Abstract
Dendritic cells (DCs) play a pivotal role in shaping antiviral immune responses in the respiratory tract. Human metapneumovirus (hMPV) is a recently identified pathogen and like its better known relative, respiratory syncytial virus (RSV), has been increasingly recognized as a major cause of respiratory morbidity in infants and in elderly persons. In the present study, we examined susceptibility as well as cellular responses of human DCs to hMPV compared with RSV. Monocyte-derived DCs (moDCs) were susceptible to infection by both viruses, but only RSV was able to induce a productive infection with release of viral progeny. Despite the fact that viral infection resulted in phenotypic maturation of moDCs, as shown by the upregulation of cell surface markers and antigen-presenting molecules (MHC I and II, CD80, CD83, CD86, CD38), RSV-infected moDCs showed a severely impaired capacity to stimulate CD4+ T cell proliferation. Compared with hMPV, RSV was a more potent inducer of inflammatory and immunomodulatory cytokines, including TNF-α, IL-6, IL-1β, IL-10, and IL-12p70 in both moDCs and plasmacytoid dendritic cells (pDCs). On the other hand, hMPV, but not RSV, was able to trigger production of IFN-α by moDCs, while both viruses strongly induced IFN-α in pDCs. Finally, both viruses strikingly suppressed IFN-α production by moDCs or pDCs stimulated with synthetic dsRNA and CpG-ODN, respectively. The findings provide novel evidence that RSV and hMPV differentially activate human DCs and may use distinct mechanisms to interfere with the host innate and adaptive immune responses.
Keywords: dendritic cells, respiratory syncytial virus, human metapneumovirus, interferon type I, innate immunity
Dendritic cells (DCs) act as a portal for virus invasion as well as potent antigen-presenting cells (APCs) involved in the antiviral host response (1, 2). Respiratory tract dendritic cells are present at high frequency within airway epithelium, submucosa, and associated lung parenchymal tissue under resting conditions (3). Their strategic localization at the site of pathogen entry makes them particularly susceptible to initial viral invasion. After detection, uptake, and degradation of viruses, DCs initiate immune responses via the secretion of interferon, chemokines, and proinflammatory cytokines, and the upregulation of a variety of costimulatory molecules and receptors, a process globally known as cell maturation. After maturation, DCs efficiently present antigens and initiate adaptive immune response by migrating into lymphoid tissue to present processed viral antigens to T lymphocytes (4–6). At least two subsets of DCs have been described in humans: the CD11c+ myeloid DCs, and the CD11c−/CD123+ plasmacytoid DCs (pDCs). The CD11c+ blood DC precursor differentiates into the classical peripheral epithelial (CD1ahi) or nonepithelial (CD1alo) tissue DCs. Blood monocytes are also capable of differentiating into dendritic cells (moDCs) (7). pDCs, also known as alpha/beta interferon (IFN-α/β)–producing cells, characteristically express high levels of the IL-3 receptor α chain (CD123), but lack the expression of myeloid-related markers. pDCs participate in innate immune responses to different types of viruses by producing large amounts of IFN-α/β (4, 7). Thus, studying the interaction of DCs with viruses and how this may influence the primary immune response is critical for understanding disease pathogenesis and immunity to viral infection.
Human metapneumovirus (hMPV) is a recently identified RNA virus belonging to the Paramyxoviridae family, which includes several major human and animal pathogens. The Paramyxoviridae family is organized into two subfamilies, the Paramyxovirinae and the Pneumovirinae, the latter taxonomically divided into the two Pneumovirus and Metapneumovirus genera. The classification of the two genera is based primarily on their gene constellation (8). Metapneumoviruses lack the nonstructural proteins NS1 and NS2, and the gene order is different from that of pneumoviruses. Respiratory syncytial virus (RSV) is the type species of the Pneumovirus genus, while hMPV has been assigned to the Metapneumovirus genus based on biological properties and genomic sequence. Epidemiologic studies indicate that like RSV, hMPV is a significant human respiratory pathogen with worldwide distribution (9). Indeed, hMPV has been found to be the second most detected pathogen in children suffering from acute respiratory tract illness, topped only by RSV (10). In young children, the clinical symptoms associated with hMPV infection are virtually indistinguishable from those caused by RSV (9, 11), although some but not all studies have reported a lower severity of disease compared with RSV (12, 13).
Since virtually no data are currently available in regard to the response of DCs to hMPV and it is not known whether this infection results in a distinct response compared with RSV, we investigated the effect of hMPV and RSV infection on human moDCs and pDCs. We show that hMPV and RSV induce different responses in moDCs and pDCs, including distinct characteristics of infection, APC function, cytokine production, and IFN-α release. Moreover, both hMPV and RSV are capable of inhibiting the production of IFN-α by moDCs and pDCs following stimulation with known agonists. These data suggest that hMPV and RSV may use distinct mechanisms to trigger and/or interfere with the immune response in the infected host.
MATERIALS AND METHODS
Culture Medium and Reagents
Mononuclear cells were cultured in complete (c) RPMI 1640 supplemented with 2 mmol/liter L-glutamine, 10% FBS, 50 μM 2-ME, and 1,000 U/I penicillin-streptomycin. TNF-α and IL-4 were purchased from R&D Systems (Minneapolis, MN) and recombinant human GM-CSF from PeproTech (Rocky Hill, NJ). pDCs were cultured in cRPMI without 2-ME. IL-3 was purchased from R&D Systems.
Establishment of moDC
The study was approved by the Institutional Review Board of the University of Texas Medical Branch. moDCs were generated from human peripheral blood mononuclear cells (PBMC) (7). Briefly, whole blood from healthy adult donors was mixed with Ficoll-hypaque, and after centrifugation the layer of mononuclear cells was collected. The mononuclear cells were laid on 25 cm2 flasks for 60–90 min at 37°C, after which nonadherent cells were removed by five washes with plain RPMI medium. Adherent cells were cultured for 7 d in cRPMI medium containing GM-CSF (100 ng/ml) and IL-4 (20 ng/ml). One-third of the medium and 100% of each cytokine were replaced every other day. In some experiments moDCs were derived from CD14+ cells, the latter isolated by immunomagnetic selection (purity > 93%) (Miltenyi, Auburn, CA). DCs obtained by either of these methods were > 97% CD11c+ (with levels of expression that were slightly different depending from the blood donor), HLA-DR, DP, DQ+, and < 1% CD14+, and therefore adherent monocytes were used in all subsequent experiments. moDCs were used on the seventh day of culture in all experiments.
Enrichment of pDCs
Isolation of pDCs from human PBMC has been greatly facilitated by the identification of pDC-specific markers. BDCA-2 is a member of the C-type lectin family of transmembrane glycoprotein. The monoclonal antibody against BDCA-2 is highly specific for human pDC. BDCA-4/ neurophilin-1 is a receptor for members of the semaphorin family that also functions as coreceptor for vascular endothelial growth factor (VEGF). Antibodies to BDCA-4 do not impair pDC function (4, 14). Human pDCs were purified from PBMC (obtained as mentioned above for DCs) by using magnetic microbeads coated with anti–BDCA-4 Mab (Miltenyi) according to the manufacturer's instructions. pDC population had a purity ⩾ 97% based on positive expression of BDCA-2 and CD123.
RSV and hMPV Preparations
RSV A2 was grown in HEp-2 cells (American Type Culture Collection, Manassas, VA) and purified by polyethylene glycol precipitation, followed by centrifugation on 35–65% discontinuous sucrose gradients as described elsewhere (15). Recombinant, red fluorescent protein (RFP)-expressing RSV (rrRSV) was generated from the full-length RSV plasmid, MP224 (16), by replacing its first gene, encoding the enhanced green fluorescent protein, with the wild-type Discosoma RFP gene from pDsRed (Clontech, Palo Alto, CA). To accomplish this, the BstXI site within the RFP gene was disrupted by PCR mutagenesis (17), and this modified RFP gene was amplified by PCR with primers that added BstXI sites to each end, in addition to the gene start and NS1 untranslated region preceding the RFP gene, and the L gene end following the RFP gene. The PCR product was inserted into the BstXI site of D13, a partial RSV clone containing the 3′ RSV genome end. The AgeI/SpeI fragment of this clone, MP230, was transferred into the full-length clone, MP224, that had been digested completely with AgeI and partially with SpeI. The resulting construct, BN1, was rescued as replicating virus by cotransfection of BN1 and 4 plasmids producing the proteins needed to initiate RSV replication and transcription, into HeLa cells infected with the recombinant vaccinia virus MVA-T7 (18, 19). For simplicity, rrRSV(BN1) is identified in this report as rrRSV. The virus titer was determined by methylcellulose plaque assay (20).
The hMPV strain CAN97–83 was obtained from the Centers for Disease Control (CDC, Atlanta, GA), with permission from Dr. Guy Boivin at the Research Center in Infectious Diseases, Regional Virology Laboratory, Laval University (Quebec City, PQ, Canada). Virus was propagated in LLC-MK2 cells (ATCC) in MEM (without serum) containing 1 μg trypsin/ml (Worthington, Lakewood, NJ). The virus titer was determined by HRP staining in LLC-MK2 cells, as previously described (21). Briefly, virus was serially diluted and added to LLC-MK2 cell monolayers. Cells were fixed and stained with anti-hMPV antibodies (kindly provided by MedImmune, Inc., Gaithersburg, MD) 48 h after infection, followed by HRP-conjugated anti–guinea pig antibody (Zymed, San Francisco, CA). The reaction was developed by the addition of 3-amino-9-ethyl-carbazole (AEC) substrate. The final viral titer was expressed as plaque-forming units (pfu)/ml.
Infection of moDCs with hMPV and RSV
moDCs (5 × 105) were resuspended in 100 μl of plain RPMI medium in 15-ml conical tubes and incubated with hMPV, RSV, or rrRSV at different multiplicity of infection (MOI) for 1–2 h at 37°C (viral adsorption phase). Cells were washed twice with plain RPMI and for the remaining time of infection were placed in a 24-well plate in a total volume of 1 ml of cRPMI with GM-CSF and IL-4. For hMPV-inoculated cells, the medium also contained 1 μg/ml of trypsin during all steps of the infection process. At different time points of infection, cells or supernatants were collected for subsequent analysis. In some experiments, moDCs were exposed to ultraviolet-inactivated (nonreplicating) preparations of RSV or hMPV (22). For viral titration, supernatant from RSV- or hMPV-exposed moDCs was taken at different time points after infection (12, 24, and 40 h), centrifuged at 14,000 RPM for 2 min, and snap-frozen in liquid nitrogen. Supernatant from RSV- and hMPV-infected moDCs was titrated in HEp-2 and LLC-MK2 cell monolayers by a methylcellulose plaque assay and HRP-based staining, respectively. For pDCs infection, 105 cells were resuspended in 50 μl of plain RPMI medium and seeded in a 96-well plate. Cells were incubated with hMPV or RSV at an MOI of 1 for 1–2 h at 37°C as described for moDCs. Infected cells were then washed twice with plain RPMI and cultured for an additional 24-h period in 200 μl of cRPMI with 10 ng/ml of IL-3. Supernatant was collected for subsequent analysis of cytokines.
Immunofluorescence Microscopy
rrRSV- and hMPV-infected moDCs, and uninfected cells, were seeded in 8-well coverglass Lab Tek tissue chambers (Nunc, Naperville, IL) at 105 cells/well. The chambers were incubated for up to 40 h at 37°C. For detection of hMPV antigen, moDCs were fixed with 1% paraformaldehyde and stained with polyclonal guinea pig anti-hMPV antibody (kindly provided by MedImmune, Inc.). After several washes, bound antibodies were detected with goat anti–guinea pig antibody directly labeled with Alexa Fluor 488 (Molecular Probes, Eugene, OR). Cells stained for hMPV antigen or directly infected with rrRSV were examined using a Zeiss LSM 510 meta-ultraviolet laser scan confocal microscope. Images were analyzed using the Zeiss LSM Image Browser software v. 3.0 (Zeiss, Thornwood, NY).
FACS Analysis of DCs
Simultaneous analysis of cell surface CD11c and intracellular hMPV or RSV antigens by moDCs was performed as follows. Cells in suspension were washed once with ice-cold wash solution (PBS with 1% heat-inactivated FCS). To block nonspecific binding, virus- and mock-infected moDCs were incubated with FcR blocking reagent (Miltenyi) for 30 min at 4°C. After washing, cells were stained with a PE-conjugated anti-human CD11c (Pharmingen, San Diego, CA). To detect expression of intracellular viral antigens, cells were then fixed with Cytofix/cytoperm (Pharmingen) and permeabilized with washing buffer Perm/wash buffer (Pharmingen). Cells were incubated with guinea pig anti-hMPV antibody, followed by a FITC-goat anti–guinea pig antibody (Zymed), or with an FITC-conjugated anti-RSV antibody (Biosource, Camarillo, CA). Cells were analyzed with a FACScan flow cytometer equipped with CellQuest software (both from Becton Dickinson Immunocytometry Systems, San Jose, CA). Analysis was performed in WinMDI software (Scripps, La Jolla, CA). For assessment of RSV replication, moDCs were exposed to rrRSV (at different MOI) for 40 h and directly analyzed by flow cytometry. For analysis of antigen-presenting molecules and other surface markers, moDCs were examined by FACS after staining with the following antibodies: anti-CD80, anti-CD86, anti-CD83, anti-CD38, anti-HLA DQ, DP, DR (Pharmingen), and anti-HLA class I (ATCC HB-95, hybridoma W6/32). Nonspecific binding was blocked as mentioned above. After the final step, cells were washed and then fixed in 200 μl of 1% paraformaldehyde in PBS.
CD4+ T Cell Proliferation Assay
Human lymphocytes were isolated from freshly collected heparinized blood by Ficoll-Paque. CD4+ T cells were purified by negative magnetic selection using a pan T isolation antibody kit (Miltenyi). The purity of CD4+ T cells was > 97% as determined by flow cytometry. Allogeneic lymphocyte mixed reaction (MLR) stimulation was performed by culturing of moDCs or virus-infected moDCs (104 cells/well) with allogeneic CD4+ T cells (105 cells/well) in 96-well round bottom microtiter plates in complete medium for 4 d. For the determination of cell proliferation the cells were pulsed with 1 μCi/well of [3H]-thymidine (ICN, Irvine, CA) for the last 18 h. Cells were harvested onto filters using a cell harvester (Brandel, Gaithersburg, MD) and incorporation of [3H]-thymidine to DNA was determined using a Wallac 1450 Microbeta/Trilux liquid scintillation counter (Perkin Elmer, Boston, MA). Results are expressed as mean counts per minute (cpm) of triplicate cultures ± SEM.
Measurement of Cytokines and IFN-α
To assess the production of cytokines and IFN-α, cell-free supernatant from virus- and mock-infected moDCs or pDCs was collected at 40 h and 24 h, respectively. Samples were tested for multiple cytokines (IL-1β, IL-2, IL-4, IL-5, IL-6, IL-7, IL-10, IL-12 p70, IL-13, IL-17, G-CSF, GM-CSF, IFN-γ, and TNF-α) using the Luminex-based Bio-Plex system (Bio-Rad Laboratories, Hercules, CA), according to the manufacturer's instructions. The lower limit of detection for all cytokines measured by this assay is 3 pg/ml. IFN-α (isoforms IFN-αA, α2, αA/D, αD, αK, and α4b) was measured by ELISA (PBL Biomedical Laboratories, Piscataway, NJ), according to the manufacturer's instructions (lower limit of detection, 12.5 pg/ml). In parallel experiments, IFN-α production was determined in the supernatant of moDCs stimulated with 2 μg/ml of poly-ICLC (Oncovir, Washinghton, DC) in the presence or absence of FuGENE (Roche, Indianapolis, IN) or in the supernatant of pDCs stimulated with 10 μg/ml of synthetic CpG oligodeoxynucleotide 2006 (ODN). CpG-ODN 2006 (5'-tcgtCGTTTTGTCGTT TTGtcgtT-3′; phosphodiester backbone with the 5′ and 3′ phosphorothioate-modified, indicated by lower case letters) was synthesized by Sigma-Genosys (The Woodlands, TX).
Statistical Analysis
Statistical analyses were performed by the Mann-Whitney U test using the InStat 3.05 biostatistics package (GraphPad, San Diego, CA). Unless otherwise indicated, mean ± SEM is shown.
RESULTS
RSV and hMPV Are Able to Infect Human moDCs
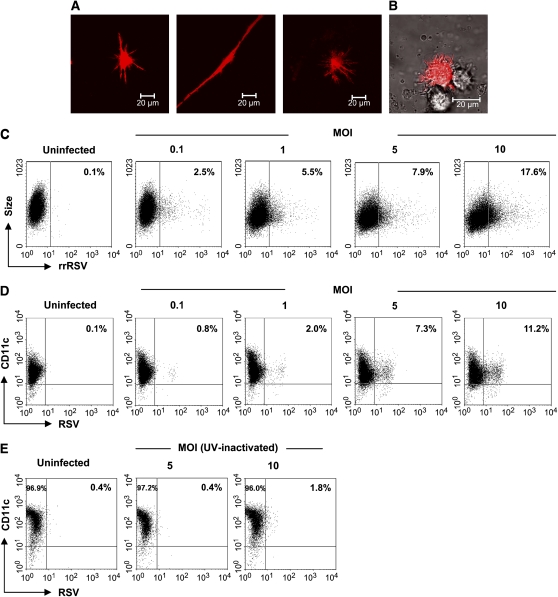
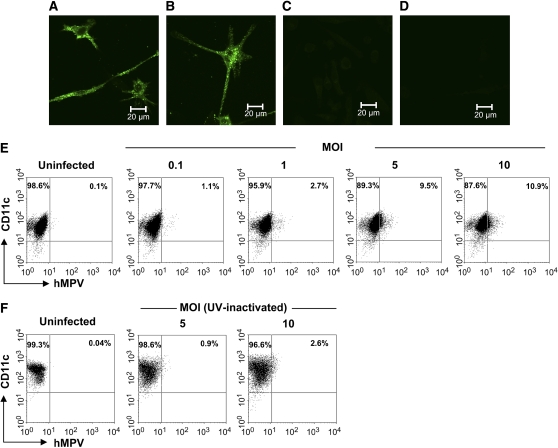
moDCs were used as model to investigate whether hMPV and RSV are able to infect human DCs. Infection of moDCs was established by directly assessing the expression of RFP in moDCs (in the case of rrRSV infection) or by staining the cells for viral antigens (in the case of wild-type RSV or hMPV infection), using either fluorescent microscopy or FACS analysis. As shown in Figures 1A and 1B, 5 to 10% of moDCs that were exposed for 40 h to rrRSV (MOI of 5) expressed DsRed protein, indicating that RSV was able to replicate in these cells. For an additional quantitative assessment of the percentage of infected cells and to establish a dose-dependent effect of the virus, moDCs were also analyzed by FACS. Indeed, we observed an increasing number of RFP-positive cells as the rrRSV MOI increased, reaching a maximum of ∼ 17% positive cells at MOI of 10 (Figure 1C). These results were confirmed by experiments in which moDCs were exposed to wild-type RSV, followed by double staining with antibodies to RSV antigens and CD11c (Figure 1D). Cells that were exposed to ultraviolet-inactivated RSV (nonreplicating virus) were virtually negative for RSV antigens (Figure 1E). Similarly, ∼ 10% of moDCs that were exposed to hMPV at an MOI of 5 for 40 h and subsequently stained with anti-hMPV antibodies showed intracytoplasmic granular fluorescence immunoreactivity (Figures 2A and 2B). No fluorescence was observed in uninfected cells (Figure 2C) or infected cells stained with an isotype control antibody (Figure 2D). FACS analysis revealed that up to 11% of moDCs were infected by hMPV infection at MOI of 10 (Figure 2E). As with RSV, there was no viral antigen expression when moDCs were exposed to ultraviolet-hMPV (Figure 2F). Overall, the results of these studies show that moDCs were susceptible to infection by both RSV and hMPV.
Figure 1.
RSV infection of human moDCs. moDCs were infected with rrRSV or RSV at different MOI for 40 h. rrRSV-infected cells (MOI of 5) were analyzed using a Zeiss LSM 510 meta-ultraviolet laser scan confocal microscope and Zeiss LSM software. (A) rrRSV-infected cells observed under fluorescent light and (B) by interference contrast (Nomarski). (C) rrRSV-infected cells were quantified in a FACScan and data were analyzed using WinMDI software. Cells infected with increasing dose of RSV (D) or exposed to ultraviolet-inactivated RSV (E) were double stained with a PE-conjugated anti-CD11c mAb and specific anti-RSV antibody. Cells were analyzed as mentioned above. Data shown are representative of one of three independent experiments.
Figure 2.
hMPV infection of human moDCs. moDCs were infected with hMPV at different MOI and cultured for 40 h. hMPV-infected cells (MOI of 5) were fixed and stained with an anti-hMPV antibody followed by anti-guinea pig Alexa 488–conjugated antibody. Stained cells were analyzed under the microscope using Zeiss LSM software. (A, B) Two different fields showing hMPV- infected moDCs; (C) uninfected cells stained with anti-hMPV antibody; (D) infected cells stained with isotype antibody. Cells infected with increasing dose of hMPV (E) or exposed to ultraviolet-inactivated hMPV (F) were double stained with a PE-conjugated anti-CD11c mAb and specific anti-hMPV antibody, and were analyzed as described in Figure 1. Data shown are representative of one of three independent experiments.
Human moDCs Are Permissive to RSV but hMPV Infection Is Restricted
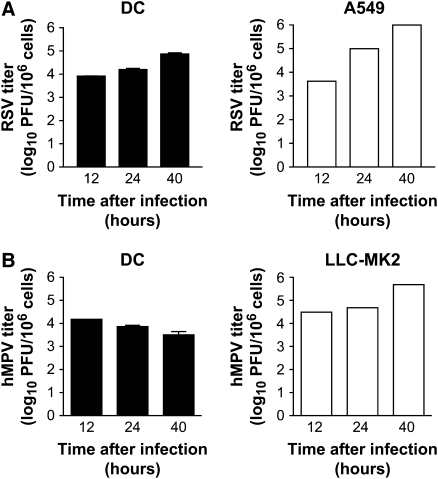
To determine whether hMPV and/or RSV infection of moDCs resulted in the release of infectious particles (i.e., productive infection), moDCs were exposed to either virus at MOI of 5 for 2 h followed by extensive washing steps to remove unbound virus. Cells were then cultured for 12, 24, and 40 h and the supernatant tested at each time point for viral replication. We observed an increase of viral titer of approximately one log (from 3.9 log10 at 12 h to 4.8 log10 at 40 h) in cell supernatant of RSV-infected moDCs (Figure 3A, left panel). For comparison, RSV titer in the supernatant of A549 cells (type II–like epithelial cells) increased from 3.62 log10 to 5.99 log10 over the same time period (Figure 3A, right panel). In contrast, hMPV replication appeared to be restricted as virus titer in the moDCs supernatant decreased over time with low levels of replicating virus detectable 40 h after infection (Figure 3B, left panel). However, when hMPV replication was assessed using a permissive cell line, LLC-MK2, an exponential increase in the amount of infectious particles was detected in the cell supernatant (from 4.0 log10 to 5.19 log10) over the same time period (Figure 3B, right panel). These results were observed despite the fact that both moDCs and LLC-MK2 were infected and cultured in the presence of trypsin (to facilitate hMPV infection). Collectively, these data suggest that RSV, but not hMPV, induces a productive infection in human moDCs.
Figure 3.
Viral production by moDC infected with RSV or hMPV. moDCs were inoculated with RSV or hMPV at MOI of 5 for 2 h (for virus adsorption). As control, the permissive cell lines A549 and LLC-MK2 were infected with RSV and hMPV, respectively. Cells were then washed and fresh medium added. Cell-free supernatant was collected at different time points and viral titer was determined. (A) RSV virus titer in moDCs (left panel) and A549 cells (right panel). (B) hMPV titer in moDCs (left panel) and LLC-MK2 cells (right panel). Bar graphs show the mean ± SEM of three different donors of moDCs and a representative experiment with A549 and LLC-MK2 cells.
hMPV and RSV Induce Maturation of moDCs
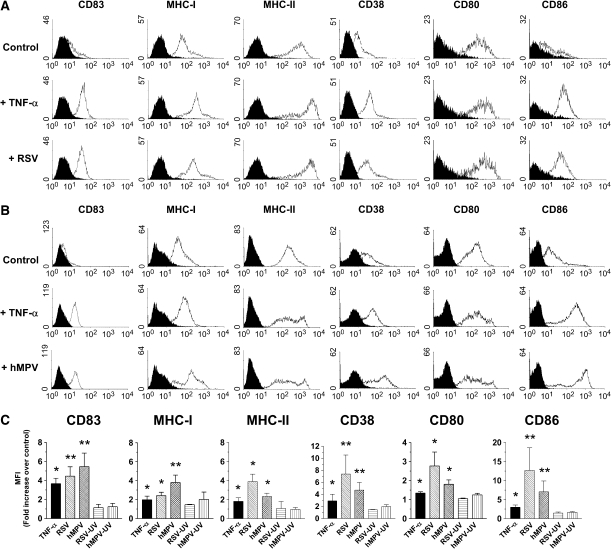
Maturation of moDCs is essential for the initiation of the immune response. Increased expression of major histocompatibility complex (MHC) antigens and costimulatory molecules on moDCs and other APCs is a critical process that results in enhanced T cell activation (23). Thus, the effect of RSV and hMPV infection on the level of expression of various molecules that are associated with a mature DC phenotype was analyzed. Untreated or TNF-α–stimulated moDCs were included as negative and positive controls, respectively. As shown in Figure 4, exposure of moDCs to RSV resulted in a significant increase in the expression of CD83, MHC-I, MHC-II, CD38, CD80, and CD86. RSV-induced upregulation of these molecules was substantially similar in terms of profile and level of expression to that observed in moDCs treated with TNF-α, as shown in the histograms (Figure 4A) and in the quantification analysis of the mean fluorescence intensity (Figure 4C). Similar to RSV, hMPV also induced a significant upregulation of the surface antigens that were examined (Figures 4B and 4C). Overall, RSV appeared to have a slightly more potent effect than hMPV in increasing the expression of the surface markers included in this work. Both ultraviolet-inactivated RSV and hMPV failed to induce any significant maturation of moDCs, as shown by lack of upregulation of surface antigens (Figure 4C).
Figure 4.
Virus-induced maturation of moDCs. moDCs were infected with RSV or hMPV at MOI of 5 for 40 h. Cells were stained with antibodies to CD83, MHC-I, MHC-II, CD38, CD80, and CD86. Cells were acquired in a FACScan and data were analyzed by WinMDI software. (A) Expression of surface molecules by moDCs was evaluated after RSV or (B) hMPV infection and compared with that of uninfected cells (Control) or cells stimulated with TNF-α. In some experiments, expression of the different antigens was determined after exposure of cells to ultraviolet-inactivated viral preparations. Data shown are representative of one out of four independent experiments. Black filled histograms represent isotype antibodies, open histograms specific antibodies. (C) Bar graphs represent mean fluorescence intensity (MFI) fold increase over control (mean ± SEM) (n = 4 donors). *P < 0.05; **P < 0.01, compared with control cells.
RSV-Infected moDCs Inhibit T Cell Proliferation
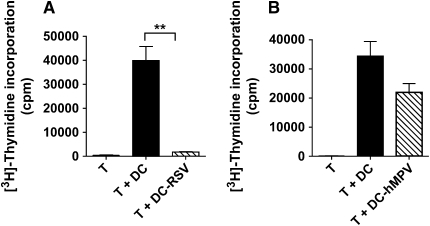
Since moDCs have a unique capacity to activate memory T cells we asked whether RSV or hMPV infection would affect their ability to induce proliferation of CD4+ T cells. To answer this question, an MLR assay was used in which uninfected or virus-infected moDCs were added to allogeneic CD4+ T cells and T cell proliferation analyzed 4 d later. While uninfected moDC were able to strongly stimulate T cell proliferation, RSV-infected moDCs induced a striking inhibition of CD4+ T cell proliferation (Figure 5A). In contrast, hMPV-infected moDCs had a much lower effect on CD4+ T cell proliferation, as the differences between infected and control cells did not reach statistical significance (Figure 5B). Therefore, despite the fact that both viruses induce maturation and upregulation of antigen-presenting molecules (Figure 4), the ability of moDCs to stimulate T cell proliferation was impaired in response to RSV and to a much lower degree to hMPV infection. The possibility that loss of the allostimulatory function of RSV-infected moDCs was the consequence of loss or reduced viability of RSV-infected cells was discarded, since the same striking inhibitory effect was observed when a small number of RSV-infected moDCs (5 × 103) were added to the co-cultures of uninfected moDCs with CD4+ T cells (data not shown).
Figure 5.
RSV-infected moDCs inhibit CD4+ T cell proliferation. moDCs where infected with either (A) RSV or (B) hMPV and cocultured with allogeneic CD4+ T cells. T cell proliferation was analyzed on Day 4 of culture by [3H]-thymidine incorporation. Data are expressed as cpm (mean ± SEM) (n = 5 donors). **P < 0.01.
Distinct Pattern of Cytokines and IFN-α Production by Viral-Infected moDCs
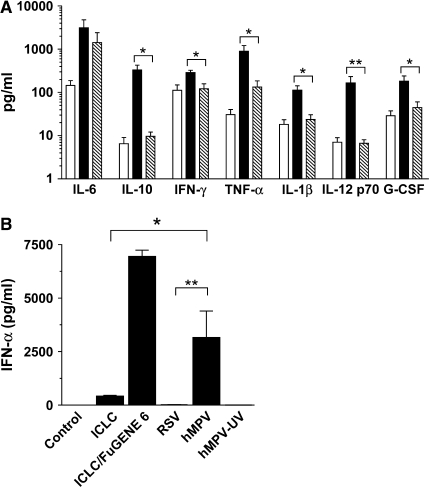
In addition to the expression of membrane molecules, matured moDCs release soluble mediators, including cytokines, that have been shown to affect both humoral and T cell–mediated immune response (1). Thus, the effect of hMPV and RSV infection on cytokine release from moDCs obtained from several healthy adult donors was assessed by testing culture supernatants for the presence of various cytokines with known inflammatory and immunomodulatory activity as well as IFN-α, a key mediator of innate immunity. Cytokine levels were determined by a Luminex-based Bio-Plex assay and IFN-α by ELISA. Overall, hMPV failed to induce cytokine production by moDCs, with the exception of IL-6 and TNF-α, which were inducible but not at concentrations significantly greater compared with control cells (Figure 6A). On the other hand, RSV infection of moDCs resulted in a robust production of IL-6, IL-10, TNF-α, IL-1β, and IL-12p70, which were induced by several fold compared with uninfected cells (Table 1). Concentrations of IFN-γ and G-CSF were also greater in RSV-infected cells compared with uninfected ones, albeit to a much lesser extent. Concentrations of IL-2, IL-5, IL-7, IL-13, and IL-17 were at the lower limit of detection in control or virus-infected moDCs, while IL-4 and GM-CSF were not tested because recombinant forms of these cytokines were exogenously added to the culture medium for cell differentiation (see Materials and Methods). Surprisingly, when we compared these two viruses for their ability to induce IFN-α by moDCs we found that, contrary to our findings in regard to cytokine production, hMPV strongly induced IFN-α secretion from moDCs, while RSV failed to induce significant levels of IFN-α in all tested donors (Figure 6B). hMPV-induced IFN-α production was dependent on viral replication, as IFN-α concentration in supernatant of moDCs exposed to ultraviolet-inactivated viral preparations was at the lower limit of detection (Figure 6B). Furthermore, dsRNA in the form of synthetic poly-ICLC strongly induced IFN-α production by moDCs when delivered intracellularly (ICLC/FuGENE 6), a finding previously reported in murine CD11c+ DCs (24).
Figure 6.
Cytokine and IFN-α production by moDCs infected with RSV or hMPV. moDCs were infected with RSV or hMPV at MOI of 5. (A) Cell-free supernatant was collected at 40 h and tested for cytokines by a multiplex bead assay. Bar graph represents cytokine concentration of uninfected cells (open bars), RSV-infected cells (black bars), and hMPV-infected cells (cross-hatched bars). (B) IFN-α production was assessed by ELISA in the supernatant of viral-infected moDCs, cells exposed to ultraviolet-inactivated virus (hMPV-UV) or cells that were stimulated with 2 μg/ml of poly-ICLC, with or without a transfecting reagent (FuGENE 6). Bar graph represents mean ± SEM (n = 5 donors). *P < 0.05, **P < 0.01.
TABLE 1.
CYTOKINE PRODUCTION BY HUMAN DENDRITIC CELLS INFECTED WITH HMPV OR RSV
| RSV | hMPV | ||
|---|---|---|---|
| IFN-α | moDC | 1.2 ± 1.1* | 252.6 ± 99.3*† |
| pDC | 581.3 ± 127.8*† | 402.8 ± 211.4*† | |
| IL-1β | moDC | 6.1 ± 1.6† | NI |
| pDC | 16.4 ± 2.3‡ | NI | |
| IL-4 | moDC | NT | NT |
| pDC | 13.9 ± 8.3‡ | 2.77 ± 1.4 | |
| IL-6 | moDC | 21.4 ± 11.4† | 9.8 ± 6.7 |
| pDC | 4.8 ± 3.3‡ | NI | |
| IL-10 | moDC | 40.9 ± 15.4‡ | NI |
| pDC | 46.4 ± 20.2‡ | NI | |
| IL-12 | moDC | 90.7 ± 75† | NI |
| pDC | 8.6 ± 1.3‡ | ND | |
| IFN-γ | moDC | 2.5 ± 0.3‡ | NI |
| pDC | 7.1 ± 1.5‡ | NI | |
| TNF-α | moDC | 29.1 ± 10.4† | 4.51 ± 1.7 |
| pDC | 44.3 ± 8.2‡ | 3.0 ± 1.5 | |
| G-CSF | moDC | 5.2 ± 2.5† | NI |
| pDC | 9.4 ± 1.1‡ | NI | |
| GM-CSF | moDC | NT | NT |
| pDC | 4.9 ± 0.9‡ | NI |
Definition of abbreviations: hMPV, human metapneumovirus; ND, not detectable; NI, not inducible; NT, not tested; RSV, respiratory syncytial virus.
Values represent fold increase in virus-infected versus uninfected cells (mean ± SEM, n = 4–6 donors).
For calculation of fold increase, IFN-α concentration in uninfected cells is extrapolated as the lower limit of detection of the ELISA (12.5 pg/ml).
P < 0.01, virus-infected versus uninfected cells.
P < 0.05, virus-infected versus uninfected cells.
Viral Regulation of Cytokine and IFN-α Production by Human pDCs
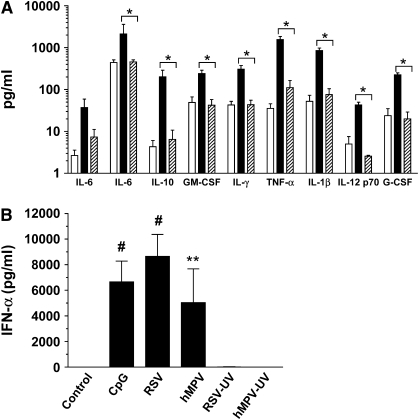
The results presented so far indicate that hMPV was a much stronger inducer of IFN-α compared with RSV. We further investigate whether these two viruses would differentially induce IFN-α also in pDCs, a DC subset considered the most potent IFN-α–producing cells. Stimulation of pDCs by viral pathogens and DNA containing unmethylated CpG sequences results in production of high levels of IFN type I and a complex array of cytokines (4). Although little is currently known regarding the response of pDCs to paramyxoviruses, this cell type has been recently shown to be susceptible to RSV infection (25). Therefore, pDCs were isolated from peripheral blood and then exposed to either hMPV or RSV (MOI of 1, for 24 h) and supernatant was tested for cytokines and IFN-α concentrations. As shown in Figure 7A and Table 1, pDCs exposed to RSV secreted several cytokines with a profile resembling that observed in moDCs. In addition, pDCs produced IL-4 and GM-CSF after stimulation with RSV. Interestingly, by comparing cytokine fold induction, RSV appeared to be a relatively stronger inducer of IL-4 than IFN-γ in this cell type (Table 1). Also in agreement with the results in moDCs, hMPV failed to induce significant levels of cytokines by pDCs. On the other hand, both intact RSV and hMPV (but not ultraviolet-inactivated viruses), as well as the CpG-ODN 2006, were all potent inducers of IFN-α in pDCs (Figure 7B). As for moDCs, viral replication was necessary for IFN-α production by pDCs in response to RSV or hMPV (Figure 7B, RSV-UV and hMPV-UV). Collectively, these data indicate that human moDCs and pDCs display virus-specific responses to paramyxovirus infection, by producing an array of proinflammatory and immunomodulatory cytokines following RSV, but not hMPV infection. In addition, these two cell types appear to have distinct characteristics in term of IFN type I response, since moDCs were able to produce IFN-α only when infected with hMPV, whereas pDCs responded with secretion of abundant IFN-α following infection with both RSV and hMPV.
Figure 7.
Cytokine and IFN-α production by human pDCs exposed to RSV or hMPV. Human pDCs were exposed to RSV or hMPV at MOI of 1. (A) Cell-free supernatant was collected at 24 h and tested for different cytokines by a multiplex bead assay. Bar graph represents cytokine concentration of uninfected cells (open bars), RSV-infected cells (black bars), and hMPV-infected cells (cross-hatched bars). (B) IFN-α production was assessed by ELISA in the supernatant of viral-infected pDCs, cells exposed to ultraviolet-inactivated viruses (RSV-UV and hMPV-UV), or cells that were stimulated with CpG-ODN 2006 (10 μg/ml). Bar graph represents mean ± SEM (n = 6 donors). *P < 0.05, **P < 0.01, #P < 0.0001.
hMPV and RSV Inhibit Poly-ICLC– and CpG-Induced IFN-α Production by DCs
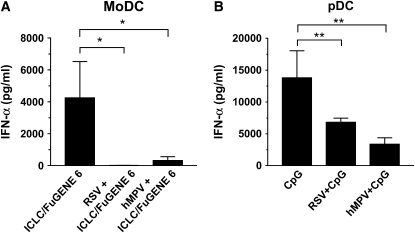
There is compelling evidence that both human and bovine RSV interfere with intracellular pathways leading to IFN-I production. In certain cell types, such as epithelial cells and macrophages, the mechanism(s) of this inhibition appears to be dependent on expression of the nonstructural viral proteins NS1/NS2 (26). Recent evidence suggests that RSV is able to block interferon production in human pDCs by inhibiting the TLR7 and TLR9 signaling pathways (25). It is currently unknown whether hMPV is capable of inhibiting IFN-α/β production. The following experiments were therefore designed to answer this question. moDCs and pDCs were infected with RSV or hMPV for 24 h, followed by stimulation with poly-ICLC/ FuGENE 6 or CpG-ODN 2006, respectively. IFN-α production was measured in cell supernatant after 24 h. As shown in Figure 8A, infection with either RSV or hMPV dramatically inhibited IFN-α production by moDCs transfected with poly-ICLC. Viral infection also completely blocked the relatively modest IFN-α by moDC stimulated with extracellular poly-ICLC (i.e., in absence of a transfection agent) (data not shown). Similarly, in pDCs, RSV and hMPV infection significantly inhibited CpG-mediated IFN-α secretion by ∼ 50% and 75%, respectively (Figure 8B). Viral replication was necessary to elicit this effect, as ultraviolet-inactivated RSV and hMPV preparations failed to inhibit IFN-α production in moDCs as well as pDCs (data not shown).
Figure 8.
Inhibition of IFN-α production in moDC and pDC by RSV and hMPV. (A) Human moDCs were infected with RSV or hMPV at MOI of 5 for 40 h, followed by stimulation with 2 μg/ml of poly-ICLC transfected with FuGENE 6. IFN-α production after 24 h was measured by ELISA. Bar graph represents mean ± SEM (n = 3 donors). (B) pDCs were exposed to RSV or hMPV at MOI of 1 for 24 h, followed by stimulation with 10 μg/ml of CpG-ODN 2006. Bar graph represents mean ± SEM (n = 6 donors). *P < 0.05, **P < 0.01.
DISCUSSION
In this work, we demonstrate that RSV and hMPV, two genetically distinct members of the Paramyxoviridae family that cause significant respiratory morbidity in infants and in the elderly, differentially activate human DCs. Both viruses are able of replicating in moDCs, but only RSV induces a productive infection. While maturation of moDCs, characterized by the upregulation of antigen-presenting molecules and costimulatory molecules, is induced by both hMPV and RSV infection, only RSV-infected moDCs are capable of inhibiting T cell proliferation. In addition, we found that a distinct profile of inflammatory and immunomodulatory cytokine production characterizes the infection of moDCs and pDCs by RSV or hMPV. Finally, we show that both viruses strikingly suppress IFN type I secretion by moDCs or pDCs exposed to the agonists poly-ICLC and CpG-ODN, respectively. Overall, the functional responses observed in RSV- or hMPV-infected DCs require viral replication (i.e., were abolished when viruses were inactivated), indicating that cell membrane signaling or uptake of virus particle by phagocytosis or other internalization processes in DCs are not sufficient to trigger these events.
We found that moDCs were susceptible to both RSV and hMPV infection, as shown by the expression of viral antigens. In the case of RSV, no major differences were observed when rrRSV or polyclonal antibodies were used to assess the percentage of susceptible cells, although the use of the recombinant virus appeared to be a more sensitive tool (Figure 1). In agreement with previous studies of cord blood–derived DCs, we found a virus dose-dependent increase in the number of RSV-positive cells, ranging from 2–17% at MOI ranging from 0.1–10 (27). In the case of hMPV infection, ∼ 10% of the whole moDCs population expressed viral antigen after exposure to MOI of 5 or 10 (Figure 2). In addition, our study provides novel evidence that RSV was able to induce a productive infection in moDCs (i.e., release of extracellular virus). Not surprisingly, we observed that the kinetics of viral release and peak titer in RSV-infected moDCs were slower and smaller than those in epithelial cells, the major target of RSV infection (Figure 3) (28). In contrast, when we examined hMPV titers in cell supernatant of moDCs compared to the susceptible LLC-MK2 reference cells, we noticed that viral replication appeared to be restricted as virus titer in the moDCs supernatant decreased over time. Based on these data we can conclude that despite the fact that moDCs are fully capable of replicating RSV and hMPV (as seen by viral antigen expression), only RSV is able to induce a productive infection in these cells.
Maturation of DCs is critical for the initiation of specific immune response. The process is characterized phenotypically by acquisition of CD83 expression, a glycoprotein with unknown function that is specifically present on mature DC (29), as well as the upregulation of important costimulatory molecules including CD80 and CD86 (30). Our results show that RSV infection resulted in a significant increase in the expression of CD83, CD80, CD86, CD38, MHC-I, and MHC-II, to a degree that was comparable to TNF-α treatment (Figure 4). These results are consistent with previous studies in RSV-infected cord blood cell–derived DCs (27). After DCs acquire a mature phenotype, they are fully competent to activate T cells (5). However, when RSV-infected moDCs were cultured with allogeneic CD4+ T cells, T cell proliferation was severely impaired (∼ 98%) compared with similar culture using uninfected moDCs (Figure 5). A similar observation has been reported following mitogen stimulation of cultured RSV-infected epithelial cells with lymphocytes (31), but this is the first study showing that RSV has a profound inhibitory activity on the antigen-presenting properties of professional APCs (DCs). The mechanism(s) underlying this inhibitory effect of RSV-infected moDCs on T cell responses is currently unknown, and investigations are currently undergoing in our laboratory. Others have not observed this inhibitory effect using RSV-infected cord blood–derived DCs cultured with naïve T cells and superantigens (32). Whether this discrepancy is related to the different phenotype of T cells (naïve versus mature) or the DCs precursors (cord blood CD34+ cells versus peripheral blood monocytes) that were used in that study compared to our remains to be determined. Nonetheless, our experimental results are consistent with the clinical observations that RSV is able to actively interfere with host immune responses, leading to incomplete immunity against the virus and repeated infections throughout life (33). Interestingly, although we observed a similar effect of the both RSV and hMPV on cell maturation, hMPV-infected moDCs did not significantly inhibit T cell proliferation. It is possible that the repertoire of cell-associated molecules that are involved in the process of antigen presentation, including negative regulators, may be differentially affected by infection with RSV or hMPV (34). Thus, the profile of antigen-presenting molecules that were analyzed in this study may only partially represent the events occurring in DCs after infection with RSV or hMPV. Future studies will have to address this possibility as well as other potential virus-specific mechanisms that could explain such difference in T cell regulation by infected DCs.
Production of cytokines is essential for modulation of the host immunity against viruses (1). In the present work we performed an extensive analysis of the immunoregulatory and inflammatory cytokines produced by moDCs infected with RSV or hMPV. After RSV infection, moDCs secreted a significant amount of IL-6, IL-10, TNF-α, IL-1β, IFN-γ, IL-12p70, and G-CSF (Figure 6 and Table 1). These data extend previous reports showing that RSV induced cytokine production in cord blood CD34+–derived DCs (27, 32). Interestingly, the profile of cytokines released by hMPV-infected moDCs was restricted only to IL-6 and TNF-α, with concentrations of these two proinflammatory cytokines that were significantly lower than those measured after RSV infection. Similar analysis of cytokines released by pDCs after exposure to RSV infection also revealed a significant increase in most of the measured cytokines, including IL-6, IL-10, GM-CSF, IFN-γ, TNF-α, IL-1β, IL-12p70, and G-CSF, compared with uninfected cells (Table 1). As in the case of moDCs, hMPV failed to induce significant levels of cytokines by pDCs. These findings are consistent with our data in an experimental mouse model in which we have recently shown that hMPV is a much weaker inducer of proinflammatory cytokines in the airways compared with RSV (35). However, in striking contrast with the cytokine results we observed that synthetic dsRNA delivered intracellularly, or hMPV infection, resulted in the release of considerable amounts of IFN-α in moDCs compared with negligible levels that were induced by RSV (Figure 6). As previously mentioned, hMPV replication was required for IFN-α induction. Failure of RSV to induce IFN-α production in moDCs has been recently reported by others (36). Moreover, these results are in agreement with our results in mice, where we observed that hMPV induced significantly higher and sustained levels of IFN-α in the lung, compared with RSV (35). In general, RSV has been reported to be a poor inducer of IFN-α/β (37, 38), and a pivotal role for the viral nonstructural proteins NS1 and NS2 in inhibiting/limiting IFN-α/β production has been claimed (39). Whether lack of these proteins in the hMPV genome would explain the distinct ability of this virus compared with RSV to induce IFN type I secretion in moDCs remains to be determined. In this regard, when pDCs (the major subset of IFN type I–producing DCs) were exposed to RSV or hMPV (replicating virus), we observed that large amounts of IFN-α were secreted by the cells in response to either virus or to the TLR9 agonist CpG-ODN (Figure 7).
To further investigate the interaction between viral infection and IFN signaling pathways in human DCs, we performed a series of experiments to determine whether RSV and/or hMPV were capable of interfering with IFN-α secretion induced by specific agonists. These studies were prompted by our recent findings in mice, which showed how RSV and hMPV infection blocked the production of IFN-α in the lung induced by synthetic dsRNA (poly-ICLC) or CpG (21). The results presented herein recapitulate our findings in vivo by showing that both RSV and hMPV were equally effective in blocking the production of IFN-α in poly-ICLC– and CpG-stimulated moDCs and pDCs, respectively (Figure 8). Depending on the cell type and the conditions that lead to the its generation, the initial steps involved in dsRNA-induced IFN-α/β production include two intracellular sensors, the IFN-inducible dsRNA-activated protein kinase R (PKR) and the recently discovered RNA helicase, retinoic acid–inducible gene I (RIG-I) (40, 41). In addition to PKR and RIG-I, dsRNA (also in the form of the analogue synthetic poly-ICLC) activates the Toll-like receptor (TLR) 3, which is expressed predominantly in the cellular endosomal compartment (42). Via the adaptor protein TRIF and the activation of the IκB kinase ε and TANK-binding kinase 1, the dsRNA/TLR3-activated pathway converges on the phosphorylation and activation of IRF-3, which is required for induction of IFN-α/β gene (43–45). As far as the CpG-mediated IFN pathway in pDCs, CpG-ODN binds to the TLR9 in the endosomal compartment (46) and, similarly to the TLR7/8 (also expressed in the cell endosomes), activates IFN production via a mechanism that requires the adaptor molecules MyD88, IRAK-1, TRAF6, and TRAF3 (47). In this pathway, IRF-5 and IRF-7, rather than IRF-3, appear to be activated (47, 48). IRF-7 in particular has been shown to be a master regulator of murine IFN-α/β production as mice deficient in irf7 gene are severely impaired in the production of IFN-α/β triggered by both MyD88-dependent and MyD88-independent pathways (49). Given the complex regulation of IFN-α/β gene, RSV and hMPV infections could interfere with one, multiple, and in a virus- or cell-specific manner, of the signal transduction pathways leading to IFN-α/β gene transcription in human moDCs or pDCs.
In conclusion, this study provides novel evidence that RSV and hMPV differentially activate human DCs. The evidence of differences in viral replication, DC-mediated T cell proliferation, expression pattern of inflammatory cytokines, and IFN-α regulation supports the notion that RSV and hMPV may use distinct mechanisms to interfere with the host innate and adaptive immune responses as well as with the pathogenesis and severity of naturally acquired infections. Ultimately, understanding the interaction between paramyxoviruses and different subsets of human DCs may be critical for developing specific vaccine strategies and therapeutic approaches against these pathogens.
Acknowledgments
The authors thank Dr. Thomas Albrecht and Eugene Knutson at the Infectious Disease and Toxicology Optical Imaging Core, UTMB, for their help with confocal microscopy imaging.
This work was supported by NIAID grant AI053785, NHLBI N01 HV28184, and American Heart Association grant 0355084Y (R.P.G.), and a pilot grant from the Sealy Center for Vaccine Development, UTMB (A.C.). A.G.-P. was supported by a fellowship from the James W. McLaughlin Fellowship Fund.
Originally Published in Press as DOI: 10.1165/rcmb.2005-0287OC on November 11, 2005
Conflict of Interest Statement: None of the authors has a financial relationship with a commercial entity that has an interest in the subject of this manuscript.
References
- 1.Banchereau J, Briere F, Caux C, Davoust J, Lebecque S, Liu YJ, Pulendran B, Palucka K. Immunobiology of dendritic cells. Annu Rev Immunol 2000;18:767–811. [DOI] [PubMed] [Google Scholar]
- 2.Rinaldo CR Jr, Piazza P. Virus infection of dendritic cells: portal for host invasion and host defense. Trends Microbiol 2004;12:337–345. [DOI] [PubMed] [Google Scholar]
- 3.Stumbles PA, Upham JW, Holt PG. Airway dendritic cells: co-ordinators of immunological homeostasis and immunity in the respiratory tract. APMIS 2003;111:741–755. [DOI] [PubMed] [Google Scholar]
- 4.Barchet W, Cella M, Colonna M. Plasmacytoid dendritic cells--virus experts of innate immunity. Semin Immunol 2005;17:253–261. [DOI] [PubMed] [Google Scholar]
- 5.Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature 1998;392:245–252. [DOI] [PubMed] [Google Scholar]
- 6.Pulendran B, Palucka K, Banchereau J. Sensing pathogens and tuning immune responses. Science 2001;293:253–256. [DOI] [PubMed] [Google Scholar]
- 7.Sallusto F, Lanzavecchia A. Efficient presentation of soluble antigen by cultured human dendritic cells is maintained by granulocyte/macrophage colony-stimulating factor plus interleukin 4 and downregulated by tumor necrosis factor alpha. J Exp Med 1994;179:1109–1118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Easton AJ, Domachowske JB, Rosenberg HF. Animal pneumoviruses: molecular genetics and pathogenesis. Clin Microbiol Rev 2004;17:390–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kahn JS. Human metapneumovirus: a newly emerging respiratory pathogen. Curr Opin Infect Dis 2003;16:255–258. [DOI] [PubMed] [Google Scholar]
- 10.Fouchier RA, Rimmelzwaan GF, Kuiken T, Osterhaus AD. Newer respiratory virus infections: human metapneumovirus, avian influenza virus, and human coronaviruses. Curr Opin Infect Dis 2005;18:141–146. [DOI] [PubMed] [Google Scholar]
- 11.van den Hoogen BG, van Doornum GJ, Fockens JC, Cornelissen JJ, Beyer WE, de Groot R, Osterhaus AD, Fouchier RA. Prevalence and clinical symptoms of human metapneumovirus infection in hospitalized patients. J Infect Dis 2003;188:1571–1577. [DOI] [PubMed] [Google Scholar]
- 12.von Linstow ML, Henrik LH, Eugen-Olsen J, Koch A, Nordmann WT, Meyer AM, Westh H, Lundgren B, Melbye M, Hogh B. Human metapneumovirus and respiratory syncytial virus in hospitalized danish children with acute respiratory tract infection. Scand J Infect Dis 2004;36:578–584. [DOI] [PubMed] [Google Scholar]
- 13.Williams JV, Harris PA, Tollefson SJ, Halburnt-Rush LL, Pingsterhaus JM, Edwards KM, Wright PF, Crowe JE Jr. Human metapneumovirus and lower respiratory tract disease in otherwise healthy infants and children. N Engl J Med 2004;350:443–450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dzionek A, Inagaki Y, Okawa K, Nagafune J, Rock J, Sohma Y, Winkels G, Zysk M, Yamaguchi Y, Schmitz J. Plasmacytoid dendritic cells: from specific surface markers to specific cellular functions. Hum Immunol 2002;63:1133–1148. [DOI] [PubMed] [Google Scholar]
- 15.Ueba O. Respiratory syncytial virus: I. concentration and purification of the infectious virus. Acta Med Okayama 1978;32:265–272. [PubMed] [Google Scholar]
- 16.Hallak LK, Spillmann D, Collins PL, Peeples ME. Glycosaminoglycan sulfation requirements for respiratory syncytial virus infection. J Virol 2000;74:10508–10513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Byrappa S, Gavin DK, Gupta KC. A highly efficient procedure for site-specific mutagenesis of full-length plasmids using Vent DNA polymerase. Genome Res 1995;5:404–407. [DOI] [PubMed] [Google Scholar]
- 18.Collins PL, Hill MG, Camargo E, Grosfeld H, Chanock RM, Murphy BR. Production of infectious human respiratory syncytial virus from cloned cDNA confirms an essential role for the transcription elongation factor from the 5′ proximal open reading frame of the M2 mRNA in gene expression and provides a capability for vaccine development. Proc Natl Acad Sci USA 1995;92:11563–11567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wyatt LS, Moss B, Rozenblatt S. Replication-deficient vaccinia virus encoding bacteriophage T7 RNA polymerase for transient gene expression in mammalian cells. Virology 1995;210:202–205. [DOI] [PubMed] [Google Scholar]
- 20.Olszewska-Pazdrak B, Casola A, Saito T, Alam R, Crowe SE, Mei F, Ogra PL, Garofalo RP. Cell-specific expression of RANTES, MCP-1, and MIP-1alpha by lower airway epithelial cells and eosinophils infected with respiratory syncytial virus. J Virol 1998;72:4756–4764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Guerrero-Plata A, Baron S, Poast JS, Adegboyega PA, Casola A, Garofalo RP. Activity and regulation of alpha interferon in respiratory syncytial virus and human metapneumovirus experimental infections. J Virol 2005;79:10190–10199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Garofalo RP, Mei F, Espejo R, Ye G, Haeberle H, Baron S, Ogra PL, Reyes VE. Respiratory syncytial virus infection of human respiratory epithelial cells up-regulates class I MHC expression through the induction of IFN-β and IL-1α. J Immunol 1997;157:2506–2513. [PubMed] [Google Scholar]
- 23.Vermaelen KY, Carro-Muino I, Lambrecht BN, Pauwels RA. Specific migratory dendritic cells rapidly transport antigen from the airways to the thoracic lymph nodes. J Exp Med 2001;193:51–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Diebold SS, Montoya M, Unger H, Alexopoulou L, Roy P, Haswell LE, Al- Shamkhani A, Flavell R, Borrow P, Reis e Sousa C. Viral infection switches non-plasmacytoid dendritic cells into high interferon producers. Nature 2003;424:324–328. [DOI] [PubMed] [Google Scholar]
- 25.Schlender J, Hornung V, Finke S, Gunthner-Biller M, Marozin S, Brzozka K, Moghim S, Endres S, Hartmann G, Conzelmann KK. Inhibition of toll-like receptor 7- and 9-mediated alpha/beta interferon production in human plasmacytoid dendritic cells by respiratory syncytial virus and measles virus. J Virol 2005;79:5507–5515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Spann KM, Tran KC, Chi B, Rabin RL, Collins PL. Suppression of the induction of alpha, beta, and lambda interferons by the NS1 and NS2 proteins of human respiratory syncytial virus in human epithelial cells and macrophages. J Virol 2004;78:4363–4369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bartz H, Buning-Pfaue F, Turkel O, Schauer U. Respiratory syncytial virus induces prostaglandin E-2, IL-10 and IL-11 generation in antigen presenting cells. Clin Exp Immunol 2002;129:438–445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wright PF, Ikizler MR, Gonzales RA, Carroll KN, Johnson JE, Werkhaven JA. Growth of respiratory syncytial virus in primary epithelial cells from the human respiratory tract. J Virol 2005;79:8651–8654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhou LJ, Tedder TF. CD14+ blood monocytes can differentiate into functionally mature CD83+ dendritic cells. Proc Natl Acad Sci USA 1996;93:2588–2592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lipscomb MF, Masten BJ. Dendritic cells: immune regulators in health and disease. Physiol Rev 2002;82:97–130. [DOI] [PubMed] [Google Scholar]
- 31.Schlender J, Walliser G, Fricke J, Conzelmann KK. Respiratory syncytial virus fusion protein mediates inhibition of mitogen-induced T-cell proliferation by contact. J Virol 2002;76:1163–1170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bartz H, Turkel O, Hoffjan S, Rothoeft T, Gonschorek A, Schauer U. Respiratory syncytial virus decreases the capacity of myeloid dendritic cells to induce interferon-gamma in naive T cells. Immunology 2003;109:49–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Crowe JE Jr, Williams JV. Immunology of viral respiratory tract infection in infancy. Paediatr Respir Rev 2003;4:112–119. [DOI] [PubMed] [Google Scholar]
- 34.Kirchberger S, Majdic O, Steinberger P, Bluml S, Pfistershammer K, Zlabinger G, Deszcz L, Kuechler E, Knapp W, Stockl J. Human rhinoviruses inhibit the accessory function of dendritic cells by inducing sialoadhesin and B7-H1 expression. J Immunol 2005;175:1145–1152. [DOI] [PubMed] [Google Scholar]
- 35.Guerrero-Plata A, Casola A, Garofalo RP. Human metapneumovirus induces a distinct profile of lung cytokines compared to respiratory syncytial virus. J Virol 2005;79:14992–14997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hornung V, Schlender J, Guenthner-Biller M, Rothenfusser S, Endres S, Conzelmann KK, Hartmann G. Replication-dependent potent IFN-alpha induction in human plasmacytoid dendritic cells by a single-stranded RNA virus. J Immunol 2004;173:5935–5943. [DOI] [PubMed] [Google Scholar]
- 37.Hall CB, Douglas RGJ, Simons RL, Geiman JM. Interferon production in children with respiratory syncytial, influenza, and parainfluenza virus infections. J Pediatr 1978;93:28–32. [DOI] [PubMed] [Google Scholar]
- 38.McIntosh K. Interferon in nasal secretions from infants with viral respiratory tract infections. J Pediatr 1978;93:33–36. [DOI] [PubMed] [Google Scholar]
- 39.Zhang W, Yang H, Kong X, Mohapatra S, Juan-Vergara HS, Hellermann G, Behera S, Singam R, Lockey RF, Mohapatra SS. Inhibition of respiratory syncytial virus infection with intranasal siRNA nanoparticles targeting the viral NS1 gene. Nat Med 2005;11:56–62. [DOI] [PubMed] [Google Scholar]
- 40.Williams BR. Signal integration via PKR. Sci STKE 2001;2001:RE2. [DOI] [PubMed] [Google Scholar]
- 41.Yoneyama M, Kikuchi M, Natsukawa T, Shinobu N, Imaizumi T, Miyagishi M, Taira K, Akira S, Fujita T. The RNA helicase RIG-I has an essential function in double-stranded RNA-induced innate antiviral responses. Nat Immunol 2004;5:730–737. [DOI] [PubMed] [Google Scholar]
- 42.Alexopoulou L, Holt AC, Medzhitov R, Flavell RA. Recognition of double-stranded RNA and activation of NF-kappaB by Toll-like receptor 3. Nature 2001;413:732–738. [DOI] [PubMed] [Google Scholar]
- 43.Fitzgerald KA, McWhirter SM, Faia KL, Rowe DC, Latz E, Golenbock DT, Coyle AJ, Liao SM, Maniatis T. IKKepsilon and TBK1 are essential components of the IRF3 signaling pathway. Nat Immunol 2003;4:491–496. [DOI] [PubMed] [Google Scholar]
- 44.Sharma S, tenOever BR, Grandvaux N, Zhou GP, Lin R, Hiscott J. Triggering the interferon antiviral response through an IKK-related pathway. Science 2003;300:1148–1151. [DOI] [PubMed] [Google Scholar]
- 45.Sato M, Suemori H, Hata N, Asagiri M, Ogasawara K, Nakao K, Nakaya T, Katsuki M, Noguchi S, Tanaka N, et al. Distinct and essential roles of transcription factors IRF-3 and IRF-7 in response to viruses for IFN-alpha/beta gene induction. Immunity 2000;13:539–548. [DOI] [PubMed] [Google Scholar]
- 46.Hemmi H, Takeuchi O, Kawai T, Kaisho T, Sato S, Sanjo H, Matsumoto M, Hoshino K, Wagner H, Takeda K, et al. A Toll-like receptor recognizes bacterial DNA. Nature 2000;408:740–745. [DOI] [PubMed] [Google Scholar]
- 47.Schoenemeyer A, Barnes BJ, Mancl ME, Latz E, Goutagny N, Pitha PM, Fitzgerald KA, Golenbock DT. The interferon regulatory factor, IRF5, is a central mediator of Toll-like receptor 7 signaling. J Biol Chem 2005;280:17005–17012. [DOI] [PubMed] [Google Scholar]
- 48.Kawai T, Sato S, Ishii KJ, Coban C, Hemmi H, Yamamoto M, Terai K, Matsuda M, Inoue J, Uematsu S, et al. Interferon-alpha induction through Toll-like receptors involves a direct interaction of IRF7 with MyD88 and TRAF6. Nat Immunol 2004;5:1061–1068. [DOI] [PubMed] [Google Scholar]
- 49.Honda K, Yanai H, Negishi H, Asagiri M, Sato M, Mizutani T, Shimada N, Ohba Y, Takaoka A, Yoshida N, et al. IRF-7 is the master regulator of type-I interferon-dependent immune responses. Nature 2005;434:772–777. [DOI] [PubMed] [Google Scholar]