Abstract
Alcohol abuse increases the incidence of acute respiratory distress syndrome and causes oxidative stress and cellular dysfunction in the lung. The mechanisms of ethanol (EtOH)-induced oxidative stress in the lung remain to be defined. Chronic alcohol ingestion has been associated with increased renin-angiotensin system (RAS) activity. Therefore, the current study investigated the ability of lisinopril, an angiotensin-converting enzyme (ACE) inhibitor, to modulate oxidative stress in the lung after chronic EtOH ingestion in a well-established rat model. Male Sprague-Dawley rats were fed liquid diets containing EtOH (36% of calories) or maltose-dextrin as an isocaloric substitution for EtOH (Control) for 6 wk. Selected animals were also treated with lisinopril (3 mg/liter) for 6 wk. Chronic EtOH ingestion increased bronchoalveolar lavage fluid glutathione disulfide levels and superoxide formation in lung parenchyma. These effects of EtOH were attenuated by lisinopril treatment. Chronic EtOH ingestion failed to increase ACE expression or angiotensin II levels in lung homogenates, but increased angiotensinogen, angiotensin II type 1 and type 2 receptor levels, and ACE activity. Chronic EtOH ingestion also increased the levels of the NADPH oxidase subunit, gp91phox, an effect that was attenuated by lisinopril, but had no effect on lung p22phox or p47phox levels. These findings suggest that EtOH-mediated RAS activation plays an important role in pulmonary oxidative stress and provide new insights into mechanisms by which EtOH causes oxidative stress in the lung and potential strategies of lung protection through ACE inhibition.
Keywords: ARDS, chronic ethanol ingestion, lung, NADPH oxidase, superoxide
The acute respiratory distress syndrome (ARDS) is a severe form of lung injury characterized by noncardiogenic pulmonary edema in response to diverse clinical scenarios such as sepsis, trauma, or aspiration that cause systemic activation of proinflammatory cascades (1). Despite extensive research, ARDS is associated with a 50% mortality rate, due in part to the lack of effective therapies (2). Therefore, identifying mechanisms that predispose the lung to acute injury has the potential to further our understanding of ARDS pathophysiology and the design of treatment strategies capable of limiting the development and/or severity of ARDS.
Chronic alcohol abuse is a comorbid variable that increases the incidence of ARDS (3). While alcohol undoubtedly predisposes to ARDS through many mechanisms, oxidative stress is a key factor (4–7). Chronic alcohol ingestion reduces the level of the antioxidant glutathione (GSH) in bronchoalveolar lavage (BAL) fluid (8) and increases renin-angiotensin system (RAS) activity (9–11).
Activation of the RAS involves renin-mediated cleavage of angiotensinogen into the decapeptide angiotensin I (Ang I). Angiotensinogen is generated mainly in the liver, but recent studies have confirmed mRNA expression in a wide range of tissues including the kidney, brain, vascular tissue, adrenal gland, and placenta (12). Renin is produced not only in the kidney in response to hypotension or volume depletion but also in vascular endothelial cells in response to more diverse stimuli (13). Ang I is converted to the octapeptide, angiotensin II (Ang II), by angiotensin-converting enzyme (ACE). Although ACE concentration is highest in the lung, it can also be found on the lumenal surface of vascular endothelial cells in other locations (14, 15). Once formed, Ang II exerts its cellular effects by binding to receptors on the cell surface, predominately Ang II Type 1 (AT1) and Type 2 (AT2) receptors (16).
Ang II is a potent inducer of oxidative stress. Ang II stimulates NADPH oxidase expression and activity as well as superoxide production in cells of the vascular wall in vitro (17–19) and in vivo (20), and increases NADPH oxidase-dependent superoxide production in coronary microvascular endothelial cells (21). Originally described in neutrophils, NADPH oxidase is a multicomponent, membrane-associated, enzyme that catalyzes the one electron reduction of oxygen to superoxide using NADH or NADPH as the electron donor (22). NADPH oxidase components include gp91phox, p22phox, p40phox, p47phox, and p67phox. The subunits gp91phox and p22phox reside in the plasma membrane and bind the components of the electron transport chain heme and FAD, forming cytochrome b558 (23, 24). The NADPH oxidase subunits p40phox, p47phox, and p67phox are cytosolic and are involved in activation of the enzyme complex (24, 25).
Recently, we determined that in a rat model of chronic EtOH ingestion, the ACE inhibitor, lisinopril, prevented EtOH-induced lung glutathione depletion and protected against endotoxin-mediated epithelial dysfunction, suggesting the RAS as a key mediator of EtOH-induced lung dysfunction (26). To our knowledge, no previous studies have examined the effects of EtOH administration on lung angiotensin peptide levels. In this study, we hypothesized that RAS-mediated activation of NADPH oxidase is one mechanism by which chronic alcohol abuse increases susceptibility of the lung to oxidative stress and ARDS. The current report demonstrates that chronic EtOH ingestion stimulates RAS-mediated increases in superoxide production and gp91phox expression in the lung, leading to increased oxidative stress.
MATERIALS AND METHODS
Animal Treatment
Male Sprague-Dawley rats were fed the liquid Lieber-DeCarli diet containing EtOH (36% of calories) for 6 wk or an isocaloric control diet without EtOH (substitution of maltose-dextrin for EtOH) (27) as previously reported (26). During the first 2 wk of the dietary regimen the EtOH-fed rats were gradually acclimated to EtOH, receiving 12% of their total calories as EtOH for 1 wk, then 24% of their total calories as EtOH for 1 wk, and then 36% of their total calories as EtOH for 4 wk. Selected animals were also treated with lisinopril (3 mg/liter) in the Control or EtOH diets. The diets are otherwise identical in protein, lipid, and essential nutrient composition.
Dihydroethidium Staining
After rats were killed, lungs were perfused blood–free, then perfused and embedded in optimal cutting temperature (OCT) compound and frozen at −80°C. Thirty-micrometer-thick sections were then prepared and stained with dihydroethidium (DHE; 10 μM) (28). In brief, 30 μl of DHE was placed over the tissue section and covered with glass coverslips. Slides were then incubated at 37°C in a humidified, 5% CO2 atmosphere for 30 min. Sections from each treatment group were examined by confocal fluorescence microscopy, and images were acquired at ×40 magnification using identical instrument settings.
ACE Activity Assay
ACE activity was determined using a commercially available kit according to the manufacturer's instructions (ALPCO Diagnostics, Windham, NH). Briefly, ACE activity was measured based on ACE-mediated cleavage of a tritiated synthetic substrate. The product of this reaction was separated from unreacted substrate by acid extraction, detected by scintillation counting, and quantitated by comparison to standards.
Analysis of Angiotensin Peptide Levels
After rats were killed with pentobarbital, lung tissue was rapidly removed and rinsed briefly in isotonic saline, weighed, and homogenized in 5 ml 4 mol/liter guanidine thiocyanate. The homogenates were then frozen at −80°C and shipped to St. Vincent's Institute of Medical Research for measurement of angiotensin peptides using high-performance liquid chromatography–based radioimmunoassays as previously reported (29).
Immunoprecipitation and Western Blotting
At killing, lungs were perfused blood–free, and sections of peripheral lung tissue were placed in lysis buffer (20 mM Tris pH 7.4, 2.5 mM EDTA, 1% TritonX-100, 1% deoxycholic acid, 0.1% SDS, 100 mM NaCl, 10 mM NaF, 1 mM Na3VO4) containing a protease inhibitor cocktail (Roche Diagnostics, Indianapolis, IN). Tissue homogenates were prepared, sonicated, and centrifuged at 15,000 × g for 10 min. The supernatants were collected, protein concentrations were determined, and 20 μg of the NADPH oxidase subunit antibody was added to 500 μg protein homogenate and incubated overnight at 4°C on a rocking platform. Samples were then incubated with sepharose beads at 4°C for 3 h. Beads were then collected by centrifugation, washed, dissolved in sample buffer at 95°C and subjected to SDS-PAGE, transferred to PVDF membranes, and immunoblotted with the same primary antibody in TBS-T (10 mM Tris-HCl pH 7.4, 150 mM NaCl, 0.1% Tween) containing 5% powdered nonfat dry milk. To examine expression of RAS components, 20 μg of lung homogenate was subjected to Western blotting as described above. Bands were identified by chemiluminescence and quantified by laser densitometry.
Real-Time PCR
Whole lung tissue was collected in RNA-Bee (Tel-test, Friendswood, TX) and homogenized. After phenol-chloroform extraction, RNA was cleaned using an RNeasy kit according to the manufacturer's (Qiagen, Valencia, CA) instructions. Total RNA (5 μg) was reverse transcribed using random nanomer primers. Real-time PCR was then performed using 18S and NADPH oxidase component–specific primers, and copy numbers were expressed per copies of 18S for normalization.
Blood Pressure Measurements
Before killing, blood pressure measurements were made in anesthetized rats. Rats were anesthetized with isofluorane and placed on a thermostatically regulated heating table for maintenance of body temperature. A Millar (Houston, TX) pressure transducer was inserted into the abdominal aorta via the femoral artery under direct visualization. Blood pressure was monitored for 15–20 min using Chart software (AD Instruments, Charlotte, NC). Blood pressure measurements were derived from the final 10 min of acquisition. Rats were killed with pentobarbital after completion of these studies.
Determination of Lung Lavage Glutathione Disulfide Concentration
Immediately after killing, bronchoalveolar lavage was performed as we have previously reported (26). In brief, high-performance liquid chromatography was employed to measure glutathione disulfide (GSSG) (30) in BAL fluid as we have previously reported (6, 7). GSSG concentrations in BAL fluid were normalized to secretory IgA an epithelial lining fluid protein not altered during acute lung injury (7) or by chronic EtOH ingestion (26).
Statistical Analysis
Overall treatment effects were examined by ANOVA. Post hoc analysis to detect differences between specific groups was accomplished with the Student-Neuman-Keuls test. The level of statistical significance was taken as P < 0.05.
RESULTS
The Impact of Chronic EtOH Ingestion on the RAS Pathway in the Lung
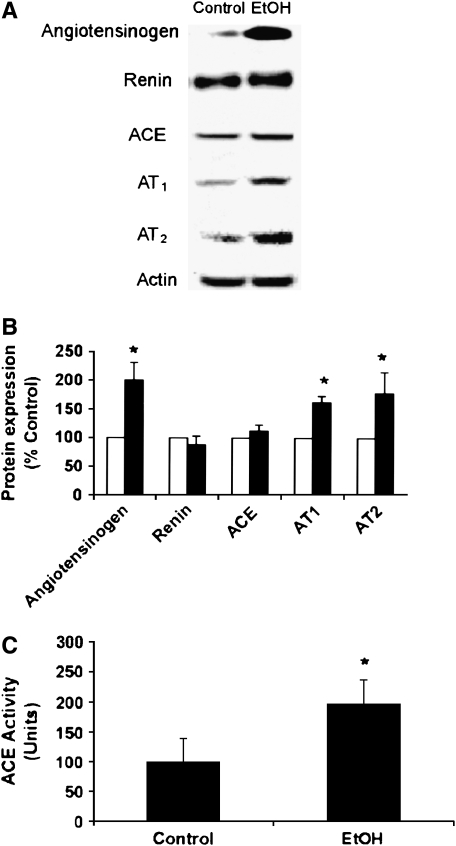
Rats were fed Control or EtOH diets for 6 wk. Homogenates prepared from lung tissue were subjected to Western blotting for specific components of the RAS pathway. As shown in Figure 1, compared with Control, chronic EtOH ingestion increased the levels of angiotensinogen and the Ang II receptors, AT1 and AT2, while having no effect on levels of ACE or renin. All blots were probed with antibodies to actin to ensure equal loading among lanes. Compared with Control, chronic EtOH ingestion produced roughly a 2-fold increase in ACE activity (Figure 1C), but did not increase lung Ang I or Ang II levels in the lung (Table 1).
Figure 1.
Chronic EtOH ingestion increased angiotensinogen, AT1, and AT2 expression as well as ACE activity in the lung. Rats were fed Control or EtOH diets for 6 wk. In A and B, equivalent amounts of lung homogenates were subjected to SDS-PAGE; probed with primary antibodies to the RAS components angiotensinogen, renin, angiotensin converting enzyme (ACE), AT1, and AT2; and quantified by laser densitometry. In B, each bar represents the mean relative density in arbitrary units ± SEM as % Control. Open bars, control; filled bars, EtOH; n = 5; *P < 0.05 versus Control. Representative Western blots are shown in A. In C, equivalent amounts of lung homogenates were subjected to ACE activity assay. Each bar represents the mean ± SEM (n = 3–4, *P < 0.05 versus Control).
TABLE 1.
LUNG ANGIOTENSIN I AND II LEVELS
| Control | Control + Lisinopril | EtOH | EtOH + Lisinopril | |
|---|---|---|---|---|
| Angiotensin I | 3.0 ± 1.6 | 4.2 ± 1.0 | 1.9 ± 0.9 | 5.4 ± 2.6 |
| Angiotensin II | 81.0 ± 12.7 | 43.6 ± 8.6* | 87.0 ± 17.1 | 36.7 ± 9.8* |
Definition of abbreviation: EtOH, ethanol.
Rats were fed Control or EtOH containing diets for 6 wk. After killing, lung tissue was collected, weighed, homogenized in guanidine thiocyanate, and frozen at −80°C. Angiotensin I and II levels were measured in each sample using high-performance liquid chromatography–based radioimmunoassays. Results are expressed as mean ± SEM peptide levels from eight animals in fmol/g tissue.
P < 0.05 versus animals on same diet without lisinopril.
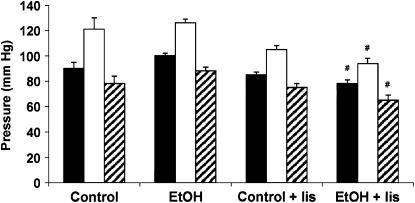
Chronic EtOH Ingestion Had No Significant Effect on Blood Pressure
Because chronic EtOH ingestion altered the levels and activity of RAS components that participate in hemodynamic regulation, blood pressure was measured after EtOH and lisinopril treatments. The results in Figure 2 illustrate that acute blood pressure measurements are comparable in the Control and EtOH-treated groups in anesthetized rats. Only the combination of the EtOH diet and lisinopril treatment (EtOH + lis) caused a small, but significant reduction in mean arterial pressure (MAP), systolic blood pressure (SBP), and diastolic blood pressure (DBP) compared with EtOH alone. As expected, lisinopril treatment was also associated with reduced lung Ang II levels (Table 1).
Figure 2.
Blood pressure in Control and EtOH fed rats ± lisinopril treatment. Rats were fed Control or EtOH diets ± lisinopril for 6 wk as described. Mean arterial (MAP; filled bars), systolic (SBP; open bars), and diastolic (DBP; striped bars) blood pressures were recorded in anesthetized rats before killing. Each bar represents the mean blood pressure ± SEM (n = 6, #P < 0.05 versus EtOH).
Chronic EtOH Ingestion Caused RAS-Dependent Oxidative Stress in the Lung
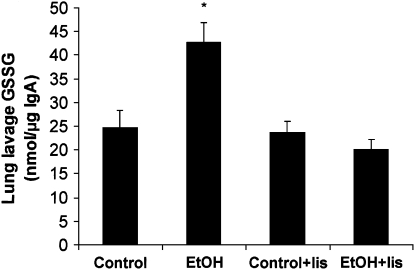
We previously reported that chronic EtOH ingestion in the rat leads to oxidative stress in the lung characterized by reductions in glutathione levels in parenchymal lung cells and in BAL fluid (4–6). In addition, treating EtOH-fed rats with lisinopril (in a protocol identical to that employed in the current study) increased levels of glutathione in BAL fluid compared with untreated, EtOH-fed rats (26). To further characterize the oxidative stress mediated by chronic EtOH ingestion, BAL fluid of Control or EtOH-fed rats treated with or without the ACE inhibitor, lisinopril, was collected, and GSSG levels were measured. As shown in Figure 3, chronic EtOH ingestion increased GSSG levels of BAL fluid in the rat, an effect attenuated by treatment with lisinopril.
Figure 3.
Chronic EtOH ingestion increased BAL fluid GSSG levels. Rats were fed Control or EtOH diets ± lisinopril (lis) for 6 wk. BAL fluid was then collected and subjected to analysis of GSSG and secretory IgA. Each bar represents the mean GSSG level in nmol/μg IgA ± SEM (n = 9–11, *P < 0.05 versus all groups).
Chronic EtOH Ingestion Increased RAS-Dependent Lung Superoxide Generation
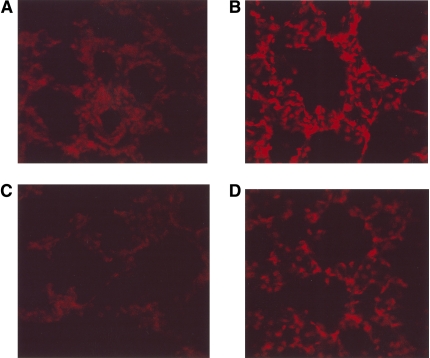
To determine if chronic EtOH ingestion amplified the production of ROS in the lung, in situ, 30-μm-thick frozen sections from OCT-embedded lungs of Control or EtOH-fed rats treated with or without lisinopril were prepared in parallel. The sections were treated with dihydroethidium (DHE) and subjected to fluorescence confocal microscopy as described in Materials and Methods. DHE fluorescence is specific for superoxide generation within the cell (28). In brief, superoxide oxidizes DHE to ethidium bromide, which binds to the DNA in the nucleus and fluoresces red. All images were taken with identical acquisition parameters. Compared with Control (Figure 4A), chronic EtOH ingestion (Figure 4B) substantially increased superoxide generation in rat lung. Inhibition of ACE with lisinopril had no effect on basal superoxide production (Figure 4C), but reduced EtOH-mediated increases in superoxide (Figure 4D).
Figure 4.
Chronic EtOH ingestion increased lung superoxide production through activation of the renin-angiotensin system. Lung tissue from (A) Control, (B) EtOH-, (C) Control + lisinopril–, and (D) EtOH + lisinopril–treated rats was frozen in OCT, sectioned at 30 μm, stained with 10 μM dihydroethidium (DHE), and evaluated using fluorescence microscopy. Representative sections from four to six animals are shown. Magnification: ×40.
EtOH-Induced Increases in Lung gp91phox Levels In Vivo Were Attenuated by Lisinopril Treatment
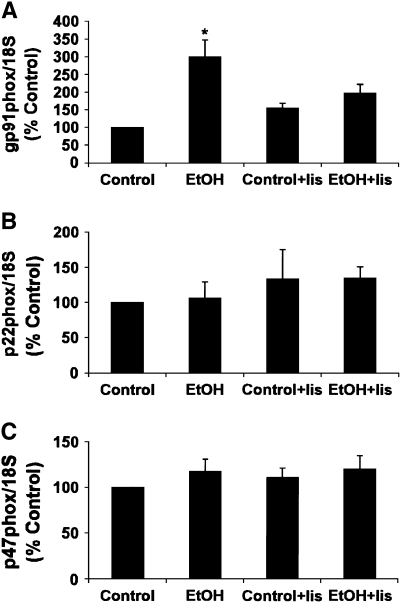
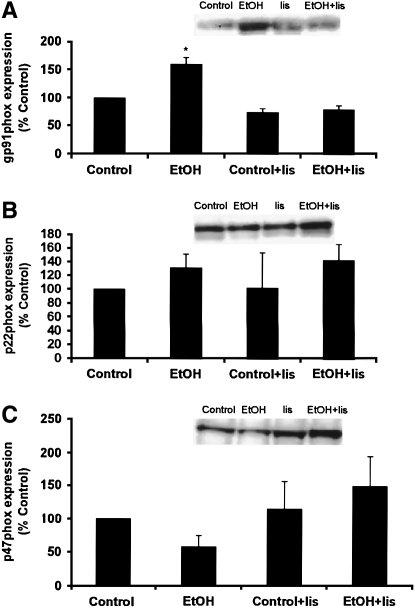
NADPH oxidase constitutes a major source of superoxide formation in the vasculature (22). To examine changes in NADPH oxidase expression in the lung as a potential source of EtOH-mediated superoxide production, RNA was isolated from lung tissue of rats fed Control or EtOH diets with or without lisinopril for 6 wk, and real-time PCR for NADPH oxidase components was performed. As shown in Figure 5A, chronic EtOH ingestion increased gp91phox message levels, an effect that was inhibited by lisinopril. This EtOH-induced alteration in NADPH oxidase subunit expression was specific for gp91phox expression in that alterations in p22phox or p47phox expression were not observed (Figures 5B and 5C). In separate experiments, NADPH oxidase components were immunoprecipitated from lung homogenates. Consistent with examination of mRNA expression, chronic EtOH ingestion caused a similar ACE-dependent increase in gp91phox protein levels (Figure 6A) without altering protein levels for p22phox or p47phox (Figures 6B and 6C). Taken together, these results demonstrate that EtOH increased the expression of gp91phox in the lung, an effect that was prevented by in vivo treatment with the ACE inhibitor, lisinopril. These findings support the hypothesis that RAS signaling plays an important role in EtOH-induced NADPH oxidase expression in the lung.
Figure 5.
Chronic EtOH ingestion increased mRNA of the gp91phox NADPH oxidase subunit. RNA was isolated from the lungs of Control and EtOH-fed rats with or without lisinopril (lis) and quantified by real-time PCR to examine the message levels of NADPH oxidase subunits (A) gp91phox, (B) p22phox, and (C) p47phox. Data are expressed as mean copies of NADPH oxidase subunit per copies of 18S ± SEM as % Control (n = 6, *P < 0.05 versus all groups).
Figure 6.
Chronic EtOH ingestion increased gp91phox protein expression. Lungs from Control and EtOH -treated rats with or without lisinopril (lis) were isolated and homogenized. After protein determination, immunoprecipitations were preformed on equivalent amounts of protein using antibodies to the NADPH oxidase subunit (A) gp91phox, (B) p22phox, and (C) p47phox. Blots were probed with the same antibody, and bands were quantified by laser densitometry. Each bar represents the mean relative density in arbitrary units ± SEM as % Control (n = 6, *P < 0.05 versus all groups). Representative Western blots are shown as insets.
DISCUSSION
Previous studies from our laboratories determined that chronic EtOH ingestion in rats renders the lung intrinsically susceptible to endotoxin-dependent edematous injury, supporting the observation that alcohol abuse predisposes the lung to injury (7). In addition, we have previously reported that EtOH ingestion markedly decreased the levels of the antioxidant glutathione in lung tissue and in BAL fluid (4–7). Recently, we found that lisinopril-mediated ACE inhibition prevented EtOH-induced glutathione depletion (26). The current study extends these observations to further clarify the mechanisms by which RAS mediates oxidative stress via NADPH oxidase activation in the lung during chronic EtOH ingestion. Consistent with EtOH-mediated reductions in BAL fluid glutathione, chronic EtOH ingestion increased GSSG levels of BAL fluid and enhanced superoxide generation in lung tissue, effects attenuated by lisinopril. Since the EtOH diet alone had no significant effect on blood pressure, these findings suggest that the effects of Ang II on oxidative stress in the lung relates more to its ability to modulate gene expression and are independent of its effects on blood pressure.
The role of the RAS in the pathogenesis of lung injury and ARDS has been suggested by studies demonstrating increased circulating levels of Ang II in patients with ARDS (31, 32). In the current study, chronic EtOH ingestion increased components of the RAS pathway in the lung, specifically angiotensinogen, AT1, and AT2 expression, as well as ACE activity. Cleavage of angiotensinogen, the only known substrate for renin, is the rate-limiting factor in the cascade of Ang II production (33), suggesting that activation of renin following inflammatory stimuli could lead to robust increases in RAS activity in the lung after chronic EtOH ingestion. Although chronic EtOH ingestion alone did not increase lung Ang I or Ang II levels, we postulate that the enhanced AT1 and AT2 receptor expression in the lung caused by chronic EtOH ingestion results in an increased sensitivity to Ang II and therefore enhanced downstream RAS effector mechanisms in the lung. As expected, lisinopril reduced lung Ang II levels and tended to increase Ang I levels in Control and ETOH-fed animals. Lisinopril-mediated reductions in Ang II may therefore provide a plausible mechanism for attenuation of downstream RAS signaling in EtOH-fed animals.
Among its many downstream targets, Ang II stimulates NADPH oxidase expression and activity, an important source of oxidative stress and ROS production in many tissues (34, 35). Chronic EtOH ingestion increased both the message and protein levels of the NADPH oxidase subunit, gp91phox, an effect attenuated by the ACE inhibitor, lisinopril. The pathophysiologic significance of gp91phox has been demonstrated in vivo where gp91phox−/− mice, compared to wild-type mice, displayed less lung microvascular injury when challenged with intraperitoneal Escherichia coli (36). A 2-fold increase in superoxide formation caused by Ang II infusion has also been reported in the aortas of wild-type animals but not in gp91phox−/− mice (37). In addition, in vitro evidence suggests that gp91phox expression provides dose-dependent regulation of superoxide generation in response to Ang II in human endothelial cells (38). Taken together, these findings suggest that EtOH-stimulated increases in RAS activity in the lung increased NADPH oxidase-dependent superoxide production by increasing gp91phox expression.
The current study does not address the mechanism by which EtOH modulates RAS activity. Regardless of the precise mechanism, our study provides novel evidence that EtOH stimulates RAS activity in the lung and stimulates lung oxidative stress through an ACE-dependent mechanism and implicates gp91phox as a downstream effector. These findings extend previous work linking EtOH ingestion to oxidative stress in the lung and susceptibility to lung injury. These findings raise the possibility that alcohol abuse increases a patient's risk for acute lung injury in part by altering the RAS and specific NADPH oxidase component expression and thereby rendering the tissue susceptible to oxidative stress. Furthermore, this study raises the possibility that lung dysfunction could be modulated by treatment with ACE inhibitors. This may be particularly beneficial in patients with a history of alcohol abuse who are at more than twice the risk of developing ARDS in response to a given insult (3).
This work is supported by grants from the Veterans Affairs Research Service (D.M.G. and C.M.H.), the National Institute of Alcohol Abuse and Alcoholism (J.A.P., L.A.S.B., D.M.G., and C.M.H.), and by a Career Development Fellowship (Award CR 02M 0829) from the National Heart Foundation of Australia (D.J.C.).
Conflict of Interest Statement: None of the authors has a financial relationship with a commercial entity that has an interest in the subject of this manuscript.
Originally Published in Press as DOI: 10.1165/rcmb.2005-0320OC on November 11, 2005
References
- 1.Ware LB, Matthay MA. The Acute Respiratory Distress Syndrome. N Engl J Med 2000;18:1334–1349. [DOI] [PubMed] [Google Scholar]
- 2.Ware LB, Matthay MA. Alveolar fluid clearance is impaired in the majority of patients with acute lung injury and the acute respiratory distress syndrome. Am J Respir Crit Care Med 2001;163:1376–1383. [DOI] [PubMed] [Google Scholar]
- 3.Moss M, Parsons PE, Steinberg KP, Hudson LD, Guidot DM, Burnham EL, Eaton S, Catsonis GA. Chronic alcohol abuse is associated with an increased incidence of ARDS and severity of multiple organ dysfunction in patients with septic shock. Crit Care Med 2003;31:869–877. [DOI] [PubMed] [Google Scholar]
- 4.Brown LAS, Harris FL, Bechara R, Guidot DM. Effect of chronic EtOH ingestion on alveolar type II cell: glutathione and inflammatory mediator-induced apoptosis. Alc Clin Exp Res 2001;25:1078–1085. [PubMed] [Google Scholar]
- 5.Guidot DM, Modelska K, Lois M, Jain L, Moss IM, Pittet JF, Brown LAS. Ethanol ingestion via GSH depletion impairs alveolar epithelial barrier function in rats. Am J Physiol 2000;279:L127–L135. [DOI] [PubMed] [Google Scholar]
- 6.Guidot DM, Brown LAS. Mitochondrial glutathione replacement restores surfactant synthesis and secretion in alveolar epithelial cells in ethanol-fed rats. Alc Clin Exp Res 2000;24:1070–1076. [PubMed] [Google Scholar]
- 7.Holguin F, Moss IM, Brown LAS, Guidot DM. Chronic ethanol ingestion impairs alveolar type II cell glutathione homeostasis and function and predisposes to endotoxin-mediated acute edamatous lung injury in rats. J Clin Invest 1998;101:761–768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Moss M, Guidot DM, Wong-Lambertina M, Ten Hoor T, Perez RL, Brown LAS. The effects of chronic alcohol abuse on pulmonary GSH homeostasis. Am J Respir Crit Care Med 2000;161:414–419. [DOI] [PubMed] [Google Scholar]
- 9.Puddey IB, Beilin LJ, Vandongen R. Regular alcohol use raises blood pressure in treated hypertensive subjects. Lancet 1987;8534:647–651. [DOI] [PubMed] [Google Scholar]
- 10.Linkola J, Fyhrquist F, Ylikahri R. Renin aldosterone and cortisol during ethanol intoxication and hangover. Acta Physiol Scand 1979;106:75–82. [DOI] [PubMed] [Google Scholar]
- 11.Wright JW, Morseth SL, Abhold RH, Harding JW. Elevations in plasma angiotensin II with prolonged ethanol treatment in rats. Pharmacol Biochem Behav 1986;24:813–818. [DOI] [PubMed] [Google Scholar]
- 12.Morgan L, Pipkin FB, Kalheker N. Angiotensinogen: molecular biology, biochemistry and physiology. Int J Biochem Cell Biol 1996;11:1211–1222. [DOI] [PubMed] [Google Scholar]
- 13.Xaio F, Puddefoot JR, Vinson GP. The expression of renin and the formation of angiotensin II in bovine aortic endothelial cells. J Endocrinol 2000;164:207–214. [DOI] [PubMed] [Google Scholar]
- 14.Igic R, Behnia R. Properties and distribution of angiotensin I converting enzyme. Curr Pharm Des 2003;9:697–706. [DOI] [PubMed] [Google Scholar]
- 15.Cziraki A, Horvath IG, Papp L. Endothelial function studies in pulmonary vascular disease: determination of ACE activity in humans. Int J Mol Med 2002;3:317–325. [PubMed] [Google Scholar]
- 16.Inagami T. Molecular biology and signaling of angiotensin receptors. J Am Soc Nephrol 1999;10:S2–S7. [PubMed] [Google Scholar]
- 17.Zafari AM, Ushio-Fukai M, Akers M, Yin Q, Shah A, Harrison DG, Taylor WR, Griendling KK. Role of NADH/NADPH oxidase derived H2O2 in angiotensin II-induced vascular hypertrophy. Hypertension 1998;32:488–495. [DOI] [PubMed] [Google Scholar]
- 18.Griendling KK, Minieri CA, Ollernshaw JD, Alexader RW. Angiotensin II stimulates NADH and NADPH oxidase in cultured vascular smooth muscle cells. Circ Res 1994;74:1141. [DOI] [PubMed] [Google Scholar]
- 19.DeKeulenaer GW, Alexander RW, Ushio-Fukai M, Ishizaka N, Griendling KK. TNFalpha actives a p22phox-based NADH oxidase in vascular smooth muscle. Biochem J 1998;329:653–657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mollnau H, Wendt M, Szocs K, Lassegue B, Schulz E, Oelze M, Li H, Bodenschatz M, August M, Kleschyov AL, et al. Effects of angiotensin II infusion on the expression and function of NADPH oxidase and components of nitric oxide/cGMP signaling. Circ Res 2002;90:e58–e65. [DOI] [PubMed] [Google Scholar]
- 21.Lang D, Mosfer SI, Shakesby A, Donaldson F, Lewis MJ. Coronary microvascular endothelial cell redox state in L ventricular hypertrophy: the role of Ang II. Circ Res 2000;86:463–469. [DOI] [PubMed] [Google Scholar]
- 22.Griendling KK, Sorescu D, Ushio-Fukai M. NADPH oxidase: role in cardiovascular biology and disease. Circ Res 2000;86:494–501. [DOI] [PubMed] [Google Scholar]
- 23.Griendling KK, Sorescu D, Lassegue B, Ushio-Fukai M. Modulation of protein kinase activity and gene expression by reactive oxygen species and their role in vascular physiology and pathophysiology. Arterioscler Thromb Vasc Biol 2000;20:2175–2183. [DOI] [PubMed] [Google Scholar]
- 24.Lassegue B, Clempus RE. Vascular NADPH oxidases: specific features, expression, and regulation. Am J Physiol Regul Integr Comp Physiol 2003;285:R277–R297. [DOI] [PubMed] [Google Scholar]
- 25.Babior BM. NADPH oxidase: an update. Blood 1999;93:1464–1476. [PubMed] [Google Scholar]
- 26.Bechara RI, Palaez A, Palacio A, Joshi PC, Hart CM, Brown LAS, Raynor R, Guidot DM. Angiotensin II mediates glutathione depletion, transforming growth factor-beta1 expression, and epithelial barrier dysfunction in the alcoholic rat lung. Am J Physiol Lung Cell Mol Physiol 2005;289:L363–L370. [DOI] [PubMed] [Google Scholar]
- 27.Lieber CS, DeCarli LM. The feeding of alcohol in liquid diets: two decades of applications and 1982 update. Alc Clin Exp Res 1982;6:523–531. [DOI] [PubMed] [Google Scholar]
- 28.Miller FJ, Gutterman DD, Rios CD, Heisted DD, Davidson BL. Superoxide production in vascular smooth muscle contributes to oxidative stress and impaired relaxation in atherosclerosis. Circ Res 1998;82:1298–1305. [DOI] [PubMed] [Google Scholar]
- 29.Campbell DJ, Lawrence AC, Kladis A, Duncan A-M. Strategies for measurement of angiotensin and bradykinin peptides and their metabolites in central nervous system and other tissues. In: Smith AI, editor. Methods in neurosciences, volume 23: peptidases and neuropeptide processing. Orlando: Academic Press; 1995. pp. 328–343.
- 30.Martni J, White JM. Fluorimetric determination of oxidized and reduced glutathione in cells and tissues by high performance liquid chromatography following derivatization with dansyl chloride. J Chromatogr 1991;568:221–225. [DOI] [PubMed] [Google Scholar]
- 31.Wenz M, Steinau R, Gerlach H, Lange M, Kaczmarczyk G. Inhaled nitric oxide does not change transpulmonary AngII formation in patients with ARDS. Chest 1997;112:478–483. [DOI] [PubMed] [Google Scholar]
- 32.Marshall RP, Webb S, Bellingan GJ, Montgomery HE, Chaudhari B, McAnulty RJ, Humphries SE, Hill MR, Laurent GJ. ACE insertion/deletion polymorphism is associated with susceptibility and outcome in ARDS. Am J Respir Crit Care Med 2002;166:646–650. [DOI] [PubMed] [Google Scholar]
- 33.Kang N, Walther T, Tian XL, Bohlender J, Fukamizu A, Ganten D, Bader M. Reduced hypertension-induced end-organ damage in mice lacking cardiac and renal angiotensinogen synthesis. J Mol Med 2002;80:359–366. [DOI] [PubMed] [Google Scholar]
- 34.Griendling KK, Ushio-Fukai M. Reactive oxygen species as mediators of angiotensin II signaling. Regul Pept 2000;9:21–27. [DOI] [PubMed] [Google Scholar]
- 35.Hanna IR, Taniyama Y, Szocs K, Rocic P, Griendling KK. NAD(P)H oxidase-derived reactive oxygen species as mediators of angiotensin II signaling. Antioxid Redox Signal 2002;4:899–914. [DOI] [PubMed] [Google Scholar]
- 36.Gao XP, Standiford TJ, Rahman A, Newstead M, Holland SM, Dinauer MC, Liu QH, Malik AB. Role of NADPH oxidase in the mechanism of lung neutrophil sequestration and microvessel injury induced by gram-negative sepsis: studies in p47 -/- and gp91 -/- mice. J Immunol 2002;168:3974–3982. [DOI] [PubMed] [Google Scholar]
- 37.Wang HD, Xu S, Johns DG, Du Y, Quinn MT, Cayatte AJ, Cohen RA. Role of NADPH oxidase in the vasculature hypertrophic and oxidative stress response to angiotensin II in mice. Circ Res 2001;88:947–953. [DOI] [PubMed] [Google Scholar]
- 38.Rueckschloss U, Quinn MT, Holtz J, Moraweitz H. Dose-dependent regulation of NADPH oxidase expression by angiotensin II in human endothelial cells. Arterioscler Thromb Vasc Biol 2002;22:1845–1851. [DOI] [PubMed] [Google Scholar]