Abstract
Pituitary adenylyl cyclase-activating polypeptide (PACAP) is a widely expressed neuropeptide originally discovered in the hypothalamus. It closely resembles vasoactive intestinal peptide (VIP), a neuropeptide well known to inhibit macrophage activity, promote Th2-type responses, and enhance regulatory T cell (Treg) production. Recent studies have shown that administration of PACAP, like VIP, can attenuate dramatically the clinical and pathological features of murine models of autoimmune diseases such as experimental autoimmune encephalomyelitis (EAE) and collagen-induced arthritis. However, specific roles (if any) of endogenous VIP and PACAP in the protection against autoimmune diseases have not been explored. Here, we subjected PACAP-deficient mice to myelin oligodendrocyte glycoprotein (MOG35–55)-induced EAE. MOG immunization of PACAP-deficient mice triggered heightened clinical and pathological manifestations of EAE compared to wild-type mice. The increased sensitivity was accompanied by enhanced mRNA expression of proinflammatory cytokines (TNFα, IL-6, IFN-γ, IL-12p35, IL-23p19, and IL-17), chemokines (MCP-1/CCL2, MIP-1α/CCL3, and RANTES/CCL5), and chemotactic factor receptors (CCR1, CCR2, and CCR5), but downregulation of the anti-inflammatory cytokines (IL-4, IL-10, and TGF-β) in the spinal cord. Moreover, the abundance of CD4+CD25+FoxP3+ Tregs in lymph nodes and levels of FoxP3 mRNA in the spinal cord were also diminished. The reduction in Tregs was associated with increased proliferation and decreased TGF-β secretion in lymph node cultures stimulated with MOG. These results demonstrate that endogenous PACAP provides protection in EAE and identify PACAP as an intrinsic regulator of Treg abundance after inflammation.
Keywords: autoimmunity, regulatory T cell, inflammation, knock-out, multiple sclerosis
Multiple sclerosis (MS) afflicts over 2.5 million people worldwide, with women affected 3 times more frequently than men. Although its precise etiology is unknown, MS has been characterized as a Th1- and/or Th17-mediated autoimmune disease (1). Experimental autoimmune encephalomyelitis (EAE), commonly induced by immunizing with various myelin autoantigens, is an experimental model of MS that allows investigators to study the pathogenesis of this disease and to test new therapeutic strategies (2). Indeed EAE is an acute or chronic-relapsing inflammatory and demyelinating autoimmune disease with resemblance to MS in humans. The major features shared are the autoimmune destruction of the myelin sheaths of the nerve fibers, the presence of multiple CNS lesions with a predominantly perivascular location, and a temporal maturation of lesions from inflammation through demyelination, and finally axonal destruction.
Pituitary adenylyl cyclase-activating polypeptide (PACAP) is a 38-amino-acid neuropeptide identified by Arimura and colleagues in 1989 from ovine hypothalamus (3) and belongs to the secretin family. Its closest family member is vasoactive intestinal peptide (VIP), to which it is 68% identical. The actions of PACAP and VIP are mediated by 3 heterotrimeric G protein-coupled receptors, 1 (PAC1), which is highly specific for PACAP except for 1 splice variant (4), and 2 (VPAC1 and VPAC2) which interact with PACAP and VIP with equally high affinity (5). Although PACAP is widely distributed in the CNS and peripheral nervous system and is classically viewed as a neurotransmitter, it is capable of eliciting a broad spectrum of biological actions (5). Numerous results indicate that it exerts growth factor-like activities in the brain during development, regeneration, and tumorigenesis (6–10). Indeed, considerable data indicate that PACAP, mainly via PAC1, regulates neural cell proliferation, survival, axon regeneration and oligodendrocyte progenitor proliferation and maturation (6–9, 11–13).
A series of studies beginning in the early 1980s implicated VIP as a regulator of immune function (14). These studies revealed that VIP can modulate both innate and adaptive immunity, showing a predominant anti-inflammatory action on macrophages, promoting a positive Th2/Th1 cytokine balance, and enhancing the production of regulatory T cells (Tregs) (15). Later it was found that PACAP could mimic many of these actions, suggesting the involvement of VPAC receptors. Moreover, it was determined that both VIP and PACAP are expressed in lymphocytes and other immune cell types (16–18), indicating that modulation of inflammation could result from either neurogenic or immunogenic neuropeptide expression. Finally, in the last decade, an increasing body of in vivo data indicates that VPAC receptors may be promising targets for the treatment of inflammatory diseases, and in particular, Th1-associated pathologies such as MS and rheumatoid arthritis. Indeed, administration of VIP and/or PACAP was found to dramatically reduce the clinical symptoms and inflammatory pathology in murine EAE (19, 20), collagen-induced arthritis (21, 22), and chemical-induced colitis models (23, 24). In the case of the EAE model, VIP and PACAP were shown to suppress myelin oligodendrocyte glycoprotein (MOG35–55)-induced disease in C57BL/6 mice, a monoepisodic model, and proteolipid protein (PLP139–151)-induced disease in SJL/J mice, a relapsing-remitting model (19, 20).
Mechanistic studies to explain the action of these peptides in inflammatory disease models have mainly focused on the action of VIP. For example, VIP administration after EAE induction resulted in decreased spinal cord levels of proinflammatory cytokines, chemokines, and chemokine receptors, and increased levels of the anti-inflammatory cytokines. Lymph nodes from VIP-treated mice showed reduced antigen-induced proliferation, and lower and higher productions of Th1 and Th2 cytokines, respectively. Subsequent work showed that administration of VIP resulted in the expansion of functional CD4+CD25+FoxP3+ expressing T cells (Tregs) in the periphery and the CNS (25). While PACAP has been shown to mimic the action of VIP on Treg production in vitro (26), no studies have addressed the modulatory role of PACAP on Treg production in vivo. Finally, despite considerable investigation of the immunomodulatory actions of administered peptide, the precise and specific roles of endogenously produced PACAP and VIP in immune system homeostasis remain entirely unknown. Here we used PACAP-deficient (KO) mice (27) and the MOG35–55 EAE model to study the potential actions of endogenous PACAP in autoimmune CNS disease. The results reveal a protective role of PACAP in this disease model, with actions impacting Th1, Th2, Th17, and Treg subsets.
Results
PACAP KO Mice Develop More Severe EAE than Wild-Type (WT) Mice After MOG Immunization.
Female C57BL/6 WT and C57BL/6-backcrossed PACAP KO mice were immunized s.c. with 100 μg of MOG35–55 peptide in complete Freund's adjuvant (CFA) followed by pertussis toxin as described in Materials and Methods section, and the severity of the disease was evaluated daily. In the case of the WT mice, clinical symptoms were first observed on day 7 postimmunization (Fig. 1A). The mean clinical score peaked on day 14, and declined from days 18 to 30 in a recovery phase. In PACAP KO mice, the clinical curve profile was almost parallel to that of the WT mice until day 14. After this time point, the average clinical score did not drop as in the WT mice, but continued increasing until day 20, when it reached a maximum in severity. Chronic and severe disease persisted until the end of the study. Overall, PACAP KO mice showed higher EAE severity, with an average maximum score of 2.25 ± 0.52, in comparison with the WT mice, which only reached an average score of 1.5 ± 0.28. Whereas 3 of 8 PACAP KO mice reached the highest score in the scale (= 4), none of the WT mice exceeded a score of 2.5 during the development of EAE. Similar results were observed in additional experiments. A combined analysis of all experiments, which included 28 WT and 28 PACAP KO mice, indicated a mean peak score (± SEM) of 1.6 ± 0.1 for WT vs. 2.5 ± 0.1 for PACAP KO mice (P < 0.01). Moreover, there was no mortality in the group of WT mice, whereas PACAP KO mice had a mortality rate of ≈30% (Fig. 1B). Thus, the absence of PACAP resulted in an exacerbated clinical course of EAE. This was consistent with histological findings of the inflamed CNS tissue on day 30, which revealed that PACAP KO mice exhibited a much higher degree and more widespread inflammation than in WT mice (Fig. 2). Collectively, PACAP KO mice developed more severe EAE, both clinically and pathologically, than WT mice.
Fig. 1.
Development of clinical symptoms of EAE in WT and PACAP KO mice. PACAP−/− and WT mice were immunized with MOG35–55 peptide in CFA and i.p. injected twice with pertussis toxin. Mice were scored daily for the EAE symptoms over 30 days using the scale described in Materials and Methods. (A) Representative data from 1 of 3 experiments (n = 8/genotype). The EAE score is shown as mean ± SEM. *, P < 0.05 (Student's t test). (B) Kaplan-Meier survival for all mice examined in the 3 experiments (n = 28/genotype), indicating for each genotype the percentage of surviving individuals with time. The survival curves were significantly different (P < 0.001, logrank test).
Fig. 2.
EAE lesions and histopathology scores of EAE in WT and PACAP KO mice. Representative spinal cord sections from WT and PACAP−/− mice showing lymphocyte and macrophage infiltrations detected by staining with hematoxiline/eosin and myelin by Luxol fast blue (A–D). (A) WT spinal cord control, vehicle injected. (B and C) MOG-induced WT and PACAP KO mice, respectively. (D) Higher magnification photo of spinal cord of a PACAP KO mouse. Arrows in B–D point to foci of inflammatory cell infiltrations. (E) Mean pathological scores in each group (n = 5/genotype). Histopathological grading was evaluated following the scale described in Materials and Methods. ND, none detected. ***, P < 0.001 (Student's t test).
Expression of Proinflammatory Cytokines and Chemokines Is Enhanced in the Spinal Cord of PACAP KO Mice After EAE Induction.
Similar to other inflammatory conditions, the pathogenesis of EAE is associated with the release of cytokines and chemokines that consolidate the disease. Therefore, we analyzed the expression of inflammatory cytokines, chemokines, and adhesion molecules in the spinal cord of MOG-immunized WT and PACAP KO mice by quantitative real time RT-PCR. On day 14 in WT mice (the peak of clinical symptoms), we observed the expected upregulated gene expression of proinflammatory cytokines TNFα, IL-6, IFN-γ, IL-17 (IL-17A), IL-12p35 and IL-23p19 (Fig. 3). Likewise, there was an increase in gene expression of IL-10, IL-4, and TGF-β, which are considered to counteract or limit the inflammatory response in some contexts. In PACAP KO mice, MOG-induction of TNFα, IFN-γ, and IL-17 mRNA were significantly enhanced over that observed in WT controls, while IL-10, IL-4, and TGF-β gene expression were remarkably diminished (Fig. 3). IL-6, IL-12p35, and IL-23p19 mRNA levels were also upregulated in PACAP KO mice, although not substantially different from the levels in WT mice at 14 days. Analysis of cytokine gene expression in spinal cord extracts on day 30 (data not shown) indicated that mRNA levels of nearly all proinflammatory and anti-inflammatory cytokines dropped in parallel to the reduction of EAE symptoms in WT mice. However, in PACAP KO mice, even though gene expression for the anti-inflammatory cytokines was also decreased on day 30, mRNA levels of proinflammatory cytokines remained as high as on day 14, with a near doubling of IL-12p35 mRNA levels (0.095 ± 0.005 vs. 0.188 ± 0.006 arbitrary units on day 14 and day 30, respectively, P < 0.001). These results show that the PACAP gene deletion results in higher proinflammatory and lower anti-inflammatory cytokine gene expression in the spinal cord during EAE, a finding that tended to become more pronounced at 30 days.
Fig. 3.
Cytokine gene expression in the spinal cord of EAE-induced WT and PACAP KO mice. EAE was induced with MOG35–55 or vehicle in WT and PACAP KO mice, and at 14 days postimmunization, cytokine gene expression was determined by real-time RT-PCR (corrected for the housekeeping gene HPRT), as described in Materials and Methods section. Data are representative of 3 experiments. *, P < 0.05; **, P < 0.01; and ***, P < 0.001. NS, nonsignificant. In WT mice, all mRNAs were significantly induced (P < 0.001).
In addition to cytokine production, the upregulation of adhesion molecules and chemokines has been shown to facilitate the inflammatory environment in the CNS by allowing the recruitment of macrophages and other leukocytes. Therefore, we examined the expression of various adhesion molecules, chemokines, and chemokine receptors in the spinal cord of EAE mice at day 14. MOG-immunization produced a significant increase in the mRNA levels of I-CAM (3.2-fold), V-CAM (4.6-fold), MCP-1/CCL2 (2.7-fold), MIP-1α/CCL3 (6.3-fold), and RANTES/CCL5 (4.4-fold) in the WT mice (Fig. 4). Similar to results on proinflammatory cytokine gene expression, the upregulation of each of these molecules was significantly more pronounced in the PACAP KO than in the WT mice, with increases of 5.4-fold for I-CAM and V-CAM, 3.1-fold for MCP-1, 11.2-fold for MIP-1α, and 9.9-fold for RANTES. These chemokines bind to receptors located on infiltrating immune cells and microglia. Thus, the expression of chemokine receptors reflects the degree of cellular infiltration in the inflamed tissue. Similar results to chemokines were obtained for CCR1, CCR2, and CCR5 mRNA levels, with greater fold inductions in PACAP KO vs. WT mice (6.3 vs. 2.9 for CCR1, 2.1 vs. 1.9 for CCR2, and 16.1 vs. 8.1 for CCR5) (Fig. 4). Overall, these results demonstrate that spinal cord gene expression of proinflammatory mediators was significantly higher, and anti-inflammatory cytokines lower, in MOG-induced PACAP KO vs. WT mice, in line with clinical observations of more severe and prolonged symptoms of EAE.
Fig. 4.
Cell adhesion molecule and chemokine gene expression in spinal cords of EAE-induced WT and PACAP KO mice. Gene expression was determined in spinal cord extracts by real-time RT-PCR 14 days after immunization with MOG or vehicle. Determinations were performed on 5 mice in each group. Data are representative of 3 experiments. *, P < 0.05; **, P < 0.01; and ***, P < 0.001. In WT mice, all mRNAs were significantly induced (P < 0.001).
Cell Proliferation and Th1/Th17 Responses Are Increased in PACAP KO Mice After Antigenic Challenge in Vitro.
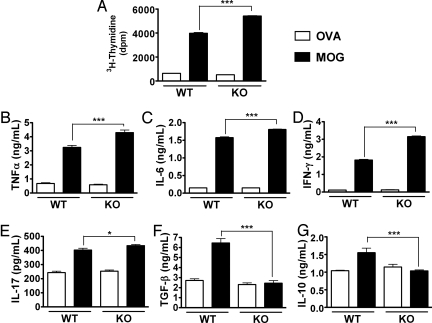
Next, we examined T lymphocyte proliferation and cytokine production in immunized PACAP KO vs. WT mice in response to MOG rechallenge in vitro. Both PACAP KO and WT mice were immunized as in previous experiments. Fourteen days later, draining lymph nodes were collected and single-cell suspensions were prepared. Suspensions were cultured in the presence of MOG or ovalbumin (OVA) as a control. In response to MOG, WT mice cells proliferated and released high levels of proinflammatory and anti-inflammatory cytokines as determined by ELISA (Fig. 5). However, the antigen-specific responses to MOG were more pronounced in cultures from PACAP KO vs. WT mice. First, PACAP KO mice cells exhibited a 40% higher degree of T cell proliferation in response to MOG (Fig. 5A). Second, the inductions of proinflammatory cytokines TNFα and IL-6 were significantly greater in PACAP KO mice compared with WT mice (4.3 ± 0.18 vs. 3.2 ± 0.14, and 1.8 ± 0.01 vs.1.6 ± 0.02 ng/mL, respectively) (Fig. 5B and 5C, P < 0.001 with Student's t test). Thirdly, PACAP KO cells released higher levels of IFN-γ and IL-17 (Fig. 5 D and E), which are the hallmarks of Th1 and Th17 responses, respectively. These results suggest that T lymphocytes from immunized PACAP KO mice exhibited a stronger Th1/Th17 proinflammatory phenotype than cells from immunized WT mice. Moreover, the antigen-specific inductions of TGF-β and IL-10, 2 Treg-associated cytokines, were completely blocked in PACAP KO mice (Fig. 5 F and G), suggesting a strongly reduced presence of regulatory T cells in these cultures. These cytokine data obtained by ELISA were mirrored qualitatively at the level of gene expression, assessed by real time RT-PCR (data not shown). Moreover, although we were unable to detect the Th2 cytokine IL-4 in culture supernatants by ELISA, we found by real time RT-PCR that IL-4 gene expression in PACAP KO cells stimulated with MOG was lower than that in stimulated cells of WT animals (0.128 ± 0.0015 vs. 0.152 ± 0.008 arbitrary units, respectively). Therefore, the absence of PACAP was associated with an increased T cell response to the priming antigen, with a more pronounced Th1/Th17, but an impaired Th2/Treg response. These results may explain in part the higher reactivity and more prolonged inflammation in MOG-treated PACAP KO mice compared to immunized WT controls.
Fig. 5.
MOG-induced proliferation and cytokine release in lymph node cultures of MOG-induced WT and PACAP KO mice. Draining lymph node cells from EAE-induced WT and PACAP KO mice (day 14) were stimulated ex vivo with MOG35–55 peptide or OVA (10 μg/mL). Proliferation (A) was measured 72 h later by [3H]-thymidine incorporation. ***, P < 0.001 (Student's t test). Cytokines (B–G) secreted into the culture supernatant were measured by ELISA 48 h after stimulation with MOG. *, P < 0.05 and ***, P < 0.001. In WT mice, all parameters measured were significantly induced by MOG (P < 0.001). Data in A–G are representative of 3 experiments.
PACAP KO Mice Exhibit a Defect in Regulatory T Cell Abundance.
Tregs play a role as intrinsic suppressors of self-reactive cells, and appear to be involved in the recovery phase of EAE (28, 29). It has been previously shown that PACAP supports Treg differentiation (26), and absence of PACAP could result in a defect in this cell subset. Indeed, the hyperproliferative response of T cells and the reduced levels of TGF-β production in the PACAP KO mice support this hypothesis. To compare the effect of the induction of EAE on Treg abundance in WT vs. PACAP KO mice, mice were killed on day 20 after MOG immunization, a time corresponding to the recovery phase in WT mice, and when Tregs are expected to have expanded and be most active (28). Draining lymph nodes (axillary and brachial) were harvested and the numbers of Tregs as CD4+CD25+FoxP3+ cells were determined by flow cytometry. The results indicate that PACAP KO mice had a 60% reduction in the percentage of CD4+ cells that were Treg (CD25+FoxP3+) (Fig. 6A−E). In parallel, we also quantified the Treg subset in naïve PACAP KO and WT lymph nodes and we found a mild diminution in PACAP KO mice that was not statistically significant (Fig. 6E, white bars). In addition, the ratio of effector T cells (Teff)/Treg (CD4+FoxP3−/CD4+FoxP3+) in the lymph nodes was higher in immunized PACAP KO vs. WT mice (9.0 vs. 4.3, data not shown). Finally, we looked for the relative mRNA expression for FoxP3, a specific marker for regulatory T cells in the spinal cord. FoxP3 mRNA levels in cords of WT and PACAP KO mice were increased around 3.5-fold compared to the respective controls at day 14 postimmunization (data not shown). However, whereas mRNA levels remained high in WT mice at day 30, FoxP3 gene expression dropped substantially in PACAP KO mice, so mRNA levels at this time in PACAP KO mice were significantly lower than in WT mice (0.148 ± 0.002 vs. 0.224 ± 0.01 arbitrary units, respectively; P < 0.001, Fig. 6F). This may reflect a deficiency of Tregs in the CNS occurring during the later course of the disease, and potentially explains the slow recovery of these mice to EAE induction.
Fig. 6.
FACS analyses of Th subsets in lymph nodes and FoxP3 spinal cord gene expression in naïve and MOG-induced WT and PACAP KO mice. Draining lymph nodes (axillary and brachial) were removed from naïve mice and EAE-induced mice 20 days after immunization with MOG (A–E). The Treg subpopulation was stained with CD4, CD25, and FoxP3 (FJK-16s) antibodies as instructed by the mouse regulatory T cell staining kit from eBioscience. (F) FoxP3 gene expression in spinal cords in naïve mice and in EAE-induced mice 30 days after MOG administration. ***, P < 0.001 (Student's t test).
The Expression of TGF-β Is Reduced in Dendritic Cells, Macrophages, and T Cells in MOG-Induced Lymph Node Cultures from Immunized PACAP KO Mice.
We found above that the EAE induction of TGF-β production in lymph node cultures was completely blocked in PACAP KO mice (Fig. 5). TGF-β, is a hallmark cytokine for Treg differentiation and can be produced by multiple immune cell types including macrophages, dendritic cells, and Tregs themselves, the latter sometimes referred to as Th3 cells (30). An important potential action of PACAP in Th differentiation might be the regulation of TGF-β production, so we examined potential cellular sources of TGF-β in FACS-sorted MOG-induced lymph node cultures from immunized and nonimmunized WT and PACAP KO mice by real time RT-PCR. In WT mice, MOG immunization resulted in an induction of TGF-β gene expression in macrophages (CD11b+), dendritic cells (CD11c+), and Th cells (CD4+) (Fig. 7). However, the upregulation in CD4+ and CD11c+ cells was completely blocked in PACAP KO mice. Curiously, in macrophages, TGF-β mRNA levels were elevated in PACAP KO mice, without EAE, but were not further induced by EAE induction. While the reduction of TGF-β gene expression in CD4+ cells might simply reflect the decreased numbers of Tregs observed in the PACAP KO mice, the attenuated reduction of TGF-β mRNA levels in macrophage and dendritic cells suggest that these cell types might be important targets of PACAP relevant to Th differentiation.
Fig. 7.
Identification of TGF-β cellular source in lymph node cultures. Draining lymph node cells from EAE-induced WT and PACAP KO mice (day 20) were stimulated ex vivo with MOG35–55 peptide or OVA (10 μg/mL). Forty-eight hours later, CD11b+, CD11c+, and CD4+ cells were sorted and analyzed for TGF-β expression by real-time RT-PCR. *, P < 0.05; **, P < 0.01; and ***, P < 0.001 (Student's t test). NS, nonsignificant. These data are representative of 2 experiments.
Discussion
Despite the interest in using PACAP and VIP as anti-inflammatory agents in human disease, the role of the endogenous peptides in the protection against autoimmune disease has not been previously explored. To begin to investigate the role of endogenous PACAP, we subjected PACAP KO mice to MOG-induced EAE. We found that PACAP-deficient mice showed significantly enhanced EAE compared to controls, with heightened clinical severity, increased mortality, and a delayed and diminished recovery phase. This was associated with an enhanced accumulation of immune cells and increased demyelination in the spinal cord accompanied by a hyperinduction of proinflammatory cytokines and chemokines. This was further associated with enhanced Th1 and Th17, diminished Th2 activity in the CNS and lymph nodes, a reduction of Treg abundance in the lymph nodes, and reduced FoxP3 gene expression in the spinal cord. PACAP could act by way of its release from either neurons or inflammatory cells. PACAP gene expression is upregulated in dorsal root ganglia neurons following target inflammation (31) and in motor neurons following application of an inflammatory stimulus directly to the nerve (32) and after axotomy (33, 34). On the other hand, while PACAP innervation of the lymph nodes and spleen has not been reported, Abad et al. demonstrated the presence of PACAP in thymocytes, T lymphocytes, B cells, and plasma cells of the lymphoid organs and peritoneum using immunohistochemistry, immunocytochemical staining, and RT-PCR on FACS-sorted cells (16). Whatever the source of PACAP, the present studies implicate PACAP as an endogenous regulator of the immune function, with potentially important implications in autoimmune disease.
The decrease in Treg abundance may be particularly important in explaining the increased severity of EAE and delayed recovery in PACAP KO mice. Indeed, FACS analysis revealed that the number of Tregs was 60% less in lymph nodes of PACAP KO vs. WT mice, and mRNA for the Treg marker FoxP3 in the spinal cord was significantly reduced. Consistent with a reduction in Treg abundance, enhanced proliferation and increased Th1/Th17 cytokine production were observed in MOG-stimulated cultures of immunized PACAP KO mice. The observed reduction of TGF-β mRNA and/or protein levels in the spinal cord and lymph nodes provided one potential mechanism to explain the decrease in Tregs. TGF-β is a pleiotropic cytokine that can act as a survival factor for naturally occurring Foxp3+ Tregs in the periphery (35), and dependent on the cytokine environment, can induce the production of Tregs. We determined that TGF-β gene expression was induced in dendritic cells, macrophages, and CD4+ cells isolated from lymph nodes of immunized WT animals, but that this induction was severely diminished in KO mice. It has been previously suggested that PACAP may control T cell differentiation through modulation of cytokine production by antigen-presenting cells. For example, it has been shown that dendritic cells obtained from bone marrow cell cultures in the presence of VIP or PACAP, exhibit a “tolerogenic” phenotype, with high secretion of IL-10 and inability to upregulate CD80, CD86, and CD40 following LPS stimulation (26). In any case, our studies suggest that endogenous PACAP may be required to induce normal Treg responses through TGF-β, and this may contribute to enhanced autoimmune inflammation.
In conclusion, the maintenance of immune homeostasis depends on a tightly controlled balance between activating and suppressing processes. A defective counterregulation and/or excessive activation of effector cells may promote tissue-damaging immune responses. Here, we demonstrate that in the absence of PACAP, the immune system shows a diminished ability to control proinflammatory responses subsequent to immunization with a self-antigen. Indeed, PACAP KO mice exhibit a heightened Th1/Th17-induced proinflammatory response with a downregulation of the anti-inflammatory Th2/Treg activities. Thus, although PACAP and VIP have formally been thought to deliver their anti-inflammatory actions mainly by inhibiting macrophage activity and skewing Th1/Th2 responses toward Th2 (36), this report provides unique in vivo evidence that endogenous PACAP modulates the production or expansion of Treg and Th17 lymphocytes. These studies thus identify PACAP as one of the few known intrinsic regulators of Treg production. Finally, while extensive evidence indicates that both VIP and PACAP can produce similar anti-inflammatory actions in vitro and in vivo, the roles of either endogenous peptide have been hitherto unknown. This report establishes PACAP as an important endogenous immunomodulatory molecule with protective actions in a well-established autoimmune disease model.
Materials and Methods
Mice.
Female 6- to 8-week-old PACAP-deficient and WT mice on a C57BL/6 background (27) (backcrossed for at least 6–12 generations) were used in our experiments. Animals were housed and fed ad libitum at the UCLA School of Medicine Gonda vivarium. All procedures were approved by UCLA's Animal Care and Use Committee and conducted in accordance with the guidelines in National Institutes of Health Guide for the Care and Use of Laboratory Animals.
EAE Induction and Clinical Evaluation.
Mice under light isoflurane anesthesia were immunized s.c. in the 2 flanks with a mix of 100 μg of myelin oligodendrocyte glycoprotein for MOG35–55 (MEVGWYRSPFSRVVHLYRNGK; GLBiochem, Shanghai, China) in 50 μL of PBS and of 50 μL of CFA (Difco, Detroit, MI) containing 5 mg/mL of Mycobacterium tuberculosis H37 Ra. These mice also received i.p. 200 ng of pertussis toxin (Sigma–Aldrich, St. Louis, MO) in 200 μL of PBS immediately after the immunization and again 48 h later. Mice were monitored daily for EAE features and clinical symptoms. The severity of disease was recorded for 14 or 30 days in a blinded fashion on a scale of 0–4: 0, no clinical signs; 1, loss of tail tone; 2, wobbly gait; 3, hind limb paralysis; and 4, moribund or death. At the end of the experiment, the clinical score and the survival of the animals were assessed.
Histopathological Study.
Spinal cords were collected on day 30 after immunization, fixed in 4% paraformaldehyde overnight (O/N), and embedded in paraffin. Six-micrometer transverse sections of the cervical, upper, and lower thoracic, and lumbar regions of the spinal cord were prepared and rehydrated for Luxol fast blue staining of myelin and counterstained with hematoxiline/eosin (H&E) according to standard protocols (37). Stained sections distributed along the cervical and thoracic cord (150/mouse) were evaluated for lymphocyte accumulation, neutrophil and macrophage infiltration, and demyelination, and scored in a blinded fashion as follows: (i) a few inflammatory cells, little demyelination; (ii) organized perivascular infiltrates, a few areas of demyelination; and (iii) increasing severity of perivascular cuffing with extension into adjacent tissue, large areas of demyelination.
RNA Extraction and Real Time RT-PCR.
Mice were killed on day 14 or 30 postimmunization. Total RNA isolation from homogenized spinal cord tissues, the quantitative real-time RT-PCR analysis, and the sequences of the primers may be found in the SI.
Ex Vivo Assay for Cytokine Measurements and Cell Proliferation.
Draining lymph nodes (axillary and brachial) were collected 14 days after EAE induction, and cell suspensions were prepared. Cells were distributed in a 96-well plate at 1 × 106/mL concentration and cultured in 200 μL of RPMI medium 1640 (Gibco BRL, Grand Island, NY) containing 2% fetal bovine serum for FBS (HyClone, Logan, UT), 2 mM L-glutamine, 100 units/mL of penicillin and 100 μg/mL of streptomycin (Gibco). Cell suspensions were restimulated with either 10 μg/mL of OVA (Sigma) or MOG for 2 days at 37 °C with 5% CO2 and humidified atmosphere. All of the cultures were run in triplicate. For mRNA cytokine expression measurement, nonadherent cells were collected and cell pellets were resuspended in guanidine thiocyanate solution for RNA extraction (see above). Supernatants were harvested and stored for cytokine assays by ELISA at −20 °C. For proliferation analysis, the 48-h cultures were pulsed with [3H]-thymidine (1 μCi/well; Amersham Biosciences, Piscataway, NJ) for an additional 16–18 h. After this treatment, cells were harvested, lysed, and acid precipitated. Finally [3H]-thymidine incorporation was determined by liquid β-scintillation counting (Beckman).
ELISA.
Kits from Peprotech (Rocky Hill, NJ) were used to measure the levels of cytokines following manufacturer's instructions. The absorbance A405 nm was measured on a microplate reader (SPECTRA max M2, Molecular Devices, Sunnyvale, CA).
Flow Cytometric Analysis.
Twenty days after MOG immunization, draining lymph nodes (axillary and brachial) were removed from MOG-induced WT and MOG-induced PACAP KO mice. Cells were harvested as described above. FACS analyses of Th subsets in lymph nodes were applied as instructed by the mouse regulatory T cell staining kit from eBioscience, by using CD4, CD25, and FoxP3 (FJK-16s) antibodies.
FACS-Sorting.
For assessment of TGF-β mRNA, lymph nodes were harvested from naïve and immunized PACAP KO and WT mice on day 20 postimmunization, and cells were cultured in the presence of OVA or MOG as above. Forty-eight hours after culture, cells were collected and labeled with CD4, CD11b, and CD11c antibodies (eBioscience; 1:100; 30 min at 4 °C), and FACS-sorted with a Becton Dickinson FACSVantage SE sorting flow cytometer, and real-time PCR was performed as previously described. Purity of sorted population was greater than 95%.
Statistical Analysis.
Differences between groups were evaluated using GraphPad Prism4 software (GraphPad Software, San Diego, CA) and Student's t tests, except in the case of the Kaplan-Meier curve, where the logrank test was used. The level of significance was set at P < 0.05. Results are expressed as mean ± SEM.
Supplementary Material
Acknowledgments.
This work was supported by the National Multiple Sclerosis Society RG3928 and National Institutes of Health Grants HD06576 and HD04612.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0812257106/DCSupplemental.
References
- 1.Sospedra M, Martin R. Immunology of multiple sclerosis. Annu Rev Immunol. 2005;23:683–747. doi: 10.1146/annurev.immunol.23.021704.115707. [DOI] [PubMed] [Google Scholar]
- 2.Steinman L, Zamvil SS. How to successfully apply animal studies in experimental allergic encephalomyelitis to research on multiple sclerosis. Ann Neurol. 2006;60(1):12–21. doi: 10.1002/ana.20913. [DOI] [PubMed] [Google Scholar]
- 3.Miyata A, et al. Isolation of a novel 38 residue-hypothalamic polypeptide which stimulates adenylate cyclase in pituitary cells. Biochem Biophys Res Commun. 1989;164(1):567–574. doi: 10.1016/0006-291x(89)91757-9. [DOI] [PubMed] [Google Scholar]
- 4.Lutz EM, et al. Characterization of novel splice variants of the PAC1 receptor in human neuroblastoma cells: consequences for signaling by VIP and PACAP. Mol Cell Neurosci. 2006;31(2):193–209. doi: 10.1016/j.mcn.2005.09.008. [DOI] [PubMed] [Google Scholar]
- 5.Vaudry D, et al. Pituitary adenylate cyclase-activating polypeptide and its receptors: from structure to functions. Pharmacol Rev. 2000;52(2):269–324. [PubMed] [Google Scholar]
- 6.Botia B, et al. Neurotrophic effects of PACAP in the cerebellar cortex. Peptides. 2007;28(9):1746–1752. doi: 10.1016/j.peptides.2007.04.013. [DOI] [PubMed] [Google Scholar]
- 7.Somogyvari-Vigh A, Reglodi D. Pituitary adenylate cyclase activating polypeptide: a potential neuroprotective peptide. Curr Pharm Des. 2004;10(23):2861–2889. doi: 10.2174/1381612043383548. [DOI] [PubMed] [Google Scholar]
- 8.Waschek JA. Multiple actions of pituitary adenylyl cyclase activating peptide in nervous system development and regeneration. Dev Neurosci. 2002;24(1):14–23. doi: 10.1159/000064942. [DOI] [PubMed] [Google Scholar]
- 9.Watanabe J, et al. Localization, characterization and function of pituitary adenylate cyclase-activating polypeptide during brain development. Peptides. 2007;28(9):1713–1719. doi: 10.1016/j.peptides.2007.06.029. [DOI] [PubMed] [Google Scholar]
- 10.Lelievre V, et al. Disruption of the PACAP gene promotes medulloblastoma in ptc1 mutant mice. Dev Biol. 2008;313(1):359–370. doi: 10.1016/j.ydbio.2007.10.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Armstrong BD, et al. Impaired nerve regeneration and enhanced neuroinflammatory response in mice lacking pituitary adenylyl cyclase activating peptide. Neuroscience. 2008;151(1):63–73. doi: 10.1016/j.neuroscience.2007.09.084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lee M, et al. Pituitary adenylyl cyclase-activating polypeptide stimulates DNA synthesis but delays maturation of oligodendrocyte progenitors. J Neurosci. 2001;21(11):3849–3859. doi: 10.1523/JNEUROSCI.21-11-03849.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Waschek JA, et al. Neural tube expression of pituitary adenylate cyclase-activating peptide (PACAP) and receptor: potential role in patterning and neurogenesis. Proc Natl Acad Sci USA. 1998;95(16):9602–9607. doi: 10.1073/pnas.95.16.9602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Delgado M, Pozo D, Ganea D. The significance of vasoactive intestinal peptide in immunomodulation. Pharmacol Rev. 2004;56(2):249–290. doi: 10.1124/pr.56.2.7. [DOI] [PubMed] [Google Scholar]
- 15.Gonzalez-Rey E, Delgado M. Vasoactive intestinal peptide and regulatory T-cell induction: a new mechanism and therapeutic potential for immune homeostasis. Trends Mol Med. 2007;13(6):241–251. doi: 10.1016/j.molmed.2007.04.003. [DOI] [PubMed] [Google Scholar]
- 16.Abad C, et al. Pituitary adenylate-cyclase-activating polypeptide expression in the immune system. Neuroimmunomodulation. 2002;10(3):177–186. doi: 10.1159/000067180. [DOI] [PubMed] [Google Scholar]
- 17.Leceta J, Martinez C, Delgado M, Garrido E, Gomariz RP. Expression of vasoactive intestinal peptide in lymphocytes: a possible endogenous role in the regulation of the immune system. Adv Neuroimmunol. 1996;6(1):29–36. doi: 10.1016/s0960-5428(96)00001-0. [DOI] [PubMed] [Google Scholar]
- 18.Martinez C, et al. Regulation of VIP production and secretion by murine lymphocytes. J Neuroimmunol. 1999;93(1–2):126–138. doi: 10.1016/s0165-5728(98)00216-1. [DOI] [PubMed] [Google Scholar]
- 19.Gonzalez-Rey E, et al. Therapeutic effect of vasoactive intestinal peptide on experimental autoimmune encephalomyelitis: down-regulation of inflammatory and autoimmune responses. Am J Pathol. 2006;168(4):1179–1188. doi: 10.2353/ajpath.2006.051081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kato H, et al. Pituitary adenylate cyclase-activating polypeptide (PACAP) ameliorates experimental autoimmune encephalomyelitis by suppressing the functions of antigen presenting cells. Mult Scler. 2004;10(6):651–659. doi: 10.1191/1352458504ms1096oa. [DOI] [PubMed] [Google Scholar]
- 21.Delgado M, Abad C, Martinez C, Leceta J, Gomariz RP. Vasoactive intestinal peptide prevents experimental arthritis by downregulating both autoimmune and inflammatory components of the disease. Nat Med. 2001;7(5):563–568. doi: 10.1038/87887. [DOI] [PubMed] [Google Scholar]
- 22.Abad C, Martinez C, Leceta J, Gomariz RP, Delgado M. Pituitary adenylate cyclase-activating polypeptide inhibits collagen-induced arthritis: an experimental immunomodulatory therapy. J Immunol. 2001;167(6):3182–3189. doi: 10.4049/jimmunol.167.6.3182. [DOI] [PubMed] [Google Scholar]
- 23.Abad C, et al. Therapeutic effects of vasoactive intestinal peptide in the trinitrobenzene sulfonic acid mice model of Crohn's disease. Gastroenterology. 2003;124(4):961–971. doi: 10.1053/gast.2003.50141. [DOI] [PubMed] [Google Scholar]
- 24.Azuma YT, et al. PACAP provides colonic protection against dextran sodium sulfate induced colitis. J Cell Physiol. 2008;216(1):111–119. doi: 10.1002/jcp.21381. [DOI] [PubMed] [Google Scholar]
- 25.Fernandez-Martin A, Gonzalez-Rey E, Chorny A, Ganea D, Delgado M. Vasoactive intestinal peptide induces regulatory T cells during experimental autoimmune encephalomyelitis. Eur J Immunol. 2006;36(2):318–326. doi: 10.1002/eji.200535430. [DOI] [PubMed] [Google Scholar]
- 26.Delgado M, Gonzalez-Rey E, Ganea D. The neuropeptide vasoactive intestinal peptide generates tolerogenic dendritic cells. J Immunol. 2005;175(11):7311–7324. doi: 10.4049/jimmunol.175.11.7311. [DOI] [PubMed] [Google Scholar]
- 27.Colwell CS, et al. Selective deficits in the circadian light response in mice lacking PACAP. Am J Physiol Regul Integr Comp Physiol. 2004;287(5):R1194–1201. doi: 10.1152/ajpregu.00268.2004. [DOI] [PubMed] [Google Scholar]
- 28.Korn T, Anderson AC, Bettelli E, Oukka M. The dynamics of effector T cells and Foxp3+ regulatory T cells in the promotion and regulation of autoimmune encephalomyelitis. J Neuroimmunol. 2007;191(1–2):51–60. doi: 10.1016/j.jneuroim.2007.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tang Q, Bluestone JA. Regulatory T-cell physiology and application to treat autoimmunity. Immunol Rev. 2006;212:217–237. doi: 10.1111/j.0105-2896.2006.00421.x. [DOI] [PubMed] [Google Scholar]
- 30.Li MO, Wan YY, Sanjabi S, Robertson AK, Flavell RA. Transforming growth factor-beta regulation of immune responses. Annu Rev Immunol. 2006;24:99–146. doi: 10.1146/annurev.immunol.24.021605.090737. [DOI] [PubMed] [Google Scholar]
- 31.Zhang Y, Danielsen N, Sundler F, Mulder H. Pituitary adenylate cyclase-activating peptide is upregulated in sensory neurons by inflammation. Neuroreport. 1998;9(12):2833–2836. doi: 10.1097/00001756-199808240-00027. [DOI] [PubMed] [Google Scholar]
- 32.Armstrong BD, et al. Induction of neuropeptide gene expression and blockade of retrograde transport in facial motor neurons following local peripheral nerve inflammation in severe combined immunodeficiency and BALB/C mice. Neuroscience. 2004;129(1):93–99. doi: 10.1016/j.neuroscience.2004.06.085. [DOI] [PubMed] [Google Scholar]
- 33.Armstrong BD, et al. Lymphocyte regulation of neuropeptide gene expression after neuronal injury. J Neurosci Res. 2003;74(2):240–247. doi: 10.1002/jnr.10750. [DOI] [PubMed] [Google Scholar]
- 34.Zhou X, et al. Axotomy-induced changes in pituitary adenylate cyclase activating polypeptide (PACAP) and PACAP receptor gene expression in the adult rat facial motor nucleus. J Neurosci Res. 1999;57(6):953–961. [PubMed] [Google Scholar]
- 35.Marie JC, Letterio JJ, Gavin M, Rudensky AY. TGF-beta1 maintains suppressor function and Foxp3 expression in CD4+CD25+ regulatory T cells. J Exp Med. 2005;201(7):1061–1067. doi: 10.1084/jem.20042276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Delgado M. VIP: a very important peptide in T helper differentiation. Trends Immunol. 2003;24(5):221–224. doi: 10.1016/s1471-4906(03)00069-3. [DOI] [PubMed] [Google Scholar]
- 37.Margolis G, Pickett JP. New applications of the Luxol fast blue myelin stain. Lab Invest. 1956;5(6):459–474. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.