Abstract
Pulmonary accumulation of fibroblasts and myofibroblasts in idiopathic pulmonary fibrosis/usual interstitial pneumonia (IFP/UIP) has been linked to (1) increased migration of a circulating pool of fibrocytes, (2) cell proliferation, and (3) resistance to apoptosis. The mechanism of physiologic apoptosis of lung fibroblasts is poorly understood. Using normal and fibrotic human lung fibroblasts and the human lung fibroblast cell line, MRC-5, we examined the regulation of Fas-induced apoptosis by the proinflammatory cytokines TNF-α and IFN-γ. Herein, we show that the basal resistance of lung fibroblasts and myofibroblasts to Fas-induced apoptosis is overcome by sensitization with TNF-α. IFN-γ did not sensitize cells to Fas-induced apoptosis, but exhibited synergistic activity with TNF-α. Sensitization by TNF-α was observed in MRC-5 cells and in fibroblasts and myofibroblasts from normal and fibrotic human lung, suggesting that this represents a conserved mechanism to engage Fas-induced apoptosis. The mechanism of sensitization was localized at the level of recruitment of the adapter protein, FADD, to the cytoplasmic domain of Fas. Collectively, these findings suggest that fibroblast apoptosis involves two steps, sensitization and induction, and that inadequate pulmonary inflammation in IPF/UIP may favor fibroblast accumulation by reducing sensitization to apoptosis.
Keywords: fibroblast, TNF-α, Fas, apoptosis, pulmonary fibrosis
Idiopathic pulmonary fibrosis/usual interstitial pneumonia (IPF/UIP) is a progressive fibrosing interstitial lung disease of unknown etiology. Invariably fatal, it has no known effective therapy. Pathologically, IPF/UIP is characterized by the identification of a pattern of usual interstitial pneumonitis (UIP) on surgical lung biopsy (1). This pattern is histopathologically heterogeneous with areas of patchy subpleural fibrosis alternating with regions of near-normal lung tissue. The fibroblast focus, a pathologic hallmark of UIP, has been suggested to represent the leading edge of the fibrotic process (2, 3) and is composed of fibroblasts and myofibroblasts enmeshed within the extracellular connective tissue matrix (ECM). Since fibroblasts and myofibroblasts synthesize and deposit collagens and other ECM components within these fibrotic lesions (4), a fuller understanding of the origin and fate of fibroblasts and myofibroblasts in IPF/UIP may provide insights into how the accumulation of these cells can be controlled or regulated in this disorder.
Recent studies have focused on a role for circulating CD45+CXCR4+ColI+ fibrocytes in the accumulation of fibroblasts and myofibroblasts in animal models of pulmonary fibrosis (5, 6). However, the mechanisms that enable these cells to persist in pulmonary fibrotic disorders remain poorly understood. Studies of dermal wound repair have suggested that at the completion of the repair process, fibroblasts and myofibroblasts undergo apoptosis and disappear from areas of remodeled tissue (7, 8), while other studies have shown that pulmonary myofibroblasts undergo apoptosis and rapidly disappear from the newborn lung at the completion of postnatal alveolarization (9). These collective studies suggest that although fibroblasts and myofibroblasts are fully capable of undergoing apoptosis, the environment of the fibrotic lung somehow prevents this from happening, an hypothesis that is consistent with previous studies documenting low levels of apoptosis in lung biopsy specimens from patients with IPF/UIP (10).
Little is known about the physiologic stimuli that promote fibroblast and myofibroblast apoptosis in the lung. Fas, a death receptor of the tumor necrosis factor receptor superfamily, has been implicated in the development of bleomycin-induced pulmonary fibrosis in mice primarily through its ability to promote apoptosis of alveolar epithelial cells (11, 12). Paradoxically, mice bearing inactivating mutations in Fas (lpr) and FasL (gld) spontaneously develop an interstitial pneumonitis and lung pathology similar to that seen in patients with connective tissue disease–associated lung fibrosis (13). These findings suggest that while Fas–FasL interactions may be important in the initiation of epithelial injury and apoptosis, they may also be important in the physiologic apoptosis of mesenchymal cells. However, previous in vitro studies have shown that lung fibroblasts are resistant to Fas-induced apoptosis (14, 15). Thus, additional mechanisms or stimuli must exist to allow lung fibroblasts and myofibroblasts to undergo Fas-induced apoptosis in vivo. Furthermore, we would expect that these additional mechanisms or stimuli are inactive or not present during the progression of pulmonary fibrosis.
Contrary to the previously held view that fibrosis in IPF/UIP arises as a consequence of chronic inflammation (16), recent studies have suggested that the progression of pulmonary fibrosis, especially within the fibroblast focus, occurs in the absence of an inflammatory cell infiltrate (17). These findings also imply that fibrosis may progress or worsen in the absence (or the presence of reduced levels) of proinflammatory cytokines, such as TNF-α and IFN-γ. Indeed, several lines of evidence support the concept that TNF-α and IFN-γ serve to limit fibrogenesis. Based on earlier observations that IFNs protect mice from bleomycin-induced pulmonary fibrosis (18, 19), Jiang and coworkers (20) recently showed that the production of IFN-γ by resident pulmonary NK cells during the first 24 h of injury limits the fibrotic response 21 d later. Recent clinical studies in patients with IPF/UIP have also suggested that pulmonary fibrosis is associated with a relative deficiency in pulmonary IFN-γ and/or IFN-γ–induced chemokine expression (21, 22). TNF-α also appears to play a dual role in fibrosis by initially participating in epithelial injury but later serving to limit the fibrogenic response. Duality in TNF-α function would thus be consistent with early studies showing an inhibition of bleomycin-induced pulmonary fibrosis in mice treated with anti–TNF-α antibody (23) and in TNF-R1 and TNF-R2 double-null mice (24). It would also be consistent with work in transgenic mice showing that pulmonary expression of TNF-α reduces the extent of bleomycin-induced pulmonary fibrosis and promotes lung repair (25, 26). Thus, while TNF-α and IFN-γ may be important in promoting initial epithelial cell injury, they also appear to play a key role in limiting the extent of subsequent fibrosis. Taking cues from these findings, we hypothesized that pulmonary fibroblasts and myofibroblasts are intrinsically resistant to Fas-induced apoptosis in the absence of the proinflammatory cytokines, TNF-α and IFN-γ, but are rendered sensitive to Fas-induced apoptosis in their presence. Herein, we show that exposure to TNF-α sensitizes human lung fibroblasts and myofibroblasts to Fas-induced apoptosis while IFN-γ augments the efficiency of TNF-α–induced sensitization. In addition, we show that the mechanism of sensitization by TNF-α is localized to the initial recruitment of the adapter protein FADD to the cytoplasmic domain of Fas.
MATERIALS AND METHODS
Materials
TNF-α and IFN-γ were purchased from R&D Systems (Minneapolis, MN). Agonistic anti-human Fas antibody (clone CH-11) was obtained from Upstate Biotechnology (Lake Placid, NY). The anti-human Fas antibody used for immunoprecipitation and Western blotting, anti-FADD, and anti-vimentin antibodies were obtained from Santa Cruz Biotechnology (Santa Cruz, CA). Anti–caspase-8 antibody was obtained from Cell Signaling Technologies (Beverly, MA). Anti–α-smooth muscle actin antibody was obtained from Sigma (St. Louis, MO). Anti–pro-collagen I was obtained from Alexis (San Diego, CA).
Cell Culture Methods
The MRC-5 (CCL-171) cell line, a human fetal lung fibroblast cell line, was obtained from ATCC (Rockville, MD) and maintained in Dulbecco's minimal essential medium (DMEM) supplemented with 100 U/ml penicillin, 100 μg/ml streptomycin, and 10% heat-inactivated fetal calf serum. Primary cultures of normal human lung fibroblasts were derived from nondiseased human lungs. Whole lung samples were obtained from Tissue Transformation Technologies (Edison, NJ), in accordance with an approved Institutional Review Board (IRB) protocol. All donors suffered brain death and were evaluated for organ donation before research consent. All lung samples failed regional lung selection criteria for transplantation. For inclusion in this study, individuals had to have no evidence of current systemic or pulmonary infection, a clear chest radiograph, and PaO2/fraction of inspired oxygen ratio > 250 mm Hg O2. Patients were excluded if they had any history of lung disease or a history of systemic disease that commonly affects the lungs (e.g., rheumatoid arthritis or systemic lupus erythematosus). The clinical characteristics for the tissue donors are shown in Table 1. All fibrotic lung fibroblasts were derived from surgical lung biopsy specimens banked with the Interstitial Lung Disease Program at National Jewish Medical and Research Center. Study subjects had been prospectively enrolled in our (IRB) approved National Institutes of Health (NIH) supported specialized center of research (SCOR) longitudinal study of interstitial lung disease. All pathologic diagnoses were determined via review by an expert pulmonary pathologist as part of the SCOR program. All clinical diagnoses were determined by the ILD Program physician who was caring for the patient. The clinical characteristics of the patients from whom fibrotic lung fibroblasts were obtained are shown in Table 1.
TABLE 1.
DEMOGRAPHICS OF HUMAN LUNG AND TISSUE DONORS
| A. Nondiseased Lung Donors
| ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Age (yr) | Sex | Race | Cause of Death | Medical History | ||||||
| HLF 01 | 59 | F | White | Stroke | Hypertension, non–insulin-dependent diabetes | |||||
| HLF 02 | 64 | M | White | Stroke | Hypertension | |||||
| HLF 03 | 53 | F | White | Stroke | Cerebral aneurysm, hypertension | |||||
| HLF 04 | 54 | F | African-American | Head trauma | Non–insulin-dependent diabetes, hypertension | |||||
| B. IPF/UIP Lung Donors
|
||||||||||
| Age (yr) | Sex | Race | Clinical-Pathologic Diagnosis | |||||||
| FHLF 01 | 66 | M | White | IPF/UIP | ||||||
| FHLF 02 | 77 | F | White | IPF/UIP | ||||||
| FHLF 03 | 48 | M | White | Familial IPF/UIP | ||||||
| FHLF 04 | 73 | M | White | IPF/UIP | ||||||
Definition of abbreviation: IPF/UIP, idiopathic pulmonary fibrosis/usual interstitial pneumonia.
Freshly harvested explants were minced into 1–2 mm3 sections and cultured in DMEM containing 10% heat-inactivated fetal calf serum media on scored tissue culture dishes. After 10–14 d, fibroblasts derived from the explants were trypsinized and maintained in tissue culture. All experiments were performed on early passage (2–4) cell cultures. MRC-5 cells or primary cultures of normal human lung fibroblasts were incubated with TGF-β (2 ng/ml) for 72 h to induce myofibroblast differentiation as described (27) and assessed for α-smooth muscle actin (α-SMA) expression and organization by Western blotting and confocal microscopy, respectively.
Apoptosis Methods
Active caspase-8 was determined by using the Fluorochrome Inhibitor of Caspase Apoptosis (FLICA) Detection Kit from Immunochemistry Technologies, LLC (Bloomington, MN) followed by analysis on a FACScalibur flow cytometer and Cell Quest software (both from BD Biosciences, San Diego, CA). Caspase-3 activity was determined by a commercially available fluorometric substrate assay used in accordance with the manufacturer's protocol (BD Biosciences-PharMingen, San Diego, CA). Mitochondrial depolarization was quantified by JC-1 staining (Molecular Probes, Eugene, OR) at 2.5 μg/ml for 1 h at 37°C. The ratio of green:red fluorescence was determined by flow cytometry and analyzed with Cell Quest software. Phosphatidylserine (PS)/7-amino-actinomycin D (7AAD) analysis was performed on trypsinized cells by staining with FITC–annexin V (BD Biosciences) and 7AAD (BD Biosciences), followed by flow cytometry as described (28). Apoptotic cells were defined as annexin V–positive and 7AAD-negative. TUNEL staining was performed as per manufacturer's protocol with a commercially available kit (Roche Diagnostics, Indianapolis, IN).
Co-immunoprecipitation
Cells were lysed in NP-40 lysis buffer (50 mM Tris-HCl, pH 7.4, 136 mM NaCl, 10% [vol/vol] glycerol, 1% [vol/vol] NP-40, 1 mM NaF, 1 mM phenylmethysufonyl fluoride, 10 μg/ml leupeptin, 5 μg/ml aprotinin, and 1 mM Na3VO4) and centrifuged at 14,000 rpm for 10 min at 4°C to obtain postnuclear supernatants. Protein content was quantitated using the BCA protein quantitation kit in accordance with the manufacturer's protocol (Pierce, Rockford, IL). A quantity of 250 μg of protein in 500 μl of lysis buffer was pre-cleared with 25 μl of a 1:1 slurry of protein G Sepharose beads. Fas was then immunoprecipitated with 1 μg of goat anti-human Fas antibody or nonimmune goat IgG as a control together with 25 μl of protein G Sepharose beads for 24 h at 4°C. The beads were washed three times in lysis buffer, boiled in Laemlli sample buffer, and resolved by electrophoresis through 12% (wt/vol) SDS-polyacrylamide gels, as described (29). Detection of specific proteins was by Western blot analysis as described (30).
Quantification of Fas
Cell surface expression of Fas was determined by flow cytometry. Cells were harvested by incubation in PBS containing 1 mM EDTA (pH 7.4) for 30 min followed by gentle scraping with a cell lifter. The cells were stained with 10 μl of APC-labeled anti-Fas antibody (clone DX2; B&D Biosciences) or 10 μl of APC-labeled nonimmune IgG (B&D Biosciences) for 4 h at 4°C. After washing, the stained cells were processed on a FACScalibur flow cytometer and analyzed with Cell Quest software. Cell viability was confirmed before flow analysis by trypan blue exclusion and was consistently > 90%. Total cellular Fas levels were determined by ELISA. Fas ELISA plates were obtained from ELISATech (Denver, CO) and used according to the manufacturer's protocol. Fas concentrations were determined by interpolation against a standard curve generated with recombinant Fas extracellular domain. The limit of sensitivity of the assay was 50 pg/ml.
Immunocytochemistry
Immunocytochemistry studies were performed as previously described (29).
Statistical Analysis
All statistical analysis was performed on Prism software (GraphPad, San Diego, CA).
RESULTS
Characterization of Fibroblast and TGF-β–Differentiated Myofibroblast Populations
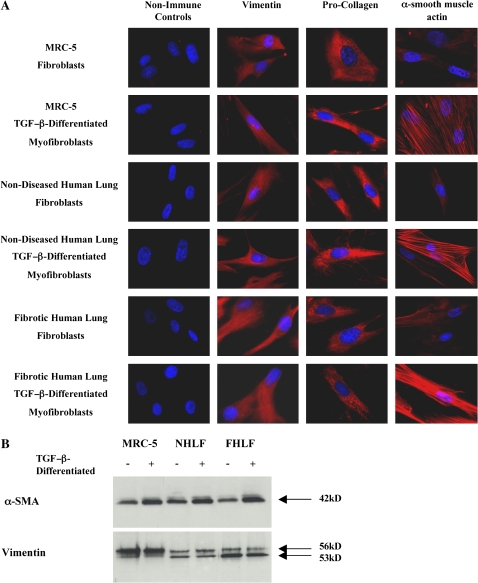
MRC-5 cells, a nontransformed human fetal lung fibroblast cell line, normal nondiseased human lung fibroblasts, and fibroblasts derived from patients with histologically proven IPF/UIP were characterized to confirm their fibroblast/myofibroblast phenotype and assure the uniformity of the populations. Figure 1 depicts representative immunocytochemical studies for both undifferentiated and TGF-β–differentiated cell populations. All fibroblast cell populations stained positive (> 95%) for both vimentin and pro-collagen–I confirming their fibroblast phenotype. Myofibroblast-like cells were obtained by incubating MRC-5 cells, nondiseased human lung fibroblasts, and fibrotic lung fibroblasts with TGF-β1 (2 ng/ml) for 72 h as previously described (27). All TGF-β–differentiated populations stained positive (> 95%) for vimentin, pro-collagen, and α-SMA. Moreover, as can be seen in Figure 1, α-SMA in the TGF-β–differentiated populations was organized into filaments, suggesting a myofibroblast phenotype. Western blotting for α-SMA showed that undifferentiated MRC-5 cells, nondiseased human lung fibroblasts, and fibrotic lung fibroblasts all express appreciable and similar levels of α-SMA at baseline and that TGF-β differentiation increased α-SMA expression in all cell populations (Figure 1B).
Figure 1.
Characterization of fibroblasts and myofibroblasts. (A) Immunocytochemistry for vimentin, pro-collagen I, and α-SMA expression in MRC-5, nondiseased human lung and fibrotic human lung fibroblast and TGF-β–differentiated myofibroblast cell populations. All fibroblast populations stained positive for vimentin and pro-collagen I with > 95% uniformity. All myofibroblast populations stained positive for vimentin, pro-collagen I, and α-SMA with > 95% uniformity and demonstrated organization of α-SMA into filaments. (B) Western blots showing relative expression of vimentin and α-SMA by MRC-5, nondiseased human lung and fibrotic human lung fibroblast and TGF-β–differentiated myofibroblast cell populations.
TNF-α Sensitizes MRC-5 Cells to Fas-Induced Apoptosis
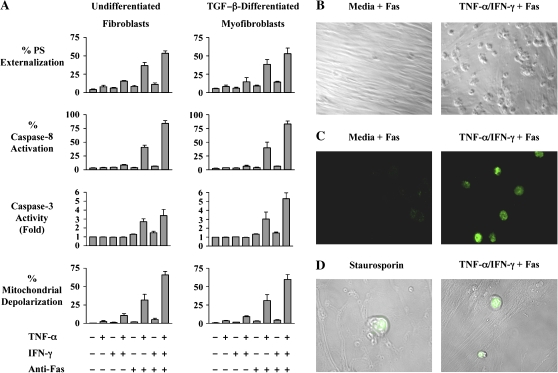
To assess the effects of cytokines on Fas-induced apoptosis, monolayers of MRC-5 cells were incubated with medium alone or were stimulated with TNF-α (20 ng/ml), IFN-γ (50 U/ml), or with both cytokines for 36 h. The cells were then stimulated with agonistic anti-Fas antibody (250 ng/ml) for 6 h and assayed for apoptosis by quantifying: (1) annexin-V binding, (2) activation of caspase-8 and -3, and (3) mitochondrial depolarization (Figure 2A). As previously reported for fibroblast cell lines and primary cultures of human dermal and lung fibroblasts (14, 31, 32), exposure of MRC-5 fibroblasts to agonistic anti-Fas antibody alone failed to induce apoptosis. Exposure to either TNF-α alone or IFN-γ alone, in the absence of Fas ligation, also failed to induce apoptosis. However, after pre-incubation with TNF-α, 41% of MRC-5 fibroblasts underwent Fas-induced apoptosis as assessed by caspase-8 activation (P < 0.001), whereas 84% were apoptotic after pre-incubation with TNF-α and IFN-γ and stimulation with anti-Fas antibody (P < 0.001). Similar results were also obtained when apoptosis was assessed by phosphatidylserine externalization, mitochondrial depolarization, and by measurement of caspase-3 activity (Figure 2A). In addition, brightfield microscopy and TUNEL staining were performed to confirm the presence of morphologic features of apoptosis, DNA cleavage, and irreversible commitment to the apoptotic pathway (Figures 2B–2D). Small increases in background apoptosis were also noted with TNF-α plus IFN-γ sensitization alone compared with control conditions but did not achieve statistical significance (P > 0.05).
Figure 2.
TNF-α–induced sensitization of MRC-5 fibroblasts and TGF-β–differentiated myofibroblasts to Fas-induced apoptosis. (A) MRC-5 fibroblasts and TGF-β–differentiated myofibroblasts were incubated in medium alone or with TNF-α (20 ng/ml), IFN-γ 50 (U/ml), or both cytokines for 36 h before challenge with agonistic anti-Fas antibody (250 ng/ml) for 6 h. The percentages of apoptotic cells were quantified by phosphatidylserine externalization, activation of caspases-8 and -3, and mitochondrial depolarization. The data shown represent the mean ± SD of at least three independent experiments. (B) Brightfield images of MRC-5 fibroblasts incubated with medium alone or TNF-α plus IFN-γ for 36 h before exposure to agonistic anti-Fas antibody for 6 h (magnification: ×10). (C) TUNEL staining of MRC-5 fibroblasts incubated with medium alone or TNF-α plus IFN-γ for 36 h before exposure to agonistic anti-Fas antibody for 6 h (magnification: ×63). (D) TUNEL staining superimposed on Nomarski images correlating morphologic changes of apoptosis with DNA cleavage. The MRC-5 fibroblasts pictured on the left were treated with 1 μM staurosporine for 12 h (positive control), whereas the cells on the right were treated with TNF-α plus IFN-γ for 36 h before exposure to agonistic anti-Fas antibody for 6 h.
Since myofibroblasts are also present in lung tissues of patients with IPF/UIP, we next determined if TNF-α and IFN-γ were capable of sensitizing myofibroblasts to Fas-induced apoptosis. As can be seen in Figure 2A, TGF-β–differentiated MRC-5 cells responded similarly to undifferentiated cells with regards to (1) basal resistance to Fas-induced apoptosis, (2) the effect of pre-incubation with TNF-α alone, and (3) the augmentation of TNF-α–induced sensitization to Fas-induced apoptosis by IFN-γ. Collectively, these findings suggest that both fibroblast-like and myofibroblast-like MRC-5 cells are resistant to Fas- induced apoptosis in the absence of cytokines but are sensitized to Fas-induced apoptosis by prior exposure to TNF-α alone, or in combination with IFN-γ.
TNF-α Sensitizes Human Lung Fibroblasts to Fas-Induced Apoptosis
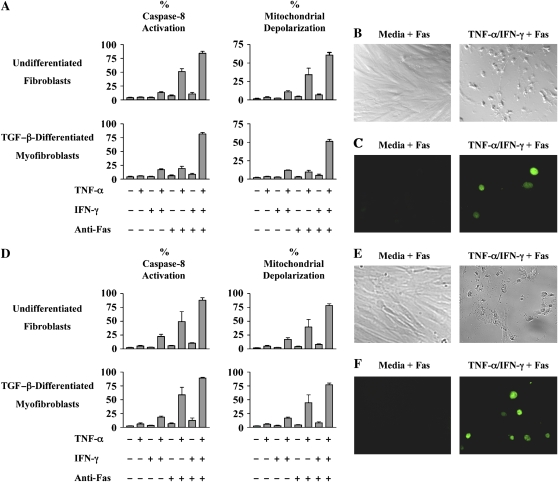
To validate the findings from the MRC-5 cells, we next determined if TNF-α sensitized primary cultures of normal human lung fibroblasts to Fas-induced apoptosis. Human lung fibroblasts were isolated from lung explants of four organ donors who died from nonpulmonary causes (Table 1). The optimal time points for sensitization and apoptosis in primary cells were determined in preliminary experiments. Accordingly, early passage fibroblasts were incubated in medium alone or were stimulated with TNF-α (20 ng/ml), IFN-γ (50 U/ml), or with both cytokines for 48 h before stimulation with agonistic anti-Fas antibody (250 ng/ml) or medium alone for 12 h. As can be seen in Figure 3, < 5% of normal human lung fibroblasts were sensitive to Fas-induced apoptosis when pre-incubated in medium alone or with IFN-γ alone. However, after pre-incubation with TNF-α, 51% of the cells were apoptotic as reflected by caspase-8 activation after Fas ligation (Figure 3A, P < 0.001). Concurrent pre-incubation with TNF-α and IFN-γ increased the level of Fas-induced apoptosis to 84% (Figure 3A, P < 0.001). Similar results were obtained when apoptosis was quantified by mitochondrial depolarization (Figure 3A, P < 0.001), TUNEL staining (Figure 3B), and by expression of the morphologic features of apoptosis (Figure 3C). Elevated background levels of apoptosis were again noted with pretreatment with TNF-α plus IFN-γ in the absence of Fas ligation, through these did not achieve statistical significance.
Figure 3.
(A) TNF-α–induced sensitization of normal human lung fibroblasts and TGF-β–differentiated myofibroblasts to Fas-induced apoptosis. Early passage normal human lung fibroblasts and TGF-β–differentiated (2 ng/ml, 72 h) myofibroblasts were incubated in medium alone or with TNF-α (20 ng/ml), IFN-γ (50 U/ml), or both cytokines for 48 h before exposure to agonistic anti-Fas antibody (250 ng/ml) for 12 h. The percentages of apoptotic cells were quantified by assays for caspase-8 activation and mitochondrial depolarization. The data shown represent the composite mean ± SD of fibroblasts derived from four donors each evaluated independently in triplicate. (B) Brightfield images of normal human lung fibroblasts incubated with medium alone or TNF-α plus IFN- γ for 48 h before exposure to agonistic anti-Fas anti-body for 12 h (magnification: ×10). (C) TUNEL staining of normal human lung fibroblasts incubated with medium alone or TNF-α plus IFN-γ for 48 h before exposure to agonistic anti-Fas antibody for 12 h (magnification: ×63). (D) TNF-α–induced sensitization of human fibrotic lung fibroblasts and TGF-β–differentiated myofibroblasts to Fas-induced apoptosis. Early passage human fibrotic lung fibroblasts and TGF-β–differentiated myofibroblasts were incubated in medium alone or with TNF-α (20 ng/ml), IFN-γ (50 U/ml), or both cytokines for 48 h before exposure to agonistic anti-Fas antibody (250 ng/ml) for 12 h. The percentages of apoptotic cells were quantified by assays for caspase-8 activation mitochondrial depolarization. The data shown represent the composite mean ± SD of fibroblasts derived from four donors each evaluated independently in triplicate. (E) Brightfield images of fibrotic human lung fibroblasts incubated with medium alone or TNF-α plus IFN- γ for 48 h before exposure to agonistic anti-Fas antibody for 12 h (magnification: ×10). (F) TUNEL staining of fibrotic human lung fibroblasts incubated with medium alone or TNF-α plus IFN-γ for 48 h before exposure to agonistic anti-Fas antibody for 12 h (magnification: ×63).
We also investigated the ability of TNF-α to sensitize normal human lung myofibroblasts to Fas-induced apoptosis. Monolayers of normal human lung fibroblasts were incubated with TGF-β1 (2 ng/ml) for 72 h and the development of a myofibroblast phenotype was confirmed (Figure 1). As can be seen in Figure 3A, < 6% of normal human lung myofibroblasts were sensitive to Fas-induced apoptosis as measured by caspase-8 activation after pretreatment with medium alone or with IFN-γ alone. Preincubation with TNF-α increased the degree of apoptosis to 19% (P < 0.01), while co-incubation with TNF-α and IFN-γ increased the level of Fas-induced apoptosis to 82% (Figure 3A, P < 0.001). When comparing the TGF-β–differentiated myofibroblasts and the undifferentiated fibroblasts, less apoptosis was seen in the TNF-α alone pretreated group after Fas ligation in the myofibroblasts compared with the fibroblasts (19 versus 51%, P < 0.01). Interestingly, the elevated background levels of apoptosis seen with pretreatment with TNF-α plus IFN-γ in the absence of Fas ligation did achieve statistical significance in the TGF-β–differentiated myofibroblasts (P < 0.05). TUNEL positivity and morphologic features of apoptosis were also confirmed in the TGF-β–differentiated myofibroblast experiments and correlated with other markers of apoptosis (data not shown).
The origin of normal human lung fibroblasts is not completely understood, through recent studies have suggested that circulating CXCR4+CD45+ColI+ fibrocytes accumulate in the lung in bleomycin-induced pulmonary fibrosis in mice and contribute to collagen deposition (5, 6). Since the origin of fibroblasts in normal and fibrotic lung may differ, we also investigated the role of TNF-α in sensitizing lung fibroblasts and myofibroblasts isolated from fibrotic human lungs to Fas-induced apoptosis. Primary early passage cultures of human fibrotic lung fibroblasts were derived from surgical biopsy specimens obtained from four separate patients with open lung biopsy proven UIP (Table 1). Fibroblasts were pre-incubated in medium alone, TNF-α (20 ng/ml), IFN-γ (50 U/ml), or both cytokines for 48 h before stimulation with agonistic anti-Fas antibody for 12 h and flow cytometric analysis of caspase-8 activation and mitochondrial depolarization. Figure 3D shows that < 7% of fibrotic human lung fibroblasts incubated in medium alone, TNF-α alone, IFN-γ alone or anti-Fas antibody alone were apoptotic. However, prior exposure to TNF-α also sensitized human fibrotic lung fibroblasts to Fas-induced apoptosis, while pre-incubation with TNF-α and IFN-γ further augmented this response (P < 0.001). Similar results were obtained when apoptosis was assessed by morphology (Figure 3E) and by TUNEL staining (Figure 3F). We also differentiated human fibrotic lung fibroblasts into myofibroblasts with TGF-β1, and then determined their response to cytokine exposure and Fas-induced apoptosis. As can be seen in Figure 3D, the results were not significantly different from the results obtained with fibrotic human lung fibroblasts. Interestingly, unlike with the normal pulmonary fibroblasts and myofibroblasts, fibrotic lung cells pretreated with TNF-α alone followed by Fas ligation did not differ in the degree of observed apoptosis between the undifferentiated fibroblasts and TGF-β–differentiated myofibroblasts (P > 0.05). A direct comparison between the TGF-β–differentiated, nondiseased human lung myofibroblasts and fibrotic human lung myofibroblasts suggested a trend toward decreased Fas-sensitivity in the nondiseased myofibroblasts, but this did not achieve statistical significance (P > 0.05).
Finally, we confirmed that the results in the nondiseased and fibrotic human lung fibroblast experiments were due to experimental conditions rather than individual patient variability. A two-way ANOVA statistical analysis was performed on each set of experiments and showed that > 85% of the variability between groups across all data sets was due exclusively to the experimental conditions (P < 0.001) and that inter-patient variability accounted for < 3% of the observed variation between groups across all data sets (P < 0.05).
Collectively, these findings indicate that primary cultures of normal human lung and fibrotic human lung fibroblasts and myofibroblasts responded similarly to MRC-5 cells in terms of basal resistance to Fas-induced apoptosis and sensitivity to Fas-induced apoptosis after pre-incubation with TNF-α, alone and in combination with IFN-γ, and that regardless of their origin, these cells undergo a fundamentally similar process of sensitization by TNF-α to Fas-induced apoptosis.
IFN-γ Potentiates TNF-α-Dependent Sensitization to Fas-Induced Apoptosis
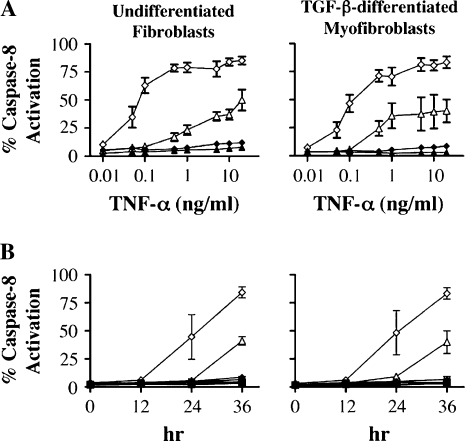
While pretreatment with IFN-γ alone did not induce sensitization to Fas-induced apoptosis, the data shown in Figures 2 and 3 indicate that IFN-γ augments sensitization by TNF-α. Dose response studies indicated that 20–50 U/ml of IFN-γ produced an optimal response (data not shown). The effect of IFN-γ on the TNF-α response in turn could plausibly be due to increases in: (1) sensitivity to TNF-α, (2) the rate of TNF-α–induced sensitization, or (3) both processes. We therefore determined the effect of IFN-γ on the dose response and time course of TNF-α–induced sensitization to Fas-induced apoptosis. Undifferentiated MRC-5 cells were pretreated with TNF-α (0.01–20 ng/ml) in the presence and absence of IFN-γ (50 U/ml) for 36 h and analyzed for Fas-induced apoptosis. Figure 4A shows that while a dose of TNF-α of 5 ng/ml was required to induce maximal apoptosis in the absence of IFN-γ, a 50-fold lower amount of TNF-α induced maximal apoptosis in the presence of IFN-γ. In addition, more apoptosis was seen in cells co-incubated with IFN-γ for any given dose of TNF-α compared with that seen in the absence of IFN-γ. Figure 4B shows the time course of pretreatment with TNF-α in the presence and absence of IFN-γ on the degree of Fas-induced apoptosis. It can be seen that co-stimulation with IFN-γ shortened the time required for TNF-α to sensitize cells to Fas-induced apoptosis by ∼ 12 h. These findings suggest that IFN-γ augments TNF-α–induced sensitization to Fas-induced apoptosis by both increasing the sensitivity to TNF-α and by decreasing the time required for sensitization.
Figure 4.
IFN-γ potentiates TNF-α sensitization to Fas-induced apoptosis in MRC-5 fibroblasts and TGF-β–differentiated myofibroblasts. (A) TNF-α dose response curve for MRC-5 fibroblasts and TGF-β–differentiated myofibroblasts. Cells were incubated with increasing doses of TNF-α (0.01–20 ng/ml) in the presence or absence of IFN-γ (50 U/ml) for 36 h followed by stimulation with agonistic anti-Fas antibody for 6 h. (B) Time course of sensitization of MRC-5 fibroblasts and TGF-β–differentiated myofibroblasts to Fas-induced apoptosis by TNF-α (20 ng/ml), IFN-γ (50 U/ml), or both cytokines. Squares, medium; triangles, TNF-α; inverted triangles, IFN-γ; diamonds, TNF-α + IFN- γ; filled symbols, no anti-Fas antibody; open symbols, with anti-Fas antibody. Apoptosis was quantified by caspase-8 activation.
Effect of TNF-α on Cell Surface Fas Expression
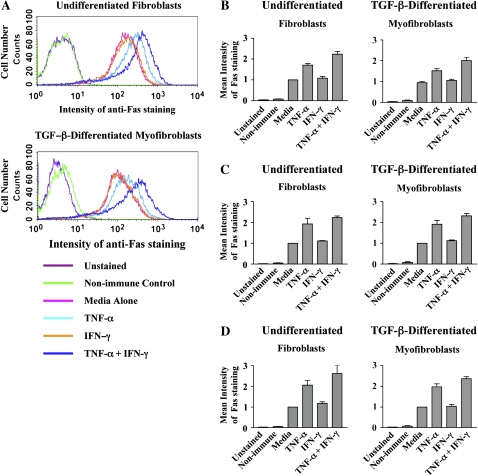
Intracellular sequestration of Fas has previously been reported as a mechanism of resistance to Fas-induced apoptosis (33). Thus, it was possible that the mechanism of TNF-α–induced sensitization to Fas-induced apoptosis may involve an increase in cell surface Fas expression. We therefore quantified Fas expression on MRC-5 fibroblasts after incubation with medium alone, TNF-α (20 ng/ml), IFN-γ (50 U/ml), or both cytokines for 36 h, by flow cytometry. Incubation with IFN-γ had no effect on cell surface Fas expression compared with medium alone (P > 0.05), whereas incubation with TNF-α led to a 70% increase in Fas expression (Figure 5A and 5B, P < 0.001), and this was further augmented in cells incubated with TNF-α and IFN-γ, which demonstrated a 124% increase over baseline Fas cell surface expression (Figures 5A and 5B, P < 0.001). We also investigated the effects of TNF-α and IFN-γ on cell surface Fas expression in normal (Figure 5C) and fibrotic (Figure 5D) human lung fibroblasts and obtained similar results. TGF-β–differentiated myofibroblasts were likewise investigated for each of the cell populations and no differences were found between the fibroblast and myofibroblast-like cells from each population (Figures 5A–5D). A two-way ANOVA statistical analysis was performed on each set of experiments and showed that > 90% of the variability between groups across all data sets was due exclusively to the experimental conditions (P < 0.001) and that inter-patient variability accounted for < 2% of the observed variation between groups. Additionally, we quantified total Fas levels by ELISA and detected similar changes in total Fas levels after stimulation with cytokines (data not shown). These data also showed that at baseline, undifferentiated and TGF-β–differentiated cells expressed abundant levels of Fas (56 ± 11 ng/mg cell protein and 67 ± 24 ng/mg cell protein, respectively), confirming that the basal resistance of fibroblasts and myofibroblasts is not due to a lack of available Fas.
Figure 5.
Effect of TNF-α and IFN-γ on cell surface Fas expression. (A) MRC-5 fibroblasts and TGF-β–differentiated myofibroblasts were incubated in medium alone or with TNF-α (20 ng/ml), IFN-γ (50 U/ml), or both cytokines for 36 h before staining with APC-labeled anti-Fas antibody and analysis by flow cytometry. Representative flow cytometry histograms for Fas cell surface expression, repeated independently three times, are depicted. (B) Mean intensity of staining ± SEM of Fas expression levels. (C) Human lung fibroblasts and TGF-β–differentiated myofibroblasts were incubated in medium alone or with TNF-α (20 ng/ml), IFN-γ (50 U/ml), or both cytokines for 48 h before staining with APC-labeled anti-Fas antibody and analysis by flow cytometry. Mean intensity of staining ± SEM of Fas expression levels of pooled data with fibroblasts obtained from four normal lung donors, with cells from each individual donor repeated independently three times. (D) Mean intensity of staining ± SEM of Fas expression levels of pooled data with fibroblasts obtained from four fibrotic lung donors, with cells from each individual donor repeated independently three times.
Exposure to TNF-α Promotes Assembly of the Fas-Induced Death-Inducing Signaling Complex
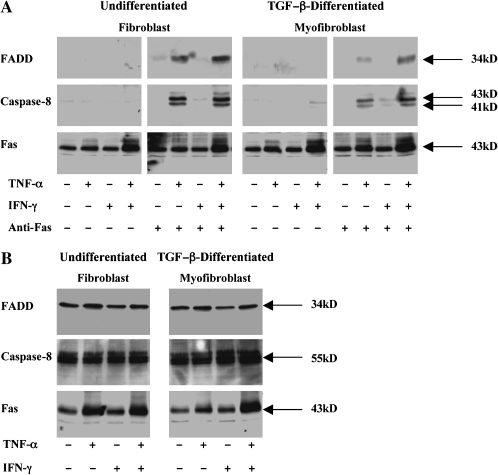
Since control of Fas-induced apoptosis appeared to be regulated above the level of caspase-8 activation, we investigated the hypothesis that TNF-α permits Fas signaling at the level of assembly of the death-inducing signaling complex (DISC). Accordingly, undifferentiated MRC-5 cells were pre-incubated in medium alone, TNF-α (20 ng/ml), IFN-γ (50 U/ml), or both cytokines for 36 h before stimulation with agonistic anti-Fas antibody for 4 h to allow optimal DISC assembly before apoptosis. The cells were then lysed, immunoprecipitated with anti-Fas antibody, and co-immunoprecipitating FADD and caspase-8 were detected by immunoblotting. As can be seen in Figure 6A, co-immunoprecipitating FADD or caspase-8 were not detected in anti-Fas immunoprecipitates from unstimulated cells or in cells pre-incubated with IFN-γ alone. However, after sensitization with TNF-α, FADD, and active caspase-8 (41- and 43-kD isoforms) were recruited to the cytoplasmic domain of Fas. Furthermore, as was seen in the apoptosis experiments described earlier, IFN-γ augmented the level of FADD recruitment (Figure 6A). We also determined the effect of sensitization with TNF-α and IFN-γ on Fas-induced DISC assembly in TGF-β1–differentiated MRC-5 cells. As can be seen in Figure 6A, neither FADD nor caspase-8 were recruited to Fas in the absence of TNF-α. However, sensitization with TNF-α, alone or in combination with IFN-γ, also stimulated FADD and caspase-8 recruitment to Fas.
Figure 6.
Effect of TNF-α and IFN-γ on the assembly of the Fas-induced death inducing signaling complex (DISC). (A) MRC-5 fibroblasts and TGF-β–differentiated myofibroblasts were incubated in medium alone or with TNF-α (20 ng/ml), IFN-γ (50 U/ml), or both cytokines for 36 h before challenge with agonistic anti-Fas antibody (250 ng/ml) for 4 h. Cells were lysed and equal amounts of total cell protein were immunoprecipitated with anti-Fas antibody, washed and separated by SDS-PAGE. Co-immunoprecipitating FADD (34 kD) and active caspase-8 (41 and 43 kD) were detected by Western blotting with specific antibodies. Immunoprecipation of Fas (43 kD) was confirmed by stripping the blots and re-probing with anti-Fas antibody. (B) Western blot analysis of total FADD, caspase-8 and Fas levels in undifferentiated and TGF-β–differentiated MRC-5 cells. Cells were stimulated as in A., lysed, and equal amounts of total cell lysate proteins were analyzed by SDS-PAGE and Western blotting with the appropriate antibody. Data shown are representative results of three independent experiments.
Western blot analyses were also performed on post-nuclear cell lysates from MRC-5 cells to determine if the levels of FADD and caspase-8 were regulated by TNF-α and IFN-γ. Figure 6B shows that FADD and caspase-8 expression were largely unaffected by exposure to either TNF-α or IFN-γ. Collectively, these findings suggest that sensitization by TNF-α to Fas-induced apoptosis occurs at the level of recruitment of FADD to ligated Fas and that the basal resistance of MRC-5 fibroblasts to Fas-induced apoptosis is associated with a failure of FADD recruitment.
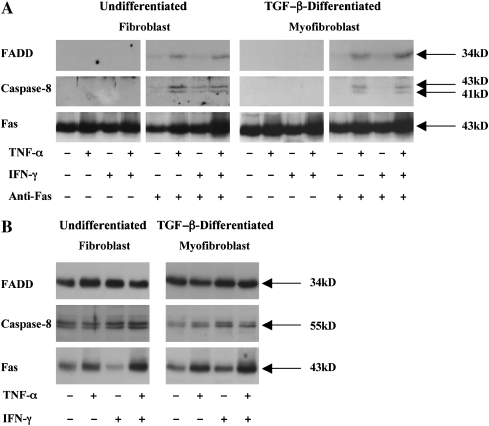
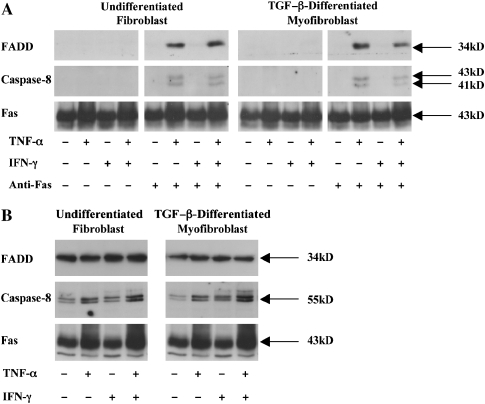
Given the mechanistic importance of these findings, we sought to confirm that the basal resistance of nondiseased human lung fibroblasts and fibrotic human lung fibroblasts was also due to a failure of FADD recruitment and that TNF-α–dependent sensitization permitted FADD recruitment upon Fas ligation. As can be seen in Figure 7A, neither FADD nor caspase-8 were recruited to Fas in the absence of TNF-α in nondiseased human lung fibroblasts and TGF-β–differentiated myofibroblasts. However, sensitization with TNF-α, alone, or in combination with IFN-γ, permitted FADD and caspase-8 recruitment to Fas upon receptor ligation. Identical results were also found in the fibrotic human lung fibroblasts and TGF-β–differentiated myofibroblasts (Figure 8A). Western blot analyses on postnuclear cell lysates from the primary human cell populations showed that FADD expression was largely unaffected by exposure to either TNF-α or IFN-γ (Figures 7B and 8B). Caspase-8 expression was similarly unaffected by exposure to either TNF-α or IFN-γ in the normal human lung fibroblasts (Figure 7B), but in the fibrotic lung fibroblasts and TGF-β–differentiated myofibroblasts, TNF-α and TNF-α plus IFN-γ increased total caspase-8 expression levels (Figure 8B).
Figure 7.
(A) Nondiseased human lung fibroblasts and TGF-β–differentiated myofibroblasts were incubated in medium alone or with TNF-α (20 ng/ml), IFN-γ (50 U/ml), or both cytokines for 48 h before challenge with agonistic anti-Fas antibody (250 ng/ml) for 6 h Cells were lysed and analyzed as described in the legend to Figure 6. Data shown are representative results of three independent experiments for each of four patients. (B) Western blot analysis of total FADD, caspase-8, and Fas levels in undifferentiated and TGF-β–differentiated nondiseased human lung cells. Data shown are representative result of three independent experiments for each of four patients.
Figure 8.
(A) Fibrotic human lung fibroblasts and TGF-β–differentiated myofibroblasts were incubated in medium alone or with TNF-α (20 ng/ml), IFN-γ (50 U/ml), or both cytokines for 48 h before challenge with agonistic anti-Fas antibody (250 ng/ml) for 6 h. Cells were lysed and analyzed as described in the legend to Figure 6. Data shown are representative results of three independent experiments for each of four patients. (B) Western blot analysis of total FADD, caspase-8, and Fas levels in undifferentiated and TGF-β–differentiated fibrotic human lung cells. Data shown are representative results of three independent experiments for each of four patients.
DISCUSSION
The mechanisms underlying the removal of fibroblasts and myofibroblasts from the lung during repair, and the consequent dysregulation of these events in IPF/UIP, remain poorly understood. Using primary cultures of human lung fibroblasts, in vitro differentiated human myofibroblasts, and MRC-5 cells, our study has revealed a novel mechanism in which TNF-α sensitizes lung fibroblasts and myofibroblasts to undergo apoptosis upon Fas ligation and that IFN-γ augments this response. We have shown that sensitization to Fas-induced apoptosis is conserved among different populations of lung fibroblasts and myofibroblasts. In addition, the mechanism of sensitization by TNF-α occurs at the level of recruitment of the adapter protein FADD to the cytoplasmic domain of Fas, thereby enabling assembly of the pro-apoptotic death-inducing signaling complex (DISC).
Our finding that lung fibroblasts and myofibroblasts express Fas at the cell surface but are basally resistant to Fas-induced apoptosis is consistent with previous studies using human lung fibroblasts (14, 31), and with data reported by Kazufumi and colleagues (34) showing that fibroblasts and myofibroblasts express Fas within the fibroblast foci of patients with biopsy-proven IPF/UIP. Although Moodley and colleagues have been able to show some degree of Fas-induced apoptosis in unsensitized pulmonary fibroblasts, their studies used very high, supraphysiologic levels of FasL (30–50 μg/ml) for 24 h and achieved modest levels of apoptosis (31, 35). In contrast to the present study, Moodley and coworkers observed differences in sensitivity to apoptosis by normal and fibrotic human lung fibroblasts (31, 35). However, given the supraphysiologic level of FasL used in their study, it is possible that the mechanism of apoptosis may be fundamentally different and not directly comparable to this study. Another possibility is that the high levels of FasL used in the studies by Moodley and colleagues promoted TNF-α expression and autocrine sensitization by the fibroblasts, similar to a mechanism implicated by Borges and coworkers in their studies of silicosis (36).
Based on recent reports suggesting that the progression of pulmonary fibrosis occurs in the face of a limited inflammatory response (17), we reasoned that the proinflammatory cytokines TNF-α and IFN-γ contribute to lung repair by sensitizing fibroblasts and myofibroblasts to undergo Fas-induced apoptosis at the completion of the repair process. This concept would thus predict that the absence (or reduced levels) of these cytokines, as has been reported in IPF/UIP (21, 37), would favor fibroblast and myofibroblast persistence and accumulation. Although initially appearing conceptually incompatible with earlier studies that indicated a profibrotic role for TNF-α in bleomycin-induced pulmonary fibrosis in mice (23, 24, 38, 39), several lines of evidence now suggest that the profibrotic role of TNF-α may be indirect and related to initial injury of the alveolar epithelium and/or an underappreciated role of the related TNF-receptor ligand lymphotoxin-α in lung injury (25). Furthermore, recent studies in mice suggest that TNF-α may serve to limit the extent of fibrosis and subpleural honeycombing. Fujita and colleagues (40) showed that enforced expression of TNF-α by alveolar type II epithelial cells in transgenic mice ameliorates pulmonary fibrosis induced by either bleomycin or TGF-β. In addition, Kuroki and coworkers (25) reported that tnf-α−/− mice develop severe pneumopathy associated with subpleural honeycombing after bleomycin instillation. They also showed that pulmonary delivery of TNF-α to tnf-α−/− mice 14 d after bleomycin instillation reversed the lung pathology and promoted lung repair and lung cell apoptosis (25). While our study is the first to report a mechanism for the sensitization of human lung fibroblasts and myofibroblasts to Fas-induced apoptosis, other studies have suggested that TNF-receptor signaling is somehow involved in coupling Fas ligation to the activation of apoptosis. Costelli and colleagues (41) showed that mice deficient in both TNF-R1 and TNF-R2 are resistant to Fas-induced liver injury; while Teh and coworkers (42) reported that TNF-R2–deficient CD8+ T cells are resistant to Fas-induced apoptosis. Thus, the ability of TNF-α to sensitize cells to Fas-induced apoptosis may represent a broad mechanism to promote and control Fas-induced apoptosis.
A number of studies have shown that IFNs, especially IFN-γ, can limit or inhibit the development of pulmonary fibrosis in mouse models (18, 19) and promote improvement in lung function and survival in patients with progressive IPF/UIP (21, 22). Although IFN-γ did not induce sensitization of human lung fibroblasts and myofibroblasts to Fas-induced apoptosis in the absence of TNF-α, it augmented sensitization by TNF-α by decreasing the dose and the time required for sensitization. Synergistic interaction between cytokines is well established, and augmentation of TNF-α–dependent responses by IFN-γ, especially NF-κB activation, has been previously reported (43–45). Thus, although TNF-α was sufficient to promote sensitization to Fas-induced apoptosis in vitro, both cytokines may be necessary to induce physiologic sensitization in vivo. This possibility is also consistent with recent studies by Jiang and colleagues (20) in which pulmonary expression of IFN-γ by resident CXCR3+ NK cells was found to play a role in limiting the progression of pulmonary fibrosis and subpleural honeycombing in bleomycin-treated mice. The current findings, together with the observed increased subpleural honeycombing in mice deficient in either TNF-α– or in IFN-γ–producing CXCR3+ pulmonary NK cells, suggest that reduced expression of either cytokine will promote fibroblast/myofibroblast accumulation by reducing their sensitivity to Fas-induced apoptosis.
Recent studies have provided important new insights into the origin and phenotype of pulmonary fibroblasts and myofibroblasts (5, 6, 46). We therefore addressed the question of whether the ability of TNF-α and IFN-γ to sensitize fibroblasts and myofibroblasts to Fas-induced apoptosis was conserved by both cell phenotypes. Using in vitro differentiation with TGF-β1 to generate myofibroblasts from fibroblasts (27), we saw no difference in the ability of normal human lung fibroblasts or myofibroblasts to undergo Fas-induced apoptosis in response to combined exposure to TNF-α and IFN-γ. Since these cells may have different origins in mesenchymal tissues and in the bone marrow in normal and fibrotic lungs (5, 6, 47, 48), we also addressed this question in primary cultures of fibroblasts isolated from normal and fibrotic lung tissues. No difference was noted in the ability of TNF-α and IFN-γ to sensitize normal or fibrotic human lung fibroblasts and myofibroblasts to undergo Fas-induced apoptosis. Similar results were also obtained with the human lung fibroblast cell line, MRC-5, and with human skin fibroblasts (data not shown). Thus, although fibroblasts and myofibroblasts exhibit phenotypic differences dependent on their origin and location (5, 6, 47, 48), these findings suggest that sensitization by TNF-α and IFN-γ is a fundamentally important mechanism to enable these cells to undergo apoptosis independent of their origin or phenotype. We did observe a trend in which normal lung myofibroblasts were less sensitive to TNF-α than fibrotic lung myofibroblasts, but this trend did not achieve statistical significance. Similar findings have also been reported for IL-1β–induced apoptosis of TGF-β–treated rat lung fibroblasts (49), and by Moodley and colleagues for high-dose FasL-induced apoptosis (35). While the molecular basis of the difference in sensitivity exhibited by normal and fibrotic lung myofibroblasts is unclear, it is conceivable that the bone marrow or mesenchymal origins of these cells may contribute to the quantitative differences in TNF-α sensitivity. Alternatively, previous studies have shown that fibroblasts maintain their in vivo phenotypic imprinting during culture in vitro (47, 50). Thus, it is also possible that the in vivo milieu of the normal and fibrotic lung may contribute to the observed difference in the response to TNF-α in vitro.
In seeking to define the mechanism by which TNF-α sensitizes fibroblasts and myofibroblasts to Fas-induced apoptosis, we investigated the hypothesis that the mechanism of sensitization by TNF-α and IFN-γ might be localized to the initial assembly of the DISC and that the basal resistance of lung fibroblasts to Fas-induced apoptosis was a result of failure to assemble the DISC. Co-immunoprecipitation data clearly showed that the basal resistance to Fas-induced apoptosis was associated with a failure of ligated Fas to recruit FADD and caspase-8. However, after sensitization with TNF-α, Fas ligation resulted in recruitment of FADD and caspase-8 and led to caspase-8 activation. While it remains possible, or even probable, that TNF-α and TNF-α + IFN-γ have other proapoptotic effects that may improve the efficiency of apoptotic induction after Fas ligation, such as the upregulation of caspase-8 expression seen in the fibrotic lung fibroblasts (or, for example, downregulation of c-IAP and Bcl-2 family members), our data suggest that the ability to recruit FADD and assemble the DISC is the critical and most proximal component of TNF-α–dependent sensitization.
Although the molecular basis for TNF-α–induced sensitization and DISC assembly remains to be determined, one possibility is that it may involve TNF-α–induced augmentation in cell surface Fas expression above a given threshold of cell surface receptor density. This hypothesis would stipulate that unstimulated cells do not have a sufficient number of cell surface receptors to transduce an apoptotic signal, but that the augmentation in cell surface Fas expression seen after cytokine treatment produces a receptor density in excess of the threshold required to transduce an apoptotic signal. Examples of this are well documented in the literature. For example, both thrombopoietin and CD46 have been shown to have dramatically different signal transduction responses depending upon whether or not a critical threshold receptor density is exceeded (51, 52). This hypothesis will be the subject of further investigation.
Second, basal resistance to Fas-induced apoptosis may be mediated by a constitutively expressed protein that inhibits the recruitment of FADD to ligated Fas. The hypothetical protein could prevent Fas signaling by forming a heteromeric complex with either Fas or FADD. In this scenario, sensitization with TNF-α is proposed to promote dissociation of the complex to enable FADD recruitment to ligated Fas. Previous studies have identified LFG and FAIM as inhibitors of Fas-induced apoptosis, though little is known about their mechanism of action (53, 54). The antiapoptotic protein FLIP has been implicated in the basal Fas resistance of normal human lung fibroblasts by Tanaka and coworkers (14) and in dermal fibroblasts by Santiago and colleagues (32). However, FLIP activity is dependent on its recruitment to FADD after Fas ligation (55, 56). Since FADD is not recruited to Fas in the absence of TNF-α sensitization, a role for FLIP appears incompatible with our data. In addition, we have found very low/undetectable levels of FLIP in unstimulated MRC-5, nondiseased, and fibrotic human lung fibroblasts and TGF-β–differentiated myofibroblasts; we have also found that these levels increase after stimulation with TNF-α and TNF-α plus IFN-γ (data not shown.) However, it is not possible to completely exclude a role for FLIP at present.
Third, TNF-α, through its ability to activate kinase cascades, may promote FADD recruitment to ligated Fas by phosphorylating Fas or FADD. Recent studies have shown that human FADD is phosphorylated on Ser 194 (57) and that phosphorylation at this site sensitizes prostate carcinoma cells to etoposide-induced apoptosis (58). The role of FADD phosphorylation in promoting Fas-induced apoptosis in Fas-insensitive cells is not known, though this does not appear to contribute to apoptosis in Fas-sensitive cells (59). Other studies have also shown that TNF-R family members, including Fas, DR3, and TNF-R1, are phosphorylated in response to TNF-α signaling and that this event is capable of regulating apoptosis (29, 60, 61). Intriguingly, as can be seen in Figure 6, sensitization with TNF-α, alone or in combination with IFN-γ, resulted in the appearance of a slower migrating species of Fas consistent with the possibility that Fas may be phosphorylated during sensitization. Finally, Fas has been shown to partition into lipid rafts (62) and may be important in promoting apoptosis (63). Thus, it is conceivable that the mechanism of sensitization by TNF-α may involve translocation of Fas to lipid rafts. These possibilities will be addressed in future studies.
In conclusion, we have identified a novel, physiologic mechanism in which the proinflammatory cytokines TNF-α and IFN-γ sensitize human lung fibroblasts and myofibroblasts to undergo Fas-induced apoptosis. The ability of TNF-α and IFN-γ to promote sensitization to Fas-induced apoptosis was conserved among fibroblasts and myofibroblasts from normal and fibrotic human lung. In addition, the mechanism of sensitization was localized to the assembly of the DISC by ligated Fas. Since FasL has been detected in lung tissues of patients with IPF/UIP, we propose that the absence of a robust inflammatory response in the lungs of patients with IPF/UIP (64), and the associated deficiencies in IFN-γ and TNF-α expression during fibroproliferation, augments fibroblast and myofibroblast accumulation in this disorder by preventing Fas-induced apoptosis. Conversely, strategies aimed at increasing the expression or function of TNF-α and IFN-γ in the lung may reduce fibroblast and myofibroblast accumulation and thus be beneficial in treating this disorder.
Acknowledgments
The authors wish to acknowledge the outstanding technical assistance provided by Linda Remigio. They are also indebted to Drs. Peter Henson, Bob Mason, Marvin Schwarz, and Ms. Amanda Kostyk for their continued interest and input into this work and for reviewing the manuscript.
This work was supported by Public Health Service grants HL68628, HL55549 (D.W.H.R.), K08-067848 (S.K.F.), and HL67671 (G.P.C. and K.K.B.) from the National Heart Lung and Blood Institute of the National Institutes of Health, Institutional Training Grant T-32 AI00048 (M.W.W.), and a Parker B. Francis Fellowship (G.P.C. and M.W.W.).
Originally Published in Press as DOI: 10.1165/rcmb.2005-0155OC on November 4, 2005
Conflict of Interest Statement: S.K.F, over the past three years, has served as either the principal investigator or a sub-investigator at the National Jewish site for multicentered clinical research trials sponsored by Intermune, Wyeth, Actelion, Fibrogen, and Genzyme. National Jewish has received research support for costs associated with these multicentered clinical research trials. In addition, S.K.F. has received speaking/lecture fees from Intermune promoting physician awareness of the INSPIRE trial totaling $2,000 in 2004 and $2,000 in 2005. G.P.C., over the past three years, has served as either the principal investigator or a sub-investigator at the National Jewish site for multicentered clinical research trials sponsored by Intermune, Wyeth, Actelion, Fibrogen, and Genzyme. National Jewish has received research support for costs associated with these multicentered clinical research trials. In addition, G.P.C. has received speaking/lecture fees from Intermune promoting physician awareness of the INSPIRE trial totaling $2,000 in 2004 and $2,000 in 2005. S.-I.C. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. C.D.C. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. M.W.W. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. B.L.E. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. K.K.B., over the past five years, has served as a consultant and/or advisory board member to the following pharmaceutical companies: Biogen, Intermune, Wyeth, Actelion, Fibrogen, Genzyme, Arizeke, and Encysive. He has also received research support for costs associated with these multicentered clinical research trials from the following companies: Biogen, Intermune, Wyeth, Actelion, Fibrogen, and Genzyme. D.W.H.R. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript.
References
- 1.American Thoracic Society. Idiopathic pulmonary fibrosis: diagnosis and treatment. International consensus statement. American Thoracic Society (ATS), and the European Respiratory Society (ERS). Am J Respir Crit Care Med 2000;161:646–664. [DOI] [PubMed] [Google Scholar]
- 2.Myers JL, Katzenstein AL. Epithelial necrosis and alveolar collapse in the pathogenesis of usual interstitial pneumonia. Chest 1988;94:1309–1311. [DOI] [PubMed] [Google Scholar]
- 3.Kuhn C III, Boldt J, King TE Jr, Crouch E, Vartio T, McDonald JA. An immunohistochemical study of architectural remodeling and connective tissue synthesis in pulmonary fibrosis. Am Rev Respir Dis 1989;140:1693–1703. [DOI] [PubMed] [Google Scholar]
- 4.Kuhn C, McDonald JA. The roles of the myofibroblast in idiopathic pulmonary fibrosis. Ultrastructural and immunohistochemical features of sites of active extracellular matrix synthesis. Am J Pathol 1991;138:1257–1265. [PMC free article] [PubMed] [Google Scholar]
- 5.Hashimoto N, Jin H, Liu T, Chensue SW, Phan SH. Bone marrow-derived progenitor cells in pulmonary fibrosis. J Clin Invest 2004;113:243–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Phillips RJ, Burdick MD, Hong K, Lutz MA, Murray LA, Xue YY, Belperio JA, Keane MP, Strieter RM. Circulating fibrocytes traffic to the lungs in response to CXCL12 and mediate fibrosis. J Clin Invest 2004;114:438–446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Clark RAF. Wound repair: overview and general considerations. In: Clark RAF, editor. The molecular and cell biology of wound repair. New York: Plenum Press; 1995. pp. 3–50.
- 8.Desmouliere A, Redard M, Darby I, Gabbiani G. Apoptosis mediates the decrease in cellularity during the transition between granulation tissue and scar. Am J Pathol 1995;146:56–66. [PMC free article] [PubMed] [Google Scholar]
- 9.Bruce MC, Honaker CE, Cross RJ. Lung fibroblasts undergo apoptosis following alveolarization. Am J Respir Cell Mol Biol 1999;18:228–236. [DOI] [PubMed] [Google Scholar]
- 10.Lappi-Blanco E, Soini Y, Paakko P. Apoptotic activity is increased in the newly formed fibromyxoid connective tissue in bronchiolitis obliterans organizing pneumonia. Lung 1999;177:367–376. [DOI] [PubMed] [Google Scholar]
- 11.Hagimoto N, Kuwano K, Nomoto Y, Kunitake R, Hara N. Apoptosis and expression of Fas/Fas ligand pathway in bleomycin-induced pulmonary fibrosis in mice. Am J Respir Cell Mol Biol 1997;16:91–101. [DOI] [PubMed] [Google Scholar]
- 12.Kuwano K, Hagimoto N, Kawasaki M, Yatomi T, Nakamura N, Nagata S, Suda T, Kunitake R, Maeyama T, Miyazaki H, et al. Essential roles of the Fas-Fas ligand pathway in the development of pulmonary fibrosis. J Clin Invest 1999;104:13–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cohen PL, Eisenberg RA. The lpr and gld genes in systemic autoimmunity: life and death in the Fas lane. Immunol Today 1992;13:427–428. [DOI] [PubMed] [Google Scholar]
- 14.Tanaka T, Yoshimi M, Maeyama T, Hagimoto N, Kuwano K, Hara N. Resistance to Fas-mediated apoptosis in human lung fibroblast. Eur Respir J 2002;20:359–368. [DOI] [PubMed] [Google Scholar]
- 15.Buhling F, Welte T, Wille A, Baier A, Pap T. Fibroblasts derived from patients with lung fibrosis are characterized by alterations in fas-ligand (FasL-) induced apoptosis. ATS 99th International Conference, Seattle, WA. 2003. B014.
- 16.Keogh BA, Crystal RG. Alveolitis: the key to the interstitial lung disorders. Thorax 1982;37:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Selman M, King TE, Pardo A. Idiopathic pulmonary fibrosis: prevailing and evolving hypotheses about its pathogenesis and implications for therapy. Ann Intern Med 2001;134:136–151. [DOI] [PubMed] [Google Scholar]
- 18.Hyde DM, Henderson TS, Giri SN, Tyler NK, Stovall MY. Effect of murine gamma interferon on the cellular responses to bleomycin in mice. Exp Lung Res 1988;14:687–704. [DOI] [PubMed] [Google Scholar]
- 19.Giri SN, Hyde DM. Ameliorating effect of an interferon inducer polyinosinic-polycytidylic acid on bleomycin-induced lung fibrosis in hamsters: morphologic and biochemical evidence. Am J Pathol 1988;133:525–536. [PMC free article] [PubMed] [Google Scholar]
- 20.Jiang D, Liang J, Hodge J, Lu B, Zhu Z, Yu S, Fan J, Gao Y, Yin Z, Homer R, et al. Regulation of pulmonary fibrosis by chemokine receptor CXCR3. J Clin Invest 2004;114:291–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ziesche R, Hofbauer E, Wittman K, Petkov V, Block LH. A preliminary study of long-term treatment with interferon-gamma-1b and low dose prenisolone in patients with idiopathic pulmonary fibrosis. N Engl J Med 1999;341:1264–1269. [DOI] [PubMed] [Google Scholar]
- 22.Raghu G, Brown KK, Bradford WZ, Starko K, Noble PW, Schwartz DA, King TE Jr. A placebo-controlled trial of interferon gamma-1b in patients with idiopathic pulmonary fibrosis. N Engl J Med 2004;350:125–133. [DOI] [PubMed] [Google Scholar]
- 23.Piguet PF, Collart MA, Grau GE, Kapanci Y, Vassalli P. Tumor necrosis factor/cachectin plays a key role in bleomycin-induced pneumopathy and fibrosis. J Exp Med 1989;170:655–663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ortiz LA, Lasky J, Hamilton RF Jr, Holian A, Hoyle GW, Banks W, Peschon JJ, Brody AR, Lungarella G, Friedman M. Expression of TNF and the necessity of TNF receptors in bleomycin-induced lung injury in mice. Exp Lung Res 1998;24:721–743. [DOI] [PubMed] [Google Scholar]
- 25.Kuroki M, Noguchi Y, Shimono M, Tomono K, Tashiro T, Obata Y, Nakayama E, Kohno S. Repression of bleomycin-induced pneumopathy by TNF. J Immunol 2003;170:567–574. [DOI] [PubMed] [Google Scholar]
- 26.Fujita M, Shannon JM, Morikawa O, Gauldie J, Hara N, Mason RJ. Overexpression of tumor necrosis factor-alpha diminishes pulmonary fibrosis induced by bleomycin or transforming growth factor-beta. Am J Respir Cell Mol Biol 2003;29:669–676. [DOI] [PubMed] [Google Scholar]
- 27.Desmouliere A, Geinoz A, Gabbiani F, Gabbiani G. Transforming growth factor-beta 1 induces alpha-smooth muscle actin expression in granulation tissue myofibroblasts and in quiescent and growing cultured fibroblasts. J Cell Biol 1993;122:103–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.O'Sullivan MP, Tyner JW, Holtzman MJ. Apoptosis in the airways, another balancing act in the epithelial program. Am J Respir Cell Mol Biol 2003;29:3–7. [DOI] [PubMed] [Google Scholar]
- 29.Cottin V, Van Linden AA, Riches DWH. Phosphorylation of the tumor necrosis factor receptor CD120a (p55) recruits bcl-2 and protects against apoptosis. J Biol Chem 2001;276:17252–17260. [DOI] [PubMed] [Google Scholar]
- 30.Chan ED, Winston BW, Uh ST, Wynes MW, Rose DM, Riches DW. Evaluation of the role of mitogen-activated protein kinases in the expression of inducible nitric oxide synthase by IFN-gamma and TNF-alpha in mouse macrophages. J Immunol 1999;162:415–422. [PubMed] [Google Scholar]
- 31.Moodley YP, Caterina P, Scaffidi AK, Misso NL, Papadimitriou JM, McAnulty RJ, Laurent GJ, Thompson PJ, Knight DA. Comparison of the morphological and biochemical changes in normal human lung fibroblasts and fibroblasts derived from lungs of patients with idiopathic pulmonary fibrosis during FasL-induced apoptosis. J Pathol 2004;202:486–495. [DOI] [PubMed] [Google Scholar]
- 32.Santiago B, Galindo M, Palao G, Pablos JL. Intracellular regulation of Fas-induced apoptosis in human fibroblasts by extracellular factors and cycloheximide. J Immunol 2004;172:560–566. [DOI] [PubMed] [Google Scholar]
- 33.Nambu Y, Hughes SJ, Rehemtulla A, Hamstra D, Orringer MB, Beer DG. Lack of cell surface Fas/APO-1 expression in pulmonary adenocarcinomas. J Clin Invest 1998;101:1102–1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kazufumi M, Sonoko N, Masanori K, Takateru I, Akira O. Expression of bcl-2 protein and APO-1 (Fas antigen) in the lung tissue from patients with idiopathic pulmonary fibrosis. Microsc Res Tech 1997;38:480–487. [DOI] [PubMed] [Google Scholar]
- 35.Moodley YP, Misso NL, Scaffidi AK, Fogel-Petrovic M, McAnulty RJ, Laurent GJ, Thompson PJ, Knight DA. Inverse effects of interleukin-6 on apoptosis of fibroblasts from pulmonary fibrosis and normal lungs. Am J Respir Cell Mol Biol 2003;29:490–498. [DOI] [PubMed] [Google Scholar]
- 36.Borges VM, Falcao H, Leite-Junior J, Alvim L, Teixeira GP, Russo M, Nobrega AF, Lopes MF, Rocco PM, Davidson WF, et al. Fas ligand triggers pulmonary silicosis. J Exp Med 2005;194:155–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Martinez JA, King TE Jr, Brown K, Jennings CA, Borish L, Mortenson RL, Khan TZ, Bost TW, Riches DW. Increased expression of the interleukin-10 gene by alveolar macrophages in interstitial lung disease. Am J Physiol 1997;273:L676–L683. [DOI] [PubMed] [Google Scholar]
- 38.Piguet PF, Kaufman S, Barazzone C, Muller M, Ryffel B, Eugster HP. Resistance of TNF/LT alpha double deficient mice to bleomycin- induced fibrosis. Int J Exp Pathol 1997;78:43–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Piguet PF, Vesin C. Treatment by human recombinant soluble TNF receptor of pulmonary fibrosis induced by bleomycin or silica in mice. Eur Respir J 1994;7:515–518. [DOI] [PubMed] [Google Scholar]
- 40.Fujita M, Shannon JM, Morikawa O, Gauldie J, Hara N, Mason RJ. Overexpression of tumor necrosis factor-alpha diminishes pulmonary fibrosis induced by bleomycin or transforming growth factor-beta. Am J Respir Cell Mol Biol 2003;29:669–676. [DOI] [PubMed] [Google Scholar]
- 41.Costelli P, Aoki P, Zingaro B, Carbo N, Reffo P, Lopez-Soriano FJ, Bonelli G, Argiles JM, Baccino FM. Mice lacking TNFalpha receptors 1 and 2 are resistant to death and fulminant liver injury induced by agonistic anti-Fas antibody. Cell Death Differ 2003;10:997–1004. [DOI] [PubMed] [Google Scholar]
- 42.Teh HS, Seebaran A, Teh SJ. TNF receptor 2-deficient CD8 T cells ar resistant to Fas/Fas ligand-induced cell death. J Immunol 2000;165:4814–4821. [DOI] [PubMed] [Google Scholar]
- 43.Cheshire JL, Williams BR, Baldwin AS Jr. Involvement of double-stranded RNA-activated protein kinase in the synergistic activation of nuclear factor-kappaB by tumor necrosis factor-alpha and gamma-interferon in preneuronal cells. J Biol Chem 1999;274:4801–4806. [DOI] [PubMed] [Google Scholar]
- 44.Cheshire JL, Baldwin AS Jr. Synergistic activation of NF-kappaB by tumor necrosis factor alpha and gamma interferon via enhanced I kappaB alpha degradation and de novo I kappaBbeta degradation. Mol Cell Biol 1997;17:6746–6754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wesemann DR, Benveniste EN. STAT-1α and IFN-γ as modulators of TNF-α signaling in macrophages: regulation and functional implications of the TNF receptor 1:STAT-1α complex. J Immunol 2003;171:5313–5319. [DOI] [PubMed] [Google Scholar]
- 46.Abe R, Donnelly SC, Peng T, Bucala R, Metz CN. Peripheral blood fibrocytes: differentiation pathway and migration to wound sites. J Immunol 2001;166:7556–7562. [DOI] [PubMed] [Google Scholar]
- 47.Chang HY, Chi JT, Dudoit S, Bondre C, van de Rijn M, Botstein D, Brown PO. Diversity, topographic differentiation, and positional memory in human fibroblasts. Proc Natl Acad Sci USA 2002;99:12877–12882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Phan SH. Fibroblast phenotypes in pulmonary fibrosis. Am J Respir Cell Mol Biol 2003;29:S87–S92. [PubMed] [Google Scholar]
- 49.Zhang H-Y, Phan SH. Inhibition of myofibroblast apoptosis by transforming growth factor−β1. Am J Respir Cell Mol Biol 1999;21:658–665. [DOI] [PubMed] [Google Scholar]
- 50.Wilborn J, Bailie M, Coffey M, Burdick M, Strieter R, Peters-Golden M. Constitutive activation of 5-lipoxygenase in the lungs of patients with idiopathic pulmonary fibrosis. J Clin Invest 1996;97:1827–1836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Millot GA, Vainchenker W, Dumenil D, Svinarchuk F. Distinct effects of thrombopoietin depending on a threshold level of activated Mpl in BaF-3 cells. J Cell Sci 2002;115:2329–2337. [DOI] [PubMed] [Google Scholar]
- 52.Anderson BD, Nakamura T, Russell SJ, Peng K-W. High CD46 receptor density determines preferential killing of tumor cells by oncolytic measles virus. Cancer Res 2004;64:4919–4926. [DOI] [PubMed] [Google Scholar]
- 53.Schneider TJ, Fischer GM, Donohoe TJ, Colarusso TP, Rothstein TL. A novel gene coding for a Fas apoptosis inhibitory molecule (FAIM) isolated from inducibly Fas-resistant B lymphocytes. J Exp Med 1999;189:949–956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Somia NV, Schmitt MJ, Vetter DE, Van Antwerp D, Heinemann SF, Verma IM. LFG: an anti-apoptotic gene that provides protection from Fas-mediated cell death. Proc Natl Acad Sci USA 1999;96:12667–12672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yeh W-C, Itie A, Elia AJ, Ng M, Shu HB, Wakeham A, Mirtsos C, Suzuki N, Bonnard M, Goeddel DV, Mak TW. 2000. Requirement for CASPER (c-FLIP) in regulation of death receptor-induced apoptosis and embryonic development. Immunity 12:633–642. [DOI] [PubMed]
- 56.Zhang J, Cado D, Chen A, Kabra NH, Winoto A. Fas-mediated apoptosis and activation-induced T-cell proliferation are defective in mice lacking FADD/Mort1. Nature 1998;392:296–300. [DOI] [PubMed] [Google Scholar]
- 57.Scaffidi C, Volkland J, Blomberg I, Hoffmann I, Krammer PH, Peter ME. Phosphorylation of FADD/ MORT1 at serine 194 and association with a 70-kDa cell cycle-regulated protein kinase. J Immunol 2000;164:1236–1242. [DOI] [PubMed] [Google Scholar]
- 58.Shimada K, Matsuyoshi S, Nakamura M, Ishida E, Kishi M, Konishi N. Phosphorylation of FADD is critical for sensitivity to anticancer drug-induced apoptosis. Carcinogenesis 2004;25:1089–1097. [DOI] [PubMed] [Google Scholar]
- 59.Hua ZC, Sohn SJ, Kang C, Cado D, Winoto A. A function of Fas-associated death domain protein in cell cycle progression localized to a single amino acid at its C-terminal region. Immunity 2003;18:513–521. [DOI] [PubMed] [Google Scholar]
- 60.Kennedy NJ, Budd RC. Phosphorylation of FADD/MORT1 and Fas by kinases that associate with the membrane-proximal cytoplasmic domain of Fas. J Immunol 1998;160:4881–4888. [PubMed] [Google Scholar]
- 61.Frankel SK, Van Linden AA, Riches DWH. Heterogeneity in the phosphorylation of human death receptors by p42mapk/erk2. Biochem Biophys Res Commun 2001;288:313–320. [DOI] [PubMed] [Google Scholar]
- 62.Scheel-Toellner D, Wnag K, Singh R, Majeed S, Raza K, Curnow SJ, Salmon M, Lord JM. The death-inducing signalling complex is recruited to lipid rafts in Fas-induced apoptosis. Biochem Biophys Res Commun 2002;297:876–879. [DOI] [PubMed] [Google Scholar]
- 63.Muppidi JR, Siegel RM. Ligand-independent redistribution of Fas (CD95) into lipid rafts mediates clonotypic T cell death. Nature Immunology 2004;5:182–189. [DOI] [PubMed] [Google Scholar]
- 64.Kuwano K, Kawasaki M, Maeyama T, Hagimoto N, Nakamura N, Shirakawa K, Hara N. Soluble form of Fas and Fas ligand in BAL fluid from patients with pulmonary fibrosis and bronchiolitis obliterans organizing pneumonia. Chest 2000;118:451–458. [DOI] [PubMed] [Google Scholar]