Abstract
Atmospheric CO2 is an important environmental cue that regulates several types of animal behavior. In mice, CO2 responses of the olfactory sensory neurons (OSNs) require the activity of carbonic anhydrase to catalyze the conversion of CO2 to bicarbonate and the opening of cGMP-sensitive ion channels. However, it remains unknown how the enhancement of bicarbonate levels results in cGMP production. Here, we show that bicarbonate activates cGMP-producing ability of guanylyl cyclase-D (GC-D), a membrane GC exclusively expressed in the CO2-responsive OSNs, by directly acting on the intracellular cyclase domain of GC-D. Also, the molecular mechanism for GC-D activation is distinct from the commonly believed model of “release from repression” for other membrane GCs. Our results contribute to our understanding of the molecular mechanisms of CO2 sensing and suggest diverse mechanisms of molecular activation among membrane GCs.
Keywords: carbonic anhydrase, CNG channels, necklace glomeruli, transduction
Carbon dioxide (CO2) is one of the major by-products of cellular metabolism. Atmospheric CO2 can be detected by the olfactory system of many animal species and mediates many fundamental behaviors including finding mates, seeking food, and avoiding predators (1–5). Recent years have witnessed substantial progresses on our understanding of the neurobiological mechanisms of CO2 sensing. One emerging feature of CO2 detection is that a subset of olfactory sensory neurons (OSNs) is specialized for sensing CO2. In flies, CO2 is detected by OSNs that project to the glomerulus V in the antennal lobe (3, 4). Similarly, in Caenorhabditis elegans, the BAG neurons are believed to be critical for CO2 sensing (5). Although CO2 is odorless to humans, our recent study shows that the mouse olfactory system sensitively detects CO2 by a subset of OSNs that project their axons to the necklace glomeruli in the olfactory bulb (1).
The exact molecular mechanisms of CO2 detection remain unclear. Guanylyl cyclase-D (GC-D) is expressed exclusively in the CO2-responsive neurons, which are thus named GC-D+ neurons (6–8). GC-D+ neurons also express other components of signaling pathway that implicate cGMP as the second messenger, including cGMP-stimulated phosphodiesterase 2A (PDE2A) and cGMP-sensitive cyclic nucleotide-gated (CNG) channels (7, 9). Interestingly, carbonic anhydrase II (CAII) is expressed only in GC-D+ neurons in the olfactory epithelium (1). As revealed by 0our previous study, CO2 responses in the GC-D+ neurons require the enzymatic activity of CAII and the opening of CNG channels (1). However, the signaling cascade that links the enzymatic activity of CAII with the opening of cGMP-sensitive CNG channels remains unknown. Presumably, CO2 is a small molecule that can diffuse across cell membrane and can in turn be converted into bicarbonate ions (HCO3−) and protons (H+) by CAII. Protons may be buffered by intracellular machinery to maintain physiological pH. Bicarbonates, the other product of CAII, may stimulate a GC, such as GC-D, to produce cGMP from GTP and open CNG channels. GC-D is a pseudogene in humans (10, 11), and CO2 is odorless to humans (12), suggesting that GC-D may be important for CO2 sensing. However, there was no evidence so far suggesting that bicarbonate could activate a GC, although it can activate soluble adenylyl cyclase (sAC) in sperm (13). In contrast, 3 membrane GCs (GC-A through GC-C) are receptors for natriuretic peptides (14–16). GC-D has also been implicated in detecting guanylin (G) and uroguanylin (UG) (17), 2 known peptide ligands of GC-C (18, 19).
In this study, we carried out biochemical assays to examine the effects of bicarbonate on GC-D. Our results demonstrate that bicarbonate directly activates GC-D by acting on its intracellular cyclase domain without the need of any accessory factors. Our data further suggest that the mechanism for GC-D activation is distinct from the commonly accepted “release from repression” model for GC-A activation (20), a well characterized receptor for atrial natriuretic peptide (ANP). Thus, our data provide a framework for understanding the molecular and cellular mechanisms of CO2 sensing.
Results
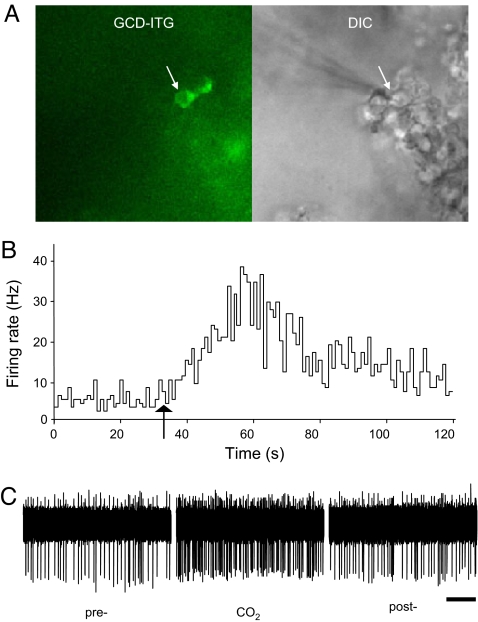
Ca2+ imaging in our previous study shows that CO2 enhances intracellular Ca2+ levels of GC-D+ neurons (1). To directly test whether CO2 indeed increases the frequency of action potential firing, we carried out targeted electrophysiological recording of GC-D+ neurons in an intact epithelial preparation (Fig. 1A) (21). All tested GC-D+ neurons responded vigorously after CO2 application (Fig. 1 B and C; basal firing rate, 4.8 ± 1.6 Hz; peak firing rate in CO2, 13.7 ± 2.9 Hz; n = 7 cells). In contrast, none of the tested GC-D− neurons were activated by CO2 (basal firing rate, 5.0 ± 0.2 Hz; peak firing rate in CO2, 4.9 ± 0.7 Hz; n = 3 cells). Thus, our recordings directly demonstrate the selective excitatory effect of CO2 on GC-D+ neurons and validate by using Ca2+ imaging to measure CO2 responses in GC-D+ neurons.
Fig. 1.
CO2 activates GC-D+ neurons. (A) Targeted recording of GC-D+ neurons. GC-D+ cells were identified by green fluorescence in both somata and knobs in an olfactory epithelial preparation from GCD-ITG mice (Left). An electrode was guided toward a GC-D+ cell (arrows) by using DIC microscopy (Right) for cell-attached extracellular recording. (B) The mean firing rate of a GC-D+ cell was dramatically enhanced after CO2 application in the perfusion solution (arrow). (C) Sample physiological traces showing the firing of the neurons shown in B before (pre), during (CO2), and after CO2 application (post). (Scale bar, 2 s.)
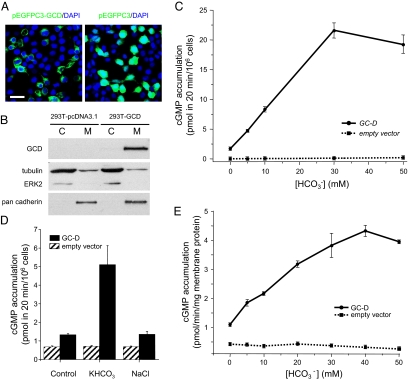
Because CO2 responses depend on both the enzymatic activity of CA and the opening of cGMP-sensitive CNG channels (1, 22), we hypothesized that bicarbonate could activate the cyclase activity of GC-D to produce cGMP. We first tested the effect of bicarbonate on cGMP production in cultured mammalian cells expressing full-length rat GC-D cDNA. GC-D expression was observed on the cell membrane of HEK-293T cells (Fig. 2 A and B). Bicarbonate in the extracellular medium can be transported into HEK-293 cells within minutes, and early studies have used this method to study the bicarbonate effects on the cyclase activity of sAC (13). Extracellular application of NaHCO3 stimulated cGMP accumulation in HEK-293T cells transfected with GC-D, but not in those with empty expression vector (Fig. 2C). This activation was dose-dependent for NaHCO3, with significant effects observed for NaHCO3 at as low as 5 mM and saturation at 30–50 mM. The effective bicarbonate concentrations are within the ranges of intracellular bicarbonate levels reported for various cells and are in accordance with earlier studies on the effective bicarbonate concentrations on sAC (13). Addition of KHCO3, but not NaCl, produced stimulatory effects similar to those by NaHCO3 (Fig. 2D), suggesting that GC-D activation was specific to bicarbonate ion, but not due to Na+ or altered ionic strength. The stimulatory effects of bicarbonate on GC-D were further corroborated by using optical imaging of FRET signals sensitive to cGMP levels [supporting information (SI) Fig. S1]. Thus, these results suggest that GC-D cyclase activity can be activated by bicarbonate in a cellular context in the absence of factors specific to GC-D+ neurons.
Fig. 2.
Bicarbonate activates the cyclase activity of GC-D. (A and B) GC-D can be heterologously expressed on cell membrane. (A) GCD-GFP fusion protein was expressed on the surface of transfected HEK-293T cells (Left), whereas GFP was expressed diffusively in cells (Right); pEGFPC3-GCD encodes the fusion protein of GFP::GC-D and pEGFPC3 encodes GFP only. DAPI indicates the DNA staining dye 4′,6-diamidino-2-phenylindole and serves to mark cell nuclei. (Scale bar, 30 μm.) (B) Western blottings showing that GC-D was present in membrane (M), but not cytosolic (C) fractions of HEK-293T cells transiently transfected with a vector containing GC-D. No GC-D was detected in HEK-293T cells transfected with empty vector pcDNA3.1. Tubulin and ERK2 were used as control for cytosolic fraction and pan cadherin was the control for membrane fraction. (C) Bicarbonate stimulated intracellular cGMP accumulation in HEK-293T cells expressing GC-D (line with circles), but had no effect on cells transfected with empty vector (dashed line with squares). (D) KHCO3 (40 mM), but not NaCl (40 mM) activated GC-D. (E) Bicarbonate dose-dependently activated GC-D in vitro. Guanylyl cyclase activity at different bicarbonate concentrations was assayed by using particulate extracts from HEK-293T cells transiently expressing GC-D (line with circles) or those from cells expressing empty vector (dashed line with squares). Data are expressed as picomoles of cGMP formed per minute per milligram of total membrane protein. In this and following figures, error bars = SEM, and data were average of triplicate measurements.
We next examined the effect of bicarbonate on GC-D protein in vitro. GC-D was transiently expressed in HEK-293T cells, and membrane protein from particulate fraction was extracted. Bicarbonate stimulated cGMP accumulation in membrane protein fraction from HEK-293T cells expressing GC-D, but not from those transfected with empty vector (Fig. 2E). This stimulation was again dose-dependent for bicarbonate, with maximum stimulatory effects at concentrations of 30–50 mM. Therefore, the cyclase activity of GC-D can be directly stimulated by bicarbonate without any accessory factors in cytosolic solutions.
Although GC-D is expressed exclusively in the CO2-responsive OSNs, other membrane GCs and soluble (s)GCs are known to function in the olfactory epithelium (23). We tested whether several other mammalian membrane GCs could be activated by bicarbonate. Bicarbonate did not increase GC activity in HEK-293T cells expressing GC-A, GC-E, and GC-F (Fig. 3A). Bicarbonate at concentrations of 25 and 50 mM appeared to inhibit GC-E and GC-F (Fig. 3A), 2 membrane GCs expressed in retina photoreceptors (24, 25). Similarly, we found that bicarbonate did not stimulate cGMP production in T84 human colonic adenocarcinoma cells (Fig. 3A), which have been used extensively for assaying GC-C activity due to their high endogenous expression of GC-C (18, 26). As a control, GC-A and GC-C were dramatically activated after the application of their respective ligands: ANP for GC-A and G/UG for GC-C (Fig. S2). We next carried out optical imaging to test whether CO2 responses of GC-D+ neurons depended on the activity of sGCs. CO2 responses were largely intact in the presence of a specific sGC inhibitor ODQ, 1H- (1, 2, 4) oxadiazolo [4, 3-a] quinoxalin-1-one (27), suggesting that bicarbonate had no stimulatory effect on sGCs (Fig. 3 B–D). Thus, our results suggest that GC-D is a unique mammalian membrane GC that can be activated by bicarbonate, and bicarbonate stimulation is not a general feature of all GCs.
Fig. 3.
Bicarbonate does not activate other membrane GCs or sGCs. (A) Bicarbonate did not stimulate the cyclase activity of cells expressing GC-A, GC-C, GC-E, or GC-F. It appeared to inhibit the basal cyclase activity of GC-E and GC-F. GC-C was assayed on T84 cells, whereas other GCs were transiently expressed in HEK-293T cells. Numbers 0, 25, and 50 in leftmost panel indicate NaHCO3 concentrations and apply to all panels. *, P < 0.05; ***, P < 0.001; t test. (B–D) Ca2+ imaging reviewed that CO2 responses of GC-D+ neurons were independent on the activity of sGCs. (B) GFP labeling of GC-D+ neurons within an intact epithelial preparation from a GCD-ITG mouse (Left) and loading of calcium-sensitive Rhod-2/AM dye into GC-D+ neurons (Right). (C) Application of a selective sGC blocker, ODQ (50 μM), had no effect on CO2-evoked Ca2+ signals. Arrows indicated the start of perfusion of CO2 solution. (Scalle Bars, 5% ΔF/F and 50 s.) (D) Group data showing that the CO2-evoked responses before and after ODQ application were not statistically different (P = 0.08; n = 27 cells; paired t test).
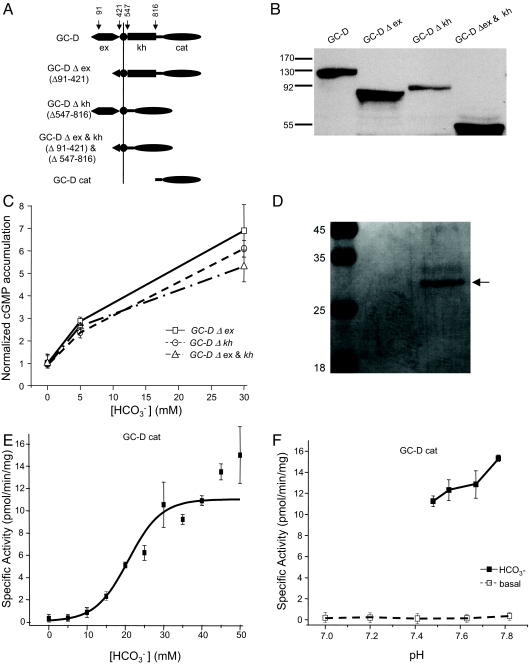
GC-D, like other membrane GCs, contains an extracellular domain (ex), a hydrophobic membrane-spanning region, an intracellular kinase-homology domain (kh), a coiled-coiled dimerization motif, and a C-terminal GC domain essential for its catalytic activity (cat) (Fig. 4A) (6, 15). To delineate the specific functional domain that mediates bicarbonate sensing, we engineered 3 constructs expressing mutant GC-D genes lacking the GC-D ex (Δex), kh (Δkh), or both of these domains (Δex and kh). Western blottings confirmed that these mutant proteins were expressed on cell membrane of HEK-293T cells (Fig. 4B). Applying bicarbonate in the extracellular medium stimulated cGMP production in HEK-293T cells expressing any of these engineered proteins (Fig. 4C), suggesting that bicarbonate sensing by GC-D is independent on its ex and kh.
Fig. 4.
Bicarbonate directly activates GC-D intracellular cyclase domain and this activation is pH-independent. (A–C) Deletions of GC-D ex and its intracellular kh had no effects on the ability of GC-D to sense bicarbonate. (A) Schematic representation showing engineered GC-D mutations with deletions of ex (GC-D Δex), intracellular kh (GC-D Δkh), and both of these domains (GC-D Δex and kh). For GC-D Δex, residues 91–421 were removed. Residues 1–90 were spared, because they were necessary for membrane expression. For GC-D Δkh, residues 547–816 were removed. GC-D cyclase domain (GC-D cat; residues 850–1,110) was purified for assay shown in E. (B) Western blotting by using an antibody against GC-D C-terminal peptide showing GC-D and its mutants of Δex, Δkh, and Δex and kh in membrane fractions of HEK-293T cells transfected with vectors of these proteins. (C) Deletion of ex, kh, or both had no obvious effects on the stimulation of cyclase activity by bicarbonate. (D) Coomassie Blue staining of purified recombinant GC-D cyclase domain (arrow). (E) Bicarbonate directly activated purified GC-D cyclase domain. Data are expressed as picomoles of cGMP formed per minute per milligram of protein. (F) The activation of purified GC-D cyclase domain was independent on pH when assayed alone (dashed line) or in the presence of 40 mM bicarbonate (solid black line). All of the pH values were measured in the final assay solutions.
To directly test whether bicarbonate acts on GC-D cyclase domain (GC-D cat) and to further exclude the possibility of accessory factors mediating activation, we expressed the GC-D cyclase domain in insect HiFive cells and purified the recombinant protein to test the effects of bicarbonate (Fig. 4D). The cyclase activity of the purified protein was dramatically enhanced by bicarbonate in a dose-dependent manner (Fig. 4E). The median effective concentration (EC50) of this activation was ≈21 mM. Bicarbonate stimulation was not due to altered pH, because neither the recombinant protein (GC-D cat) alone nor bicarbonate-stimulated GC activities of the recombinant protein were sensitive to pH changes over the range 7.0 to 7.8 (Fig. 4F). Therefore, bicarbonate activates GC-D by directly acting on GC-D cyclase domain without the need of any accessory factors in the cell.
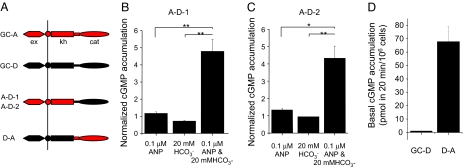
For GC-A, an extensively characterized membrane GC, the kh is believed to repress the cat of the cyclase domain and binding of ANP to its ex relieves this repression (15). Deleting kh from GC-A results in a constitutively active GC (20, 28). However, GC-D cyclase domain is normally inactive and can be activated by bicarbonate without the application of external peptide ligands (Fig. 4 C and E). To test whether the exs and khs in GC-A and GC-D have different roles in regulating their respective cyclase domain, we engineered constructs expressing chimera proteins containing domains from these 2 proteins (Fig. 5A and Fig. S3). Replacement of GC-A cyclase domain with that of GC-D resulted in chimera proteins (A-D-1 and A-D-2) that were activated only by combined application of both bicarbonate and ANP, whereas neither bicarbonate nor ANP alone had effects (Fig. 5 B and C). In contrast, replacement of GC-D cyclase domain with that of GC-A resulted in a chimera protein (D-A) that was constitutively active without the presence of bicarbonate or ANP (Fig. 5D). Thus, GC-D cyclase domain is directly activated by bicarbonate, and GC-D activation may use a mechanism different from the release from repression model proposed for GC-A.
Fig. 5.
Stimulation of GC activity of GC-D by bicarbonate is independent on the release of repression by ex. (A) Schematic representations showing the domain organization of native GC-A, GC-D, and chimera GC with domains from either GC-A or GC-D. A-D-1 and A-D-2 were engineered by replacing the cyclase domain of GC-A with that of GC-D starting at residue 830 or 850, respectively. D-A was engineered by replacing the cyclase domain of GC-D with that of GC-A. (B and C) The cyclase activity of both A-D-1 (B) and A-D-2 (C) was activated only by applying both bicarbonate and ANP, the peptide that binds to GC-A ex. *, P < 0.05; **, P < 0.01; t test. Data were normalized to the cGMP levels for A-D-1 and A-D-2 samples without the application of either ANP or bicarbonate. (D) D-A exhibited high basal GC activity. Unlike that of GC-A, the N-terminal region of GC-D does not repress the C-terminal cyclase domain, indicating that there may be no needs for any peptide as coactivator to release any potential repression in GC-D.
Discussion
GC-D+ neurons express rich amount of CAII and are important for sensitive detection of atmospheric CO2 (1). CO2 responses of these neurons also require the opening of cGMP-sensitive CNG channels (1). Our data in this study show that bicarbonate stimulates the production of cGMP by GC-D by directly acting on its intracellular cyclase domain, uncovering a key event within the signaling transduction pathway for CO2 sensing.
Intracellular Signal Transduction Cascade for CO2-Sensing.
Bicarbonate stimulates the cyclase activity of GC-D when it is heterologously expressed in cultured cells. This activation can be similarly observed when GC-D protein is assayed in vitro. Most importantly, bicarbonate directly activates the purified recombinant protein containing the intracellular cyclase domain in dose-dependent and pH-insensitive manner. Therefore, GC-D can be directly activated by bicarbonate without the need of accessory factors. Bicarbonate is believed to be an important cellular “first messenger” that can be sensed by sAC (13, 29, 30). Now, GC-D should be added to the list of bicarbonate sensors.
Our results lead us to propose the following scenario of signal transduction cascade after CO2 diffusion into the GC-D+ OSNs (Fig. 6A). CAII catalyzes the conversion of CO2 into bicarbonate ion and proton. Bicarbonate activates GC-D by directly acting on its cyclase domain, whereas proton may be quickly buffered by the cell to maintain stable intracellular pH. GC-D produces cGMPs from GTPs; cGMPs in turn open cGMP-sensitive CNG channels to allow cation influx into the GC-D+ cells, resulting in neuronal depolarization to fire action potentials. This model suggests that CO2 is sensed by mechanisms different from those for typical odorants. In typical OSNs, odorant molecules bind to odorant receptors, which are G protein-coupled receptors, to activate Golf, which in turn activates adenylyl cyclase to produce cAMPs; thus, opening cAMP-sensitive CNG channels (31–33). Our model on CO2 sensing can be further tested by future studies on the behavioral and physiological effects of both GC-D-knockout mice and the cGMP-sensitive CNG channel-knockout mice. Nevertheless, the fact that GC-D is a pseudogene in human provides an attractive explanation on why the human olfactory system lacks ability to sense CO2 (10). Recent bioinformatics analysis indicates that GC-D is functional in most of mammals but degenerates in many primate species (11), suggesting that CO2 sensing ability might be preserved in most of mammals but largely lost in primates.
Fig. 6.
Our models on the intracellular signal transduction for CO2 detection by GC-D+ neurons and the molecular mechanism of bicarbonate sensing by GC-D. (A) Intracellular signal-transduction cascades in GC-D+ neurons. After CO2 arrival, CAII catalyzes the conversion of CO2 into bicarbonate, which activates GC-D to produce cGMP; cGMP in turn opens the cGMP-sensitive CNG channels to activate GC-D+ neurons. PDE2A restores cGMP levels by hydrolyzing it to 5′-GMP. (B) Comparison of the molecular mechanisms of activation between GC-D and GC-A. GC-D cyclase domain is normally inactive and can be activated by bicarbonate. In contrast, GC-A cyclase domain is constitutively active, but its activity is blocked by GC-A ex and kh. Binding of ANP to GC-A ex releases this blockade and activates GC-A cyclase activity.
Our data also suggest that the molecular mechanisms of CO2 sensing by mammals differ from those by insects. In fly, a pair of membrane receptors, Gr21a and Gr63a, are necessary and sufficient for CO2 detection (4, 34). These 2 receptors, like other fly odorant receptors, are presumably channels themselves (35, 36). In C. elegans, DAF-11 (a membrane GC) and cGMP-sensitive CNG channels are essential for CO2-mediated avoidance behavior (5). It will be interesting to test whether the mechanisms of CO2 sensing are conserved between mammals and worms, and whether DAF-11 or other membrane GCs in C. elegans could be activated by bicarbonate.
Different Mechanisms of Molecular Activation Among Membrane GCs.
Our data on domain deletions and swaps indicate that the molecular mechanism for GC-D activation differ from that for GC-A. Removal of GC-A kh results in high constitutive activity, consistent with the concept that GC-A kh represses a normally active cyclase domain (14, 28). Binding of ANP to GC-A ex is believed to induce a conformational change to release the repression; thus, activating the enzyme (Fig. 6B Right) (15). In contrast, deleting GC-D kh neither increases its basal cyclase activity nor disrupts the activating effects of bicarbonate. Similarly, GC-D without ex can be activated by bicarbonate. More importantly, purified recombinant protein of GC-D cyclase domain itself is normally inactive, but can be directly activated by bicarbonate. These results indicate that GC-D is activated by direct action of bicarbonate, rather than by binding a ligand to its ex to release the repression from kh (Fig. 6B Left). The chimera protein with the ex and kh from GC-A and the cyclase domain from GC-D can be activated only with combined application of ANP and bicarbonate. However, the chimera protein with the ex and kh from GC-D and the cyclase domain from GC-A is constitutively active. These results are consistent with the model of release from repression for GC-A (15), and a model of direct activation by bicarbonate for GC-D, suggesting diverse molecular mechanisms of activation among membrane GCs.
A study using electrophysiological recordings and Ca2+ images suggests that GC-D can be activated by G and UG (17), 2 peptide natriuretic hormones that are known to activate GC-C. Biochemical assays from another study suggest that UG but not G activates GC-D by binding to its ex (37). Also, this study suggests that Ca2+ and neurocalcin can activate GC-D by binding to its intracellular domains (37). Thus, GC-D may be activated by factors other than bicarbonate. In this study, we have identified the stimulatory effects of bicarbonate on the activation of GC-D. It will be interesting to examine the effects of these additional factors on GC-D and their potential roles in behavioral and physiological functions of CO2 sensing.
Materials and Methods
Electrophysiological Recording, Ca2+ Imaging, and FRET Imaging.
The preparation of intact olfactory epithelia and calcium imaging was carried out by procedures as described previously (1, 21). Briefly, adult GCD-ITG (GCD-ires-tauGFP) mice was anesthetized and decapitated. Swatches of olfactory epithelium were peeled off and placed in oxygenated Hepes based Ringer solution (140 mM NaCl/1.5 mM KCl/1 mM MgCl2/2.5 mM CaCl2/11 mM glucose/10 mM Hepes, pH 7.4). In these mice, GFP is coexpressed with GC-D by bicistronic strategy that leaves the coding region of GC-D intact (8). For cell-attached patch recording, intracellular pipette solution contained (in mM) 130 K-gluconate, 5 KCl, 10 Hepes, 13 Na-phosphocreatine, and 4 MgCl2 and 0.6 EGTA. Recordings were carried out at room temperature (RT; 25 ± 2 °C).
For Ca2+ imaging, Rhod-2/AM Ca2+-sensitive dye was loaded at 34 °C. Tissues were then transferred to an imaging chamber, stabilized with a nylon net, and continuously perfused with oxygenated Ringer solution at RT. For CO2 delivery, oxygenated Ringer solution was bubbled with pure CO2 and then added into the chamber by superfusion, resulting in a final CO2 concentration of 6.4 mM.
Imaging of FRET signals for cGMP was carried out as described previously (38). Briefly, HEK-293T cells were transfected with cGMP probe cGES-DE5 either with GC-D or control empty vector. FRET signals were expressed as the intensity of YFP/CFP ratio. Transfected cells were continuously perfused with Hepes-based Ringer solution, and imaging was performed at RT.
Unless specified otherwise, all inorganic salts and peptides in this study were purchased from Sigma.
Cell Culture and Western Blottings.
HEK-293T cells were maintained in DMEM containing 10% FBS. Transfections of HEK-293T cells were performed by using Lipofectamine2000 (Invitrogen). To examine bicarbonate sensitivity of GCs in cellular systems, transfected cells were grown in bicarbonate-free L-15 medium (GIBCO) in CO2-deprived incubator for 24 h. Full-length GC-D cDNA was cloned from rat olfactory epithelium. For Western blottings, an anti-GC-D antibody (GeneTex) was used, because it reacted with an epitope from the GC-D C-terminal and labeled both intact and engineered GC-D proteins.
Protein Purification and Particulate Membrane Fraction Preparation.
His-tagged intracellular cyclase domain of GC-D (His-GC-D-cyclase), consisting of GC-D residues 850–1,110, was heterologously expressed in insect HiFive cells by using the Bac-to-Bac Baculovirus Expression System (Invitrogen), and protein was purified by chromatography over Ni2+-NTA Sepharose Resin (Qiagen) followed by a Superdex200 HR10/30 column (GE Healthcare). The purity of the recombinant protein was confirmed by Coomassie Blue staining (Fig. 4D). Membrane fraction of HEK-293T cells transfected with GC-D is prepared as described previously (14).
Guanylyl Cyclase Activity Assay.
The concentrations of cGMP were determined with the cGMP ELISA Assay Kit from Assay Designs. For cell lysate assay, cells were incubated for 18–24 h after transfection before the medium was changed to serum-free medium; 24 h after transfection, cells were incubated at 37 °C for 20 min with NaHCO3 at the indicated concentrations as well as 1 mM phosphodiesterase inhibitor IBMX (3-isobutyl-1-methylxanthine). We empirically determined the dilution factor for each cell lysate so that the experimental readings resided in the optimal assay range of the cGMP Assay Kit.
For GC activity assay in vitro, purified recombinant proteins or particulate fractions containing heterologously expressed GCs were mixed in 50 μL of reaction buffer (20 mM Hepes, pH 7.4/100 mM NaCl/0.4 mM MgCl2/0.1 mM GTP) and incubated for 20 min at 30 °C. The reaction was terminated by boiling for 5min before its cGMP content was determined with the cGMP Assay Kit.
Supplementary Material
Acknowledgments.
We thank Peter Mombaerts (MPI Biophysics, Frankfurt) for GCD-ITG mice and Xiaodong Wang for discussion and advice. This work is supported by the China Ministry of Science and Technology and a National Natural Science Foundation of China Young Investigator grant (M.L.); a Human Frontier Science Program grant (M.L. and H.M.); and by grants from the National Institutes of Health (H.M.).
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/cgi/content/full/0812220106/DCSupplemental.
References
- 1.Hu J, et al. Detection of near-atmospheric concentrations of CO2 by an olfactory subsystem in the mouse. Science. 2007;317:953–957. doi: 10.1126/science.1144233. [DOI] [PubMed] [Google Scholar]
- 2.Youngentob SL, Hornung DE, Mozell MM. Determination of carbon dioxide detection thresholds in trained rats. Physiol Behav. 1991;49:21–26. doi: 10.1016/0031-9384(91)90224-c. [DOI] [PubMed] [Google Scholar]
- 3.Suh GS, et al. A single population of olfactory sensory neurons mediates an innate avoidance behaviour in Drosophila. Nature. 2004;431:854–859. doi: 10.1038/nature02980. [DOI] [PubMed] [Google Scholar]
- 4.Jones WD, Cayirlioglu P, Kadow IG, Vosshall LB. Two chemosensory receptors together mediate carbon dioxide detection in Drosophila. Nature. 2007;445:86–90. doi: 10.1038/nature05466. [DOI] [PubMed] [Google Scholar]
- 5.Hallem EA, Sternberg PW. Acute carbon dioxide avoidance in Caenorhabditis elegans. Proc Natl Acad Sci USA. 2008;105:8038–8043. doi: 10.1073/pnas.0707469105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fulle HJ, et al. A receptor guanylyl cyclase expressed specifically in olfactory sensory neurons. Proc Natl Acad Sci USA. 1995;92:3571–3575. doi: 10.1073/pnas.92.8.3571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Juilfs DM, et al. A subset of olfactory neurons that selectively express cGMP-stimulated phosphodiesterase (PDE2) and guanylyl cyclase-D define a unique olfactory signal transduction pathway. Proc Natl Acad Sci USA. 1997;94:3388–3395. doi: 10.1073/pnas.94.7.3388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Walz A, Feinstein P, Khan M, Mombaerts P. Axonal wiring of guanylate cyclase-D-expressing olfactory neurons is dependent on neuropilin 2 and semaphorin 3F. Development. 2007;134:4063–4072. doi: 10.1242/dev.008722. [DOI] [PubMed] [Google Scholar]
- 9.Meyer MR, Angele A, Kremmer E, Kaupp UB, Muller F. A cGMP-signaling pathway in a subset of olfactory sensory neurons. Proc Natl Acad Sci USA. 2000;97:10595–10600. doi: 10.1073/pnas.97.19.10595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Torrents D, Suyama M, Zdobnov E, Bork P. A genome-wide survey of human pseudogenes. Genome Res. 2003;13:2559–2567. doi: 10.1101/gr.1455503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Young JM, Waters H, Dong C, Fulle HJ, Liman ER. Degeneration of the olfactory guanylyl cyclase D gene during primate evolution. PLoS ONE. 2007;2:e884. doi: 10.1371/journal.pone.0000884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shusterman D, Avila PC. Real-time monitoring of nasal mucosal pH during carbon dioxide stimulation: Implications for stimulus dynamics. Chem Senses. 2003;28:595–601. doi: 10.1093/chemse/bjg050. [DOI] [PubMed] [Google Scholar]
- 13.Chen Y, et al. Soluble adenylyl cyclase as an evolutionarily conserved bicarbonate sensor. Science. 2000;289:625–628. doi: 10.1126/science.289.5479.625. [DOI] [PubMed] [Google Scholar]
- 14.Chinkers M, et al. A membrane form of guanylate cyclase is an atrial natriuretic peptide receptor. Nature. 1989;338:78–83. doi: 10.1038/338078a0. [DOI] [PubMed] [Google Scholar]
- 15.Potter LR, Abbey-Hosch S, Dickey DM. Natriuretic peptides, their receptors, and cyclic guanosine monophosphate-dependent signaling functions. Endocr Rev. 2006;27:47–72. doi: 10.1210/er.2005-0014. [DOI] [PubMed] [Google Scholar]
- 16.Schulz S, Green CK, Yuen PS, Garbers DL. Guanylyl cyclase is a heat-stable enterotoxin receptor. Cell. 1990;63:941–948. doi: 10.1016/0092-8674(90)90497-3. [DOI] [PubMed] [Google Scholar]
- 17.Leinders-Zufall T, et al. Contribution of the receptor guanylyl cyclase GC-D to chemosensory function in the olfactory epithelium. Proc Natl Acad Sci USA. 2007;104:14507–14512. doi: 10.1073/pnas.0704965104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Currie MG, et al. Guanylin: An endogenous activator of intestinal guanylate cyclase. Proc Natl Acad Sci USA. 1992;89:947–951. doi: 10.1073/pnas.89.3.947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hamra FK, et al. Uroguanylin: Structure and activity of a second endogenous peptide that stimulates intestinal guanylate cyclase. Proc Natl Acad Sci USA. 1993;90:10464–10468. doi: 10.1073/pnas.90.22.10464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chinkers M, Garbers DL. The protein kinase domain of the ANP receptor is required for signaling. Science. 1989;245:1392–1394. doi: 10.1126/science.2571188. [DOI] [PubMed] [Google Scholar]
- 21.Ma M, Chen WR, Shepherd GM. Electrophysiological characterization of rat and mouse olfactory receptor neurons from an intact epithelial preparation. J Neurosci Methods. 1999;92:31–40. doi: 10.1016/s0165-0270(99)00089-8. [DOI] [PubMed] [Google Scholar]
- 22.Coates EL. Olfactory CO2 chemoreceptors. Respir Physiol. 2001;129:219–229. doi: 10.1016/s0034-5687(01)00292-4. [DOI] [PubMed] [Google Scholar]
- 23.Moon C, et al. Calcium-sensitive particulate guanylyl cyclase as a modulator of cAMP in olfactory receptor neurons. J Neurosci. 1998;18:3195–3205. doi: 10.1523/JNEUROSCI.18-09-03195.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shyjan AW, de Sauvage FJ, Gillett NA, Goeddel DV, Lowe DG. Molecular cloning of a retina-specific membrane guanylyl cyclase. Neuron. 1992;9:727–737. doi: 10.1016/0896-6273(92)90035-c. [DOI] [PubMed] [Google Scholar]
- 25.Yang RB, Foster DC, Garbers DL, Fulle HJ. Two membrane forms of guanylyl cyclase found in the eye. Proc Natl Acad Sci USA. 1995;92:602–606. doi: 10.1073/pnas.92.2.602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schulz S, Chrisman TD, Garbers DL. Cloning and expression of guanylin. Its existence in various mammalian tissues. J Biol Chem. 1992;267:16019–16021. [PubMed] [Google Scholar]
- 27.Schrammel A, Behrends S, Schmidt K, Koesling D, Mayer B. Characterization of 1H-[1,2,4]oxadiazolo[4,3-a]quinoxalin-1-one as a heme-site inhibitor of nitric oxide-sensitive guanylyl cyclase. Mol Pharmacol. 1996;50:1–5. [PubMed] [Google Scholar]
- 28.Koller KJ, de Sauvage FJ, Lowe DG, Goeddel DV. Conservation of the kinaselike regulatory domain is essential for activation of the natriuretic peptide receptor guanylyl cyclases. Mol Cell Biol. 1992;12:2581–2590. doi: 10.1128/mcb.12.6.2581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Buck J, Sinclair ML, Schapal L, Cann MJ, Levin LR. Cytosolic adenylyl cyclase defines a unique signaling molecule in mammals. Proc Natl Acad Sci USA. 1999;96:79–84. doi: 10.1073/pnas.96.1.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Steegborn C, Litvin TN, Levin LR, Buck J, Wu H. Bicarbonate activation of adenylyl cyclase via promotion of catalytic active site closure and metal recruitment. Nat Struct Mol Biol. 2005;12:32–37. doi: 10.1038/nsmb880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bakalyar HA, Reed RR. The second messenger cascade in olfactory receptor neurons. Curr Opin Neurobiol. 1991;1:204–208. doi: 10.1016/0959-4388(91)90079-m. [DOI] [PubMed] [Google Scholar]
- 32.Ma M. Encoding olfactory signals via multiple chemosensory systems. Crit Rev Biochem Mol Biol. 2007;42:463–480. doi: 10.1080/10409230701693359. [DOI] [PubMed] [Google Scholar]
- 33.Zufall F, Munger SD. From odor and pheromone transduction to the organization of the sense of smell. Trends Neurosci. 2001;24:191–193. doi: 10.1016/s0166-2236(00)01765-3. [DOI] [PubMed] [Google Scholar]
- 34.Kwon JY, Dahanukar A, Weiss LA, Carlson JR. The molecular basis of CO2 reception in Drosophila. Proc Natl Acad Sci USA. 2007;104:3574–3578. doi: 10.1073/pnas.0700079104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sato K, Pellegrino M, Nakagawa T, Vosshall LB, Touhara K. Insect olfactory receptors are heteromeric ligand-gated ion channels. Nature. 2008;452:1002–1006. doi: 10.1038/nature06850. [DOI] [PubMed] [Google Scholar]
- 36.Wicher D, et al. Drosophila odorant receptors are both ligand-gated and cyclic-nucleotide-activated cation channels. Nature. 2008;452:1007–1011. doi: 10.1038/nature06861. [DOI] [PubMed] [Google Scholar]
- 37.Duda T, Sharma RK. ONE-GC membrane guanylate cyclase, a trimodal odorant signal transducer. Biochem Biophys Res Commun. 2008;367:440–445. doi: 10.1016/j.bbrc.2007.12.153. [DOI] [PubMed] [Google Scholar]
- 38.Nikolaev VO, Gambaryan S, Lohse MJ. Fluorescent sensors for rapid monitoring of intracellular cGMP. Nat Methods. 2006;3:23–25. doi: 10.1038/nmeth816. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.