Abstract
Deletion of phenylalanine 508 (ΔF508) accounts for nearly 70% of all mutations that occur in the cystic fibrosis transmembrane conductance regulator (CFTR). The ΔF508 mutation is a class II processing mutation that results in very little or no mature CFTR protein reaching the apical membrane and thus no cAMP-mediated Cl− conductance. Therapeutic strategies have been developed to enhance processing of the defective ΔF508 CFTR molecule so that a functional cAMP-regulated Cl− channel targets to the apical membrane. Sarcoplasmic/endoplasmic reticulum calcium (SERCA) inhibitors, curcumin and thapsigargin, have been reported to effectively correct the CF ion transport defects observed in the ΔF508 CF mice. We investigated the effect of these compounds in human airway epithelial cells to determine if they could induce ΔF508 CFTR maturation, and Cl− secretion. We also used Baby Hamster Kidney cells, heterologously expressing ΔF508 CFTR, to determine if SERCA inhibitors could interfere with the interaction between calnexin and CFTR and thereby correct the ΔF508 CFTR misfolding defect. Finally, at the whole animal level, we tested the ability of curcumin and thapsigargin to (1) induce Cl− secretion and reduce hyperabsorption of Na+ in the nasal epithelia of the ΔF508 mouse in vivo, and (2) induce Cl− secretion in intestine (jejunum and distal colon) and the gallbladder of the ΔF508 CF mouse. We conclude that curcumin and thapsigargin failed to induce maturation of ΔF508 CFTR, or induce Cl− secretion, as measured by biochemical and electrophysiologic techniques in a variety of model systems ranging from cultured cells to in vivo studies.
Keywords: ΔF508 CF mouse, CFTR trafficking, Cl− channel, curcumin
The ΔF508 mutation is the most common cystic fibrosis (CF) mutation and results in the deletion of phenylalanine at the 508 position in the CF transmembrane conductance regulator (CFTR) protein (1). Abnormal processing of the ΔF508 CFTR protein leads to its retention in the endoplasmic reticulum (2) and ultimately rapid intracellular degradation. Failure to reach the plasma membrane is largely responsible for the inability of ΔF508 CFTR to function as a cAMP activated Cl channel (3). However, under specific experimental conditions, such as low temperature or addition of chemical chaperones, trafficking of ΔF508 CFTR to the plasma membrane can be enhanced (2–4). Although the ion channel properties of ΔF508 CFTR are not identical to those of wild-type (WT)-CFTR, the magnitude of normal ΔF508 CFTR trafficking produced by these maneuvers may be sufficient to partially restore Cl− secretion to the CF epithelium (2–4).
It has been previously reported that sarcoplasmic/endoplasmic reticulum calcium (SERCA) pump inhibitors decrease the calcium concentration within the endoplasmic reticulum (ER), interfering with the ability of Ca++-dependent chaperone proteins to retain the misfolded protein in the ER (5). It has been suggested that blockade of this chaperone interaction allows misfolded ΔF508 CFTR to escape from the ER, localize to the cell surface, and function as a Cl− channel (6).
Recently, it has been reported that the low-affinity SERCA pump inhibitor (curcumin), after oral administration, corrects the abnormal nasal and rectal PDs of ΔF508 homozygous CF mice (6). In addition to the observed electrophysiologic correction, the ΔF508 CF mice treated with curcumin (in a 10-wk study) gained weight and exhibited normal survival compared with untreated Δ508 CF mice. The authors also reported that curcumin treatment of baby hamster kidney (BHK) cells, heterologously expressing ΔF508 CFTR, produced plasma membrane localization and Cl− channel function of the ΔF508 CFTR protein (6). A study by Dragomir and coworkers reported a small but significant Cl− efflux in ΔF508 CFTR expressing BHK cells treated with curcumin (7). The authors of this study concluded that since comparable experiments failed to demonstrate an effect of curcumin on ΔF508 CFTR airway epithelial cells, the BHK observation was likely due to the overexpression artifact of this model system. Likewise, other researchers have investigated this curcumin-mediated phenomenon and reported no evidence for curcumin-induced correction of ΔF508 CFTR trafficking (7–10). However, none of these studies have carefully replicated the original methods and used all of the model systems reported by Egan and colleagues (5, 6).
Because curcumin or other SERCA pump inhibitors have been proposed as a validated therapeutic class for CF therapy, we have investigated the effect of SERCA pump inhibitors (curcumin and thapsigargin) at multiple levels. At the molecular level, we investigated: (1) the interaction between the Ca++-dependent chaperone calnexin and wild-type CFTR; and (2) the ability of these compounds to produce mature ΔF508 CFTR protein. At the cellular/tissue level, the efficacy of these two SERCA pump inhibitors to induce Cl− secretion in cultured CF human bronchial epithelia was investigated. Finally, at the whole animal level, we tested the ability of curcumin to (1) induce Cl− secretion and reduce hyperabsorption of Na+ in the nasal epithelia of the ΔF508 CF mouse in vivo; and (2) induce Cl− secretion in the intestine (jejunum and distal colon) and the gallbladder of the ΔF508 CF mouse.
MATERIALS AND METHODS
In Vitro Studies
Cell cultures.
BHK.
Baby hamster kidney (BHK-21) cells expressing ΔF508 CFTR or extope ΔF508 CFTR were grown at 37°C in 5% CO2 as described previously (11). To allow maturation of the mutant protein, BHK-21 cells expressing Extope-F508 CFTR were grown at 27°C for 2 d in the presence of 2 mM butyrate. For comparison, BHK-21 cells expressing Extope-CFTR were grown under the same conditions.
Human airway epithelial cells.
Bronchial specimens were obtained from donor and CF patients (ΔF508) at transplantation, and epithelial cells isolated, cultured, and studied at 27–28 d, as previously described (12).
Immunoblotting.
Cells were washed in ice-cold phosphate-buffered saline (PBS) and lysed with NP-40 lysis buffer (1% NP-40, 150 mM NaCl, 50 mM Tris, pH 7.4, 10 mM NaMoO4) at 4°C for 30 min. Protease inhibitors were added to NP-40 lysis buffer to a final concentration of 1 μg/ml leupeptin, 2 μg/ml aprotinin, 50 μg/ml Pefabloc, 121 μg/ml benzamidine, and 3.5 μg/ml E64. Cell lysates were centrifuged at maximal speed in an Eppifuge at 4°C, and supernatants were collected. Cell lysates (25 μg) were loaded, separated on 6% SDS-PAGE minigels, and transferred to nitrocellulose. Blots were probed with anti-CFTR antibody 596 (1:2,000), anti-calnexin antibody SPA-860 (Stressgen, Ann Arbor, MI), anti-CFTR monoclonal mouse antibody M3A7, or rabbit anti-calnexin antibody SPA-860.
Immunoprecipitation of the CFTR–calnexin complex.
BHK lysates were prepared as described above. Soluble fractions of the lysates were incubated with protein G beads coupled with anti-CFTR antibody 596. The immunocomplexes were washed and eluted with 50 mM Tris-HCl, pH 6.8, and 1% SDS. The protein components of the complexes were separated by SDS-PAGE. CFTR and calnexin were detected by immunoblot.
SERCA inhibitors.
Curcumin (VitaminShoppe.com) was diluted in a stock DMSO solution and a 0.1% concentration added to both the luminal and serosal surface of cultures for the designated intervals (3 or 24 h). Airway cells were treated for 3 h with 0–50 μM curcumin, followed by incubation for 3 h in regular medium or treated for 24 h with 0 or 50 μM curcumin. Airway cells were also exposed to 1 μM thapsigargin (Molecular Probes, Eugene, OR) for 1.5 h, followed by a 2-h incubation in regular media, or treated for 24 h.
Primary airway epithelial cell cultures.
Human lung tissue (7 non-CF and 7 CF lungs) was procured under a protocol approved by the University of North Carolina Committee on the Protection of the Rights of Human Subjects. Epithelial cell harvest and culture was performed as previously described (13). All primary CF airway epithelial cells used in this study were genotyped as ΔF508/ΔF508 by usual clinical testing methods. Cryopreserved passage 1 cells were cultured in bronchial epithelial growth medium on Vitrogen-coated plastic dishes (14). At 75–90% confluence, passage 2 cells were transferred to type IV collagen-coated Snapwell membranes (Corning Costar, Cambridge, MA) for use in Ussing chambers, or 30-mm-diameter Millicell CM membranes (Millipore, Bedford, MA) for biochemical studies. Beginning at Days 4–7, visibly confluent cultures were maintained at an air–liquid interface (ALI) (14). Human airway epithelial cultures grown under these conditions demonstrate a well-differentiated histology. Furthermore, those cultured that generated a transepithelial resistance (Rt) of at least 200 Ωcm2, after the resistance of the permeable support was subtracted, were used for Ussing chamber studies. Typically, these criteria were achieved at 14–21 d after plating onto Snapwell inserts. Rt was not different between WT and CF monolayers, nor was Rt affected by exposure to curcumin at any dose for any length of time.
Well-differentiated ALI cultures were treated with varying concentrations of curcumin in DMSO (0.05–0.1%) or vehicle alone for the indicated time periods. For the majority of the experiments, the curcumin was from Fluka (Steinheim, Switzerland), but in a subset of experiments an alternative source of curcumin was used (AFI curcumanoids, Piscataway, NJ).
Electrical measurements.
Electrical measurements (i.e., Rt, transepithelial potential [Vt], and short-circuit current [Isc]), were made on cell monolayers mounted in Ussing chambers, as previously described (12). Monolayers were bathed in a bilateral Krebs Bicarbonate Ringer solution (KBR) bubbled with 95% O2, 5% CO2 and maintained at 37°C. Vt was clamped to zero, and pulsed to ± 10 mV for 0.5 s every 60 s. The electrometer output was digitized online and Isc, Rt, and calculated Vt were displayed on a video monitor and stored on a computer hard drive. Drugs were added from concentrated stock solutions to either lumenal and/or serosal surfaces of the tissue.
In Vivo and In Vitro Murine Studies
Most of the ΔF508 CF mice (Cftrtm1Kth) (congenic C57BL6/J) were obtained from the colony at Case Western Reserve University. However, a small number of ΔF508 CF mice were obtained from the colony at Yale University. Most of the wild-type control mice were littermates to the CF mice. However, as we lacked several wild-type mice, these controls were obtained from Jackson Laboratory (Bar Harbor, ME) and were strain- (C57BL/6J), age-, and sex-matched. All mice were maintained on a high-fat diet (9%) and given Colyte in place of water. The genotypes of all mice were confirmed by PCR.
Curcumin dosing In vivo.
The mice were dosed orally three times per day (45 mg/kg) for 3 d at ∼ 8-h intervals with curcumin (Curcuminoids [AFI lot number A20132]; VitaminShoppe.com). This is the same source, lot number, and curcumin dose as used to obtain the in vivo data reported by Egan and coworkers (6) (personal communication with Dr. Egan). The curcumin was removed from the capsule and suspended in infant formula (Alimentum; Ross Pediatrics, Abbot Park, IL). Each dose was suspended in an ∼ 25-μl volume (per 20 g mouse body mass) of the infant formula and given orally by pipette. The mice readily swallowed the formula and virtually all the curcumin was ingested. The control mice were dosed identically except that the Alimentum contained no curcumin. Exactly 2 h after the final dose, the nasal PDs were measured. This dosing regime and source of curcumin (and lot number) reflected personal communications with Egan and Caplan subsequent to the publication of their study, and some aspects of the protocols varied from those originally published.
Nasal PD.
For the nasal PD measurements, the mice were anesthetized with a combination of ketamine/xylazine (86.95 mg/kg and 9.89 mg/kg, respectively). The body temperature of the mouse was continually monitored with a rectal thermocouple (Physitemp, Clifton, NJ) and maintained at 37°C with a heat lamp. All details of the nasal PD technique have been previously published (15), the only modification being that the perfusion flow rate was reduced to 0.5 μl/min. When solutions were switched, the new solution reached the mouse's nose within ∼ 1.5 min. The normal and low Cl− buffers have been described previously. Amiloride (10−4 M) and isoproterenol (10−5 M) were made fresh daily, and added to the buffers as indicated. Continuous recording of the PD tracing is necessary to obtain an accurate measurement of PD response times when solutions are changed. In particular, continuous trace recordings are valuable in distinguishing between signal and noise responses after a solution change. All of the experiments reported in this study were performed by continuous trace recording. In contrast, the study by Egan and colleagues (6) did not routinely use continuous recording (M. Egan, personal communication).
Ussing chamber studies.
Immediately after nasal PDs were measured, the mice were killed with an anesthetic overdose and the distal colon, jejunum, and gallbladder were removed for Ussing chamber study. The Ussing chamber studies were conducted as previously described (15).
RESULTS
Heterologous Cell Studies
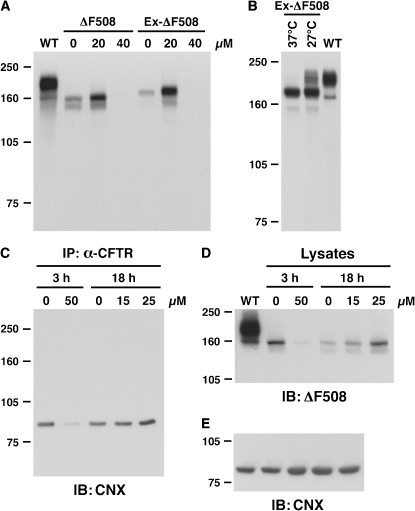
When BHK 21 cells stably expressing ΔF508 CFTR or an epitope-tagged CFTR variant (EX-Δ508) were grown in the presence of 0, 20, and 40 uM curcumin for 18 h, we found no evidence of mature ΔF508 CFTR in any of the curcumin-treated preparations (Figure 1A). Neither the unmodified ΔF508 CFTR nor the epitope-tagged version showed any detectable maturation as a consequence of the curcumin exposure. In contrast, the Ex ΔF508 cells grown at 27°C for 48 h exhibited mature ΔF508 CFTR (Figure 1B), demonstrating that low temperature was able to partially circumvent the ΔF508 CFTR folding defect. Thus, the tagged version of ΔF508 CFTR is able to detect conditions that promote maturation.
Figure 1.
Effect of curcumin on ΔF508 CFTR expression and interaction with calnexin. (A) BHK-21 cells stably expressing ΔF508 CFTR or Extope-ΔF508 CFTR (Ex-ΔF508), an epitope-tagged variant, were grown in the presence of 0, 20, and 40 μM curcumin for 18 h. Cell lysates were separated by SDS-PAGE, transferred to nitrocellulose, and detected with mouse monoclonal anti-CFTR antibody 596. Wild-type CFTR is shown in the left lane (WT). (B) Lysates of Extope-ΔF508 CFTR-expressing cells grown at 37°C or 27°C for 48 h. (C) Cells were grown in the presence of 0 and 50 μM curcumin for 3 h, followed by incubation for 3 h in regular media or in the presence of 0, 15, and 25 μM curcumin for 18 h. ΔF508 CFTR was immunoprecipitated (IP) from cell lysates by anti-CFTR mAb 596 crosslinked to protein G–coupled Dynabeads. Calnexin interacting with immunoprecipitated CFTR was detected by immunoblotting (IB) with rabbit anti-calnexin antibody SPA-860 (Stressgen). The same cell lysates were analyzed by immunoblotting using anti-CFTR monoclonal mouse antibody M3A7 (D) or rabbit anti-calnexin antibody SPA-860 (E) to detect ΔF508 CFTR and calnexin, respectively.
Next, we tested the hypothesis that curcumin mediates ΔF508 CFTR maturation by interfering with the interaction of a Ca2+-dependent ER chaperone, calnexin, with ΔF508 CFTR. After incubation of BHK cells expressing Ex-ΔF508 with curcumin (0–50 μM, for either 3 or 18 h), cell lysates were first precipitated by an anti-CFTR antibody and then blotted for expression of calnexin using a calnexin-specific antibody (Figure 1C). None of the curcumin doses tested significantly reduced the CFTR–calnexin interaction, nor did they result in ΔF508 CFTR maturation (Figure 1D) or alteration of free calnexin levels (Figure 1E).
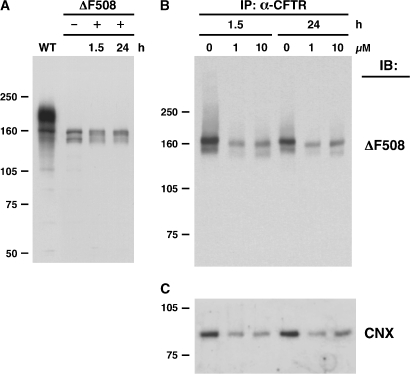
Egan and colleagues have shown in an earlier article (6) that another SERCA inhibitor (thapsigargin) also mediated similar efficacy in correcting the ΔF508 CFTR maturation defects (5). We therefore examined the ability of thapsigargin to induce ΔF508 CFTR maturation in BHK cells as well as its ability to interfere with calnexin binding (Figure 2). Using doses and incubations identical to those used by Egan and coworkers (6), we detected no maturation of ΔF508 CFTR as measured by the lack of a fully gylcosylated band “c” (Figure 2A), alteration in the co-precipitation pattern of ΔF508 CFTR with calnexin (Figure 2C), or difference in the blotting patterns for ΔF508 CFTR (Figure 2B) compared with calnexin (Figure 2C). Thus, neither SERCA inhibitor (curcumin or thapsigargin) promoted maturation of nascent ΔF508 CFTR by dissociation from calnexin, nor by other means. Indeed, ΔF508 CFTR did not progress beyond the ER, even when calnexin binding was completely prevented by glycosylation inhibitors or by mutagenesis (M. Gentzsch, unpublished observations). Thus, in addition to the probable lack of specificity of targeting CFTR/calnexin interactions, the strategy appears to be ineffective in the case of ΔF508 CFTR.
Figure 2.
Effect of thapsigargin on ΔF508 CFTR expression and interaction with calnexin. (A) ΔF508 CFTR–expressing BHK cells were treated with 10 μM thapsigargin for 0, 1.5, or 24 h (+) or left untreated (−). Western blot of lysates, including untreated WT, is shown. (B) Cells were grown in 1 or 10 μM thapsigargin for 1.5 or 24 h. ΔF508 CFTR was immunoprecipitated from the cell lysates and immunoblotted with anti-CFTR as in A. (C) Same blot probed with anti-calnexin, showing that amount of CFTR-associated calnexin reflects amount of CFTR.
Cultured Airway Epithelial Studies
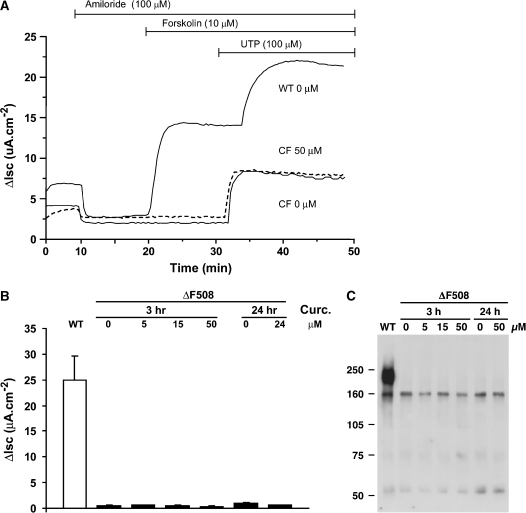
We next studied the effect of curcumin on forskolin-stimulated Cl− secretion by cultured primary human airway epithelia (normal and ΔF508 CF) in Ussing chambers. The curcumin doses chosen (0–50 uM, 3 or 24 h) spanned the dose ranges reported to be effective by Egan and colleagues (6). Representative traces, shown in Figure 3A, demonstrate the lack of a forskolin-stimulated Cl− current following the incubation of cultured CF airway epithelia with curcumin. In contrast, normal (WT) airway cultures exhibit a robust response to forskolin (Figure 3A). Summary data reveal that normal (WT) airway cells exhibited a robust forskolin response and that curcumin was without effect in ΔF508 airway cells (Figure 3B). Biochemical analyses of ΔF508 airway cells exposed to the same doses of curcumin for either 3 or 24 h failed to detect mature ΔF508 CFTR (Figure 3C).
Figure 3.
Effect of curcumin on normal and ΔF508 human airway epithelial cultures. (A) Representative Isc responses of WT and CF human airway epithelial cultures exposed to curcumin. WT (upper line) and CF (lower two lines, solid and dashed) well-differentiated cultures were exposed to either vehicle, 0.1% DMSO (solid lines), or 50 μM curcumin (dashed line) for 24 h before mounting in Ussing chambers. (B) Dose–effect studies in ΔF508 CF airway epithelia of curcumin (3 h or 24 h exposures; 0–50 μM) on forskolin-stimulated Cl− secretion. Doses used spanned the dose range reported by Egan and coworkers (6). Histobars represent the mean ± SEM of at least six different human airway epithelial cultures from at least three different donor tissue samples for each dose tested. In a separate series of experiments, genistein (100 μM) was added to enhance the forskolin-stimulated current; no significant difference was detected between nontreated and curcumin-treated cultures (n = 4). WT (normal control) response depicted in the open bar. (C) Biochemical analyses of human airway epithelia exposed to curcumin. CFTR was immunoprecipitated and immobilized with anti-CFTR mAb 596 as in Figure 1, separated by SDS-PAGE, and transferred to nitrocellulose, which was probed with the same antibody.
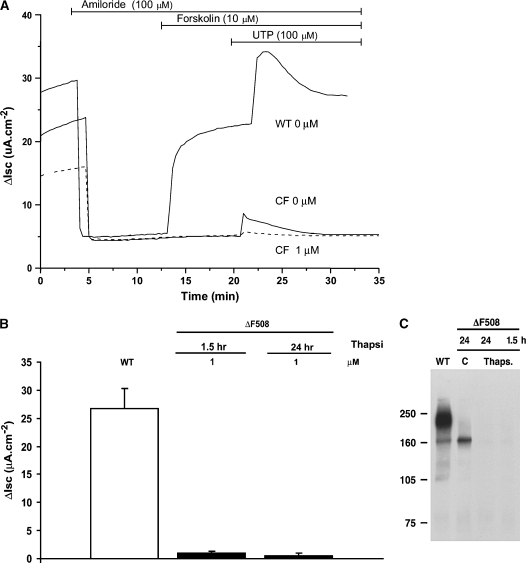
Identical experiments were performed with thapsigargin, using a dose and incubation time identical to that reported in an earlier study by Egan and coworkers (5). This compound also failed to induce Cl− secretion in response to forskolin addition in ΔF508 cells, whereas normal airway cells exhibited the typical secretory response to forskolin (Figure 4A). Also note that the subsequent UTP-mediated Cl− secretion is greatly reduced following exposure to thapsigargin (consistent with depletion of intracellular Ca2+ stores). Immunoprecipitation and immunoblotting again indicated that no mature ΔF508 CFTR was present in the thapsigargin-treated ΔF508 cells (Figure 4C). Moreover, ΔF508 CFTR band “B” appears to be significantly reduced by this exposure to thapsigargin.
Figure 4.
Effect of thapsigargin on normal and ΔF508 human airway epithelial cultures. (A) Representative Isc responses of WT and CF human airway epithelial cultures exposed to thapsigargin. WT (upper line) and CF (lower two lines, solid and dashed) well-differentiated cultures were exposed to either vehicle, 0.1% DMSO (solid lines), or 1 μM thapsigargin (dashed line) for 90 min before mounting in Ussing chambers. (B) Duration–effect (1.5 h; 24 h) studies of thapsigargin (1 μM) on forskolin-stimulated Cl− secretion in homozygous ΔF508 human airway epithelia. Thapsigargin dose and times span those reported by Egan and coworkers in heterologous systems (5). Histobars depict mean ± SEM of at least six different cultures from two donor samples. (C) Cells were treated with 1 μM thapsigargin for 1.5 or 24 h or with vehicle control (0.1% DMSO) for 24 h, lysed, and analyzed by sequential immunoprecipitation and immunoblotting, as in Figure 3C. Results with normal airway cells expressing wild-type CFTR (WT) are shown in the left lane.
In Vivo Studies
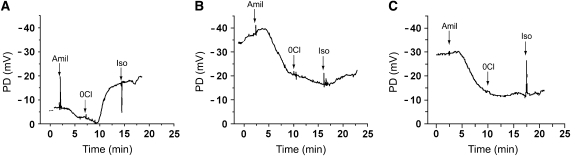
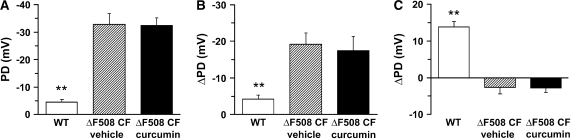
The basal in vivo PDs in the ΔF508 CF mice receiving vehicle alone were significantly raised compared with the basal PDs of the WT mice (Figures 5 and 6A). No reduction of the CF-specific raised PD was observed after curcumin treatment (Figures 5 and 6). As a control for nonspecific effects of curcumin on nasal PD, a group of WT mice were also dosed with curcumin, and the bioelectric data did not differ from that of the WT mice receiving vehicle treatment (data not shown). As a second measure of the Na+ transport rate, amiloride was included in the PD perfusate. Again, the amiloride-sensitive PD response of vehicle-exposed ΔF508 CF mice was significantly greater than that of WT mice, and curcumin treatment of CF mice was without effect (Figures 5 and 6B). Collectively, these data indicate that curcumin did not slow the Na+ hyperabsorption that is a hallmark of CF airways disease (16).
Figure 5.
Nasal PD recorder traces. Representative PD traces from WT mouse (A), ΔF508 CF mouse given vehicle (B), and a ΔF508 CF mouse treated with curcumin (C). Amiloride, 10−4 M, (Amil) was added at the times indicated. Then, the system was switched to a 0 chloride plus amiloride buffer (0 Cl), and finally, isoproterenol (10−5 M) (Iso) was added to the 0 Cl plus amiloride buffer at the times indicated.
Figure 6.
Mean nasal PD data. (A) Mean basal PD in WT, ΔF508 CF vehicle-treated, and ΔF508 CF mice treated with curcumin. (B) Change in PD in response to amiloride. (C) Change in PD in response to low Cl− substitution. All bars are means ± SEM. Open bars are WT mice (n = 10), hatched bars are ΔF508 CF mice given vehicle (n = 5), and filled bars are ΔF508 CF mice treated with curcumin (n = 7).
The nasal epithelium of the normal mouse exhibits a large apical membrane Cl− conductance. This pathway may be examined in vivo by luminal Cl− substitution (containing amiloride), which typically results in a significant hyperpolarization of the nasal PD (Figures 5A and 6C). The vehicle-treated ΔF508 CF mice exhibited a small depolarization in the post-amiloride PD, which differed significantly from that of the WT mice. The curcumin-treated CF mice exhibited Cl− substitution responses virtually identical to those of vehicle-treated CF mice. Following the low Cl− response, isoproterenol (10−4 M) was added to the perfusate. All three groups of mice exhibited a small hyperpolarization (ΔmV, WT 1.8 ± 0.73 [10], ΔF508 CF vehicle treatment 3.5 ± 2.0 [2], and ΔF508 CF curcumin RX 1.08 ± 0.8 [3]) in response to the isoproterenol perfusion that did not differ significantly among the three groups. Three additional ΔF508 mice were dosed with curcumin (45 mg/kg) for 5–7 d and PDs measured 6–8 h after the final dose. This experimental alteration was designed to allow for the possibility of a longer curcumin-mediated transit time for CFTR maturation. The longer incubation period had no effect on basal PD, amiloride-sensitive PD, or Cl− secretion (data not shown). Additional ΔF508 CF mice were obtained from the Yale colony (same strain as used by Egan and coworkers [6]) and studied at Case Western Reserve University following the dosing protocol described in our paper. Nasal PDs were studied before and after curcumin treatment in these mice. These studies indicated that there was no evidence of a correction in the Cl− transport defect in response to the curcumin treatment (Cl− diffusion potential 0.47 ± 0.43 mV before curcumin treatment and 0.3 ± 0.99 mV after curcumin treatment; P < 0.54, n = 5). Together, these data confirm that CF mice exhibit defective apical membrane Cl− conductance and Na+ hyperabsorption and that curcumin did not modify these defects.
Murine In Vitro Studies
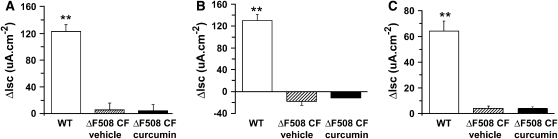
After the PD measurements, the mice were killed, and tissues were immediately excised and mounted on Ussing chambers. The WT jejuna exhibited a robust Cl− secretory response (ΔIsc in response to forskolin [17]), whereas the forskolin response exhibited by the tissue from the vehicle- or curcumin-treated ΔF508 CF mice did not differ from zero (Figure 7A). The distal colon from the WT mice also exhibited a robust Cl− secretory response to forskolin, whereas the colons from both the vehicle- and curcumin-treated ΔF508 CF mice exhibited a small response of reversed polarity, likely reflecting K+ secretion (18) (Figure 7B). The gallbladders of the WT mice also exhibited a significant forskolin induced Cl− secretory response, whereas neither vehicle- nor curcumin-treated ΔF508 CF mice exhibited significant responses to forskolin (Figure 7C). In no study was there a difference in response between the curcumin- and vehicle-treated ΔF508 CF mice.
Figure 7.
Change in short circuit in response to forskolin for freshly excised tissue studied on the Ussing chamber. (A) Forskolin response of WT, ΔF508 CF vehicle-treated, and ΔF508 CF curcumin-treated jejuna. (B) Forskolin response of distal colons. (C) Forskolin response of gallbladders. All bars are means ± SEM. Open bars are WT mice (n = 8), hatched bars are ΔF508 CF mice given vehicle (n = 3), and filled bars are ΔF508 CF mice treated with curcumin (n = 6).
Finally, PD studies and Ussing chamber studies (intestine and gallbladder) were repeated on vehicle- or curcumin-treated UNC-null CF mice (CFTRtm1Unc) and WT control mice. Again, curcumin was completely without effect and responses of the curcumin-treated CF mice did not differ from those of vehicle-treated CF mice, whereas both groups of CF mice differed significantly from WT (data not shown).
DISCUSSION
An article recently published by Egan and colleagues reported that curcumin given to ΔF508 CF mice in doses similar to those which can be safely administered to humans induced striking corrections of ion transport in ΔF508 CF mice (6). This article had important clinical implications for treatment of patients with CF, but there were divergent opinions as to the implementation of a clinical development program. As a result of this article, some advocated clinical trials with curcumin on patients with CF (19, 20). Others suggested that the data must first be independently replicated and confirmed (21, 22). We and others opted to undertake a comprehensive study to examine on the efficacy of SERCA compounds on reversing the trafficking/transport defect of ΔF508 CFTR.
Egan and coworkers (6), using BHK cells as a heterologous host for human WT and ΔF508 CFTR expression, reported that curcumin induced a modest maturation of ΔF508 CFTR, which they suggested reflected blockade of the interaction of the Ca++-dependent ER chaperone calnexin with ΔF508 CFTR. A more recently published study failed to find a significant effect of curcumin or thapsigargin on ΔF508 CFTR maturation in BHK cells (9). In control experiments, these investigators found that incubating ΔF508 CFTR–expressing BHK cells for 18 h at 27°C markedly increased the yield of mature ΔF508 CFTR protein (9). Another study, employing ΔF508-BHK and a human CF airway epithelial cell line (CFBE), reported that curcumin treatment caused no noticeable trafficking of ΔF508 CFTR to the plasma membrane (7). Likewise, our studies revealed no evidence of curcumin- or thapsigargin-induced ΔF508 CFTR maturation, and we extended previous studies by failing to detect interactions between calnexin and ΔF508 CFTR protein (Figures 1 and 2).
Because ultimately the importance of SERCA pump inhibitors (curcumin) as therapeutic agents will be reflected in organ level function, we measured the Cl− secretory as well as biochemical correlates of curcumin and thapsigargin administration in a well-differentiated human bronchial epithelial culture system that has been useful in predicting efficacy of other forms of therapy in vivo (23, 24). We detected neither bioelectric evidence of forskolin-induced activation of ΔF508 CFTR chloride secretory currents in curcumin- or thapsigargin-treated cultures, nor any evidence that curcumin or thapsigargin induced maturation of ΔF508 CFTR protein in these cultures (Figures 3 and 4). Again, the doses and duration of exposure spanned, or even exceeded, those reported by Egan and colleagues in murine or BHK cell systems (6). Further, our techniques detected large forskolin-induced Cl− secretory currents in WT cells (Figures 3A and 4A), and our highly sensitive immunoblots or immunoprecipitates from these cultures detected distinctive immature ΔF508 CFTR protein bands both in the absence and presence of these agents (Figures 1–4).
Others have also reported negative results on the effect of curcumin on human CF airway cells. Song and coworkers reported that curcumin treatment failed to induce a forskolin-mediated Cl− secretion in primary human airway cells homozygous for ΔF508 CFTR (10). As a control, these investigators reported that ΔF508 CFTR human cells grown at 27°C responded to forskolin with a substantial increase in Isc (10). Similarly, Dragomir and colleagues reported that curcumin failed to induce a forskolin-mediated Cl− efflux in either CFBE cells or CF nasal epithelial cells (7). However, they did report a small increase in net cAMP-activated Cl− efflux after curcumin treatment of ΔF508 CFTR BHK cells (7). Berger and coworkers reported that in human CF bronchus (homozygous ΔF508) curcumin treatment failed to induce a response to forskolin in the CF preparations when studied in Ussing chambers (8). However, these investigators did report an acute effect of curcumin on WT CFTR channel activity measured by inside-out membrane patch clamp (8). Finally, in a heterologous expression system, Fischer rat thyroid cells expressing ΔF508 CFTR, exposure to curcumin (1–40 μM up to 24 h incubation) showed no evidence of an enhanced iodide influx compared with the untreated ΔF508 CFTR cells (10).
Egan and colleagues detected only modest maturation of ΔF508 CFTR as a result of curcumin treatment. Conceivably, our failure to detect maturation of CFTR, either by immunochemistry in BHK or airway cells or by Cl− transport in the human airway epithelium, could reflect a sensitivity issue. The large dynamic range for Cl− secretion in our culture assay (Figures 3 and 4), and the very high sensitivity of the newer anti-CFTR monoclonal antibodies (25), argue against this possibility.
An approach to more directly test the sensitivity issue was to study curcumin effects in mice because of the striking magnitude of curcumin-induced correction in the ΔF508 CF mouse reported by Egan and coworkers (6). First, we attempted to reproduce the data of Egan and coworkers demonstrating curcumin-induced correction of sodium transport in the nasal cavity, but we could find no evidence that curcumin corrected Na+ hyperabsorption in the ΔF508 CF mouse (Figure 6B). Moreover, we used a spectrum of protocols, varying the doses and duration of curcumin treatment, but detected no evidence that curcumin corrected chloride transport in the nasal cavity of ΔF508 CF mice (Figure 6C). Finally, the in vivo PD studies of Cl− transport in vehicle- versus curcumin-treated ΔF508 CF mice, conducted at Case Western Reserve University, produced similar negative results. The absence of curcumin-induced nasal Cl− transport is consistent with a recent report of Song and colleagues showing no correction of the nasal epithelial Cl− secretion in curcumin-treated ΔF508 CF mice (10). In the study by Song and coworkers (10), serum curcumin was measured 2 h after dosing mice at 15 mg/kg. At this dose, no detectable serum curcumin was found. However at a dose of 100 mg/kg, 2 h after dosing, very low levels of curcumin (36 nm) were detected (10), indicating very low levels of curcumin bioavailabilty.
It is difficult to reconcile the unequivocally negative results obtained in the present investigations (as well as those in the literature) with the strikingly positive results obtained in the study by Egan and colleagues. We would emphasize that our studies in CF mice spanned the doses and duration originally described in that study (6), and we expanded the studies (after consultation with the authors) to include other sources of curcumin, extended durations of dosing, and the apparent requirement to measure the in vivo PD within 2 h of the last dosing. Most of the ΔF508 CF mice in our studies were of the same genetic background, but the strain backgrounds were slightly different than those studied by Egan and coworkers (UNC/CWRU: congenic C57BL/6; Egan and colleagues: C57BL/6 with ∼ 25% 129 SvEv). However, we also obtained ΔF508 CF mice from the Yale colony. The nasal bioelectrics of these mice were also not corrected with curcumin treatment. The mice used for the in vivo PD studies by Song and coworkers (10) were on a CD1 genetic background. The strain differences do not, therefore, appear to explain the disparity in the data. Possibly with respect to the PD recording, technical issues—for examples, continuous (UNC) versus intermittent PD recording (M. Egan, personal communication)—may be the more relevant technical differences (see Materials and Methods).
Because Egan and colleagues reported that curcumin corrected abnormal rectal PDs, produced weight gain, and extended survival of ΔF508 CF mice, we performed extensive Ussing chamber studies to determine whether we could reproduce the correction of Cl− transport of the GI tract in curcumin-treated ΔF508 CF mice. Again, we could find no evidence of even a minimal restoration of Cl− transport in jejuna, distal colon, or gallbladders after curcumin treatment. It is possible that the improved weight gain and extended survival of the curcumin-treated mice reported by Egan and coworkers (6) may have been due to a laxative effect of the curcumin (or Alimentum alone), as no vehicle controls were performed in their study. However, this difference would not explain the reported correction of rectal PD of the ΔF508 mice in the curcumin-treated mice reported by Egan and colleagues.
It may not be surprising that we observed no correction in the intestine of the ΔF508 CF mouse, since the mice that we (and Egan's group) studied have a marked decrease in ΔF508 CFTR mRNA compared with the ΔF508 CFTR mRNA levels in other tissues as well as compared with WT CFTR mRNA in the intestine (27). This finding, coupled with the report that curcumin treatment resulted in surface expression of ΔF508 CFTR (BHK cells) that was only 25% of that obtained after low-temperature incubation (6) (we found none), would suggest that little if any ΔF508 CFTR would be localized to the plasma membrane of enterocytes in curcumin-treated ΔF508 CF mice.
Our results, performed in three separate institutes, employing several different model systems (a heterologous cell system, human ΔF508 CF primary cells, and ΔF508 CF mice), with high sensitivity and positive controls (e.g., low temperature), demonstrated that SERCA pump inhibitors are not effective in trafficking ΔF508 CFTR to the membrane and correcting function in CF tissue. Given our results, coupled with the data in the literature that fail to detect any effect of curcumin in correcting transport defects associated with ΔF508 CFTR, we would suggest that it is premature to examine the efficacy of curcumin in CF human clinical trials.
Acknowledgments
The authors thank Nicole Kyle, Troy D. Rogers, and Tarra Wasilchen for excellent technical assistance, and Ms. Lisa Brown for manuscript assistance.
This work was supported by awards from the NIH (P30 DK 065988-021 to B.R.G., S.E.G., and R.C.B.; P50 HL 60280-07 to B.R.G., S.E.G., J.R.R., and R.C.B.) and HL68883 (M.L.D.).
Originally Published in Press as DOI: 10.1165/rcmb.2005-0286OC on November 11, 2005
Conflict of Interest Statement: B.R.G. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. S.E.G. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. A.M. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. M.G. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. S.H.R. has provided consulting services to Novartis Institutes for Biomedical Research Inc. and Vertex Pharmaceuticals Inc. on matters not directly related to the use of SERCA pump inhibitors. A.M.V.H. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. M.R.K. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. M.L.D. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. J.R.R. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. R.C.B. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript.
References
- 1.Riordan JR, Rommens JM, Kerem B-T, Alon N, Rozmahel R, Grzelczak Z, Zielenski J, Lok S, Plavsic N, Chou J-L, et al. Identification of the cystic fibrosis gene: cloning and characterization of complementary DNA. Science 1989;245:1066–1073. [DOI] [PubMed] [Google Scholar]
- 2.Cheng SH, Gregory RJ, Marshall J, Paul S, Souza DW, White GA, O'Riordan C, Smith AE. Defective intracellular transport and processing of CFTR is the molecular basis of most cystic fibrosis. Cell 1990;63:827–834. [DOI] [PubMed] [Google Scholar]
- 3.Denning GM, Anderson MP, Amara JF, Marshall J, Smith AE, Welsh MJ. Processing of mutant cystic fibrosis transmembrane conductance regulator is temperature-sensitive. Nature 1992;358:761–764. [DOI] [PubMed] [Google Scholar]
- 4.Drumm ML, Wilkinson DJ, Smith LS, Worrell RT, Strong TV, Frizzell RA, Dawson DC, Collins FS. Chloride conductance expressed by delta F508 and other mutant CFTRs in Xenopus oocytes. Science 1991;254:1797–1799. [DOI] [PubMed] [Google Scholar]
- 5.Egan ME, Gloeckner-Pagel J, Ambrose CA, Cahill PA, Pappoe L, Balamuth N, Cho E, Canny S, Wagner CA, Geibel J, et al. Calcium-pump inhibitors induce functional surface expression of deltaF508-CFTR protein in cystic fibrosis epithelial cells. Nat Med 2002;8:485–492. [DOI] [PubMed] [Google Scholar]
- 6.Egan ME, Pearson M, Weiner SA, Rajendran V, Rubin D, Gloeckner-Pagel J, Canny S, Du K, Lukacs GL, Caplan MJ. Curcumin, a major constituent of turmeric, corrects cystic fibrosis defects. Science 2004;304:600–602. [DOI] [PubMed] [Google Scholar]
- 7.Dragomir A, Bjoerstad J, Hjelte L, Roomans GM. Curcumin does not stimulate cAMP-mediated chloride transport in cystic fibrosis airway epithelial cells. Biochem Biophys Res Commun 2004;322:447–451. [DOI] [PubMed] [Google Scholar]
- 8.Berger AL, Randak CO, Ostedgaard LS, Karp PH, Vermeer DW, Welsh MJ. Curcumin stimulates cystic fibrosis transmembrane conductance regulator Cl− channel activity. J Biol Chem 2005;280:5221–5226. [DOI] [PubMed] [Google Scholar]
- 9.Loo TW, Bartlett MC, Clarke DM. Thapsigargin or curcumin does not promote maturation of processing mutants of the ABC transporters, CFTR, and P-glycoprotein. Biochem Biophys Res Commun 2004;325:580–585. [DOI] [PubMed] [Google Scholar]
- 10.Song Y, Sonawane ND, Salinas D, Qian L, Pedemonte N, Galietta LJV, Verkman AS. Evidence against the rescue of defective deltaF508-CFTR cellular processing by curcumin in cell culture and mouse models. J Biol Chem 2004;279:40629–40633. [DOI] [PubMed] [Google Scholar]
- 11.Gentzsch M, Cui L, Mengos A, Chang XB, Chen JH, Riordan JR. The PDZ-binding chloride channel ClC-3B localizes to the Golgi and associates with cystic fibrosis transmembrane conductance regulator-interacting PDZ proteins. J Biol Chem 2003;278:6440–6449. [DOI] [PubMed] [Google Scholar]
- 12.Donaldson SH, Hirsh A, Li DC, Holloway G, Chao J, Boucher RC, Gabriel SE. Regulation of the epithelial sodium channel by serine proteases in human airways. J Biol Chem 2002;277:8338–8345. [DOI] [PubMed] [Google Scholar]
- 13.Bernacki SH, Nelson AL, Abdullah L, Sheehan JK, Harris A, Davis CW, Randell SH. Mucin gene expression during differentiation of human airway epithelia in vitro: muc4 and muc5b are strongly induced. Am J Respir Cell Mol Biol 1999;20:595–604. [DOI] [PubMed] [Google Scholar]
- 14.Fulcher ML, Gabriel S, Burns KA, Yankaskas JR, Randell SH. Well-differentiated human airway epithelial cell cultures. Methods Mol Med 2004;107:183–206. [DOI] [PubMed] [Google Scholar]
- 15.Grubb BR. Bioelectric measurement of CFTR function in mice. Methods Mol Med 2002;70:525–535. [DOI] [PubMed] [Google Scholar]
- 16.Grubb BR, Vick RN, Boucher RC. Hyperabsorption of Na+ and raised Ca2+-mediated Cl− secretion in nasal epithelia of CF mice. Am J Physiol 1994;266:C1478–C1483. [DOI] [PubMed] [Google Scholar]
- 17.Grubb BR. Ion transport across the jejunum in normal and cystic fibrosis mice. Am J Physiol 1995;268:G505–G513. [DOI] [PubMed] [Google Scholar]
- 18.Grubb BR. Ion transport across the normal and CF neonatal murine intestine. Am J Physiol 1999;277:G167–G174. [DOI] [PubMed] [Google Scholar]
- 19.Croft NM. The spice of life for cystic fibrosis. Gastroenterology 2004;127:1639–1640. [DOI] [PubMed] [Google Scholar]
- 20.Zeitlin P. Can curcumin cure cystic fibrosis? N Engl J Med 2004;351:606–608. [DOI] [PubMed] [Google Scholar]
- 21.Accurso F. Curcumin and cystic fibrosis. J Pediatr Gastroenterol Nutr 2005;39:235. [DOI] [PubMed] [Google Scholar]
- 22.Davis PB, Drumm ML. Some like it hot: curcumin and CFTR. Trends Mol Med 2004;10:473–475. [DOI] [PubMed] [Google Scholar]
- 23.Grubb BR, Pickles RJ, Ye H, Yankaskas JR, Vick RN, Engelhardt JF, Wilson JM, Johnson LG, Boucher RC. Inefficient gene transfer by adenovirus vector to cystic fibrosis airway epithelia of mice and humans. Nature 1994;371:802–806. [DOI] [PubMed] [Google Scholar]
- 24.Tarran R, Grubb BR, Parsons D, Picher M, Hirsh AJ, Davis CW, Boucher RC. The CF salt controversy: In vivo observations and therapeutic approaches. Mol Cell 2001;8:149–158. [DOI] [PubMed] [Google Scholar]
- 25.Mall M, Kreda SM, Mengos A, Jensen TJ, Hirtz S, Seydewitz HH, Yankaskas J, Kunzelmann K, Riordan JR, Boucher RC. The DeltaF508 mutation results in loss of CFTR function and mature protein in native human colon. Gastroenterology 2004;126:32–41. [DOI] [PubMed] [Google Scholar]
- 26.Zeiher BG, Eichwald E, Zabner J, Smith JJ, Puga AP, McCray PB Jr, Capecchi MR, Welsh MJ, Thomas KR. A mouse model for the deltaF508 allele of cystic fibrosis. J Clin Invest 1995;96:2051–2064. [DOI] [PMC free article] [PubMed] [Google Scholar]