Abstract
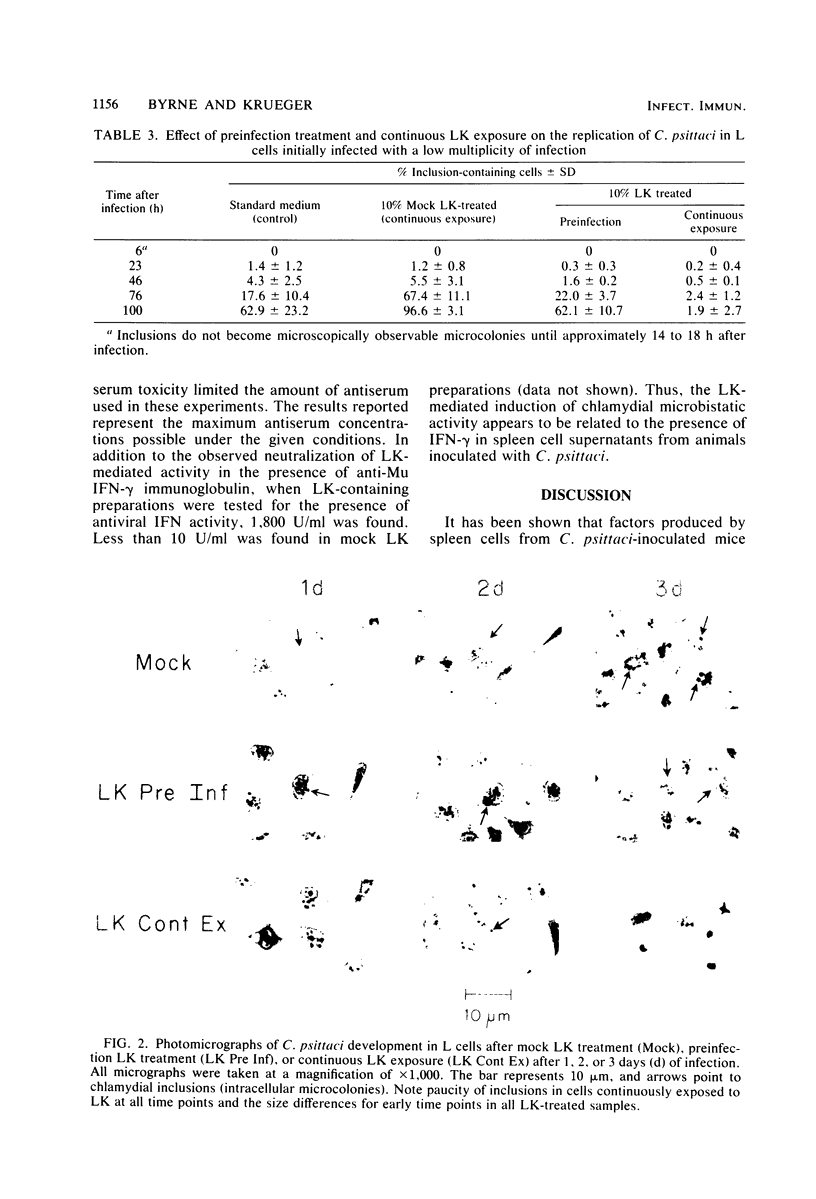
Experiments were carried out to characterize immunologically mediated chlamydial persistence in cell culture. Mouse fibroblasts were activated to restrict Chlamydia psittaci 6BC replication by including mitogen (concanavalin A)-induced spleen cell supernatant fluids from immunized animals in the growth medium. When mouse fibroblasts were incubated with lymphokine for 24 h before infection and then with growth medium after infection (preinfection treatment), chlamydial replication was delayed but eventually detected. No substantial chlamydial growth occurred, even with extended incubation times when mouse fibroblasts were continuously exposed to lymphokine before and after infection. Low levels of infectious chlamydiae were produced in preinfection-treated mouse fibroblasts but not in mouse fibroblasts subjected to continuous lymphokine exposure. Incubation of lymphokine with anti-murine gamma interferon immunoglobulin neutralized the observed lymphokine-mediated activity, but incubation in the presence of anti-murine alpha plus beta interferon serum did not alter lymphokine activity. We conclude that the lymphokine components responsible for activating fibroblasts to restrict C. psittaci replication exhibits properties similar to gamma interferon.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Byrne G. I., Faubion C. L. Inhibition of Chlamydia psittaci in oxidatively active thioglycolate-elicited macrophages: distinction between lymphokine-mediated oxygen-dependent and oxygen-independent macrophage activation. Infect Immun. 1983 May;40(2):464–471. doi: 10.1128/iai.40.2.464-471.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byrne G. I., Faubion C. L. Lymphokine-mediated microbistatic mechanisms restrict Chlamydia psittaci growth in macrophages. J Immunol. 1982 Jan;128(1):469–474. [PubMed] [Google Scholar]
- Byrne G. I., Rothermel C. D. Differential susceptibility of chlamydiae to exogenous fibroblast interferon. Infect Immun. 1983 Feb;39(2):1004–1005. doi: 10.1128/iai.39.2.1004-1005.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grayston J. T., Wang S. P. The potential for vaccine against infection of the genital tract with Chlamydia trachomatis. Sex Transm Dis. 1978 Apr-Jun;5(2):73–77. doi: 10.1097/00007435-197804000-00011. [DOI] [PubMed] [Google Scholar]
- Hanna L., Dawson C. R., Briones O., Thygeson P., Jawetz E. Latency in human infections with TRIC agents. J Immunol. 1968 Jul;101(1):43–50. [PubMed] [Google Scholar]
- Hatch T. P. Competition between Chlamydia psittaci and L cells for host isoleucine pools: a limiting factor in chlamydial multiplication. Infect Immun. 1975 Jul;12(1):211–220. doi: 10.1128/iai.12.1.211-220.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee C. K., Moulder J. W. Persistent infection of mouse fibroblasts (McCoy cells) with a trachoma strain of Chlamydia trachomatis. Infect Immun. 1981 May;32(2):822–829. doi: 10.1128/iai.32.2.822-829.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormack W. M., Alpert S., McComb D. E., Nichols R. L., Semine D. Z., Zinner S. H. Fifteen-month follow-up study of women infected with Chlamydia trachomatis. N Engl J Med. 1979 Jan 18;300(3):123–125. doi: 10.1056/NEJM197901183000305. [DOI] [PubMed] [Google Scholar]
- Moulder J. W. Inhibition of onset of overt multiplication of Chlamydia psittaci in persistently infected mouse fibroblasts (L cells). Infect Immun. 1983 Feb;39(2):898–907. doi: 10.1128/iai.39.2.898-907.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moulder J. W., Levy N. J., Schulman L. P. Persistent infection of mouse fibroblasts (L cells) with Chlamydia psittaci: evidence for a cryptic chlamydial form. Infect Immun. 1980 Dec;30(3):874–883. doi: 10.1128/iai.30.3.874-883.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothermel C. D., Rubin B. Y., Murray H. W. Gamma-interferon is the factor in lymphokine that activates human macrophages to inhibit intracellular Chlamydia psittaci replication. J Immunol. 1983 Nov;131(5):2542–2544. [PubMed] [Google Scholar]
- Schachter J., Caldwell H. D. Chlamydiae. Annu Rev Microbiol. 1980;34:285–309. doi: 10.1146/annurev.mi.34.100180.001441. [DOI] [PubMed] [Google Scholar]
- Schachter J., Sugg N., Sung M. Psittacosis: the reservoir persists. J Infect Dis. 1978 Jan;137(1):44–49. doi: 10.1093/infdis/137.1.44. [DOI] [PubMed] [Google Scholar]



