Abstract
Ozone exposure produces acute inflammation and neutrophil influx in the distal lung. Alveolar epithelial cells cover a large surface area, secrete chemokines, and may initiate or modify the inflammatory response. The effect of ozone on chemokine production by these cells has not been defined. Isolated rat type II cells were cultured in different conditions to express the morphologic appearance and biochemical markers for the type I and the type II cell phenotypes. These cells were exposed to ozone at an air/liquid interface. The type I–like cells were more susceptible to injury than the type II cells and showed signs of injury at exposure levels of 100 ppb ozone for 60 min. Both phenotypes showed evidence of lipid peroxidation after ozone exposure as measured by 8-isoprostane production, but neither phenotype secreted increased amounts of MIP-2 (CXCL3), CINC-1 (CXCL1), or MCP-1 (CCL2) in response to ozone. Both cell phenotypes secreted MIP-2 and MCP-1 in response to IL-1β or lipopolysaccharide, but there was no priming or synergy with ozone. It is likely that the inflammatory response to ozone in the alveolar compartment is not due to the direct effect of ozone on epithelial cells.
Keywords: alveolar epithelium, LPS, 8-isoprostane, MCP-1, MIP-2
Ozone penetrates deep into the lung and produces acute inflammation and adverse respiratory health effects in animals and humans. Ozone causes injury primarily in alveolar ducts and terminal bronchioles but also in conducting airways and proximal alveolar regions (1–3). Alveolar and airway epithelial cells are among the first cells to come in contact with inhaled ozone and thus constitute one of the primary targets for ozone toxicity. Exposure to ozone induces acute inflammation and a neutrophil influx in vivo (4–6). In humans and monkeys, the neutrophil influx has been suggested to be due to secretion of IL-8 (7–9). Despite these reproducible studies on acute inflammation in vivo, little is known about the primary cell type responsible for initiation of the inflammatory response or if the effect of ozone is direct or indirect.
Ozone is thought to stimulate resident lung cells to secrete a variety of chemoattractants (10–12). The resident cells responsible and the determination of the signaling molecules and pathways involved have not been defined. Isolated alveolar macrophages and THP-1 cells derived from monocytic leukemic cells failed to secrete chemokines in response to ozone in some studies (13, 14) but were reported to secrete IL-8 in another study (15). These results suggest that macrophages may not be the prime target for induction of the inflammatory response or that a more complicated signaling process may be involved. Although there have been toxicity studies of the effect of ozone on alveolar epithelial cells, the effect of ozone on chemokine production has not been reported (16). A human lung cancer cell line (A549 cells), bronchial epithelial cells (BEAS-2B), and human nasal epithelial cells have been reported to secrete chemokines and cytokines in response to ozone in vitro (9, 13, 17–19). However, the chemokine stimulation is modest, and there are observations that ozone did not increase chemokine secretion (20). In vivo there is good evidence that monkey bronchial epithelial cells express and secrete IL-8 in response to ozone and that the neutrophil chemotactic activity is inhibited by a neutralizing antibody to IL-8 (9). Hence, the epithelium is likely to participate in the inflammatory response to ozone in vivo, but the direct effects of ozone on epithelial cells in vitro is modest, and the signaling pathways may be complex.
Alveolar epithelial cells are likely targets of ozone and a source of inflammatory chemokines. Type I cells comprise 95% of the surface area of the alveolar epithelium, and, although they do not produce surfactant, they are metabolically active and may play physiologically important roles in alveolar homeostasis (21, 22). Type II cells transdifferentiated in culture to display morphologic and surface markers of type I–like cells have been reported to secrete chemokines (23, 24). Type II cells produce pulmonary surfactant and have been reported to secrete or express chemokines in vitro and in vivo (25–28). Hence, both cell types could initiate or modify the inflammatory response to ozone. The ability of these two phenotypes to produce chemokines in response to ozone has not been reported.
In this study, we sought to determine if alveolar type I–like cells or type II cells secrete chemokines in direct response to ozone and if exposure to IL-1β or LPS would enhance this effect. We focused on secretion of MIP-2 (CINC-3/CXCL3) and MCP-1 (CCL2) because their mRNA levels are increased after exposure to ozone and show synergy with LPS and ozone in vivo (29). We hypothesized that type II cells would secrete chemokines in response to ozone and that this effect would be greatly enhanced by pre-exposure to IL-1β or LPS.
MATERIALS AND METHODS
Type II Cell Isolation
Alveolar type II cells were isolated from pathogen-free adult male Sprague-Dawley rats (Harlan Sprague Dawley, Indianapolis, IN) by dissociation with porcine pancreatic elastase (Roche Molecular Biochemicals, Indianapolis, IN) and partial purification on discontinuous density gradients by standard methods (30–32). The only major difference from previous reports was that the density gradient was made from Optiprep (Axis-Shield Poc As, Oslo, Norway) instead of Metrizamide.
Culture in Apical-Access System
Type II cells were plated on a filter insert (0.4-μm pore, 30 mm diameter) (Millicell-CM; Millipore Corp., Bedford, MA) that had been coated with 0.4 ml of a 4:1 (vol/vol) mixture of rat-tail collagen and Engelbreth-Holm-Swarm (EHS) tumor matrix (Matrigel; Collaborative Biochemedical Products, Bedford, MA) (31, 32). The mixture was prepared at 4°C and allowed to polymerize at 37°C and contained ∼ 0.8 mg rat tail collagen and 2 mg EHS protein per milliliter. Freshly isolated viable type II cells (2.5 × 106) were plated in 1 ml of Dulbecco's modified Eagle's medium (DMEM) containing 5% rat serum (RS) (Pel-Freez Biologicals, Rogers, AR), 2 mM glutamine, 2.5 μg/ml amphotericin B, 100 μg/ml streptomycin, 100 μg/ml penicillin G (all from GIBCO BRL, Life Technologies Inc., Rockville, MD), and 10 μg/ml gentamicin (Sigma-Aldrich, St. Louis, MO). Two milliliters of the same medium were added in the basolateral compartment of each well. After attachment for 24 h, the monolayers were rinsed twice with DMEM. For the type I–like cell phenotype, the alveolar epithelial cells were cultured in DMEM supplemented with 5% FBS but without keratinocyte growth factor (KGF). For the type II phenotype, the cells were cultured in DMEM containing 5% RS and 10 ng/ml KGF. Each well contained 0.4 ml of apical medium and 2.0 ml of basolateral medium. The six-well plates were incubated on a rocking platform inside a humidified incubator gassed with 10% CO2 after 48-h medium was replaced with new medium with the addition of 10−8 M dexamethasone (dex). This was done to maximally differentiate type II cells. The dex was added to the type I–like cells to keep the basal media similar for the two cell phenotypes. In later experiments involving LPS, dex was omitted from all cultures.
In Vitro Ozone Exposure
Cells were exposed to ozone in an in vitro exposure chamber that has a precisely regulated concentration of ozone and is fully humidified (33). Ozone was generated by passing oxygen through an ozone generator (Model OZ2SS-SS; Ozotech, Yreka, CA). Four specifically designed 3.6-l glass chambers were used to expose the cultured cells. One of these chambers was used as a control chamber and received humidified and warm air/CO2 mixtures. The other three chambers received a specified concentration of ozone. All chambers were fitted with rocking platforms to ensure uniform exposure of the monolayer. Before the exposures, medium from the apical surface was completely removed, and 1 ml of medium was added to the basolateral compartment. Cultured cells were exposed to different concentrations of ozone or air for 60 min. Ozone concentration in the ozone exposure chamber was precisely analyzed by an ozone analyzer (Model MD-050-12-f-4; Perma Pure Inc., Toms River, NJ) and regulated by a computerized feedback system.
LPS Stimulation of Alveolar Epithelial Cells
Type II and type I–like cells were exposed to LPS (Escherichia coli serotype 055:B5; Sigma Chemical Co., St. Louis, MO) diluted in DMEM with 5% RS or DMEM with 5% FBS, respectively, for type II and type I–like cells. LPS was dissolved in DMEM and sonicated for 20 min at 25°C in a Branson 1510 water bath sonicator (Branson Ultrasonics Corp., Danbury, CT) before dilution.
Experimental Design
The cells were exposed to ozone on the seventh day of culture (Day 6 under air/liquid conditions). Rat alveolar cells were primed with IL-1β (10 ng/ml) 4 h before ozone exposure (100 ppb) or stimulated with IL-1β (10 ng/ml) immediately after ozone exposure. In LPS-priming experiments, cells were incubated with LPS (10 ng/ml) for 18 h before ozone exposure. The cells were exposed to variable concentrations of LPS for 18 h to stimulate the cells; medium was changed; and cells were incubated for 24 h for protein, nitrite, and 8-isoprostane detection. Apical and basolateral media were pooled, collected, and spun at 2,700 × g for 10 min and kept frozen at −20°C until assayed.
Immunocytochemistry and Western Blot Analysis for T1α
Immunocytochemistry staining was performed on cultured type I–like cells as previously described (34). The cultured cells were fixed in 4% paraformaldehyde, embedded in paraffin, and sectioned. Sections were stained with hematoxylin and eosin for light microscopy. For immunofluorescence staining, paraffin-embedded sections or directly fixed monolayer cultures on filters were subjected for localization of T1α with a mouse anti-rat T1α IgG (gift of Dr. Mary Williams, Boston University, Boston, MA) and CY3-labeled Alexa Fluor 594 donkey anti-mouse IgG (Molecular Probes Inc., Eugene, OR). The sections were counterstained with 4′,6-diamidino-2-phenylindole for nuclear morphology, visualized by fluorescent microscopy, and photographed.
For immunoblot analyses, cell lysates from different culture conditions were separated by SDS-PAGE using precast 18% acrylamide Tris-glycine gels (Invitrogen Life Technologies, Carlsbad, CA). Proteins were transferred to nitrocellulose membranes for 2 h at 30 V at 4°C, and the membrane was blocked with 5% nonfat dry milk in Tris-Tween buffered saline (TTBS) (20 mM Tris-Hcl, 137 mM NaCl, and 0.05% Tween 20 [pH 7.5]) for 1 h. The blot was incubated with a mouse monoclonal anti-T1α antibody (a gift of Dr. Mary Williams), diluted in 5% nonfat dry milk in TTBS (1:500 vol/vol), and incubated overnight at 4°C with rocking. The blot was washed three times with TTBS and developed using secondary antibody conjugated with anti-mouse horseradish peroxidase (Jackson ImmunoResearch Laboratories, Inc., West Grove, PA) at a 1/5,000 dilution in TTBS, and antigen–antibody complexes were detected by chemiluminescence (ECL Plus; Amersham Pharmacia Biotech, Piscataway, NJ) and exposure to Hyperfilm (Amersham Pharmacia Biotech).
Measurement of DNA
To harvest type I–like cells and type II cells for DNA assay, the collagen-EHS gels were teased off the insert and placed in a polypropylene tube. The gel was digested with 1 ml of a 1:4 (vol/vol) mixture of 5 mg/ml type I collagenase (Worthington Biochemical Corp., Lakewood, NJ) in DMEM and dispase (BD Biosciences, Bedford, MA) for 60 min at 37°C. The cells were diluted in saline, sedimented, resuspended, and washed once before resuspension in phosphate buffer containing EDTA for the DNA analysis. The suspension was frozen and stored at −20°C. After thawing, cells were sonicated, and DNA content was measured fluorometrically (35).
Measurement of Chemokine Secretion
Chemokines were measured by commercially available ELISA kits MCP-1 and MIP-2 (BioSource, Camarillo, CA) and CINC-1 (Assay Design, Inc., Ann Arbor, MI) according to the manufacturer's instructions. CINC-2 and LIX (CXCL5) were measured with an ELISA based on standard techniques, with standards and antibodies provided by R&D Systems (Minneapolis, MN). The antibody for CINC-2β (R&D Systems) used in this ELISA shows significant crossreactivity to CINC-2α; hence, the product of this ELISA is referred to as CINC-2. The CINC-2 and LIX ELISA assays were developed by ELISA Tech (Aurora, CO).
Measurement of 8-Isoprostane
8-Isoprostane, a marker of lipid peroxidation, was measured with a commercially available enzyme immunoassay kit (Cayman Chemical Co., Ann Arbor, MI) by a competitive enzyme immunoassay according to the manufacturer's instructions.
Determination of Nitrite
After LPS treatment, the amount of nitrite, an oxidative product of nitric oxide, in culture medium of type I–like and type II cells was detected by a colorimetric assay using the Griess reaction. Briefly, 100 μl of supernatant medium was reacted for 10 min at room temperature with an equal volume of Griess reagent (1% sulfanilamide, 0.1% naphthylethylene diaminedihydrochloride, and 2.5% phosphoric acid). Optical density was measured at 550 nm. Nitrite content was quantified by comparison with a standard curve generated with sodium nitrite in the range of 0–100 μM.
Statistical Analyses
Data are represented as the mean ± SEM. For statistical analysis, the test used depended on the experimental design. For the effect of ozone on the two phenotypes, a paired two-tailed t test was used. For the dose–response relationships and the effect of pre- and post-treatment with IL-1β, the results were analyzed by ANOVA and Dunnett's test. A P value of < 0.05 was considered to indicate a significant difference between the groups.
RESULTS
Characterization of Type II and Type I–Like Cells
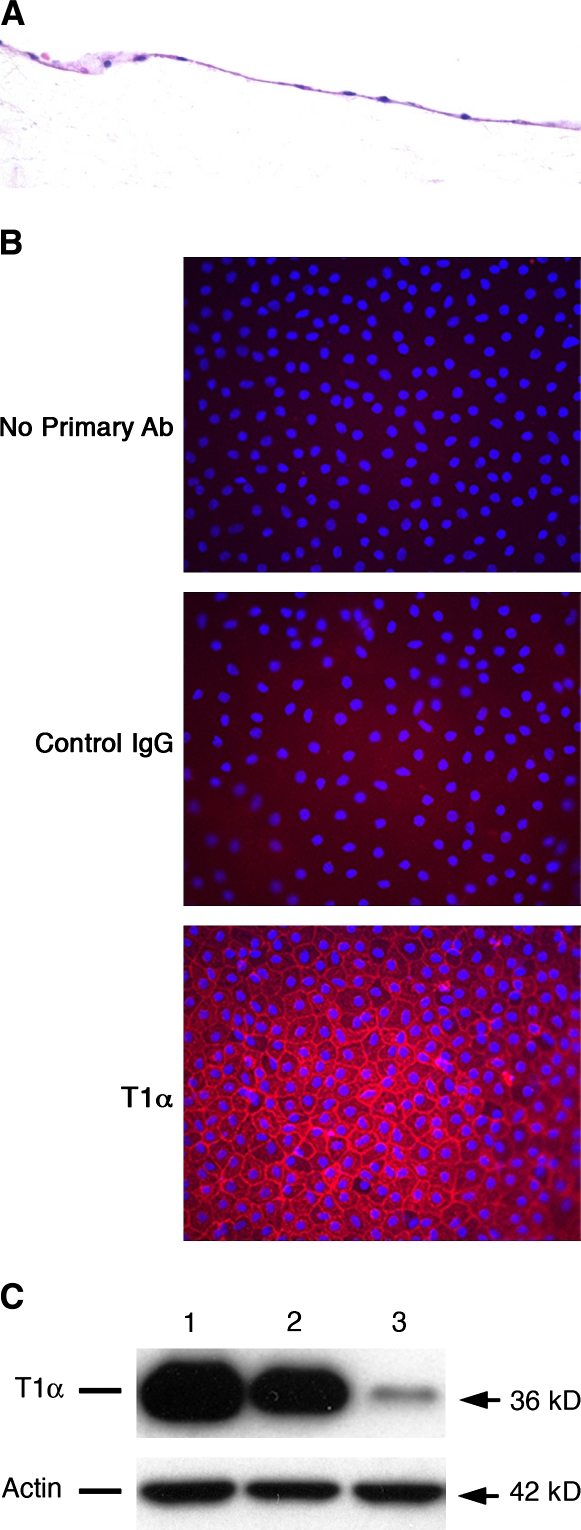
The type II cell cultures have been described previously (31, 32). These cells secrete surfactant components and have a cuboidal shape, apical microvilli, and numerous intracellular organelles typical of type II cells (31). The isolated type II cells were also cultured to transdifferentiate into type I–like cells that express T1α (Figure 1). These cells are flat, spread out, and express genes typical of type I cells and have a markedly reduced expression of genes of the type II phenotype.
Figure 1.
Characterization of rat alveolar type I-like cells. Rat alveolar type I–like cells were cultured as described in Materials and Methods. (A) Sections from paraffin-embedded cells were stained with hematoxylin and eosin. (B) Immunofluorescence detection of T1α in type I–like cells cultured on matrix-coated inserts was done after cell fixation in 4% paraformaldehyde. (C) Western blot analysis for T1α protein in rat alveolar epithelial cells cultured on different culture conditions for 6 d. Lane 1: Cells cultured on tissue culture plastic in 5% FBS supplemented-DMEM. Lane 2: Cells cultured on insert coated with 0.4 ml of a 4:1(vol/vol) mixture of rat tail collagen and Matrigel in 5% FBS-supplemented media. dex (10−8 M) was added for last 4 d of culture (type I–like cell phenotype). Lane 3: Cells cultured on insert coated with a mixture of rat-tail collagen and Matrigel in media supplemented with 5% RS, 10 ng/ml KGF, and 10−8 M dex (type II cell phenotype). The results are representative of three independent experiments.
Type I–Like Cells Are More Susceptible to Ozone than Type II Cells
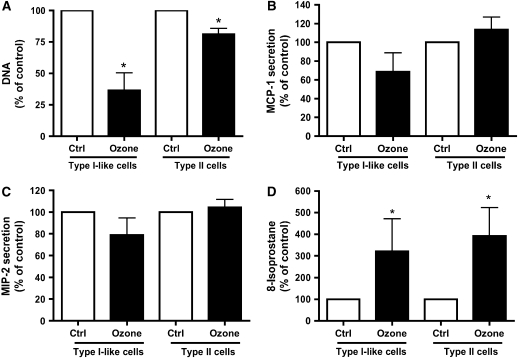
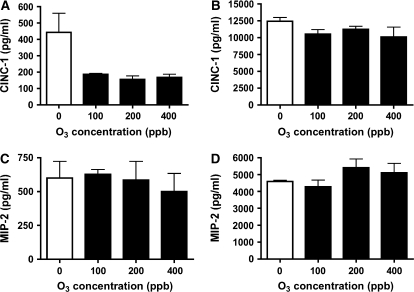
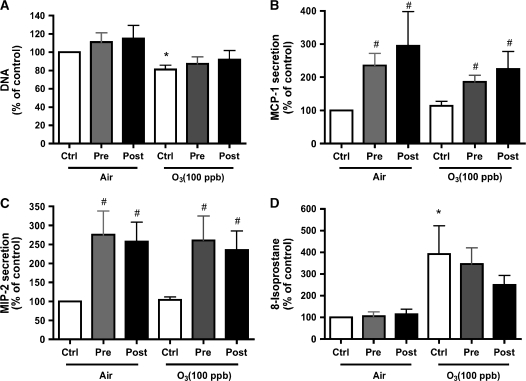
The type II cells and type I–like cells were exposed to air or ozone (100 ppb) for 1 h. Cells and medium were harvested 24 h after exposure. Immediately after exposure, there was no obvious morphologic effect due to the ozone exposure. However, by 24 h, the type I–like cells were visibly injured, whereas the type II cell phenotype showed only minor changes. In the type I–like cells, ozone caused a significant detachment as measured by decrease in DNA/well (Figure 2A). For type I–like cells exposed to air, there were 3.89 ± 0.34 μg DNA/well; for type I–like cells exposed to ozone, there were 1.51 ± 0.62 μg DNA/well (n = 5). For type II cells exposed to air, there were 21.45 ± 1.80 μg DNA/well; for type II cells exposed to ozone, there were 17.2 ± 1.3 μg DNA/well. Both phenotypes secreted MCP-1 (CCL2) and MIP-2 (CXCL3, CINC-3) into the medium (Figures 2B and 2C). However, there was no increase in secreted MIP-2 or MCP-1 that could be attributed to ozone exposure. For the type I–like cell phenotype, the levels of MCP-1 and MIP-2 in the medium were 736 ± 296 pg/ml and 824 ± 150 pg/ml, respectively. For the type II cell phenotype, the levels of MCP-1 and MIP-2 in the medium were 670 ± 290 pg/ml and 7,640 ± 2,450 pg/ml. Concentrations of ozone up to 400 ppb for 60 min did not elicit a chemokine response in either phenotype (Figure 3). In the studies of direct ozone exposure, we did not find any additional secretion of MCP-1, CINC-1, CINC-2, MIP-2 (CINC-3), and LIX (CXCL5) by either alveolar epithelial phenotype. In spite of a lack of chemokine secretion, ozone exposure induced lipid peroxidation as measured by 8-isoprostane release into the medium in both phenotypes (Figure 2D). For type I–like cells, the 8-isoprostane level was 31.88 ± 8.09 pg/ml; for type II cells, the 8-isoprostane level was 460.8 ± 243.7 pg/ml.
Figure 2.
Ozone produces toxicity but no chemokine secretion. Cells were cultured and exposed to air (control [ctrl]) or to ozone 100 ppb for 60 min and cultured for an additional 24 h. (A) DNA/well, which was selected as a marker of toxicity as a measure of cell detachment. (B and C) Secretion of the chemokines MCP-1 and MIP-2 into the media. (D) 8-Isoprostane levels in the medium as a marker of lipid peroxidation. The values are normalized to the air control, and values represent mean ± SEM for five different experiments. *P < 0.05 compared with control.
Figure 3.
High levels of ozone fail to elicit a chemokine response in either phenotype. Type I–like and type II cells were cultured as described in Materials and Methods and exposed to air and different concentrations of ozone (100, 200, and 400 ppb) for 60 min and cultured for an additional 24 h. (A and C) Secretion of CINC-1 and MIP-2 in type I–like cells. (B and D) Secretion of CINC-1 and MIP-2 protein in type II cells in response to different doses of ozone. These results are representative of two independent experiments.
IL-1β Stimulates MCP-1 and MIP-2 in Type I–Like Cells and Type II Cells
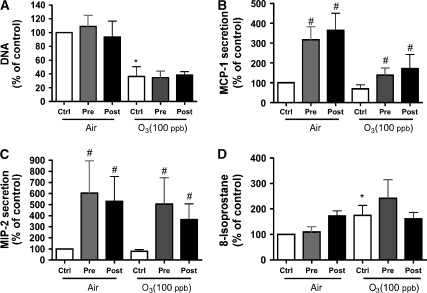
Because of insignificant changes in secretion of MCP-1 and MIP-2 in response to ozone alone, we used IL-1β (10 ng/ml) to prime and stimulate both cell phenotypes before and after ozone exposure (Figures 4 and 5). Both phenotypes secreted MCP-1 and MIP-2 in response to IL-1β, but there was no synergism with ozone exposure (Figures 4B, 4C, 5B, and 5C). After ozone exposure, both cell types increased secretion of MIP-2 and MCP-1 in response to IL-1β, which indicates that the ozone exposure did not prevent a chemokine response. In these experiments, there was no chemokine response due to ozone exposure alone in either phenotype. Similar results were seen for CINC-1, LIX, and CINC-2 (data not shown). IL-1β did not alter isoprostane production in air or after ozone exposure (Figures 4D and 5D). IL-1β also did not alter detachment or DNA/well compared with wells without IL-1β (Figures 4A and 5A).
Figure 4.
IL-1β stimulates type I–like cells to secrete MCP-1 and MIP-2. Cultured type I–like cells were incubated with 10 ng/ml IL-1β 4 h before (pre) or immediately after (post) exposure to filtered air or ozone. Cells without IL-1β are indicated as controls (ctrl). (A) Cell attachment as measured by DNA/well. There was no effect by IL-1β treatment, but there was an ozone effect. (B and C) There was increased secretion of chemokines by IL-1β but no effect of ozone. (D) The 8-isoprostane level was increased with ozone. Values represent mean ± SEM for five different experiments. *P < 0.05 for the untreated ozone exposure compared with air. The pound sign (#) signifies the effect of IL-1β compared with the appropriate air or ozone controls. In this series of experiments, the air control values were as follows: (A) 3.90 ± 0.34 μg DNA/well; (B) 736 ± 296 pg MCP-1/ml; (C) 825 ± 151 pg MIP-2/ml; and (D) 31.9 ± 8.1 pg 8-isoprostane/ml.
Figure 5.
IL-1β stimulates type II cells to secrete MCP-1 and MIP-2. Cultured type II cells were incubated with 10 ng/ml IL-1β 4 h before (pre) or immediately after (post) exposure to filtered air or ozone. Cells without IL-1β are indicated as controls (ctrl). (A) Cell attachment as measured by DNA/well. (B and C) There was increased secretion of MCP-1 and MIP-2 by IL-1β but no effect of ozone. (D) 8-Isoprostane levels, which are increased by ozone. Values represent mean ± SEM for five different experiments. In this series of experiments, the air control values were as follows: (A) 21.4 ± 1.8 μg DNA/well; (B) 670 ± 290 pg MCP-1/ml; (C) 7,640 ± 2,450 pg MIP- 2/ml; and (D) 961 ± 244 pg 8-isoprostane/ml. *P < 0.05 for the untreated ozone exposure compared with air. The pound sign (#) signifies the effect of IL-1β compared with the appropriate air or ozone control.
In the absence of effects of ozone exposure on cytokine production and the lack of synergy with IL-1β, we ran controls with A549 cells. These cells have been reported to secrete IL-8 in response to ozone (36). We also found that ozone increased IL-8 secretion from 2.3 ng/ml (air) to 4.0 ng/ml after 400 ppb ozone for 60 min, but this response was minimal compared with the effect of IL-1β, which increased A549 cell secretion of IL-8 to 172 ng/ml. Hence, we concluded that our exposure system was valid and produced similar effects to that in the literature for A549 cells but that the chemokine response to ozone in A549 cells was minimal compared with chemokine stimulation with IL-1β.
Type I–Like Cells Secrete MCP-1 and MIP-2 in Response to LPS, but There Is No Synergy with Ozone
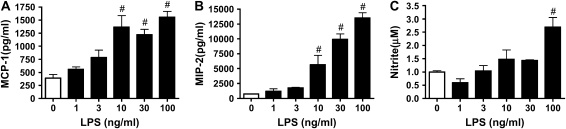
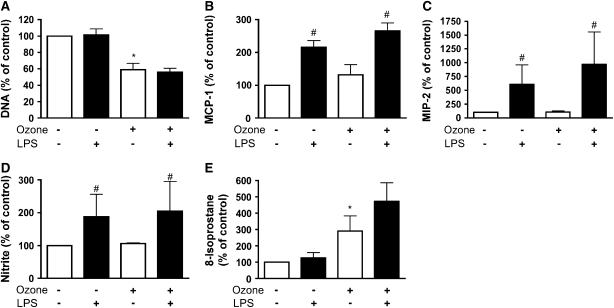
Because LPS has been reported to increase the chemokine response to ozone in vivo, we evaluated the effect of LPS pretreatment in vitro (29). In type I–like cells, LPS stimulated MCP-1 and MIP-2 in a dose-dependent manner (Figures 6A and 6B). For the type I–like cells, maximum stimulation for MCP-1 and MIP-2 was elicited with 100–300 ng/ml LPS. In addition, LPS increased nitrite production in a dose-dependent manner (Figure 6C). LPS caused no change in isoprostane level in the medium or changes in DNA per well (data not shown). However, previous exposure to 10 ng/ml LPS for 18 h did not prime the cells for a chemokine response to ozone (Figures 7B and 7C). LPS increased nitrite secretion, but there was no further increase with ozone (Figure 7D). 8-Isoprostane production increased in response to ozone as in previous experiments but was not altered by exposure to LPS (Figure 7E). Similarly, LPS did not alter the loss of DNA per well after ozone exposure (Figure 7A).
Figure 6.
LPS stimulates the type I–like phenotype to secrete MCP-1 and MIP-2. Type I–like cells were stimulated with increasing concentrations of LPS (1–100 ng/ml) for 18 h. The medium was changed, and cells were cultured for 24 h before harvest for different assays. (A and B) The amount of MCP-1 and MIP-2 secreted in response to LPS. (C) The increase in nitrite in the medium. Each value represents the mean ± SEM of triplicate determinations. The results are representative of two independent experiments. #P < 0.05 compared with control.
Figure 7.
Pretreatment with LPS does not alter the ozone effect on the type I–like phenotype. Type I–like cells were primed with 10 ng /ml of LPS for 18 h before filtered air or ozone exposure. Cells were cultured in fresh media for 24 h after exposure and before collection of media and cells for different assays. (A) Cell by DNA/well, which is a measure of cell detachment or toxicity. (B and C) MCP-1 and MIP-2 secretion. (D and E) Nitrite and 8-isoprostane release into the medium. Each value represents the mean ± SEM for three experiments. *P < 0.05 for the untreated ozone exposure compared with air. The pound sign (#) signifies the effect of LPS compared with the appropriate air or ozone controls (P < 0.05).
Type II Cells Secrete MCP-1 and MIP-2 in Response to LPS, but There Is No Synergy with Subsequent Ozone Exposure
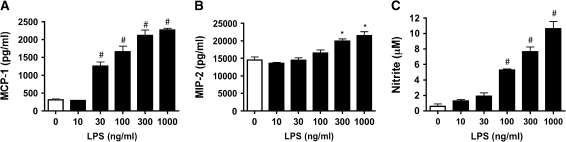
Rat type II cells were cultured in 5% RS and KGF to determine the response of LPS on chemokine secretion. LPS stimulated MCP-1 and MIP-2 in a dose-dependent manner (Figures 8A and 8B). For the type II cell phenotype, maximum stimulation for MCP-1 and MIP-2 was elicited with 300 ng/ml–1 μg/ml LPS. Nitrite production also increased with increasing LPS concentration in a dose-dependent manner (Figure 8C). LPS did not alter 8-isoprostane levels in these experiments (data not shown).
Figure 8.
LPS stimulates type II cells to secrete MCP-1 and MIP-2. Type II cells were stimulated with increasing concentrations of LPS (10 ng/ml– 1 μg/ml) for 18 h. Medium was changed, and cells were cultured for 24 h before harvest. (A and B) Amount of MCP-1 and MIP-2 secreted in response to LPS. (C) Increase in nitrite in the medium. Each value represents the mean ± SEM for n = 3. #P < 0.05 compared with control.
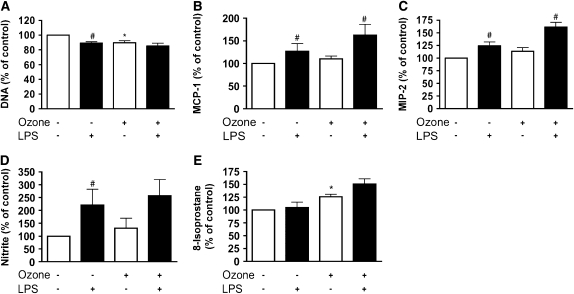
Because LPS stimulated chemokine secretion, we sought to determine whether ozone exposure would increase the effect of a previous low dose of LPS (Figure 9). However, previous exposure to 10 ng/ml LPS for 18 h did not prime the cell for chemokine response to ozone (Figures 9B and 9C). LPS also did not alter the DNA per well or 8-isoprostane production (Figures 9A and 9E). However, LPS did alter nitrite secretion, but there was no synergy with ozone exposure given after the LPS treatment (Figure 9D).
Figure 9.
Pretreatment with LPS does not alter the ozone effect on the type II cell phenotype. Type II cells were primed with 10 ng /ml of LPS for 18 h before filtered air or ozone exposure. Cells were grown in fresh media for 24 h after exposure and before collection of media and cells for different assays. (A) Cell DNA/well, which is a measure of cell attachment or toxicity. (B and C) MCP-1 and MIP-2 secretion. (D and E) Nitrite and 8-isoprostane release into the medium. Each value represents mean ± SEM for n = 3. *P < 0.05 for the untreated ozone exposure compared with air. The pound sign (#) signifies the effect of LPS compared with the appropriate air or ozone control (P < 0.05).
DISCUSSION
Ozone initiates an inflammatory response in the distal lung, and it was our hypothesis that direct ozone exposure would increase chemokine secretion by type II cells or type I–like cells in vitro. However, this did not occur. We then tested the hypothesis that there would be a synergistic effect of ozone with IL-1β or LPS. Although we demonstrated a direct effect of IL-1β and LPS on MCP-1 and MIP-2 secretion, we observed no synergy with IL-1β given before or after ozone exposure or LPS given before ozone exposure. We conclude that direct effect of ozone on these epithelial cells is unlikely to be responsible for the neutrophil influx seen in vivo.
We found no evidence that ozone directly stimulates chemokine secretion from alveolar epithelial cells in vitro. The result was seen in the original experiments (Figures 2 and 3) and with pretreatment and post-treatment with IL-1β (Figures 4 and 5) and post-treatment with LPS (Figures 7 and 9). The dose of ozone was sufficient to produce ozone toxicity as measured by cell appearance, 8-isoprostane production, and detachment of epithelial cells (type I–like cell phenotype). However, the exposure to ozone did not prevent a chemokine response. Both phenotypes increased chemokine production after ozone exposure in response to IL-1β. Moreover, doses up to 400 ppb did not evoke a chemokine response (Figure 3). Although the medium was removed, there likely remained some surface liquid, which could provide an alternative substrate for ozone. We chose to keep the medium for the exposures the same as the culture medium to reduce the number of potential confounding variables for the chemokine response. It is unlikely that any of the potential targets for ozone in the residual medium blocked the effect of ozone because we observed toxicity and because there was no chemokine response with ozone exposures up to 400 ppb for 60 min.
Although a direct effect of ozone on the epithelium seems unlikely to explain in vivo observations, we believe that it is probable that the alveolar epithelium participates in regulation of the inflammatory response to ozone. Ozone may produce signaling molecules by reaction with surfactant or cause complex cell–cell signaling pathways. Ozone is a very reactive gas, and it is estimated that it may penetrate only 0.1 μm in biologic fluids (37). There are substrates for ozone oxidation in alveolar fluid that include surfactant, glutathione, urate, and ascorbate (38–41). Oxidation of these substances by themselves could produce inflammatory reaction products, or such substances might stimulate resident cells to secrete neutrophil chemokines.
Recently, ozone-induced reaction products of surfactant, especially 1-palmitoyl-2-(9′-oxo-nananoyl)-glycerophosphocholine (9-al PC), 5 β,6β-epoxycholesterol, and cholestan-6-oxo-3β,5α-diol, have been shown to be cytotoxic and could be signaling molecules (42, 43). The effects of these potential signaling molecules were not tested in our studies. The oxidized phosphatidylcholine (9-al PC) initiates apoptosis in A549 cells and can be generated at physiologic concentrations in vitro with as little as 125 ppb ozone for 4 h reacting with pulmonary surfactant (42). The oxidized cholesterol products due to ozone are also very biologically active on cultured bronchial epithelial cells and can be produced by ozone exposure in vivo and recovered in alveolar lavage fluid (43, 44). Another possibility for the in vivo effects of ozone is that ozone alters alveolar epithelial cells, especially type I cells, to secrete a factor that stimulates other resident cells, such as alveolar type II cells or alveolar macrophages, to secrete chemokines. This possibility can be tested but is beyond the scope of this article.
We may have selected the wrong chemokine to measure. We chose to measure MCP-1 and MIP-2 (CCL2) and MIP-2 (CINC-3, CXCL3) because the mRNAs for these chemokines are increased by ozone in vivo (45). In addition, we measured CINC-1, CINC-2, and CXCL5. In other studies, type II cells have been reported to secrete a variety of chemokines, including IL-8, MIP-1α, and RANTES, in addition to MCP-1 and MIP-2 (28). Although we think that this is unlikely, alveolar epithelial cells could be secreting rat homologs of these other proteins. We also did not test for leukotriene B4 (LTB4) or platelet activating factor secretion, which have been reported to be possible chemoattractants for neutrophils after ozone exposure.
We were surprised to find no synergy with LPS or IL-1β. In vivo LPS treatments have been reported to increase the neutrophilic response to ozone (29). More recently, Johnston and colleagues (46) reported that LPS given before ozone produced a synergistic response, whereas LPS given after ozone produced a blunted response. However, in this study, only mRNA abundance was measured, and protein levels of chemokines and neutrophil influx were not reported. Again the differences between in vivo and in vitro observations suggest a more complex signaling process.
To our knowledge, this is the first report demonstrating that two different primary alveolar cell types, which differ morphologically and in expression of their respective markers, were studied simultaneously for ozone toxicity. The type I–like cell phenotype was more sensitive than the type II cell phenotype to ozone exposure as measured by cell detachment at 24 h (47). This observation is compatible with the in vivo studies. It is not clear if this represents a fundamental susceptibility to injury based on reduced antioxidant or repair capability or is due to the larger surface area exposed to ozone. The cell density for the type II cell phenotype is about five times that of the type I– like cells. In vivo, type I cells represent about half the number of type II cells and cover ∼ 95% of the alveolar surface. The sensitivity of our cultures to ozone is also similar to the sensitivity of rat alveolar epithelial cells reported by Check and colleagues (16), who demonstrated an increase in epithelial permeability with ozone exposure.
In conclusion, the elicitation of the chemokine response after ozone exposure in vivo is likely to be complex. We believe that it involves oxidation products of surfactant and complex cell–cell interactions. Type I–like and type II cell phenotypes can produce chemokines and may participate in the inflammatory response in vivo. However, the simple direct effect of ozone on the epithelium is unlikely to be the mechanism for chemokine production observed in vivo.
Acknowledgments
The authors thank Shuanglin Wang and Lynn Cunningham for with help with the immunocytochemistry and Teneke Warren for her help in preparing this manuscript.
This research was supported by grants from the National Institutes of Health (HL-29891) and from the Environmental Protection Agency (CRX-83084601).
Originally Published in Press as DOI: 10.1165/rcmb.2005-0205OC on October 20, 2005
Conflict of Interest Statement: None of the authors has a financial relationship with a commercial entity that has an interest in the subject of this manuscript.
References
- 1.Rajini P, Witschi H. Cumulative labeling indices in epithelial cell populations of the respiratory tract after exposure to ozone at low concentrations. Toxicol Appl Pharmacol 1995;130:32–40. [DOI] [PubMed] [Google Scholar]
- 2.Chang LY, Huang Y, Stockstill BL, Graham JA, Grose EC, Menache MG, Miller FJ, Costa DL, Crapo JD. Epithelial injury and interstitial fibrosis in the proximal alveolar regions of rats chronically exposed to a simulated pattern of urban ambient ozone. Toxicol Appl Pharmacol 1992;115:241–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pino MV, Levin JR, Stovall MY, Hyde DM. Pulmonary inflammation and epithelial injury in response to acute ozone exposure in the rat. Toxicol Appl Pharmacol 1992;112:64–72. [DOI] [PubMed] [Google Scholar]
- 4.Hyde DM, Hubbard WC, Wong V, Wu R, Pinkerton K, Plopper CG. Ozone-induced acute tracheobronchial epithelial injury: relationship to granulocyte emigration in the lung. Am J Respir Cell Mol Biol 1992;6:481–497. [DOI] [PubMed] [Google Scholar]
- 5.Tsukagoshi H, Haddad EB, Sun J, Barnes PJ, Chung KF. Ozone-induced airway hyperresponsiveness: role of superoxide anions, NEP, and BK receptors. J Appl Physiol 1995;78:1015–1022. [DOI] [PubMed] [Google Scholar]
- 6.Takahashi N, Yu XY, Schofield BH, Kleeberger SR, Scott AL, Hasegawa S, Spannhake EW. Expression of ICAM-1 in airway epithelium after acute ozone exposure in the mouse. J Appl Physiol 1995;79:1753–1761. [DOI] [PubMed] [Google Scholar]
- 7.Aris RM, Christian D, Hearne PQ, Kerr K, Finkbeiner WE, Balmes JR. Ozone-induced airway inflammation in human subjects as determined by airway lavage and biopsy. Am Rev Respir Dis 1993;148:1363–1372. [DOI] [PubMed] [Google Scholar]
- 8.McBride DE, Koenig JQ, Luchtel DL, Williams PV, Henderson WR Jr. Inflammatory effects of ozone in the upper airways of subjects with asthma. Am J Respir Crit Care Med 1994;149:1192–1197. [DOI] [PubMed] [Google Scholar]
- 9.Chang MM, Wu R, Plopper CG, Hyde DM. IL-8 is one of the major chemokines produced by monkey airway epithelium after ozone- induced injury. Am J Physiol 1998;275:L524–L532. [DOI] [PubMed] [Google Scholar]
- 10.Haddad EB, Salmon M, Koto H, Barnes PJ, Adcock I, Chung KF. Ozone induction of cytokine-induced neutrophil chemoattractant (CINC) and nuclear factor-kappa b in rat lung: inhibition by corticosteroids. FEBS Lett 1996;379:265–268. [DOI] [PubMed] [Google Scholar]
- 11.Koto H, Salmon M, el Haddad B, Huang TJ, Zagorski J, Chung KF. Role of cytokine-induced neutrophil chemoattractant (CINC) in ozone-induced airway inflammation and hyperresponsiveness. Am J Respir Crit Care Med 1997;156:234–239. [DOI] [PubMed] [Google Scholar]
- 12.Zhao Q, Simpson LG, Driscoll KE, Leikauf GD. Chemokine regulation of ozone-induced neutrophil and monocyte inflammation. Am J Physiol 1998;274:L39–L46. [DOI] [PubMed] [Google Scholar]
- 13.Devlin RB, McKinnon KP, Noah T, Becker S, Koren HS. Ozone-induced release of cytokines and fibronectin by alveolar macrophages and airway epithelial cells. Am J Physiol 1994;266:L612–L619. [DOI] [PubMed] [Google Scholar]
- 14.Janic B, Umstead TM, Phelps DS, Floros J. An in vitro cell model system for the study of the effects of ozone and other gaseous agents on phagocytic cells. J Immunol Methods 2003;272:125–134. [DOI] [PubMed] [Google Scholar]
- 15.Arsalane K, Gosset P, Vanhee D, Voisin C, Hamid Q, Tonnel AB, Wallaert B. Ozone stimulates synthesis of inflammatory cytokines by alveolar macrophages in vitro. Am J Respir Cell Mol Biol 1995;13:60–68. [DOI] [PubMed] [Google Scholar]
- 16.Cheek JM, Buckpitt AR, Li C, Tarkington BK, Plopper CG. Ozone injury to alveolar epithelium in vitro does not reflect loss of antioxidant defenses. Toxicol Appl Pharmacol 1994;125:59–64. [DOI] [PubMed] [Google Scholar]
- 17.Jaspers I, Chen LC, Flescher E. Induction of interleukin-8 by ozone is mediated by tyrosine kinase and protein kinase A, but not by protein kinase C. J Cell Physiol 1998;177:313–323. [DOI] [PubMed] [Google Scholar]
- 18.Nichols BG, Woods JS, Luchtel DL, Corral J, Koenig JQ. Effects of ozone exposure on nuclear factor-kappaB activation and tumor necrosis factor-alpha expression in human nasal epithelial cells. Toxicol Sci 2001;60:356–362. [DOI] [PubMed] [Google Scholar]
- 19.Rusznak C, Devalia JL, Sapsford RJ, Davies RJ. Ozone-induced mediator release from human bronchial epithelial cells in vitro and the influence of nedocromil sodium. Eur Respir J 1996;9:2298–2305. [DOI] [PubMed] [Google Scholar]
- 20.Lang DS, Jorres RA, Mucke M, Siegfried W, Magnussen H. Interactions between human bronchoepithelial cells and lung fibroblasts after ozone exposure in vitro. Toxicol Lett 1998;96–97:13–24. [DOI] [PubMed]
- 21.Williams MC. Alveolar type i cells: molecular phenotype and development. Annu Rev Physiol 2003;65:669–695. [DOI] [PubMed] [Google Scholar]
- 22.Gonzalez R, Yang YH, Griffin C, Allen L, Tigue Z, Dobbs L. Freshly isolated rat alveolar type I cells, type II cells, and cultured type II cells have distinct molecular phenotypes. Am J Physiol Lung Cell Mol Physiol 2005;288:L179–L189. [DOI] [PubMed] [Google Scholar]
- 23.Nishina K, Zhang F, Nielsen LD, Edeen K, Wang J, Mason RJ. Expression of CINC-2beta is related to the state of differentiation of alveolar epithelial cells. Am J Respir Cell Mol Biol 2005;33:505–512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Paine R III, Rolfe MW, Standiford TJ, Burdick MD, Rollins BJ, Strieter RM. MCP-1 expression by rat type II alveolar epithelial cells in primary culture. J Immunol 1993;150:4561–4570. [PubMed] [Google Scholar]
- 25.Jeyaseelan S, Manzer R, Young SK, Yamamoto M, Akira S, Mason RJ, Worthen GS. Induction of CXCL5 during inflammation in the rodent lung involves activation of alveolar epithelium. Am J Respir Cell Mol Biol 2005;32:531–539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vanderbilt JN, Mager EM, Allen L, Sawa T, Wiener-Kronish J, Gonzalez R, Dobbs LG. CXC chemokines and their receptors are expressed in type II cells and upregulated following lung injury. Am J Respir Cell Mol Biol 2003;29:661–668. [DOI] [PubMed] [Google Scholar]
- 27.Armstrong L, Medford AR, Uppington KM, Robertson J, Witherden IR, Tetley TD, Millar AB. Expression of functional toll-like receptor-2 and -4 on alveolar epithelial cells. Am J Respir Cell Mol Biol 2004;31:241–245. [DOI] [PubMed] [Google Scholar]
- 28.Witherden IR, Vanden Bon EJ, Goldstraw P, Ratcliffe C, Pastorino U, Tetley TD. Primary human alveolar type II epithelial cell chemokine release: effects of cigarette smoke and neutrophil elastase. Am J Respir Cell Mol Biol 2004;30:500–509. [DOI] [PubMed] [Google Scholar]
- 29.Johnston CJ, Oberdorster G, Gelein R, Finkelstein JN. Endotoxin potentiates ozone-induced pulmonary chemokine and inflammatory responses. Exp Lung Res 2002;28:419–433. [DOI] [PubMed] [Google Scholar]
- 30.Dobbs LG, Mason RJ. Pulmonary alveolar type II cells isolated from rats: release of phosphatidylcholine in response to beta-adrenergic stimulation. J Clin Invest 1979;63:378–387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mason RJ, Lewis MC, Edeen KE, McCormick-Shannon K, Nielsen LD, Shannon JM. Maintenance of surfactant protein A and D secretion by rat alveolar type II cells in vitro. Am J Physiol Lung Cell Mol Physiol 2002;282:L249–L258. [DOI] [PubMed] [Google Scholar]
- 32.Mason RJ, Pan T, Edeen KE, Nielsen LD, Zhang F, Longphre M, Eckart MR, Neben S. Keratinocyte growth factor and the transcription factors C/EBP alpha, C/EBP delta, and SREBP-1c regulate fatty acid synthesis in alveolar type II cells. J Clin Invest 2003;112:244–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Allen CB. An automated system for exposure of cultured cells and other materials to ozone. Inhal Toxicol 2003;15:1039–1052. [DOI] [PubMed] [Google Scholar]
- 34.Xu X, McCormick-Shannon K, Voelker DR, Mason RJ. KGF increases SP-A and SP-D mRNA levels and secretion in cultured rat alveolar type II cells. Am J Respir Cell Mol Biol 1998;18:168–178. [DOI] [PubMed] [Google Scholar]
- 35.Labarca C, Paigen K. A simple, rapid, and sensitive DNA assay procedure. Anal Biochem 1980;102:344–352. [DOI] [PubMed] [Google Scholar]
- 36.Jaspers I, Flescher E, Chen LC. Ozone-induced IL-8 expression and transcription factor binding in respiratory epithelial cells. Am J Physiol 1997;272:L504–L511. [DOI] [PubMed] [Google Scholar]
- 37.Pryor WA. How far does ozone penetrate into the pulmonary air/tissue boundary before it reacts? Free Radic Biol Med 1992;12:83–88. [DOI] [PubMed] [Google Scholar]
- 38.Sun G, Crissman K, Norwood J, Richards J, Slade R, Hatch GE. Oxidative interactions of synthetic lung epithelial lining fluid with metal-containing particulate matter. Am J Physiol Lung Cell Mol Physiol 2001;281:L807–L815. [DOI] [PubMed] [Google Scholar]
- 39.Postlethwait EM, Cueto R, Velsor LW, Pryor WA. O3-induced formation of bioactive lipids: estimated surface concentrations and lining layer effects. Am J Physiol 1998;274:L1006–L1016. [DOI] [PubMed] [Google Scholar]
- 40.Mudway IS, Kelly FJ. Modeling the interactions of ozone with pulmonary epithelial lining fluid antioxidants. Toxicol Appl Pharmacol 1998;148:91–100. [DOI] [PubMed] [Google Scholar]
- 41.Putman E, van Golde LMG, Haagsman HP. Toxic oxidant species and their impact on the pulmonary surfactant system. Lung 1997;175:75–103. [DOI] [PubMed] [Google Scholar]
- 42.Uhlson C, Harrison K, Allen CB, Ahmad S, White CW, Murphy RC. Oxidized phospholipids derived from ozone-treated lung surfactant extract reduce macrophage and epithelial cell viability. Chem Res Toxicol 2002;15:896–906. [DOI] [PubMed] [Google Scholar]
- 43.Pulfer MK, Murphy RC. Formation of biologically active oxysterols during ozonolysis of cholesterol present in lung surfactant. J Biol Chem 2004;279:26331–26338. [DOI] [PubMed] [Google Scholar]
- 44.Pulfer MK, Taube C, Gelfand E, Murphy RC. Ozone exposure in vivo and formation of biologically active oxysterols in the lung. J Pharmacol Exp Ther 2005;312:256–264. [DOI] [PubMed] [Google Scholar]
- 45.Johnston CJ, Stripp BR, Reynolds SD, Avissar NE, Reed CK, Finkelstein JN. Inflammatory and antioxidant gene expression in C57BL/6J mice after lethal and sublethal ozone exposures. Exp Lung Res 1999;25:81–97. [DOI] [PubMed] [Google Scholar]
- 46.Johnston CJ, Holm BA, Finkelstein JN. Sequential exposures to ozone and lipopolysaccharide in postnatal lung enhance or inhibit cytokine responses. Exp Lung Res 2005;31:431–447. [DOI] [PubMed] [Google Scholar]
- 47.Chang L, Miller FJ, Ultman J, Huang Y, Stockstill BL, Grose E, Graham JA, Ospital JJ, Crapo JD. Alveolar epithelial cell injuries by subchronic exposure to low concentrations of ozone correlate with cumulative exposure. Toxicol Appl Pharmacol 1991;109:219–234. [DOI] [PubMed] [Google Scholar]