Abstract
Olfactory-like chemosensory signaling occurs outside of the olfactory epithelium. We find that major components of olfaction, including olfactory receptors (ORs), olfactory-related adenylate cyclase (AC3) and the olfactory G protein (Golf), are expressed in the kidney. AC3 and Golf colocalize in renal tubules and in macula densa (MD) cells which modulate glomerular filtration rate (GFR). GFR is significantly reduced in AC3−/− mice, suggesting that AC3 participates in GFR regulation. Although tubuloglomerular feedback is normal in these animals, they exhibit significantly reduced plasma renin levels despite up-regulation of COX-2 expression and nNOS activity in the MD. Furthermore, at least one member of the renal repertoire of ORs is expressed in a MD cell line. Thus, key components of olfaction are expressed in the renal distal nephron and may play a sensory role in the MD to modulate both renin secretion and GFR.
Keywords: adenylate cyclase 3, glomerular filtration rate, Golf, macula densa, renin
Olfactory receptors (ORs) are 7 transmembrane domain G protein-coupled receptors that function in the olfactory epithelium as chemosensors for the detection of odorants (1). The capacity of the olfactory system to distinguish among a staggeringly large universe of chemical compounds depends in large measure on the fact that ≈1,000 ORs are encoded in the mammalian genome. The odor information gathered by this multiplicity of ORs is funneled through a single common signaling pathway. When an OR binds to its odorant, it activates a single species of G protein, the olfactory trimeric G protein (Golf), which then activates the olfactory isoform of adenylate cyclase (AC3). Both AC3 and Golf are obligate constituents of the olfactory machinery, as demonstrated by the fact that mice that are null for the expression of either AC3 or Golf are severely compromised in their ability to smell (2, 3).
We wondered whether OR signaling pathways might be exploited by the epithelial cells of the kidney to detect and respond to changes in the chemical composition of the “internal environment” that is constituted by the extracellular body fluid compartment. Olfactory receptors are known to play chemosensory roles outside of the olfactory epithelium, most notably in sperm (4). The kidney would appear to be an ideal organ in which the capabilities of the olfactory chemosensory machinery could be brought to bear, because it must keep careful track of the chemical composition of the tubular fluid as it moves through the different segments of the nephron. For example, it may be beneficial for the kidney to adjust its filtration, reabsorption, or secretion rates in response to changes in metabolite levels, as well as in response to levels of xenobiotics (to promote clearance of such substances) or dicarboxylic acids (to avoid renal stone formation). It may be that the kidney employs well-designed chemosensors, such as ORs, to regulate the excretion of these substances.
In the present study we examined whether olfactory-like signaling may play a role in the kidney by assaying for the presence of proteins necessary for olfactory signaling (AC3, Golf, and ORs) in the kidney, and by assaying renal function in mice lacking a protein necessary for olfaction (AC3). We find that the olfactory machinery is expressed in the distal nephron and macula densa (MD) and that it may play a physiologically critical role in regulating fundamental aspects of renal function.
Results
AC3 and Golf Are Expressed in the Kidney.
Although there are >1,000 ORs, each of these ORs initiates downstream signaling by activating Golf and AC3. The mRNAs encoding AC3 and Golf were found to be present in the kidney by RT-PCR (Fig. 1 A and B). The products of these RT-PCRs were cloned and sequenced and found to be indistinguishable from previously published sequences from the olfactory epithelium.
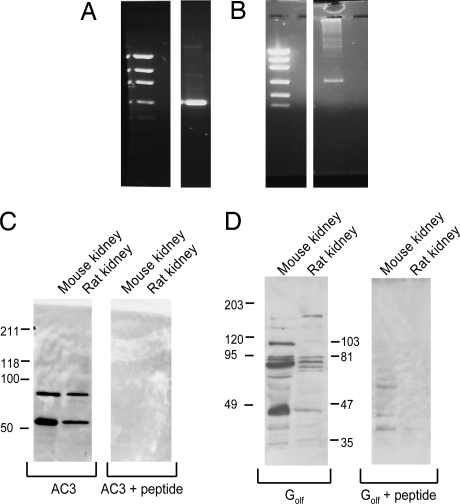
Fig. 1.
Two primary components of the olfaction pathway, AC3 and Golf, are expressed in kidney. (A and B) Both AC3 (A) and Golf (B) are detected in mouse kidneys by RT-PCR. The left lane is a low DNA mass ladder (Invitrogen 10068–013). The primers used for both AC3 and Golf span introns, and products were cloned and sequenced to confirm identity. In addition, AC3 and Golf proteins were detected in both mouse and rat kidneys by Western blot. (C and D) Bands of the expected sizes were obtained for AC3 (55 kDa, 90kDa) (C) and Golf (43/46 kDa, 77 kDa) (D), and antibody binding was competitively blocked by preincubating each antibody with an excess of its antigenic peptide.
In addition, by using well-characterized, commercially available antibodies that have been widely and routinely used to detect AC3 and Golf in the olfactory epithelium as well as in sperm (2, 3, 5–9), we were able to detect the presence of the AC3 and Golf proteins in lysates prepared from kidneys by Western blot analysis (Fig. 1 C and D). Importantly, the AC3 antibody has been demonstrated to not cross-react with AC1, 2, 4, 5, 6, or 9 (9). Western Blot analysis using lysates from both mouse and rat kidneys showed that AC3 and Golf yielded bands of sizes consistent with those reported in the literature. Furthermore, detection of these bands is completely prevented by preincubation of each antibody with its antigenic peptide. AC3 is typically found at 130 kDa in olfactory tissues (10). In nonolfactory tissues (such as sperm), however, this protein is reported to migrate with a molecular mass of 55 kDa (11), occasionally accompanied by additional bands at either 90 (11) or 130 kDa (9). As shown in Fig. 1C, we detect the 55-kDa AC3 band in both mouse and rat kidneys along with the additional 90-kDa band. Golf is typically detected as a band of 43–46 kDa, and an additional band at 77 kDa has been reported (6, 12). As shown in Fig. 1D, we detect the 46-kDa Golf band in mouse and rat kidneys, along with the additional 77-kDa band. Thus, our Western blot results clearly demonstrate that AC3 and Golf proteins are expressed in the renal tissue of both the mouse and the rat.
Immunolocalization of AC3 and Golf Within the Kidney.
We next performed immunohistochemistry using AC3 and Golf antibodies to confirm the expression of these proteins in the kidney and to examine their localizations. Fig. 2 shows immunohistochemical detection of Golf expression in the cortex (Fig. 2A), which is completely eliminated when the antibody is preincubated with its antigenic peptide (Fig. 2B). Golf expression was also seen in the outer medulla (data not shown). The cortical Golf staining clearly localized to the distal convoluted tubule (DCT) as shown by extensive colocalization (serial sections) with the NaCl cotransporter (NCC) (see Fig. 2 C and D and ref. 13). Because a minority of the Golf signal was found in tubule segments that did not label with NCC, we also doublestained using several other markers of the distal nephron. We found that Golf also colocalized (to a lesser extent than it did with NCC) with calbindin, which is a marker of the late DCT, connecting tubule, and collecting duct (data not shown) (13). Based on our colocalizations, Golf does not appear to be expressed in the thick ascending limb. We occasionally, but inconsistently, noted expression of Golf in the proximal tubule (as seen in Fig. 3A).
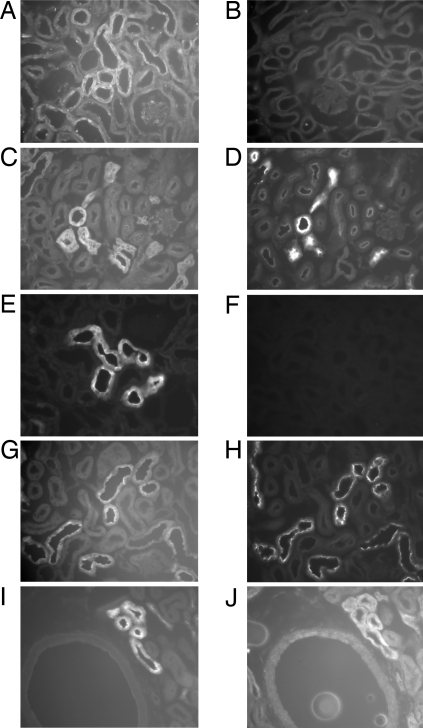
Fig. 2.
Golf and AC3 are expressed together in renal DCT. (A and B) Golf is expressed in distal tubule segments in the cortex (A), and this staining is no longer present when the antibody is preincubated with its antigenic peptide (B). (C and D) Golf expression was localized to the DCT by examining serial sections of mouse renal tissue stained for Golf (C) and for NCC, a DCT marker (D). (E and F) AC3 also localizes to cortical distal tubule segments (E); this staining is completely competitively blocked when the AC3 antibody is preincubated with its anitgenic peptide (F). Although the AC3 staining pattern appears to be distributed throughout the cytoplasm, it frequently exhibited particularly strong labeling along the apical edge of renal epithelial cells. (E) Similar to Golf, AC3 is expressed in the DCT, as demonstrated by analysis of serial sections of mouse kidneys stained with antibodies directed against AC3 (G) and NCC (H). (I and J) Furthermore, and most importantly, AC3 and Golf colocalize with one another, as shown in serial sections of mouse kidney stained with antibodies directed against AC3 (I) and Golf (J).
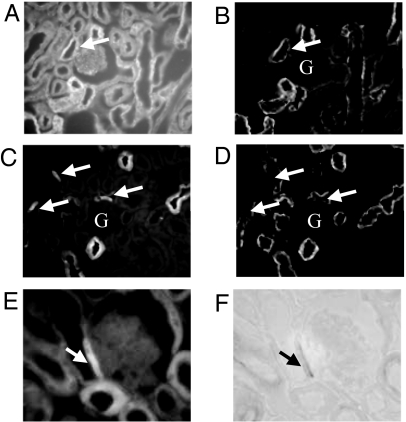
Fig. 3.
AC3 and Golf both localize to the cells of the MD. An antibody against the Na+-K+-ATPase was used to highlight MD cells because MD cells exhibit dramatically less Na+-K+-ATPase expression than their neighboring cells. (A and B) Golf (A) is expressed in the MD, as identified by the Na+-K+-ATPase staining pattern (B, arrows indicate MD). However, Golf is also well-expressed in the surrounding cells of the DCT. (C and D) In contrast, AC3 is strongly and specifically expressed in MD cells (C), which are once again identified by their relative lack of Na+-K+-ATPase staining and their position directly opposite the vascular pole of a glomerulus (D). (E and F) AC3 also colocalizes to the MD (E) as identified by NADPH diaphorase staining (F). Glomeruli are labeled by “G” in B–D.
The localization of AC3 in the kidney had a pattern of expression very similar to that of Golf. AC3 clearly stained distal tubules (Fig. 2E), typically along the apical surfaces of the tubule epithelial cells. Once again, competitive preincubation of the antibody with its antigenic peptide (Fig. 2F) was able to completely block the signal. AC3 also showed excellent colocalization with NCC in serial sections (Fig. 2 G and H), and colocalized to a lesser extent with calbindin (data not shown), but not with a marker of the thick ascending limb. Importantly, Fig. 2 I and J demonstrate that AC3 and Golf colocalized with one another in the cortical distal nephron.
Initial immunolocalization experiments showed a staining pattern that was consistent with the expression of AC3 and Golf in the MD. MD cells were positively identified by the relatively low expression of Na+-K+-ATPase compared with the surrounding cells of the thick ascending limb (14), and by NADPH diaphorase staining (Fig. 3 E and F). In Fig. 3 A and B, it can be seen that Golf is indeed expressed in the MD, although it also appears to be equally well-expressed in the surrounding tubular cells. In contrast, AC3 has a striking pattern of expression specifically in MD cells (Fig. 3 C, D, E, and F).
Identification of ORs in Mouse Kidney and in a MD Cell Line.
We used a PCR approach employing degenerate primers capable of amplifying the entire repertoire of mouse ORs to identify potential ORs expressed in the kidney. Renal expression of each candidate OR was further analyzed by RT-PCR employing specific primers. By using these approaches, we have confirmed the expression of 6 different ORs in the kidney (Olfr78, Olfr90, Olfr1373, Olfr1392, Olfr1393, and Olfr NP_TR6JSE50FPA).
Because AC3 and Golf localized to the MD by immunohistochemistry, we also performed PCR on a MD cell line (15) to determine whether any of the renal ORs were expressed in the MD. The MD cell line was generated by FACS sorting cells from mice transgenic for the SV40 large T antigen, and has been previously characterized (15). By using specific primers for each renal OR, we found that Olfr 90 is expressed in the MD cell line. The full-length sequence of Olfr 90 from the MD was cloned and sequenced, and found to be identical to the sequence previously obtained from both olfactory epithelium and from mouse kidney.
Characterization of Renal Function in AC3−/− Mice.
Because the olfactory signaling machinery is expressed in several segments of the renal epithelium, we assayed renal function in mice null for a necessary component of the olfaction pathway (AC3). AC3−/− mice have been studied previously (2, 5, 7, 16, 17), and are known to be anosmic (unable to smell) and to have a very high fatality rate after birth. It is believed that the neonatal mortality is due to the inability of the pups to smell their mother, compromising their ability to nurse. With vigilant care, some AC3−/− pups are able to survive this initial period, and then to survive into adulthood. By adulthood, AC3−/− mice become quite obese (Table 1).
Table 1.
Renal physiology of AC3+/+ and AC3−/− mice
| Characteristics | Genotype |
|
|---|---|---|
| AC3+/+ | AC3−/− | |
| BW, g | 34.1 ± 1.4 | 44.3 ± 0.6* |
| BP, mmHg | 91.6 ± 2.9 | 97.1 ± 5.4 |
| UV, mL/min | 1.21 ± 0.27 | 2.00 ± 0.52 |
| GFR, mL/min | 0.31 ± 0.02 | 0.19 ± 0.04* |
| PNa, mM | 154.0 ± 2.8 | 152.1 ± 1.7 |
| PK, mM | 4.22 ± 0.17 | 4.47 ± 0.20 |
| ENa, mEq/min/100 g BW | 0.23 ± 0.05 | 0.39 ± 0.14 |
| EK, mEq/min/100 g BW | 0.76 ± 0.14 | 0.38 ± 0.04* |
| FENa, % | 0.17 ± 0.03 | 0.53 ± 0.17 |
| FEK, % | 20.5 ± 3.7 | 24.6 ± 4.1 |
Renal function in AC3+/+ and AC3−/− mice (n = 7). Values are mean ± SEM. The GFR was significantly lower in AC3−/− mice as compared to the wild-type controls. BW, body weight; BP, blood pressure; UV, urine volume; GFR, glomerular filtration rate; PNa, plasma sodium; PK, plasma potassium; ENa, excretion of sodium; EK, excretion of potassium; FENa, fractional excretion of sodium; FEK, fractional excretion of potassium.
*, P < 0.05 versus AC3+/+
Initial studies of metabolic parameters in AC3−/− mice showed normal basal values of blood gases, electrolytes, water balance (hematocrit and hemoglobin), plasma glucose, urine pH, and urine osmolarity (data not shown). AC3−/− mice do not exhibit proteinuria. We next performed studies on anesthetized animals to examine directly the renal clearance of inulin as well as the renal clearance of Na+ and K+. Renal clearance of inulin, a polymer commonly used in glomerular filtration rate (GFR) measurement that is freely filtered but not reabsorbed or secreted, was measured to calculate the GFR. The results of these studies are shown in Table 1. The mean arterial blood pressure (MAP) appears to trend toward a modest elevation in AC3−/− animals as compared with AC3+/+ controls (Table 1). However, subsequent studies using radiotelemetry to monitor MAP in freely moving, conscious mice clearly showed no difference in MAP in the AC3−/− vs. wild-type mice, and they demonstrated that AC3−/− mice have normal circadian rhythms (n = 3 for each genotype, see Table 2). Similarly, blood pressures of the anesthetized mice used for micropuncture were not different between genotypes.
Table 2.
Telemetry values of AC3+/+ and AC3−/− mice
| Characteristics | Genotype |
|||
|---|---|---|---|---|
| AC3+/+ |
AC3−/− |
|||
| Day | Night | Day | Night | |
| MAP, mmHg | 104.17 ± 5.75 | 114.72 ± 7.81 | 106.82 ± 4.99 | 120.12 ± 5.53 |
| HR | 498.15 ± 23.55 | 561.00 ± 10.57 | 493.47 ± 37.23 | 570.03 ± 31.55 |
| Time active, % | 31.53 ± 4.05 | 52.43 ± 4.74* | 21.57 ± 5.26 | 46.62 ± 3.30* |
Telemetry values for AC3+/+ and AC3−/− mice. Values are mean ± SEM. AC3−/− had normal blood pressure and circadian rhythms. HR, heart rate.
*, P < 0.05 versus ″day″ for the same genotype
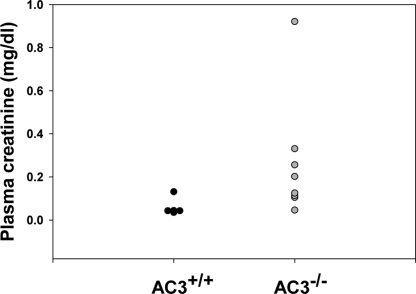
Table 1 shows that the GFR in anesthetized AC3−/− animals is significantly reduced compared with that measured in controls. In addition, measurements of GFR in conscious animals by single injection FITC inulin clearance confirmed the presence of a lower GFR in AC3−/− mice (238.4 ± 16.6 μL/min) than in wild-type mice (323.4 ± 35.1 μL/min; P = 0.046). Assessment of plasma creatinine levels by HPLC also supports this finding (Fig. 4). We found that although some of the AC3−/− mice are able to maintain near normal plasma creatinine values, a substantial number possessed quite elevated values. This finding is consistent with the fact that, although plasma creatinine levels rise as GFR falls, plasma creatinine is not detectably elevated until GFR has fallen by at least 50% (18). This phenomenon is due to increased secretion of creatinine by the proximal tubule in response to decreased GFR (19). Because AC3−/− mice exhibit GFRs that are on average ≈60% of normal as measured by inulin clearance, it is to be expected that a subset of these animals will manifest relatively normal plasma creatinine values. The marked elevation of plasma creatinine in a subset of the AC3−/− mice indicates a chronic and substantial reduction in GFR.
Fig. 4.
AC3−/− mice tend to manifest increased plasma creatinine levels as compared with their wild-type littermates. Although AC3+/+ mice (n = 5) all exhibit appropriately low plasma creatinine values, AC3−/− mice (n = 8) manifest a wide range of plasma creatinine values, extending from near normal to quite elevated. This range of values is consistent with the ≈40% reduction in inulin clearance detected in these animals, because plasma creatinine values only begin to rise once GFR is reduced by 50% or more.
To rule out an anatomical basis for this difference in GFR, a histological assessment revealed that AC3−/− mice had normal numbers of glomeruli and no obvious histological abnormalities (histology was examined in a blinded fashion by M. Kashgarian, Yale University). Furthermore, despite the marked differences in body weight, kidney weights were not significantly different between wild-type and AC3−/− mice (441 ± 34 mg vs. 400 ± 33.3 mg; n = 6 vs. n = 5). Thus, the decreased GFR is unlikely to be due to a reduction in nephron number but instead, is likely because of alterations in the activity or effectiveness of the control mechanisms that regulate renal hemodynamics.
The ion clearances for AC3+/+ and AC3−/− mice were not significantly different (Table 1), with the exception of the K+ excretion. The significantly decreased K+ excretion is likely to be, at least in part, because of the reduced GFR. A low GFR would be expected to lead to less flow-mediated K+ secretion in the distal nephron, as well as to less distal Na+ delivery and therefore also to less K+ secretion, because Na+ absorption and K+ secretion are electrically coupled in the collecting tubule.
Measurement of Tubuloglomerular Feedback and of Plasma Levels of Renin.
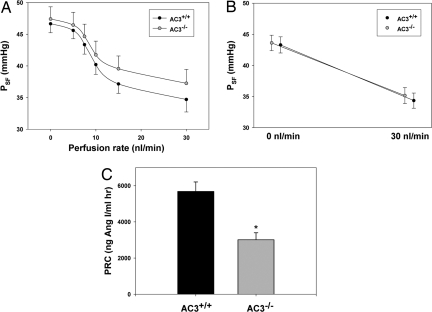
The localization of AC3 to MD cells, coupled with the dysregulation of GFR in AC3−/− mice, raised the question of whether AC3 may be involved in the roles of the MD to regulate tubuloglomerular feedback (TGF) or renin secretion. To assess TGF, micropuncture experiments were performed in which the flow rate in the distal segment of a nephron was manipulated while the proximal stop-flow pressure (PSF) in that same nephron was measured as an index of glomerular capillary pressure. As shown in Fig. 5 A and B, the TGF response magnitude was not measurably different between wild-type and AC3−/− mice. Furthermore, flow rates inducing half-maximal responses were statistically indistinguishable between genotypes (9.6 nL/min in wild-type and 9.5 nL/min in AC3−/− mice). As noted above, arterial blood pressure at the time of micropuncture was similar between wild-type and AC3−/− mice.
Fig. 5.
Although tubuloglomerular feedback (TGF) is normal, plasma renin concentration is significantly reduced in AC3−/− mice. TGF was measured by single nephron micropuncture. Distal flow rates were varied in individual nephrons and the stop flow technique was used to assess consequent changes in stop-flow pressures. (A) The traces presented demonstrate that over a range of distal flow rates, AC3−/− mice (n = 10) are able to properly regulate proximal stop-flow pressure in a manner similar to that of wild-type littermates (n = 10). (B) Similarly, (with a larger n) there is no difference in the proximal stop-flow rates between AC3+/+ (n = 20) and AC3−/− mice (n = 25) at the minimal and maximal distal flow rates investigated. (C) Although TGF is normal, plasma renin is significantly reduced in AC3−/− mice. The plasma renin concentrations in AC3−/− mice (n = 11) are reduced by nearly 50% as compared with their wild-type littermates (n = 9; * indicates P < 0.001).
The MD's other primary role in controlling GFR and body fluid volume is to regulate renin secretion. Measurements of plasma levels of renin in conscious AC3+/+ and AC3−/− mice (Fig. 5C) showed significantly lower levels, by ≈50%, in AC3−/− mice as compared with those found in their wild-type littermates.
COX-2 and Neuronal Nitric Oxide Synthase (nNOS) in MD Cells.
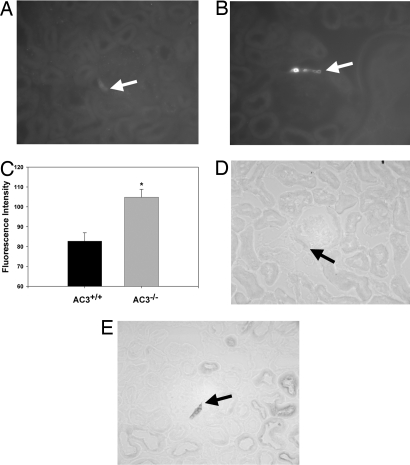
Because both the COX-2 and nNOS enzymes have been previously shown to be critical upstream factors in the signaling cascade that controls renin secretion (20–23), we examined the expression of COX-2 in the MD of AC3+/+ and AC3−/− mice by using immunohistochemistry. The intensity of COX-2 stain in the MD of AC3−/− mice was significantly greater than that of AC3+/+ (Fig. 6 A–C). In addition, we stained both AC3+/+ and AC3−/− mouse kidneys by using NADPH-diaphorase to determine the level of nNOS activity, which also appeared to be up-regulated in AC3−/− (Fig. 6 D and E).
Fig. 6.
COX-2 expression and nNOS activity are up-regulated in the MD of AC3−/−. (A–C) COX-2 staining in AC3−/− (B) is significantly greater than in wild-type littermates (A), as quantified in C. (D and E) nNOS activity, as indicated by NADPH-diaphorase staining, also appeared to be increased in AC3−/− (D) as compared with AC3+/+ (E). Arrows indicate MD.
Discussion
In this paper, we report that elements of the machinery necessary for olfaction are present in the kidney and that mice deficient in one of the key components of olfactory signal transduction are unable to properly regulate GFR. We first demonstrated that AC3 and Golf are expressed in the kidney both at the RNA and protein levels, and that they localize to common segments of the nephron (the DCT, the early portion of the connecting tubule, and the MD). In addition to these obligate downstream components of olfactory signaling, we have found that the sensory components of olfactory signaling, the ORs themselves, are also expressed in the kidney, and that at least one of these ORs is present in a MD cell line. Furthermore, AC3−/− mice are unable to properly regulate GFR and plasma renin.
Intriguingly, it has recently been observed that several ciliopathies are associated with olfactory deficits. These ciliopathies include diseases such as Bardet–Biedl Syndrome (BBS) (24, 25) and Joubert and Meckel syndromes (26). Diseases such as BBS hint at a connection between the nose and the kidney because BBS patients present with both anosmia (the inability to smell) and renal cysts, as well as obesity. Recently, it has been found that several ciliary proteins, when disrupted, lead to an obese phenotype. This mechanism appears to involve the proopiomelanocortin neurons in the hypothalamus, which cannot respond to satiety signals unless they possess properly formed cilia (27). We speculate that the obesity seen in AC3−/− mice may be due to a similar mechanism.
Hoffert et al. (28) previously examined the renal expression of adenylate cyclases, and also found that AC3 is present in the kidney. However, the Hoffert study focused on AC3 expression in the inner medullary collecting duct (IMCD) (demonstrated by PCR and Western blot using samples enriched for IMCD), but did not comment on its expression in the cortex. Although the present study has used the same AC3 antibody as Hoffert et al., we have not been able to detect AC3 in the IMCD by histochemistry. It is possible that species differences (rat vs. mouse) are responsible for this discrepancy.
In this study, we have determined that 6 olfactory receptors are expressed in the kidney, and that one of these 6 ORs can be detected in a MD cell line. Although we have likely not identified the full complement of renal ORs, it is interesting to note that, by using the degenerate OR primer method, at least one OR was cloned multiple times, implying that either the renal complement of ORs is fairly small or that the ORs we have identified constitute a relatively abundant subpopulation. Unfortunately, the great majority (≈90%) of ORs are orphan receptors (29, 30), and none of the renal ORs that we have identified have known ligands. In future studies, we plan to elucidate the specific ligands which are recognized by the renal ORs.
We observed that the GFR was significantly lower in AC3−/− mice as compared with wild-type mice, a finding that was confirmed in both anesthetized and conscious animals. The causes for the reduction in GFR are not entirely clear, and although AC3−/− mice do not exhibit gross anatomical changes, we are unable to exclude the possibility that the obesity and associated metabolic phenotype could have unexpected effects on renal function. Although it is possible that AC3 expression in renal blood vessels could contribute to this phenotype, we have been unable to detect AC3 in the renal vasculature by immunohistochemistry. Nevertheless, signaling by any of a number of vasodilating agonists may be impaired in AC3-deficient mice. These agonists could be contributed by renal autonomic nerves or may be produced in association with intrinsic renal mechanisms that regulate glomerular blood flow, such as TGF.
Because the MD is thought to be a key controller of GFR, and because AC3 and Golf both localized to the MD, we tested the possibility that the reduction of GFR is the consequence of a change in TGF signaling, resulting from a reduction in MD cAMP levels. There is previous evidence to indicate that cAMP in the MD diminishes TGF responses (31). However, we found that the absence of AC3 did not alter the response characteristics of glomerular capillary pressure to changes in flow past the MD cells (Fig. 5 A and B). These data indicate that the signaling mechanism across the juxtaglomerular apparatus beyond the MD-sensing step is not measurably modified by AC3. However, it is to be noted that the present data were generated by perfusing loops of Henle with an artificial perfusate. It is possible that this artificial perfusate lacks one or more of the critical ligands that would normally induce AC3 activation, thereby eliminating possible differences between AC3-deficient and wild-type mice. However, previous studies comparing native tubular fluid with artificial perfusates failed to find any differences in TGF (32–34), except in salt-loaded animals (33, 34). It should be noted, however, that the “native tubular fluid” used in these studies was collected from a proximal site and would therefore not contain substances that may normally be secreted in other segments proximal to the MD.
The present data show that plasma renin is decreased in the absence of AC3, implying that AC3 acts as a regulator of renin secretion. Although a reduction of plasma renin could be the result of extracellular volume expansion, there is little reason to think that AC3−/− mice are in fact volume-expanded. It must be noted that, despite the fact that the MAP was normal by telemetry (indicating the mice were euvolemic), it trended toward being elevated. Coupled with the observed trend toward decreased physical activity (also insignificant), which would be expected to lower measured MAP, it remains possible that the actual MAP of AC3−/− is slightly elevated, which would be consistent with very mild volume expansion. In addition, however, to the statistically indistinguishable MAP measurements, AC3−/− mice also had normal hematocrits and plasma Na+, and a normal urine volume excretion when given an exogenous infusion of saline for clearance studies (as in Table 1), all of which argue strongly against volume expansion. In addition, the increase of COX-2 and nNOS activity in AC3−/− MD is precisely the opposite of what would be expected if the mice were volume-expanded. Furthermore, because arterial blood pressure was not statistically different between genotypes, it is unlikely that baroreceptor activation could be the underlying cause for the change in renin. A direct effect of a reduction in cAMP in juxtaglomerular (JG) granular cells of AC3-deficient mice could explain the observed effect; however, AC3 has not been detected in freshly isolated JG cells, making such a direct effect unlikely (35). Furthermore, our immunohistochemistry staining clearly showed AC3 localizing to MD, but not to JG cells.
As noted above, we also observed an up-regulation of COX-2 and nNOS activity in the MD. This phenomenon is probably a feedback response to the low renin, because decreased renin and angiotensin II levels should lead to decreased angiotensin II-mediated inhibition of COX-2 (36). What, then, is causing the renin to be low? One possibility is that renal sympathetic nerve activity is inappropriately low in these mice. At this time, we have no data to indicate the presence or absence of AC3 in renal sympathetic nerves. Alternatively, it is possible that the COX-2/nNOS/renin pathway is “broken” at the level of the MD in the absence of AC3. For example, prostaglandin E (PGE) synthase is necessary to generate PGE2 from COX-2-derived prostaglandin H2. If PGE synthase expression or activity were dependent on AC3-generated cAMP, this could potentially explain why renin remains low despite the elevation of COX-2/nNOS.
In conclusion, we have uncovered the presence of the olfactory OR/Golf/AC3 signaling system in the kidney and demonstrated its specific localization in the distal nephron. Our data suggest a use of the olfactory machinery in the regulation of renin secretion and glomerular filtration rate. In future studies, it will be intriguing to further examine the role of individual ORs in the kidney to better understand what activates these signaling pathways and what physiological roles they serve.
Methods
RT-PCR for AC3 and Golf.
RT-PCR was performed using standard methodologies; please see the supporting information (SI) for details.
Western Blot for AC3 and Golf.
Mouse and rat kidneys were removed after euthanasia, and microsomal kidney protein was extracted. Details of protein extraction and Western blot procedures can be found in the SI.
Immunohistochemistry.
Immunohistochemistry experiments were performed using standard procedures. For details and antibodies used, please see the SI.
In Vivo Studies.
All experiments were conduced in accordance with the policies and procedures of the Yale Institutional Animal Care and Use Committee and the National Institutes of Health principles and guidelines for the Care and Use of Laboratory Animals. Please see the SI for details.
Detection of Renal ORs.
Because there are ≈1,000 olfactory receptors, and only a few antibodies are available, we took a screening approach to identify ORs in the kidney. To screen for renal OR expression, we used degenerate OR primers (dOR), published previously by several groups (37, 38). The dOR primer mix is sufficient to PCR any OR in the genome, and subsequent to the initial screen, gene-specific primers were used to confirm renal expression for each OR. For details of these procedures, please see the SI.
Supplementary Material
Acknowledgments.
We thank SueAnn Mentone for preparation of histochemistry slides; David Geller and Junhui Zhang for measurement of blood pressure by telemetry and of plasma aldosterone; Laszlo Furu and Stephen Somlo for measurement of plasma creatinine; Michael Kashgarian for assistance with the histological analysis; the members of the M.J.C., S.F., and C.A.G. laboratories for helpful discussions; and Drs. Gerhard Giebisch and John Carlson for careful readings of the manuscript. This work was supported by a postdoctoral fellowship from the Polycystic Kidney Disease Foundation (to J.L.P.), National Institutes of Health Grant DK-17433 (Core E; to T.W.), intramural funds from National Institute of Diabetes and Digestive and Kidney Diseases (to J.S.), and National Institutes of Health Grants DK-57328 and DK-17433 (to M.J.C.).
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/cgi/content/full/0812859106/DCSupplemental.
References
- 1.Buck L, Axel R. A novel multigene family may encode odorant receptors: A molecular basis for odor recognition. Cell. 1991;65:175–187. doi: 10.1016/0092-8674(91)90418-x. [DOI] [PubMed] [Google Scholar]
- 2.Wong ST, et al. Disruption of the type III adenylyl cyclase gene leads to peripheral and behavioral anosmia in transgenic mice. Neuron. 2000;27:487–497. doi: 10.1016/s0896-6273(00)00060-x. [DOI] [PubMed] [Google Scholar]
- 3.Belluscio L, Gold GH, Nemes A, Axel R. Mice deficient in G(olf) are anosmic. Neuron. 1998;20:69–81. doi: 10.1016/s0896-6273(00)80435-3. [DOI] [PubMed] [Google Scholar]
- 4.Spehr M, et al. Identification of a testicular odorant receptor mediating human sperm chemotaxis. Science. 2003;299:2054–2058. doi: 10.1126/science.1080376. [DOI] [PubMed] [Google Scholar]
- 5.Livera G, et al. Inactivation of the mouse adenylyl cyclase 3 gene disrupts male fertility and spermatozoon function. Mol Endocrinol. 2005;19:1277–1290. doi: 10.1210/me.2004-0318. [DOI] [PubMed] [Google Scholar]
- 6.Itakura S, Ohno K, Ueki T, Sato K, Kanayama N. Expression of Golf in the rat placenta: Possible implication in olfactory receptor transduction. Placenta. 2006;27:103–108. doi: 10.1016/j.placenta.2004.12.006. [DOI] [PubMed] [Google Scholar]
- 7.Zou DJ, et al. Absence of adenylyl cyclase 3 perturbs peripheral olfactory projections in mice. J Neurosci. 2007;27:6675–6683. doi: 10.1523/JNEUROSCI.0699-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wei J, et al. Phosphorylation and inhibition of olfactory adenylyl cyclase by CaM kinase II in Neurons: A mechanism for attenuation of olfactory signals. Neuron. 1998;21:495–504. doi: 10.1016/s0896-6273(00)80561-9. [DOI] [PubMed] [Google Scholar]
- 9.Defer N, et al. The olfactory adenylyl cyclase type 3 is expressed in male germ cells. FEBS Lett. 1998;424:216–220. doi: 10.1016/s0014-5793(98)00178-1. [DOI] [PubMed] [Google Scholar]
- 10.Fend F, et al. Immuno-LCM: Laser capture microdissection of immunostained frozen sections for mRNA analysis. Am J Pathol. 1999;154:61–66. doi: 10.1016/S0002-9440(10)65251-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Baxendale RW, Fraser LR. Evidence for multiple distinctly localized adenylyl cyclase isoforms in mammalian spermatozoa. Mol Reprod Dev. 2003;66:181–189. doi: 10.1002/mrd.10344. [DOI] [PubMed] [Google Scholar]
- 12.Herve D, et al. G(olf) and Gs in rat basal ganglia: Possible involvement of G(olf) in the coupling of dopamine D1 receptor with adenylyl cyclase. J Neurosci. 1993;13:2237–2248. doi: 10.1523/JNEUROSCI.13-05-02237.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Loffing J, et al. Distribution of transcellular calcium and sodium transport pathways along mouse distal nephron. Am J Physiol. 2001;281:F1021–F1027. doi: 10.1152/ajprenal.0085.2001. [DOI] [PubMed] [Google Scholar]
- 14.Kashgarian M, Biemesderfer D, Caplan M, Forbush B., III Monoclonal antibody to Na,K-ATPase: Immunocytochemical localization along nephron segments. Kidney Int. 1985;28:899–913. doi: 10.1038/ki.1985.216. [DOI] [PubMed] [Google Scholar]
- 15.Yang T, et al. Low chloride stimulation of prostaglandin E2 release and cyclooxygenase-2 expression in a mouse macula densa cell line. J Biol Chem. 2000;275:37922–37929. doi: 10.1074/jbc.M006218200. [DOI] [PubMed] [Google Scholar]
- 16.Esposito G, et al. Mice deficient for soluble adenylyl cyclase are infertile because of a severe sperm-motility defect. Proc Natl Acad Sci USA. 2004;101:2993–2998. doi: 10.1073/pnas.0400050101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Xie F, et al. Soluble adenylyl cyclase (sAC) is indispensable for sperm function and fertilization. Dev Biol. 2006;296:353–362. doi: 10.1016/j.ydbio.2006.05.038. [DOI] [PubMed] [Google Scholar]
- 18.Shemesh O, Golbetz H, Kriss JP, Myers BD. Limitations of creatinine as a filtration marker in glomerulopathic patients. Kidney Int. 1985;28:830–838. doi: 10.1038/ki.1985.205. [DOI] [PubMed] [Google Scholar]
- 19.Schwartz GJ, Furth SL. Glomerular filtration rate measurement and estimation in chronic kidney disease. Pediatr Nephrol. 2007;22:1839–1848. doi: 10.1007/s00467-006-0358-1. [DOI] [PubMed] [Google Scholar]
- 20.Kim SM, et al. Low plasma renin and reduced renin secretory responses to acute stimuli in conscious COX-2-deficient mice. Am J Physiol. 2007;292:F415–F422. doi: 10.1152/ajprenal.00317.2006. [DOI] [PubMed] [Google Scholar]
- 21.Traynor TR, Smart A, Briggs JP, Schnermann J. Inhibition of macula densa-stimulated renin secretion by pharmacological blockade of cyclooxygenase-2. Am J Physiol. 1999;277:F706–F710. doi: 10.1152/ajprenal.1999.277.5.F706. [DOI] [PubMed] [Google Scholar]
- 22.Matzdorf C, Kurtz A, Hocherl K. COX-2 activity determines the level of renin expression but is dispensable for acute up-regulation of renin expression in rat kidneys. Am J Physiol. 2007;292:F1782–F1790. doi: 10.1152/ajprenal.00513.2006. [DOI] [PubMed] [Google Scholar]
- 23.Beierwaltes WH. Macula densa stimulation of renin is reversed by selective inhibition of neuronal nitric oxide synthase. Am J Physiol. 1997;272:R1359–R1364. doi: 10.1152/ajpregu.1997.272.5.R1359. [DOI] [PubMed] [Google Scholar]
- 24.Kulaga HM, et al. Loss of BBS proteins causes anosmia in humans and defects in olfactory cilia structure and function in the mouse. Nat Genet. 2004;36:994–998. doi: 10.1038/ng1418. [DOI] [PubMed] [Google Scholar]
- 25.Iannaccone A, et al. Clinical evidence of decreased olfaction in Bardet-Biedl syndrome caused by a deletion in the BBS4 gene. Am J Med Genet A. 2005;132:343–346. doi: 10.1002/ajmg.a.30512. [DOI] [PubMed] [Google Scholar]
- 26.McEwen DP, et al. Hypomorphic CEP290/NPHP6 mutations result in anosmia caused by the selective loss of G proteins in cilia of olfactory sensory neurons. Proc Natl Acad Sci USA. 2007;104:15917–15922. doi: 10.1073/pnas.0704140104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Davenport JR, et al. Disruption of intraflagellar transport in adult mice leads to obesity and slow-onset cystic kidney disease. Curr Biol. 2007;17:1586–1594. doi: 10.1016/j.cub.2007.08.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hoffert JD, Chou CL, Fenton RA, Knepper MA. Calmodulin is required for vasopressin-stimulated increase in cyclic AMP production in inner medullary collecting duct. J Biol Chem. 2005;280:13624–13630. doi: 10.1074/jbc.M500040200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Saito H, Kubota M, Roberts RW, Chi Q, Matsunami H. RTP family members induce functional expression of mammalian odorant receptors. Cell. 2004;119:679–691. doi: 10.1016/j.cell.2004.11.021. [DOI] [PubMed] [Google Scholar]
- 30.Malnic B, Godfrey PA, Buck LB. The human olfactory receptor gene family. Proc Natl Acad Sci USA. 2004;101:2584–2589. doi: 10.1073/pnas.0307882100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bell PD. Cyclic AMP-calcium interaction in the transmission of tubuloglomerular feedback signals. Kidney Int. 1985;28:728–732. doi: 10.1038/ki.1985.191. [DOI] [PubMed] [Google Scholar]
- 32.Bell PD, Thomas C, Williams RH, Navar LG. Filtration rate and stop-flow pressure feedback responses to nephron perfusion in the dog. Am J Physiol. 1978;234:F154–F165. doi: 10.1152/ajprenal.1978.234.2.F154. [DOI] [PubMed] [Google Scholar]
- 33.Schnermann J, Schubert G, Briggs J. Tubuloglomerular feedback responses with native and artificial tubular fluid. Am J Physiol. 1986;250:F16–F21. doi: 10.1152/ajprenal.1986.250.1.F16. [DOI] [PubMed] [Google Scholar]
- 34.Haberle DA, Davis JM. Resetting of tubuloglomerular feedback: Evidence for a humoral factor in tubular fluid. Am J Physiol. 1984;246:F495–F500. doi: 10.1152/ajprenal.1984.246.4.F495. [DOI] [PubMed] [Google Scholar]
- 35.Grunberger C, Obermayer B, Klar J, Kurtz A, Schweda F. The calcium paradoxon of renin release: Calcium suppresses renin exocytosis by inhibition of calcium-dependent adenylate cyclases AC5 and AC6. Circ Res. 2006;99:1197–1206. doi: 10.1161/01.RES.0000251057.35537.d3. [DOI] [PubMed] [Google Scholar]
- 36.Cheng HF, et al. Angiotensin II attenuates renal cortical cyclooxygenase-2 expression. J Clin Invest. 1999;103:953–961. doi: 10.1172/JCI5505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Malnic B, Hirono J, Sato T, Buck LB. Combinatorial receptor codes for odors. Cell. 1999;96:713–723. doi: 10.1016/s0092-8674(00)80581-4. [DOI] [PubMed] [Google Scholar]
- 38.Otaki JM, Yamamoto H, Firestein S. Odorant receptor expression in the mouse cerebral cortex. J Neurobiol. 2004;58:315–327. doi: 10.1002/neu.10272. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.