Abstract
Catecholamines can suppress production of inflammatory mediators in different cell types, including airway epithelium, but downstream signaling mechanisms involved in regulation of these antiinflammatory effects are largely unknown. We theorized that acute β2-adrenergic stimulation of airway epithelial cells with albuterol could suppress the production and release of inflammatory mediators, specifically granulocyte macrophage–colony stimulating factor (GM-CSF) via a pathway involving inducible nitric oxide synthase (iNOS). Normal human bronchial epithelial (NHBE) cells in primary culture were exposed to a cytokine mixture (10 ng/ml each IFN-γ and IL-1β) to induce iNOS expression. (R)- and (S)-enantiomers of albuterol, as well as racemic mixtures, were added with these cytokines, and effects on GM-CSF expression and production were assessed. Specific inhibitors and activators of protein kinases (PKs), β2-adrenergic receptor antagonists, and small interfering RNAs against iNOS were used to delineate signaling pathways involved. iNOS message was significantly upregulated in a concentration-dependent manner by the active (R)-enantiomer of albuterol. (R)-albuterol also attenuated cytokine-induced increases in GM-CSF steady-state mRNA expression and protein release. The (S)-enantomer of albuterol had no effect on these parameters. PKC, specifically, the δ isoform, was required for iNOS message increase, but PKA and PKG were not involved in the pathway. Overall, this study identifies a novel pathway by which β2-adrenergic agonists may exhibit antiinflammatory effects in airway epithelium and surrounding milieu.
Keywords: albuterol, asthma, cytokines, epithelium, inducible nitric oxide synthase
β2-Adrenergic agonists are potent bronchodilators widely used clinically to treat patients with asthma. The β2-adreneregic receptor mediates smooth muscle relaxation by activating adenylate cyclase via a trimeric Gs protein signaling pathway. In addition, β2-adrenergic agonists in the airway can regulate ciliary beat frequency (1, 2), ion transport (3), and repair of injury (4). β2-adreneregic agonists also may suppress proinflammatory responses in several airway cell types, including attenuation of IL-8 production by monocytes and neutrophils (5, 6), downregulation of granulocyte macrophage–colony stimulating factor (GM- CSF) and eotaxin production in airway smooth muscle cells (7), and suppression of intercellular adhesion molecule (ICAM)–1 and GM-CSF production by airway epithelial cells (8, 9).
As the first line of defense against inhaled pathogens, irritants, and environmental particulates, airway epithelium plays a critical role in coordinating inflammatory responses. A prominent mechanism by which airway epithelial cells regulate inflammatory responses is through the pluripotent radical, nitric oxide (NO). Airway epithelial cells in vivo uniquely express the inducible isoform of NO synthase (iNOS) constitutively (10). In these cells, iNOS appears to be involved intimately in local inflammatory responses, as it is upregulated in response to injury (11) or by exposure to proinflammatory cytokines, such as TNF-α, IL-1β, or IFN-γ (12–14). There is some controversy as to whether iNOS activity is beneficial or detrimental in inflamed airways. Mice that have been genetically altered so as to not express the iNOS gene exhibit enhanced susceptibility to ovalbumin (OVA) challenge, with increased fibrosis and inflammation compared with wild-type OVA-challenged mice (15). Conversely, patients with asthma have increased levels of peroxynitrite, an active and potentially toxic reactive nitrogen species that forms in the presence of NO and superoxide (16). Increased peroxynitrite in the lung can significantly enhance airway inflammation (17). Thus, NO can elicit differing effects depending on production, location, metabolite formation, and antioxidant contribution.
Interestingly, NOS expression and activity increase in bronchial epithelium in response to β-adrenergic agonists in both rats and dogs (18, 19). As NO can attenuate production of the proinflammatory cytokine, GM-CSF, in bronchial epithelial cells (20), it is possible that β2-adrenergic agonists elicit some of their antiinflammatory properties through an iNOS-mediated pathway. The present study examines the effects of the short-acting β2-adrenergic agonist, (R)-albuterol, on expression of iNOS in primary cultures of normal human bronchial epithelial (NHBE) cells in vitro. The results indicate that cytokine-induced expression of iNOS is enhanced by the (R)-enantiomer of albuterol in these cells. (R)-albuterol also suppresses cytokine-induced expression of GM-CSF, and this suppression is attenuated if iNOS is inhibited, suggesting that (R)-albuterol's antiinflammatory effects may be mediated by NO. Additional studies demonstrated that (R)-albuterol–mediated enhancement of iNOS expression requires protein kinase (PK) C, but not PKA or PKG, activity.
MATERIALS AND METHODS
Primary Culture of NHBE Cells
Expansion and cryopreservation of NHBE cells were performed as described previously (21). After expansion, passage-2 NHBE cells were seeded on plastic 6- or 12-well culture plates at a density of 1.0 × 104 cells/cm2. Medium (modified bronchial epithelial cell basal medium) was changed every other day until cultures were 80–90% confluent, at which time the cells were incubated in medium, from which bovine pituitary extract and human recombinant epidermal growth factor were removed for 24 h to force cells into a quiescent, nonproliferative state. Confluent cultures were then used for the experiments described subsequently here. Unless otherwise described, agents added to cells were prepared in warmed (37°C), serum-free medium before addition to the culture surface. See the online supplement for treatment details.
TaqMan Real-Time RT-PCR
Total RNA was isolated from culture lysates using an RNeasy Mini Kit according to the manufacturer's instructions (Qiagen, Valencia, CA). Each sample was treated with RNase-free DNase for 15 min to reduce genomic DNA contamination. One microgram of RNA from each sample was reverse-transcribed using an iScript cDNA synthesis kit according to recommended thermal conditions (Bio-Rad, Hercules, CA). Real-time PCR, using a hydrolysis probe, was performed as described previously (22). Primer and probe sequences were designed using Beacon Designer software (Premier Biosoft, Palo Alto, CA) based on published mRNA sequences for human iNOS, GM-CSF, and β-actin obtained from the National Center for Biotechnology Information sequence online database (www.ncbi.nlm.nih.gov). Relative quantification of targets, normalized to β-actin message, was achieved according to a method described by Peirson and colleagues (23). See the online supplement for additional details.
GM-CSF ELISA
GM-CSF protein released from NHBE cells was assayed with a commercially available enzyme-linked immunosorbent assay (BD-Biosciences, San Diego, CA) according to the manufacturer's instructions. GM-CSF values were normalized to total protein (Bradford method; Bio-Rad).
NHBE Transfection and RNA Silencing
NHBE cells were transfected using a 0.45% final concentration of fugene 6 transfection reagent (Roche, Indianapolis, IN). To silence iNOS message, 225 nM annealed 21-bp small interfering RNA (siRNA; catalog no. 8678; Ambion, Austin, TX) was added during the transfection procedure. Transfection was optimized from the manufacturer's general protocol. Additional control cultures were exposed to scrambled siRNA (catalog no. 4611; Ambion) or transfection reagent alone. For complete details, see the online supplement.
PKC Activity Assay
PKC activity was assessed using a PepTag (Promega, Madison, WI) assay for nonradioactive detection of PKC, following the manufacturer's protocol. Reaction mixtures were separated on 0.8% agarose gels, and phosphorylated peptides were quantified by Labworks image acquisition and analysis software (Ultra Violet Products, Ltd., Upland, CA). Values were extrapolated from a linear standard curve based on a series dilution of purified PKC supplied with the kit (0–20 ng). For details of additional studies with PKC-related reagents, see the online supplement.
Cytotoxicity Assay
All reagents were tested for cytotoxicity to NHBE cells by measuring lactate dehydrogenase release/retention. The assay was performed using a commercially available kit (Promega) according to the manufacturer's instructions. All treatments shown in this study elicited lactate dehydrogenase release comparable to that of nontreated cultures (3–7%), and therefore were not considered cytotoxic.
Statistical Analysis
Data were analyzed for significance using one-way ANOVA with Tukey's post-test for multiple comparisons or Student's t test. Data were considered significant at P < 0.05.
RESULTS
IL-1β + IFN-γ Increases iNOS Message in NHBE Cells
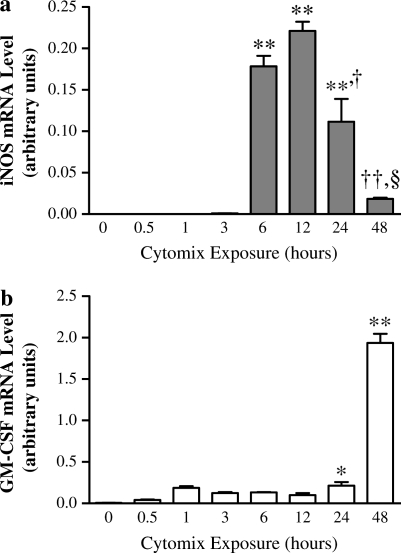
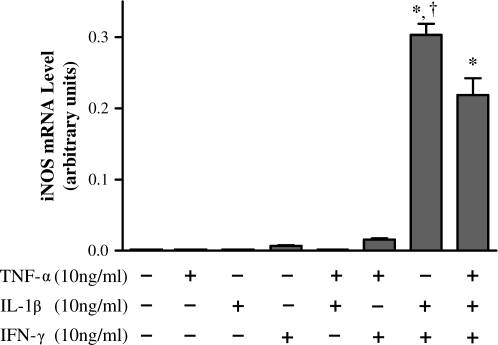
To mimic proinflammatory conditions, NHBE cells were exposed to a cytokine mixture (“cytomix”: 10 ng/ml each of TNF-α, IL-1β, and IFN-γ) over a time course of 0–48 h. iNOS message was induced after 6 h of cytomix exposure, peaked at ∼ 12 h, and then fell to near-undetectable levels by 48 h (Figure 1a). Cytomix exposure also increased GM-CSF message after 1 h, but then seemed to decrease it slightly over the next 12-h period. At 24 h, GM-CSF production began to rise again, and, at 48 h, GM-CSF message levels were ∼ 15-fold higher than the 6-h level and 10-fold higher than the 24-h level (Figure 1b). To determine the optimal cytokine mixture that would synergistically induce iNOS message in NHBE cells, different combinations of cytokines were tested. The combination of IL-1β and IFN-γ induced the highest levels of iNOS expression (Figure 2), so the combination of 10 ng/ml IL-1β and 10 ng/ml IFN-γ (IL-1β + IFN-γ) was used for the remainder of the experiments described subsequently here.
Figure 1.
Exposure to 10 ng/ml each of TNF-α, IL-1β, and IFN-γ (cytomix) upregulates inducible nitric oxide synthase (iNOS) and granulocyte macrophage–colony stimulating factor (GM-CSF) message in normal human bronchial epithelial (NHBE) cells. iNOS and GM-CSF message were measured after total RNA isolation using TaqMan real-time RT-PCR and normalized to β-actin message. (a) iNOS message levels rise at 6 h and peak after ∼ 12 h of cytomix exposure, then fall to barely detectable levels by 48 h. ** Significantly increased iNOS expression (P < 0.001); † significantly less iNOS expression than 6 and 12 h cytomix exposure (P < 0.01; ††P < 0.001; §P < 0.001). (b) GM-CSF message levels rise slightly after initial cytomix exposure, then rise by nearly 15-fold after 48 h. * Significantly greater than constitutive iNOS expression (P < 0.05); ** significantly greater iNOS expression than any other time point (P < 0.001). All data are presented as mean ± SEM (n = 6).
Figure 2.
A combination of IL-1β + IFN-γ elicits greatest synergistic upregulation of iNOS transcription in NHBE cells. Cells were exposed to combinations of TNF-α, IL-1β, and IFN-γ, all at 10 ng/ml, for 12 h. iNOS message was normalized to β-actin message. The combination of the cytokines IL-1β + IFN-γ induced the highest levels of iNOS transcription. Data are presented as mean ± SEM (n = 3). * Significantly greater expression compared with all other treatments (P < 0.001); † significantly greater than cytomix-induced expression (P < 0.01).
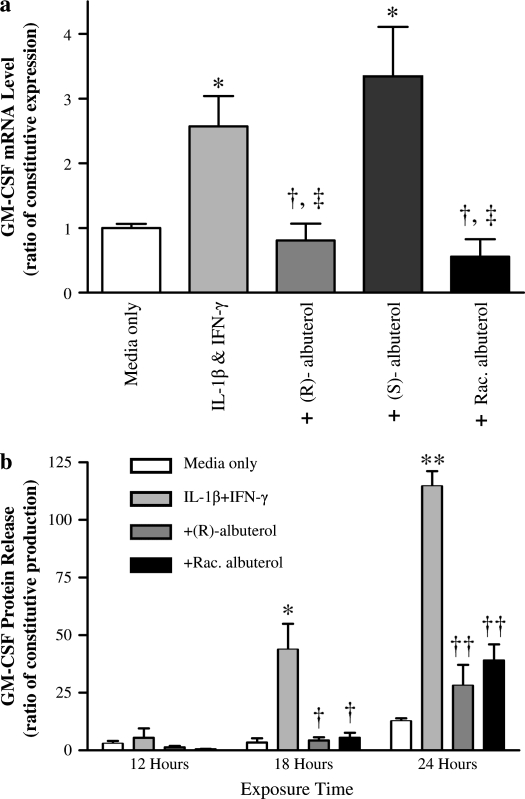
(R)-Albuterol Suppresses Cytokine-Induced GM-CSF Augmentation
As illustrated in Figure 3, (R)- and racemic albuterol at 10−6 M attenuated the increase in GM-CSF message in NHBE cells exposed to IL-1β + IFN-γ after 12 h. Conversely, (S)-albuterol did not decrease GM-CSF message at any time point. Cytokine-stimulated GM-CSF protein release was also attenuated by (R)- and racemic albuterol at all time points tested, with statistically significant suppression (P < 0.01) at 18 and 24 h, whereas (S)-albuterol had no effect (data not shown).
Figure 3.
(R)- and racemic albuterol significantly suppress IL-1β + IFN-γ– mediated upregulation of GM-CSF message and protein release. (a) NHBE cells were stimulated with 10 ng/ml each of IL-1β and IFN-γ for 12 h in the presence of either control medium, 10−6 M (R)-albuterol, 10−6 M (S)-albuterol, or 10−6 M racemic (R + S) albuterol. GM-CSF message was normalized to β-actin message. Both (R)- and racemic albuterol significantly suppressed GM-CSF upregulation by the cytokines, whereas (S)-albuterol had no significant effect. Data are presented as mean ± SEM (n = 3–7). * Significantly greater than constitutive GM-CSF mRNA expression (P < 0.05); † significantly lower than cytokine-induced GM-CSF mRNA expression (P < 0.01); ‡ significantly lower than cytokine-induced GM-CSF mRNA expression with exposure to (S)-albuterol (P < 0.01). (b) NHBE cells were stimulated with 10 ng/ml each of IL-1β and IFN-γ over a range of times in the presence of control medium, 10−5 M (R)-albuterol, or 10−5 M racemic albuterol. GM-CSF protein in culture media was measured by ELISA and normalized to total protein of cell lysates. Both (R)- and racemic albuterol significantly attenuated the increase in GM-CSF protein release elicited by IL-1β + IFN-γ after 12 h. Data are presented as mean ± SEM (n = 4–6). * Significantly greater than constitutive GM-CSF protein release (P < 0.01; ** = P < 0.001). † Significantly lower than cytokine-induced GM-CSF protein release (P < 0.01, †† = P < 0.001). White bars, media only; light gray bars, IL-1β + IFN-γ; dark gray bars, + (R)-albuterol; black bars, + racemic albuterol.
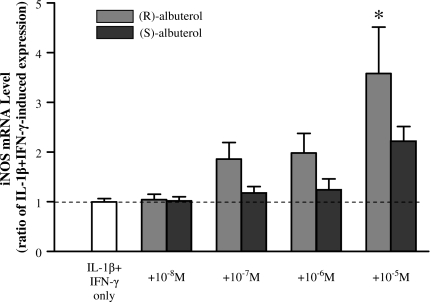
(R)-Albuterol Augments Cytokine-Induced iNOS Transcription via β2-Adrenergic Receptor Activation
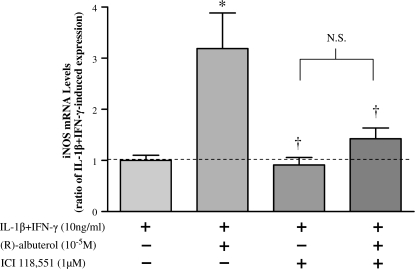
NHBE cells were exposed to IL-1β + IFN-γ in the presence of (R)- or (S)-albuterol over a range of concentrations (10−8 to 10−5 M) for 12 h. As illustrated in Figure 4, (R)-albuterol augmented cytokine-induced iNOS message in a concentration-dependent manner, reaching statistical significance (P < 0.001) at 10−5 M. (S)-albuterol, in contrast, did not significantly affect iNOS message, even at the highest concentration tested (10−5 M). To ensure that (R)-albuterol was mediating its suppressive effects through the β2-adrenergic receptor, selected cultures were incubated with the specific β2-adrenergic receptor antagonist, ICI 118551. As illustrated in Figure 5, 1 μM ICI 116551 negated (R)-albuterol–mediated augmentation of iNOS message at 18 h, suggesting that β2-adrenergic receptor activation is required for this action.
Figure 4.
(R)-albuterol augments, in a concentration-dependent manner, iNOS message in NHBE cells exposed to IL-1β + IFN-γ. Cells were exposed to 10 ng/ml each of IL-1β and IFN-γ for 12 h together with either (R)- or (S)-albuterol over a range of concentrations. (R)-albuterol (light gray bars) significantly augmented iNOS message at 10−5 M, whereas (S)-albuterol (dark gray bars) did not have a significant effect at this or lower concentrations. Dashed line represents IL-1β + IFN-γ–induced iNOS expression. Data are presented as mean ± SEM (n = 6). * Significantly greater than cytokine-induced iNOS expression (P < 0.001).
Figure 5.
β2-adrenergic receptor activation is required for (R)-albuterol–mediated increase in iNOS expression. NHBE cells were stimulated with 10 ng/ml of IL-1β + IFN-γ with or without 10−5 M (R)-albuterol for 18 h in the presence or absence of the β2-adrenergic antagonist, ICI 118551. iNOS message, normalized to β-actin message, was assayed by TaqMan real-time RT-PCR. β2-adrenergic receptor antagonism alone did not affect IL-1β + IFN-γ–induced iNOS transcription, but (R)-albuterol augmentation of iNOS message was blocked by ICI 118551. NHBE cells incubated in medium alone exhibited no measurable iNOS message. Dashed line represents IL-1β + IFN-γ–induced iNOS expression. Data are presented as mean ± SEM (n = 4). * Significantly greater than cytokine-induced iNOS expression (P < 0.01); † significantly lower than iNOS message expression after cytokine + (R)-albuterol treatment (P < 0.01). N.S., no significant difference (P > 0.05).
GM-CSF Suppression by (R)-Albuterol Is Mediated through iNOS
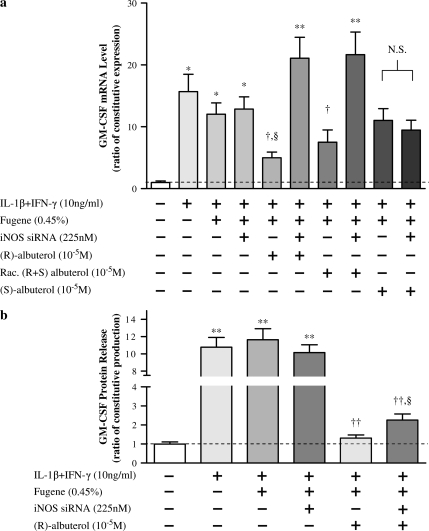
To link (R)-albuterol–mediated iNOS augmentation and GM-CSF suppression, RNA silencing of iNOS message was used. Although we have observed that chemical inhibition of iNOS can augment GM-CSF message (24), RNA silencing is a more exact method to specifically target a particular transcript. NHBE cells were transfected using fugene 6 reagent and a 21-base annealed siRNA designed using human iNOS mRNA sequence and verified to be effective in silencing experiments (Ambion). Fugene 6 reagent alone or a scrambled siRNA did not affect iNOS transcription in cytokine-stimulated NHBE cells, but addition of iNOS siRNA inhibited transcription by ∼ 60% (see online supplement, Figure E1). As illustrated in Figure 6a, silencing iNOS message negated the suppressive effect of (R)- and racemic albuterol on GM-CSF message levels. (S)-albuterol had no effect on GM-CSF message, with or without silencing. As illustrated in Figure 6b, iNOS silencing only partially negated (R)-albuterol–mediated suppression of GM-CSF protein release, suggesting that (R)-albuterol may suppress GM-CSF post-transcriptionally through an additional pathway not involving iNOS, or that ∼ 60% silencing still left sufficient iNOS for partial GM-CSF suppression.
Figure 6.
Silencing iNOS transcription decreases attenuation of GM-CSF message and protein release elicited by (R)-albuterol. (a) NHBE cells were transfected for 24 h with iNOS-silencing RNA, as described in the text. After the 24-h transfection period, cells were stimulated with 10 ng/ml each of IL-1β and IFN-γ for 18 h to augment GM-CSF message levels. iNOS silencing had little effect on IL-1β + IFN-γ augmentation of GM-CSF message (lanes 1–4), but it did negate (R)- and racemic albuterol–mediated suppression of GM-CSF message (lanes 5–8). (S)-albuterol, with or without silencing, did not affect GM-CSF message expression (lanes 9 and 10). These results suggest that iNOS is necessary for (R)- and racemic albuterol–mediated GM-CSF message suppression in NHBE cells exposed to cytokines. Dashed line represents constitutive GM-CSF expression. Data are presented as mean ± SEM (n = 3–4). * Significantly greater than constitutive GM-CSF expression (P < 0.05, ** = P < 0.001); † significantly lower than GM-CSF expression after treatment with iNOS silencing (P < 0.01); § significantly lower than cytokine-stimulated GM-CSF expression (P < 0.05). N.S., no significant difference (P > 0.05). (b) NHBE cells were transfected and treated as described previously here, and GM-CSF protein in culture medium was measured by ELISA. The addition of iNOS small interfering RNA (siRNA) did not significantly affect cytokine-mediated GM-CSF protein release. However, (R)-albuterol (10−5 M) significantly suppressed cytokine-mediated GM-CSF protein release, and iNOS silencing partially suppressed this effect. Dashed line represents constitutive GM-CSF protein release. Data are presented as mean ± SEM (n = 12). ** Significantly greater than constitutive GM-CSF protein release (P < 0.001); †† significantly lower than cytokine-induced GM-CSF protein release (P < 0.001); § significantly greater GM-CSF protein release compared with cytokine + fugene 6 + (R)-albuterol–treated cell cultures (P < 0.05).
PKCδ Mediates iNOS Expression
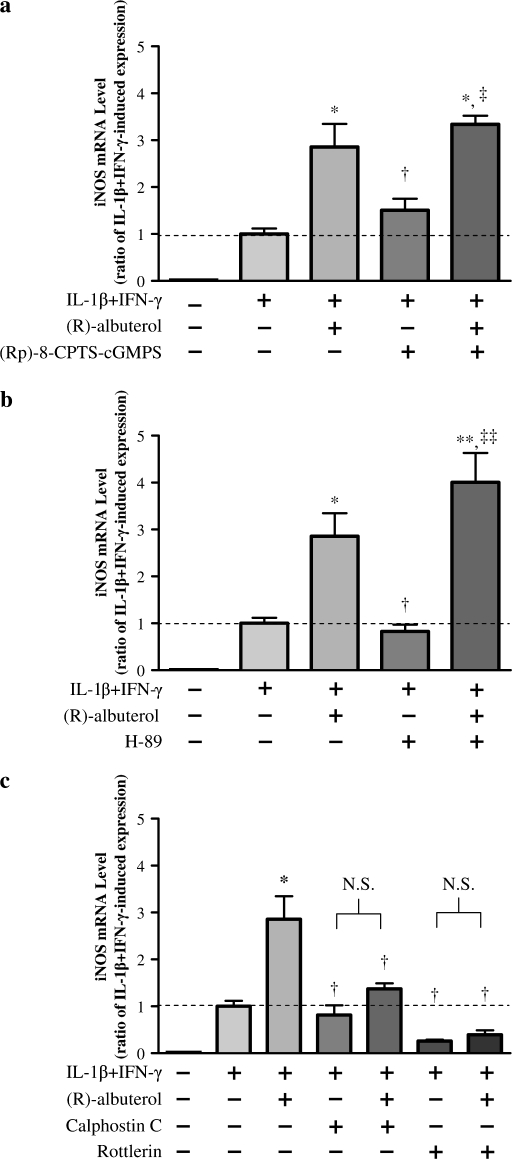
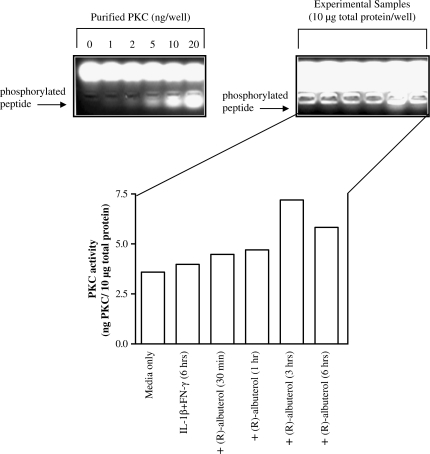
To determine PK involvement in (R)-albuterol upregulation of iNOS message, chemical inhibitors of PKA, PKC, PKCδ, and PKG (H-89, calphostin C, rottlerin, and [Rp]-8-CPTS-cGMPS, respectively) were used (PKA, PKC, and PKG activity have previously been shown to induce iNOS transcription) (25–27). The results indicated that PKA and PKG inhibition did not block (R)-albuterol–mediated augmentation of iNOS message in cytokine-stimulated NHBE cells (Figures 7a and 7b). The PKC inhibitor calphostin C (500 nM), however, did attenuate the (R)-albuterol–mediated increase in iNOS expression. In addition, PKCδ inhibition with rottlerin (3 μM) inhibited (R)-albuterol augmentation of iNOS message, and partially inhibited IL-1β + IFN-γ induction of iNOS message (Figure 7c). To further implicate PKC involvement in this signaling pathway, PKC activity was assessed. As shown in Figure 8, 10−5 M (R)-albuterol augmented PKC activity in NHBE cells. Maximal PKC activity was obtained when cells were incubated with IL-1β + IFN-γ for 3 h, and then 10−5 M (R)-albuterol added for another 3 h (of note, longer exposures to [R]-albuterol resulted in decreased PKC activity, possibly due to proteolytic regulatory mechanisms).
Figure 7.
(R)-albuterol–mediated augmentation of iNOS message involves protein kinase (PK) C, but not PKA or PKG. NHBE cells were exposed to 10 ng/ml each of IL-1β and IFN-γ for 12 h, together with either the PKG inhibitor (Rp)-8-pCPT-cGMPS (1 μM), the PKA inhibitor H-89 (2 μM), the PKC inhibitor calphostin C (500 nM), or the PKCδ inhibitor rottlerin (3 μM), in the presence or absence of 10−5 M (R)-albuterol. iNOS message, normalized to β-actin message, was assayed by TaqMan real-time RT-PCR after total RNA isolation. Inhibition of PKG (a) or PKA (b) did not affect (R)-albuterol–mediated iNOS message augmentation in cytokine-stimulated cells. (c) The PKC inhibitor calphostin C attenuated (R)-albuterol–mediated augmentation of iNOS message, suggesting PKC involvement in the response. Inhibition of PKCδ with rottlerin negated both the (R)-albuterol–mediated increase in iNOS transcription and also IL-1β + IFN-γ–induced iNOS transcription. There was no detectable constitutive iNOS expression in these cells. Dashed line represents IL-1β + IFN-γ–induced iNOS expression. Data are presented as mean ± SEM (n = 3–7). * Significantly greater than cytokine-induced iNOS expression (P < 0.01, ** P < 0.001); ‡ significantly greater than inhibitor treated control (P < 0.01, ‡‡ = P < 0.001); † significantly lower than cytokine + (R)-albuterol–treated iNOS expression (P < 0.05). N.S., no significant difference (P > 0.05).
Figure 8.
PKC activity in NHBE cells is increased by (R)-albuterol. Cells were stimulated with 10 ng/ml each of IL-1β and IFN-γ for 6 h. (R)-albuterol (10−5 M) was added to cytokine-stimulated cultures at the indicated time points, at which time cells were lysed and immediately assayed for PKC activity. (R)-albuterol appeared to augment PKC activity maximally at 3 h exposure, followed by a diminution of the effect. Band intensity reflects phosphorylation activity by PKC, which was quantified by imaging software (UVP, Ltd., Upland, CA). Values were extrapolated from a linear standard curve based on a series dilution of purified PKC supplied with the assay kit (gel on left). Data are representative of three individual experiments.
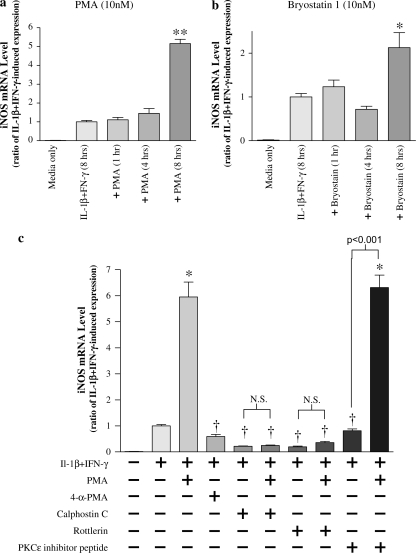
To demonstrate that PKC activation can increase iNOS message, specific chemical activators were added to cytokine-stimulated NHBE cells. As illustrated in Figure 9a, activation of PKC with either phorbol 12-myristate 13-acetate (PMA), or with the specific PKCδ/ε activator bryostatin 1 augmented iNOS message over cytokine-induced levels at 8 h. Further evidence of PKCδ involvement in mediation of iNOS message is illustrated in Figure 9b: both IL-1β + IFN-γ– and PMA-induced iNOS expression were completely blocked with PKCδ inhibition (rottlerin), whereas inhibition of PKCε had no effect.
Figure 9.
iNOS gene expression is reversibly enhanced by PKC activators. (a) NHBE cells were stimulated with 10 ng/ml each of IL-1β and IFN-γ for 8 h. The general PKC activator phorbol 12-myristate 13-acetate (PMA, 10 nM), or the specific PKCδ/ε activator bryostatin 1 (10 nM), were added for 1, 4, and 8 h, at which times iNOS message levels also were measured. Both PMA and bryostatin 1 augmented iNOS message at 8 h. Data are presented as mean ± SEM (n = 6). * Significantly more than cytokine-induced iNOS expression (P < 0.01, ** = P < 0.001). (b) PMA (10 nM) was added to NHBE cells for 8 h in the presence of either control medium, calphostin C (500 nM), rottlerin (3 μM), or PKCε translocation inhibitor peptide (300 μM), and iNOS message was assessed. In selected wells, the PMA negative control, 4α-PMA (10 nM), was added in place of PMA. The increase in iNOS message in response to PMA was attenuated by either calphostin C or rottlerin, suggesting that PKCδ was involved. Neither the PKCε translocation inhibitor nor 4α-PMA affected iNOS expression. PKC activation alone with bryostatin 1 (10 nM) in the absence of IL-1β + IFN-γ did not affect iNOS expression (data not shown). Data are presented as mean ± SEM (n = 4–8). * Significantly greater than cytokine-induced iNOS expression (P < 0.001); † significantly lower than cytokine + PMA–induced iNOS expression (P < 0.001). N.S., no significant difference (P > 0.05).
DISCUSSION
Nebulized administration of β2-agonists is a common treatment for bronchoconstriction associated with asthma. Activation of β2 receptors by both long- and short-acting agonists has been linked to antiinflammatory actions in various lung cells. For example, β2-adrenergic agonists suppress GM-CSF production in macrophages, smooth muscle cells, and epithelial cell lines (7, 9, 28–30). Modulation of GM-CSF is particularly interesting because the epithelium has been suggested as a major source of GM-CSF production, especially in asthmatic airways (31), and GM-CSF levels in bronchoalveolar lavage fluid of individuals with asthma correlate with eosinophilic infiltration and disease severity (32). Mechanisms whereby GM-CSF production is regulated in airway epithelial cells have not been elucidated. The results described here suggest the existence of a novel signaling pathway involving iNOS (and its product, NO) in this mechanism, and suggest possible target(s) by which β2-agonists may elicit their suppressive effects on GM-CSF production and release in airway epithelium.
The short-acting β2-adrenergic agonist (R)-albuterol is commonly administered to patients in racemic form, containing equal parts of its enantiomers, (R)- and (S)-albuterol. In vitro binding experiments have demonstrated that (R)-albuterol is a potent ligand for the β2-adrenergic receptor, exhibiting over 100-fold greater affinity than that of (S)-albuterol (33). Based on this, many of the beneficial effects of racemic albuterol have been attributed to the “active” enantiomer, (R)-albuterol, discounting the (S)-enantiomer, which has been considered largely pharmacologically inactive. However, several recent reports suggest that (S)-albuterol may have deleterious effects, counteracting many of the therapeutic effects of (R)-albuterol when the racemic mixture is administered to patients (34–36). A recent study in cultured airway smooth muscle cells showed that (S)-albuterol could partially counteract the suppression of GM-CSF production mediated by the corticosteroid, dexamethasone, whereas (R)-albuterol elicited additional suppression (37). In our studies with bronchial epithelial cells, (S)-albuterol did not significantly affect the increase in GM-CSF expression provoked by exposure of the cells to cytokines. In addition, suppression of GM-CSF protein release by racemic albuterol was similar to the effect of (R)-albuterol alone. At shorter time points, however, 10−5 M (S)-albuterol appears to downregulate iNOS message and subsequent cGMP production in NHBE cells (24, 38) suggesting that this enantiomer is pharmacologically active in airway epithelial cells.
In vivo, iNOS is expressed in bronchial epithelium of normal adults (10). This constitutive expression appears to be lost when these cells are placed in tissue culture (10, 13), but iNOS expression can be “recovered” with exogenous application of cytomix (TNF-α, IL-1β, and IFN-γ [39]). In the present study, the combination of 10 ng/ml IL-1β and IFN-γ elicited comparable (or even larger) levels of iNOS expression in NHBE cells after 12-h exposure compared with total cytomix, which includes TNF-α. These data agree somewhat with findings reported by Kwon and colleagues (40), in which substantial synergy between IL-1β and IFN-γ in iNOS monomer production was observed in the A549 alveolar epithelial cell line. The root of this synergy may stem from co-activation by IL-1β and IFN-γ of two separate transcription factors that promote iNOS expression, NF-κB and interferon regulatory factor-1 (IRF-1), respectively (41, 42). However, in contrast to the study by Kwon and coworkers, these specific cytokines did not elicit significant iNOS message expression in NHBE cells when administered singly, nor was there any synergistic effect with addition of TNF-α. This may simply be due to differences between immortalized, transformed, or cancer-derived cell lines and the primary cell cultures used in our studies. In addition, the cytokine concentrations used in our study were ∼ 10-fold less than those used with A549 cells, which may “dilute” a potential effect of TNF-α on iNOS expression in these cells.
Nitric oxide and its inducible synthase have previously been reported to play a role in GM-CSF expression in human bronchial epithelial cells. Sanders and colleagues (20) elegantly demonstrated that administration of exogenous NO (using the chemical NO donor, NONOate) reduced GM-CSF message and protein levels that were enhanced in human rhinovirus–infected BEAS-2B bronchial epithelial cells via a transcriptionally regulated event. In addition, we have previously demonstrated that GM-CSF expression is increased in NHBE cells in which iNOS is chemically inhibited, suggesting that iNOS plays a role in suppression of GM-CSF expression (38). In the studies reported here, the kinetics of the GM-CSF response of NHBE cells to cytokine stimulation (Figure 1b) are consistent with a role for one or more autocrine or paracrine factors from airway epithelial cells that could have acted subsequently to affect GM-CSF production. For example, airway epithelial cells can produce an array of eicosanoids that have been shown to affect expression of GM-CSF, such as PGE2 (43, 44).
In light of findings of previous studies demonstrating that β-adrenergic agonists increase iNOS activity and NO production in airway epithelium (18, 19, 45), it was hypothesized that β2-adrenergics may elicit suppressive effects on GM-CSF expression through an iNOS-dependent pathway. We found that (R)-albuterol increased iNOS message levels in IL-1β + IFN-γ–stimulated NHBE cells. We further explored this regulation by investigating the potential roles of specific protein kinases shown in other studies to regulate iNOS. Cyclic AMP–dependent PKA is known to modulate many effects mediated by the β2-adrenergic receptor, but PKA did not appear to be involved in GM-CSF modulation by albuterol, as treatment with the PKA inhibitor isoquinolinesulfoamide (H-89) did not prevent (R)-albuterol–mediated upregulation of iNOS. Previous studies with rottlerin, calphostin C, and most of the inhibitors/agonists used in these experiments have indicated that they do affect their “target” molecules (21, 46). However, there is always the possibility that any of these pharmacologic agents can also affect other molecules and pathways that may not be apparent. For example, the specificity of isoquinolinesulfonamides has recently been questioned, and these compounds may possibly influence actions of other enzymes. Although a relatively low concentration of H-89 was used in this study, the possibility that PKA may still be involved in β2-adrenergic receptor–mediated augmentation of iNOS expression in NHBE cells cannot entirely be ruled out (28, 47).
Our results do suggest, however, that PKC, in particular the δ isoform, seems to be involved in (R)-albuterol–mediated iNOS expression. Rottlerin is a compound that specifically inhibits PKCδ and θ, but, because NHBE cells do not contain the PKCθ isoform (46), it serves as a specific inhibitor of PKCδ in these cells. In our studies, rottlerin not only prevented (R)-albuterol (and PMA) upregulation of iNOS, but also attenuated full induction of iNOS by IL-1β + IFN-γ. This finding is supported by results of a study by Carpenter and colleagues that demonstrated that IL-1β activation of PKCδ stabilized iNOS message in pancreatic β-cells (48). In our studies, (R)-albuterol augmented PKC activity initially, but the activity of PKC began to decrease after 6-h exposure. This was not surprising, as prolonged PKC activation can lead to downregulation through proteolytic cleavage, especially for PKC isozymes that bind biologically active phorbol esters (for review see Ref. 49). Increased PKC activity with exposure to PMA did increase iNOS expression, implicating involvement of these PKC isozymes. The PKCδ activator bryostatin 1 also increased iNOS expression in a similar manner. Overall, the results suggest that PKCδ plays an important role in this pathway.
iNOS message silencing, involving transfection of double-stranded iNOS siRNA into primary NHBE cells, was used to investigate involvement of iNOS in GM-CSF suppression. Although we show that iNOS silencing significantly negated (R)-albuterol–induced suppression of GM-CSF message, alteration of GM-CSF protein release was not as dramatic (although still statistically significant). There are several explanations for this discrepancy. First, siRNA transfection into primary cultures is exceedingly difficult, which is why most silencing studies involve established cell lines that better tolerate the invasive reagents used to transfect siRNA or plasmid constructs. Primary cultures are much more sensitive to their environmental surroundings, so a fine balance between the amount of transfection reagent that does not deleteriously alter normal cell function and the largest achievable message knockdown must be established. It was found that only an average of ∼ 60% knockdown of iNOS message could be realized without transfection procedures being toxic to the cell or without altering baseline gene expression of our measured targets. Thus, there still existed a significant level of iNOS activity in “silenced” NHBE cells that could affect iNOS-mediated alteration of the measured endpoints. Thus, alteration of (R)-albuterol–induced suppression of GM-CSF protein release with iNOS silencing could have been somewhat underestimated. Second, there are likely multiple signaling pathways involved in β2-adrenergic–mediated suppression of GM-CSF, including both transcriptional and post-transcriptional mechanisms. There is indeed evidence for post-transcriptional regulation of GM-CSF protein release in A549 cells in response to IL-1β stimulation (50), so this is a potential possibility in NHBE cells.
In summary, the results of this study indicate that β2-adrenergic receptor activation by the short-acting agonist (R)-albuterol can effectively suppress GM-CSF expression and protein release in cytokine-stimulated NHBE cells in primary culture. In addition, we show, for the first time, that this potential antiinflammatory activity is mediated through iNOS, where its expression relies on the activity of the PKCδ isoform. (R)-albuterol–mediated augmentation of iNOS expression also appears to be independent of PKA. Interestingly, GM-CSF production has been positively correlated with eosinophilic influx and function in vivo (51), and patients with asthma who take β-agonists do have reduced numbers of activated eosinophils in serum and bronchial mucosa (52). A possible beneficial antiinflammatory effect, then, of β2-adrenergic receptor agonists could be to downregulate epithelial production of GM-CSF via a pathway dependent on the function of iNOS.
Supplementary Material
Acknowledgments
This work is in partial fulfillment of the requirements for the degree of Doctor of Philosophy in Comparative Biomedical Sciences (B.N.C.). The authors thank Nancy Akley for her excellent technical assistance.
This work was supported by grant R37 HL36982 from the National Institutes of Health (K.B.A) and a grant from Sepracor, Inc., Marlborough, MA.
This article has an online supplement, which is accessible from this issue's table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1165/rcmb.2005-0338OC on September 29, 2005
Conflict of Interest Statement: B.N.C. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. Y.L. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. S.F. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. J.-A.P. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. K.B.A. received $68,000 in 2004 as a research grant from AstraZeneca and ∼ $40,000 in research grants from Sepracor since 2002. K.B.A. serves on the scientific advisory board of Sepracor and received $2,500 for this service in 2004, and served as a consultant to Immunogen and received $1,500 in 2004. K.B.A. holds 150,000 founders shares of a start-up biotech company, BioMarck.
References
- 1.Yanaura S, Imamura N, Misawa M. Effects of beta-adrenoceptor stimulants on the canine tracheal ciliated cells. Jpn J Pharmacol 1981;31:951–956. [DOI] [PubMed] [Google Scholar]
- 2.Frohock JI, Wijkstrom-Frei C, Salathe M. Effects of albuterol enantiomers on ciliary beat frequency in ovine tracheal epithelial cells. J Appl Physiol 2002;92:2396–2402. [DOI] [PubMed] [Google Scholar]
- 3.Knowles M, Murray G, Shallal J, Askin F, Ranga V, Gatzy J, Boucher R. Bioelectric properties and ion flow across excised human bronchi. J Appl Physiol 1984;56:868–877. [DOI] [PubMed] [Google Scholar]
- 4.Spurzem JR, Gupta J, Veys T, Kneifl KR, Rennard SI, Wyatt TA. Activation of protein kinase A accelerates bovine bronchial epithelial cell migration. Am J Physiol Lung Cell Mol Physiol 2002;282:L1108–1116. [DOI] [PubMed] [Google Scholar]
- 5.Farmer P, Pugin J. Beta-adrenergic agonists exert their “anti-inflammatory” effects in monocytic cells through the IkappaB/NF-kappaB pathway. Am J Physiol Lung Cell Mol Physiol 2000;279:L675–L682. [DOI] [PubMed] [Google Scholar]
- 6.Reid DW, Ward C, Wang N, Zheng L, Bish R, Orsida B, Walters EH. Possible anti-inflammatory effect of salmeterol against interleukin-8 and neutrophil activation in asthma in vivo. Eur Respir J 2003;21:994–999. [DOI] [PubMed] [Google Scholar]
- 7.Hallsworth MP, Twort CH, Lee TH, Hirst SJ. beta(2)-adrenoceptor agonists inhibit release of eosinophil-activating cytokines from human airway smooth muscle cells. Br J Pharmacol 2001;132:729–741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Oddera S, Silvestri M, Lantero S, Sacco O, Rossi GA. Downregulation of the expression of intercellular adhesion molecule (ICAM)-1 on bronchial epithelial cells by fenoterol, a beta2-adrenoceptor agonist. J Asthma 1998;35:401–408. [DOI] [PubMed] [Google Scholar]
- 9.Korn SH, Jerre A, Brattsand R. Effects of formoterol and budesonide on GM-CSF and IL-8 secretion by triggered human bronchial epithelial cells. Eur Respir J 2001;17:1070–1077. [DOI] [PubMed] [Google Scholar]
- 10.Guo FH, Erzurum SC. Characterization of inducible nitric oxide synthase expression in human airway epithelium. Environ Health Perspect 1998;106:1119–1124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liu HW, Anand A, Bloch K, Christiani D, Kradin R. Expression of inducible nitric oxide synthase by macrophages in rat lung. Am J Respir Crit Care Med 1997;156:223–228. [DOI] [PubMed] [Google Scholar]
- 12.Robbins RA, Springall DR, Warren JB, Kwon OJ, Buttery LD, Wilson AJ, Adcock IM, Riveros-Moreno V, Moncada S, Polak J, et al. Inducible nitric oxide synthase is increased in murine lung epithelial cells by cytokine stimulation. Biochem Biophys Res Commun 1994;198:835–843. [DOI] [PubMed] [Google Scholar]
- 13.Guo FH, Uetani K, Haque SJ, Williams BR, Dweik RA, Thunnissen FB, Calhoun W, Erzurum SC. Interferon gamma and interleukin 4 stimulate prolonged expression of inducible nitric oxide synthase in human airway epithelium through synthesis of soluble mediators. J Clin Invest 1997;100:829–838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Warner RL, Paine R III, Christensen PJ, Marletta MA, Richards MK, Wilcoxen SE, Ward PA. Lung sources and cytokine requirements for in vivo expression of inducible nitric oxide synthase. Am J Respir Cell Mol Biol 1995;12:649–661. [DOI] [PubMed] [Google Scholar]
- 15.Kenyon NJ, Gohil K, Last JA. Susceptibility to ovalbumin-induced airway inflammation and fibrosis in inducible nitric oxide synthetase–deficient mice: mechanisms and consequences. Toxicol Appl Pharmacol 2003; 191:2–11. [DOI] [PubMed] [Google Scholar]
- 16.Saleh D, Ernst P, Lim S, Barnes PJ, Giaid A. Increased formation of the potent oxidant peroxynitrite in the airways of asthmatic patients is associated with induction of nitric oxide synthase: effect of inhaled glucocorticoid. FASEB J 1998;12:929–937. [PubMed] [Google Scholar]
- 17.Muijsers RB, van der Veeken A, Habernickel J, Folkerts G, Postma DS, Nijkamp FP. Intra-luminal exposure of murine airways to peroxynitrite causes inflammation but not hyperresponsiveness. Inflamm Res 2002; 51:33–37. [DOI] [PubMed] [Google Scholar]
- 18.Eliseeva EV, Romanova NA, Maistrovskaya YV. Regulation of NO-producing function of the lungs with salmeterol. Bull Exp Biol Med 2001;132:709–712. [DOI] [PubMed] [Google Scholar]
- 19.Tamaoki J, Kondo M, Takemura H, Chiyotani A, Yamawaki I, Konno K. Cyclic adenosine monophosphate–mediated release of nitric oxide from canine cultured tracheal epithelium. Am J Respir Crit Care Med 1995;152:1325–1330. [DOI] [PubMed] [Google Scholar]
- 20.Sanders SP, Kim J, Connolly KR, Porter JD, Siekierski ES, Proud D. Nitric oxide inhibits rhinovirus-induced granulocyte macrophage colony–stimulating factor production in bronchial epithelial cells. Am J Respir Cell Mol Biol 2001;24:317–325. [DOI] [PubMed] [Google Scholar]
- 21.Krunkosky TM, Fischer BM, Martin LD, Jones N, Akley NJ, Adler KB. Effects of TNF-α on expression of ICAM-1 in human airway epithelial cells in vitro: signaling pathways controlling surface and gene expression. Am J Respir Cell Mol Biol 2000;22:685–692. [DOI] [PubMed] [Google Scholar]
- 22.Heid CA, Stevens J, Livak KJ, Williams PM. Real time quantitative PCR. Genome Res 1996;6:986–994. [DOI] [PubMed] [Google Scholar]
- 23.Peirson SN, Butler JN, Foster RG. Experimental validation of novel and conventional approaches to quantitative real-time PCR data analysis. Nucleic Acids Res 2003;31:e73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chorley BN, Martin LD, Crews AR, Li Y, Adler KB. Differential effects of albuterol isomers on normal human bronchial epithelial cells in vitro. Am J Respir Crit Care Med 2003;167:A205. (Abstr.) [Google Scholar]
- 25.Choi SH, Shin KH, Kang SW, Chun YS, Chun BG. Guanosine 5′,3′-cyclic monophosphate enhances lipopolysaccharide-induced nitric oxide synthase expression in mixed glial cell cultures of rat. Neurosci Lett 1999;276:29–32. [DOI] [PubMed] [Google Scholar]
- 26.Dobashi K, Asayama K, Shirahata A. Differential effects of cyclic AMP on induction of nitric oxide synthase in 3T3–L1 cells and brown adipocytes. Free Radic Biol Med 2003;35:94–101. [DOI] [PubMed] [Google Scholar]
- 27.Banan A, Farhadi A, Fields JZ, Zhang LJ, Shaikh M, Keshavarzian A. The delta-isoform of protein kinase C causes inducible nitric-oxide synthase and nitric oxide up-regulation: key mechanism for oxidant-induced carbonylation, nitration, and disassembly of the microtubule cytoskeleton and hyperpermeability of barrier of intestinal epithelia. J Pharmacol Exp Ther 2003;305:482–494. [DOI] [PubMed] [Google Scholar]
- 28.Meja KK, Catley MC, Cambridge LM, Barnes PJ, Lum H, Newton R, Giembycz MA. Adenovirus-mediated delivery and expression of a cAMP-dependent protein kinase inhibitor gene to BEAS-2B epithelial cells abolishes the anti-inflammatory effects of rolipram, salbutamol, and prostaglandin E2: a comparison with H-89. J Pharmacol Exp Ther 2004;309:833–844. [DOI] [PubMed] [Google Scholar]
- 29.Johnson M. Effects of beta2-agonists on resident and infiltrating inflammatory cells. J Allergy Clin Immunol 2002;110:S282–S290. [DOI] [PubMed] [Google Scholar]
- 30.Butchers PR, Vardey CJ, Johnson M. Salmeterol: a potent and long-acting inhibitor of inflammatory mediator release from human lung. Br J Pharmacol 1991;104:672–676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Park CS, Choi YS, Ki SY, Moon SH, Jeong SW, Uh ST, Kim YH. Granulocyte macrophage colony-stimulating factor is the main cytokine enhancing survival of eosinophils in asthmatic airways. Eur Respir J 1998;12:872–878. [DOI] [PubMed] [Google Scholar]
- 32.Woolley KL, Adelroth E, Woolley MJ, Ellis R, Jordana M, O'Byrne PM. Effects of allergen challenge on eosinophils, eosinophil cationic protein, and granulocyte-macrophage colony-stimulating factor in mild asthma. Am J Respir Crit Care Med 1995;151:1915–1924. [DOI] [PubMed] [Google Scholar]
- 33.Penn RB, Frielle T, McCullough JR, Aberg G, Benovic JL. Comparison of R-, S-, and RS-albuterol interaction with human beta 1- and beta 2-adrenergic receptors. Clin Rev Allergy Immunol 1996;14:37–45. [DOI] [PubMed] [Google Scholar]
- 34.Templeton AG, Chapman ID, Chilvers ER, Morley J, Handley DA. Effects of S-salbutamol on human isolated bronchus. Pulm Pharmacol Ther 1998;11:1–6. [DOI] [PubMed] [Google Scholar]
- 35.Cho SH, Hartleroad JY, Oh CK. (S)-albuterol increases the production of histamine and IL-4 in mast cells. Int Arch Allergy Immunol 2001;124:478–484. [DOI] [PubMed] [Google Scholar]
- 36.Baramki D, Koester J, Anderson AJ, Borish L. Modulation of T-cell function by (R)- and (S)-isomers of albuterol: anti-inflammatory influences of (R)-isomers are negated in the presence of the (S)-isomer. J Allergy Clin Immunol 2002;109:449–454. [DOI] [PubMed] [Google Scholar]
- 37.Ameredes BT, Gligonic WJ, Calhoun WJ. Enantiomers of beta-agonists exhibit differential effects in combination with dexamethasone on GM-CSF production in human airway smooth muscle cells. J Allergy Clin Immunol 2004;113:S190–S191. [Google Scholar]
- 38.Chorley BN, Adler KB. Inducible nitric oxide synthase/protein kinase G signaling pathway suppresses granulocyte macrophage colony stimulating factor transcription in normal human bronchial epithelial cells. Am J Respir Crit Care Med 2004;169:A241. (Abstr.) [Google Scholar]
- 39.Robbins RA, Barnes PJ, Springall DR, Warren JB, Kwon OJ, Buttery LD, Wilson AJ, Geller DA, Polak JM. Expression of inducible nitric oxide in human lung epithelial cells. Biochem Biophys Res Commun 1994;203:209–218. [DOI] [PubMed] [Google Scholar]
- 40.Kwon S, Newcomb RL, George SC. Mechanisms of synergistic cytokine-induced nitric oxide production in human alveolar epithelial cells. Nitric Oxide 2001;5:534–546. [DOI] [PubMed] [Google Scholar]
- 41.Xie QW, Kashiwabara Y, Nathan C. Role of transcription factor NF-kappa B/Rel in induction of nitric oxide synthase. J Biol Chem 1994; 269:4705–4708. [PubMed] [Google Scholar]
- 42.Taylor BS, de Vera ME, Ganster RW, Wang Q, Shapiro RA, Morris SM Jr, Billiar TR, Geller DA. Multiple NF-kappaB enhancer elements regulate cytokine induction of the human inducible nitric oxide synthase gene. J Biol Chem 1998;273:15148–15156. [DOI] [PubMed] [Google Scholar]
- 43.Daffern PJ, Jagels MA, Saad JJ, Fischer W, Hugli TE. Upper airway epithelial cells support eosinophil survival in vitro through production of GM-CSF and prostaglandin E2: regulation by glucocorticoids and TNF-alpha. Allergy Asthma Proc 1999;20:243–253. [DOI] [PubMed] [Google Scholar]
- 44.Clarke DL, Belvisi MG, Catley MC, Yacoub MH, Newton R, Giembycz MA. Identification in human airway smooth muscle cells of the prostanoid receptor and signalling pathway through which PGE2 inhibits the release of GM-CSF. Br J Pharmacol 2004;141:1141–1150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jain B, Rubinstein I, Robbins RA, Leise KL, Sisson JH. Modulation of airway epithelial cell ciliary beat frequency by nitric oxide. Biochem Biophys Res Commun 1993;191:83–88. [DOI] [PubMed] [Google Scholar]
- 46.Park J-A, He F, Martin LD, Li Y, Adler KB. Human neutrophil elastase induces hypersecretion of mucin from human bronchial epithelial cells in vitro via a PKCδ-mediated mechanism. Am J Pathol 2005;167:651–661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Davies SP, Reddy H, Caivano M, Cohen P. Specificity and mechanism of action of some commonly used protein kinase inhibitors. Biochem J 2000;351:95–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Carpenter L, Cordery D, Biden TJ. Protein kinase Cdelta activation by interleukin-1beta stabilizes inducible nitric-oxide synthase mRNA in pancreatic beta-cells. J Biol Chem 2001;276:5368–5374. [DOI] [PubMed] [Google Scholar]
- 49.Liu WS, Heckman CA. The sevenfold way of PKC regulation. Cell Signal 1998;10:529–542. [DOI] [PubMed] [Google Scholar]
- 50.Bergmann M, Barnes PJ, Newton R. Molecular regulation of granulocyte macrophage colony–stimulating factor in human lung epithelial cells by interleukin (IL)-1β, IL-4, and IL-13 involves both transcriptional and post-transcriptional mechanisms. Am J Respir Cell Mol Biol 2000; 22:582–589. [DOI] [PubMed] [Google Scholar]
- 51.Woolley KL, Adelroth E, Woolley MJ, Ellis R, Jordana M, O'Byrne PM. Granulocyte-macrophage colony–stimulating factor, eosinophils and eosinophil cationic protein in subjects with and without mild, stable, atopic asthma. Eur Respir J 1994;7:1576–1584. [DOI] [PubMed] [Google Scholar]
- 52.Pedersen B, Dahl R, Venge P. The effect of salmeterol on the early and late phase reaction to bronchial hyperreactivity, blood eosinophils, and serum eosinophil cationic protein. Am Rev Respir Dis 1991;143:A649. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.