Abstract
Previous studies have demonstrated a robust pulmonary expression of hypoxia-induced mitogenic factor (HIMF) during the perinatal period, when surfactant protein (SP) synthesis begins. We hypothesized that HIMF modulates SP expression and participates in lung development and maturation. The temporal-spatial expression of HIMF, SP-B, and SP-C in developing mouse lungs was examined by immunohistochemical staining, Western blot, and RT-PCR. The expression and localization of SP-B and SP-C were investigated in mouse lungs after intratracheal instillation of HIMF in adult mice. The effects of HIMF on SP-B and SP-C transcription activity, and on mRNA degradation, were investigated in mouse lung epithelial (MLE)-12 and C10 cells using the promoter-luciferase reporter assay and actinomycin D incubation. The activation of Akt, extracellular signal-regulated kinase (ERK)1/2, and p38 mitogen-activated protein kinase was explored by Western blot. Intratracheal instillation of HIMF resulted in significant increases of SP-B and SP-C production, predominantly localized to alveolar type II cells. In MLE-12 and C10 cells, HIMF enhanced SP-B and SP-C mRNA levels in a dose-dependent manner. Meanwhile, HIMF increased transcription activity and prevented actinomycin D–facilitated SP-B and SP-C mRNA degradation in MLE-12 cells. Incubation of cells with LY294002, PD098059, or U0126 abolished HIMF-induced Akt and ERK1/2 phosphorylation and suppressed HIMF-induced SP-B and SP-C production, whereas SB203580 had no effect. These results indicate that HIMF induces SP-B and SP-C production in mouse lungs and alveolar type II–like cell lines via activations of phosphatidylinositol 3-kinase/Akt and ERK1/2 mitogen-activated protein kinase, suggesting that HIMF plays critical roles in lung development and maturation.
Keywords: gene expression, lung development, lung epithelial cells, mRNA stability, signal transduction
Surfactant is mainly synthesized by alveolar type II (ATII) cells (1). In recent years, there have been major advances in our knowledge concerning the differentiation of developing lungs and expression of the genes encoding lung surfactant proteins (SP). There are four surfactant-specific proteins: SP-B and SP-C are small and hydrophobic, whereas SP-A and SP-D are large, hydrophilic, and participate in the innate immune process (2). Inherited deficiency of SP-B in infants leads to congenital alveolar proteinosis and fatal respiratory failure (3). Targeted disruption of SP-B causes respiratory failure in newborn mice (4). A lack of SP-C is associated with respiratory distress syndrome (5, 6). Regulation of SP gene expression is multifactorial, involving glucocorticoid hormones (8), retinoic acid (9), fibroblast growth factor (FGF)-2 (10), transforming growth factor–β (11), nitric oxide, and keratinocyte growth factor (12).
In a mouse model of hypoxia-induced pulmonary hypertension, we previously found a highly upregulated gene that we named hypoxia-induced mitogenic factor (HIMF) (13). The amino acid sequence of HIMF is identical to that of the protein found in inflammatory zone 1 (FIZZ1), identified in a mouse lung inflammation model (14), and the protein, resistin-like molecule (RELM)-α, in adipose tissue (15). Studies from our laboratory have demonstrated that HIMF has mitogenic, angiogenic, and vasoconstrictive effects (13). In the developing lung, it has been shown that HIMF is highly expressed in the perinatal period, and possesses an antiapoptotic function in cultured embryonic lungs (16). Intratracheal instillation of recombinant HIMF protein induces widespread cell proliferation in the lung, including airway epithelial cells, ATII cells, and vascular endothelial cells (17). These findings indicate that HIMF may be a lung-specific growth factor participating in lung development. We hypothesize that HIMF may participate in the regulation of SP-B and SP-C expression and promote lung development and maturation. In this study, we investigated the roles of HIMF in SP-B and SP-C production in mouse lungs, and explored its signal transduction pathways in cultured mouse lung epithelial cells.
MATERIALS AND METHODS
Mouse Lung Samples
Embryonic, neonatal, and adult lungs were obtained and processed as previously reported (16). For the experiments on HIMF intratracheal instillation, male C57Bl/6 mice (Jackson Laboratories, Bar Harbor, ME) were killed by halothane overdose. All experiments followed the protocols approved by the Animal Care and Use Committee of Johns Hopkins University.
Immunohistochemical Staining for HIMF, SP-B, and SP-C
Lung samples were processed and immunostained as previously described (13, 17). Polyclonal anti–SP-B and anti–SP-C antibodies (1:1,000 dilutions; Santa Cruz Biotechnology, Inc., Santa Cruz, CA) were raised against human SP-B or SP-C and recognized active forms of mouse and rat SP-B and SP-C, respectively.
Western Blot for HIMF, SP-B, SP-C, and Glyceraldehyde Phosphate Dehydrogenase
Tissue collection, homogenization and protein electrophoresis were performed as described previously (16). For the HIMF expression assay of cultured cell lines, 40 μl of medium supernatant from each sample was subjected to 4–20% precast polyacrylamide gel (Bio-Rad, Hercules, CA) electrophoresis and transferred to nitrocellulose membranes (Bio-Rad). HIMF was detected with 1:1,000 dilution of the anti-HIMF antiserum. For SP-B, SP-C, and glyceraldehyde phosphate dehydrogenase (GAPDH) (Santa Cruz Biotechnology) detections, the primary antibody dilution was 1:500, 1:500, and 1:1,000, respectively, and were followed by 1:3,000 dilution of goat anti-rabbit horseradish peroxidase–labeled antibody (Bio-Rad). An ECL substrate kit (Amersham, Piscataway, NJ) was used for the chemiluminscent detection of the signals with autoradiography film (Amersham).
Semiquantitative RT-PCR for HIMF, SP-B, and SP-C
For the quantification of gene transcripts of HIMF, SP-B, and SP-C in the lung tissues and epithelial cells, total RNA was isolated with RNeasy Mini Kit (Qiagen Inc., Valencia, CA) as specified by the manufacturer. The reverse transcription reactions were conducted using Transcriptor First Strand cDNA Synthesis Kit (Roche, Indianapolis, IN). The PCR primers used for mouse HIMF 5′-ATGAAGACTACAACTTG TTCCC-3′ (positions 104–125 of the second exon) and 5′-TTAGGA CAGTTGGCAGCAGCG-3′ (positions 419–439 of the fourth exon), amplifying a 336–base-pair (bp) fragment; for mouse SP-B, 5′-CTAT CACGTC GGCCTCATC-3′ (positions 403–421 of second exon) and 5′-CTGGCTCTGGAAGTAG TCAAT-3′ (positions 705–725 of fourth exon), amplifying a 323-bp fragment; for SP-C, 5′-GCCTTCTCA TCGTG GTTGT-3′ and 5′-CCAGTATCATGCCCTTCCT-3′, amplifying a 388-bp fragment between positions 131 and 518; for mouse GAPDH, 5′-GCCAAGGTCATCCATGACAACTTTGG-3′ and 5′-GCCTGCTTCACCACCTTCTTGATGTC-3′, amplifying a 314-bp fragment between positions 532 and 845. The PCR conditions for SP-B, HIMF, and GAPDH were initial denaturation at 94°C for 3 min, and thereafter, 30 cycles of 94°C for 30 s, 55°C for 30 s, and 72°C for 1 min, followed by a 7-min extension at 72°C. For SP-C, 25 cycles of PCR amplification was conducted under the same conditions. PCR bands were separated on ethidium bromide–stained agarose gels and quantified by Phoretix 1 D software (Phoretix International Ltd., Newcastle-upon-Tyne, UK). GAPDH was used to normalize the initial variations in sample concentration, and served as a control for reaction efficiency. The ratio between the amplified DNA fragments and GAPDH of each sample RNA was determined.
Intratracheal Instillation of HIMF
Recombinant HIMF protein was produced and isolated as described previously (13). To examine potential effects of HIMF on SP production in vivo, HIMF protein or BSA (Sigma, St. Louis, MO) was intratracheally administered to adult mice (200 ng/animal in 40 μl saline) (17). The vehicle controls were instilled with saline (40 μl/animal). Mouse lungs were collected at a range of days of embryonic (E) and postnatal (P) growth (see Figure Legends), . The immunohistochemistry, Western blot, and RT-PCR for HIMF, SP-B, and SB-C were performed as described previously here.
Cell Culture and Stimulation with HIMF
Mouse lung epithelial (MLE)-12 cells (American Type Culture Collection [ATCC], Manassas, VA; CRL-2110) were grown on plastic tissue-culture dishes in RPMI 1640 medium (Gibco Laboratories, Grand Island, NY) containing 10% FBS (Life Technologies, Inc., Gaithersburg, MD), penicillin (100 U/ml), and streptomycin (100 μg/ml). C10, a nonmalignant murine ATII-like cell line (18), was a kind gift of Dr. Alvin M. Malkinson (University of Colorado at Denver and Health Science Center, Aurora, CO), and was grown in CRML-1066 medium (Gibco Laboratories) supplemented with 10% FBS and antibiotics. Confluent monolayers of MLE-12 and C10 were trypsinized, and 2 × 105 viable cells suspended in 2 ml of culture medium supplemented with 10% FBS were added to each well of a 6-well plate. When the wells reached 80–90% confluence, the cell culture medium was replaced by medium supplemented with 0.1% FBS and 2 mM L-glutamine. After 24 h, cells were pretreated with different signal transduction inhibitors for 1 h and stimulated with different concentrations of HIMF protein for the indicated periods, with or without incubation in actinomycin D (5 μg/ml; Sigma).
Stable Cell Lines for HIMF Overexpression
Mouse HIMF cDNA was amplified from mouse lung tissue and subcloned into pcDNA3.1/Zeo(+) (Invitrogen, Carlsbad, CA) to form pcDNA3.1-HIMF. The primers used for the HIMF cDNA amplification were sense 5′-CACCATGAAGACTACAACTTGTTCCC-3′ and antisense 5′-TTAGGACAGTTGGCAGCAGCG-3′. HIMF cDNA and its control vector were transfected into MLE-12 cells with Lipofectamine 2000 (Life Technologies). Stable cell lines, named MLE-HIMF and MLE-Zeo, were screened based on resistance to Zeocin (Invitrogen; 400 μg/ml), and HIMF overexpression was validated by both Western blot and RT-PCR analyses.
Dual-Luciferase Assays for SP-B and SP-C Promoter Activities
The mouse SP-B and SP-C promoter–reporter constructs, pBL-SPB-CAT and pGL-SPC-Luc, were kind gifts from Dr. Jeffrey A. Whitsett (Cincinnati Children's Hospital Medical Center, Ohio) (19, 20). With the pBL-SPB-CAT construct as a template, the 1,838-bp fragment of DNA, including 1,826 bp of mouse SP-B promoter, was generated with PCR using primers 5′-GCCTAGGTCGACGGTACCATGTCTATCCTGACCC-3′ and 5′-CGCTGCAAG CTTAGCCACTGCAGTAGGTGCGACT-3′. The PCR fragments were purified with Qiagen PCR purification kit (Qiagen), digested with SalI/HindIII and cloned into pGL2-Basic vector (Promega, Madison, WI) to give pGL-SPB-Luc luciferase reporter construct, which was validated with restriction enzyme digestion and sequencing. When wells reached 80–90% confluency, cells were cotransfected with either pGL-SPB-Luc or pGL-SPC-Luc, and pRL-TK (Promega). After being cultured for a variety of durations, cells were treated with passive lysis buffer, according to the dual-luciferase assay manual (Promega), and luciferase activity was measured with a luminometer (Lumat LB9507; Berthold Tech., Bad Wildbad, Germany). The firefly luciferase signal was normalized to the renilla luciferase signal for each individual well. All experiments were performed three times with triplicate in each treatment.
Phosphorylation Assay for Akt, Extracellular Signal-Regulated Kinase, and p38 Mitogen-Activated Protein Kinase
MLE-12 and C10 cells were cultured in each well of 6-well plates to 70–80% confluence with complete culture medium. The cell culture medium was changed to either RPMI 1640 or CRML-1066 supplemented with 0.1% FBS and 2 mM L-glutamine for 33 h, and then changed to serum-free medium for 2–3 h. Finally, different signal transduction inhibitors were added to the serum-free medium for an additional 1-h incubation before treatment with HIMF protein was begun. Cells were then washed once with ice-cold PBS and extracted with cell lysis buffer. Protein (50 μg) from each sample was subjected to 4–20% precast polyacrylamide gel (Bio-Rad) electrophoresis, transferred to nitrocellulose membranes (Bio-Rad), and probed with rabbit antibodies against phospho-specific and nonphosphorylated Akt, extracellular signal-regulated kinase (Erk) and p38 mitogen-activated protein kinase (MAPK) (Santa Cruz Biotechnology), followed by goat anti-rabbit horseradish peroxidase–labeled antibody (1:3000; Bio-Rad). ECL substrate kit (Amersham, Piscataway, NJ) was used for the chemiluminscent detection of the signals with autoradiography film (Amersham).
Statistical Analysis
Unless otherwise stated, all data were shown as mean ± SEM. Statistical significance (P < 0.05) was determined by t test or analysis of variance, followed by assessment of differences using SigmaStat 2.03 software (Jandel, Erkrath, Germany).
RESULTS
Temporal–Spatial Expression of HIMF, SP-B, and SP-Cin Developing Mouse Lungs
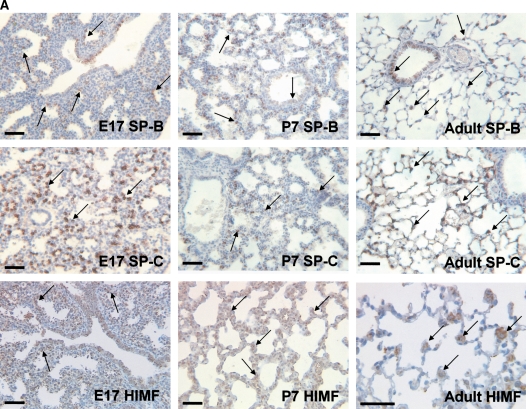
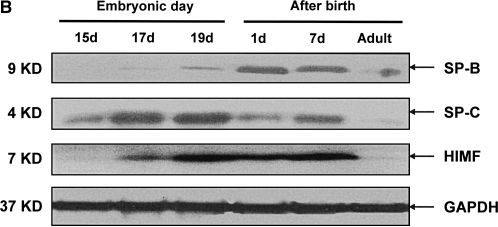
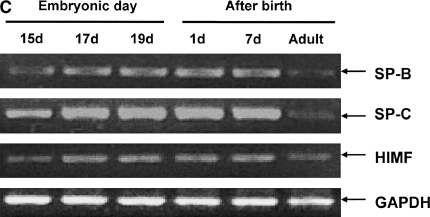
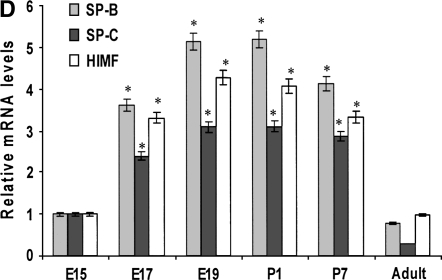
Our previous studies have indicated that a robust HIMF expression was found in mouse lungs at E16 through P30, a period during which SP start to be synthesized, and only a low-level expression of HIMF was observed at E14 and E15 (16). Because mouse pregnancy has a duration of 20 d, it is conceivable that HIMF upregulated in the last trimester of pregnancy and in the perinatal period indicates important roles of HIMF in embryonic and perinatal lung maturation. Because SP-B and SP-C also play important roles in the maintenance of lung function and participate in lung development and maturation, we hypothesize that HIMF expression may be correlated with that of SP-B and SP-C. To answer this question, the mouse embryonic and developing lungs at E15, E17, E19, P1, P7, and adult were harvested for immunohistochemical staining. As shown in Figure 1A, from E17 and thereafter, the intensity of SP-B staining was increased and restricted to the precursors of ATII cells in the distal lungs. It was also detectable in tracheal and bronchiolar epithelial cells. In adult lungs, SP-B was detected only in ATII cells, but not in the alveolar lumen. The expression pattern of SP-C was similar to that of SP-B, being detected at E17 and restricted to the cytoplasm of epithelial cells in distal lung buds. From E17 and thereafter, SP-C was restricted to ATII precursor cells, and the intensity of staining increased with advancing gestation. The increase in SP-B and SP-C is consistent with the finding that SP is required for adaptation to air breathing at birth, as demonstrated previously (21–23). Moreover, HIMF-positive signals were present in E17 airway epithelial cells and ATII cells; in P7 and adult lungs, HIMF is mainly in ATII cells. The results from Western blot (Figure 1B) and RT-PCR (Figures 1C and 1D) show that the temporal–spatial expression pattern of HIMF is closely correlated with that of SP-B and SP-C in developing mouse lungs, especially during the perinatal period, indicating the possibility of HIMF regulating the expression of these two SPs to participate in lung maturation.
Figure 1.
Temporal–spatial expression of hypoxia-induced mitogenic factor (HIMF), surfactant protein (SP)-B, and SP-C in developing mouse lungs. The lungs from C57Bl/6 mice at embryonic (E) Days 15, 17, 19, postnatal (P) Days 1 and 7, and adult were harvested (n = 3 for each time point). (A) Immunohistochemical staining revealed that a robust HIMF expression was found in mouse E17 and P7 lungs, whereas the intensity of SP-B staining increased from E17, and was restricted to alveolar type II (ATII) cells and bronchiolar epithelial cells. The expression pattern of SP-C was similar to that of SP-B, detectable at E17 and restricted to the cytoplasm of epithelial cells in distal lung buds. Arrows indicate positively stained cells for SP-B, SP-C, and HIMF. Scale bars: 60 μm. Western blot with proteins from lung homogenates (B) and RT-PCR from lungs at different developmental stages (C and D) indicated that HIMF expression is closely correlated with that of SP-B and SP-C in developing lungs, especially during perinatal period. * Significant increase from mouse E15 lungs (P < 0.05). Shown is one representative experiment of three, all with similar results.
HIMF-Enhanced SP-B and SP-C Expression in Mouse Lungs
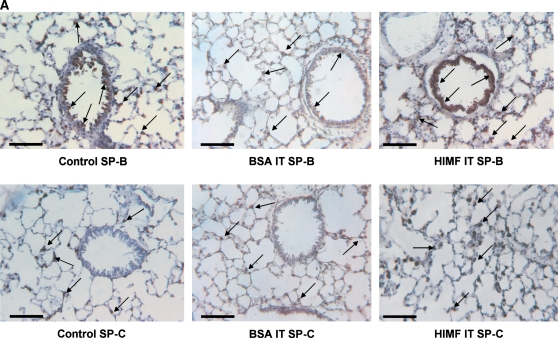
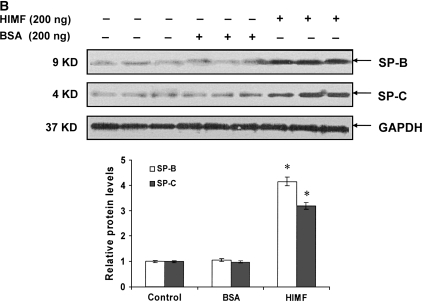
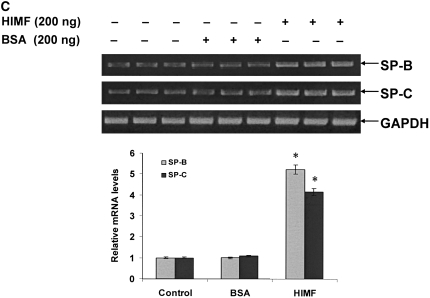
To examine the hypothesis that HIMF may regulate SP-B and SP-C expression, we intratracheally instilled HIMF protein into adult mouse lungs. As demonstrated by immunohistochemical staining (Figure 2A), HIMF protein instillation resulted in a significant increase in SP-B production, mainly localized to ATII and airway epithelial cells. Similarly, HIMF protein enhanced the SP-C expression of ATII cells. No significant increase of SP-B and SP-C expression was observed in control mouse lungs treated with either saline or BSA. This HIMF-specific induction of SP-B and SP-C in mouse lungs was confirmed by Western blot and RT-PCR (Figures 2B and 2C). Combined with the findings that SP-B and SP-C play important roles in the metabolism of surfactant and in lung maturation (22, 23), these results strongly suggest that HIMF may participate in lung function through modulating SP-B and SP-C production.
Figure 2.
HIMF specifically enhanced SP-B and SP-C expression in mouse lungs. Recombinant HIMF protein or BSA was intratracheally instilled into adult mouse lungs (200 ng/animal in 40 μl saline; n = 3 for each group). The vehicle controls were instilled with saline (40 μl/animal; n = 3). After 6 h, the mouse lungs were collected. (A) Results of immunohistochemical staining indicated that instillation of HIMF protein, but not BSA, resulted in a significant increase in SP-B and SP-C production, mainly located at ATII and bronchiolar epithelial cells (arrows). Scale bars: 60 μm. Western blot with proteins from lung homogenates (B) and RT-PCR from lung tissues (C) indicated that SP-B and SP-C production was specifically enhanced in HIMF- instilled, but not in BSA- or saline-instilled, mouse lungs. * Significant increase from control mouse lungs instilled with saline only (P < 0.05). Triplicate experiments were performed with essentially identical results.
HIMF-Induced SP-B and SP-C Expression in Mouse Lung Epithelial MLE-12 and C10 Cells
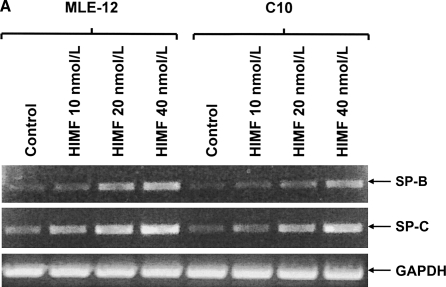
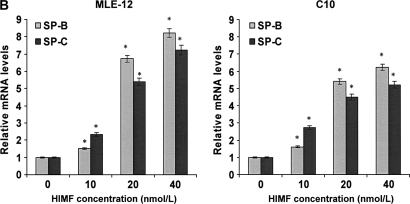
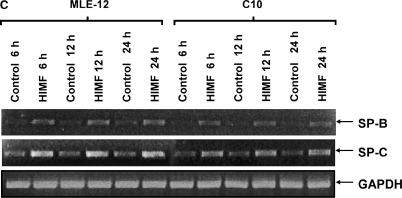
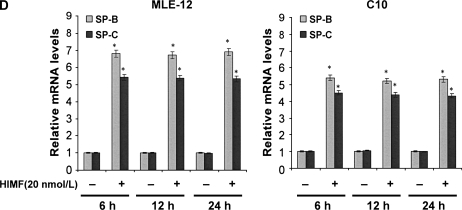
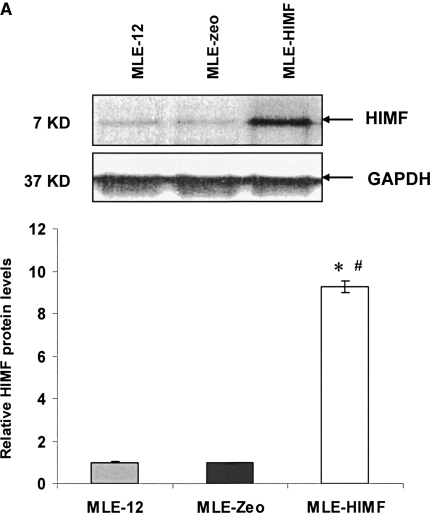
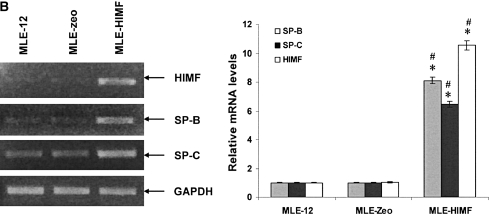
To further investigate the molecular mechanisms of HIMF- induced SP-B and SP-C production, we used cultured mouse ATII- like cell lines MLE-12 and C10 as models, and examined their mRNA changes of SP-B and SP-C. Incubation of MLE-12 and C10 cells with 10–40 nmol/L of HIMF dose-dependently increased SP-B and SP-C mRNA (Figures 3A and 3B). A time-course study indicated that HIMF-induced SP-B and SP-C production started as early as 6 h, and sustained through 24 h (Figures 3C and 3D). To further validate the HIMF-elicited SP-B and SP-C expression in cultured ATII-like cells, we established the HIMF-overexpressing cell line MLE-HIMF by transfection of HIMF cDNA into MLE-12 cells and selected with Zeocin. Figures 4A and 4B demonstrate that MLE-HIMF cells overexpressed HIMF mRNA and protein as compared with their parent MLE-12 and transfection control MLE-Zeo cells. More importantly, the SP-B and SP-C mRNA levels of MLE-HIMF were also increased significantly compared with those of their control counterparts (Figure 4B). These results indicated that HIMF, either extragenously administered into the culture medium, or expressed by the stable transfectants themselves, induced SP-B and SP-C production.
Figure 3.
HIMF-induced SP-B and SP-C production in mouse lung epithelial cell lines MLE-12 and C10. Confluent monolayers of MLE-12 and C10 were starved with culture medium supplemented with 0.1% FBS and 2 mM L-glutamine. After 24 h, cells were treated with different concentrations of HIMF for various periods, as indicated. Semiquantitative RT-PCR was performed for HIMF mRNA expression. (A and B) Incubation of cells with 10, 20, and 40 nmol/L of HIMF protein resulted in SP-B and SP-C production in a dose-dependent manner. (C and D) Time-course study indicated that HIMF-induced SP-B and SP-C production started at 6 h, and was sustained for 24 h. * Significant increase from untreated control cells (P < 0.05). Three separated experiments were performed with essentially identical results.
Figure 4.
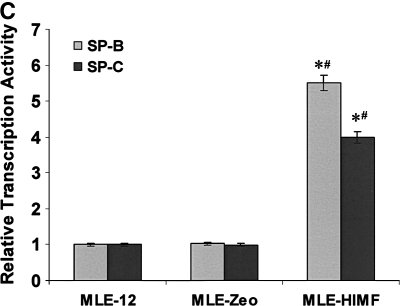
Generation of HIMF overexpressing mouse lung epithelial cells. Confluent monolayers of MLE-12 cells were transfected by HIMF cDNA or control vector with Lipofectamine 2000. Stable cell lines, MLE-HIMF and MLE-Zeo, were screened based on resistance to Zeocin (400 μg/ml). (A) Western blot from culture medium indicated that MLE-HIMF cells produce higher level of HIMF protein than their parent and transfection counterparts. (B) RT-PCR demonstrated that MLE-HIMF cells have overexpressed HIMF mRNA, and enhanced SP-B and SP-C mRNA levels compared with their parent and transfection controls. (C) Luciferase assay indicated that the promoter activity of SP-B and SP-C in MLE-HIMF cells was enhanced compared with that of their parent and transfection controls (all groups are in triplicate). * Significant increase from MLE-12 parent; # significant increase from transfection controls (P < 0.05). Shown is one representative experiment of three, all with similar results.
HIMF Modulated the Transcriptional Activities and mRNA Stability of SP-B and SP-C in MLE-12 Cells
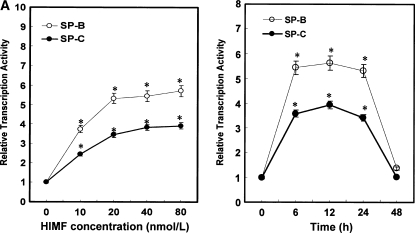
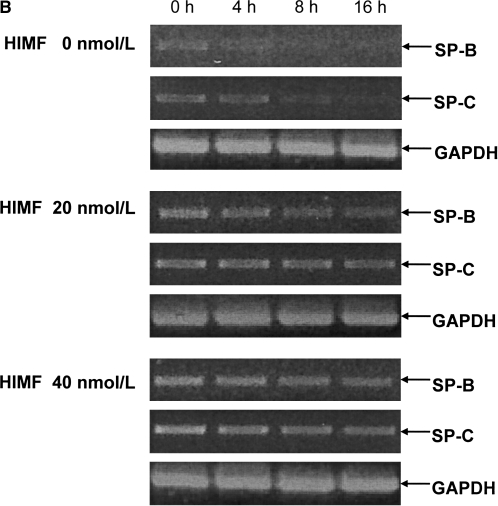
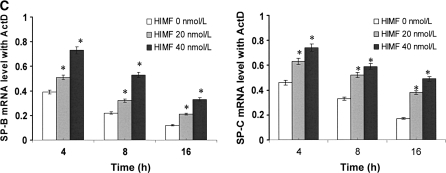
To elucidate the modulation patterns of HIMF on SP-B and SP-C mRNA in mouse epithelial cells, we used the SP-B and SP-C promoter-luciferase reporter constructs. As shown in Figure 4C, dual-luciferase assay demonstrated that the promoter activities of SP-B and SP-C in MLE-HIMF cells were stronger than those of their parent cells and vector-transfection controls. Similarly, in cultured MLE-12 cells, HIMF protein stimulation also enhanced SP-B and SP-C promoter activity in a dose-dependent manner (Figure 5A). The time-course study demonstrated that HIMF-induced SP-B and SP-C promoter activity started as early as 6 h, and persisted for 24 h (Figure 5A). Because modulation of mRNA stability is an important post-transcriptional mechanism for SP-B and SP-C gene expression, we used the transcription inhibitor actinomycin D, and examined the potential protective effects of HIMF on SP-B and SP-C mRNA stability. As shown in Figures 5B and 5C, pretreatment of MLE-12 cells with HIMF protein prevented actinomycin D–facilitated SP-B and SP-C mRNA degradation. Combined with the finding that HIMF enhanced SP-B and SP-C protein production in vivo, we believe that HIMF modulates SP-B and SP-C expression in mouse lung epithelial cells by both increasing transcriptional activities and strengthening mRNA stability, resulting in increased transcriptional levels of these two proteins.
Figure 5.
HIMF increased both promoter activities and mRNA stability of SP-B and SP-C in mouse lung epithelial MLE-12 cells. (A) Confluent monolayers of MLE-12 were cotransfected with either pGL-SPB-Luc or pGL-SPC-Luc, and pRL-TK. After 24 h, cells were incubated with HIMF protein as indicated. Cells were then lysed, and luciferase activity was measured according to the dual-luciferase assay manual. The firefly luciferase signal was normalized to the renilla luciferase signal for each individual well. After incubation with 10–80 nmol/liter of HIMF, SP-B and SP-C promoter activity in MLE-12 cells was enhanced in a dose-dependent manner. The time-course study demonstrated that the SP-B and SP-C promoter activity induced by HIMF (20 nmol/liter) started at 6 h and persisted for 24 h. (B and C) MLE-12 cells were treated with different concentrations of HIMF, and incubated with 5 μg/ml of actinomycin D for 4 , 8, and 16 h. RT-PCR was performed, and the results indicated that HIMF prevented actinomycin D–facilitated SP-B and SP-C degradation in MLE-12 cells. * Significant increase compared with MLE-12 controls treated without HIMF (P < 0.05). All experiments were in triplicate and performed three times with similar results.
Phosphatidylinositol 3-Kinase/Akt and ERK MAPK Were Involved in HIMF-Induced SP-B and SP-C Production
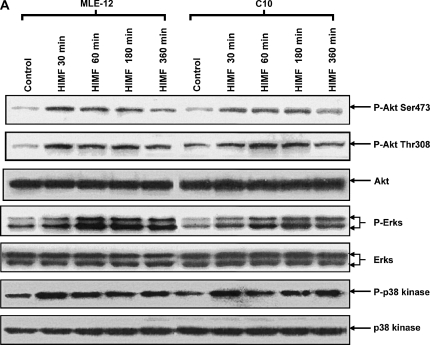
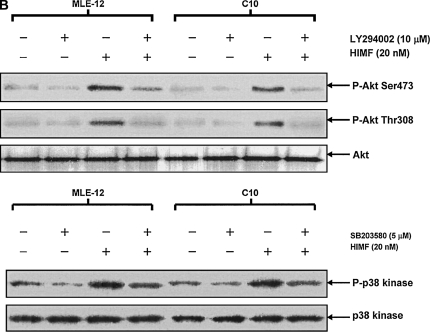
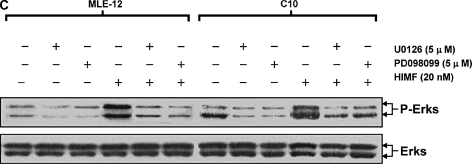
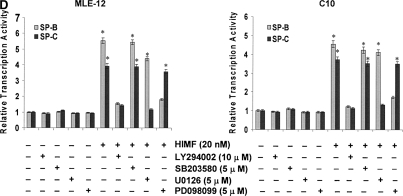
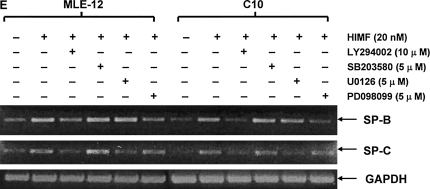
To better elucidate the molecular mechanisms underlying HIMF-induced SP-B and SP-C production in mouse lung epithelial cells, we first explored HIMF-mediated signaling pathways in MLE-12 and C10 cells. As shown in Figure 6A, HIMF strongly activated the phosphorylation of Akt at Ser473 and Thr308, and the phosphorylation of ERK1/2 and p38 MAPK. The phosphatidylinositol 3-kinase (PI-3K) inhibitor LY294002 (10 μmol/liter) inhibited HIMF-activated Akt phosphorylation (Figure 6B). Moreover, incubation of cells with p38 MAPK inhibitor SB203580 (5 μmol/liter), and ERK1/2 inhibitors PD098059 (5 μmol/liter) or U0126 (5 μmol/liter), blocked HIMF-induced phosphorylation of p38 and ERK1/2 MAPK (Figures 6B and 6C). However, luciferase assay revealed that HIMF-induced SP-B and SP-C production was not abolished by SB203580 (Figure 6D). Incubation of MLE-12 cells with LY294002 prevented HIMF-induced upregulation of both SP-B and SP-C (Figure 6D). Meanwhile, PD098059 and U0126 suppressed HIMF-induced SP-B and SP-C production, respectively (Figure 6D). These results were further confirmed by RT-PCR (Figure 6E). Therefore, we believe that the activation of PI-3K/Akt and ERK1/2 MAPK, rather than p38 MAPK, is involved in the signaling cascade triggered by HIMF to stimulate SP-B and SP-C production in mouse ATII-like cells.
Figure 6.
Phosphatidylinositol 3-kinase (PI-3K)/Akt and extracellular signal-regulated kinase (ERK)1/2 mitogen-activated protein kinase (MAPK) were involved in HIMF-induced SP-B and SP-C production. Confluent monolayers of MLE-12 and C10 were starved with medium supplemented with 0.1% FBS and 2 mM L-glutamine. After 33 h, cells were incubated in serum-free medium for 3–4 h at 37°C. Cells were then pretreated with different signal transduction inhibitors for 1 h, and then stimulated with HIMF protein for various periods, as indicated. (A) Western blot with proteins from cell lysate indicated that HIMF strongly activated Akt phosphorylation at Ser473 and Thr308, and phosphorylation of ERK1/2 and p38 MAPK. (B and C) The PI-3K inhibitor LY294002 (10 μmol/liter) inhibited HIMF-activated Akt phosphorylation. Moreover, incubation of cells with SB203580, inhibitor against p38 (5 μmol/liter), and either PD098059 (5 μmol/liter) or U0126 (5 μmol/liter), inhibitors of ERK1/2 MAPK pathways, also blocked HIMF-induced phosphorylation of p38 and ERK1/2. (D) Luciferase assay revealed that HIMF-induced SP-B and SP-C expression was not abolished by p38 inhibitor SB203580. However, incubation of MLE-12 cells with the PI-3K inhibitor, LY294002, prevented HIMF-induced upregulation of both SP-B and SP-C. Meanwhile, the inhibitors of ERK1/2 MAPK, PD098059 and U0126, also blocked HIMF-induced SP-B and SP-C production, respectively, which was further confirmed by RT-PCR (E). * Significant increase compared with MLE-12 controls treated without HIMF (P < 0.05). Three experiments with triplicate for each treatment were performed, and all with similar results.
DISCUSSION
Surfactant is a developmentally and hormonally regulated, phospholipid-rich lipoprotein, synthesized predominantly by ATII cells (24–26). A number of hormones, growth factors, and cytokines influence surfactant synthesis, and, in turn, modulate fetal lung development and maturation (7). The cyclic AMP analog 8-bromo cAMP modestly increases SP-B and SP-C mRNA levels in fetal human, fetal rat, and fetal rabbit lung tissues in vitro (8, 27, 28). Similarly, and the adenylate cyclase activator forskolin increases SP-B and SP-C mRNA expression in fetal rat and rabbit lung tissues (27, 28). Glucocorticoids generally increase SP-B and SP-C mRNA expression in human (8), rat (27), and rabbit (29) fetal lung tissues. Targeted deletion of the gene encoding transforming growth factor–β decreases SP-C expression and delays pulmonary development in mice (11). By contrast, all-trans retinoic acid and 9-cis-retinoic acid inhibit SP-C mRNA levels, but increase SP-B mRNA levels in fetal human lung in vitro preparations (9). The aim of this study was to understand the molecular mechanisms of HIMF-induced SP-B and SP-C expression. The present study provides several lines of evidence indicating that HIMF-induced SP-B and SP-C expression is dependent upon activated PI-3K/Akt and ERK1/2 MAPK, but not p38 MAPK. These results also suggest that HIMF may play critical roles in mouse SP-B and SP-C production, and promote lung development and maturation.
HIMF, also named as FIZZ1 (14), belongs to a novel class of cysteine-rich secreted proteins known as RELMs (15). Previous studies from our laboratory demonstrated that HIMF is a lung-specific growth factor participating in lung cell proliferation and modulation of compensatory lung growth (13, 17). Intratracheal instillation of HIMF protein induces widespread proliferation of airway epithelial cells, ATII cells, and cells in the lung parenchyma (17). Our published data have also demonstrated that HIMF is a potent vasoconstrictor that can significantly raise pulmonary arterial pressure (13). HIMF-induced pulmonary arterial constriction is more potent than that produced by endothelin-1, angiotensin II, or serotonin, but less potent than that produced by the thromboxane mimetic U-46619 (13). HIMF possesses an angiogenic function that promotes vascular tube formation in a matrigel plug model (13). More recently, we reported that HIMF is developmentally regulated in mouse lung, particularly during the perinatal period, and that HIMF has an antiapoptotic effect in cultured embryonic lungs (16). However, the relationship between HIMF expression and the production of SP-B and SP-C in alveolar epithelial cells of developing lungs has not previously been investigated.
During the canalicular phase of mouse lung development (E16 and E17), rapid growth rate is replaced by differentiation of epithelial cells lining the ducts (30). This phase is characterized by the initiation of capillary growth and the first appearance of ATII cells containing lamellar bodies, the cellular organelle compartment for lung surfactant (30). Our previous studies indicated a robust HIMF expression in mouse lungs at E16 through P30, and only a low-level expression at E14 and E15. This temporal course indicates a potential role of HIMF during the canalicular phase of mouse lung development (16). The pattern of temporal– spatial distributions of HIMF, SP-B, and SP-C demonstrated in the present study raises the possibility that HIMF regulates the participation of these two surfactants in lung maturation. Our observation that intratracheally instilled HIMF protein in adult mice specifically enhances SP-B and SP-C expression supports a cause–effect relationship. Together with our previous findings that HIMF has an antiapoptotic effect in cultured embryonic lung (16), our data suggest that HIMF plays important roles in mouse lung maturation through stimulating SP-B and SP-C production in ATII cells.
To better understand the regulation mechanisms of HIMF on SP-B and SP-C production in mouse lung epithelial cells, the effects of HIMF on mRNA levels and stability of SP-B and SP-C were explored in MLE-12 and C10 cells. The results indicate that HIMF stimulation leads to increased transcriptional activities of SP-B and SP-C in both MLE-12 and C10 cells. Previous studies have demonstrated that mouse SP-B promoter activity in MLE-12 cells is dependent on sequences within the −842 bp region (19) that contain binding sites for thyroid transcription factor-1, hepatocyte nuclear factor-3, specificity protein 1/3, activator protein-1, and activator transcription factor/cAMP responsive element transcription factors. Deletion mapping studies of mouse SP-C 5′ flanking DNA have identified DNA sequences within 320 bp upstream of the transcription start site as necessary for expression in MLE-15 cells, a mouse lung cell line with characteristics of ATII cells (20). Post-transcriptional and pretranslational mechanisms also regulate the expression of SP-B and SP-C. In the present study, we found that pretreatment of MLE-12 cells with HIMF protein prevents actinomycin D–facilitated SP-B and SP-C mRNA degradation, indicating that HIMF may be involved in stimulating the modification of a protein factor that stabilizes SP-B and SP-C mRNA. Thus, we believe that HIMF modulates both transcriptional and post-transcriptional processes of SP-B and SP-C, enhancing their protein production.
Peptide growth factors, such as FGF, that activate PI-3K and MAPK signal transduction pathways, play an important role in mediating lung branching morphogenesis. Using specific inhibitors to signaling molecules downstream of FGF receptors, Matsui and colleagues identified PI-3K as one of the critical components mediating surfactant gene expression after FGF-2 stimulation (10). In lung explants, terminal bud growth was increased by FGF-1 treatment and decreased by the ERK1/2 inhibitor PD98059 and by the PI-3K–specific inhibitors LY294002 and wortmannin (31). Treatment of lung explants with LY294002 and wortmannin also decreased SP-C expression in these studies, suggesting participation of the FGF/PI-3K/Akt pathway in the regulation of SP-C expression (31). Other investigators have shown that SP-A activates the PI-3K/Akt signaling pathway through cell surface SP-A receptors to upregulate SP-B and SP-C (32). In the present study, we found that HIMF strongly induces phosphorylation of Akt, ERK1/2, and p38 MAPK in both MLE-12 and C10 cells. Furthermore, inhibition of PI-3K or ERK1/2 MAPK blocked HIMF-induced Akt activation as well as HIMF-induced SP-B and SP-C expression in these cells. However, the stress-activated protein kinase p38 MAPK does not appear to be involved in this process. Thus, we believe that HIMF-induced SP-B and SP-C expression in mouse epithelial cells is mediated through the PI-3K/Akt and ERK1/2 MAPK pathways.
In summary, the present study shows that HIMF can enhance SP-B and SP-C production by increasing their transcription and mRNA stability in mouse lung tissues and epithelial cell lines. This process is dependent upon PI-3K/Akt and ERK1/2 MAPK activity. Together with our previous findings that HIMF has an antiapoptotic effect in cultured embryonic lung, these results suggest that HIMF plays critical roles in mouse SP production and lung development. However, the HIMF-induced increase in p38 MAPK activation did not participate in SP-B and SP-C upregulation, and the exact role of HIMF-induced p38 MAPK activation warrants further investigation.
Acknowledgments
to the authors thank Dr. Alvin M. Malkinson (University of Colorado at Denver and Health Science Center, Aurora, CO) for his kind gift of mouse epithelial C10 cell line. They also appreciate Dr. Jeffrey A. Whitsett (Cincinnati Children's Hospital Medical Center, Cincinnati, OH) for providing the mouse SP-B and SP-C promoter-reporter constructs.
This work was supported by National Institutes of Health grant HL075755.
Originally Published in Press as DOI: 10.1165/rcmb.2005-0172OC on September 15, 2005
Conflict of Interest Statement: None of the authors has a financial relationship with a commercial entity that has an interest in the subject of this manuscript.
References
- 1.Haagsman HP, van Golde LM. Synthesis and assembly of lung surfactant. Annu Rev Physiol 1991;53:441–464. [DOI] [PubMed] [Google Scholar]
- 2.Mallory GB Jr. Surfactant proteins: role in lung physiology and disease in early life. Paediatr Respir Rev 2001;2:151–158. [DOI] [PubMed] [Google Scholar]
- 3.Nogee LM, Garnier G, Dietz HC, Singer L, Murphy AM, deMello DE, Colten HR. A mutation in the surfactant protein B gene responsible for fatal neonatal respiratory disease in multiple kindreds. J Clin Invest 1994;93:1860–1863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Clark JC, Wert SE, Bachurski CJ, Stahlman MT, Stripp BR, Weaver TE, Whitsett JA. Targeted disruption of the sufactant protein B gene disrupts surfactant homeostasis, causing respiratory failure in newborn mice. Proc Natl Acad Sci USA 1995;92:7794–7798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Glasser SW, Burhans MS, Korfhagen TR, Na CL, Sly PD, Ross GF, Ikegami M, Whitsett JA. Altered stability of pulmonary surfactant in SP-C–deficient mice. Proc Natl Acad Sci USA 2001;98:6366–6371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Danlois F, Zaltash S, Johansson J, Robertson B, Haagsman HP, van Eijk M, Beers MF, Rollin F, Ruysschaert JM, Vandenbussche G. Very low surfactant protein C contents in newborn Belgian white and blue calves with respiratory distress syndrome. Biochem J 2000;351:779–787. [PMC free article] [PubMed] [Google Scholar]
- 7.Boggaram V. Regulation of lung surfactant protein gene expression. Front Biosci 2003;8:d751–d764. [DOI] [PubMed] [Google Scholar]
- 8.Whitsett JA, Weaver TE, Clark JC, Sawtell N, Glasser SW, Korfhagen TR, Hull WM. Glucocorticoid enhances surfactant proteolipid Phe and pVal synthesis and RNA in fetal lung. J Biol Chem 1987;262: 15618–15623. [PubMed] [Google Scholar]
- 9.Metzler MD, Snyder JM. Retinoic acid differentially regulates expression of surfactant-associated proteins in human fetal lung. Endocrinology 1993;133:1990–1998. [DOI] [PubMed] [Google Scholar]
- 10.Matsui R, Brody JS, Yu Q. FGF-2 induces surfactant protein gene expression in foetal rat lung epithelial cells through a MAPK-independent pathway. Cell Signal 1999;11:221–228. [DOI] [PubMed] [Google Scholar]
- 11.Kaartinen V, Voncken JW, Shuler C, Warburton D, Bu D, Heisterkamp N, Groffen J. Abnormal lung development and cleft palate in mice lacking TGF-[beta]3 indicates defects of epithelial-mesenchymal interaction. Nat Genet 1995;11:415–421. [DOI] [PubMed] [Google Scholar]
- 12.Mouhieddine-Gueddiche OB, Pinteur C, Chailley-Heu B, Barlier-Mur AM, Clement A, Bourbon JR. Dexamethasone potentiates keratinocyte growth factor–stimulated SP-A and SP-B gene expression in alveolar epithelial cells. Pediatr Res 2003;53:231–239. [DOI] [PubMed] [Google Scholar]
- 13.Teng X, Li D, Champion HC, Johns RA. FIZZ1/RELM{alpha}, a novel hypoxia-induced mitogenic factor in lung with vasoconstrictive and angiogenic properties. Circ Res 2003;92:1065–1067. [DOI] [PubMed] [Google Scholar]
- 14.Holcomb IN, Kabakoff RC, Chan B, Baker TW, Gurney A, Henzel W, Nelson C, Lowman HB, Wright BD, Skelton NJ, et al. FIZZ1, a novel cysteine-rich secreted protein associated with pulmonary inflammation, defines a new gene family. EMBO J 2000;19:4046–4055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Steppan CM, Brown EJ, Wright CM, Bhat S, Banerjee RR, Dai CY, Enders GH, Silberg DG, Wen X, Wu GD, et al. A family of tissue-specific resistin-like molecules. Proc Natl Acad Sci USA 2001;98: 502–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wagner KF, Hellberg AK, Balenger S, Depping R, Dodd O, Johns RA, Li D. Hypoxia-induced mitogenic factor has antiapoptotic action and is upregulated in the developing lung: coexpression with hypoxia-inducible factor-2α. Am J Respir Cell Mol Biol 2004;31:276–282. [DOI] [PubMed] [Google Scholar]
- 17.Li D, Fernandez LG, Dodd-o J, Langer J, Wang D, Laubach VE. Upregulation of hypoxia-induced mitogenic factor in compensatory lung growth after pneumonectomy. Am J Respir Cell Mol Biol 2005;32: 185–191. [DOI] [PubMed] [Google Scholar]
- 18.Thompson DC, Porter SE, Bauer AK, Das KC, Ou B, Dwyer-Nield L, White CW, Malkinson AM. Cytokine-induced nitric oxide formation in normal but not in neoplastic murine lung epithelial cell lines. Am J Physiol Lung Cell Mol Physiol 1998;274:L922–L932. [DOI] [PubMed] [Google Scholar]
- 19.Bruno MA, Bohinski RJ, Carter JE, Foss KA, Whitsett JA. Structure and function of the mouse surfactant protein B gene. Am J Physiol Lung Cell Mol Physiol 1995;268:L381–L389. [DOI] [PubMed] [Google Scholar]
- 20.Kelly SE, Bachurski CJ, Burhans MS, Glasser SW. Transcription of the lung-specific surfactant protein C gene is mediated by thyroid transcription factor 1. J Biol Chem 1996;271:6881–6888. [DOI] [PubMed] [Google Scholar]
- 21.Zhou L, Lim L, Costa RH, Whitsett JA. Thyroid transcription factor-1, hepatocyte nuclear factor-3beta, surfactant protein B, C, and Clara cell secretory protein in developing mouse lung. J Histochem Cytochem 1996;44:1183–1193. [DOI] [PubMed] [Google Scholar]
- 22.Nogee LM. Alterations in SP-B and SP-C expression in neonatal lung disease. Annu Rev Physiol 2004;66:601–623. [DOI] [PubMed] [Google Scholar]
- 23.Jaskoll TF, Phelps D, Taeusch HW, Smith BT, Slavkin HC. Localization of pulmonary surfactant protein during mouse lung development. Dev Biol 1984;106:256–261. [DOI] [PubMed] [Google Scholar]
- 24.Frerking I, Gunther A, Seeger W, Pison U. Pulmonary surfactant: functions, abnormalities and therapeutic options. Intensive Care Med 2001; 27:1699–1717. [DOI] [PubMed] [Google Scholar]
- 25.Piknova B, Schram V, Hall SB. Pulmonary surfactant: phase behavior and function. Curr Opin Struct Biol 2002;12:487–494. [DOI] [PubMed] [Google Scholar]
- 26.Liley HG, White RT, Warr RG, Benson BJ, Hawgood S, Ballard PL. Regulation of messenger RNAs for the hydrophobic surfactant proteins in human lung. J Clin Invest 1989;83:1191–1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Floros J, Gross I, Nichols KV, Veletza SV, Dynia D, Lu HW, Wilson CM, Peterec SM. Hormonal effects on the surfactant protein B (SP-B) mRNA in cultured fetal rat lung. Am J Respir Cell Mol Biol 1991;4: 449–454. [DOI] [PubMed] [Google Scholar]
- 28.Margana RK, Boggaram V. Transcription and mRNA stability regulate developmental and hormonal expression of rabbit surfactant protein B gene. Am J Physiol Lung Cell Mol Physiol 1995;268:L481–L490. [DOI] [PubMed] [Google Scholar]
- 29.Boggaram V, Margana RK. Rabbit surfactant protein C: cDNA cloning and regulation of alternatively spliced surfactant protein C mRNAs. Am J Physiol Lung Cell Mol Physiol 1992;263:L634–L644. [DOI] [PubMed] [Google Scholar]
- 30.Snyder JM, Mendelson CR. The morphology of lung development in the human fetus. In: Pulmonary development: transition from intrauterine to extrauterine life. New York: Dekker Press; 1985. pp. 19–46.
- 31.Wang J, Ito T, Udaka N, Okudela K, Yazawa T, Kitamura H. PI3K-AKT pathway mediates growth and survival signals during development of fetal mouse lung. Tissue Cell 2005;37:25–35. [DOI] [PubMed] [Google Scholar]
- 32.Strayer DS, Korutla L. Activation of surfactant protein-B transcription: signaling through the SP-A receptor utilizing the PI3 kinase pathway. J Cell Physiol 2000;184:229–238. [DOI] [PubMed] [Google Scholar]