeny, and the common myeloid progenitors (CMPs), which retain full erythromyeloid potential (2, 3). The CMPs further differentiate to form the granulocyte/macrophage progenitors (GMPs) that differentiate to the myelomonocytic lineage and the megakaryocytic/erythrocyte progenitors (MEPs) that eventually differentiate to form red blood cells and platelets.
Other studies have suggested alternative pathways for the differentiation of the megakaryocytic/erythroid versus lymphoid/myeloid cell lineages. Specifically, these studies have indicated that the separation of the myeloid versus megakaryocytic/erythroid cell lineages may occur at an earlier branch point before the development of CMPs. This work has suggested the presence of lymphoid-primed (L) MPPs in the bone marrow, which have the ability to develop into lymphoid and myeloid progeny, but cannot give rise to erythroid and megakaryocytic cells (4). Also, more recent studies have used additional markers, including CD105 and CD41, to separate the myeloerythroid progenitors into Pre GM, GMP, premegakaryocytic/erythroid (Pre MegE), Pre CFU-E, CFU-E, and MkP compartments. These studies suggested a hierarchy, in which the Pre GM population gives rise to the GMP compartment, whereas the Pre MegE progenitors act upstream of the MkP and the Pre CFU-E (5).
Transcriptional regulation is a key mechanism controlling HSC homeostasis, development, and lineage commitment (6). For example, the commitment of hematopoietic progenitors to the B cell lineage and their development to mature B cells depends on combined activities of the transcription factors E2A, EBF, and Pax5 (7, 8). E2A is a member of the E-protein family of basic helix–loop–helix (bHLH) proteins. The E2A gene encodes 2 E proteins, E12 and E47, which are generated by differential splicing of the exon encoding the DNA binding and dimerization domain (9). Along with E12 and E47, the E-proteins include E2–2, HEB, and the Drosophila gene product Daughterless. E-proteins have the ability to bind canonical E-box [G(orA)CAXXTGG(orA)] elements as either homodimers or heterodimers with other members of the bHLH family (10, 11). Within the hematopoietic compartment, the E2A proteins form heterodimers with SCL. SCL becomes expressed in mesodermal cells that develop into embryonic blood cells and continues to be expressed in fetal and adult HSCs (12). Although SCL is not required for the maintenance of HSC self-renewal, it is critical for proper erythroid and megakaryocyte development in the adult (13).
Here, we show the E2A proteins are ubiquitously expressed in HSCs and in subsets of hematopoietic progenitor cells, but that their expression levels are dynamic. We show that the E2A proteins act to promote the developmental progression of the entire spectrum of early hematopoietic progenitors. The observations also mechanistically connect the E2A proteins, cell cycle regulators, and the maintenance of the HSC pool in a common pathway.
Results
Reduced Numbers of HSCs in E2A-Deficient Bone Marrow.
To examine E2A expression in HSCs and early hematopoietic progenitors, we used a knock-in mouse mutant, in which the coding sequence for GFP was fused to the C terminus of the E2A gene in-frame through homologous recombination (14). As previously reported, E2A levels are high in the LSK (Lin−/c-kit+/Sca-1+) population [supporting information (SI) Fig. S1] (14). Within the LSK population, the LMPP compartment exhibited higher levels of E2A expression than the LT- and ST-HSC populations (Fig. S1). Given that E2A is expressed throughout the HSC compartment, we investigated whether loss of E2A affects the numbers of the various HSC populations in the bone marrow. By using multiparameter flow cytometry, we compared the number of HSCs in the bone marrow of wild-type and E2A mutant mice. We analyzed mice <2 months of age, because, at later ages, E2A −/− mice readily develop lymphoma (15).
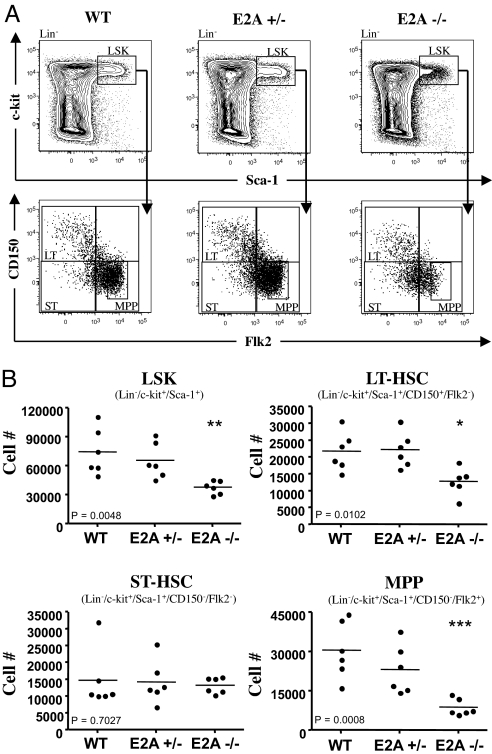
We observed a significant decrease in the LSK compartment of E2A −/− mice, compared with wild type (2.5-fold) (Fig. 1 A and B). The LSK compartment can be further separated on the basis of CD150 and Flk2 expression (16–19). The CD150+/Flk2− population comprises the LT-HSC compartment, the CD150−/Flk2− population the ST-HSC and the CD150−Flk2+ compartment consists of MPPs. Loss of E2A activity resulted in a 2-fold decrease in the number of LT-HSCs (Fig. 1B). The cellularity of MPPs in the bone marrow of E2A −/− mice was reduced 4-fold, compared with wild-type mice (Fig. 1B). To determine whether the fraction of LMPPs was affected by the absence of E2A, LSK cells were examined for the expression of Flk2 as described previously (4, 20). Strikingly, the proportion and cellularity of Flk2 expressing cells was severely reduced in E2A −/− bone marrow (Fig. 1A). We note that Flk2 expression is not down-regulated at the CLP or Pre GM stage in E2A −/− bone marrow, indicating that Flk2 is not a direct target of E2A. Collectively, these data indicate that E2A acts during early hematopoiesis to maintain the HSC compartment and to promote the development of MPPs as well as LMPPs.
Fig. 1.
Effects of E2A deletion on the numbers of HSCs in adult mouse bone marrow (BM). Total BM cells from wild-type, E2A +/−, and E2A −/− mice were harvested and prepared for analysis by flow cytometry. (A) Representative staining profiles for LSK cells and the LT-HSC, ST-HSC, and MPP subpopulations. The small gate in the MPP quadrant is representative of the LMPP population. (B) Reduced HSC numbers in the BM of E2A −/− mice. Shown are the absolute numbers of the LSK, LT-HSC, ST-HSC, and MPP populations in the BM of wild-type, E2A +/−, and E2A −/− mice (n = 6). Horizontal bars show the mean values. Statistical significance determined by unpaired t test, 2-tailed, between E2A −/− and wild type.
Reduced Numbers of Lymphoid and Myeloid Progenitors in E2A-Deficient Bone Marrow.
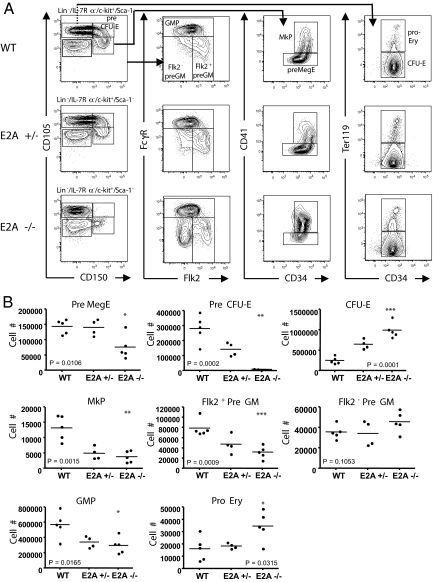
To determine whether the loss of E2A also affects the numbers of hematopoietic progenitors, we examined bone marrow cells for the presence of lymphoid and myeloerythroid progenitors. Consistent with previous observations, loss of E2A resulted in a significant decrease in the number of CLPs (Lin−/IL-7Rα+/ckitint/Sca-1int) (Fig. S2 A and B). Also, we examined bone marrow cells for the presence of erythroid/megakaryocyte and GMPs by using markers recently described (5). The Pre MegE compartment (Lin−/IL-7Rα−/c-kit+/Sca-1−/CD105lo/CD150hi/CD41−) was substantially decreased (Fig. 2 A and B). Similarly, the megakaryocyte progenitor (MkP) immediately downstream of the Pre MegE was also reduced (Fig. 2 A and B). However, the erythroid progenitor immediately downstream of the Pre MegE, the Pre CFU-E, was almost absent. Despite the near absence of the Pre CFU-E population, the CFU-E (Lin−/c-kit+/Sca-1−/IL-7Rα−/CD105hi/CD150lo/Ter119−) compartment immediately downstream was not reduced (Fig. 2 A and B, and Fig. 3D).
Fig. 2.
Effects of E2A deletion on the numbers of myeloerythroid progenitors in adult mouse BM. Total BM cells from wild-type, E2A +/−, and E2A −/− mice were harvested and prepared for analysis by flow cytometry. (A) Representative staining profiles for myeloerythroid progenitors. (B) Reduced numbers of myeloerythroid progenitor subsets in the BM of E2A −/− mice. Shown are the absolute numbers of the myeloerythroid progenitor subsets in the BM of wild-type, E2A +/−, and E2A −/− mice (n = 5). Horizontal bars show the mean values. Statistical significance determined by unpaired t test, 2-tailed, between E2A −/− and wild type.
Fig. 3.
Increased cycling by E2A-deficient HSCs. To investigate cycling of E2A −/− HSCs, BrdU was administered to 6 week-old wild-type and E2A −/− mice and BrdU incorporation by the LT-HSC, ST-HSC, and MPP cell fractions was analyzed by flow cytometry. (A) Representative staining profiles for the LT-HSC (LSK/CD150+/Flk2−) fraction. (B) Increased incorporation of BrdU by E2A −/− HSCs. Shown are the percentages of BrdU incorporation by the LT-HSC (LSK/CD150+/Flk2−), ST-HSC (LSK/CD150−/Flk2−), and MPP (LSK/CD150−/Flk2+) fractions in the bone marrow of wild-type and E2A −/− mice (n = 4). Statistical significance determined by unpaired t test, 2-tailed, between E2A −/− and wild type. (C) Cell cycle distribution in E2A −/− HSCs. Shown are the percentages of LSKFlk2+ and LSKFlk2− fractions in G0, G1, and SG2M in the BM of wild-type and E2A −/− mice (n = 3). Statistical significance determined by 2-sided Student's t test, E2A −/−, compared with wild-type. (D) Schematic representation depicting the roles of E2A in early hematopoiesis. The blue arrow indicates the importance of E2A proteins in HSC self-renewal. Decreases in the indicated hematopoietic populations detected in E2A −/− mice are shown by solid red down arrows. In the absence of E2A, significant decreases in LMPPs, CLPs, and GMPs are detected. Also, the Pre MegE, Pre CFU-E, and MkP compartments are significantly decreased. However, E2A −/− mice have near wild-type levels of Pre GMs and CFU-Es. We propose that the E2A proteins act to promote the development of the LMPPs and the Pre MegE progenitors, and to suppress the development of the Pre GM stage into CFU-Es. Data represent the mean ± SD. Statistical significance determined by unpaired t test, 2-tailed, between E2A −/− and wild type.
The GMPs were examined as described previously, with the addition of Flk2 (5). Although E2A −/− bone marrow showed no overall change in the Pre GM (Lin−/c-kit+/Sca-1−/CD105−/CD150−/FcγR−) compartment, the population was skewed toward the Flk2− side, such that the number of Flk2− Pre GM was increased and Flk2+ Pre GM decreased, compared with wild type (Fig. 2 A and B). The GMP compartment was reduced as well (Fig. 2B). Also, we performed an in vitro colony-forming unit-granulocyte (CFU-G) assay. In methylcellulose cultures, bone marrow from E2A −/− mice demonstrated a statistically significant decrease in the number of CFU-G (Fig. S3). Collectively, these data show that the E2A proteins perform a wide spectrum of activities during early hematopoiesis to modulate the developmental progression of megakaryocyte/erythroid and myeloid progenitors.
Defective Long-Term Repopulating Ability of E2A-Deficient HSCs.
To determine whether loss of E2A affects the functional capabilities of HSCs, we evaluated the repopulating capacity of purified LSK cells by using a competitive reconstitution assay. For this purpose, FACS-purified LSK cells from wild-type (CD45.1+) and E2A −/− (CD45.2+) mice were mixed at a 1:1 ratio (1 WT:1 E2A −/− mixed chimera) and transplanted into lethally irradiated CD45.1+ recipient mice. Also, separate cohorts of CD45.1+ recipient mice were transplanted with LSK cells purified from wild-type, E2A +/−, or E2A −/− (CD45.2+) mice, respectively. FACS-purified LSK cells were used instead of whole bone marrow for this assay to control for differences in the number of bone marrow mononuclear cells and the distribution of lineage positive subsets in the bone marrow of wild-type and E2A −/− mice (Fig. S4 A and B). Although the overall number of LT-HSCs was reduced in E2A −/− bone marrow, the percentage of LT-HSCs within the LSK gate were the same between mutant and wild type; thus, the number of LT-HSCs transplanted between mutant and wild-type were equivalent. After transplantation, peripheral blood from the transplanted recipients was analyzed monthly by flow cytometry to assess the contribution of CD45.2+ versus CD45.1+ to the myeloid and lymphoid cell lineages. As expected, wild-type or E2A +/− LSK cells alone successfully reconstituted the myeloid and lymphoid cell lineages in irradiated recipients, whereas E2A −/− LSK cells, whether alone or in competition with wild-type LSK cells, demonstrated a considerable defect in their ability to contribute to the lymphoid cell lineage (Fig. S5A and Fig. S6A). Conversely, E2A −/− LSK cells made a significant contribution to reconstitution of the myeloid compartment. At 4 and 8 weeks posttransplantation, the myeloid lineage repopulating ability of E2A −/− LSK cells was near predicted levels in the 1 WT: 1 E2A −/− mixed chimeras (Fig. S5A). However, analysis of the myeloid lineage in the 1 WT:1 E2A −/− mixed chimeras at later time points showed a significant decrease in the long-term repopulating ability of E2A −/− LSK cells (Fig. S5A).
Because of the decrease in GMPs in the E2A −/− bone marrow, the granulocyte output of E2A −/− LSKs may be reduced; thus, skewing an accurate assessment of LSK chimerism in a competitive setting. Therefore, we examined the LSK compartment in the bone marrow of the transplanted recipient mice to directly measure LSK chimerism and self-renewal. At 24 weeks posttransplantation, mice transplanted with E2A −/− LSK cells alone demonstrated a significant reduction in the number of LSK cells in the bone marrow, compared with mice transplanted with only wild-type or E2A +/− LSK cells (Fig. S5 B and C). Also, in the 1 WT:1 E2A −/− mixed chimeras, the contribution of E2A −/− derived (CD45.2+) cells was 25 ± 4% of a theoretically possible 50% of cells in the LSK compartment (Fig. S5C). These data indicate that the E2A proteins have a critical role in the maintenance of the LSK compartment.
To examine the serial reconstituting ability of E2A −/− LSK cells we performed secondary bone marrow transplants. Within 24 weeks, 6 of 8 secondary recipients transplanted with bone marrow from primary E2A −/− LSK cell transplants died (Fig. S5D). Importantly, none of the transplant recipients receiving E2A −/− bone marrow cells demonstrated any evidence of developing a thymic lymphoma during this study. In contrast, all secondary recipients transplanted with bone marrow from primary wild-type LSK cell transplants survived the 24 weeks (Fig. S5D). Similar to the primary LSK cell transplants, analysis of peripheral blood indicated wild-type and E2A +/− bone marrow cells efficiently re-populated the lymphoid and myeloid lineages of the secondary transplants, whereas E2A −/− bone marrow cells made a minimal contribution to the myeloid and lymphoid compartments (Fig. S5E and Fig. S6B). Also, analysis of the LSK compartment in the bone marrow of the 2 remaining mice transplanted with only E2A −/− bone marrow (70 ± 29% primary to 17 ± 4% secondary), and the mice that received bone marrow from the 1 WT:1 E2A −/− mixed chimeras (25 ± 4% primary to 13 ± 4% secondary) demonstrated a significant reduction in E2A −/− contribution to the LSK population (Fig. 5F). To determine whether the absence of E2A affects the number of hematopoietic progenitors in the bone marrow on serial transplantation, the bone marrow of the primary and secondary transplant recipients was evaluated for contribution of E2A −/− cells to the lineage-committed hematopoietic progenitor subsets. Similar to the HSC compartment, the contribution from E2A −/− bone marrow to the hematopoietic progenitor compartment was significantly reduced (Fig. S7 A–D). Collectively, these data support a major role for the E2A proteins in the self-renewal activity of HSCs.
The E2A Proteins Modulate the Expression of Cell Cycle Regulators to Control Cell Cycle Progression of the HSC Compartment.
To address the mechanism by which E2A maintains the HSC pool, we examined E2A −/− hematopoietic progenitors for their ability to migrate to the bone marrow after transplantation. However, the reconstitution defect observed in E2A −/− cells is not due to the inability of E2A −/− HSCs to home to the hematopoietic niches of the bone marrow, because homing of E2A −/− HSC subsets to the bone marrow of lethally irradiated recipients is comparable with wild-type HSCs (Fig. S8). A number of studies have indicated that the maintenance of HSC self-renewal activity is mediated in part by cell cycle regulators (21–24). E2A has been shown to modulate cell cycle progression by regulating the expression of cell cycle regulators, raising the possibility that E2A maintains HSC self-renewal activity by modulating cell cycle entry (15, 25). As a first approach to this question, we examined the cell cycle state of HSCs in wild-type and E2A −/− bone marrow. Wild-type and E2A −/− mice were labeled with BrdU, bone marrow was isolated and immunostained for BrdU, and the LT-HSC, ST-HSC, and MPP subsets analyzed by flow cytometry. Interestingly, the proportion of cycling HSCs was significantly increased in E2A −/− bone marrow (Fig. 3 A and B). In agreement with the increased uptake of BrdU by E2A −/− HSCs, we show, by using Ki-67 and DAPI staining, that E2A −/− mice have fewer LSKFlk2− HSCs in G0 (Fig. 3C and Fig. S9). Also, the LSKFlk2+ MPP subset displayed a similar pattern of enhanced cell cycling (Fig. 3C). Based on these observations, we propose that the E2A proteins act in murine bone marrow by regulating the rate and capability of cell cycle progression in the HSC compartment.
To determine how the E2A proteins act to modulate cell cycle progression and self-renewal in LSK cells, we examined the expression of a number of known E2A targets and/or regulators of cell cycle progression. To accomplish this objective, we purified LSK, LSKFlk2−, and LSKFlk2+ cells from the bone marrow of wild-type and E2A −/− mice and analyzed the expression of p18INK4C, p19INK4D, p21Cip1, p27Kip1, Cdk6, Bmi-1, Gfi-1, HoxB4, Mpl, and Notch-1 by quantitative PCR (Fig. S10). Interestingly, p21Cip1 and Gfi-1 levels were decreased significantly in LSK cells derived from E2A −/− bone marrow and have been demonstrated to modulate the self-renewal activity of HSCs (Fig. S10 A and B) (21–24). Also, previous studies have demonstrated that the E2A proteins directly regulate p21Cip1 and Gfi-1 expression (15, 26). In addition to their significantly decreased expression in LSK cells, p21Cip1, Gfi-1, and Notch1 demonstrated decreased expression in purified E2A −/− LSKFlk2− and LSKFlk2+ subsets, compared with wild type (Fig. S10C). Together, these data suggest that the E2A proteins mechanistically regulate HSC self-renewal by modulating the expression of genes involved in HSC cell proliferation.
Discussion
Previous data indicate that the initial stages of B- and T-lineage development require the activities of E-proteins (27–29). Also, recent studies have revealed a role for the E-proteins in the CLP compartment and it has been suggested that E2A becomes transcriptionally active in the CLP cell stage (30). Once E2A is activated, it acts in concert with PU.1 and IL7Rα-mediated signaling to induce the expression of EBF transcription, which, in turn, activates Pax5 gene expression (31). In addition to the activation of a B-lineage specific program of gene expression, E2A also acts to repress the expression of genes involved in the commitment of alternative cell fates, including GATA-1 and GATA-3 (32). Thus, the E2A proteins initiate and maintain a B-lineage specific program of gene expression and repress transcription of non-B lineage specific genes. However, although these studies revealed a role for the E2A proteins in B cell specification, it has remained unclear whether and how the E2A proteins act in HSCs and in hematopoietic progenitors.
Roles of Id1 and E2A in Early Hematopoiesis.
Recent studies demonstrated that Id1 acts to constrain myeloid commitment. Specifically, it was demonstrated that Id1-ablated LSK cells showed a premature induction of the myeloid differentiation program (33). These data are consistent with our observations indicating a defect in myeloid maturation in E2A −/− hematopoietic progenitors. Thus, high levels of E2A would favor commitment toward the myeloid cell lineage, whereas inactivation of E2A DNA binding would suppress the development toward the myeloid/lymphoid lineages. Surprisingly, however, are the observations that both E2A and Id1 deficiencies result in higher levels of GATA-1 expression within the LSK compartment (data not shown) (33). Similarly, the expression of Gfi-1, Bmi-1, and Hoxb4 were slightly decreased in both E2A- and Id1-deficient bone marrow (33). How can we reconcile these observations? We propose that the dosage of E- and Id-proteins is carefully calibrated in developing hematopoietic progenitors. The loss of one component of the E/Id ensemble of proteins may dramatically affect the equilibrium of interactions. For example, it is conceivable that in the absence of Id1, HEB and/or E2–2 might be released from their inhibitor to complex with E2A, converting its ability from a transcriptional repressor into an activator. Such a scenario is not that unlikely, because the E2A proteins can act to both activate and repress transcription, depending on the recruitment of a coactivator or corepressor (34). Such a model may also explain the strong dosage effects that we observed during early hematopoiesis in E2A +/− mice.
Role of E2A in Specification of the Granulocyte/Macrophage and Lymphoid Lineages.
Striking abnormalities in E2A −/− bone marrow were observed within the Pre GM progenitors, which were further separated on the basis of Flk2 expression. Pre GM progenitors in the E2A−/− bone marrow were heavily skewed toward Flk2 negativity, such that the absolute number of Flk2− cells was increased in these mice, compared with wild-type, and the Flk2+ were decreased. These data bring into question how the E2A proteins act to promote developmental progression of both lymphoid and myeloid progenitors from a common MPP or LMPP precursor. The decrease in the number of intermediates like the CLP and the Flk2+ Pre GM populations may simply reflect a decrease in the number of CD150−Flk2+ progenitors. Thus, the E2A proteins may act at the CD150−Flk2+ cell stage to induce, at low levels, the expression of a myeloid and common lymphoid, but not B and T cell specific, program of gene expression. In MPPs the E2A proteins act to induce the expression of common lymphoid genes and to suppress the expression of genes, such as GATA-1, that are involved in erythroid differentiation (32). The priming of a program of myeloid specific gene expression is likely to be induced by PU.1, which has been demonstrated to act at the branch point separating the MegE and lymphoid/myeloid cell lineages (35). Thus, based on these previous observations and the data described here, we suggest that E2A and PU.1 both act in the CD150−Flk2+ compartment to induce the priming of a common myeloid and lymphoid program of gene expression.
Role of the E2A in Erythroid and Megakaryocytic Development.
It has been previously proposed that the LSK CD150−Flk2lo compartment may directly feed into the Pre MegE population (5). The Pre MegE, in turn, feeds into the CFU-E via the Pre CFU-E. However, the Pre CFU-E is almost absent in E2A −/− bone marrow, but the CFU-E population is present at elevated levels. How are erythrocytes generated in E2A −/− bone marrow in the absence of erythroid and megakaryocytic progenitors? It is conceivable that the Pre GM cells are contributing to the CFU-E. In such a scenario, the E2A −/− Flk2− Pre GM would function as a CMP. In the absence of E2A, how does the Pre GM differentiate into the erythroid lineage? We have previously shown that the E2A proteins in MPPs act to suppress the transcription of GATA-1 (32). GATA-1 is a critical transcriptional regulator for erythroid/megakaryocyte development, because GATA-1-ablated mice lack the erythroid/megakaryocytic lineages (36, 37). Also, GATA-1 exerts instructive signals for erythroid/megakaryocytic lineage commitment and has the ability to antagonize the transactivation capacity of PU.1 to promote erythroid differentiation at the expense of myeloid maturation (38–41). Thus, although the induction of GATA-1 expression in the absence of E2A is merely 2-fold, it is conceivable that an increase of such abundance in conjunction with the induction of additional regulators yet to be identified would promote the development of CFU-E cells through a pathway independent of the Pre MegE and Pre CFU-E intermediates. Little if any GATA-1 expression is present in wild-type Pre GM cells, compared with Pre MegE and Pre CFU-E (5). Therefore, we propose that E2A suppresses GATA-1 expression in the Pre GM, preventing this population from differentiating down the erythroid lineage. We also note that, within the CD150+CD105− gate, which contains both Pre MegE and MkP progenitors, a higher percentage within this gate were MkP progenitors in E2A −/− mice. This suggests that the Pre MegE population in the E2A −/− mice is skewed toward megakaryocyte development, possibly again mediated by increased levels of GATA-1 expression.
The role of the E2A Proteins in Maintaining the HSC Pool.
E2A −/− bone marrow also shows a diminished steady-state abundance of LSKs and a defect in the ability to self-renew in serial transplantation assays. How do the E2A proteins act to maintain the HSC compartment? Our data show that the absence of E2A activity results in a substantial increase in the rate of cell cycle progression of the HSC compartment. Previous observations have indicated that the maintenance of the stem cell pool is controlled, among others, by the thrombopoietin receptor (Mpl), Gfi-1, p21Cip1, and p27Kip1 (21–24). We demonstrate here that the expression of Mpl, Gfi-1, p21Cip1, and p27Kip1 is significantly decreased in E2A −/− LSK cells (Fig. S10). It is unlikely that the reduction in p27 levels is responsible for the increase in cycling HSCs, because p27 has been demonstrated not to modulate HSC cell cycling or self-renewal, but rather controls the expansion and pool size of progenitors (21). In contrast, p21Cip1 and Mpl have been shown to have a critical role in maintaining the HSC pool. Thus, we propose that the E2A proteins mechanistically enforce the maintenance of HSCs by directly modulating the expression of Mpl and p21Cip1.
Materials and Methods
Competitive Repopulation Assay.
Lethally γ-irradiated (single dose of 1,000 cGy) CD45.1 congenic C57/B6 mice at 8–10 weeks of age were used as recipients. We injected 1,000 sorted LSK cells from wild-type (CD45.2+), E2A +/− (CD45.2+), E2A −/− (CD45.2+), or a 1 to 1 ratio of wild-type (CD45.1+) to E2A −/− (CD45.2+) i.v. into each lethally irradiated recipient mouse. To establish secondary transplants, single-cell suspensions from bone marrow of primary LSK cell transplant recipients were prepared. Bone marrow cells were resuspended in α-MEM containing 10% FBS. Lethally irradiated CD45.1+ secondary recipients were injected intravenously with a total of 2 × 106 cells.
Quantitative Real-Time PCR.
Total RNA was extracted from sorted LSK cells by using an Rneasy kit (Qiagen) according to manufacturer's protocol. For real-time PCR, cDNA synthesis was performed by using SuperScript III (Invitrogen). Real-time PCR was performed by using SYBR Green Master Mix (Stratagene) and analyzed by Mx3005P instrumentation (Stratagene). The reactions were performed in triplicate at 95 °C for 10 min, followed by 40 cycles of 95 °C for 30 s, 55 °C for 1 min, and 72 °C for 30 s. The primer sequences used are available on request.
Cell Cycle Assays.
To examine BrdU incorporation, mice were given a single i.p. injection of BrdU (Sigma) (1 mg/6 g) in PBS and maintained on 1 mg/mL of BrdU in the drinking water for 72 h. After 72 h, the bone marrow was harvested and stained with antibodies against lineage markers, c-kit, Sca-1, CD150, and Flk2. BrdU incorporation was measured by using a FITC BrdU Flow Kit (BD PharMingen).
To analyze the cell cycle status of the LSK, LSKFlk2−, and LSKFlk2 HSC subsets, bone marrow cells from wild-type and E2A −/− mice were initially stained with antibodies against Lin+ cells, c-kit, Sca-1, and Flk2. After incubation with the cell surface antibodies, the cells underwent fixation with a Cytofix/Cytoperm kit (BD Biosciences). After fixation, the cells were incubated with FITC-anti-Ki-67, washed, and stained with DAPI. Analysis was performed on a FACS LSRII (BD Biosciences).
For more details, see SI Materials and Methods.
Supplementary Material
Acknowledgments.
We thank Yuan Zhuang (Duke University Medical Center, Durham, NC) for providing E2A/GFP mice, and members of the C.M. and I.L.W. laboratories for reagents and stimulating discussions. C.M. and I.L.W. were supported in part by grants from the National Institutes of Health and the California Institute for Regenerative Medicine (CIRM); C.L.S. by a Hemoglobin and Blood Training grant and by CIRM; E.M.M. by an Endocrine Training grant; and M.A.I. by CIRM Fellowship T1-00001.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0808866106/DCSupplemental.
References
- 1.Spangrude GJ, Heimfeld S, Weissman IL. Purification and characterization of mouse hematopoietic stem cells. Science. 1988;241:58–62. doi: 10.1126/science.2898810. [DOI] [PubMed] [Google Scholar]
- 2.Akashi K, Traver D, Miyamoto T, Weissman IL. A clonogenic common myeloid progenitor that gives rise to all myeloid lineages. Nature. 2000;404:193–197. doi: 10.1038/35004599. [DOI] [PubMed] [Google Scholar]
- 3.Kondo M, Weissman IL, Akashi K. Identification of clonogenic common lymphoid progenitors in mouse bone marrow. Cell. 1997;91:661–672. doi: 10.1016/s0092-8674(00)80453-5. [DOI] [PubMed] [Google Scholar]
- 4.Adolfsson J, et al. Identification of Flt3+ lympho-myeloid stem cells lacking erythro-megakaryocytic potential a revised road map for adult blood lineage commitment. Cell. 2005;121:295–306. doi: 10.1016/j.cell.2005.02.013. [DOI] [PubMed] [Google Scholar]
- 5.Pronk CJ, et al. Elucidation of the phenotypic, functional, and molecular topography of a myeloerythroid progenitor cell hierarchy. Cell Stem Cell. 2007;1:428–442. doi: 10.1016/j.stem.2007.07.005. [DOI] [PubMed] [Google Scholar]
- 6.Pimanda JE, et al. Gata2, Fli1, and Scl form a recursively wired gene-regulatory circuit during early hematopoietic development. Proc Natl Acad Sci USA. 2007;104:17692–17697. doi: 10.1073/pnas.0707045104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Busslinger M. Transcriptional control of early B cell development. Annu Rev Immunol. 2004;22:55–79. doi: 10.1146/annurev.immunol.22.012703.104807. [DOI] [PubMed] [Google Scholar]
- 8.Singh H, Medina KL, Pongubala JM. Contingent gene regulatory networks and B cell fate specification. Proc Natl Acad Sci USA. 2005;102:4949–4953. doi: 10.1073/pnas.0500480102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Murre C, McCaw PS, Baltimore D. A new DNA binding and dimerization motif in immunoglobulin enhancer binding, daughterless, MyoD, and myc proteins. Cell. 1989;56:777–783. doi: 10.1016/0092-8674(89)90682-x. [DOI] [PubMed] [Google Scholar]
- 10.Lazorchak A, Jones ME, Zhuang Y. New insights into E-protein function in lymphocyte development. Trends Immunol. 2005;26:334–338. doi: 10.1016/j.it.2005.03.011. [DOI] [PubMed] [Google Scholar]
- 11.Sun XH. Multitasking of helix-loop-helix proteins in lymphopoiesis. Adv Immunol. 2004;84:43–77. doi: 10.1016/S0065-2776(04)84002-1. [DOI] [PubMed] [Google Scholar]
- 12.Shivdasani RA, Mayer EL, Orkin SH. Absence of blood formation in mice lacking the T-cell leukaemia oncoprotein tal-1/SCL. Nature. 1995;373:432–434. doi: 10.1038/373432a0. [DOI] [PubMed] [Google Scholar]
- 13.Mikkola HK, et al. Haematopoietic stem cells retain long-term repopulating activity and multipotency in the absence of stem-cell leukaemia SCL/tal-1 gene. Nature. 2003;421:547–551. doi: 10.1038/nature01345. [DOI] [PubMed] [Google Scholar]
- 14.Zhuang Y, Jackson A, Pan L, Shen K, Dai M. Regulation of E2A gene expression in B-lymphocyte development. Mol Immunol. 2004;40:1165–1177. doi: 10.1016/j.molimm.2003.11.031. [DOI] [PubMed] [Google Scholar]
- 15.Schwartz R, Engel I, Fallahi-Sichani M, Petrie HT, Murre C. Gene expression patterns define novel roles for E47 in cell cycle progression, cytokine-mediated signaling, and T lineage development. Proc Natl Acad Sci USA. 2006;103:9976–9981. doi: 10.1073/pnas.0603728103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Adolfsson J, et al. Upregulation of Flt3 expression within the bone marrow Lin(−)ScaI(+)c-kit(+) stem cell compartment is accompanied by loss of self-renewal capacity. Immunity. 2001;15:659–669. doi: 10.1016/s1074-7613(01)00220-5. [DOI] [PubMed] [Google Scholar]
- 17.Chen CZ, et al. Identification of endoglin as a functional marker that defines long-term repopulating hematopoietic stem cells. Proc Natl Acad Sci USA. 2002;99:15468–15473. doi: 10.1073/pnas.202614899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Christensen JL, Weissman IL. Flk-2 is a marker in hematopoietic stem cell differentiation: A simple method to isolate long-term stem cells. Proc Natl Acad Sci USA. 2001;98:14541–14546. doi: 10.1073/pnas.261562798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yang L, et al. Identification of Lin(-)ScaI(+)kit(+)CD34(+)Flt3- short-term hematopoietic stem cells capable of rapidly reconstituting and rescuing myeloablated transplant recipients. Blood. 2005;105:2717–2723. doi: 10.1182/blood-2004-06-2159. [DOI] [PubMed] [Google Scholar]
- 20.Yoshida T, Ng SY, Zuniga-Pflucker JC, Georgopoulos K. Early hematopoietic lineage restrictions directed by Ikaros. Nat Immunol. 2006;7:382–391. doi: 10.1038/ni1314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cheng T, Rodrigues N, Dombkowski D, Stier S, Scadden DT. Stem cell repopulation efficiency but not pool size is governed by p27(kip1) Nat Med. 2000;6:1235–1240. doi: 10.1038/81335. [DOI] [PubMed] [Google Scholar]
- 22.Cheng T, et al. Hematopoietic stem cell quiescence maintained by p21cip1/waf1. Science. 2000;287:1804–1808. doi: 10.1126/science.287.5459.1804. [DOI] [PubMed] [Google Scholar]
- 23.Hock H, et al. Gfi-1 restricts proliferation and preserves functional integrity of haematopoietic stem cells. Nature. 2004;431:1002–1007. doi: 10.1038/nature02994. [DOI] [PubMed] [Google Scholar]
- 24.Walkley CR, Fero ML, Chien WM, Purton LE, McArthur GA. Negative cell-cycle regulators cooperatively control self-renewal and differentiation of haematopoietic stem cells. Nat Cell Biol. 2005;7:172–178. doi: 10.1038/ncb1214. [DOI] [PubMed] [Google Scholar]
- 25.Engel I, Murre C. E2A proteins enforce a proliferation checkpoint in developing thymocytes. EMBO J. 2004;23:202–211. doi: 10.1038/sj.emboj.7600017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Prabhu S, Ignatova A, Park ST, Sun XH. Regulation of the expression of cyclin-dependent kinase inhibitor p21 by E2A and Id proteins. Mol Cell Biol. 1997;17:5888–5896. doi: 10.1128/mcb.17.10.5888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bain G, et al. Both E12 and E47 allow commitment to the B cell lineage. Immunity. 1997;6:145–154. doi: 10.1016/s1074-7613(00)80421-5. [DOI] [PubMed] [Google Scholar]
- 28.Barndt RJ, Dai M, Zhuang Y. Functions of E2A-HEB heterodimers in T-cell development revealed by a dominant negative mutation of HEB. Mol Cell Biol. 2000;20:6677–6685. doi: 10.1128/mcb.20.18.6677-6685.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Heemskerk MH, et al. Inhibition of T cell and promotion of natural killer cell development by the dominant negative helix loop helix factor Id3. J Exp Med. 1997;186:1597–1602. doi: 10.1084/jem.186.9.1597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Borghesi L, et al. E47 is required for V(D)J recombinase activity in common lymphoid progenitors. J Exp Med. 2005;202:1669–1677. doi: 10.1084/jem.20051190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pongubala JM, et al. Transcription factor EBF restricts alternative lineage options and promotes B cell fate commitment independently of Pax5. Nat Immunol. 2008;9:203–215. doi: 10.1038/ni1555. [DOI] [PubMed] [Google Scholar]
- 32.Ikawa T, Kawamoto H, Wright LY, Murre C. Long-term cultured E2A-deficient hematopoietic progenitor cells are pluripotent. Immunity. 2004;20:349–360. doi: 10.1016/s1074-7613(04)00049-4. [DOI] [PubMed] [Google Scholar]
- 33.Jankovic V, et al. Id1 restrains myeloid commitment, maintaining the self-renewal capacity of hematopoietic stem cells. Proc Natl Acad Sci USA. 2007;104:1260–1265. doi: 10.1073/pnas.0607894104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Murre C. Helix-loop-helix proteins and lymphocyte development. Nat Immunol. 2005;6:1079–1086. doi: 10.1038/ni1260. [DOI] [PubMed] [Google Scholar]
- 35.Scott EW, Simon MC, Anastasi J, Singh H. Requirement of transcription factor PU. 1 in the development of multiple hematopoietic lineages. Science. 1994;265:1573–1577. doi: 10.1126/science.8079170. [DOI] [PubMed] [Google Scholar]
- 36.Fujiwara Y, Browne CP, Cunniff K, Goff SC, Orkin SH. Arrested development of embryonic red cell precursors in mouse embryos lacking transcription factor GATA-1. Proc Natl Acad Sci USA. 1996;93:12355–12358. doi: 10.1073/pnas.93.22.12355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shivdasani RA, Fujiwara Y, McDevitt MA, Orkin SH. A lineage-selective knockout establishes the critical role of transcription factor GATA-1 in megakaryocyte growth and platelet development. EMBO J. 1997;16:3965–3973. doi: 10.1093/emboj/16.13.3965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Iwasaki H, et al. GATA-1 converts lymphoid and myelomonocytic progenitors into the megakaryocyte/erythrocyte lineages. Immunity. 2003;19:451–462. doi: 10.1016/s1074-7613(03)00242-5. [DOI] [PubMed] [Google Scholar]
- 39.Kulessa H, Frampton J, Graf T. GATA-1 reprograms avian myelomonocytic cell lines into eosinophils, thromboblasts, and erythroblasts. Genes Dev. 1995;9:1250–1262. doi: 10.1101/gad.9.10.1250. [DOI] [PubMed] [Google Scholar]
- 40.Nerlov C, Querfurth E, Kulessa H, Graf T. GATA-1 interacts with the myeloid PU. 1 transcription factor and represses PU. 1-dependent transcription. Blood. 2000;95:2543–2551. [PubMed] [Google Scholar]
- 41.Rekhtman N, Radparvar F, Evans T, Skoultchi AI. Direct interaction of hematopoietic transcription factors PU. 1 and GATA-1: Functional antagonism in erythroid cells. Genes Dev. 1999;13:1398–1411. doi: 10.1101/gad.13.11.1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.