Abstract
The electrical gradient across the mitochondrial inner membrane (Ψm) is established by electron transport chain (ETC) activity and permits mitochondrial Ca2+ sequestration. Using rhodamine-123, we determined how repetitive nerve stimulation (100 Hz) affects Ψm in motor terminals innervating mouse levator auris muscles. Stimulation-induced Ψm depolarizations in wild-type (WT) terminals were small (<5 mV at 30 °C) and reversible. These depolarizations depended on Ca2+ influx into motor terminals, as they were inhibited when P/Q-type Ca2+ channels were blocked with ω-agatoxin. Stimulation-induced Ψm depolarization and elevation of cytosolic [Ca2+] both increased when complex I of the ETC was partially inhibited by low concentrations of rotenone (25–50 nmol/l). This finding is consistent with the hypothesis that acceleration of ETC proton extrusion normally limits the magnitude of Ψm depolarization during mitochondrial Ca2+ uptake, thereby permitting continued Ca2+ uptake. Compared with WT, stimulation-induced increases in rhodamine-123 fluorescence were ≈5 times larger in motor terminals from presymptomatic mice expressing mutations of human superoxide dismutase I (SOD1) that cause familial amyotrophic lateral sclerosis (SOD1-G85R, which lacks dismutase activity; SOD1-G93A, which retains dismutase activity). Ψm depolarizations were not significantly altered by expression of WT human SOD1 or knockout of SOD1 or by inhibiting opening of the mitochondrial permeability transition pore with cyclosporin A. We suggest that an early functional consequence of the association of SOD1-G85R or SOD1-G93A with motoneuronal mitochondria is reduced capacity of the ETC to limit Ca2+-induced Ψm depolarization, and that this impairment contributes to disease progression in mutant SOD1 motor terminals.
Keywords: amyotrophic lateral sclerosis, calcium, mitochondria, motor nerve terminals
Mitochondria sequester significant amounts of stimulation-induced Ca2+ loads in many cell types (1–9). This mitochondrial Ca2+ uptake occurs via a Ca2+ uniporter/channel (10) down a potential gradient (Ψm, 150–200 mV, matrix negative) established by ETC activity (11, 12). Entry of Ca2+ into mitochondria depolarizes Ψm, which would be expected to reduce the gradient driving Ca2+ uptake. However, in motor nerve terminals, mitochondrial Ca2+ uptake continues throughout prolonged trains of action potentials (13). One possible explanation for this apparent paradox is that the Ψm depolarization produced by Ca2+ entry reduces the gradient against which ETC complexes I, III and IV extrude protons, thus accelerating ETC proton extrusion (14). This acceleration would limit the net Ψm depolarization, thereby allowing mitochondria to continue taking up Ca2+ even during prolonged stimulation (15).
We tested this hypothesis in mouse motor terminals, and found that the Ψm depolarizations produced by repetitive stimulation at 50–100 Hz were Ca2+ dependent and reversible, and were small (or undetectable) at near-physiological temperatures (30 °C). Partially inhibiting ETC activity with low concentrations of rotenone or low temperature increased the amplitude of these depolarizations.
We also tested motor terminals in transgenic mice expressing mutant versions of human SOD1 (G85R, G93A) that produce familial amyotrophic lateral sclerosis (fALS) (16–18). In these and other transgenic mice expressing fALS-inducing SOD1 mutations, motor terminals begin to degenerate well before motor neuron somata in the spinal cord begin to die, and some of the earliest reported morphological changes occur in motor terminal mitochondria (19–24). Misfolded fALS-linked mutant SOD1s associate with mitochondria (cytoplasmic face, intermembrane space, inner membrane, matrix) (25–34), and impaired ETC activity has been reported in the spinal cord of mice expressing SOD1-G93A (25, 30, 35–37). Damiano et al. (38) demonstrated a reduction in Ca2+ loading capacity in spinal cord mitochondria from both SOD1-G93A and SOD1-G85R mice. We hypothesized that this reduced ability to sequester Ca2+ loads might be caused by reduced ability to accelerate mitochondrial proton extrusion in response to the Ψm depolarization produced by Ca2+ entry. Consistent with this hypothesis, we demonstrate that at 30 °C stimulation-induced Ψm depolarizations are greater in SOD1-G85R and SOD1-G93A mice than in WT mice, and that this increase does not depend on dismutase activity. This is the first demonstration in presymptomatic fALS mice of an altered response to Ca2+ loads in mitochondria located exclusively within motor neurons (as distinct from surrounding glial cells).
Results
During repetitive stimulation at near-physiological temperatures, Ψm depolarization is small.
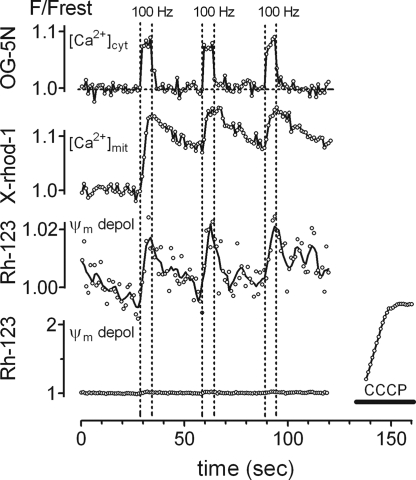
Fig. 1 shows representative changes in cytosolic [Ca2+], mitochondrial [Ca2+], and Ψm recorded in WT motor nerve terminals stimulated with three trains of action potentials (100 Hz, 5 seconds). The topmost trace shows the elevation of cytosolic [Ca2+], measured as the normalized increase in fluorescence (F/Frest) of Oregon Green 488 BAPTA-5N (OG-5N) that had been ionophoretically injected into the axoplasm. After an initial rapid increase at the onset of stimulation, the rate of rise slows, due mainly to Ca2+ sequestration by mitochondria (5, 9). When stimulation stops, cytosolic [Ca2+] shows a rapid initial decrease. Measurements of cytosolic [Ca2+] made with a higher affinity Ca2+ indicator than that used here reveal an additional slowly-decaying phase caused in part by Ca2+ extrusion from mitochondria via a Na+/Ca2+ exchanger (39).
Fig. 1.
Stimulation at 100 Hz increases cytosolic and mitochondrial [Ca2+] and depolarizes Ψm in WT mouse motor terminals at 30 °C. (A) Upper trace: Elevation of cytosolic [Ca2+] in response to three stimulus trains at 100 Hz, monitored as normalized increases (F/Frest) in the fluorescence of intra-axonally injected OG-5N (vertical lines indicate duration of stimulation). The average increase in cytosolic [Ca2+] is 1.0 μmol/l above an assumed resting value of 0.1 μmol/l (64). Second trace: Elevations of mitochondrial matrix [Ca2+], monitored as increases in the fluorescence of mitochondrially-loaded X-rhod-1 (mean of five traces). The average increase in mitochondrial [Ca2+] is ≈1–2 μmol/l above an assumed resting value of 0.05–0.1 μmol/l (13, 40, 41). Third trace: Depolarization of Ψm, monitored as increases in the fluorescence of Rh-123. The line is a smoothed moving bin average of three neighboring points. Lower trace shows (using a different vertical scale) these same data, along with the much larger Ψm depolarization produced in this terminal by the proton carrier CCCP (2 μmol/l). Cytosolic [Ca2+], mitochondrial [Ca2+], and Ψm were recorded from different preparations, aged 64–88 days.
The second trace in Fig. 1 shows the stimulation-induced increase in mitochondrial [Ca2+], measured as the fluorescence of mitochondrially-loaded X-rhod-1. Mitochondrial [Ca2+] exhibits a slower rate of rise and a slower decay than cytosolic [Ca2+] (5, 39). Mitochondrial [Ca2+] responses evoked by the second and third stimulus trains begin during the decaying phase of the response to previous trains, but the peak fluorescence evoked by subsequent trains does not exceed that evoked by the first train. This “cap” on the maximal increase in mitochondrial [Ca2+] is not due to saturation of the indicator dye (40), and is unlikely to be caused by decreased mitochondrial Ca2+ entry during subsequent trains, because both the sustained elevation of cytosolic [Ca2+] and recordings of transmitter release indicate that action potentials continue to admit Ca2+ into the cytosol throughout the train (9, 39), and the increase in cytosolic [Ca2+] produced by each train is similar. Also, if mitochondrial Ca2+ sequestration is blocked, cytosolic [Ca2+] rises progressively during stimulation (5, 9). Rather, the maximal increase in matrix [Ca2+] is limited by formation of an insoluble complex containing Ca and phosphate that reversibly sequesters matrix Ca2+ (41). The maximal increase in mitochondrial [Ca2+] is only ≈1–2 μmol/l (13, 40, 41).
The third trace in Fig. 1 shows stimulation-induced depolarizations of Ψm, recorded as increases in rhodamine 123 (Rh-123) fluorescence. In this motor terminal, each stimulus train reversibly increased fluorescence by ≈2%. The bottom trace shows these same data on a different vertical scale, along with the much larger increase in Rh-123 fluorescence (≈140%) measured during the near-complete Ψm depolarization produced by the proton carrier carbonyl cyanide m-chloro phenyl hydrazone (CCCP, 2 μmol/l). At 28–30 °C the mean increase in Rh-123 fluorescence after 500 stimuli at 100 Hz was only 0.92% ± 0.13% (SEM, range 0–4.5%, n = 47 terminals). These measurements are described further in SI Text Item #1 and Fig. S1), including analysis suggesting that this fluorescence increase corresponds to a Ψm depolarization of 3–5 mV.
Stimulation-induced Ψm depolarizations are Ca2+ dependent.
To study mechanisms underlying stimulation-induced Ψm depolarizations, we needed to increase the magnitude of the recorded Rh-123 signal. Fig. 2A demonstrates that signal magnitude increased when the temperature was reduced, or when action potential duration (and thus Ca2+ entry) was prolonged using 3,4-diaminopyridine (3,4-DAP, 20 μmol/l), which blocks certain depolarization-activated K+ channels in motor axons and terminals (42–44).
Fig. 2.
The stimulation-induced Ψm depolarization increases with cooling or addition of 3,4-diaminopyridine (3,4-DAP) and requires Ca2+ entry through plasma membrane Ca2+ channels. (A) The Ψm depolarization produced by 100 Hz stimulation at 30 °C (left) was increased by cooling to 18 °C (upper right) or by prolonging the action potential with 20 μmol/l 3,4-DAP (lower right). (B) Ψm depolarizations (the magnitudes of which were enhanced by both cooling to 20 °C and 3,4-DAP, open circles) were reduced by omitting Ca2+ from the bath (filled circles, left) or (in a different terminal) by adding 0.6 μmol/l ω-agatoxin-TK (filled circles, right). The effects of low bath [Ca2+] were readily reversible; reversal of agatoxin effects was slow and incomplete. Each record in (A) and (B) is the mean of two to nine traces. The effects of cooling, 3,4-DAP, and removal of bath Ca2+ were observed in 10, 5, and 4 additional terminals, respectively. Exposures to 3,4-DAP and agatoxin were 7–62 minutes and 60 minutes, respectively.
Fig. 2B shows that stimulation-induced Ψm depolarizations were inhibited both by replacing bath Ca2+ with Mg2+ and by ω-agatoxin TK (0.6 μmol/l), which blocks the P/Q-type (Cav2.1) Ca2+ channels that mediate most Ca2+ entry into motor terminals in mice (45, 46). Thus the Na+ influx associated with axonal action potential propagation, which continues in low [Ca2+] and ω-agatoxin, is not by itself sufficient to produce stimulation-induced Ψm depolarizations. Rather, these depolarizations require Ca2+ influx into motor terminals.
The elevation of cytosolic [Ca2+] increases with increasing frequencies of stimulation (5) (Fig. S2A). In isolated mitochondria, Ca2+ influx through the uniporter/channel exhibits a greater-than-linear dependence on external [Ca2+] (11). Thus one would predict faster Ψm depolarization with higher stimulation frequencies. As predicted, Ψm depolarized more rapidly during 100 Hz than during 25 Hz stimulation (Fig. S2B).
Partial inhibition of ETC complex I increases the stimulation-induced Ψm depolarization.
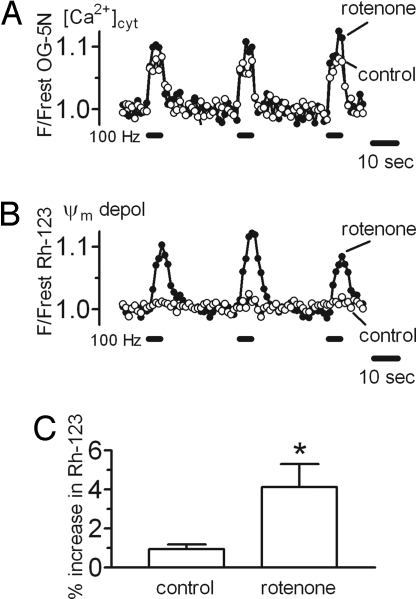
Results in Fig. 2 link stimulation-induced Ψm depolarizations to Ca2+ entry into motor terminals but do not prove a linkage to Ca2+ influx into mitochondria. It is difficult to block mitochondrial Ca2+ influx selectively, because Ru360, which blocks the uniporter/channel (47), has limited permeability across plasma membranes and appears to reduce Ca2+ influx across motor terminal membranes (13) (Fig. S3). Fig. 3B and 3C link stimulation-induced Ψm depolarizations to mitochondrial function by demonstrating that partial inhibition of complex I of the ETC with low concentrations of rotenone (25–50 nmol/l) produced a 4-fold increase in the Rh-123 signal evoked by 100 Hz stimulation. This result is consistent with the prediction that reducing mitochondrial ability to accelerate ETC activity in response to stimulation-induced Ca2+ influx will increase the magnitude of the resulting Ψm depolarization. In cell lines, neurons, and isolated nerve terminals, these low rotenone concentrations have been reported to decrease complex I activity by 20–85% but to have little effect on resting Ψm (48–53). Likewise, we found that these low rotenone concentrations had no significant effect on resting Rh-123 fluorescence, suggesting little or no effect on resting Ψm (SI Text Item #2).
Fig. 3.
A low concentration of rotenone increases stimulation-induced elevations of cytosolic [Ca2+] and Ψm depolarizations. Cytosolic [Ca2+] (A) and Ψm depolarizations (B) produced by three trains at 100 Hz before and after addition of rotenone (50 nM). [Ca2+] and Ψm traces came from different terminals. (C) Paired data from eight terminals studied before and after rotenone exposure show that in mice expressing normal SOD1 (WT or human) rotenone increased the average change in Rh-123 fluorescence from 0.96% ± 0.22% (SEM) in control medium to 4.13% ± 1.18% in rotenone (* P < 0.05). Only measurements from the initial 100 Hz train were included in the averages. The duration of rotenone exposure was 17–30 minutes. Mice were 50–375 days old.
Greater Ψm depolarization during stimulation would be predicted to reduce the electrical gradient that permits Ca2+ influx into mitochondria and thus increase the elevation of cytosolic [Ca2+]. Fig. 3A demonstrates the predicted increase of cytosolic [Ca2+] in rotenone.
Stimulation-induced Ψm depolarizations are enhanced in mice expressing mutant human superoxide dismutase 1.
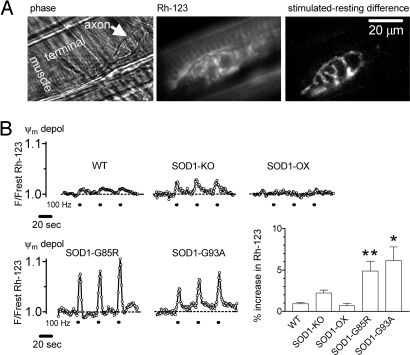
If motor terminal mitochondria in presymptomatic SOD1-G85R and SOD1-G93A mice have a reduced ability to accelerate ETC activity (see Introduction), then one would predict a greater stimulation-induced Ψm depolarization. Fig. 4A presents phase and resting Rh-123 fluorescence images of a terminal in a presymptomatic SOD1-G85R mouse. The difference image highlights those regions in which Rh-123 fluorescence increased during stimulation, showing signals specific to motor terminal mitochondria. Fig. 4B shows Rh-123 recordings from this SOD1-G85R mouse, as well as representative traces from presymptomatic SOD1-G93A and WT terminals. The bar graph in Fig. 4B shows that the average stimulation-induced increase in Rh-123 fluorescence in SOD1-G85R and SOD1-G93A motor terminals was ≈5-fold greater than that in WT terminals.
Fig. 4.
Stimulation-induced Ψm depolarizations are increased in presymptomatic SOD1-G85R and SOD1-G93A mice. (A) Phase (left) and Rh-123 fluorescence (middle) images show a resting motor terminal in a 121-day-old SOD1-G85R mouse. At right is a difference image of the same region, calculated by subtracting the resting fluorescence from the fluorescence during 100 Hz stimulation. (B) Representative stimulation-induced Ψm depolarizations evoked by repeated brief 100 Hz trains in a WT mouse (mean of two traces), a mouse lacking SOD1 (SOD1-KO), an “overexpressor” mouse with both normal mouse and normal human SOD1 (SOD1-OX), the SOD1-G85R terminal in (A), and an SOD1-G93A terminal. Bars shows mean peak amplitude (±SEM) for the first 100-Hz train for each of these groups. The Ψm depolarizations in SOD1-G85R (P < 0.01) and SOD1-G93A (P < 0.05) mice were significantly different from WT at 30 °C (analysis of variance followed by Dunnett's multiple comparison test). These differences were not significant at lower temperatures, where mitochondrial Ca2+ uptake may be reduced (64). Data were averaged from 47 terminals in 16 WT mice 50–145 days old; comparable values for SOD-KO mice were 19, 3, and 253–355; for SOD1-OX 5, 4, and 89–375; for SOD1-G85R mice 60, 6, and 121–161; for SOD1-G93A 13, 6, and 43–85. At these ages, all endplates were fully innervated. Additional details in SI Text, Item #3.
SOD1 helps to defend against oxidative damage by catalyzing the conversion of superoxide to hydrogen peroxide. SOD1-G93A retains SOD1 enzymatic activity, but SOD1-G85R lacks this activity (54), although heterodimers of G85R and wt SOD1 show activity (55). The fact that both types of mutant SOD1 terminals showed increased Ψm depolarizations suggests that this increase did not depend on the level of SOD1 activity. This suggestion was tested further by conducting similar experiments on mice lacking SOD1 activity altogether, and on mice with excess SOD1 activity due to expression of normal human SOD1. Fig. 4B shows that stimulation-induced Ψm depolarizations in these knockout and overexpressing mice were not significantly different from those recorded in WT terminals.
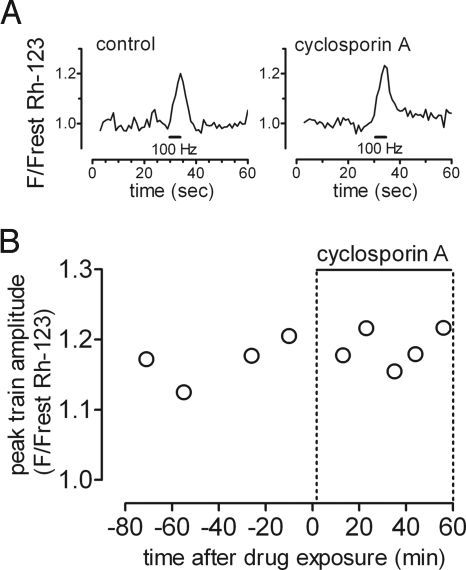
Another possible mechanism underlying the increased stimulation-induced Ψm depolarizations measured in mutant SOD1 mice is transient opening of the mitochondrial permeability transition pore (MPTP). However, Fig. 5 shows that cyclosporin A, which inhibits pore opening, did not reduce the magnitude of these depolarizations in SOD1-G85R mice.
Fig. 5.
Stimulation-induced Ψm depolarizations in SOD1-G85R mice are not reduced by cyclosporin A. (A) Representative traces show the Rh-123 fluorescence response evoked by a 100-Hz stimulus train before and 56 minutes after exposure to 5 μmol/l cyclosporin. (B) Graph plots peak amplitudes (normalized to resting fluorescence) before and during drug exposure for this terminal. Similar experiments on three additional SOD1-G85R mice (130–150 days) also showed no significant change in Ψm depolarizations in cyclosporin A (paired Wilcoxon signed rank test, P > 0.50). Similar experiments in additional mouse types (e.g., SOD1 knockout treated with 50 μmol/l 3,4-diaminopyridine) confirmed the lack of effect of 8–10 μmol/l cyclosporin A.
Discussion
Determinants of the stimulation-evoked Ψm depolarization in mouse motor terminals.
Data presented here demonstrate that the Ψm depolarization produced by repetitive stimulation depends on Ca2+ influx into motor terminals. In WT mice this Ψm depolarization is small, reversible, and repeatable, in contrast to the large Ψm depolarizations measured after applying large Ca2+ loads to isolated mitochondria (56). Some of the difference in the magnitude and reversibility of the Ca2+-induced Ψm depolarization may be due to the fact that the Ca2+ load was delivered physiologically via repetitive nerve stimulation to mitochondria in situ. Isolated mitochondria can take up larger amounts of Ca2+ with minimal Ψm depolarization when the Ca2+ load is presented in increments rather than as a bolus (41).
To determine whether the magnitude of the stimulation-induced Ψm depolarization is consistent with electrical models of mitochondrial function, we estimated the current entering mitochondria using the initial slope of the mitochondrial [Ca2+] response to stimulation (as in Fig. 1). The depolarization expected from this current was then estimated using an electrical model of the mitochondrial inner membrane consisting of the resistance of the ETC (in series with a battery representing the electron motive force) in parallel with the resistance of the mitochondrial membrane (SI Text Item #4). These calculations yielded a predicted Ψm depolarization of ≈2 mV, similar to the value estimated independently from the magnitude of the stimulation-induced change in Rh-123 fluorescence (SI Text Item #1). Ψm depolarizations of this magnitude are sufficient to accelerate ETC activity significantly (57).
According to this electrical model of the mitochondrial membrane, the magnitude of the stimulation-induced Ψm depolarization can be varied in at least two ways. The first is to alter the magnitude of the Ca2+ current that enters mitochondria: this is the likely explanation for the changes in the magnitude of the Ψm depolarization produced by reducing (with ω-agatoxin) or increasing (with 3,4-DAP) the magnitude of the stimulation-induced Ca2+ influx into the motor terminal. The second way is to change ETC activity; this is the likely mechanism underlying the increase in the stimulation-induced Ψm depolarization produced by partial blockade of complex I with rotenone. Partial ETC inhibition produces a greater Ψm depolarization in stimulated than in resting terminals (15). Synergistic damaging effects of partial complex I inhibition and Ca2+ challenges have also been documented in other preparations (58, 59).
The larger stimulation-induced Ψm depolarizations in SOD1-G85R and SOD1-G93A motor terminals likely reflect reduced ability to accelerate ETC activity after Ca2+ entry.
The Introduction section cited morphological evidence for early mitochondrial damage in motor terminals and motor neurons of mutant SOD1 models of fALS, as well as functional evidence for reduced activity of certain ETC complexes and reduced capacity for Ca2+ uptake in spinal cord mitochondria. Work presented here demonstrates that rotenone (low concentration) mimics the effect of fALS mutations on stimulation-induced Ψm depolarizations, and that these depolarizations are not reduced by cyclosporin A. Given these past and present findings, a logical explanation for the larger Ψm depolarizations recorded in motor terminals of SOD1-G85R and SOD1-G93A mice is that their mitochondria have a defect in the ETC (or its regulation) that reduces their ability to accelerate ETC activity in response to the depolarization produced by Ca2+ entry. Synaptic mitochondria are more sensitive than somatic mitochondria to both complex I inhibition and Ca2+ overload (60, 61). This might explain the finding that, in fALS mice, motor terminal damage precedes damage to the motor neuron cell body.
Deficits in mitochondrial Ca2+ handling would be expected to be most evident in motor terminals innervating fast muscles, whose tendency to discharge in high frequency bursts (62) would expose them to larger Ca2+ loads and Ψm depolarizations than motor terminals innervating slow muscle. Consistent with this idea, motor terminals innervating fast limb muscles are the earliest to degenerate in fALS mice (20, 24, 63).
In summary, mitochondria have multiple features that permit them to sequester the Ca2+ loads introduced by repetitive stimulation, including powerful Ca2+ buffering within the matrix, and acceleration of ETC activity in response to the depolarization produced by Ca2+ entry, hence preserving the electrical gradient that favors Ca2+ entry. Evidence presented here suggests that the Ca2+-dependent Ψm depolarization produced by repetitive stimulation is <5 mV in WT motor terminals, but increases in mitochondria of presymptomatic SOD1-G85R and SOD1-G93A terminals. These Ψm depolarizations would be expected to increase in terminals of older mutant SOD1 mice as ETC function deteriorates, impairing both mitochondrial Ca2+ sequestration and ATP synthesis.
Materials and Methods
Experiments used neuromuscular junctions from the levator auris longus muscle of WT, hSOD1-G85R, and hSOD1-G93A mice, as well as mice that express normal human SOD1 and mice lacking SOD1. Most experiments were conducted at 30 °C, the warmest temperature at which the dissected preparation could remain functional for several hours. Action potentials were evoked in the motor nerve by applying suprathreshold 0.3 ms depolarizing pulses (500–2000 at 50–100 Hz) via a suction electrode. Muscle contractions were blocked with d-tubocurarine (7–15 μmol/l).
Stimulation-induced changes in cytosolic [Ca2+] were monitored using OG-5N (Kd 60 μmol/l) injected ionophoretically into an internodal axon (64). Stimulation-induced changes in mitochondrial [Ca2+] were measured using the indicator X-rhod-1, loaded as described (39). Changes in Ψm were measured using Rh-123, as described (15). The preparation was imaged using a spinning disk confocal microscope system designed to use low excitation light intensities. Spatially averaged changes in fluorescence were plotted as F/Frest, where Frest is the average net, background-subtracted fluorescence calculated from 20–30 images obtained before stimulation began. SI Text Item #5 contains additional methodological details.
Supplementary Material
Acknowledgments.
This work was supported by grants from the Amyotrophic Lateral Sclerosis Association, Muscular Dystrophy Association, and National Institutes of Health (fellowship 1F31NS054606 to L.G.C., R01s NS 12404, and NS 58888). We thank Don Cleveland and Carlos Moraes for providing transgenic mice.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0810934106/DCSupplemental.
References
- 1.Friel DD, Tsien RW. An FCCP-sensitive Ca2+ store in bullfrog sympathetic neurons and its participation in stimulus-evoked changes in [Ca2+]i. J Neurosci. 1994;14:4007–4024. doi: 10.1523/JNEUROSCI.14-07-04007.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stuenkel EL. Regulation of intracellular calcium and calcium buffering properties of rat isolated neurohypophysial nerve endings. J Physiol. 1994;481:251–271. doi: 10.1113/jphysiol.1994.sp020436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Herrington J, Park YB, Babcock DF, Hille B. Dominant role of mitochondria in clearance of large Ca2+ loads from rat adrenal chromaffin cells. Neuron. 1996;16:219–228. doi: 10.1016/s0896-6273(00)80038-0. [DOI] [PubMed] [Google Scholar]
- 4.Babcock DF, Herrington J, Goodwin PC, Park YB, Hille B. Mitochondrial participation in the intracellular Ca2+ network. J Cell Biol. 1997;136:833–844. doi: 10.1083/jcb.136.4.833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.David G, Barrett JN, Barrett EF. Evidence that mitochondria buffer physiological Ca2+ loads in lizard motor nerve terminals. J Physiol. 1998;509:59–65. doi: 10.1111/j.1469-7793.1998.059bo.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pivovarova NB, Hongpaisan J, Andrews SB, Friel DD. Depolarization-induced mitochondrial Ca accumulation in sympathetic neurons: Spatial and temporal characteristics. J Neurosci. 1999;19:6372–6384. doi: 10.1523/JNEUROSCI.19-15-06372.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kaftan EJ, Xu T, Abercrombie RF, Hille B. Mitochondria shape hormonally induced cytoplasmic calcium oscillations and modulate exocytosis. J Biol Chem. 2000;275:25465–25470. doi: 10.1074/jbc.M000903200. [DOI] [PubMed] [Google Scholar]
- 8.Suzuki S, et al. Ca2+-dependent Ca2+ clearance via mitochondrial uptake and plasmalemmal extrusion in frog motor nerve terminals. J Neurophysiol. 2002;87:1816–1823. doi: 10.1152/jn.00456.2001. [DOI] [PubMed] [Google Scholar]
- 9.David G, Barrett EF. Mitochondrial Ca2+ uptake prevents desynchronization of quantal release and minimizes depletion during repetitive stimulation of mouse motor nerve terminals. J Physiol. 2003;548:425–438. doi: 10.1113/jphysiol.2002.035196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kirichok Y, Krapivinsky G, Clapham DE. The mitochondrial calcium uniporter is a highly selective ion channel. Nature. 2004;427:360–364. doi: 10.1038/nature02246. [DOI] [PubMed] [Google Scholar]
- 11.Gunter TE, Pfeiffer DR. Mechanisms by which mitochondria transport calcium. Am J Physiol. 1990;258:C755–C786. doi: 10.1152/ajpcell.1990.258.5.C755. [DOI] [PubMed] [Google Scholar]
- 12.Nicholls DG, Chalmers S. The integration of mitochondrial calcium transport and storage. J Bionenerg Biomembr. 2004;36:277–281. doi: 10.1023/B:JOBB.0000041753.52832.f3. [DOI] [PubMed] [Google Scholar]
- 13.David G. Mitochondrial clearance of cytosolic Ca2+ in stimulated lizard motor nerve terminals proceeds without progressive elevation of mitochondrial matrix [Ca2+] J Neurosci. 1999;19:7495–7506. doi: 10.1523/JNEUROSCI.19-17-07495.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nicholls DG, Ferguson SJ. Bioenergetics 3. London, UK: Academic Press; 2002. [Google Scholar]
- 15.Talbot J, Barrett JN, Barrett EF, David G. Stimulation-induced changes in NADH fluorescence and mitochondrial membrane potential in lizard motor nerve terminals. J Physiol. 2007;579:783–798. doi: 10.1113/jphysiol.2006.126383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bruijn LI, et al. ALS-linked SOD1 mutant G85R mediates damage to astrocytes and promotes rapidly progressive disease with SOD1-containing inclusions. Neuron. 1997;18:327–338. doi: 10.1016/s0896-6273(00)80272-x. [DOI] [PubMed] [Google Scholar]
- 17.Gurney ME, et al. Motor neuron degeneration in mice that express a human Cu/Zn superoxide dismutase mutation. Science. 1994;264:1772–1775. doi: 10.1126/science.8209258. [DOI] [PubMed] [Google Scholar]
- 18.Chiu AY, et al. Age-dependent penetrance of disease in a transgenic mouse model of familial amyotrophic lateral sclerosis. Mol Cell Neurosci. 1995;6:349–362. doi: 10.1006/mcne.1995.1027. [DOI] [PubMed] [Google Scholar]
- 19.Kong J, Xu Z. Massive mitochondrial degeneration in motor neurons triggers the onset of amyotrophic lateral sclerosis in mice expressing a mutant SOD1. J Neurosci. 1998;18:3241–3250. doi: 10.1523/JNEUROSCI.18-09-03241.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Frey D, Schneider C, Xu L, Borg J, Spooren W, Caroni P. Early and selective loss of neuromuscular synapse subtypes with low sprouting competence in motoneuron diseases. J Neurosci. 2000;20:2534–2542. doi: 10.1523/JNEUROSCI.20-07-02534.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fischer LR, et al. Amyotrophic lateral sclerosis is a distal axonopathy: Evidence in mice and man. Exp Neurol. 2004;185:232–240. doi: 10.1016/j.expneurol.2003.10.004. [DOI] [PubMed] [Google Scholar]
- 22.Schaefer AM, Sanes JR, Lichtman JW. A compensatory subpopulation of motor neurons in a mouse model of amyotrophic lateral sclerosis. J Comp Neurol. 2005;490:209–219. doi: 10.1002/cne.20620. [DOI] [PubMed] [Google Scholar]
- 23.Gould TW, et al. Complete dissociation of motor neuron death from motor dysfunction by Bax deletion in a mouse model of ALS. J Neurosci. 2006;26:8774–8786. doi: 10.1523/JNEUROSCI.2315-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pun S, Santos AF, Saxena S, Xu L, Caroni P. Selective vulnerability and pruning of phasic motoneuron axons in motoneuron disease alleviated by CNTF. Nat Neurosci. 2006;9:408–419. doi: 10.1038/nn1653. [DOI] [PubMed] [Google Scholar]
- 25.Mattiazzi M, et al. Mutated human SOD1 causes dysfunction of oxidative phosphorylation in mitochondria of transgenic mice. J Biol Chem. 2002;277:29626–29633. doi: 10.1074/jbc.M203065200. [DOI] [PubMed] [Google Scholar]
- 26.Higgins CM, Jung C, Xu Z. ALS-associated mutant SOD1G93A causes mitochondrial vacuolation by expansion of the intermembrane space and by involvement of SOD1 aggregation and peroxisomes. BMC Neurosci. 2003;4:16. doi: 10.1186/1471-2202-4-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sasaki S, Warita H, Murakami T, Abe K, Iwata M. Ultrastructural study of mitochondria in the spinal cord of transgenic mice with a G93A mutant SOD1 gene. Acta Neuropathol. 2004;107:461–474. doi: 10.1007/s00401-004-0837-z. [DOI] [PubMed] [Google Scholar]
- 28.Vijayvergiya C, Beal MF, Buck J, Manfredi G. Mutant superoxide dismutase I forms aggregates in the brain mitochondrial matrix of amyotrophic lateral sclerosis mice. J Neurosci. 2005;25:2463–2470. doi: 10.1523/JNEUROSCI.4385-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ferri A, et al. Familial ALS-superoxide dismutases associate with mitochondria and shift their redox potentials. Proc Natl Acad Sci USA. 2006;103:13860–13865. doi: 10.1073/pnas.0605814103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Goldsteins G, et al. Deleterious role of superoxide dismutase in the mitochondrial intermembrane space. J Biol Chem. 2008;283:8446–8452. doi: 10.1074/jbc.M706111200. [DOI] [PubMed] [Google Scholar]
- 31.Kawamata H, Manfredi G. Different regulation of wild type and mutant Cu,Zn superoxide dismutase localization in mammalian mitochondria. Hum Mol Genet. 2008;17:3303–3317. doi: 10.1093/hmg/ddn226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ahtoniemi T, Jaronen M, Keksa-Goldsteine V, Goldsteins G, Koistinaho J. Mutant SOD1 from spinal cord of G93A rats is destabilized and binds to inner mitochondrial membrane. Neurobiol Dis. 2008;32:479–485. doi: 10.1016/j.nbd.2008.08.010. [DOI] [PubMed] [Google Scholar]
- 33.Vande Velde C, Miller TM, Cashman NR, Cleveland DW. Selective association of misfolded ALS-linked mutant SOD1 with the cytoplasmic face of mitochondria. Proc Natl Acad Sci USA. 2008;105:4022–4027. doi: 10.1073/pnas.0712209105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Liu J, et al. Toxicity of familial ALS-linked SOD1 mutants from selective recruitment to spinal mitochondria. Neuron. 2004;43:5–17. doi: 10.1016/j.neuron.2004.06.016. [DOI] [PubMed] [Google Scholar]
- 35.Jung C, Higgins CMJ, Xu Z. A quantitative histochemical assay for activities of mitochondrial electron transport chain complexes in mouse spinal cord sections. J Neurosci Methods. 2002;114:165–172. doi: 10.1016/s0165-0270(01)00524-6. [DOI] [PubMed] [Google Scholar]
- 36.Kirkinezos IG, et al. Cytochrome c association with the inner mitochondrial membrane is impaired in the CNS of G93A-SOD1 mice. J Neurosci. 2005;25:164–172. doi: 10.1523/JNEUROSCI.3829-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Son M, et al. Isolated cytochrome c oxidase deficiency in G93A SOD1 mice over-expressing CCS protein. J Biol Chem. 2008;283:12267–12275. doi: 10.1074/jbc.M708523200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Damiano M, et al. Neural mitochondrial Ca2+ capacity impairment precedes the onset of motor symptoms in G93A Cu/Zn-superoxide dismutase mutant mice. J Neurochem. 2006;96:1349–1361. doi: 10.1111/j.1471-4159.2006.03619.x. [DOI] [PubMed] [Google Scholar]
- 39.García-Chacón LE, Nguyen KT, David G, Barrett EF. Extrusion of Ca2+ from mouse motor terminal mitochondria via a Na+-Ca2+ exchanger increases post-tetanic evoked release. J Physiol. 2006;574:663–675. doi: 10.1113/jphysiol.2006.110841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.David G, Talbot JD, Barrett EF. Quantitative estimate of mitochondrial [Ca2+] in stimulated motor nerve terminals. Cell Calcium. 2003;33:197–206. doi: 10.1016/s0143-4160(02)00229-4. [DOI] [PubMed] [Google Scholar]
- 41.Chalmers S, Nicholls DG. The relationship between free and total calcium concentrations in the matrix of liver and brain mitochondria. J Biol Chem. 2003;278:19062–19070. doi: 10.1074/jbc.M212661200. [DOI] [PubMed] [Google Scholar]
- 42.Tabti N, Bourret C, Mallart A. Three potassium currents in mouse motor nerve terminals. Pflugers Arch. 1989;413:395–400. doi: 10.1007/BF00584489. [DOI] [PubMed] [Google Scholar]
- 43.Morita K, Barrett EF. Evidence for two calcium-dependent potassium conductances in lizard motor nerve terminals. J Neurosci. 1990;10:2614–2625. doi: 10.1523/JNEUROSCI.10-08-02614.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.David G, Modney B, Scappaticci KA, Barrett JN, Barrett EF. Electrical and morphological factors influencing the depolarizing after-potential in rat and lizard myelinated axons. J Physiol. 1995;489:141–157. doi: 10.1113/jphysiol.1995.sp021037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Teramoto T, et al. A novel peptide from funnel web spider venom, omega-Aga-TK, selectively blocks P-type calcium channels. Biochem Biophys Res Commun. 1993;196:134–140. doi: 10.1006/bbrc.1993.2225. [DOI] [PubMed] [Google Scholar]
- 46.Westenbroek RE, Hoskins L, Catterall WA. Localization of Ca2+ channel subtypes on rat spinal motor neurons, interneurons, and nerve terminals. J Neurosci. 1998;18:6319–6330. doi: 10.1523/JNEUROSCI.18-16-06319.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Matlib MA, et al. Oxygen-bridged dinuclear ruthenium amine complex specifically inhibits Ca2+ uptake into mitochondria in vitro and in situ in single cardiac myocytes. J Biol Chem. 1998;273:10223–10231. doi: 10.1074/jbc.273.17.10223. [DOI] [PubMed] [Google Scholar]
- 48.Higgins DS, Jr., Greenamyre JT. [3H]dihydrorotenone binding to NADH: Ubiquinone reductase (complex I) of the electron transport chain: An autoradiographic study. J Neurosci. 1996;16:3807–3816. doi: 10.1523/JNEUROSCI.16-12-03807.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Barrientos A, Moraes CT. Titrating the effects of mitochondrial complex I impairment in the cell physiology. J Biol Chem. 1999;274:16188–16197. doi: 10.1074/jbc.274.23.16188. [DOI] [PubMed] [Google Scholar]
- 50.Sipos I, Tretter L, Adam-Vizi V. Quantitative relationship between inhibition of respiratory complexes and formation of reactive oxygen species in isolated nerve terminals. J Neurochem. 2003;84:112–118. doi: 10.1046/j.1471-4159.2003.01513.x. [DOI] [PubMed] [Google Scholar]
- 51.Li N, et al. Mitochondrial complex I inhibitor rotenone induces apoptosis through enhancing mitochondrial reactive oxygen species production. J Biol Chem. 2003;278:8516–8525. doi: 10.1074/jbc.M210432200. [DOI] [PubMed] [Google Scholar]
- 52.Kweon GR, et al. Distinct mechanisms of neurodegeneration induced by chronic complex I inhibition in dopaminergic and non-dopaminergic cells. J Biol Chem 2004. 2004;279:51783–51792. doi: 10.1074/jbc.M407336200. [DOI] [PubMed] [Google Scholar]
- 53.Kilbride SM, Telford JE, Tipton KF, Davey GP. Partial inhibition of complex I activity increases Ca2+-independent glutamate release rates from depolarized synaptosomes. J Neurochem. 2008;106:826–834. doi: 10.1111/j.1471-4159.2008.05441.x. [DOI] [PubMed] [Google Scholar]
- 54.Valentine JS, Doucette PA, Zittin Potter S. Copper-zinc superoxide dismutase and amyotrophic lateral sclerosis. Annu Rev Biochem. 2005;74:563–593. doi: 10.1146/annurev.biochem.72.121801.161647. [DOI] [PubMed] [Google Scholar]
- 55.Witan H, et al. Heterodimer formation of wild-type and amyotrophic lateral sclerosis-causing mutant Cu/Zn-Superoxide dismutase induces toxicity independent of protein aggregation. Hum Mol Genet. 2008;17:1373–1385. doi: 10.1093/hmg/ddn025. [DOI] [PubMed] [Google Scholar]
- 56.Vergun O, Reynolds IJ. Distinct characteristics of Ca2+-induced depolarization of isolated brain and liver mitochondria. Biochim Biophys Acta. 2005;1709:127–137. doi: 10.1016/j.bbabio.2005.07.006. [DOI] [PubMed] [Google Scholar]
- 57.Johnson-Cadwell LI, Jekabsons MB, Wang A, Polster BM, Nicholls DG. ‘Mild uncoupling’ does not decrease mitochondrial superoxide levels in cultured cerebellar granule neurons but decreases spare respiratory capacity and increases toxicity to glutamate and oxidative stress. J Neurochem. 2007;101:1619–1631. doi: 10.1111/j.1471-4159.2007.04516.x. [DOI] [PubMed] [Google Scholar]
- 58.Votyakova TV, Reynolds IJ. Ca2+-induced permeabilization promotes free radical release from rat brain mitochondria with partially inhibited complex I. J Neurochem. 2005;93:526–537. doi: 10.1111/j.1471-4159.2005.03042.x. [DOI] [PubMed] [Google Scholar]
- 59.Kwong JQ, Henning MS, Starkov AA, Manfredi G. The mitochondrial respiratory chain is a modulator of apoptosis. J Cell Biol. 2007;179:1163–1177. doi: 10.1083/jcb.200704059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Davey GP, Canevari L, Clark JB. Threshold effects in synaptosomal and nonsynaptic mitochondria from hippocampal CA1 and paramedian neocortex brain regions. J Neurochem. 1997;69:2564–2570. doi: 10.1046/j.1471-4159.1997.69062564.x. [DOI] [PubMed] [Google Scholar]
- 61.Brown MR, Sullivan PG, Geddes JW. Synaptic mitochondria are more susceptible to Ca2+ overload than nonsynaptic mitochondria. J Biol Chem. 2006;281:11658–11668. doi: 10.1074/jbc.M510303200. [DOI] [PubMed] [Google Scholar]
- 62.Burke RE. The structure and function of motor units. In: Engel AG, Franzini-Armstrong C, editors. Myology. 3rd Ed. New York: McGraw-Hill; 2004. pp. 104–118. [Google Scholar]
- 63.Hegedus J, Putman CT, Tyreman N, Gordon T. Preferential motor unit loss in the SOD1G93A transgenic mouse model of amyotrophic lateral sclerosis. J Physiol. 2008;586:3337–3351. doi: 10.1113/jphysiol.2007.149286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.David G, Barrett EF. Stimulation-evoked increases in cytosolic [Ca2+] in mouse motor nerve terminals are limited by mitochondrial uptake and are temperature-dependent. J Neurosci. 2000;20:7290–7296. doi: 10.1523/JNEUROSCI.20-19-07290.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.