Abstract
Oxidant stress–mediated regulation of extracellular signal-regulated kinases (ERK1/2) is linked to pathologic outcomes in lung epithelium, yet a role for Ca2+ and Ca2+/cAMP-response element binding protein (CREB) in ERK1/2 signaling has not been defined. In this study, we tested the hypotheses that oxidants induce Ca2+-mediated phosphorylation of ERK and CREB, and that CREB is required for oxidant-induced proliferation and apoptosis. H2O2 initiated an influx of extracellular Ca2+Ca2+Ca2+Ca2+ that was required for phosphorylation of both ERK and CREB in C10 lung epithelial cells. H2O2-mediated CREB phosphorylation was sensitive to MEK inhibition, suggesting that crosstalk between Ca2+, ERK, and CREB signaling pathways contributes to the oxidant-induced response. Reduction of CREB activity, using a dominant-negative CREB construct, inhibited c-fos steady-state mRNA levels, but unexpectedly enhanced bcl-2 steady-state mRNA levels after H2O2 exposure. Whereas inhibition of CREB activity had no detectable effect on H2O2 stimulation of cell cycle, loss of CREB activity significantly reduced the number of cells undergoing apoptosis. These data support a novel communication between Ca2+-ERK1/2 and CREB elicited by H2O2, and further provide evidence that CREB is an important regulator of apoptosis in oxidant-mediated responses of lung epithelial cells.
Keywords: calcium signaling, lung pathology, mitogen-activated protein kinase, reactive oxygen species
Reactive oxygen species (ROS) play a significant role in the pathogenesis of lung diseases and are involved in multiple physiological processes through their capacity to regulate the expression of several protein kinases and phosphatases. In lung epithelial cells, the target cells of inhaled oxidants, H2O2 leads to the activation of Ser/Thr and Tyr protein kinases, including protein kinase C (1) and the mitogen-activated protein kinases (MAPK) p38, c-Jun amino-terminal kinase, and extracellular signal-regulated kinase 1 and 2 (ERK1/2) (2, 3). H2O2 also serves as an important signaling molecule to activate the expression of transcription factors, including members of the activator protein family, c-jun and c-fos (4), resulting in proliferation (2) and apoptosis (5) of lung epithelial cells.
Oxidant stress–mediated phosphorylation of ERK1/2 is believed to be integral to pathologic signaling in lung epithelium. Upon phosphorylation, ERK1/2 can translocate to the nucleus, where it induces c-fos and c-jun, leading to the activation of transcription factors intrinsic to proliferation and apoptosis (6). ERK1/2 also mediates the actions of both Ca2+ and growth factors, resulting in activation of the Ca2+/cAMP-response element binding protein (CREB) (7).
CREB is a 43 kD transcription factor belonging to the basic-leucine zipper family (7, 8). The function of CREB is regulated by the phosphorylation of 133Ser, a serine at position 133. Phosphorylation, in turn, initiates the recruitment of cofactors to the Ca2+/cAMP-response element (CRE) that are necessary for transcriptional activation, including CREB-binding protein (CBP) 300 (9). Multiple kinase signaling pathways contribute to the phosphorylation of CREB, including protein kinase A, Ca2+/calmodulin-dependent kinase (CaMK), protein kinase C, and p90 ribosomal kinase (7, 10). Phosphorylation of CREB by ERK1/2 is proposed to be indirect through the phosphorylation of p90 ribosomal kinase (11).
CREB phosphorylation is important in the control of gene transcription driven by CREs. Many genes important for regulation of proliferation and apoptosis, including c-fos and bcl-2, respectively, contain CREs in their promoter (9). Although CREB has the capacity to regulate these gene targets (9), its role in oxidant-mediated signaling has not been assessed.
There is little information regarding the role of Ca2+ and Ca2+-mediated transcription in oxidant-mediated signaling pathways in lung epithelium, an initial target of inhaled oxidants. Because of known crosstalk between ERK1/2, Ca2+, and CREB in other cell types, the goal of this study was to determine their relative importance in H2O2-induced signaling in lung epithelial cells. In the present study, we found that H2O2 exposure resulted in Ca2+ influx that was required for phosphorylation of both ERK1/2 and CREB in lung epithelial cells. Reduction of CREB activity, by introducing a dominant-negative CREB construct, led to a decrease in apoptosis corresponding with enhanced bcl-2 transcription. These data suggest signaling crosstalk between Ca2+, ERK1/2, and CREB in lung epithelial cells, and reveal an important role for CREB in H2O2-mediated apoptosis.
MATERIALS AND METHODS
Cell Culture and Treatment
C10 cells, a contact-inhibited, nontransformed murine alveolar type II epithelial cell line (12), were grown in CMRL 1066 medium supplemented with L-glutamine (2 mM), penicillin/streptomycin (100 μg/ml), and 10% FBS (GIBCO BRL, Rockville, MD). Cells were grown to near confluence, then complete medium was replaced with CMRL 1066 medium supplemented with L-glutamine, penicillin/streptomycin, and 0.5% FBS for 48 h before exposure to agents.
H2O2 (Sigma, St. Louis, MO) was added as a bolus to the medium at the indicated concentrations. Where indicated, recombinant glucose oxidase (15 mU/ml) (Roche, Indianapolis, IN) was used to provide low-level fluxes of H2O2 (13). Forskolin and ATP (Sigma) (10 μM for 10 min) were used as positive controls for induction of phospho-CREB and phospho-ERK1/2, respectively. Control cultures received medium without agents and were treated identically.
The MAP or ERK kinase (MEK) inhibitor, U0126 (10 μM), and CaMKII inhibitor, KN93 (30 μM), or its inactive isomer, KN92 (30 μM), were obtained from Calbiochem (La Jolla, CA). Inhibitors were added 30 min before H2O2. For “low Ca2+” conditions, cells were maintained in low-calcium HEPES-buffered saline solution (10 mM HEPES [pH 7.4], 6 mM KCl, 140 mM NaCl, 100 μM CaCl2, 1 mM MgCl2, 10 mM glucose, 1 mM EGTA, effectively 100 nM Ca2+) 5 min before experiments. All experiments were performed in triplicate.
Stable Dominant-Negative CREB C10 Lung Epithelial Cells
A 133Ser phosphorylation mutant CREB construct (CREB133) (BD Biosciences, Palo Alto, CA) was ligated into pCMV-Tag5a (Stratagene, La Jolla, CA) through a BamHI restriction site. A total of 8 μg of CREB133 or pCMV-Tag5a were transfected into cells using Lipofectamine 2000 (Invitrogen, Carlsbad, CA), following the manufacturer's instructions for creating stable cell lines. Transfected cells were selected using 0.5 mg/ml geneticin (GIBCO BRL) for 14 d. The presence of the dominant-negative construct was confirmed by immunofluorescence and Western blot anaylsis.
Ca2+ Imaging Using Fluorescence Resonance Energy Transfer Constructs
Cells were grown on glass coverslips. After 48 h in low serum medium, 8 μg of the cameleon fluorescence resonance energy transfer (FRET) construct, YC2.1, in pRSETB (a gift from Roger Tsien, University of California at San Diego), was transfected into the cells using Lipofectamine 2000, following the manufacturer's instructions. After 4 h, the medium was replaced with low-serum medium and cells were incubated for 24 h before use in experiments.
FRET Ca2+ imaging was performed as described previously (14). Coverslips were placed into an SA-NIK chamber (Warner Instruments, Hamden, CT) on a Nikon Diaphot 200 inverted microscope (Nikon, Melville, NY). Agents were perfused at room temperature into the chamber containing HEPES-buffered saline solution (10 mM HEPES, 6 mM KCl, 140 mM NaCl, 2 mM CaCl2, 1 mM MgCl2, 10 mM glucose). ATP was used as a positive control, as it has been previously shown to stimulate an increase in Ca2+ levels in epithelial cells (14). Ionomycin (10 μmol/l; Calbiochem) and EGTA (2 mmol/l) were used to determine the maximum and minimum values of intracellular Ca2+, respectively. To record Ca2+-induced FRET, cells were excited at a wavelength of 440 nm, and the emission ratio of 535/480 nm was recorded every 10 s with an acquisition time of 200 ms. A neutral density filter of 1.0 nm was used. Images were captured using a Hamamatsu camera (Hamamatsu, Bridgewater, NJ) and analyzed with Metafluor Imaging System 3.0 software (Universal Imaging Corporation, West Chester, PA). Data were normalized by subtracting out background from both 535 and 480 wavelengths and then dividing 535 by 480 to obtain the emission ratio.
Detection of Phospho-CREB, Phospho-ERK, and Ki-67 by Immunofluorescence
C10 cells were grown on glass coverslips for all experiments. After experimental exposures, immunofluorescence to detect phospho-CREB, phospho-ERK, and Ki-67, a marker of cell cycle progression, was performed as previously described (2, 10). Briefly, cells were washed with PBS, fixed in 3.7% paraformaldehyde, permeablized with −20°C methanol, and incubated with blocking solution containing 2% BSA in PBS. Cells were incubated with primary antibody (rabbit polyclonal phospho-p44/42 MAPK or rabbit polyclonal phospho-CREB [Cell Signaling Technologies, Beverly, MA], 1:250, or monoclonal rat Ki-67 [Dako, Carpinteria, CA], 1:50) diluted in 2% BSA plus 0.1% Triton X-100 in PBS (BSA/PBS-T) for 1 h at 37°C. Secondary antibody (Cy3-conjugated goat anti-rabbit IgG, 1:500, or AlexaFuor568-conjugated goat anti-rat IgG [Jackson Laboratories, West Grove, PA], 1:400), diluted in BSA/PBS-T, was applied for 1 h at 37°C, followed by incubation with nuclear counterstain, YOYO-1 iodide (1:10,000; Molecular Probes, Carlsbad, CA) and 1 unit/ml RNase in BSA/PBS-T for 30 min at 37°C. Coverslips were mounted onto slides with AquaPolyMount (Polysciences Inc., Warrington, PA). For each sample, confocal images were collected in fluorescence modes using a MRC1024ES confocal scanning laser microscope (Bio-Rad, Hercules, CA).
Western Blot Analysis for Phospho-ERK1/2
After C10 cells were exposed to agents as described previously here, the medium was aspirated and the cells were washed twice with cold wash buffer (250 mM sucrose, 25 mM Tris [pH 7.4], 2.5 mM MgOAc, and 2 mM DTT), collected in lysis buffer (250 mM sucrose, 25 mM Tris [pH 7.4], 2.5 mM MgOAc, 2.5 mM EGTA, 2 mM DTT, 1 mM NaVO3, 1 mM Na4O7P2, 10 μg/ml leupeptin, 1 μg/ml aprotinin, and 1 mM PMSF), sonicated 5× for 5 s, and centrifuged at 28,000 × g for 3 min to remove debris. Protein concentrations were determined using the Bradford protein assay (Bio-Rad). A total of 20 μg of protein was separated by 10% SDS-PAGE and transferred to nitrocellulose. Western blots were performed as described previously (2). In brief, transfer blots were blocked in Tris-buffered saline containing 5% nonfat powdered milk (Sigma), then incubated with antibodies specific to total and phosphorylated ERK1/2 (rabbit polyclonal total or phospho-p44/42 MAPK [Cell Signaling Technology], 1:1000). Antibody binding was detected using a horseradish peroxidase–conjugated anti-rabbit secondary antibody (1:5,000; Santa Cruz Biotechnology, Santa Cruz, CA), followed by chemiluminescence (Kirkgaard and Perry Laboratories, Gaithersburg, MD). QuantityOne (Bio-Rad) was used to quantify band density. Band densities were normalized to total ERK.
Western Blot Analysis for Phospho-CREB
After C10 cells were exposed to agents as described previously here, the medium was aspirated, and the cells were washed twice with cold PBS and collected in 4X sample buffer (200 μM Tris [pH 6.8], 4% SDS, 4 mg/ml β-mercaptoethanol, 40% glycerol, 2 μM pyronin-Y). The amount of protein in each sample was determined using the RC/DC protein assay (Bio-Rad). A total of 20 μg of protein was separated by 10% SDS-PAGE and transferred to nitrocellulose. Western blots were performed as described previously here, using antibodies specific to total and phosphorylated CREB (rabbit polyclonal CREB, 1:1000, and phospho-CREB, 1:500; Cell Signaling Technology). QuantityOne was used to quantify band density, and values were normalized to total CREB.
RNase Protection Assay
After C10 cells were exposed to agents as described previously, total RNA was prepared and quantitated as described by Shukla and colleagues (15). Steady-state mRNA levels of cdk5, c-fos, PCNA, cyclin D1, and bcl-2, the ribosomal probe L32, and glyceraldehyde-3-phosphate dehydrogenase were examined with a RiboQuant multiprobe RNase protection assay system and a custom-made multiprobe template set (Pharmingen, San Diego, CA) according to the manufacturer's protocol. Autoradiograms were developed and quantitated with a Bio-Rad phosphoimager. Results were normalized to the expression of the housekeeping gene, L32.
Detection and Quantitation of Apoptosis
Detection of apoptosis was performed as previously described (16). Briefly, cell monolayers grown on glass coverslips were treated with 300 μM H2O2 for 4, 8, or 24 h, then fixed in methanol for 24 h at −20°C, boiled for 5 min in PBS containing 5 mM MgCl2, and then immersed in ice-cold PBS for 10 min. Cells were blocked with 40% FBS and then incubated with Apostain F7–26 (10 μg/ml; Alexis Biochemicals, San Diego, CA) followed by AlexaFluor568-conjugated secondary antibody (goat anti-mouse IgM, 1:400; Jackson Labortories, West Grove, PA). Nuclei were counterstained with YOYO-1 iodide (1:10,000; Molecular Probes) and 1 unit/ml RNase in BSA/PBS-T for 30 min at room temperature. Coverslips were mounted onto slides with AquaPolyMount (Polysciences). For each sample, confocal images were collected in fluorescence modes using a Bio-Rad MRC1024ES confocal scanning laser microscope. To determine the number of apoptotic cells and total number of cells per field, five random fields were evaluated per experimental condition.
Statistical Analysis
Differences between treatment groups were assessed by two-way ANOVA in a randomized block design. The analyses were based on non-normalized data, and pair-wise comparisons between treatment groups were performed using Fisher's least significant difference procedure. Differences were considered statistically significant at P < 0.05.
RESULTS
H2O2 Initiates a Rise in Cytoplasmic Calcium that Is Prevented by Reducing Extracellular Calcium
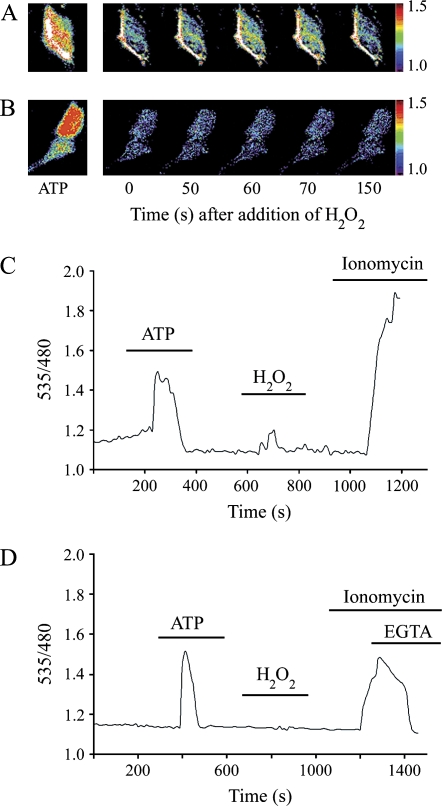
To measure calcium signaling in lung epithelial cells, the FRET-based chameleon Ca2+ indicator, YC2.1, was expressed in C10 cells. YC2.1 senses changes in intracellular Ca2+ levels through a Ca2+-dependent conformational change, resulting in FRET between yellow fluorescent protein (YFP) (480 nm emission) and cyan fluorescent protein (CFP) (535 nm emission) that can be detected by live cell fluorescence imaging (14). Intracellular Ca2+ levels are thus directly correlated to the 535/480 FRET ratio.
ATP stimulation resulted in a rapid and transient rise in intracellular Ca2+ levels. H2O2 stimulation resulted in a response that was consistent but less pronounced (Figures 1A and 1C). To determine the role of extracellular calcium in the H2O2-mediated Ca2+ response, cells were bathed in low-Ca2+ buffer (100 nM), and then intracellular Ca2+ concentration was monitored after the addition of ATP and H2O2. Although the ATP response was not dependent on extracellular Ca2+ levels, the H2O2-mediated Ca2+ signal was abolished by lowering extracellular Ca2+ (Figures 1B and 1D), suggesting that H2O2-mediated Ca2+ signaling requires entry of extracellular Ca2+.
Figure 1.
H2O2 initiates a rise in cytoplasmic calcium that requires extracellular Ca2+. C10 cells were transfected with the fluorescence resonance energy transfer (FRET)–based Ca2+ indicator, YC2.1, and the emission ratio of 535/480 nm (yellow fluorescent protein/cyan fluorescent protein [YFP/CFP]) was acquired every 10 s. (A) Pseudocolor images of the FRET emission ratio recorded live from a single cell expressing YC2.1. The color range represents a ratio of 535/480 from 1.0 (low Ca2+) to 1.5 (high Ca2+). ATP was used at 10 μM. Time 0 represents the addition of 200 μM H2O2. (B) Time-lapse images of the Ca2+ responses as in (A) using buffer containing low Ca2+ (100 nM). (C) Percent basal ratio (YFP/CFP) plot of the Ca2+ response from the live emission recording of a representative cell. Treatments were added to the bath as indicated by the lines on the plot. (D) Percent basal ratio (YFP/CFP) plot of the Ca2+ responses as in (C) using buffer containing low Ca2+ (100 nM).
H2O2 Stimulates Phosphorylation of CREB that Requires Extracellular Ca2+ and Is Regulated by ERK1/2
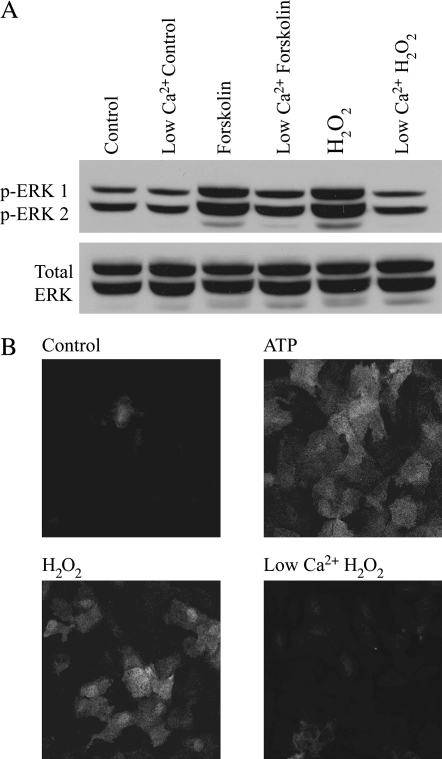
We have previously shown that H2O2 leads to ERK1/2 phosphorylation in lung epithelial cells (2). To determine if the observed Ca2+ influx stimulated by H2O2 is required for the activation of ERK1/2, phospho-ERK1/2 levels were detected by Western blot and immunofluorescence. The level of phospho-ERK1/2 increased in response to H2O2 when compared with control (100 ± 11.90 versus 658.27 ± 91.71; n = 3), and the response was reduced by lowering extracellular Ca2+ (658.27 ± 91.71 versus 348.96 ± 51.22; n = 3) (Figures 2A and 2B).
Figure 2.
Extracellular signal-regulated kinase 1 and 2 (ERK1/2) phosphorylation stimulated by H2O2 is dependent on Ca2+. (A, B) C10 cells were treated with 10 μM forskolin, 10 μM ATP, or 200 μM H2O2 for 10 min in buffer containing normal (2 mM) or low (100 nM) Ca2+ and analyzed by Western blot (A) or immunofluorescence (B) using an antibody specific for phospho-ERK1/2 (p-ERK1/2). An antibody recognizing total ERK was used as a control for protein loading in (A).
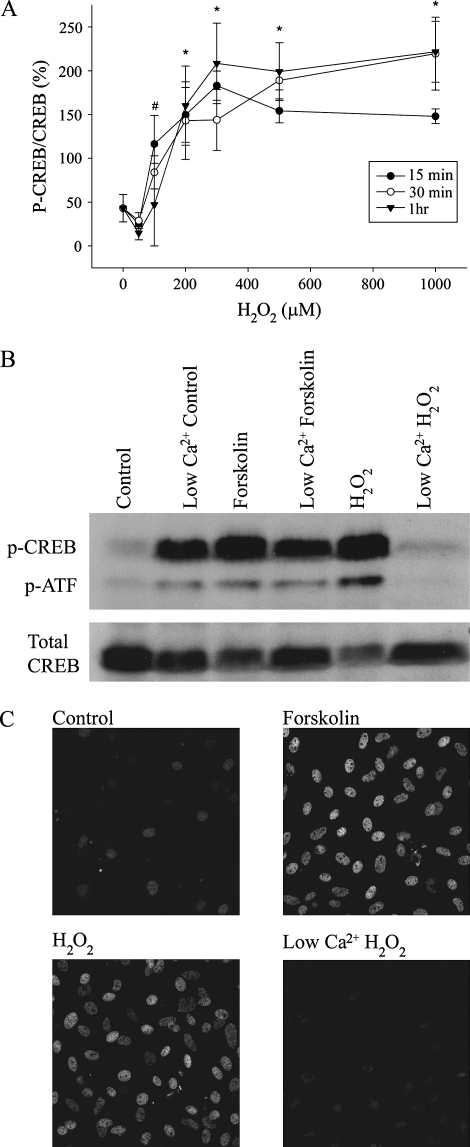
Because Ca2+ and ERK are important regulators of the transcription factor, CREB (7, 17), we investigated the possibility that H2O2 mediates CREB phosphorylation, using Western blot and immunofluorescence analyses. Exposure to bolus addition of H2O2 led to CREB phosphorylation in a dose- and time-dependent manner, with maximal activation occurring at 200 μM H2O2 (Figure 3A). Similar results were observed when glucose oxidase was used as the H2O2-generating system (data not shown). The induction of phospho-CREB was significantly reduced by lowering extracellular Ca2+ (281.82 ± 16.07 versus 155.09 ± 22.47; P < 0.05, n = 3) (Figure 3B, C), and the pattern of CREB phosphorylation in response to H2O2 closely paralleled that seen in response to activation of protein kinase A using forskolin.
Figure 3.
H2O2 mediates cAMP response element binding protein (CREB) phosphorylation in a dose- and time-dependent manner. (A) Protein expression of phospho-CREB was determined using Western blot analysis after C10 cells were exposed to H2O2 at the indicated dose and time points. The graph represents data normalized to the control response. #P < 0.05 at 15 min time point when compared with control; *P < 0.05 at all time points when compared with control. H2O2 induces CREB phosphorylation in a Ca2+-dependent manner. (B, C) C10 cells were treated with 10 μM forskolin or 200 μM H2O2 for 10 min in normal (2 mM) or low (100 nM) Ca2+ buffer and analyzed by Western blot (B) or immunofluorescence (C) using an antibody specific for phospho-CREB (p-CREB). An antibody recognizing total CREB was used as a control for protein loading in (B).
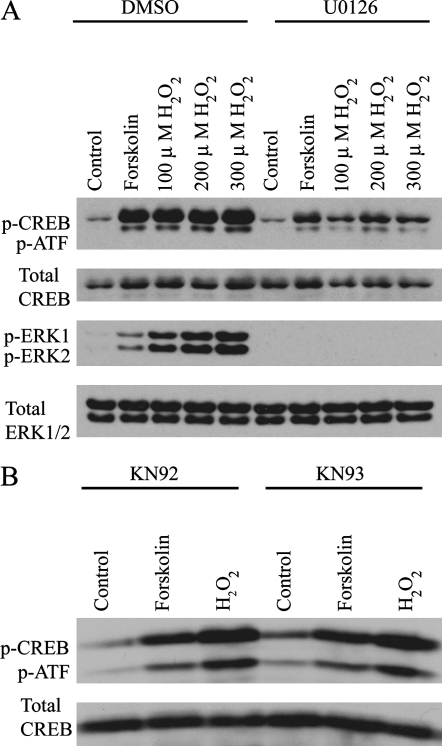
To determine the contributions of ERK1/2 and CaMKII in the observed H2O2-induced CREB phosphorylation, cells were evaluated with or without pretreatment with the MEK inhibitor, U0126, or the CaMKII inhibitor, KN93. H2O2-mediated CREB activation was partially inhibited by U0126 at 100, 200, and 300 μM concentrations of H2O2 (83.23 ± 13.41 versus 44.08 ± 11.95, 104.64 ± 10.61 versus 66.32 ± 10.44, and 100.40 ± 7.17 ver- sus 61.81 ± 9.77, respectively; n = 5) (Figure 4A). KN93 did not affect H2O2-mediated CREB activation (199.45 ± 18.72 versus 165.92 ± 29.37; n = 4) (Figure 4B), but did inhibit H2O2-mediated phospho-CaMKII activity (data not shown). Taken together, these data suggest that H2O2-mediated CREB phosphorylation is partially mediated by the MAPK/ERK pathway and is independent of CaMKII function.
Figure 4.
H2O2-mediated CREB activation is inhibited by the MEK inhibitor, U0126, but is unaffected by the CaMKII inhibitor, KN93. (A) C10 cells, preincubated with 10 μM U0126 for 30 min, were treated with 10 μM forskolin or 100, 200, or 300 μM H2O2 for 10 min, and then analyzed by Western blot for p-CREB, total CREB, phospho-ERK1/2 (p-ERK1/2), and total ERK1/2. (B) C10 cells were preincubated with 30 μM KN93 or its inactive isomer, KN92, for 30 min, and then treated with 10 μM forskolin or 200 μM H2O2 for 10 min. p-CREB was then detected by Western blot. An antibody recognizing total CREB was used as a control for protein loading in A and B.
Reduction of CREB Activity Inhibits c-fos Steady-State mRNA Levels, Enhances bcl-2 Steady-State mRNA Levels, and Significantly Reduces the Number of Cells Undergoing Apoptosis after H2O2 Exposure
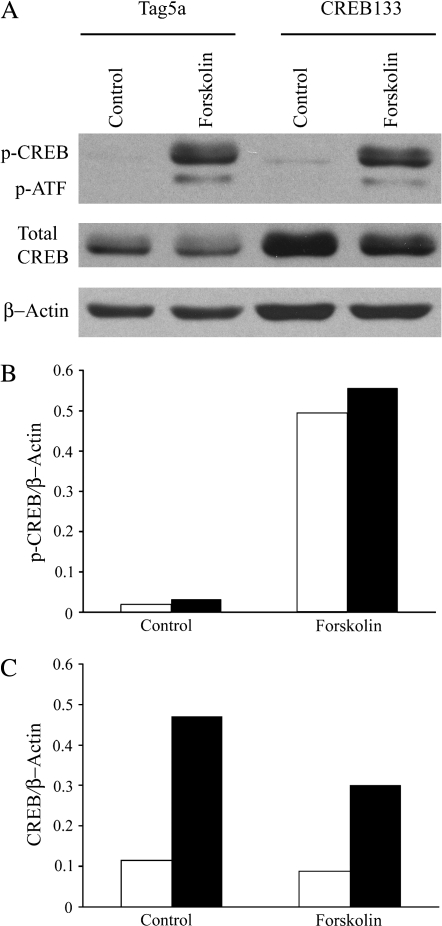
CREB has the capacity to regulate multiple genes involved in proliferation and apoptosis (9). To examine the role of CREB in H2O2-mediated proliferation and apoptosis, stable lines of C10 cells were generated that express a CREB phosphorylation mutant construct (CREB133) or empty vector (Tag5a). A mutation in 133Ser results in inactivation of CREB transcriptional activity (18). Expression of CREB133 resulted in an elevation of CREB expression, but did not change the forskolin response of endogenous CREB (Figure 5). Thus, as expected, the ratio of phospho-CREB/CREB is greatly reduced by the mutant (from 5.571 to 1.851 in response to forskolin), leading to inhibition of active dimer formation.
Figure 5.
Expression of CREB133 mutant results in a decreased p-CREB/CREB ratio. (A) C10 cells stably transfected with CREB133 or empty vector (Tag5a) were treated with 10 μM forskolin for 10 min, where indicated, and then analyzed by Western blot for p-CREB, total CREB, and β-actin. (B) Quantification of p-CREB in (A) normalized to β-actin. (C) Quantification of CREB in (A) normalized to β-actin.
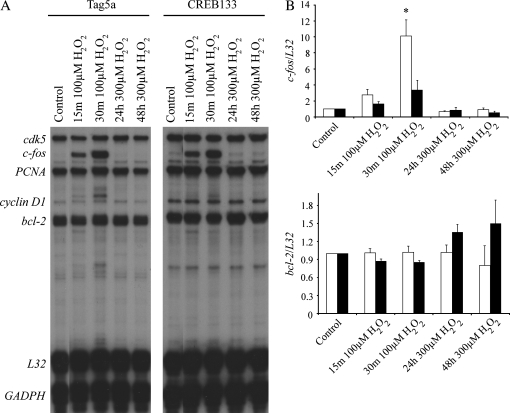
An RNase protection assay was used to measure the effect of CREB inhibition on H2O2-mediated induction of select CRE-containing genes, including c-fos and bcl-2. Exposure of cells to 100 μM H2O2, a concentration leading to proliferation at early time points (data not shown), led to an increase in c-fos steady-state mRNA levels that was reduced by expression of CREB133 (10.12 ± 0.108 versus 1.00 ± 0.023; P < 0.05, n = 3) (Figure 6). Exposure of cells to 300 μM H2O2, a concentration resulting in apoptosis after 24 h (2), resulted in a small but consistent increase in the steady-state mRNA level of bcl-2 only in cells expressing CREB133 cells (Figure 6), suggesting that CREB may exert negative regulation of bcl-2 in response to high concentrations of oxidants.
Figure 6.
Reduction of CREB activity using a CREB phosphorylation mutant leads to inhibition of c-fos steady-state mRNA levels, but enhanced bcl-2 steady-state mRNA levels after exposure to H2O2 at the indicated times and doses. (A) C10 cells stably transfected with CREB133 or empty vector (Tag5a) were treated with H2O2 at the indicated dose and time and then analyzed by RNase protection assay using a custom-made template of genes indicated on left. (B) Quantification of c-fos and bcl-2 transcription in response to H2O2. *P < 0.05 when compared with control.
To assess whether CREB activity is necessary for H2O2-induced proliferation, C10 cells stably transfected with Tag5a or CREB133 treated with 100 μM H2O2 were examined with the proliferation marker, Ki-67. After exposure to H2O2 for 1 h, both Tag5a and CREB133 cells showed a significant increase in the percentage of Ki-67-positive cells (3.72 ± 2.11% versus 11.69 ± 3.24% and 1.22 ± 1.21% versus 8.74 ± 1.49%, respectively; P < 0.05, n = 3). Although CREB133 cells exhibited a trend toward less proliferation when compared with Tag5a cells, it was not significant, suggesting that the reduction of CREB activity by CREB133 was not sufficient to inhibit H2O2-mediated proliferation.
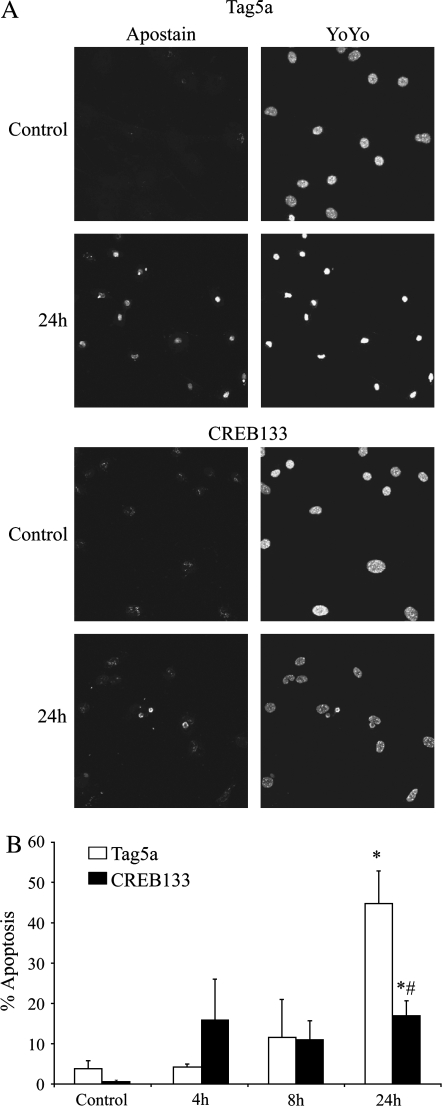
To determine whether CREB activation is essential for H2O2-induced apoptosis, C10 cells stably transfected with Tag5a or CREB133 were treated with 300 μM H2O2 and apoptotic cells were identified by Apostain. H2O2 induced a significant increase in the percentage of cells undergoing apoptosis compared with the control in both Tag5a and CREB133 cells (44.8 ± 8.1% versus 3.8 ± 1.8% and 16.9 ± 3.7% versus 0.5 ± 0.4%; P < 0.05, n = 3) (Figures 7A and 7B). However, CREB133 cells had significantly lower levels of apoptosis at 24 h than the Tag5a cells (16.9 ± 3.7% versus 44.8 ± 8.1%; P < 0.05, n = 3) (Figures 7A and 7B), supporting a role for CREB in the regulation of apoptosis induced by H2O2.
Figure 7.
Reduction of CREB activity significantly reduces the number of cells undergoing apoptosis after H2O2 exposure. (A) C10 cells stably transfected with CREB133 or empty vector (Tag5a) were treated with 300 μM H2O2 for the indicated times and analyzed by immunofluorescence using an antibody specific for single-stranded DNA (Apostain). Nuclei were stained with YOYO-1. (B) Quantification of percent apoptosis (number of Apostain-positive cells/total number of cells) in response to H2O2. *P < 0.05 when compared with control; #P < 0.05 when compared with Tag5a, 24 h, 300 μM H2O2.
DISCUSSION
Lung epithelial cells are exposed to ROS through direct inhalation, through the production of oxidants during inflammation, or through by-products generated during cell metabolism. Excessive production of oxidants plays a role in the pathogenesis of many pulmonary diseases, including asthma, pulmonary fibrosis, and cancers in lung epithelial cells (19). Here, we present the first evidence for a link between oxidants and apoptosis through Ca2+-mediated activation of ERK1/2 and the CREB transcription factor in lung epithelial cells.
An increase in ROS has been linked to effects on Ca2+ signaling in primary macrophages (20), vascular endothelium, and smooth muscle cells (21). However, a relationship between ROS and Ca2+ signaling has not been described previously in lung epithelial cells. Using a FRET-based Ca2+ indicator, we examined the effects of ROS on intracellular Ca2+ signals in single lung epithelial cells. We show that H2O2 elicits a transient increase in the intracellular Ca2+ level. The dependence of the H2O2-mediated Ca2+ signal on extracellular Ca2+ suggests that entry of Ca2+ is necessary, and that the signal is not likely generated through release of intracellular Ca2+ stores (22).
We have previously demonstrated that ERK1/2 is transiently phosphorylated in response to ROS in lung epithelial cells (2). Here, we show that H2O2-mediated ERK1/2 activation is partially regulated by Ca2+, indicating that Ca2+ is important for downstream signaling by ERK1/2 in cells responding to oxidants. Changes in intracellular Ca2+ levels have been shown to regulate the MAPK/ERK pathway through upstream activation of Ras in PC12 cells and primary neurons (17, 23). Rusanescu and colleagues (24) demonstrated that increased intracellular Ca2+ levels induced Shc phosphorylation, which ultimately leads to the activation of Ras. Ca2+ has also been implicated in regulating Ras by promoting enhanced GTPase activity directly or indirectly through calmodulin (23).
Recent studies show that H2O2 regulates the activity of CREB in astrocytes (25), glial cells (26), macrophages (27), and smooth muscle cells (28). The present study indicates that H2O2 also serves to activate CREB in lung epithelial cells. In addition, similar to H2O2-mediated ERK phosphorylation, CREB phosphorylation in response to H2O2 is regulated by extracellular Ca2+.
Ca2+ signaling has been implicated in the control of many cellular processes, including the activation of the CREB transcription factor (7). Although the CaMK pathway is often thought to be the primary Ca2+-mediated activator of CREB (29), we found that CREB phosphorylation after H2O2 exposure was not affected by blocking CaMKII activity, but was significantly reduced by inhibiting ERK1/2 signaling. These data are similar to the finding that H2O2 leads to CREB activation through the activation of ERK1/2 and p38 in vascular smooth muscle cells (28). Taken together, these data suggest that Ca2+ may be an important regulator of CREB through the ERK1/2 signaling pathway in lung epithelium exposed to oxidants.
CRE sequences are found in the promoters of many genes related to gene transcription, growth factors, and cell cycle (9). Modulation of c-fos is partially Ca2+- and CREB-dependent, suggesting that altered Ca2+ can affect expression of genes that contain a CRE (8). The mechanisms by which Ca2+-dependent signaling pathways regulate lung epithelial cell gene transcription are not yet known. We show that reduction of CREB activity inhibits c-fos steady-state mRNA levels after H2O2 exposure, indicating that CREB plays an essential role in the regulation of oxidant-induced c-fos steady-state mRNA levels. Although c-Fos has been implicated in the signal transduction between ROS and proliferation (30), our data indicate that CREB activity does not play a significant role in H2O2-induced proliferation. In contrast to vascular smooth muscle cells, these data indicate that although CREB regulates c-fos steady-state mRNA levels, CREB activity is not necessary for H2O2-mediated proliferation in lung epithelial cells (31, 32).
Inhibition of CREB function resulted in the desensitization of lung epithelial cells to induction of apoptosis after extended exposure to high concentrations of H2O2. A small, but consistent increase in bcl-2 transcript levels, when CREB function was suppressed, was also observed. These data thus support a role for CREB in the promotion of apoptosis after oxidant exposure. Although the observed effect on apoptosis is likely due to loss of CREB function, expression of the dominant-negative mutant has the potential to disrupt other signaling pathways not detected by the assays employed. These results are in contrast to findings in both neurons and vascular smooth muscle cells, where CREB plays a necessary role in growth factor–induced survival by stimulating expression of Bcl-2 through CREB. Furthermore, in those cells, reduction of CREB activity induces apoptosis, which can be rescued by overexpression of Bcl-2 (9, 33). The reasons for the discrepancies between lung epithelial cells, neurons, and smooth muscle cells are not clear, but in light of the multiple signaling pathways that regulate CREB, it is not surprising that different cell types and stimuli could lead to different physiologic outcomes.
In context of the findings of others, our data indicate that responses of lung epithelial cells to ROS include signaling through Ca2+- and ERK-dependent activation of CREB. Furthermore, subsequent changes in CREB-dependent gene expression play an integral role in oxidant-induced apoptosis. These data thus reveal an important role for CREB in oxidant-mediated signaling pathways in lung epithelial cells, and suggest a potential role for CREB in the development of pathologies related to oxidant-mediated lung injury.
Acknowledgments
The authors thank Douglas Taatjes, Ph.D., and Maria Stern from the University of Vermont Cell Imaging and Analysis Core, Tim Hunter from the Vermont Cancer Center DNA Analysis Facility for technical assistance, and Pam Vacek, Ph.D., for assistance with statistical analysis. The YC2.1 construct was generously supplied by Roger Tsien, Ph.D., University of California at San Diego.
This work was supported by National Institutes of Health Grant PO1 HL67004. C.A.B. was supported by Environmental Pathology Training Grant NIEHS T3207122.
Originally Published in Press as DOI: 10.1165/rcmb.2005-0153OC on September 8, 2005
Conflict of Interest Statement: None of the authors has a financial relationship with a commercial entity that has an interest in the subject of this manuscript.
References
- 1.Konishi H, Tanaka M, Takemura Y, Matsuzaki H, Ono Y, Kikkawa U, Nishizuka Y. Activation of protein kinase C by tyrosine phosphorylation in response to H2O2. Proc Natl Acad Sci USA 1997;94:11233–11237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Buder-Hoffmann S, Palmer C, Vacek P, Taatjes D, Mossman B. Different accumulation of activated extracellular signal-regulated kinases (ERK1/2) and role in cell-cycle alterations by epidermal growth factor, hydrogen peroxide, or asbestos in pulmonary epithelial cells. Am J Respir Cell Mol Biol 2001;24:405–413. [DOI] [PubMed] [Google Scholar]
- 3.Ishikawa Y, Kitamura M. Anti-apoptotic effect of quercetin: intervention in the JNK- and ERK-mediated apoptotic pathways. Kidney Int 2000;58:1078–1087. [DOI] [PubMed] [Google Scholar]
- 4.Janssen YM, Matalon S, Mossman BT. Differential induction of c-fos, c-jun, and apoptosis in lung epithelial cells exposed to ROS or RNS. Am J Physiol 1997;273:L789–L796. [DOI] [PubMed] [Google Scholar]
- 5.Jimenez LA, Zanella C, Fung H, Janssen YM, Vacek P, Charland C, Goldberg J, Mossman BT. Role of extracellular signal-regulated protein kinases in apoptosis by asbestos and H2O2. Am J Physiol 1997;273: L1029–L1035. [DOI] [PubMed] [Google Scholar]
- 6.Karin M. The regulation of AP-1 activity by mitogen-activated protein kinases. J Biol Chem 1995;270:16483–16486. [DOI] [PubMed] [Google Scholar]
- 7.Shaywitz AJ, Greenberg ME. CREB: a stimulus-induced transcription factor activated by a diverse array of extracellular signals. Annu Rev Biochem 1999;68:821–861. [DOI] [PubMed] [Google Scholar]
- 8.Lonze BE, Ginty DD. Function and regulation of CREB family transcription factors in the nervous system. Neuron 2002;35:605–623. [DOI] [PubMed] [Google Scholar]
- 9.Mayr B, Montminy M. Transcriptional regulation by the phosphorylation-dependent factor CREB. Nat Rev Mol Cell Biol 2001;2:599–609. [DOI] [PubMed] [Google Scholar]
- 10.Stevenson AS, Cartin L, Wellman TL, Dick MH, Nelson MT, Lounsbury KM. Membrane depolarization mediates phosphorylation and nuclear translocation of CREB in vascular smooth muscle cells. Exp Cell Res 2001;263:118–130. [DOI] [PubMed] [Google Scholar]
- 11.Roux PP, Richards SA, Blenis J. Phosphorylation of p90 ribosomal S6 kinase (RSK) regulates extracellular signal-regulated kinase docking and RSK activity. Mol Cell Biol 2003;23:4796–4804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Malkinson AM, Dwyer-Nield LD, Rice PL, Dinsdale D. Mouse lung epithelial cell lines: tools for the study of differentiation and the neoplastic phenotype. Toxicology 1997;123:53–100. [DOI] [PubMed] [Google Scholar]
- 13.Burch PM, Yuan Z, Loonen A, Heintz NH. An extracellular signal-regulated kinase 1– and 2–dependent program of chromatin trafficking of c-Fos and Fra-1 is required for cyclin D1 expression during cell cycle reentry. Mol Cell Biol 2004;24:4696–4709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Miyawaki A, Llopis J, Heim R, McCaffery JM, Adams JA, Ikura M, Tsien RY. Fluorescent indicators for Ca2+ based on green fluorescent proteins and calmodulin. Nature 1997;388:882–887. [DOI] [PubMed] [Google Scholar]
- 15.Shukla A, Timblin C, BeruBe K, Gordon T, McKinney W, Driscoll K, Vacek P, Mossman BT. Inhaled particulate matter causes expression of nuclear factor (NF)-kappaB–related genes and oxidant-dependent NF-kappaB activation in vitro. Am J Respir Cell Mol Biol. 2000;23:182–187. [DOI] [PubMed] [Google Scholar]
- 16.Shukla A, Stern M, Lounsbury KM, Flanders T, Mossman BT. Asbestos-induced apoptosis is protein kinase C delta–dependent. Am J Respir Cell Mol Biol 2003;29:198–205. [DOI] [PubMed] [Google Scholar]
- 17.Xing J, Ginty DD, Greenberg ME. Coupling of the RAS-MAPK pathway to gene activation by RSK2, a growth factor–regulated CREB kinase. Science 1996;273:959–963. [DOI] [PubMed] [Google Scholar]
- 18.Gonzalez GA, Yamamoto KK, Fischer WH, Karr D, Menzel P, Biggs W III, Vale WW, Montminy MR. A cluster of phosphorylation sites on the cyclic AMP–regulated nuclear factor CREB predicted by its sequence. Nature 1989;337:749–752. [DOI] [PubMed] [Google Scholar]
- 19.Mossman BT, Churg A. Mechanisms in the pathogenesis of asbestosis and silicosis. Am J Respir Crit Care Med 1998;157:1666–1680. [DOI] [PubMed] [Google Scholar]
- 20.Brown DM, Donaldson K, Borm PJ, Schins RP, Dehnhardt M, Gilmour P, Jimenez LA, Stone V. Calcium and ROS-mediated activation of transcription factors and TNF-{alpha} cytokine gene expression in macrophages exposed to ultrafine particles. Am J Physiol Lung Cell Mol Physiol 2004;286:L344–L353. [DOI] [PubMed] [Google Scholar]
- 21.Lounsbury KM, Hu Q, Ziegelstein RC. Calcium signaling and oxidant stress in the vasculature. Free Radic Biol Med 2000;28:1362–1369. [DOI] [PubMed] [Google Scholar]
- 22.Putney JW Jr. A model for receptor-regulated calcium entry. Cell Calcium 1986;7:1–12. [DOI] [PubMed] [Google Scholar]
- 23.Rosen LB, Ginty DD, Weber MJ, Greenberg ME. Membrane depolarization and calcium influx stimulate MEK and MAP kinase via activation of Ras. Neuron 1994;12:1207–1221. [DOI] [PubMed] [Google Scholar]
- 24.Rusanescu G, Qi H, Thomas SM, Brugge JS, Halegoua S. Calcium influx induces neurite growth through a Src-Ras signaling cassette. Neuron 1995;15:1415–1425. [DOI] [PubMed] [Google Scholar]
- 25.Rosenberger J, Petrovics G, Buzas B. Oxidative stress induces proorphanin FQ and proenkephalin gene expression in astrocytes through p38- and ERK-MAP kinases and NF-kappaB. J Neurochem 2001;79:35–44. [DOI] [PubMed] [Google Scholar]
- 26.Iwata E, Asanuma M, Nishibayashi S, Kondo Y, Ogawa N. Different effects of oxidative stress on activation of transcription factors in primary cultured rat neuronal and glial cells. Brain Res Mol Brain Res 1997;50:213–220. [DOI] [PubMed] [Google Scholar]
- 27.Kurata S. Selective activation of p38 MAPK cascade and mitotic arrest caused by low level oxidative stress. J Biol Chem 2000;275:23413–23416. [DOI] [PubMed] [Google Scholar]
- 28.Ichiki T, Tokunou T, Fukuyama K, Iino N, Masuda S, Takeshita A. Cyclic AMP response element–binding protein mediates reactive oxygen species–induced c-fos expression. Hypertension 2003;42:177–183. [DOI] [PubMed] [Google Scholar]
- 29.Bito H, Deisseroth K, Tsien RW. CREB phosphorylation and dephosphorylation: a Ca(2+)- and stimulus duration–dependent switch for hippocampal gene expression. Cell 1996;87:1203–1214. [DOI] [PubMed] [Google Scholar]
- 30.Han MJ, Kim BY, Yoon SO, Chung AS. Cell proliferation induced by reactive oxygen species is mediated via mitogen-activated protein kinase in Chinese hamster lung fibroblast (V79) cells. Mol Cells 2003;15:94–101. [PubMed] [Google Scholar]
- 31.Klemm DJ, Watson PA, Frid MG, Dempsey EC, Schaack J, Colton LA, Nesterova A, Stenmark KR, Reusch JE. cAMP response element–binding protein content is a molecular determinant of smooth muscle cell proliferation and migration. J Biol Chem 2001;276:46132–46141. [DOI] [PubMed] [Google Scholar]
- 32.Reusch JE, Klemm DJ. Cyclic AMP response element–binding protein in the vessel wall: good or bad? Circulation 2003;108:1164–1166. [DOI] [PubMed] [Google Scholar]
- 33.Riccio A, Ahn S, Davenport CM, Blendy JA, Ginty DD. Mediation by a CREB family transcription factor of NGF-dependent survival of sympathetic neurons. Science 1999;286:2358–2361. [DOI] [PubMed] [Google Scholar]