Abstract
Caveolin-1 has been reported to regulate apoptosis, lipid metabolism, and endocytosis in macrophages. In the present study, we demonstrate that caveolin-1 can act as a potent immunomodulatory molecule. We first observed caveolin-1 expression in murine alveolar macrophages by Western blotting and immunofluorescence microscopy. Loss-of-function experiments using small interfering RNA showed that downregulating caveolin-1 expression in murine alveolar and peritoneal macrophages increased LPS-induced proinflammatory cytokine TNF-α and IL-6 production but decreased anti-inflammatory cytokine IL-10 production. Gain-of-function experiments demonstrated that overexpression of caveolin-1 in RAW264.7 cells decreased LPS-induced TNF-α and IL-6 production and augmented IL-10 production. p38 mitogen-activated protein kinase (MAPK) phosphorylation was increased by overexpressing caveolin-1 in RAW264.7 cells, whereas c-Jun N-terminal kinase, extracellular signal-regulated kinase MAPK, and Akt phosphorylation were inhibited. The antiinflammatory modulation of LPS-induced cytokine production by caveolin-1 was significantly abrogated by the administration of p38 inhibitor SB203580 in RAW264.7 cells. Peritoneal macrophages isolated from MKK3 null mice did not demonstrate any modulation of LPS-induced cytokine production by caveolin-1. LPS-induced activation of NF-κB and AP-1 determined by electrophoretic mobility shift assay were significantly reduced by overexpressing caveolin-1 in RAW264.7 cells. The reductions were attenuated by the administration of p38 inhibitor SB203580. Taken together, our data suggest that caveolin-1 acts as a potent immunomodulatory effecter molecule in immune cells and that the regulation of LPS-induced cytokine production by caveolin-1 involves the MKK3/p38 MAPK pathway.
Keywords: caveolin-1, cytokines, inflammation, lipopolysaccharide, macrophages
Caveolae are flask-shaped membrane invaginated vesicular organelles with a diameter of 50–100 nm (1). Besides a distinctive lipid composition that is rich in glycosphingolipids, sphingomyelin, and cholesterol (2, 3), caveolae are characterized by the existence of the 22- to 24-kD scaffolding proteins termed caveolins (4, 5). Three caveolins have been identified. Caveolin-1 is most extensively characterized. It exists as oligomeric complex of 14–16 monomers and forms complexes with caveolin-2 (4). Caveolin-1 and caveolin-2 are coexpressed in many cell types and are abundant in endothelial cells, type I pneumocytes, fibroblasts, and adipocytes (6, 7). Caveolin-3 expression is restricted to muscle cells (8, 9). Caveolae have many diverse functions, including membrane trafficking, endocytosis, regulation of cholesterol and calcium homeostasis, and signal transduction in cellular growth and apoptosis (2). Known as signalosome, caveolae concentrate many signaling molecules within one specialized plasma membrane organelle, such as H-Ras, Src family, heterotrimeric G protein α subunits, and endothelial nitric oxide synthase (eNOS) (10). Caveolin-1 interacts with other signaling molecules and regulates their activation. For example, caveolin-1 inhibits the activation of growth factor receptor (e.g., epidermal growth factor receptor and platelet-derived growth factor receptor), and the downstream mitogen-activated protein kinase (MAPK) and PI3K pathway, resulting in reduced cell growth and increased apoptosis (11–13).
The expression of caveolin-1 and the existence of caveolae in immune cells have been debated for a long time. Although it was originally thought that macrophages did not express caveolin-1 (14), recent evidence suggests that caveolin-1 is expressed in macrophages, dendritic cells, and lymphocytes (15, 16). Immunoblot analysis showed that caveolin-1 was expressed in THP-1 macrophages (17, 18), RAW264.7 cells (19), and peritoneal macrophages (PMs) (20). Caveolin-1 mRNA was also detected in RAW264.7 cells, J774 cells, and PMs by RT-PCR or ribonuclease protection assay (19, 21). For murine PMs, electron microscopy and immunofluorescence studies demonstrated the existence of caveolae and caveolin-1 in the plasma membrane (20–22).
The biological significance of caveolin-1 in macrophages is not clear. Caveolin-1 has been shown to play a role in apoptosis in macrophages (23). When treated with various unrelated apoptotic agents, including simvastatin, camptothecin, and glucose deprivation, in glycolate-elicited murine PMs, caveolin-1 expression was specifically and markedly increased, whereas caveolin-2 expression remained unaffected (23). Caveolae is also believed to be involved in lipid metabolism in macrophages (24), which is important in the development of atherosclerosis (25). Caveolin-1 can directly bind to cholesterol and lipid acid (26). CD36 and SR-BI, two receptors that bind high-density lipoprotein (HDL) and selectively transfer core cholesteryl esters to the cells, are localized in caveolae (27). Transfection of caveolin-1 into J774 and RAW264.7 inhibits HDL-mediated cholesteryl ester uptake but not efflux (28). Upregulation of the caveolin-1 level, which is the result of THP-1 differentiation, is associated with the increase of HDL cholesteryl ester uptake by CD36 and cholesterol efflux (18). Caveolae is known to be involved in non–clathrin-coated endocytosis and may regulate the internalization of particles such as viruses and bacteria (29, 30). When treated with caveolae inhibitor filipin, the speed of fluid-phase and receptor-mediated endocytosis was decreased. In macrophages and mast cells, FimH-mediated internalization of bacteria is dependent on caveolae-like vesicles. The compounds that disrupt caveolae formation can effectively block the bacterial uptake (31, 32).
Macrophages exist in different functional subtype according to environmental conditions (33). Alveolar macrophages (AMs) and PMs are bone-marrow–derived resident macrophages and play pivotal roles in phagocytosis and inflammation (33, 34). However, the function of caveolin-1 with respect to innate immunity in macrophages is not clear. Here we hypothesize that caveolin-1 act as an immunomodulatory molecule and directly regulate the inflammatory response. Furthermore, we hypothesize that caveolin-1 mediates the anti-inflammatory effect via the mitogen-activated protein kinase (MAPK) pathway. We tested our hypothesis in vitro in murine macrophages using LPS-induced inflammatory responses.
Materials and Methods
Cell Culture, Animals, and Reagents
Primary PMs and AMs were maintained in 10% FBS Dulbecco's modified Eagle's medium (DMEM). RAW264.7 cells were grown in 5% FBS DMEM (Invitrogen-Gibco, Carlsbad, CA). Caveolin-1 stably transfected RAW264.7 cells, and vector control cells were generated and maintained as described previously (35). Cultures were maintained at 37°C in a humidified atmosphere of 5% CO2 and 95% air. The cells were serum starved before 100 ng/ml LPS stimulation. C57BL/6 mice were purchased from Jackson Laboratory (Bar Harbor, ME) and acclimated 1 wk before experiments. MKK3 (−/−) mice were generated as described previously (36). Wortmannin (100 nM), SB203580 (10 μM), SP600125 (10 μM), and UO126 (10 μM) (Calbiochem, Darmstadt, Germany) were dissolved in DMSO. All reagents were added to the culture medium 1 h before other treatments.
Isolation of PM and AM
The experimental protocols were performed in accordance with the guidelines of Institutional Animal Care and Use Committee of the University of Pittsburgh. Briefly, after the mice were anesthetized with isoflurane, PMs were isolated by peritoneal lavage with 2 ml PBS each time for three times. For AM isolation, mice were tracheotomized after transection of the abdomen. A blunt 21-gauge needle was secured into the trachea. Bronchoalveolar lavage (BAL) was performed five times with 0.3 ml PBS. Cell pellets were collected after centrifuging at 1,000 × g for 10 min at 4°C. The supernatant was discarded, and the cells were resuspended in 10% FBS DMEM. Unattached cells were washed off twice with PBS after 3 h in culture, and the remaining macrophages were incubated overnight in DMEM supplemented with 10% FBS. On the following day, cells were washed twice before treatment as indicated.
Synthesis and Transfection of Small Interfering RNA
Small interfering RNA (siRNA) sequences targeting caveolin-1 gene were designed using software (Dharmacon, Inc., Lafayette, CO). Among the five siRNAs synthesized, two sequences with better effects (AAA GAUGUGAUUGCAGAACCA and AAUCAGCCGCGUCUA CUCCAU) were used. The sequence (AACGCGCACACCAAGGA GAUU) with no apparent effects was used as a negative control. For transfection, cells were plated on 6-well plates or 24-well plates. Transfections were performed with TransIT-TKO reagent (Mirus Corp., Madison, WI) as directed by the manufacturer. For 6-well plates, each well received 10 nM siRNA in a volume of 1 ml in triplicate. For 24-well plates, each well received 2 nM siRNA in a volume of 200 μl in triplicate. LPS was added after 24 h of transfection.
Cytokine Analysis
The levels of cytokines (TNF-α, IL-6, and IL-10) released by LPS-stimulated macrophages into the culture supernatant were measured by ELISA kits (R&D Systems, Minneapolis, MN) according to the manufacturer's instructions.
Western Blot Analysis
Cells were rinsed with ice-cold PBS and homogenized in lyses buffer (1% NP-40, 20 mM Tris [pH 8.0], 137.5 mM NaCl, 1 mM Na3VO4, and 1 mM PMSF) with complete protease inhibitor mixture (Roche Diagnostics, Indianapolis, IN). The supernatant was collected after centrifugation for 20 min at 14,000 × g at 4°C. The protein concentrations were determined by Coomassie (Bradford) protein assay kit (Pierce Biotechnology Inc., Rockford, IL) with BSA as a standard. A total of 50–100 μg proteins in each sample were subjected to electrophoresis through a 4–12% SDS-polyacrylamide gel (Invitrogen, Carlsbad, CA). The proteins were electro-transferred onto polyvinylidene diflouride membrane (Invitrogen). Membranes were blocked in Tris-buffered saline with 0.1% Tween 20 (TTBS) containing 5% low-fat milk for 1 h and incubated overnight at 4°C with the corresponding primary antibody. After being washed with TTBS, membranes were incubated for 2 h at room temperature with the corresponding secondary antibody. Membranes were washed again and developed with ECL reagent (Amersham Biosciences, Piscataway, NJ). Anti–caveolin-1 polyclonal antibody was purchased from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA). Anti-mouse β-actin monoclonal antibody was purchased from Sigma Chemical Co. (St. Louis, MO). Total and phosphorylated forms of MAPK were from Cell Signaling Technology (Beverly, MA).
Immunofluorescence Microscopy
BAL cells were seeded on cover slips at a density of 5 × 104/well (24-well). Cells were rinsed three times with PBS and fixed in 2% paraformaldehyde. The cover slips were sequentially incubated at room temperature in PBS containing 4% goat serum for 60 min and immunostained with polyclonal rabbit caveolin-1 and Mac-3 antibodies in 0.5% BSA/PBS for 60 min. Bound primary antibodies were visualized with Cy-3 and Alexa 488–conjugated secondary antibodies (Jackson ImmunoResearch Laboratories, West Grove, PA). Cells were viewed with an Olympus Fluoview BX 61 confocal microscope, and images were collected using a DC-330S cooled CCD camera (DAGE-MTI Inc., Michigan City, IN).
Electrophoretic Mobility Shift Assay
Nuclear protein extracts were made as described previously (37). Double-stranded oligonucleotides containing the transcription factor-binding site for AP-1 (5′-CGCTTGATGAGTCAGCCGGAA-3′) and NF-κB (5′-AGTTGAGGGGACTTTCCCAGGC-3′) (Promega, Madison, WI) were labeled with 32P-ATP using T4 polynucleotide kinase (Promega) as described (37). Four micrograms of nuclear proteins were incubated with the probe in binding buffer (Promega) for 20 min at 37°C. The reaction mixture was subjected to electrophoresis through 5% native polyacrylamide gels. The gels were dried and exposed.
Statistical Analysis
The differences in cytokine expression were compared by unpaired Student's t test because cytokine production follows a normal distribution as tested by the Anderson-Darling goodness-of-fit test. The variances are equal across groups as verified by the Bartlett test. All analyses were performed with SPSS 12.0.1 for Windows and were considered significant at P < 0.05.
RESULTS
Caveolin-1 Expression in Murine AMs
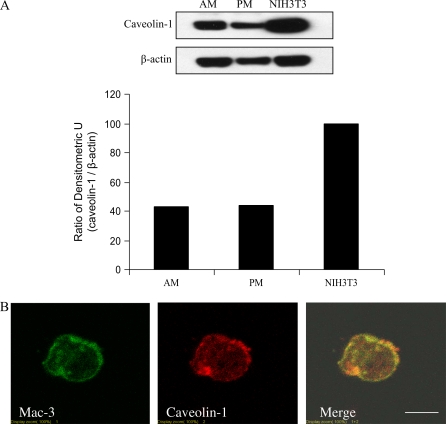
We first examined caveolin-1 expression in murine AMs by Western blot analysis. The levels of caveolin-1 expressed in AMs and PMs are similar, with increased levels observed in NIH3T3 murine fibroblasts (Figure 1A). To better characterize the expression of caveolin-1 expression in murine AMs, we performed colocalization studies using antibodies specific for the murine macrophage-specific marker Mac-3 (38) and caveolin-1. Mac-3 immunostaining established that approximately 94% of cells derived from BAL cells were macrophages (data not shown). Double immunofluorescent staining of BAL macrophages with Mac-3 and caveolin-1 demonstrated caveolin-1 expression in AMs (Figure 1B). Caveolin-1 staining is mainly present on the surface and perinuclear compartments (Figure 1B) and extensively colocalizes with the Mac-3 antigen (Figure 1B), a known macrophage plasma membrane marker.
Figure 1.
Caveolin-1 expression in murine AMs. Murine AMs were isolated and cultured. (A) Caveolin-1 and β-actin expressions were determined by Western blot analysis along with murine PMs and NIH3T3 fibroblasts. (B) Immunofluorescent staining of caveolin-1 and Mac-3 were performed in murine AMs. To investigate the quality of the AMs, we performed murine macrophage specific marker Mac-3 staining. Isolated cells were subjected to immunofluorescent microscopy. Cells (5 × 100) were counted, and the green-staining cells were regarded as macrophages. The positive staining cell of percentage is 94 ± 2%. Scale bar: 7 μm.
Caveolin-1 Knockdown Modulates LPS-Induced Cytokine Production in Murine AMs
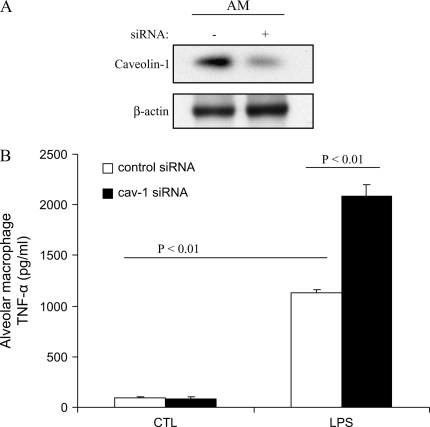
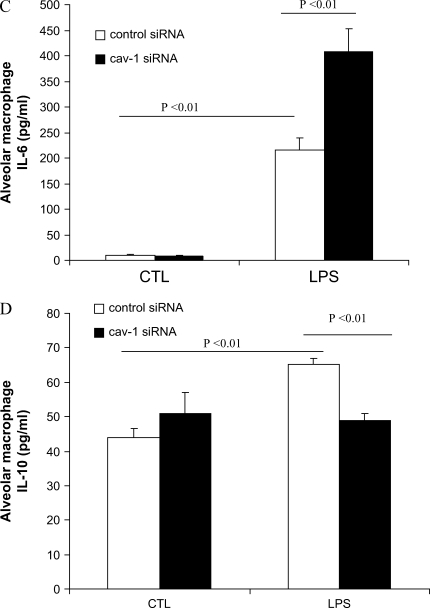
To assess the function of caveolin-1 in macrophages, we isolated murine AMs and investigated the modulation of cytokines production in response to LPS. We transfected siRNA targeting specific caveolin-1 mRNA sequences into these macrophages. At 24 h post-transfection, caveolin-1 protein levels were significantly decreased compared with that of the control, as evaluated by Western blot analysis (Figure 2A). AMs subjected to caveolin-1 knockdown exhibited significant and selective augmentation of proinflammatory cytokines TNF-α and IL-6 production and significant suppression of the antiinflammatory cytokine IL-10 production after LPS stimulation (Figures 2B–2D).
Figure 2.
Modulation of LPS-induced cytokine production by downregulating caveolin-1 expression in murine AMs. Murine AMs were isolated and cultured. (A) The cells were transfected with siRNA of caveolin-1 and control siRNA for 24 h. Caveolin-1 and β-actin expressions were determined by Western blot analysis. After 24 h post-trasfection, the culture medium of transfected PMs was harvested with or without LPS treatment for 4 h. TNF-α (B), IL-6 (C), and IL-10 (D) cytokine production was determined by ELISA. Values are mean ± SE (n = 3). Data are representative of three independent experiments.
Overexpressing Caveolin-1 in RAW264.7 Modulates LPS-Induced Cytokine Production
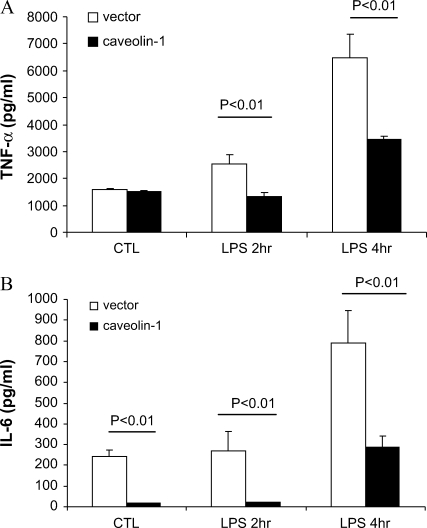
To further confirm whether caveolin-1 can modulate LPS-induced cytokine production, we used caveolin-1 cell line overexpressing RAW264.7 macrophages. In agreement with our observations from the siRNA experiments that demonstrated augmented production of proinflammatory cytokines and suppression of antiinflammatory cytokine by knockdown of caveolin-1, we show the reverse effect by overexpressing caveolin-1 (Figure 3). Overexpression of caveolin-1 caused marked attenuation of LPS-induced proinflammatory cytokines TNF-α and IL-6 and augmentation of antiinflammatory cytokine IL-10 (Figure 3).
Figure 3.
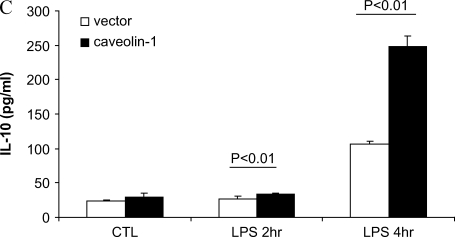
Overexpressing caveolin-1 in RAW264.7 modulates LPS-induced cytokines production. RAW264.7 cells stably transfected with caveolin-1, were serum starved for 24 h and administered LPS for 2 and 4 h. The culture medium was harvested. (A) TNF-α, (B) IL-6, and (C) IL-10 were determined by ELISA. Values were mean ± SE (n = 3). Data are representative of three independent experiments.
Modulation of the MAPK and PI3K Pathway by Caveolin-1
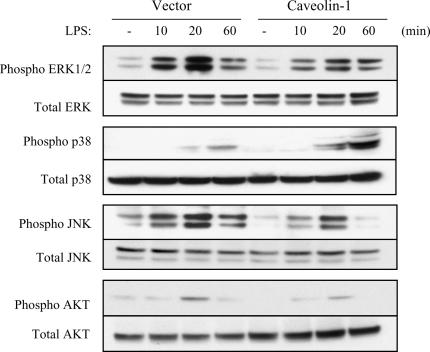
We attempted to delineate the possible mechanism by which caveolin-1 exerts its antiinflammatory effects. Given that MAPK (ERK1/2) and PI3K mediate cytokine production and are regulated by caveolin-1 in fibroblasts and endothelial cells (13, 39), we examined whether MAPK and PI3K are modulated in caveolin-1 overexpressing RAW264.7 cells. LPS-induced activation of ERK1/2 MAPK, JNK MAPK, and PI3K was significantly decreased, whereas p38 MAPK activation was significantly increased in caveolin-1 overexpressing cells (Figure 4).
Figure 4.
Modulation of MAPK and PI3K pathway by caveolin-1. After serum starvation for 24 h, RAW264.7 cells stably transfected with caveolin-1 gene and with control vector were treated with or without LPS for 10 min, 20 min, and 60 min. The cells were harvested and subjected to Western blot analysis for phosphorylated ERK1/2, JNK, p38, and Akt. The same blots were washed and blotting for total ERK1/2, JNK, p38, and Akt as the loading control. Data are representative of three independent experiments.
p38 and JNK MAPK Pathways Are Involved in the Regulation of Caveolin-1 on Cytokine Production
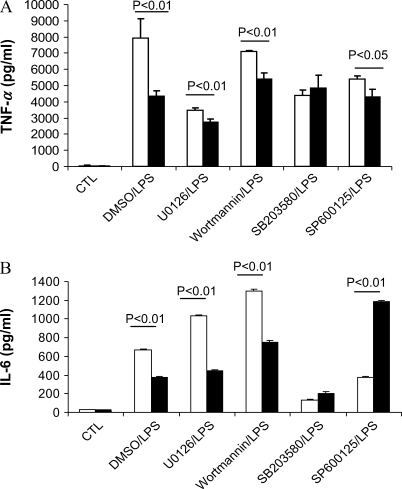
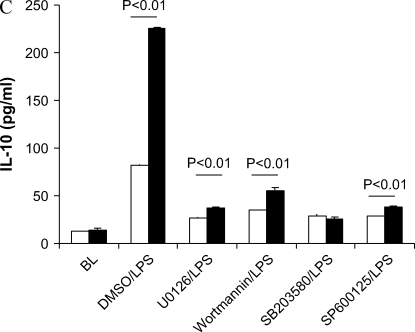
Because PI3K and all three MAPK pathways were regulated by caveolin-1, we tested which pathway attenuation would lead to a loss of cytokine's modulation by caveolin-1. UO126 (the inhibitor of ERK1/2 MAPK) decreased TNF-α and IL-10 production compared with DMSO/LPS treatment (Figure 5). Wortmannin (the inhibitor of PI3K) decreased IL-10 production compared with DMSO/LPS treatment. SB203580 (the inhibitor of p38 MAPK) and SP600125 (JNK inhibitor) decreased TNF-α, IL-6, and IL-10 cytokine production. We observed significant differences in cytokine production (TNF-α, IL-6, and IL-10) between caveolin-1 overexpressing and control vector RAW264.7 cells in DMSO/LPS, UO126/LPS, and wortmannin/LPS treatments. These findings suggest that ERK1/2 and PI3K pathways are not involved in the regulation of cytokine productions by caveolin-1. However, SB203580, the inhibitor of p38 MAPK, eliminated the differences of TNF-α, IL-6, and IL-10 production between caveolin-1 overexpressing RAW264.7 and vector control cells. Administration of JNK inhibitor SP600125 had no effect on the modulation of the cytokine TNF-α and IL-10 production by caveolin-1 while reversing the modulation of caveolin-1 on IL-6 production.
Figure 5.
p38 and JNK pathway involved in the regulation of caveolin-1 on cytokines production. RAW264.7 cells stably transfected with caveolin-1 gene (filled bars) and with control vector (open bars) were serum starved for 24 h. The cells were pretreated for 2 h with DMSO with or without different chemicals dissolved, including UO126, Wortmannin, SB203580, and SP600125. The treated cells were then administrated LPS for 4 h. The culture media were harvested. Cytokines levels in culture medium (A) TNF-α, (B) IL-6, and (C) IL-10 were determined by ELISA. Values were means ± SE (n = 3). Data are representative of three independent experiments.
MKK3/p38 MAPK Mediates the Modulation of LPS-Induced Cytokine Production in Mouse Macrophages
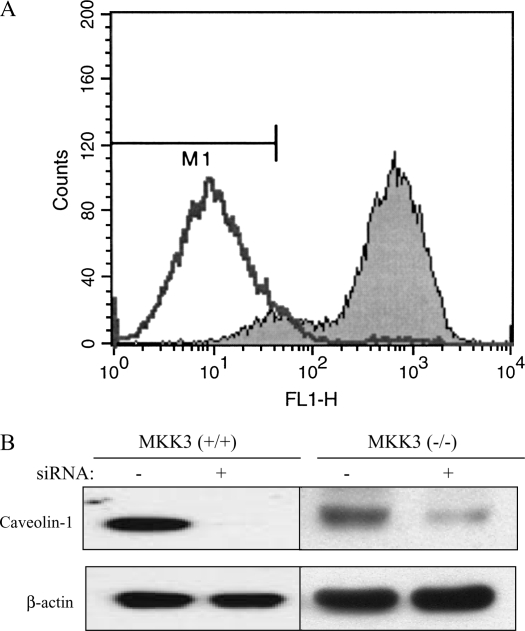
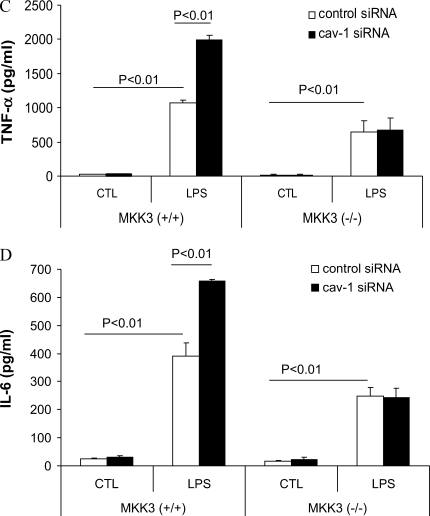
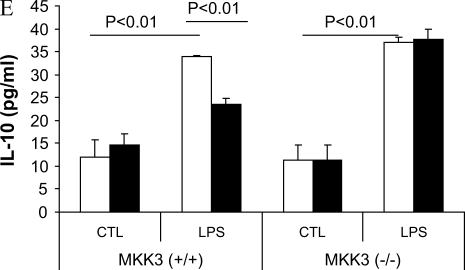
We observed that caveolin-1 increased p38 phosphorylation (Figure 4) while decreasing other MAPKs and PI3K activation. Inhibition of p38 activation chemically eliminated the modulation of LPS-induced TNF-α, IL-6, and IL-10 production by caveolin-1. To explore further the role of p38 MAPK, we isolated mouse PMs from MKK3 (a major upstream kinase of p38) null mice and wild-type littermates and transfected with siRNA of caveolin-1. Decreasing caveolin-1 in MKK3 (−/−) macrophages had no effect on LPS-induced TNF-α, IL-6, and IL-10 production compared with the control siRNA transfectants (Figure 6).
Figure 6.
MKK3/p38 mediates the modulation of LPS induced cytokine production by caveolin-1. PMs were isolated and cultured from MKK3 (+/+) and MKK3 (−/−) mice. (A) To investigate the quality of the macrophages, we performed murine macrophage–specific marker Mac-3 staining. For PMs, the cells were subjected to flow cytometry. The positive staining cell of percentage is 95%. The cells were transfected with siRNA for caveolin-1 and the control siRNA. At 24 h post-transfection, the cells were treated with or without LPS for 4 h. The effects of siRNA transfection were determined by Western blot analysis (B). The culture medium was harvested. Medium cytokine levels TNF-α (C), IL-6 (D), and IL-10 (E) were determined by ELISA (bars in E are as for those in C and D). Values are means ± SE (n = 3). Data are representative of three independent experiments.
The Effects of Caveolin-1 on LPS-Induced AP-1 and NF-κB Activation
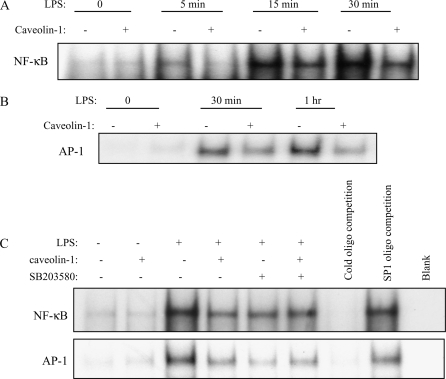
Because the cytokines studied in this study are largely regulated by transcription factors NF-κB and AP-1 (40), we hypothesized that caveolin-1 attenuates upstream LPS-induced NF-κB and AP-1 activation. Caveolin-1 overexpressing and vector control RAW264.7 macrophages were treated with LPS, and nuclear extracts were obtained at 5 min, 15 min, 30 min, and 1 h later and analyzed for NF-κB and AP-1 binding activities by electrophoretic mobility shift assay (EMSA). Cells treated with LPS showed a predicted increase in NF-κB and AP-1 binding activities, whereas caveolin-1 overexpressing cells treated with LPS showed a marked reduction in NF-κB– and AP-1–binding activities (Figures 7A and 7B).
Figure 7.
Effects of caveolin-1 on LPS induced NF-κB and AP-1 activation. RAW264.7 cells stably transfected with caveolin-1 gene and with control vector were serum starved for 24 h. After treatment with LPS for 0 min, 5 min, 15 min, 30 min, and 1 h, the cells were harvested. Nuclear proteins were extracted and subjected to EMSA for (A) NF-κB and (B) AP-1 activation. (C) Effects of SB203580 on LPS induced NF-κB and AP-1 activation. After serum starvation, RAW264.7 cells stably transfected with caveolin-1 gene and with control vector were pretreated with SB203580 or DMSO for 1 h. The nuclear proteins were extracted and subjected to EMSA for NF-κB and AP-1 activation. Cold oligonucleotides containing the transcription factor-binding site for NF-κB and AP-1 were added as a competition control, and cold oligonucleotides containing SP1 binding site were added as a negative competition control. Data are representative of three independent experiments.
We next examined whether p38 MAPK was involved in the suppression of transcriptional factor AP-1 and NF-κB activation by caveolin-1. SB203580, the inhibitor of p38, was administered to RAW264.7 cells stably transfected with caveolin-1 gene and vector. Caveolin-1 attenuated the LPS-induced activation of NF-κB and AP-1 (Figure 7C). When SB203580 was added, the activation of NF-κB and AP-1 were similarly inhibited in vector-transfected cells as in caveolin-1 overexpressing cells. SB203580 eliminated the attenuation of caveolin-1 on NF-κB and AP-1 activation, suggesting that p38 was involved in the suppression of caveolin-1 on LPS-induced inflammatory transcriptional factor activation.
DISCUSSION
Caveolin-1, first identified about one decade ago, was reported to have many functions, including the formation of caveolae, membrane trafficking, signal transduction pathways, apoptosis, calcium and lipid homeostasis in fibroblasts, adipocytes, and endothelial cells (2). Recently, caveolin-1 was reported to be expressed in PMs and involved in the pathogenesis of atherosclerosis and inflammation (24). Although Kasper and colleagues found caveolin-1 staining in rat AMs by immunohistochemistry, they hypothesized it was the result of phagocytosis of other cell-type debris (41). Thus, studies of caveolin-1 in AMs are absent, and little is known about the expression and function of caveolin-1 in AMs. In this study, we used two independent methods to detect caveolin-1 expression in murine AMs. Using Western blot analysis, we demonstrate that mouse AM expression of caveolin-1 is similar to that of the PM. Consistent with the notion that caveolin-1 is primarily a membrane bound protein (4), immunofluorescence staining demonstrated that caveolin-1 mainly colocalized with Mac-3 on the plasma membrane of primary mouse AMs.
The macrophage is one of the major host immune defensive cells. It plays pivotal roles in phagocytosis, cytokine production, and antigen presentation (33). Many investigators are interested in the function of caveolin-1 in macrophages (15, 42–44). Besides lipid metabolism, it is becoming more accepted that caveolin-1 modulates inflammation. Caveolin-1 suppresses inflammation via its interaction with and inhibition of eNOS activity (45). Leonard and colleagues reported that filipin and nystatin, drugs that specifically disrupt the caveolae microdomain by cholesterol chelation, impaired the entry of Chlamydia trachomatis serovar K. into J774A.1 cells (42). Satizo and colleagues showed that downregulation of caveolin-1 combined with upregulation of eNOS decreased leukocyte adhesion in pial venules of ovariectomized female rats (45). Bucci and colleagues used the scaffolding domain of caveolin-1 to inhibit edema formation and vascular leakage to the same extent as glucocorticoid in rats (43). In our LPS-induced inflammation model, caveolin-1 has a direct effect on cytokine production in AMs and PMs. We chose siRNA technology to manipulate caveolin-1 levels in primary macrophages, resulting in a marked suppression of caveolin-1 expression. Because primary macrophages do not divide, the concentration of siRNA in each cell may remain high for a longer time than in fast-growing cells. Production of the proinflammatory cytokines TNF-α and IL-6 was greatly increased by the treatment of siRNA targeting caveolin-1, whereas production of the antiinflammatory cytokine IL-10 was markedly attenuated. Consistently, RAW264.7 cells stably transfected with caveolin-1 demonstrated a markedly attenuated proinflammatory cytokine (TNF-α and IL-6) production and increased antiinflammatory cytokine (IL-10) production.
The MAPK and PI3K pathways are activated by many cellular stresses, including LPS, and are intimately linked with the regulation of cytokine production (45). ERK2 and PI3K were reported to localize in caveolae (2). These findings suggest that the MAPK and PI3K signaling cascades were attractive target candidates for caveolin-1. We have shown in this study, in a mouse macrophage cell line RAW264.7, that caveolin-1 selectively augmented p38 phosphorylation but inhibited JNK, ERK1/2, and Akt phosphorylation. p38 inhibitor (SB203580) blocked the effects of caveolin-1 on LPS-induced cytokines production. In contrast, inhibition of the ERK1/2 and PI3K/Akt kinase cascades did not affect the modulation of cytokine production by caveolin-1. The pivotal role of p38 activation was further confirmed by studies on macrophages isolated from MKK3-gene–deleted mice. There are three MKKs that can phosphorylate the p38 MAPKs: MKK3, MKK4, and MKK6 (46, 47). Given that MKK3 is the major activator of p38, we administered LPS to MKK3 null macrophages and found that attenuation of the p38 by MKK3 knockout led to a complete loss of the response. These results indicate that caveolin-1 affects LPS-induced cytokine production through the activation of MKK3/p38. Using a chemical inhibitor, we also showed that inhibition of the JNK pathway reverses the modulation of proinflammatory cytokine IL-6 production by caveolin-1, while not affecting TNF-α and IL-10 production. Our data, along with the previous implication of the JNK pathway in the reduction of macrophage cytokines production by carbon monoxide and heat shock (48), suggest that the JNK pathway is involved in the regulation of inflammatory cytokine IL-6 production. Although the conclusion drawn from chemical inhibitor experiments alone is not convincing, it is likely that caveolin-1 has effects via multiple signaling pathways.
It has been previously demonstrated that the effects of LPS on macrophages require recruitment of LPS receptor complex (TLR4 and MD-2) to lipid rafts containing CD14 (49). Walton and colleagues reported that LPS was capable of inducing membrane translocation of TLR4 and MD-2 to caveolar fraction in human aortic endothelial cells with cell fractionation and immunofluorescence analyses (50). Caveolin-1 has been reported to regulate membrane receptor signaling through directly binding or through the interaction of the downstream signal molecules (11). TLR4 contains a putative caveolin-1 binding motif (739FIQSRW CIF747) (5). It is possible that caveolin-1 may interact with TLR4 complex and regulate downstream effector functions.
NK-κB and AP-1 are well-known transcriptional factors that regulate LPS-induced cytokine production (40). We explored the effects of caveolin-1 on NK-κB and AP-1 activation after LPS treatment. The results demonstrated that caveolin-1 markedly suppressed the activation of these two transcriptional factors as measured by EMSA. Given previous reports that p38 regulates NK-κB and AP-1 activation in macrophages (51, 52), we hypothesized that p38 is involved in the suppression of NK-κB and AP-1 activation by caveolin-1. SB203580, the inhibitor of p38, consistently abolished the suppression of the transcriptional factors activation by caveolin-1. The exact mechanisms by which caveolin-1 and p38 modulate NK-κB and AP-1 will be subject of ongoing studies.
In summary, we have demonstrated for the fist time caveolin-1 expression in mouse AMs. Caveolin-1 has a protective role for inflammation by suppression of proinflammatory cytokine (TNF-α and IL-6) production and augmentation of antiinflammatory cytokine (IL-10) production in the LPS model. We have also presented evidence that the effect of caveolin-1 involves the p38 MAPK signal pathway. We speculate that increased LPS-mediated activation of p38 and decreased activation of JNK, NK-κB, and AP-1 by caveolin-1 lead to protection against inflammation. Caveolin-1 is an important mediator of anti-inflammatory effects.
Acknowledgments
The authors thank Dr. Xuehui Geng for the advice and help on the immunofluorescence microscopy.
This work was supported by grant AHA0515312U (X.M.W.), grant AHA0525552U (H.P.K.), and by NIH grants R01-HL060234, R01-HL55330, R01-HL079904, and P01-HL70807 (A.M.K.C.).
Originally Published in Press as DOI: 10.1165/rcmb.2005-0376OC on December 15, 2005
Conflict of Interest Statement: None of the authors has a financial relationship with a commercial entity that has an interest in the subject of this manuscript.
References
- 1.Palade GE. The fine structure of blood capillaries. J Appl Phys 1953; 24:1424. [Google Scholar]
- 2.Anderson RG. Caveolae: where incoming and outgoing messengers meet. Proc Natl Acad Sci USA 1993;90:10909–10913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Smart EJ, Ying YS, Mineo C, Anderson RG. A detergent-free method for purifying caveolae membrane from tissue culture cells. Proc Natl Acad Sci USA 1995;92:10104–10108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rothberg KG, Heuser JE, Donzell WC, Ying YS, Glenney JR, Anderson RG. Caveolin, a protein component of caveolae membrane coats. Cell 1992;68:673–682. [DOI] [PubMed] [Google Scholar]
- 5.Schlegel A, Volonte D, Engelman JA, Galbiati F, Mehta P, Zhang XL, Scherer PE, Lisanti MP, Silva W, Maldonado H, et al. Crowded little caves: structure and function of caveolae. Cell Signal 1998;10:457–463. [DOI] [PubMed] [Google Scholar]
- 6.Scherer PE, Okamoto T, Chun M, Nishimoto I, Lodish HF, Lisanti MP. Identification, sequence, and expression of caveolin-2 defines a caveolin gene family. Proc Natl Acad Sci USA 1996;93:131–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Scherer PE, Lewis RY, Volonte D, Engelman JA, Galbiati F, Couet J, Kohtz DS, van Donselaar E, Peters P, Lisanti MP. Cell-type and tissue-specific expression of caveolin-2: caveolins 1 and 2 co-localize and form a stable hetero-oligomeric complex in vivo. J Biol Chem 1997; 272:29337–29346. [DOI] [PubMed] [Google Scholar]
- 8.Tang Z, Scherer PE, Okamoto T, Song K, Chu C, Kohtz DS, Nishimoto I, Lodish HF, Lisanti MP. Molecular cloning of caveolin-3, a novel member of the caveolin gene family expressed predominantly in muscle. J Biol Chem 1996;271:2255–2261. [DOI] [PubMed] [Google Scholar]
- 9.Song KS, Scherer PE, Tang Z, Okamoto T, Li S, Chafel M, Chu C, Kohtz DS, Lisanti MP. Expression of caveolin-3 in skeletal, cardiac, and smooth muscle cells: caveolin-3 is a component of the sarcolemma and co-fractionates with dystrophin and dystrophin-associated glycoproteins. J Biol Chem 1996;271:15160–15165. [DOI] [PubMed] [Google Scholar]
- 10.Okamoto T, Schlegel A, Scherer PE, Lisanti MP. Caveolins, a family of scaffolding proteins for organizing “preassembled signaling complexes” at the plasma membrane. J Biol Chem 1998;273:5419–5422. [DOI] [PubMed] [Google Scholar]
- 11.Mineo C, James GL, Smart EJ, Anderson RG. Localization of epidermal growth factor-stimulated Ras/Raf-1 interaction to caveolae membrane. J Biol Chem 1996;271:11930–11935. [DOI] [PubMed] [Google Scholar]
- 12.Liu J, Oh P, Horner T, Rogers RA, Schnitzer JE. Organized endothelial cell surface signal transduction in caveolae distinct from glycosylphosphatidylinositol-anchored protein microdomains. J Biol Chem 1997; 272:7211–7222. [DOI] [PubMed] [Google Scholar]
- 13.Liu P, Ying Y, Ko YG, Anderson RG. Localization of platelet-derived growth factor-stimulated phosphorylation cascade to caveolae. J Biol Chem 1996;271:10299–10303. [DOI] [PubMed] [Google Scholar]
- 14.Fielding CJ, Fielding PE. Intracellular cholesterol transport. J Lipid Res 1997;38:1503–1521. [PubMed] [Google Scholar]
- 15.Harris J, Werling D, Hope JC, Taylor G, Howard CJ. Caveolae and caveolin in immune cells: distribution and functions. Trends Immunol 2002;23:158–164. [DOI] [PubMed] [Google Scholar]
- 16.Harris J, Werling D, Koss M, Monaghan P, Taylor G, Howard CJ. Expression of caveolin by bovine lymphocytes and antigen-presenting cells. Immunology 2002;105:190–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Arakawa R, Abe-Dohmae S, Asai M, Ito JI, Yokoyama S. Involvement of caveolin-1 in cholesterol enrichment of high density lipoprotein during its assembly by apolipoprotein and THP-1 cells. J Lipid Res 2000;41:1952–1962. [PubMed] [Google Scholar]
- 18.Matveev S, van der Westhuyzen DR, Smart EJ. Co-expression of scavenger receptor-BI and caveolin-1 is associated with enhanced selective cholesteryl ester uptake in THP-1 macrophages. J Lipid Res 1999;40:1647–1654. [PubMed] [Google Scholar]
- 19.Lei MG, Morrison DC. Differential expression of caveolin-1 in lipopolysaccharide-activated murine macrophages. Infect Immun 2000;68:5084–5089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kiss AL, Turi A, Mullner N, Timar J. Caveolin isoforms in resident and elicited rat peritoneal macrophages. Eur J Cell Biol 2000;79:343–349. [DOI] [PubMed] [Google Scholar]
- 21.Gargalovic P, Dory L. Caveolin-1 and caveolin-2 expression in mouse macrophages: high density lipoprotein 3-stimulated secretion and a lack of significant subcellular co-localization. J Biol Chem 2001;276:26164–26170. [DOI] [PubMed] [Google Scholar]
- 22.Kiss AL, Turi A, Muller N, Kantor O, Botos E. Caveolae and caveolin isoforms in rat peritoneal macrophages. Micron 2002;33:75–93. [DOI] [PubMed] [Google Scholar]
- 23.Gargalovic P, Dory L. Cellular apoptosis is associated with increased caveolin-1 expression in macrophages. J Lipid Res 2003;44:1622–1632. [DOI] [PubMed] [Google Scholar]
- 24.Gargalovic P, Dory L. Caveolins and macrophage lipid metabolism. J Lipid Res 2003;44:11–21. [DOI] [PubMed] [Google Scholar]
- 25.Ross R. The pathogenesis of atherosclerosis: a perspective for the 1990s. Nature 1993;362:801–809. [DOI] [PubMed] [Google Scholar]
- 26.Trigatti BL, Anderson RG, Gerber GE. Identification of caveolin-1 as a fatty acid binding protein. Biochem Biophys Res Commun 1999; 255:34–39. [DOI] [PubMed] [Google Scholar]
- 27.Babitt J, Trigatti B, Rigotti A, Smart EJ, Anderson RG, Xu S, Krieger M. Murine SR-BI, a high density lipoprotein receptor that mediates selective lipid uptake, is N-glycosylated and fatty acylated and colocalizes with plasma membrane caveolae. J Biol Chem 1997;272:13242–13249. [DOI] [PubMed] [Google Scholar]
- 28.Matveev S, Uittenbogaard A, van Der Westhuyzen D, Smart EJ. Caveolin-1 negatively regulates SR-BI mediated selective uptake of high-density lipoprotein-derived cholesteryl ester. Eur J Biochem 2001;268:5609–5616. [DOI] [PubMed] [Google Scholar]
- 29.Mulvey MA, Hultgren SJ. Cell biology: bacterial spelunkers. Science 2000;289:732–733. [DOI] [PubMed] [Google Scholar]
- 30.Kiss AL, Geuze HJ. Caveolae can be alternative endocytotic structures in elicited macrophages. Eur J Cell Biol 1997;73:19–27. [PubMed] [Google Scholar]
- 31.Baorto DM, Gao Z, Malaviya R, Dustin ML, van der Merwe A, Lublin DM, Abraham SN. Survival of FimH-expressing enterobacteria in macrophages relies on glycolipid traffic. Nature 1997;389:636–639. [DOI] [PubMed] [Google Scholar]
- 32.Shin JS, Gao Z, Abraham SN. Involvement of cellular caveolae in bacterial entry into mast cells. Science 2000;289:785–788. [DOI] [PubMed] [Google Scholar]
- 33.van Furth R, Cohn ZA, Hirsch JG, Humphrey JH, Spector WG, Langevoort HL. The mononuclear phagocyte system: a new classification of macrophages, monocytes, and their precursor cells. Bull World Health Organ 1972;46:845–852. [PMC free article] [PubMed] [Google Scholar]
- 34.Hocking WG, Golde DW. The pulmonary-alveolar macrophage (first of two parts). N Engl J Med 1979;301:580–587. [DOI] [PubMed] [Google Scholar]
- 35.Kim HP, Wang X, Galbiati F, Ryter SW, Choi AM. Caveolae compartmentalization of heme oxygenase-1 in endothelial cells. FASEB J 2004; 18:1080–1089. [DOI] [PubMed] [Google Scholar]
- 36.Lu HT, Yang DD, Wysk M, Gatti E, Mellman I, Davis RJ, Flavell RA. Defective IL-12 production in mitogen-activated protein (MAP) kinase kinase 3 (Mkk3)-deficient mice. EMBO J 1999;18:1845–1857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sarady JK, Otterbein SL, Liu F, Otterbein LE, Choi AM. Carbon monoxide modulates endotoxin-induced production of granulocyte macrophage colony-stimulating factor in macrophages. Am J Respir Cell Mol Biol 2002;27:739–745. [DOI] [PubMed] [Google Scholar]
- 38.Shibata Y, Zsengeller Z, Otake K, Palaniyar N, Trapnell BC. Alveolar macrophage deficiency in osteopetrotic mice deficient in macrophage colony-stimulating factor is spontaneously corrected with age and associated with matrix metalloproteinase expression and emphysema. Blood 2001;98:2845–2852. [DOI] [PubMed] [Google Scholar]
- 39.Engelman JA, Chu C, Lin A, Jo H, Ikezu T, Okamoto T, Kohtz DS, Lisanti MP. Caveolin-mediated regulation of signaling along the p42/44 MAP kinase cascade in vivo: a role for the caveolin-scaffolding domain. FEBS Lett 1998;428:205–211. [DOI] [PubMed] [Google Scholar]
- 40.Takeda K, Kaisho T, Akira S. Toll-like receptors. Annu Rev Immunol 2003;21:335–376. [DOI] [PubMed] [Google Scholar]
- 41.Kasper M, Seidel D, Knels L, Morishima N, Neisser A, Bramke S, Koslowski R. Early signs of lung fibrosis after in vitro treatment of rat lung slices with CdCl2 and TGF-beta1. Histochem Cell Biol 2004;121:131–140. [DOI] [PubMed] [Google Scholar]
- 42.Norkin LC, Wolfrom SA, Stuart ES. Association of caveolin with Chlamydia trachomatis inclusions at early and late stages of infection. Exp Cell Res 2001;266:229–238. [DOI] [PubMed] [Google Scholar]
- 43.Bucci M, Gratton JP, Rudic RD, Acevedo L, Roviezzo F, Cirino G, Sessa WC. In vivo delivery of the caveolin-1 scaffolding domain inhibits nitric oxide synthesis and reduces inflammation. Nat Med 2000;6:1362–1367. [DOI] [PubMed] [Google Scholar]
- 44.DeFranco AL, Crowley MT, Finn A, Hambleton J, Weinstein SL. The role of tyrosine kinases and map kinases in LPS-induced signaling. Prog Clin Biol Res 1998;397:119–136. [PubMed] [Google Scholar]
- 45.Santizo RA, Xu HL, Galea E, Muyskens S, Baughman VL, Pelligrino DA. Combined endothelial nitric oxide synthase upregulation and caveolin-1 downregulation decrease leukocyte adhesion in pial venules of ovariectomized female rats. Stroke 2002;33:613–616. [DOI] [PubMed] [Google Scholar]
- 46.Derijard B, Raingeaud J, Barrett T, Wu IH, Han J, Ulevitch RJ, Davis RJ. Independent human MAP-kinase signal transduction pathways defined by MEK and MKK isoforms. Science 1995;267:682–685. [DOI] [PubMed] [Google Scholar]
- 47.Raingeaud J, Whitmarsh AJ, Barrett T, Derijard B, Davis RJ. MKK3- and MKK6-regulated gene expression is mediated by the p38 mitogen-activated protein kinase signal transduction pathway. Mol Cell Biol 1996;16:1247–1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Morse D, Pischke SE, Zhou Z, Davis RJ, Flavell RA, Loop T, Otterbein SL, Otterbein LE, Choi AM. Suppression of inflammatory cytokine production by carbon monoxide involves the JNK pathway and AP-1. J Biol Chem 2003;278:36993–36998. [DOI] [PubMed] [Google Scholar]
- 49.Triantafilou M, Miyake K, Golenbock DT, Triantafilou K. Mediators of innate immune recognition of bacteria concentrate in lipid rafts and facilitate lipopolysaccharide-induced cell activation. J Cell Sci 2002; 115:2603–2611. [DOI] [PubMed] [Google Scholar]
- 50.Walton KA, Cole AL, Yeh M, Subbanagounder G, Krutzik SR, Modlin RL, Lucas RM, Nakai J, Smart EJ, Vora DK, et al. Specific phospholipid oxidation products inhibit ligand activation of toll-like receptors 4 and 2. Arterioscler Thromb Vasc Biol 2003;23:1197–1203. [DOI] [PubMed] [Google Scholar]
- 51.Woo CH, Lim JH, Kim JH. Lipopolysaccharide induces matrix metalloproteinase-9 expression via a mitochondrial reactive oxygen species-p38 kinase-activator protein-1 pathway in Raw 264.7 cells. J Immunol 2004;173:6973–6980. [DOI] [PubMed] [Google Scholar]
- 52.Matsuyama W, Wang L, Farrar WL, Faure M, Yoshimura T. Activation of discoidin domain receptor 1 isoform b with collagen up-regulates chemokine production in human macrophages: role of p38 mitogen-activated protein kinase and NF-kappa B. J Immunol 2004;172:2332–2340. [DOI] [PubMed] [Google Scholar]