Abstract
Autism spectrum disorder (ASD) is a heterogeneous group of neurodevelopmental disorders that share deficits in sociability, communication, and restrictive and repetitive interests. ASD is likely polygenic in origin in most cases, but we presently lack an understanding of the relationships between ASD susceptibility genes and the neurobiological and behavioral phenotypes of ASD. Two genes that have been implicated as conferring susceptibility to ASD are PTEN and Serotonin transporter (SLC6A4). The PI3K and serotonin pathways, in which these genes respectively act, are both potential biomarkers for ASD diagnosis and treatment. Biochemical evidence exists for an interaction between these pathways; however, the relevance of this for the pathogenesis of ASD is unclear. We find that Pten haploinsufficient (Pten+/−) mice are macrocephalic, and this phenotype is exacerbated in Pten+/−; Slc6a4+/− mice. Furthermore, female Pten+/− mice are impaired in social approach behavior, a phenotype that is exacerbated in female Pten+/−; Slc6a4+/− mice. While increased brain size correlates with decreased sociability across these genotypes in females, within each genotype increased brain size correlates with increased sociability, suggesting that epigenetic influences interact with genetic factors in influencing the phenotype. These findings provide insight into an interaction between two ASD candidate genes during brain development and point toward the use of compound mutant mice to validate biomarkers for ASD against biological and behavioral phenotypes.
Keywords: autism, brain development, brain growth
Autism spectrum disorder (ASD) is highly heritable, with a 2–3% recurrence rate in siblings and a 60–90% concordance rate in monozygotic twins. However, known genetic causes—for example, single gene disorders such as fragile-X or tuberous sclerosis—account for ≈10% of ASD cases. Thus, the majority of cases of ASD are of unknown cause at present. Current estimates are that ASD susceptibility is conferred by numerous genes interacting with one another and with environmental factors.
Two genes that give insight into idiopathic autism are PTEN and SLC6A4. PTEN acts as a negative regulator of the PI3-kinase (PI3K) pathway (1). Heterozygous PTEN mutations have been identified in a subset of individuals with autism and macrocephaly, thus rendering affected individuals PTEN haploinsufficient (2–5). The clinical-phenotypic presentation of cognitive impairment in PTEN haploinsufficient individuals is varied. Thus, it has been suggested that individuals with ASD who carry PTEN mutations may represent a sensitized group in which to screen for second-site genetic modifiers of the ASD clinical phenotype (4). SLC6A4 encodes membrane-bound transporter of serotonin that influences extracellular levels of this neurotransmitter. SLC6A4 has been implicated as both an ASD candidate susceptibility gene and a second-site genetic modifier in ASD (6, 7). Brain overgrowth (8) and severe social behavioral impairments (9) have been reported in individuals with ASD carrying low-expressing Slc6a4 promoter polymorphism alleles. Furthermore, SLC6A4 regulates extracellular serotonin levels, and one of the most replicated reports of a peripheral biomarker in ASD is increased levels of extracellular serotonin in individuals with ASD (6).
Given their implications for ASD, both PTEN and SLC6A4 are potential peripheral biomarkers in that both genes are pleiotropic, with expression and function outside of the CNS. However, the effects of altered levels of expression of these markers need to be validated against biological and behavioral measures. There is evidence suggesting that the serotonin pathway (in which Slc6a4 acts) intersects with the PI3K pathway (in which Pten acts) in the brain. Evidence has been found for a physical interaction between Pten and the serotonin receptor 5-HT2c, with the phosphatase activity of Pten regulating the activity of this receptor (10). Furthermore, several studies in neural and nonneural cells have demonstrated that Akt is activated by serotonin receptor agonists and that this activation occurs in a PI3K-dependent manner (reviewed in ref. 11). However, while the serotonin and PI3K pathways are both strongly implicated in the pathogenesis of ASD, the significance of these interactions for ASD-relevant biological and behavioral phenotypes is not clear at present.
In Cowden syndrome patients, a subset of whom have ASD, missense mutations in PTEN tend to cluster in the core catalytic phosphatase domain of exon 5, and these tend to inactivate the phosphatase function of the protein (12). As a mouse model that approximates these genetic lesions, we have made use of a previously generated Pten mutant line in which exon 5, and thus the core catalytic phosphatase domain, is deleted (13). Mice homozygous for this mutant Pten allele are not viable; however, mice heterozygous for this allele survive into adulthood. This factor makes Pten heterozygous mice a tenable tool for investigating the developmental outcomes of Pten haploinsufficiency on brain structure and function and for screening for second-site genetic and environmental modifiers of such phenotypes. In addition to PTEN, mutations in other repressors of the PI3-kinase pathway have also been associated with ASD, specifically the tuberous sclerosis complex genes TSC1 and TSC2 (14) and Neurofibromin 1 (15, 16). Thus, Pten haploinsufficient mice represent a generally useful tool in which a signaling pathway enriched among ASD candidate genes, the PI3-kinase pathway, is sensitized. These mice also provide an opportunity to explore broader issues of gene–environment interactions in influencing brain size and behavioral measures relevant to autism.
Results
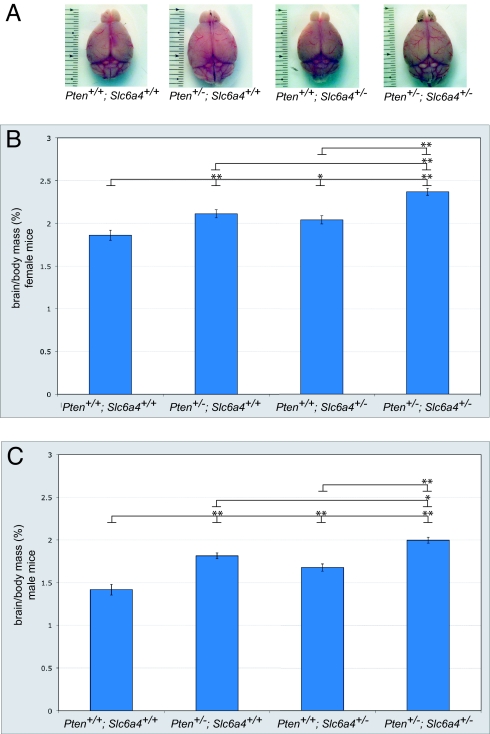
To test for a potential interaction between Pten and Slc6a4, we first needed to identify a phenotypic readout relevant to ASD. One of the most widely reported neuroanatomical abnormalities reported in ASD is macrocephaly, with an incidence of 10–30% in adulthood and up to 60% during development (17). Studies in ASD patients have shown that brain size is positively correlated with the severity of behavioral phenotypes in ASD-relevant measures (18, 19). Reports from conditional knockouts of Pten in the mouse brain describe macrocephaly phenotypes, with increases in cell soma size and neurite hypertrophy likely contributing to these phenotypes (20–22). We generated Pten and Slc6a4 compound heterozygous mutant mice by crossing Pten haploinsufficient mice to a line carrying a previously described Slc6a4 loss-of-function allele (23). We focused on germline heterozygous mice to maximize clinical relevance. To examine whether Pten or Slc6a4 haploinsufficient mice exhibit brain overgrowth, we obtained measures of overall brain mass, normalized to body mass to account for variations in body size. These data showed that haploinsufficiency for Pten or Slc6a4 results in a macrocephaly phenotype in both males and females (Fig. 1 A–C). Furthermore, Pten+/−; Slc6a4+/− mice had an additive brain overgrowth phenotype more severe than that seen in either Pten or Slc6a4 haploinsufficient mice (Fig. 1 A–C). Although the cellular mechanism underlying the effect on growth remains to be identified, these data indicate that Pten and Slc6a4 act cooperatively to influence a phenotype relevant to ASD, brain overgrowth.
Fig. 1.
Macrocephaly in Pten+/−; Slc6a4+/− mice. (A) Representative dorsal-view images of brains from male Pten+/+; Slc6a4+/+, Pten+/−; Slc6a4+/+, Pten+/+; Slc6a4+/−, and Pten+/−; Slc6a4+/− mice. Brains were collected at 12 weeks of age. (B and C) As compared to wild-type controls, Pten and Slc6a4 haploinsufficient mice show a significant increase in brain mass. (B) Brain mass in females is significantly increased in Pten+/−; Slc6a4+/− mice as compared to Pten+/+; Slc6a4+/+, Pten+/−; Slc6a4+/+, and Pten+/+; Slc6a4+/− mice (F3,42 = 20.6; P < 0.001). n = 10 Pten+/+; Slc6a4+/+, 10 Pten+/−; Slc6a4+/+, 10 Pten+/+; Slc6a4+/−, and 13 Pten+/−; Slc6a4+/− mice. (C) Brain mass in males is significantly increased in Pten+/−; Slc6a4+/− mice compared to Pten+/+; Slc6a4+/+, Pten+/−; Slc6a4+/+, and Pten+/+; Slc6a4+/− mice (F3,33 = 30.0; P < 0.001). n = 6 Pten+/+; Slc6a4+/+, 6 Pten+/−; Slc6a4+/+, 11 Pten+/+; Slc6a4+/−, and 11 Pten+/−; Slc6a4+/− mice.*, P < 0.05; **, P < 0.01 [Tukey's honestly significant difference (HSD) test]. Ages were 8–12 weeks. Data are normalized to body mass to account for differences in body mass between animals.
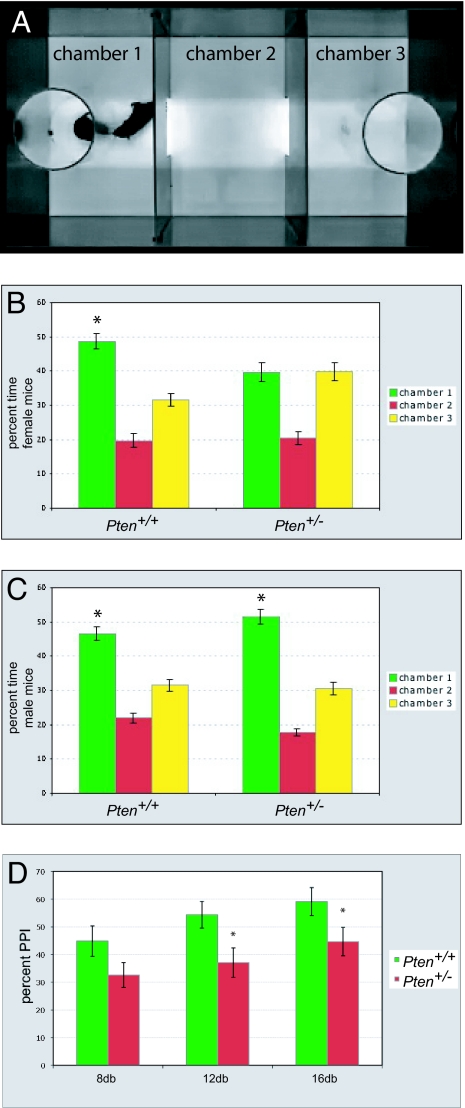
The above results argue that Pten haploinsufficient mice may be a useful tool to examine second-site genetic modifiers that interact with Pten in a manner relevant to polygenic ASD and for testing the effects of environmental modifications and the effects of therapeutic compounds. To investigate whether Pten haploinsufficient mice have surface validity for behavioral phenotypes relevant to ASD, we tested 12-week-old mice using several behavior assays. The assays we used tested sociability, which reflects a core diagnostic deficit seen in individuals with ASD (24), and prepulse inhibition, a measure of sensorimotor gating that has been reported to be abnormal in individuals with ASD (25–27). To test for a possible confounding factor of olfactory function in social behavior, we exposed Pten haploinsufficient mice and controls to an olfactory habituation–dishabituation test and found that these mice responded normally (Fig. S1), indicating that they do not have a gross impairment in olfactory function. To measure social approach behavior, we used a variation of an apparatus in which mice have to choose between spending time interacting with an unfamiliar gender- and age-matched stimulus mouse (social chamber) or an inanimate object (nonsocial chamber) (28, 29) (Fig. 2A). Whereas wild-type mice of both genders showed a significant preference for spending time in the social chamber, Pten haploinsufficient female mice did not show this preference and spent roughly equal time in the social and nonsocial chambers (Fig. 2B). Male Pten haploinsufficient mice did not show this same deficit in social approach behavior (Fig. 2C). In tests of sensorimotor gating in Pten haploinsufficient mice and controls, we found that both genders had deficits in prepulse inhibition of the acoustic startle response (Fig. 2D). Our results with Pten haploinsufficient mice generally agree with behavioral results from CNS-specific conditional Pten knockout mice (21), in which animals were tested for a variety of ASD-relevant phenotypes. Differences are seen in the initial tendency for social approach in males, where conditional knockouts show a deficit and Pten haploinsufficient mice do not. These differences are most likely attributable to the different nature of the genetic manipulation in these 2 complementary models. Identifying the neurobiological basis for different behavioral phenotypes in these models should provide insight into how Pten influences the development of neural circuitry relevant to ASD.
Fig. 2.
Behavioral characterization of Pten+/− mice. (A) Still images of the apparatus used to assay social approach behavior. A social stimulus mouse resides in an acrylic cage in chamber 1 and the acrylic cage in chamber 3 remains empty as a control. The subject mouse begins the assay in chamber 2, the assay is video recorded for 10 min, and the percentage of time spent in each chamber is then quantified. (B and C) Data showing percentage of time spent in a chamber containing a cage that holds a stimulus mouse (chamber 1), an empty chamber (chamber 2), or a chamber containing a cage with no stimulus mouse (chamber 3). All mice tested were 12 weeks of age. (B) In females, Pten+/+ mice show a significant preference for chamber 1 over chamber 3, whereas this preference is not seen in Pten+/− mice. (C) In males, both Pten+/+ and Pten+/− mice show a preference for chamber 1 over chamber 3. *, P < 0.05, ANOVA within-group comparison between chamber 1 and chamber 3. n = 17 males, 12 females for each genotype. Error bars indicate SEM. (D) Prepulse inhibition of the acoustic startle response in Pten+/− mice. All mice tested were 12 weeks of age. As compared to Pten+/+ mice, Pten+/− mice have significant deficits in startle inhibition at prepulses 12 or 16 db above background. *, P < 0.05, ANOVA comparison between genotypes for given prepulse intensity. n = 12 mice (6 females) for each genotype.
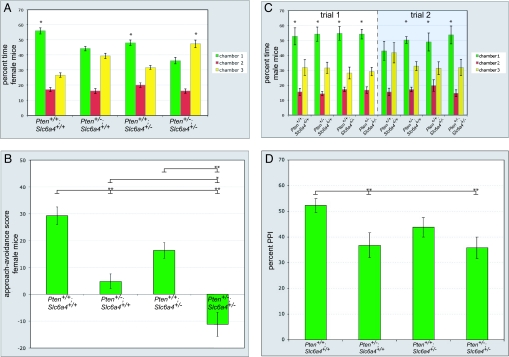
To test whether the social approach phenotype we observe in Pten+/− female mice may be modified by haploinsufficiency for Slc6a4, as happens with brain size, we examined 8-week-old female wild-type, Pten haploinsufficient, Slc6a4 haploinsufficient, and Pten+/−; Slc6a4+/− compound mutant mice. Slc6a4 haploinsufficient and Pten+/−; Slc6a4+/− mice responded normally in a test of olfactory habituation–dishabituation (Fig. S1), indicating no gross impairment of olfaction in these mice. We found that Slc6a4 haploinsufficient mice displayed a decreased preference for interacting with a stimulus mouse in the social approach assay as compared with wild-type mice, although this did not fall below the threshold for significance. However, Pten+/−; Slc6a4+/− mice did show a significant decrease in preference for interacting with a stimulus mousee (Fig. 3A). Analyzing these data using a social approach–avoidance score (28) (time in social chamber 1 minus time in nonsocial chamber 3), we found that the time spent interacting with a stimulus mouse was significantly less in Pten+/−; Slc6a4+/− mice than in wild-type, Pten haploinsufficient, or Slc6a4 haploinsufficient mice (Fig. 3B). This result is consistent with haploinsufficiency for Pten and Slc6a4 acting additively to influence an ASD-relevant behavioral phenotype, social approach.
Fig. 3.
Social behavior and prepulse inhibition in Pten and Slc6a4 haploinsufficient mice. (A) Social approach data for 8-week-old female Pten+/+; Slc6a4+/+ (n = 13), Pten+/−; Slc6a4+/+ (n = 13), Pten+/+; Slc6a4+/− (n = 11), and Pten+/−; Slc6a4+/− (n = 13) mice. *, P < 0.05, ANOVA within-group comparison between chamber 1 and chamber 3. Error bars indicate SEM. (B) Social approach data from A presented as approach–avoidance score for analysis across genotypes. Time spent with a social stimulus mouse in chamber 1 is significantly decreased in Pten+/−; Slc6a4+/− mice as compared to Pten+/+; Slc6a4+/+, Pten+/−; Slc6a4+/+, and Pten+/+; Slc6a4+/− mice (F3,49 = 25.3; P < 0.001). *, P < 0.05; **, P < 0.01 (Tukey's HSD test). (C) Social approach and recognition data for 8-week-old male mice. During trial 1, the stimulus (located in chamber 1) and the subject mouse interacted for 10 min. Mice were then separated for 30 min. Trial 2 then took place, during which the subject and the stimulus mouse interacted for 5 min. Pten+/+; Slc6a4+/+ (n = 12), Pten+/−; Slc6a4+/+ (n = 10), Pten+/+; Slc6a4+/− (n = 8), and Pten+/−; Slc6a4+/− (n = 8) mice are shown. *, P < 0.05, ANOVA within-group comparison between chamber 1 and chamber 3. Error bars indicate SEM. (D) Prepulse inhibition of the acoustic startle response in 8-week-old Pten+/−; Slc6a4+/− mice. Pten+/−; Slc6a4+/+ and Pten+/−; Slc6a4+/− mice have significant deficits in startle inhibition at a prepulse 16 db above background (F3,46 = 3.6; P < 0.05). *, P < 0.05 (Tukey's HSD test). n = 11 Pten+/+; Slc6a4+/+ (8 female), 10 Pten+/−; Slc6a4+/+ (7 female), 13 Pten+/+; Slc6a4+/− (7 female), and 13 Pten+/−; Slc6a4+/− (9 female) mice.
To further examine social behavior in Pten+/− male mice, we tested 8-week-old male wild-type, Pten haploinsufficient, Slc6a4 haploinsufficient, and Pten+/−; Slc6a4+/− mice for social approach and recognition behavior. To do this, we adapted our 3-chamber apparatus to a protocol used to test social recognition in Oxytocin knockout mice, which show normal initial social investigation behavior but fail to recognize the same social target upon reexposure after 30 min (30–32). Consistent with our findings for 12-week-old male wild-type and Pten haploinsufficient mice (Fig. 2C), we did not observe a significant change in preference for social interaction in any of these genotypes in the male groups for the first 10-min trial (trial 1) (Fig. 3C). Upon reexposure to the same social target in trial 2 (5 min), wild-type mice showed equal interest in investigating the control and stimulus mouse-containing chambers, with this effect presumably due to habituation (Fig. 3C). In contrast, Pten haploinsufficient, Slc6a4 haploinsufficient, and Pten+/−; Slc6a4+/− mice did not show attenuation of preference for social investigation between the first and second trials (Fig. 3D). This finding indicates that haploinsufficiency for Pten and Slc6a4 may impair social recognition in male mice; however, further testing will be necessary to separate out a specific deficit in the social recognition pathway (i.e., oxytocin–vasopressin–dopamine) vs. sustained interest in social interaction.
In tests of prepulse inhibition, the relationship between Pten and Slc6a4 haploinsufficiency appeared to be epistatically dominant rather than additive, with Pten haploinsufficient and Pten+/−; Slc6a4+/− mice each being significantly impaired as compared to wild type but not as compared to one another (Fig. 3D). This suggests that interactions between Pten and Slc6a4 may have a degree of specificity in regard to the development of brain circuitry underlying behavior. We speculate that cooperative function of Pten and Slc6a4 is particularly important for the assembly and function of circuitry involved in social behavior, as it is for brain size control, but not necessarily for circuitry involved in other ASD-relevant behaviors such as sensorimotor gating.
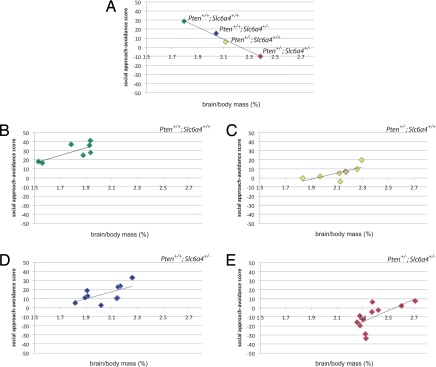
Finally, we asked whether the social approach phenotype related to brain size across genotypes and within each genotype. Analyzing population means revealed a negative correlation between brain size (relative to body mass) and social approach–avoidance score across genotypes (Fig. 4A). Surprisingly, analysis of individual subjects within each genotype showed a significant positive correlation between these measures in each group, with the exception of Slc6a4 haploinsufficient mice, which showed more variability (Fig. 4 B–E). This indicates that haploinsufficiency for Pten and Slc6a4 leads to a decrease in sociability with increase in brain size. Within each of these genotypes, however, intragroup factors appear to mediate an increase in sociability with increase in brain size.
Fig. 4.
Correlation between brain mass and sociability across and within genotypes. (A) Plot of population means for brain mass (normalized to body mass) (X-axis) and social approach–avoidance scores (Y-axis) for 8-week-old female Pten+/+; Slc6a4+/+ (n = 7), Pten+/−; Slc6a4+/+ (n = 8), Pten+/+; Slc6a4+/− (n = 10), and Pten+/−; Slc6a4+/− (n = 11) mice. r = −0.98. (B–E) Individual subjects from A, arranged by genotype, plotted for brain mass (normalized to body mass) (X-axis) and social approach–avoidance scores (Y-axis). (B) Pten+/+; Slc6a4+/+: r = 0.77; P < 0.05 (r to P conversion). (C) Pten+/−; Slc6a4+/+: r = 0.70; P < 0.05. (D) Pten+/+; Slc6a4+/−: r = 0.60; P = 0.07. (E) Pten+/−; Slc6a4+/−: r = 0.65; P < 0.05. Group sizes are different from those reported in Fig. 3 A and B because not all animals assayed for social approach were measured for brain mass.
Discussion
In regard to brain structure and function, we find that Pten haploinsufficient mice of both genders have brain overgrowth and that females have deficits in social approach behavior. We find that these phenotypes can be modified in an additive fashion by Slc6a4 haploinsufficiency. These findings are striking given that autism affects males over females at a ratio of ≈4:1. At present we do not know why we see differential effects of gender in social behavior in Pten haploinsufficient mice. It is important to emphasize that the social approach assay we have used captures only one aspect of social behavior in rodents that may be relevant to ASD. Further testing of behaviors such as ultrasonic vocalization or social reward will be necessary to fully characterize the extent and nature of social impairment in these mice. However, one interesting possible clue for these gender differences in our assays comes from reports of evidence for a link between immune system dysfunction, particularly autoimmunity, and ASD (19, 33, 34). Postmortem studies have also shown an increase in the expression of several markers for neuroinflammation in ASD (35). Importantly, Pten+/− mice have immune system abnormalities, including autoimmunity, and these effects are more pronounced in female mice as compared to male mice (36). This argues that Pten haploinsufficient mice will be a useful model to study the influence of gender and immune system dysfunction on neural development.
Mechanisms by which serotonin signaling (including Slc6a4) and the PI3K pathway (including Pten) may interact to influence brain development are illustrated in Fig. S2. One possibility is that Pten and serotonin receptors may physically interact in a regulatory manner to influence brain development. In neurons, Pten binds the 5-HT2c receptor and, via its phosphatase activity, limits agonist-induced activation of this receptor and modulates the firing rate of dopaminergic neurons in the ventral tegmental area (10). It is interesting to note that 3 drugs that have been reported as alleviating symptoms of autism—the atypical antipsychotics risperidone (37) and olanzapine (38) and the antidepressant fluoxetine (39)—all have antagonistic effects on the 5-HT2c receptor, in addition to well-known effects targeting other members of the serotonin and dopamine pathways. The degree to which antagonism of the 5-HT2c receptor is relevant for the action of these drugs in autism will be an interesting area for future investigation. It is also possible that the PI3K/Akt pathway is directly modulated by serotonin signaling. In cultured rodent hippocampal neurons, addition of 5-HT1A receptor agonist can activate Akt and this activation can be blocked via pharmacological inhibition of PI3K (40). 5-HT1A receptor is expressed in the brain as early as midembryogenesis (41), suggesting that altered activity of this receptor could modify the course of brain development even from early stages of morphogenesis. Stimulation of 5-HT1B receptor is also capable of activating Akt (42). There is evidence that this receptor modulates axonal responses to guidance cues in the developing neocortex (43) and that excess serotonin acting on this receptor is responsible for specific cytoarchitechtonic abnormalities in Slc6a4 knockout mice (44). Either mechanism could converge on molecules capable of influencing morphogenesis, growth, and neuronal function, including mTOR, GSK-3beta, Creb, and NF-kappa-B. Interestingly, serotonergic stimulation increases (45), and serotonin deficiency decreases (46), levels of GSK-3beta phosphorylated at Ser 9 in the mouse brain. Regulation of GSK-3beta at Ser 9 by Pten via Akt is involved in establishing and maintaining neuronal polarity (47), and levels of phospho-GSK-3beta are reported as elevated in the brains of mice in which Pten has been conditionally knocked out in the CNS (21). While further experiments will be necessary to verify this model, molecules upon which the serotonin and Pten-PI3K pathways converge may prove to be useful biomarkers, and therapeutic targets, for a subset of individuals with ASD.
Our data also suggest that a negative correlation exists between brain size and sociability across the genotypes examined in this study. However, at the level of individual animals within a given genotypic group, we find a significant positive correlation between brain size and sociability. We interpret this finding as indicating that, in reference to sociability, there are both beneficial and detrimental ways of changing brain size. We have identified haploinsufficiency for Pten and Slc6a4 as additively leading to correlated increases in brain size and decreased sociability. Perhaps acting secondary to this, and possibly reflecting environmental effects on neural plasticity, we speculate that specific pathways influence brain size in a beneficial manner in reference to sociability. While in autism, clinical data indicate a negative correlation between head circumference and sociability (18, 19), evidence exists from numerous studies that a positive correlation exists between brain size and social complexity in primates and other animals (reviewed in ref. 48). The possibility that distinct biological pathways influence brain size and lead to differential outcomes in regard to sociability may help explain this paradox. We hypothesize that these pathways may act as evolutionary substrates for changes in social behavior within and across species.
Our results also argue that overall brain size may serve as a convenient phenotypic readout for screening for interactions with the serotonin and PI3K pathways. It would be interesting to examine whether proposed mechanisms of ASD pathophysiology, including increased excitation/inhibition ratios (49) and altered local vs. long-distance connectivity in the frontal cortex (50), are apparent in this model. An elevated rate of de novo genomic copy number variation has been observed in ASD (51). Given that Pten has a role in the maintenance of genomic stability and that a loss of Pten results in an accumulation of double-stranded DNA breaks (52, 53), it is possible that a background of PTEN haploinsufficiency may increase the probability of a secondary modifying event, such as a copy number variation in a gene relevant to ASD, occurring. In regard to environmental modifiers, polychlorinated biphenyls (PCBs) are a class of organic compounds capable of disrupting neocortical development and are candidates for interacting with genetic susceptibility in ASD etiology (54). Experimental evidence exists for a PCB, PCB77, altering nitric oxide signaling and NF-kappa-B activity via the PI3K pathway, and this effect can be offset by inhibiting PI3K (55). Given the role of PTEN as a negative regulator of PI3K signaling, we hypothesize that haploinsufficiency for PTEN may be a genetic risk factor for abnormal brain development in response to exposure to environmental toxins that impinge on the PI3K pathway, such as PCBs.
Materials and Methods
Animals.
Strains used were B6.129-Ptentm1Rps (13) (from the National Cancer Institute) and B6.129-Slc6a4tm1Kpl (23) (from Taconic). At their respective facilities, each line was crossed to a C57BL/6 background for 10 generations to reach congenicity. We generated mice for this study from two crosses: Pten+/−; Slc6a4+/+ × Pten+/+; Slc6a4+/+ mice to yield Pten+/+; Slc6a4+/+ (“wild type”) and Pten+/−; Slc6a4+/+ (“Pten haploinsufficient”) mice, and Pten+/−; Slc6a4+/+ × Pten+/+; Slc6a4−/− mice to yield Pten+/+; Slc6a4+/− (“Slc6a4 haploinsufficient”) and Pten+/−; Slc6a4+/− (“Pten and Slc6a4 haploinsufficient”) mice. We found this approach advantageous over a Pten+/− × Slc6a4+/− mating strategy due to elevated rates of embryonic and early postnatal lethality in Pten+/−; Slc6a4+/− mice.
Behavioral testing occurred at 8 weeks or 12 weeks of age. All animals were housed in groups of 2–5 mice per cage, with no differences in housing between genotypes. Food and water were freely available and animals were kept on a 12-h light/dark cycle. All behavioral testing was carried out near the end of the light cycle. Experiments were performed according to a protocol approved by the Massachusetts Institute of Technology Committee on Animal Care and in accordance with National Institutes of Health guidelines.
Social Approach.
A mouse was placed in an open top acrylic box [24 in. long (L) × 12 in. wide (W) × 12 in. high (H)] with opaque walls. The box was divided into 3 (8 in. L × 12 in. W) chambers separated by opaque acrylic panels with holes providing passage between chambers. For reference, the chambers were numbered, from left to right 1, 2, and 3. In chamber 1, a wild-type unfamiliar sex- and strain-matched mouse was held in a clear cylindrical acrylic cage (4 in. diameter × 8 in. H) fitted with numerous holes that allow for proper ventilation and visual, tactile, and olfactory contact between the 2 mice. An identical cage was placed in chamber 3 but left empty. The orientation of the apparatus in the testing room was kept consistent from trial to trial. We have previously found that randomizing the orientation of the apparatus relative to the testing room does not alter results. Before testing, the stimulus mouse and the subject mouse were acclimated to the social approach apparatus individually for 5 min per day for 3 days. On the day of testing, the mice were again acclimated to the apparatus for 5 min. During this time, the subject mouse was observed for bias toward chamber 1 or 3—no such bias was observed for any genotype. The stimulus mouse was then added to the apparatus and a video recording was taken of a 10-min trial. The resulting video was scored with computer assistance by importing into ImageJ software (http://rsb.info.nih.gov/ij/), where each chamber was defined as a region of interest using a script written in house, and the number of frames in which the mouse was in each region of interest was quantified. All data sets were hand checked by a trained observer blind to genotype to ensure accuracy.
Olfaction.
Protocol was adapted from refs. 30 and 56. Each mouse was placed into a clean plastic cage identical to the home cage and allowed 5 min to acclimate. A cotton swab moistened with 10 μl of distilled water was inserted through the lid of the cage at a height of 10 cm for 1 min. The swab was replaced with a fresh swab twice, for a total of 3 presentations, and then followed with three 1-min presentations of swabs moistened with 10 μl vanilla extract (Frontier). After presentation of the vanilla, swabs moistened with 5 μl of lemon extract (Simply Organic) were presented 3 times for 1 min each. For each swab presentation, the frequency and duration (in seconds) of sniffs <3 cm from the swab were recorded.
Prepulse Inhibition.
We used an ASR-PRO1 acoustic startle reflex test apparatus (Med Associates). Before testing, mice were acclimated to the testing room for 1 h. Mice were acclimated to the apparatus for 2 min before the start of trials. Trials were given at an interval of 3–8 s (randomized). Trials consisted of either a 40-ms startle stimulus alone (110 db white noise) or a startle stimulus preceded 100 ms earlier by a 20-ms white noise prepulse that was 8, 12, or 16 db above background (60 db white noise). Five trials for each stimulus configuration were recorded using Startle Reflex Software (Med Associates).
Acknowledgments.
We thank Adelaide Fuller for technical assistance with experiments. We thank Ramon Parsons and Dennis Murphy for generating the Pten and Slc6a4 lines used in this paper, respectively. We thank Peter Lawrence and Birgitta Haraldson for many helpful discussions. We also thank Jacqueline Crawley for useful advice on social behavioral testing. This work was supported by funding from the Nancy Lurie Marks Family Foundation (D.P.), the Simons Foundation, and the Autism Consortium.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0804428106/DCSupplemental.
References
- 1.Cully M, You H, Levine AJ, Mak TW. Beyond PTEN mutations: the PI3K pathway as an integrator of multiple inputs during tumorigenesis. Nat Rev Cancer. 2006;6:184–192. doi: 10.1038/nrc1819. [DOI] [PubMed] [Google Scholar]
- 2.Butler MG, et al. Subset of individuals with autism spectrum disorders and extreme macrocephaly associated with germline PTEN tumour suppressor gene mutations. J Med Genet. 2005;42:318–321. doi: 10.1136/jmg.2004.024646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Goffin A, Hoefsloot LH, Bosgoed E, Swillen A, Fryns JP. PTEN mutation in a family with Cowden syndrome and autism. Am J Med Genet. 2001;105:521–524. doi: 10.1002/ajmg.1477. [DOI] [PubMed] [Google Scholar]
- 4.Herman GE, et al. Increasing knowledge of PTEN germline mutations: two additional patients with autism and macrocephaly. Am J Med Genet A. 2007;143:589–593. doi: 10.1002/ajmg.a.31619. [DOI] [PubMed] [Google Scholar]
- 5.Herman GE, et al. Genetic testing in autism: How much is enough? Genet Med. 2007;9:268–274. doi: 10.1097/gim.0b013e31804d683b. [DOI] [PubMed] [Google Scholar]
- 6.Bartlett CW, Gharani N, Millonig JH, Brzustowicz LM. Three autism candidate genes: a synthesis of human genetic analysis with other disciplines. Int J Dev Neurosci. 2005;23:221–234. doi: 10.1016/j.ijdevneu.2004.10.004. [DOI] [PubMed] [Google Scholar]
- 7.Hessl D, et al. Brief report: aggression and stereotypic behavior in males with fragile X syndrome-moderating secondary genes in a “single gene” disorder. J Autism Dev Disord. 2008;38:184–189. doi: 10.1007/s10803-007-0365-5. [DOI] [PubMed] [Google Scholar]
- 8.Wassink TH, et al. Cerebral cortical gray matter overgrowth and functional variation of the serotonin transporter gene in autism. Arch Gen Psychiatry. 2007;64:709–717. doi: 10.1001/archpsyc.64.6.709. [DOI] [PubMed] [Google Scholar]
- 9.Brune CW, et al. 5-HTTLPR genotype-specific phenotype in children and adolescents with autism. Am J Psychiatry. 2006;163:2148–2156. doi: 10.1176/ajp.2006.163.12.2148. [DOI] [PubMed] [Google Scholar]
- 10.Ji SP, et al. Disruption of PTEN coupling with 5-HT2C receptors suppresses behavioral responses induced by drugs of abuse. Nat Med. 2006;12:324–329. doi: 10.1038/nm1349. [DOI] [PubMed] [Google Scholar]
- 11.Cowen DS. Serotonin and neuronal growth factors—a convergence of signaling pathways. J Neurochem. 2007;101:1161–1171. doi: 10.1111/j.1471-4159.2006.04420.x. [DOI] [PubMed] [Google Scholar]
- 12.Eng C. PTEN: one gene, many syndromes. Hum Mutat. 2003;22:183–198. doi: 10.1002/humu.10257. [DOI] [PubMed] [Google Scholar]
- 13.Podsypanina K, et al. Mutation of Pten/Mmac1 in mice causes neoplasia in multiple organ systems. Proc Natl Acad Sci USA. 1999;96:1563–1568. doi: 10.1073/pnas.96.4.1563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Muhle R, Trentacoste SV, Rapin I. The genetics of autism. Pediatrics. 2004;113:e472–e486. doi: 10.1542/peds.113.5.e472. [DOI] [PubMed] [Google Scholar]
- 15.Marui T, et al. Association between the neurofibromatosis-1 (NF1) locus and autism in the Japanese population. Am J Med Genet B Neuropsychiatr Genet. 2004;131:43–47. doi: 10.1002/ajmg.b.20119. [DOI] [PubMed] [Google Scholar]
- 16.Mbarek O, et al. Association study of the NF1 gene and autistic disorder. Am J Med Genet. 1999;88:729–732. [PubMed] [Google Scholar]
- 17.Courchesne E, Pierce K. Brain overgrowth in autism during a critical time in development: implications for frontal pyramidal neuron and interneuron development and connectivity. Int J Dev Neurosci. 2005;23:153–170. doi: 10.1016/j.ijdevneu.2005.01.003. [DOI] [PubMed] [Google Scholar]
- 18.Courchesne E, Carper R, Akshoomoff N. Evidence of brain overgrowth in the first year of life in autism. J Am Med Assoc. 2003;290:337–344. doi: 10.1001/jama.290.3.337. [DOI] [PubMed] [Google Scholar]
- 19.Sacco R, et al. Clinical, morphological, and biochemical correlates of head circumference in autism. Biol Psychiatry. 2007;62:1038–1047. doi: 10.1016/j.biopsych.2007.04.039. [DOI] [PubMed] [Google Scholar]
- 20.Backman SA, et al. Deletion of Pten in mouse brain causes seizures, ataxia and defects in soma size resembling Lhermitte-Duclos disease. Nat Genet. 2001;29:396–403. doi: 10.1038/ng782. [DOI] [PubMed] [Google Scholar]
- 21.Kwon CH, et al. Pten regulates neuronal arborization and social interaction in mice. Neuron. 2006;50:377–388. doi: 10.1016/j.neuron.2006.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kwon CH, et al. Pten regulates neuronal soma size: a mouse model of Lhermitte-Duclos disease. Nat Genet. 2001;29:404–411. doi: 10.1038/ng781. [DOI] [PubMed] [Google Scholar]
- 23.Bengel D, et al. Altered brain serotonin homeostasis and locomotor insensitivity to 3, 4-methylenedioxymethamphetamine (“Ecstasy”) in serotonin transporter-deficient mice. Mol Pharmacol. 1998;53:649–655. doi: 10.1124/mol.53.4.649. [DOI] [PubMed] [Google Scholar]
- 24.Crawley JN. Designing mouse behavioral tasks relevant to autistic-like behaviors. Ment Retard Dev Disabil Res Rev. 2004;10:248–258. doi: 10.1002/mrdd.20039. [DOI] [PubMed] [Google Scholar]
- 25.Frankland PW, et al. Sensorimotor gating abnormalities in young males with fragile X syndrome and Fmr1-knockout mice. Mol Psychiatry. 2004;9:417–425. doi: 10.1038/sj.mp.4001432. [DOI] [PubMed] [Google Scholar]
- 26.McAlonan GM, et al. Brain anatomy and sensorimotor gating in Asperger's syndrome. Brain. 2002;125:1594–1606. doi: 10.1093/brain/awf150. [DOI] [PubMed] [Google Scholar]
- 27.Perry W, Minassian A, Lopez B, Maron L, Lincoln A. Sensorimotor gating deficits in adults with autism. Biol Psychiatry. 2007;61:482–486. doi: 10.1016/j.biopsych.2005.09.025. [DOI] [PubMed] [Google Scholar]
- 28.Brodkin ES, Hagemann A, Nemetski SM, Silver LM. Social approach-avoidance behavior of inbred mouse strains towards DBA/2 mice. Brain Res. 2004;1002:151–157. doi: 10.1016/j.brainres.2003.12.013. [DOI] [PubMed] [Google Scholar]
- 29.Nadler JJ, et al. Automated apparatus for quantitation of social approach behaviors in mice. Genes Brain Behav. 2004;3:303–314. doi: 10.1111/j.1601-183X.2004.00071.x. [DOI] [PubMed] [Google Scholar]
- 30.Crawley JN, et al. Social approach behaviors in oxytocin knockout mice: comparison of two independent lines tested in different laboratory environments. Neuropeptides. 2007;41:145–163. doi: 10.1016/j.npep.2007.02.002. [DOI] [PubMed] [Google Scholar]
- 31.Ferguson JN, Aldag JM, Insel TR, Young LJ. Oxytocin in the medial amygdala is essential for social recognition in the mouse. J Neurosci. 2001;21:8278–8285. doi: 10.1523/JNEUROSCI.21-20-08278.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ferguson JN, et al. Social amnesia in mice lacking the oxytocin gene. Nat Genet. 2000;25:284–288. doi: 10.1038/77040. [DOI] [PubMed] [Google Scholar]
- 33.Cabanlit M, Wills S, Goines P, Ashwood P, Van de Water J. Brain-specific autoantibodies in the plasma of subjects with autistic spectrum disorder. Ann NY Acad Sci. 2007;1107:92–103. doi: 10.1196/annals.1381.010. [DOI] [PubMed] [Google Scholar]
- 34.Sweeten TL, Bowyer SL, Posey DJ, Halberstadt GM, McDougle CJ. Increased prevalence of familial autoimmunity in probands with pervasive developmental disorders. Pediatrics. 2003;112:e420. doi: 10.1542/peds.112.5.e420. [DOI] [PubMed] [Google Scholar]
- 35.Vargas DL, Nascimbene C, Krishnan C, Zimmerman AW, Pardo CA. Neuroglial activation and neuroinflammation in the brain of patients with autism. Ann Neurol. 2005;57:67–81. doi: 10.1002/ana.20315. [DOI] [PubMed] [Google Scholar]
- 36.Di Cristofano A, et al. Impaired Fas response and autoimmunity in Pten+/− mice. Science. 1999;285:2122–2125. doi: 10.1126/science.285.5436.2122. [DOI] [PubMed] [Google Scholar]
- 37.McCracken JT, et al. Risperidone in children with autism and serious behavioral problems. N Engl J Med. 2002;347:314–321. doi: 10.1056/NEJMoa013171. [DOI] [PubMed] [Google Scholar]
- 38.Malone RP, Cater J, Sheikh RM, Choudhury MS, Delaney MA. Olanzapine versus haloperidol in children with autistic disorder: an open pilot study. J Am Acad Child Adolesc Psychiatry. 2001;40:887–894. doi: 10.1097/00004583-200108000-00009. [DOI] [PubMed] [Google Scholar]
- 39.Cook EH, Jr, Rowlett R, Jaselskis C, Leventhal BL. Fluoxetine treatment of children and adults with autistic disorder and mental retardation. J Am Acad Child Adolesc Psychiatry. 1992;31:739–745. doi: 10.1097/00004583-199207000-00024. [DOI] [PubMed] [Google Scholar]
- 40.Cowen DS, Johnson-Farley NN, Travkina T. 5-HT receptors couple to activation of Akt, but not extracellular-regulated kinase (ERK), in cultured hippocampal neurons. J Neurochem. 2005;93:910–917. doi: 10.1111/j.1471-4159.2005.03107.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bonnin A, Peng W, Hewlett W, Levitt P. Expression mapping of 5-HT1 serotonin receptor subtypes during fetal and early postnatal mouse forebrain development. Neuroscience. 2006;141:781–794. doi: 10.1016/j.neuroscience.2006.04.036. [DOI] [PubMed] [Google Scholar]
- 42.Hsu EH, Lochan AC, Cowen DS. Activation of Akt1 by human 5-hydroxytryptamine (serotonin)1B receptors is sensitive to inhibitors of MEK. J Pharmacol Exp Ther. 2001;298:825–832. [PubMed] [Google Scholar]
- 43.Bonnin A, Torii M, Wang L, Rakic P, Levitt P. Serotonin modulates the response of embryonic thalamocortical axons to netrin-1. Nat Neurosci. 2007;10:588–597. doi: 10.1038/nn1896. [DOI] [PubMed] [Google Scholar]
- 44.Salichon N, et al. Excessive activation of serotonin (5-HT) 1B receptors disrupts the formation of sensory maps in monoamine oxidase a and 5-ht transporter knock-out mice. J Neurosci. 2001;21:884–896. doi: 10.1523/JNEUROSCI.21-03-00884.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Li X, et al. In vivo regulation of glycogen synthase kinase-3beta (GSK3beta) by serotonergic activity in mouse brain. Neuropsychopharmacology. 2004;29:1426–1431. doi: 10.1038/sj.npp.1300439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Beaulieu JM, et al. Role of GSK3 beta in behavioral abnormalities induced by serotonin deficiency. Proc Natl Acad Sci USA. 2008;105:1333–1338. doi: 10.1073/pnas.0711496105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jiang H, Guo W, Liang X, Rao Y. Both the establishment and the maintenance of neuronal polarity require active mechanisms: critical roles of GSK-3beta and its upstream regulators. Cell. 2005;120:123–135. doi: 10.1016/j.cell.2004.12.033. [DOI] [PubMed] [Google Scholar]
- 48.Dunbar RI, Shultz S. Evolution in the social brain. Science. 2007;317:1344–1347. doi: 10.1126/science.1145463. [DOI] [PubMed] [Google Scholar]
- 49.Rubenstein JL, Merzenich MM. Model of autism: increased ratio of excitation/inhibition in key neural systems. Genes Brain Behav. 2003;2:255–267. doi: 10.1034/j.1601-183x.2003.00037.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Courchesne E, Pierce K. Why the frontal cortex in autism might be talking only to itself: local over-connectivity but long-distance disconnection. Curr Opin Neurobiol. 2005;15:225–230. doi: 10.1016/j.conb.2005.03.001. [DOI] [PubMed] [Google Scholar]
- 51.Sebat J, et al. Strong association of de novo copy number mutations with autism. Science. 2007;316:445–449. doi: 10.1126/science.1138659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Puc J, Parsons R. PTEN loss inhibits CHK1 to cause double stranded-DNA breaks in cells. Cell Cycle. 2005;4:927–929. doi: 10.4161/cc.4.7.1795. [DOI] [PubMed] [Google Scholar]
- 53.Shen WH, et al. Essential role for nuclear PTEN in maintaining chromosomal integrity. Cell. 2007;128:157–170. doi: 10.1016/j.cell.2006.11.042. [DOI] [PubMed] [Google Scholar]
- 54.Kenet T, Froemke RC, Schreiner CE, Pessah IN, Merzenich MM. Perinatal exposure to a noncoplanar polychlorinated biphenyl alters tonotopy, receptive fields, and plasticity in rat primary auditory cortex. Proc Natl Acad Sci USA. 2007;104:7646–7651. doi: 10.1073/pnas.0701944104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lim EJ, Smart EJ, Toborek M, Hennig B. The role of caveolin-1 in PCB77-induced eNOS phosphorylation in human-derived endothelial cells. Am J Physiol Heart Circ Physiol. 2007;293:H3340–H3347. doi: 10.1152/ajpheart.00921.2007. [DOI] [PubMed] [Google Scholar]
- 56.Luo AH, et al. Impaired olfactory behavior in mice deficient in the alpha subunit of G(o) Brain Res. 2002;941:62–71. doi: 10.1016/s0006-8993(02)02566-0. [DOI] [PubMed] [Google Scholar]