Abstract
Long-term success in lung transplantation is limited by obliterative bronchiolitis, whereas T cell effector mechanisms in this process remain incompletely understood. Using the mouse heterotopic allogeneic airway transplant model, we studied T cell effector responses during obliterative airways disease (OAD). Allospecific CD8+IFN-γ+ T cells were detected in airway allografts, with significant coexpression of TNF-α and granzyme B. Therefore, using IFN-γ as a surrogate marker, we assessed the distribution and kinetics of extragraft allo-specific T cells during OAD. Robust allospecific IFN-γ was produced by draining the lymph nodes, spleen, and lung mononuclear cells from allograft, but not isograft recipients by Day 14, and significantly decreased by Day 28. Although the majority of allospecific T cells were CD8+, allospecific CD4+ T cells were also detected in these compartments, with each employing distinct allorecognition pathways. An influx of pluripotent CD8+ effector cells with a memory phenotype were detected in the lung during OAD similar to those seen in the allografts and secondary lymphoid tissue. Antibody depletion of CD8+ T cells markedly reduced airway lumen obliteration and fibrosis at Day 28. Together, these data demonstrate that allospecific CD8+ effector T cells play an important role in OAD and traffic to the lung after heterotopic airway transplant, suggesting that the lung is an important immunologic site, and perhaps a reservoir, for effector cells during the rejection process.
Keywords: effector T cells, lung allograft rejection, lung transplantation, obliterative airways disease
Lung transplantation is the final therapeutic option for selected patients with end-stage lung disease. Chronic allograft rejection is the major factor limiting long-term survival resulting in obliterative bronchiolitis (OB) (1, 2). Despite the marked prevalence of OB after transplant and a critical role for adaptive immune responses, little is known regarding the T-cell effector mechanisms in OB. To address this issue, we used the murine heterotopic allogeneic airway transplant model to study effector mechanisms during obliterative airways disease (OAD) (3). A previous study by Higuchi and colleagues in this model indicated an important role for CD8+ T cells, with a reduction in airway fibrosis noted at 30 d after tranplant in an OAD model in which tracheal airways from mice congenic for the human leukocyte antigen– A2 gene (on a B6 background [H-2b]) were transplanted into wild-type (WT) B6 mice (4). Interestingly, CD8+ T cell–deficient mice were found to reject at pace with WT animals in this study, though compensatory CD4+ T cell–mediated cytotoxicity has been well described in viral immunity, and may account for this observation (5). A recent report in this model demonstrated the presence and retention of CD8+ IFN-γ+ effector T cells infiltrating major histocompatibility complex (MHC)–fully mismatched airway allografts (BALB/c [H-2d] into B6 [H-2b]) during OAD development, although, unexpectedly, these cells were not detectable in secondary lymphoid tissue (6). These investigators also used the 2C T cell receptor (TCR) transgenic (Tg) system in which CD8+ T cells recognize the BALB/c alloantigen Ld. Using adoptively transferred Ld-specific TCR-Tg 2C CD8+ T cells, these investigators demonstrated these cells to be sufficient to induce OAD in BALB/c allografts transplanted into B6 nu/nu recipients (lacking thymus and T cells), while displaying a highly activated (CD69+CD44high) phenotype and secreting IFN-γ. However, the role of IFN-γ remains controversial in allograft rejection based on observations that IFN-γ–deficient mice are capable of allograft rejection (7, 8), and anti–IFN-γ treatment does not appear to significantly alter the rejection process (9, 10). CD8+ T cells are known to predominantly mediate their effector mechanisms via the production of the effector cytokines IFN-γ and TNF-α and/or the cytolytic effector molecules (e.g., perforin and granzyme) (11). Taking these studies into account, we hypothesized that allospecific CD8+ effector T cells infiltrating the airway allografts were pluripotent, and capable of producing other important inflammatory mediators during the rejection process. To test this, we studied OAD in fully mismatched mice (BALB/c airways into B6 recipients), because fully mismatched MHC alleles are the usual occurrence in lung transplantation. Herein, we report an important role for allospecific CD8+ effector T cells in the pathogenesis of OAD, and provide evidence for the systemic allosensitization of these cells in airway rejection, including the trafficking of these cells to the lung.
MATERIALS AND METHODS
Mice
WT female C57BL/6 (I-ab, H-2b), and BALB/c (I-ad, H-2d) mice, 5–8 wk old, were purchased from the National Cancer Institute (Frederick, MD). C57BL/6 congenic CD45.1 mice (B6.SJL-Ptprca Pep3b/BoyJ) were purchased from Jackson Laboratories (Bar Harbor, ME). All mice were housed in the Bayview Laboratory Animal Resource Center at the Johns Hopkins University Asthma and Allergy Center, and experiments conducted under a protocol approved by the Johns Hopkins Animal Care and Use Committee.
Heterotopic Tracheal Transplant
Briefly, donor mice were killed via CO2 asphyxiation. Tracheae were excised and placed in medium. Recipient mice were anesthetized with ketamine/xylazine (60 and 10 mg/kg, respectively, intraperitoneally) (Phoenix Pharmaceuticals, St. Joseph, MO), and the upper back of the neck shaved and prepped with 50% betadine solution. A small incision was made, and 1–2 tracheal grafts were placed in a subcutaneous pocket formed with forceps. The wound was closed with two Clay Adams surgical wound clips (Samuel Perkins, Quincy, MA). Recipient mice were given an intraperitoneal injection of cefazolin 25 mg/kg (Apothecon, Barneveld, The Netherlands). Similar surgical methods were used for heterotopic liver tissue transplantation.
Medium and Reagents
Cell culture medium RPMI 1640 (Biofluids, Rockville, MD) was supplemented with 10% FCS (Sigma-Aldrich, St. Louis, MO), 2 mM glutamine, 1 mM sodium pyruvate, 1% nonessential amino acids (NEAA), 100 U/ml Penicillin, 100 MGC/ml Streptomycin, 50μM β-mercaptoethanol, and 25 mM Hepes (Biofluids). Concanavalin (Con A) was purchased from Sigma-Aldrich and used at 3 μg/ml.
Cell Preparations, Stimulation, and Cytokine Detection
Spleen, draining lymph nodes (LN), lungs, and grafts were harvested from mice on Days 14 and 28. Lungs and grafts were minced and incubated at 37°C in an enzyme cocktail of RPMI containing 2.4 mg/ml of collagenase I and 20μg/ml DNase (Invitrogen, Carlsbad, CA), then mashed through a 70 μm nylon cell strainer (BD Falcon, Franklin Lakes, NJ). A 23 and 70% bilayer percoll (Amersham Biosciences, Uppsala, Sweden) gradient was performed, the interface collected, and lymphocytes were counted using a Levy hemocytometer (Hausser Scientific, Horsham, PA) and trypan blue staining. CD4+ and CD8+ T cells were isolated using magnetic beads (Miltenyi Biotec, Auburn, CA) with purity > 97%. Splenic dendritic cells were isolated (CD80+, CD86+, CD11c+) as previously described (12). For flow cytometry experiments, isolated cells from allografts, LN, spleen, or lung mononuclear cells (LMNC; responder cells) were cocultured for 6 h in 48-well plates in medium alone, or with B6 or BALB/c splenocytes previously irradiated with 3,000 rads (at a 1:3 ratio, based on titration experiments to determine maximum IFN-γ production). Responder cells were plated at 1 × 106 cells/ml in either 96-well (200 μl) round-bottom plates (Nunc, Roskilde, Denmark) or 48-well (1 ml) flat-bottom plates (Costar, Cambridge, MA). At 72 h after in vitro stimulation, culture supernatants were harvested from 96-well plates and IFN-γ was assayed by sandwich ELISA using matched-antibody (Ab) pairs (Pierce-Endogen, Rockford, IL).
Flow Cytometry
The following antibodies were purchased from BD Pharmingen (San Diego, CA): FITC-, PE-labeled anti-CD4; FITC-, PerCP Cy5.5–labeled anti-mouse CD8; FITC-labeled and PE-labeled anti-CD45.1; allophycocyanin (APC)-labeled CD44; APC-labeled anti–IFN-γ; FITC-labeled anti–TNF-α; PE-labeled anti–granzyme B; and respective isotype Abs. Intracellular cytokine staining (ICS) was performed as previously described (13). Brefeldin A (10 μg/ml; Sigma) was added for the final 3 h of stimulation. FACS analysis was performed, typically with acquisition of 3–5 × 105 gated events on the homogenous lymphocyte population, with characteristic low forward scatter/side scatter, using a FACSCalibur instrument and CellQuest for data acquisition (Becton Dickinson, San Jose, CA), and Flowjo for analysis (Tree Star Inc, San Carlos, CA).
Immunohistochemistry
Excised tracheas were placed in plastic molds (Sakura Finetek, Torrance, CA) and frozen in liquid nitrogen. Ten micron cryostat sections were collected on poly-L-lysine–coated slides and air dried overnight before fixation for 10 min in −20°C acetone. CD8 immunostaining was performed by sequential application of Avidin-Biotin block (Invitrogen), 5% donkey serum (Jackson ImmunoResearch Laboratories, West Grove, PA) for 30 min, 6.25 μg/ml rat anti-mouse CD8a (Ly-2) monoclonal Ab (mAb) (BD Pharmingen) for 60 min, 10 μg/ml biotinylated goat anti-rat Ab (BD Pharmingen) for 30 min, and avidin-alkaline phosphatase conjugate (Vector Laboratories, Burlingame, CA) for 30 min. CD8-positive cells were visualized by incubation for 15 min with DAKO Cytomation Permanent Red alkaline phosphatase substrate (DAKO Cytomation, Carpinteria, CA). 2.5 μg/ml purified rat IgG2b (BD Pharmingen) was used as negative control. Sections were counterstained lightly with hematoxylin and coverslipped.
In Vivo CD8+ T Cell Depletion
C57/BL6 mice were given intravenous injection via the tail vein of either 1 mg anti-CD8 mAb (clone 2.43, rat IgG2b) or saline immediately before heterotopic BALB/c transplant, and repeated every 7 d for a total of 4 doses. At 28 d, mice were killed and tracheal grafts were excised for histologic examination. Mice receiving anti-CD8 mAb treatment had a 90% reduction in CD8+ cells in the lymphocyte gate of pooled spleens at 28 d compared with saline injected animals. Anti-CD8 Ab (clone 2.43) was a generous gift from Dr. Fred Finkelman (Cincinnati, Ohio).
Statistical Analysis
Normally distributed continuous variable comparisons were done using Student's t test using Microsoft Excel (Microsoft Corporation, Redmond, WA).
RESULTS
Pluripotent Allospecific CD8+ Effector T Cells in the Graft Produce IFN-γ, TNF-α, and Granzyme B during OAD
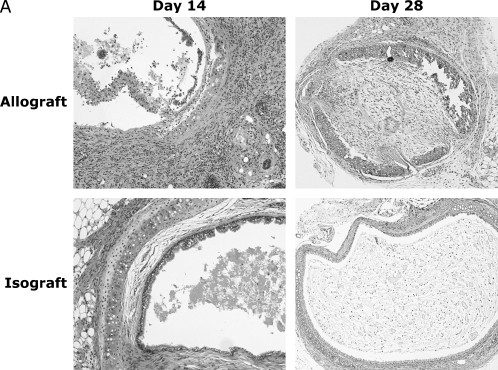
Previous studies of OAD after airway allotransplantion in MHC–fully mismatched mice have demonstrated maximal mononuclear infiltration of the allografts by 2 wk after transplant with a predominance of CD8+ T cells, followed by airway obliteration and fibrosis by 4 wk (14). We similarly assessed airway allografts (BALB/c trachea into B6) and isografts (B6 into B6) at these time points (Figure 1A). By Day 14, a striking mononuclear infiltration was present in the allografts, with severe airway epithelial denudation and sloughing into the airway lumen. In contrast, isografts displayed a relatively normal airway epithelial architecture, with intact cilia and minimal cellularity. At 28 d, 100% of BALB/c allografts demonstrated complete airway lumen obliteration with fibrosis, decreased cellularity, and airway cartilage breakdown consistent with OAD, whereas the isografts appeared normal. Similar to previous studies, we consistently detected an increased ratio of ∼ 2.5:1 CD8+ to CD4+ T cells in rejecting allografts (data not shown).
Figure 1.
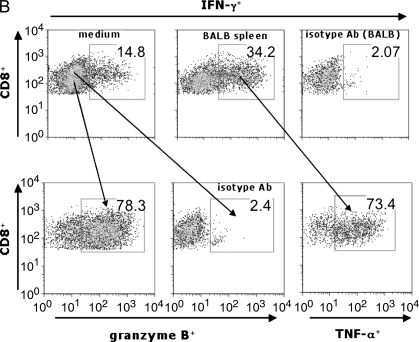
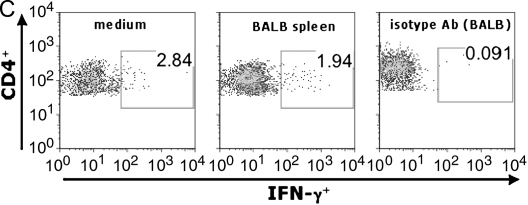
Murine obliterative airways disease develops by Day 28 with pluripotent allospecific CD8+ effector T cells infiltrating rejecting tracheal grafts by Day 14. (A) Obliterative airways disease (OAD) develops in major histocompatability complex (MHC)–mismatched mice by 28 d after transplant. B6 female recipient mice were heterotopically transplanted with BALB/c (allograft) or B6 (isograft) trachea. On Days 14 and 28, animals were killed, and the tracheal allografts were harvested, fixed in 10% formalin, and H&E stained. On Day 14, allograft mononuclear cells were isolated and cocultured (see MATERIALS AND METHODS), and gated CD8+ (B) and CD4+ (C) populations were stained with allophycocyanin (APC)-conjugated anti–IFN-γ, FITC-labeled anti–TNF-α, phycoerythrin (PE)-labeled anti–granzyme B, or isotype control antibodies (Abs) using intracellular cytokine staining (ICS). Numbers in upper right of panels are the percentage of positive staining cells for gated populations. Results are representative of three separate experiments (n = 3–5 mice per group).
Using irradiated BALB/c splenocytes for in vitro restimulation (stimulator to responder ratio of 1:3), we assessed graft-infiltrating CD8+ T cells for effector function(s). We found that a substantial population of graft CD8+ T cells constitutively produced IFN-γ after culture in medium alone (14.8%), and that the frequency of CD8+IFN-γ+ cells consistently increased 2- to 3-fold upon 6 h restimulation with BALB/c splenocytes (34.2%), as shown in Figure 1B. Similarly, graft CD8+ T cells constitutively produced TNF-α (∼ 10%; data not shown), with the majority of graft-allospecific CD8+IFN-γ+ T cells coexpressing TNF-α. Furthermore, the majority of graft CD8+ T cells were granzyme B+ (78.3%), including the majority (> 70%) of CD8+IFN-γ+ cells (data not shown). Thus, a substantial proportion of airway allograft CD8+ T cells were pluripotent, exhibiting multiple effector functions during OAD. Additionally, a smaller percentage of graft CD4+ T cells produced IFN-γ, either spontaneously in medium (2.84%) or in response to alloantigen (1.94%) (Figure 1C).
Secondary Lymphoid Tissue Cells from Allograft, but Not Isograft Recipients, Produce Alloantigen-Specific IFN-γ upon In Vitro Restimulation
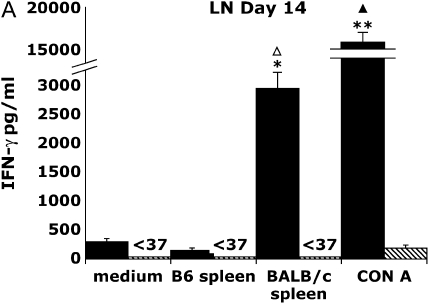
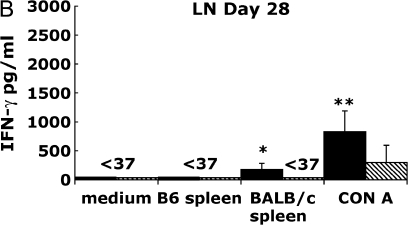
Because graft CD8+ effector T cells produce both IFN-γ and TNF-α in response to alloantigen restimulation, we used IFN-γ production as a surrogate marker to study the distribution and kinetics of allospecific responses during OAD. We next determined whether cells from the secondary lymphoid tissue were capable of IFN-γ production after restimulation with BALB/c splenocytes or the mitogen, Con A. To test this, we isolated splenocytes and draining LN (cervical, axillary, and brachial) from allograft and isograft recipients on Days 14 and 28 after transplant, and placed them in culture under various stimulatory conditions described, and assessed IFN-γ production from culture supernatants at 72 h. In Figure 2A, Day 14 allograft LN cultures stimulated with irradiated BALB/c spleen produced large amounts of IFN-γ (2,961 pg/ml) at a level 10- to 20-fold above that of allograft LN cultures in medium alone or those cocultured with syngeneic B6 splenocytes (P ⩽ 0.05). In marked contrast, IFN-γ was not detected (< 37 pg/ml, limit of assay) in isograft LN cultures (P = 0.01) under the same culture conditions. To compare the global IFN-γ producing capacity of allograft LN to isograft LN, we stimulated cultures with the Con A and found that allograft LN cultures produced strikingly higher IFN-γ (> 100-fold) at 72 h (mean 17,468 pg/ml ± 630 [SEM]; P < 0.0001) compared with isograft LN cultures (187 pg/ml ± 57). However, by Day 28 after transplant (Figure 2B), IFN-γ production from allograft LN cultures in response to alloantigen was significantly less (179 pg/ml ± 100) than at Day 14 (16-fold; P ⩽ 0.03); nonetheless, this diminished alloantigen-specific IFN-γ production was still significantly higher when compared with isograft LN cultures at that time point (P ⩽ 0.02). In addition, there was a significant reduction in Con A–induced IFN-γ production (21-fold; P ⩽ 0.0001) in allograft LN cultures at Day 28 compared with Day 14, whereas isograft LN cultures produced similar amounts of IFN-γ in response to Con A at both time points. Thus, allograft LN cultures, but not isograft, produced large amounts of IFN-γ in response to alloantigen or mitogen stimulation at Day 14, and these responses significantly decreased late in OAD.
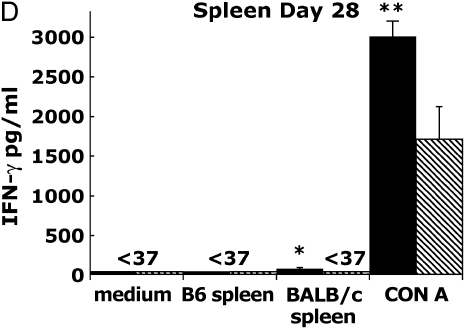
Figure 2.
Allograft lymph node (LN) and spleen produce alloantigen-specific IFN-γ in response to allogeneic BALB/c spleen at Days 14 and 28 after transplant. Allograft (BALB/c into B6) or isograft (B6 into B6) LN (A, B) and spleen (C, D) were cultured (see MATERIALS AND METHODS). At 72 h, culture supernatants were assessed using ELISA for IFN-γ (limit of assay, 37 pg/ml). Values represent the mean of the samples performed in triplicate, and the error bars represent the SEM. Statistically significant differences between allograft cells stimulated with BALB/c sp. compared with medium or syngeneic spleen or isograft cell cultures are indicated by (*P ⩽ 0.01 in [A], P ⩽ 0.02 in [B], P ⩽ 0.05 in [C], P ⩽ 0.001 in [D]), and with Con A–stimulated cultures by (**P ⩽ 0.0001 in [A], P ⩽ 0.0001 in [B], P ⩽ 0.0001 in [C], P ⩽ 0.001 in [D]), whereas (P ⩽ 0.03 in [A], P ⩽ 0.0001 in [C]) and (P ⩽ 0.03 in [A], P ⩽ 0.0001 in [C]) indicate significant differences between Day 14 compared with Day 28 alloantigen and Con A responses, respectively. Results are representative of eight independent experiments (n = 3–5 mice per group).
In the same experiments, we assessed spleen cultures for alloantigen-specific IFN-γ production. Day 14 allograft spleen cultures produced 615 pg/ml upon restimulation with BALB/c splenocytes at 72 h (Figure 2C), which was significantly higher than spleen from isograft animals under similar conditions (141 pg/ml ± 40; P ⩽ 0.05) or allograft spleen cultures in medium or in coculture with B6 spleen (P ⩽ 0.05). Similar to LN, Day 14 allograft spleen cultures produced sixfold higher levels of IFN-γ in response to Con A (11,451 pg/ml ± 569) compared with isograft spleen cultures (1,977 pg/ml ± 386; P ⩽ 0.0001). Moreover, whereas alloantigen-specific or Con A–induced IFN-γ production was still detectable in allograft spleen cultures at Day 28 compared with isograft spleen cultures (P ⩽ 0.001), these responses were significantly reduced compared with Day 14 responses (P ⩽ 0.03 and P ⩽ 0.0001, respectively) (Figure 2D). Thus, both LN and spleen cultures from allograft recipients, but not isograft recipients, produced significant IFN-γ upon restimulation with BALB/c alloantigen or mitogen, with these cellular responses decreasing by Day 28, consistent with the notion that effector/memory responses contract over time after a primary immune response. We also assessed TNF-α production from the same supernatants using ELISA (limit of detection 30 pg/ml), but were unable to detect significant levels over the course of eight experiments, particularly in response to alloantigen restimulation. Finally, in contrast to LN or spleen cultures, we found that graft cell cultures in medium alone constitutively produced large amounts of IFN-γ (range, 600–2,800 pg/ml; data not shown) over four separate experiments, most likely due to the presence of alloantigen in these cultures leading to persistent activation.
CD8+ T Cells Are the Primary Source of Alloantigen-Specific IFN-γ in the Secondary Lymphoid Tissue of Allograft Recipients
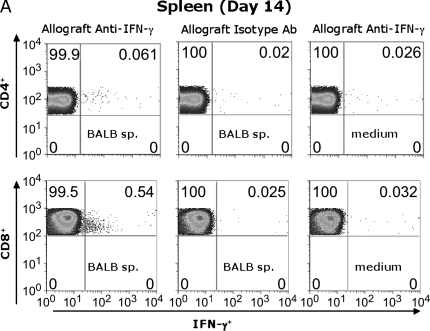
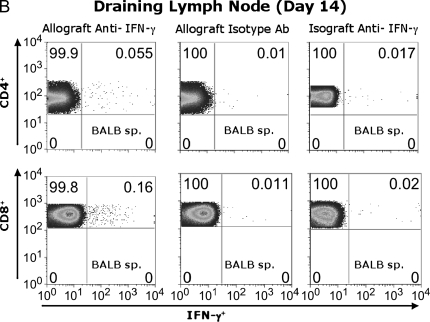
To determine whether allo-specific IFN-γ is produced by CD8+ and/or CD4+ T cells, Day 14 spleen and LN cells were stimulated for 6 h in medium alone, or with BALB/c irradiated splenocytes and assessed for IFN-γ using ICS. As seen in Figure 3A, the frequency of splenic IFN-γ+ CD4+ T cells in response to BALB/c alloantigen stimulation was 0.061%, representing a modest two- to threefold increase over the anti–IFN-γ isotype Ab control (0.02%) or unstimulated cells (medium) (0.026%). However, the frequency of splenic alloantigen-specific IFN-γ+CD8+ T cells was substantially higher (0.54%) in alloantigen-stimulated cultures than in those stained with isotype Ab (0.025%) or cells cultured in medium alone (0.032%). In a separate experiment, CD45.1 congenic B6 mice received BALB/c tracheal transplants, allowing us to gate on CD45.1+ recipient T cells after alloantigen stimulation (Figure 3B). Although both CD4+ and CD8+ T cells from the allograft LN were IFN-γ+ in response to BALB/c alloantigen stimulation above the isotype control background (5- to 15-fold), the majority of allospecific IFN-γ+ T cells in the LN were CD8+. These responses were not detected in isograft animals. (Figure 3B). Thus, CD8+ effector T cells are the primary source of alloantigen-specific IFN-γ from the secondary lymphoid tissue during OAD.
Figure 3.
Both CD4+ and CD8+ T cells contribute to alloantigen-specific IFN-γ production in allograft spleen and LN. Pooled Day 14 cells from allograft (BALB/c into B6 wild-type) spleen (A) and allograft (BALB/c into CD45.1 congenic B6) or isograft (B6 into CD45.1-congeneic B6) draining LN (B) cells were cultured in 48-well plates (see MATERIALS AND METHODS), and CD45.1+ gated CD4+ (FITC-conjugated) and CD8+ (PerCP Cy5.5–conjugated) populations were analyzed after staining with APC-conjugated anti–IFN-γ or isotype control Ab after ICS. Numbers in upper right of panels are the percentage of positive staining cells for gated populations. Results are representative of three separate experiments (n = 3–5 mice per group).
CD4+ and CD8+ Effector T Cells Employ Distinct Allorecognition Pathways during OAD
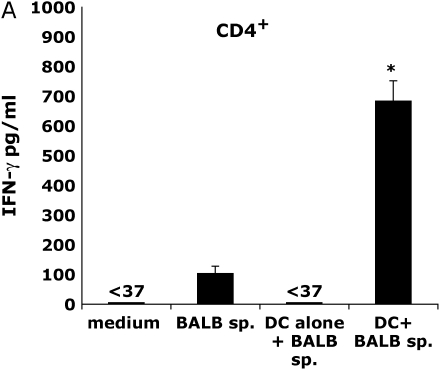
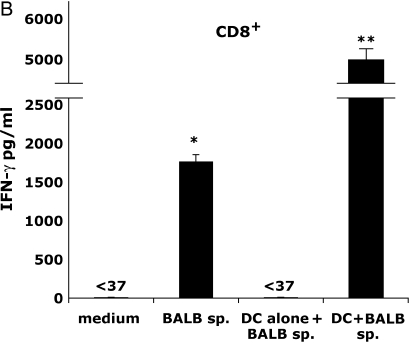
Recent studies suggest that several allorecognition pathways contribute to cellular responses during OAD (15, 16). To examine this in regard to alloantigen-specific IFN-γ production, we purified both CD4+ and CD8+ T cells from pooled allograft recipient spleens, and tested these populations via the direct and indirect allorecognition pathways. As shown in Figure 4A, allograft CD4+ T cells in the presence of BALB/c spleen produced small amounts of IFN-γ (103 pg/ml ± 25); however, when syngeneic dendritic cells (DC) from naive B6 mice were added to cultures (DC to T cell ratio of 1:3), significantly higher levels of IFN-γ were produced (684 pg/ml ± 68; P ⩽ 0.02) via the indirect allorecognition pathway (employing host APC). In contrast, direct allostimulation of allograft CD8+ T cells with BALB/c spleen (Figure 4B) induced substantial IFN-γ (1,681 pg/ml ± 84) compared with unstimulated CD8+ T cells (P ⩽ 0.0005). The addition of syngeneic DC to CD8+ T-cell cultures stimulated with BALB/c spleen markedly enhanced IFN-γ production (P ⩽ 0.005), consistent with the notion that accessory cell signals augment CD8+ T cell effector function; however, these cells are not required for allo-specific cytokine induction. Thus, alloantigen-specific production of IFN-γ by CD4+ T cells during OAD occured predominantly through indirect allorecognition, whereas CD8+ T cells were capable of robust responses via direct allorecognition, with substantial enhancement in the presence of accessory cells.
Figure 4.
CD4+ T cells produce alloantigen-specific IFN-γ, predominantly through indirect allorecognition, whereas CD8+ T cells are capable of IFN-γ production via direct allorecognition. CD4+ (A) and CD8+ (B) T cells (> 97% pure) were isolated from allograft (BALB/c into B6) spleen (Day 14) and cultured (2 × 105 cells/200 μl) in the presence or absence of irradiated BALB/c splenocytes (7 × 104) with/without syngenic dendritic cells (DC) (7 × 104) isolated from naive B6 mice. DC alone without T cells were also tested with alloantigen. At 72 h, culture supernatants were assessed for IFN-γ using ELISA. Statistically significant differences between alloantigen-induced IFN-γ responses compared with medium responses are indicated by (*P ⩽ 0.02 in [A] and P ⩽ 0.005 in [B]). The enhancement of IFN-γ production in cultures of alloantigen-stimulated CD8+ T cells in the presence of syngeneic DC is indicated by (**P ⩽ 0.005 in [B]) compared with alloantigen stimulation without syngeneic DC. Results are representative of three independent experiments (n = 3–5 mice per group).
Pluripotent Allospecific CD8+ Effector T Cells Traffic to the Lung during OAD
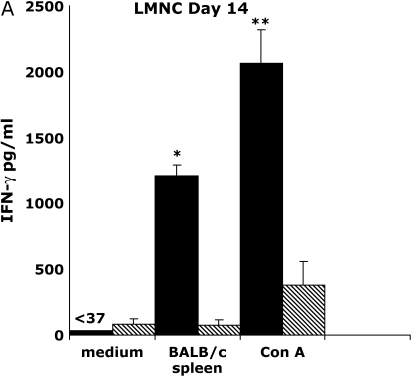
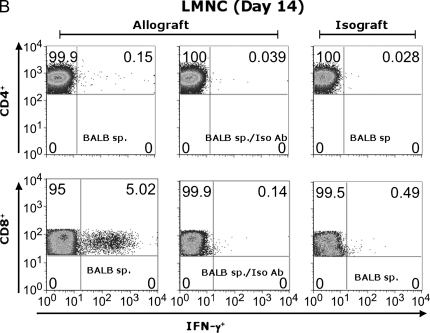
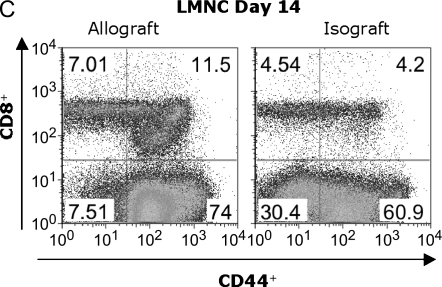
Previous reports have demonstrated the presence of effector T-cell populations in nonlymphoid tissue, including the lung (17, 18). To test whether LMNC are capable of producing alloantigen-specific IFN-γ after allogeneic airway transplant, we isolated LMNC from B6 allograft and isograft recipients. Allograft LMNC produced substantial amounts of IFN-γ by 72 h after in vitro stimulation with BALB/c spleen that was significantly higher than that of allograft LMNC in medium alone or isograft LMNC in medium or in the presence of alloantigen (P ⩽ 0.001; Figure 5A). The global IFN-γ producing capacity of LMNC, assessed by stimulation with Con A, was 5-fold higher in allograft LMNC cultures compared with isograft LMNC cultures, similar to spleen and LN cultures. Flow cytometric analysis of allograft LMNC cultures demonstrated that, similar to secondary lymphoid organs, a small population of CD4+ T cells produced IFN-γ in response to alloantigen stimulation (0.15%), representing a 5-fold increase above background staining with the anti–IFN-γ isotype Ab (0.039%) (Figure 5B). However, a striking percentage of CD8+ T cells (5.02%) were IFN-γ+ in response to BALB/c spleen restimulation. As expected, histologic examination of the lungs of allograft or isograft recipients revealed evidence of mononuclear inflammation (data not shown). Moreover, an influx of CD44high CD8+ T cells was detected in the LMNC from allograft animals (11.5%) compared with isograft LMNC (4.2%), consistent with an activated memory phenotype (Figure 5C), and similar to cytokine-producing effector cells seen in rejecting allografts. Collectively, these data demonstrate that a substantial number of alloantigen-specific IFN-γ+ CD8+ effector memory T cells traffic to the lung during OAD.
Figure 5.
Lung mononuclear cells (LMNC) produce alloantigen-specific IFN-γ. On Day 14 after transplant, allograft (BALB/c into B6) and isograft (B6 into B6) LMNC were isolated and cultured (see MATERIALS AND METHODS). At 72 h, culture supernatants were assessed for IFN-γ using ELISA. Statistically significant differences between alloantigen-induced IFN-γ in allograft cultures compared with medium alone or isograft cultures are indicated by (*P ⩽ 0.001 in [A]). Differences in Con A–induced IFN-γ between allograft and isograft cultures are indicated by (**P ⩽ 0.04 in [A]). (B) Day 14 allograft (BALB/c into B6) LMNC cultures were stimulated similar to above, and gated CD4+ (PE-conjugated) and CD8+ (FITC-conjugated) populations were stained with APC-conjugated anti–IFN-γ or isotype control Ab. Allograft and Isograft CD8+ LMNC were also assessed for CD44 expression (C). Numbers in upper right of panels are the percentage of positive staining cells for gated populations. Results are representative of five separate experiments (n = 3–5 mice per group).
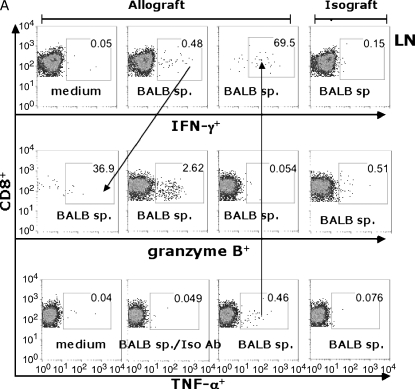
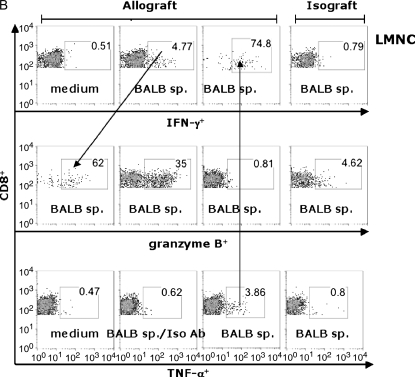
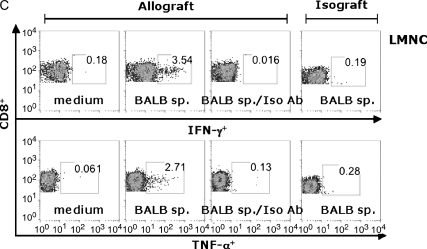
Using the B6 CD45.1 congenic mice as allograft recipients, we compared LMNC CD8+ effector T cells to those in the LN (Figure 6). At 14 d after transplant, LN cells (Figure 6A) or LMNC (Figure 6B) from allograft recipients or isograft recipients were cultured for 6 h in the presence or absence of irradiated BALB/c spleen and assessed for IFN-γ, TNF-α, and granzyme B using ICS. Restimulation of LN or LMNC with BALB/c spleen resulted in an approximately 10-fold increase in the frequency of IFN-γ+ and TNF-α+ CD8+ T cells compared with medium alone or isotype Ab staining. Interestingly, there were approximately 10-fold higher allospecific cytokine-producing cells in the LMNC cultures compared with LN cultures, indicating a substantial population of allospecific effector cells distributed in the lung during allograft rejection. To determine whether the trafficking of allospecific CD8+ T cells to the lung was related to airway transplantation itself, we transplanted a section of BALB/c or B6 liver tissue, and found similar populations of allospecific LMNC (Figure 6C), suggesting that allospecific CD8+ T cells traffic to the lung regardless of the transplanted tissue type. The majority of allospecific CD8+ TNF-α+ T cells were copositive for IFN-γ in both compartments (69.5–74.8%), as well as for granzyme B (data not shown). Additionally, a significant proportion of CD8+ IFN-γ+ T cells were positive for the cytolytic effector molecule granzyme B in both the LN (36.9%) and LMNC (62%). We consistently found that the frequencies of CD8+ granzyme B+ cells, although 5- to 8-fold higher in allograft recipients compared with isograft recipients, did not change after restimulation with alloantigen above the frequencies seen with medium alone (data not shown). Unexpectedly, the frequency of granzyme B+ CD8+ effector T cells was 10-fold higher in the LMNC of allograft recipients compared with the LN, further supporting the notion that allospecific cytotoxic T lymphocyte (CTL) traffic to the lung during allograft rejection. Together, these data show that pluripotent allospecific CD8+ T cells with the capacity for CTL effector functions are distributed in both the secondary lymphoid tissue and the lung during OAD.
Figure 6.
Pluripotent allospecific CD8+ effector T cells from the lung and LN produce IFN-γ, TNF-α, and granzyme B during OAD. Isolated LN (A) and LMNC (B) from allograft (first three columns) or isograft (last column) recipients at 14 d after transplant were cultured as described in MATERIALS AND METHODS. Surface staining was performed using PerCP Cy5.5-labeled anti-CD8, PE- or FITC-labeled CD45.1, and ICS using APC-labeled anti–IFN-γ, PE-labeled anti–granzyme B, FITC-labeled anti– TNF-α, and the respective isotype control Abs were done as described previously here. ICS was analyzed by gating on CD45.1+CD8+ cell populations. Results are representative of three separate experiments. Allospecific CD8+ effector T cells traffic to lung independent of transplant tissue type (C). BALB/c liver tissue was transplanted heterotopically into B6/CD45.1-congenic mice, and CD45.1+ CD8+ LMNC were assessed for allospecific IFN-γ and TNF-α responses at 14 d after transplant using the same surface and intracellular cytokine staining methods as described previously here.
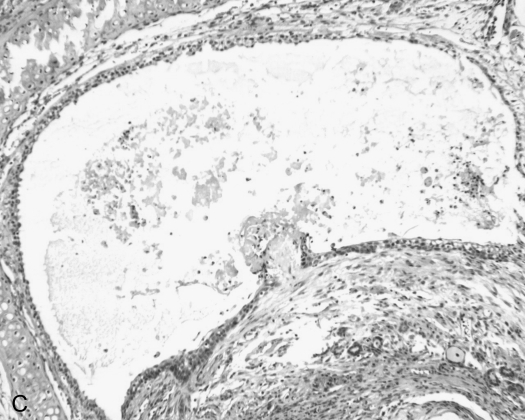
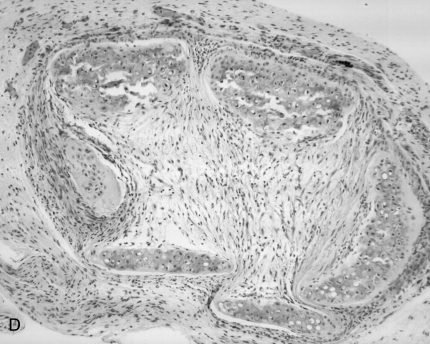
In Vivo Depletion of CD8+ T Cells Reduces Airway Graft Lumen Obliteration and Fibrosis
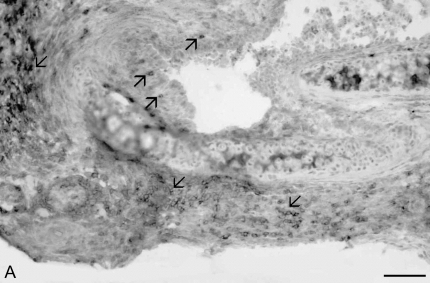
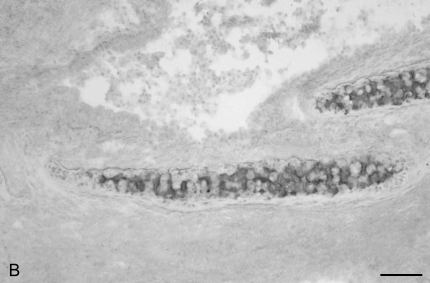
We next determined the sites of CD8+ T cell infiltration in rejecting tracheal allografts at Day 14, using immunohistochemistry. Extensive CD8+ T cell infiltration was observed in the subepithelia, the lamina propria, and the tracheal adventitia (Figures 7A and 7B). To assess the functional role of CD8+ T cells after tracheal allograft transplant, we treated mice with an anti-CD8 mAb (clone 2.43) via tail vein injection at a dose of 1 mg every 7 d beginning immediately before transplant. Control mice were injected with saline. Day-28 tracheal allografts from CD8-depleted animals demonstrated a marked reduction in lumenal obliteration and fibrosis compared with control mice (Figures 7C and 7D). Interestingly, however, anti-CD8 mAb–treated mice still developed significant mononuclear cell infiltration in their allografts with evidence of epithelial injury.
Figure 7.
CD8+ T cells infiltrate rejecting allogeneic tracheal grafts and Ab-mediated CD8+ T cell depletion reduces airway obliteration and fibrosis at Week 4 after allogeneic heterotopic tracheal transplant. Day 14 tracheal allografts were snap-frozen in liquid nitrogen and immunostained for CD8 (A) or isotype control Ab (B). Examples of extensive CD8+ infiltration of the subepithelia, lamina propria, and tracheal adventitia are marked by arrows. Scale bar in lower right corner = 50 μm. Anti-CD8 Ab (clone 2.43, rat IgG2b) was administered by tail vein injection at a dose of 1 mg per mouse at transplant and every 7 d thereafter, for a total of 4 doses. Mice were killed at Day 28 and H&E staining performed on tracheal grafts. A representative tracheal graft (n = 5 per group) from CD8+-depleted mice (C) and saline control (D) are shown.
DISCUSSION
Adaptive cellular immune responses are critical for the development of OAD after allogeneic airway transplantation (19, 20). However, the T-cell effector mechanisms and distribution of allospecific effector T cells during OAD remain incompletely understood. In this report, we demonstrate that pluripotent CD8+ effector T cells are the predominant effector T cell in fully mismatched (BALB/c into B6) airway allografts that develop OAD by 4 wk, and play an important role in immunopathogenesis, with depletion of this population resulting in a marked reduction in airway lumenal obliteration and fibrosis. These intragraft pluripotent CD8+ effectors produce IFN-γ and TNF-α in response to alloantigen, with a substantial degree of coproduction of these effector cytokines. Moreover, the majority of intragraft CD8+ effectors are constitutively granzyme B+, consistent with the potential for CTL function. We also detected CD4+ T helper (Th) type 1 (Th1) effectors in rejecting allografts, though to a lesser extent than CD8+ effector T cells. This is consistent with our observations of a 2- to 3-fold predominance of CD8+ compared with CD4+ T cells in the allografts, as previously described (14). Interestingly, there is growing evidence that the contribution of CD4+ T cells during OAD appears to be more prominent in transplants where there are only discrete minor histocompatibility differences between the donor and recipient (16, 19, 21). Nevertheless, a critical role for CD4+ T cell help in fully mismatched airway transplants is supported by studies showing that CD154-deficient recipients do not develop OAD, and that CD4+ T-cell–deficient mice have delayed onset of OAD in the setting of fully mismatched MHC (4, 22).
Recently, a study by Richards and colleagues demonstrated the presence of CD8+ IFN-γ+ effector T cells in airway allografts during OAD (6); however, these investigators did not detect evidence of activated IFN-γ+ effector T cells in the draining LN of allograft recipients, ex vivo, suggesting the retention of class I alloantigen molecules and alloreactive CD8+ T cells within the rejecting grafts. We hypothesized that allospecific effector T cells were present in the secondary lymphoid tissue of allograft recipients, but were not likely activated to the same degree as intragraft effector cells. Therefore, we used alloantigen restimulation of the draining LN or spleen cells from allograft and isograft recipients to determine whether allospecific effector T-cell responses were detectable beyond the allograft during OAD. Because we observed a substantial degree of overlap in effector function among intragraft CD8+ efffector T cells, we used IFN-γ production as a surrogate to assess the distribution and kinetics of allospecific responses in OAD. We found significant allospecific IFN-γ production in the LN and spleen cultures from allograft recipients, but not isograft recipients, after in vitro alloantigen restimulation, consistent with systemic allosensitization in these animals. Moreoever, the magnitude of allospecific (and global capacity, as measured by Con A stimulation) responses corresponded with the peak of mononuclear infiltration in the allografts, with declining cytokine responses evident during late OAD. This may be due to several factors, including a contraction of the primary allospecific CD8+ effector pool, resulting in decreased cytokine production and/or a decreasing ability of APC to export alloantigen to the LN with progressive graft fibrosis (23). Although both of these factors may play a role, the significant decrease in global IFN-γ producing capacity in response to Con A stimulation is also consistent with significant contraction of the primary alloimmune response, as Con A responses were unchanged in isograft recipients between Days 14 and 28. Similar to our intragraft findings, there was a predominance of allospecific CD8+ effector T cells that produce IFN-γ upon restimulation with alloantigen. However, unlike infiltrating graft effector cells, ex vivo allograft LN and spleen cultures in medium alone failed to produce IFN-γ in the absence of alloantigen restimulation. This suggests that insufficient alloantigen, necessary for activation of these effector cells, remained in the secondary lymphoid tissue by Day 14. Nonetheless, we demonstrate that highly purified populations of both CD4+ and CD8+ T cells from the spleen are capable of robust allospecific cytokine production in the presence of alloantigen via distinct allorecognition pathways. These data are consistent with previous reports indicating a role for indirect allostimulation for CD4+ T cells during OAD and bronchiolitis obliterans syndrome (15, 21, 24), as well as other models of allograft rejection (25–27), and support the concept that both CD4+ and CD8+ T cells cooperate in the cellular pathogenesis of OAD. Taken together, differences in the in vitro culture conditions of secondary lymphoid cells may account for our observed experimental differences from previous investigators in the detection of extragraft allospecific cytokine responses during OAD.
The presence of effector/memory T-cell populations in nonlymphoid tissues, such as the lung, has been described for both CD4+ and CD8+ T cells in several infectious and Tg models (17, 18, 28–31). Nonlymphoid T cells have similarities and differences with lymphoid T cells in that they have the capacity to produce substantial effector cytokines (both Th1 and Th2) upon antigen challenge, but appear unable to divide or migrate back to LN (30). This is the first report to demonstrate that allospecific CD8+ effector cells traffic to the lung during heterotopic tracheal transplantation, with LMNC from allograft recipients capable of producing significant amounts of IFN-γ upon alloantigen restimulation during OAD. Our data demonstrate that the lung is an important site for allospecific CD8+ effector/memory cells, perhaps serving as a reservoir for allospecific effector cells, as a significant influx of CD44high CD8+ T cells into the lung occurs during OAD. In the case of allogeneic airway transplant, it is unclear at this time whether these allospecific effector/memory T cells in the lung have previously passed through the allograft, or alternatively, upon expansion in the LN, home to the lung, where they serve as resting effector/memory cells capable of effector function upon rechallenge with alloantigen. Similarly, evidence in a respiratory viral infection model has demonstrated that recruitment of memory CD8+ T cells to the lung occurs with viral challenge with effector cytokine production upon rechallenge with antigen (32, 33). Furthermore, our finding that allospecific effector/memory T cells traffic to the lung may not only account for higher rates of lung allograft rejection compared with those of other solid organ transplants (2), but also provide a potential mechanism for the development of OB in bone marrow transplant recipients (34).
There are several caveats to point out regarding these studies. For one, the role of IFN-γ in allograft rejection is controversial. Several studies in other transplant models, in addition to the heterotopic tracheal transplant model, have demonstrated allograft rejection in IFN-γ−/− mice or after anti–IFN-γ Ab treatment, suggesting that this cytokine is not required for rejection (7–10). However, it was noted in the later study (9) that local IFN-γ in the graft site was incompletely neutralized. Indeed, observations of early allograft necrosis in the absence of IFN-γ have led some investigators to argue for potential protective effects of this cytokine (35, 36). Conversely, evidence for a role for IFN-γ in airway allograft rejection is provided in the rat tracheal allograft model, including the observation that B7 costimulation blockade, using the fusion protein CTLA4Ig, significantly delayed the development of airway occlusion, and was associated with a decrease in intragraft IFN-γ mRNA expression (37, 38). In fact, we observed an IFN-γ–associated gene signature throughout the course of OAD using gene expression profiling, with IFN-γ–associated genes upregulated at Days 14 and 28 (data not shown). Furthermore, two recent studies have shown that IFN-γ–associated chemokines, acting through CXC receptor 3, specifically CXCL9 (monokine induced by IFN-γ), CXCL10 (interferon-inducible protein 10), and CXCL11 (IFN-inducible T-cell α-chemoattractant) recruit mononuclear cells to the site of rejection during OAD, with blockade of these factors resulting in diminished pathology (39, 40). Therefore, because of the controversy regarding IFN-γ in airway allograft rejection, we examined other cellular mediators, such as TNF-α and granzyme B, and found a high degree of coexpression of these factors with IFN-γ in CD8+ effector T cells. Interestingly, two previous reports have shown an amelioration of OAD in mice treated with an anti–TNF-α Ab or anti–human TNF-α Receptor:Fc Ab, although the cellular sources of TNF-α were not elucidated in these studies (10, 41). These data show that pluripotent CD8+ effector T cells in the graft, LN, and lung are a major source of allospecific TNF-α secretion, in addition to IFN-γ and granzyme B, suggesting a role for CD8+ CTL producing these effector cytokines during OAD. Thus, although the role of IFN-γ remains controversial in the setting of allograft rejection, we find IFN-γ useful as a surrogate marker for the alloimmune response during OAD, due to its specificity of production in allograft recipients.
In summary, our findings show that pluripotent allospecific CD8+ effector T cells are distributed in the airway graft, secondary lymphoid tissue, and traffic to the lung during OAD in the heterotopic tracheal allograft model, with depletion of these cells resulting in reduced airway obliteration and fibrosis. Although these findings indicate an important role for CD8+ T cells in OAD, it remains unclear whether these cells directly mediate airway lumen obliteration and/or fibrosis. Alternatively, these cells may produce critical factors that indirectly lead to airway fibrosis via other cell populations. These murine experiments were specifically conducted under fully mismatched histocompatible conditions, which is typically the case in human lung transplantation. Although caution must be used in extrapolating mechanisms in the murine heterotopic airway transplant model to post-transplant bronchiolitis obliterans in humans, these studies do provide plausible cellular mechanisms for carrying out future investigations. The heterotopic tracheal transplant model is limited in that the graft is not directly vascularized, and is not functional lung tissue. Furthermore, although the tracheal transplant model is limited in its capacity to study nonalloimmune factors implicated in bronchiolitis obliterans, nevertheless, this model is useful to study basic alloimmune mechanisms of airway obliteration and fibrosis (42). To that end, we are currently conducting studies in human lung transplant recipients to determine whether allospecific CD8+ effector memory T cells contribute to alloimmune responses during acute and chronic lung allograft rejection.
Acknowledgments
The authors thank Robert A. Seder for helpful discussions and careful review of this manuscript, and James Watkins and Brian Schofield for their assistance with tissue staining.
This work was supported by National Institutes of Health grant HL68682 (J.F.M.), HL66583 (J.G.N.G.), AI55848 (F.D.F.), and Department of Veterans Affairs Merit Award (F.D.F.) and ESO3819.
Originally Published in Press as DOI: 10.1165/rcmb.2005-0164OC on September 29, 2005
Conflict of Interest Statement: None of the authors has a financial relationship with a commercial entity that has an interest in the subject of this manuscript.
References
- 1.Trulock EP. Lung transplantation. Am J Respir Crit Care Med 1997;155: 789–818. [DOI] [PubMed] [Google Scholar]
- 2.Arcasoy SM, Kotloff RM. Lung transplantation. N Engl J Med 1999;340: 1081–1091. [DOI] [PubMed] [Google Scholar]
- 3.Hertz MI, Jessurun J, King MB, Savik SK, Murray JJ. Reproduction of the obliterative bronchiolitis lesion after heterotopic transplantation of mouse airways. Am J Pathol 1993;142:1945–1951. [PMC free article] [PubMed] [Google Scholar]
- 4.Higuchi T, Jaramillo A, Kaleem Z, Patterson GA, Mohanakumar T. Different kinetics of obliterative airway disease development in heterotopic murine tracheal allografts induced by CD4+ and CD8+ T cells. Transplantation 2002;74:646–651. [DOI] [PubMed] [Google Scholar]
- 5.Doherty PC, Allan W, Eichelberger M, Carding SR. Roles of alpha beta and gamma delta T cell subsets in viral immunity. Annu Rev Immunol 1992;10:123–151. [DOI] [PubMed] [Google Scholar]
- 6.Richards DM, Dalheimer SL, Hertz MI, Mueller DL. Trachea allograft class I molecules directly activate and retain CD8+ T cells that cause obliterative airways disease. J Immunol 2003;171:6919–6928. [DOI] [PubMed] [Google Scholar]
- 7.Saleem S, Konieczny BT, Lowry RP, Baddoura FK, Lakkis FG. Acute rejection of vascularized heart allografts in the absence of IFNgamma. Transplantation 1996;62:1908–1911. [DOI] [PubMed] [Google Scholar]
- 8.Ring GH, Saleem S, Dai Z, Hassan AT, Konieczny BT, Baddoura FK, Lakkis FG. Interferon-gamma is necessary for initiating the acute rejection of major histocompatibility complex class II–disparate skin allografts. Transplantation 1999;67:1362–1365. [DOI] [PubMed] [Google Scholar]
- 9.Halloran PF, Miller LW, Urmson J, Ramassar V, Zhu LF, Kneteman NM, Solez K, Afrouzian M. IFN-gamma alters the pathology of graft rejection: protection from early necrosis. J Immunol 2001;166:7072–7081. [DOI] [PubMed] [Google Scholar]
- 10.Smith CR, Jaramillo A, Lu KC, Higuchi T, Kaleem Z, Mohanakumar T. Prevention of obliterative airway disease in HLA-A2–transgenic tracheal allografts by neutralization of tumor necrosis factor. Transplantation 2001;72:1512–1518. [DOI] [PubMed] [Google Scholar]
- 11.Seder RA, Ahmed R. Similarities and differences in CD4+ and CD8+ effector and memory T cell generation. Nat Immunol 2003;4:835–842. [DOI] [PubMed] [Google Scholar]
- 12.Inaba K, Swiggard W, Steinman R, Romani N, Schuler G. Isolation of dendritic cells. In: Coligan JE, Bierer BE, Margulies DH, Shevach EM, Strober W, editors. Current Protocols in Immunology. New York: Green Publishing Associates; 1998. pp. 3.7.1–3.7.15.
- 13.Prussin C, Metcalfe DD. Detection of intracytoplasmic cytokine using flow cytometry and directly conjugated anti-cytokine antibodies. J Immunol Methods 1995;188:117–128. [DOI] [PubMed] [Google Scholar]
- 14.Belperio JA, Keane MP, Burdick MD, Lynch JP III, Xue YY, Berlin A, et al. Critical role for the chemokine MCP-1/CCR2 in the pathogenesis of bronchiolitis obliterans syndrome. J Clin Invest 2001;108:547–556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chalermskulrat W, Neuringer IP, Brickey WJ, Felix NJ, Randell SH, Ting JP, Aris RM. Hierarchical contributions of allorecognition pathways in chronic lung rejection. Am J Respir Crit Care Med 2003;167:999–1007. [DOI] [PubMed] [Google Scholar]
- 16.Richards DM, Dalheimer SL, Ehst BD, Vanasek TL, Jenkins MK, Hertz MI, Mueller DL. Indirect minor histocompatibility antigen presentation by allograft recipient cells in the draining lymph node leads to the activation and clonal expansion of CD4+ T cells that cause obliterative airways disease. J Immunol 2004;172:3469–3479. [DOI] [PubMed] [Google Scholar]
- 17.Reinhardt RL, Khoruts A, Merica R, Zell T, Jenkins MK. Visualizing the generation of memory CD4 T cells in the whole body. Nature 2001;410:101–105. [DOI] [PubMed] [Google Scholar]
- 18.Masopust D, Vezys V, Marzo AL, Lefrancois L. Preferential localization of effector memory cells in nonlymphoid tissue. Science 2001;291:2413–2417. [DOI] [PubMed] [Google Scholar]
- 19.Kelly KE, Hertz MI, Mueller DL. T-cell and major histocompatibility complex requirements for obliterative airway disease in heterotopically transplanted murine tracheas. Transplantation 1998;66:764–771. [DOI] [PubMed] [Google Scholar]
- 20.Neuringer IP, Mannon RB, Coffman TM, Parsons M, Burns K, Yankaskas JR, Aris RM. Immune cells in a mouse airway model of obliterative bronchiolitis. Am J Respir Cell Mol Biol 1998;19:379–386. [DOI] [PubMed] [Google Scholar]
- 21.Smith MA, Jaramillo A, SivaSai KS, Naziruddin B, Kaleem Z, Patterson GA, Mohanakumar T. Indirect recognition and antibody production against a single mismatched HLA-A2–transgenic molecule precede the development of obliterative airway disease in murine heterotopic tracheal allografts. Transplantation 2002;73:186–193. [DOI] [PubMed] [Google Scholar]
- 22.Rumbley CA, Silver SJ, Phillips SM. Dependence of murine obstructive airway disease on CD40 ligand. Transplantation 2001;72:1616–1625. [DOI] [PubMed] [Google Scholar]
- 23.Badovinac VP, Porter BB, Harty JT. Programmed contraction of CD8(+) T cells after infection. Nat Immunol 2002;3:619–626. [DOI] [PubMed] [Google Scholar]
- 24.SivaSai KS, Smith MA, Poindexter NJ, Sundaresan SR, Trulock EP, Lynch JP, Cooper JD, Patterson GA, Mohanakumar T. Indirect recognition of donor HLA class I peptides in lung transplant recipients with bronchiolitis obliterans syndrome. Transplantation 1999;67:1094–1098. [DOI] [PubMed] [Google Scholar]
- 25.Auchincloss H Jr, Lee R, Shea S, Markowitz JS, Grusby MJ, Glimcher LH. The role of “indirect” recognition in initiating rejection of skin grafts from major histocompatibility complex class II–deficient mice. Proc Natl Acad Sci USA 1993;90:3373–3377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ciubotariu R, Liu Z, Colovai AI, Ho E, Itescu S, Ravalli S, et al. Persistent allopeptide reactivity and epitope spreading in chronic rejection of organ allografts. J Clin Invest 1998;101:398–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Reed AJ, Noorchashm H, Rostami SY, Zarrabi Y, Perate AR, Jeganathan AN, Caton AJ, Naji A. Alloreactive CD4 T cell activation in vivo: an autonomous function of the indirect pathway of alloantigen presentation. J Immunol 2003;171:6502–6509. [DOI] [PubMed] [Google Scholar]
- 28.Cauley LS, Cookenham T, Miller TB, Adams PS, Vignali KM, Vignali DA, Woodland DL. Cutting edge: virus-specific CD4+ memory T cells in nonlymphoid tissues express a highly activated phenotype. J Immunol 2002;169:6655–6658. [DOI] [PubMed] [Google Scholar]
- 29.Ely KH, Roberts AD, Woodland DL. Cutting edge: effector memory CD8+ T cells in the lung airways retain the potential to mediate recall responses. J Immunol 2003;171:3338–3342. [DOI] [PubMed] [Google Scholar]
- 30.Harris NL, Watt V, Ronchese F, Le Gros G. Differential T cell function and fate in lymph node and nonlymphoid tissues. J Exp Med 2002;195: 317–326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wu CY, Kirman JR, Rotte MJ, Davey DF, Perfetto SP, Rhee EG, Freidag BL, Hill BJ, Douek DC, Seder RA. Distinct lineages of T(H)1 cells have differential capacities for memory cell generation in vivo. Nat Immunol 2002;3:852–858. [DOI] [PubMed] [Google Scholar]
- 32.Ely KH, Cauley LS, Roberts AD, Brennan JW, Cookenham T, Woodland DL. Nonspecific recruitment of memory CD8+ T cells to the lung airways during respiratory virus infections. J Immunol 2003;170:1423–1429. [DOI] [PubMed] [Google Scholar]
- 33.Chen HD, Fraire AE, Joris I, Brehm MA, Welsh RM, Selin LK. Memory CD8+ T cells in heterologous antiviral immunity and immunopathology in the lung. Nat Immunol 2001;2:1067–1076. [DOI] [PubMed] [Google Scholar]
- 34.Dudek AZ, Mahaseth H, DeFor TE, Weisdorf DJ. Bronchiolitis obliterans in chronic graft-versus-host disease: analysis of risk factors and treatment outcomes. Biol Blood Marrow Transplant 2003;9:657–666. [DOI] [PubMed] [Google Scholar]
- 35.Halloran PF, Afrouzian M, Ramassar V, Urmson J, Zhu LF, Helms LM, Solez K, Kneteman NM. Interferon-gamma acts directly on rejecting renal allografts to prevent graft necrosis. Am J Pathol 2001;158:215–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fairchild RL. The Yin and Yang of IFN-gamma in allograft rejection. Am J Transplant 2003;3:913–914. [DOI] [PubMed] [Google Scholar]
- 37.Boehler A, Bai XH, Liu M, Cassivi S, Chamberlain D, Slutsky AS, Keshavjee S. Upregulation of T-helper 1 cytokines and chemokine expression in post-transplant airway obliteration. Am J Respir Crit Care Med 1999;159:1910–1917. [DOI] [PubMed] [Google Scholar]
- 38.Tikkanen JM, Lemstrom KB, Koskinen PK. Blockade of CD28/B7–2 costimulation inhibits experimental obliterative bronchiolitis in rat tracheal allografts: suppression of helper T cell type 1–dominated immune response. Am J Respir Crit Care Med 2002;165:724–729. [DOI] [PubMed] [Google Scholar]
- 39.Belperio JA, Keane MP, Burdick MD, Lynch JP III, Xue YY, Li K, Ross DJ, Strieter RM. Critical role for CXCR3 chemokine biology in the pathogenesis of bronchiolitis obliterans syndrome. J Immunol 2002;169:1037–1049. [DOI] [PubMed] [Google Scholar]
- 40.Belperio JA, Keane MP, Burdick MD, Lynch JP III, Zisman DA, Xue YY, Li K, Ardehali A, Ross DJ, Strieter RM. Role of CXCL9/CXCR3 chemokine biology during pathogenesis of acute lung allograft rejection. J Immunol 2003;171:4844–4852. [DOI] [PubMed] [Google Scholar]
- 41.Aris RM, Walsh S, Chalermskulrat W, Hathwar V, Neuringer IP. Growth factor upregulation during obliterative bronchiolitis in the mouse model. Am J Respir Crit Care Med 2002;166:417–422. [DOI] [PubMed] [Google Scholar]
- 42.Estenne M, Hertz MI. Bronchiolitis obliterans after human lung transplantation. Am J Respir Crit Care Med 2002;166:440–444. [DOI] [PubMed] [Google Scholar]