Abstract
Asbestos causes pulmonary toxicity in part by generating reactive oxygen species that cause DNA damage. We previously showed that the mitochondria-regulated (intrinsic) death pathway mediates alveolar epithelial cell (AEC) DNA damage and apoptosis. Because p53 regulates the DNA damage response in part by inducing intrinsic cell death, we determined whether p53-dependent transcriptional activity mediates asbestos-induced AEC mitochondrial dysfunction and apoptosis. We show that inhibitors of p53-dependent transcriptional activation (pifithrin and type 16-E6 protein) block asbestos-induced AEC mitochondrial membrane potential change (ΔΨm), caspase 9 activation, and apoptosis. We demonstrate that asbestos activates p53 promoter activity, mRNA levels, protein expression, and Bax and p53 mitochondrial translocation. Further, pifithrin, E6, phytic acid, or ρ0-A549 cells (cells incapable of mitochondrial reactive oxygen species production) block asbestos-induced p53 activation. Finally, we show that asbestos augments p53 expression in cells at the bronchoalveolar duct junctions of rat lungs and that phytic acid prevents this. These data suggest that p53-dependent transcription pathways mediate asbestos-induced AEC mitochondria-regulated apoptosis. This suggests an important interactive effect between p53 and the mitochondria in the pathogenesis of asbestos-induced pulmonary toxicity that may have broader implications for our understanding of pulmonary fibrosis and lung cancer.
Keywords: asbestos, DNA damage, epithelium, free radicals, p53
Asbestos is a naturally occurring group of mineral silicate fibers that cause pulmonary and pleural fibrosis, lung cancer, and mesothelioma by mechanisms that are not fully established (1, 2). Alveolar epithelial cell (AEC) apoptosis is one important early event implicated in the pathogenesis of pulmonary fibrosis from a variety of agents, including asbestos (1–3). Asbestos fibers are internalized by AEC soon after exposure, resulting in the production of reactive oxygen species (ROS), DNA damage, and apoptosis (1, 2). We previously showed that mitochondria-derived ROS mediate asbestos-induced AEC DNA damage and apoptosis via the mitochondria-regulated (intrinsic) death pathway (4–6). Electron spin resonance trapping methods have also demonstrated that iron derived from the asbestos fibers augment the formation of the highly reactive hydroxyl radical in the presence of H2O2 and a reducing agent (7). However, the molecular mechanism by which asbestos-induced AEC DNA damage triggers mitochondrial dysfunction and apoptosis is unclear.
The tumor suppressor protein p53, a transcriptional factor that is critically involved in the DNA damage cellular response, causes mitochondrial dysfunction and apoptosis. Activated p53 affects numerous genes that inhibit cell growth to allow time for DNA repair and, if DNA damage is extensive, augment apoptosis in part by the mitochondria-regulated death pathway (8–10). A normal functioning p53 response after exposure to DNA damaging agents prevents mutations from accumulating. The most common mutations in human tumors involve the p53 gene family members (8, 9). One mechanism by which p53 regulates apoptosis involves activating the mitochondria-regulated death pathway by increasing gene expression of pro-apoptotic stimuli (e.g., BAX, PUMA, and others) while inhibiting the expression of anti-apoptotic Bcl-2 family members (8–10). There is also a transcriptional-independent mechanism by which DNA damaging agents trigger an initial rapid mitochondrial p53 translocation (within 60 min) and an early wave of apoptosis (10). The DNA binding domain of wild-type p53 protein interacts with Bcl-xl to promote Bax/Bak-induced outer mitochondrial membrane permeabilization. Tumor-derived p53 mutants that block the interaction between p53 and Bcl-xl cause a “double hit” on the mitochondria-regulated apoptotic pathway by preventing the transcriptional-dependent and direct mitochondrial affects of p53 (9, 10). A mechanistic link between mitochondria-derived ROS and p53-induced apoptosis has recently been suggested (11). Further, p53 DNA binding is blocked by metal chelators and is augmented by reducing agents (12, 13). It is unknown whether any of these mechanisms are involved with mediating asbestos-induced pulmonary toxicity or AEC apoptosis.
Altered p53 expression is implicated in the pathophysiology of pulmonary fibrosis, including that caused by asbestos exposure, and asbestos-associated malignancies. Increased p53 protein expression occurs in the bronchiolar and alveolar epithelium of humans with idiopathic pulmonary fibrosis and in rodents exposed to asbestos (14–16). It is established that asbestos induces p53 and p21 expression in lung epithelial and mesothelial cells and that this results in cell cycle arrest (17–19). Crocidolite asbestos induces p53 gene mutations predominantly in exons 9 through 11 in BALB/c-3T3 cells (20). Further, p53 levels accumulate in lung cancers of patients with asbestosis (21, 22). Collectively, these data suggest that p53 has an important pathophysiologic role in regulating the lung epithelial cell DNA damage response after exposure to asbestos.
Given these findings, we reasoned that p53 mediates asbestos-induced AEC apoptosis by the mitochondria-regulated death pathway. We show that inhibitors of p53-dependent transcription completely block asbestos-induced reductions in AEC mitochondrial membrane potential change (ΔΨm), caspase 9 activation, and apoptosis. Furthermore, asbestos increases A549 cell p53 promoter activity, mRNA levels, protein expression, and mitochondrial translocation of p53 and Bax, and these effects are prevented by inhibitors of p53-dependent transcription. A role for mitochondrial-derived ROS is suggested by our findings that iron chelators block asbestos-induced p53 promoter activity and protein expression and that p53 promoter activity is reduced in ρ0-A549 cells that are incapable mitochondrial ROS production. Finally, we show that an iron chelator attenuates asbestos-induced p53 expression in distal alveolar cells in a rat model of asbestosis. Taken together, these data suggest that p53 mediates asbestos-induced AEC mitochondrial dysfunction and apoptosis and suggest that iron-derived ROS from the mitochondria have an important role in activating p53.
MATERIALS AND METHODS
Asbestos and Reagents
Amosite asbestos fibers were Union International Centere le Cancer reference standard samples supplied by Dr. Timbrell as described (23). All reagents were purchased from Sigma (St. Louis, MO) unless otherwise noted.
Cell Culture
A549 cells, which are human malignant cells with some features of alveolar epithelial type II cells (AT2), and a wild-type p53 (24), were obtained from the American Type Culture Collection (Rockville, MD). A549-E6 and A549-vector cells were a gift from Dr. K. J. Russell (University of Washington School of Medicine, Seattle, WA). ρ0-A549 cells were prepared as previously described (6). In general, A549 cells were plated in 6-well plates at a seeding density of 1 × 106 and grown to confluence over 24 h in Dulbecco's modified Eagle's medium supplemented with 10% FBS while in a humidified 37°C incubator containing 5% CO2. To limit cell proliferation, the media was changed to Dulbecco's modified Eagle's medium with 0.5% FBS for an additional 24 h. Primary rat AT2 cells were by obtained by elastase digestion as described (4–6).
Mitochondria Assays
AEC ΔΨm and caspase 9 activation were assessed as previously described by our laboratory (5). Briefly, AEC ΔΨm was based upon the percentage difference in the ratio of tetramethylrhodamine ethyl ester and mitotracker green fluorescence corrected for the background fluorescence as previously defined (5). Caspase 9 activity was assessed by a commercially available fluorometric assay kit (Roche Diagnostics, Indianapolis, IN) according to the manufacturer's protocol using a fluorescent microplate reader and normalized to the total protein concentration as determined by the Bio-Rad (Hercules, CA) protein assay. In some experiments, inhibitors of p53 transcription (pifithrin), an iron chelator (phytic acid or deferoxamine), or an antioxidant (sodium benzoate) were added before fiber exposure.
Apoptosis Assays
Asbestos-induced A549 cell apoptosis was assessed by terminal deoxynucleotidyl transferase–mediated deoxyuridine-5′-triphosphate-biotin nick end labeling (TUNEL)–stained nuclear morphology and DNA fragmentation (Roche Diagnostics) assays as previously described (4–6). TUNEL-stained cells were assessed under a fluorescent microscope by an investigator who was blinded to the experimental protocol.
Western Analysis
Western blots were done by conventional techniques using primary antibodies to p53 and bax (Cell Signaling Technology, Beverly, MA) and horseradish peroxidase-conjugated secondary antibody (Cell Signaling Technology). For localization studies, we separated the total cellular protein into the mitochondrial and the cytosolic fractions using an Apoalert fractionation kit (BD Biosciences Clontech, Palo Alto, CA). The same blots were stripped and probed with antibodies to beta tubulin (1:1,000) (Santa Cruz Biotechnologies; Santa Cruz, CA) or cytochrome oxidase IV (1:1,000) (COXIV; Cell Signaling Technology) to verify equal loading of cytosolic or mitochondrial protein, respectively. The protein bands were visualized by enhanced chemiluminescence reaction (Amersham Biosciences, Indianapolis, IN).
p53 Luciferase Promoter Assay
A549 cells were transfected cells with a p53 promoter–luciferase construct (BD Biosciences, San Jose, CA) using Lipofectamine (Invitrogen Life Technologies, Carlsbad, CA). Transfection efficiency was normalized to renilla luciferase activity, which remained consistent under all conditions studied (data not shown).
Quantitative Analysis of mRNA by Real-Time RT-PCR
Total RNA was extracted from treated cells using an RNAquos 4-PCR kit (Ambion, Austin, TX) following the manufacturer's instructions. The amount of RNA was measured spectrophotometrically by the absorbance at 260 nm. An aliquot of the total RNA (2 μg) was reverse-transcribed to cDNA by Retroscript-MMLV reverse transcriptase and stored at −20°C until use. The oligonucleotide primers used for the detection of cDNA specific for p53 and the ribosomal subunit 18s were derived from sequences available from the GeneBank databases using the Primer3 software (Whitehead Institute for Biomedical Research, Cambridge, MA). The forward and reverse PCR primer sequences for RT-PCR gene expression analysis include p53 forward 5′-GTG GTT TCA AGG CCA GAT GT-3′, p53 reverse 5′-GGC CCA CTT CAC CGT ACT AA-3′, 18s forward 5′-AAA CGG CTA CCA CAT CCA AG-3′, and 18s reverse 5′-CCT CCA ATG GAT CCT CGT TA-3′. A 1-ml aliquot of first-strand cDNA or H2O (as a negative control) was put into Bio-Rad Cycler with 1 ml of 20 pM primer for target DNA and 18 ml of mixture of SYBRgreen PCR reaction mix (Bio-Rad). Various concentrations of standard sample cDNA were also used to construct a standard curve. Real-time RT-PCR assay was carried out under the following conditions: 10 min at 95°C and 40 cycles of 95°C for 10 s, 59.8°C for 30 s, and 72°C for 40 s. The melt curve was performed after final amplification by increasing the temperature to 95°C over 1 min, decreasing to 55°C over 1 min, and increasing 0.5°C every 10 s to a final temperature of 95°C. The standard curve was shown as a straight line of linear regression with cycle number versus log-concentration of standard samples. This standard curve was used to estimate the concentration of each sample. The relative expression of p53 was normalized to the expression value of the 18s product using the comparative threshold method as previously described (25).
Immunofluorescence and Confocal Microscopy
A549 cells were grown on chamber slides, treated with asbestos for 24 h, washed, and incubated with 1 μM Mitotracker Red (Molecular Probes) to stain mitochondria. The slides were rinsed three times with PBS, fixed with cold 99.8% methanol for 15 min at −20°C, rehydrated twice with PBS, and blocked with 1% BSA for 10 min at 25°C. The cells were incubated overnight with a specific antibody (p53 or Bax; Cell Signaling), rinsed extensively with PBS, and incubated with a FITC secondary antibody for 1 h at 25°C in the dark. To visualize nuclear DNA, we used DAPI staining. The cells were rinsed successively with PBS, distilled water, and ethanol and mounted on a microslide. Visualization was performed using an Axioskop2 plus (Carl Zeiss Microimaging, Inc., Thornwood, NY) fluorescence microscope coupled to a color charge-coupled device camera or to Confocal Microradiance (Bio-Rad) equipment. The images were digitized with Metamorph, Lasershap2000 version 4, Adobe Photoshop version 7.0, Adobe Illustrator version 10 (Adobe Systems, Inc., Beaverton, OR), and Microsoft PowerPoint SP-2 software (Microsoft Corp., Redmond, WA).
Immunohistochemistry
To determine whether asbestos induces p53 activation to cells at the bronchoalveolar duct junctions, we used archived paraffin-embedded lungs from a rat model of asbestosis previously described by our laboratory (26) using a mouse monoclonal p53 primary antibody (DAKO Corporation, Carpentaria, CA). The Animal Care and Use Committee approved our rat model of asbestosis.
Statistical Analysis
The results of each experimental condition were determined from the mean of triplicate trials. The data are expressed as mean ± SE. A two-tailed Student's t test was used to assess the significance of differences between two groups. ANOVA was used when comparing more than two groups; differences between two groups within the set were analyzed by a Fisher' s protected least significant difference test. Probability values < 0.05 were considered significant.
RESULTS
Pifithrin Blocks Asbestos-Induced AEC Mitochondrial Dysfunction and Apoptosis
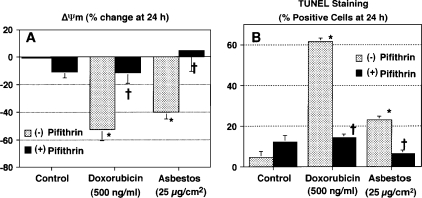
To determine whether p53-dependent transcription is important in mediating asbestos-induced AEC mitochondrial dysfunction and apoptosis, we assessed the protective effects of pifithrin (30 μM for 24 h), a well described p53 inhibitor that blocks p53 DNA binding (27). We used human A549 cells, which are malignant cells with AT2-like features and a wild-type p53 function (24), and nonmalignant, primary, isolated rat AT2 cells. Pifithrin completely blocks asbestos-induced A549 cell mitochondrial dysfunction, as assessed by reductions in ΔΨm, and apoptosis, as assessed by TUNEL staining (Figure 1). Similar protective effects with pifithrin were noted with our positive control, doxorubicin, which causes apoptosis via a p53-dependent mechanism (28). Moreover, pifithrin prevents asbestos-induced apoptosis and caspase 9 activation in primary rat AT2 (Table 1). Collectively, these findings suggest that p53-dependent transcription has an important role in mediating asbestos-induced AEC mitochondrial dysfunction and apoptosis.
Figure 1.
Pifithrin blocks asbestos-induced ΔΨm and apoptosis. A549 cells were treated with pifithrin (30 μM × 24 h) and exposed to doxorubicin (500 ng/ml) or asbestos (25 μg/cm2) for 24 h. ΔΨm (A) and TUNEL staining (B) were determined as described in Materials and Methods. Data are expressed as mean ± SEM. *P < 0.05 versus control; †P < 0.05 versus (−) pifithrin (n = 6).
TABLE 1.
PIFITHRIN BLOCKS ASBESTOS-INDUCED AT2 CELL APOTOSIS AND CASPASE 9 ACTIVATION*
| Condition | TUNEL (% positive cells at 24 h) | DNA Fragmentation (fold control at 24 h) | Caspase 9 (% control RU at 24 h) |
|---|---|---|---|
| Control | 2.6 ± 0.6 | 1.0 ± 0 | 100 ± 0 |
| Pifithrin (30 μM) | 4.1 ± 0.8 | 0.9 ± 0.3 | 110 ± 10 |
| Doxorubicin (500 ng/ml) | 60.6 ± 4.6† | 5.2 ± 1.7† | 240 ± 30† |
| Asbestos (25 μg/cm2) | 39.9 ± 2.0† | 4.3 ± 1.0† | 200 ± 30† |
| Pifithrin + doxorubicin | 25.7 ± 2.0†‡ | 1.9 ± 0.2†‡ | 140 ± 10†‡ |
| Pifithrin + asbestos | 18.0 ± 1.9‡ | 1.3 ± 0.3‡ | 130 ± 20‡ |
n = 6 for each group.
P < 0.05 versus control.
P < 0.05 versus doxorubicin or asbestos.
E6-Transfected A549 Cells Prevents Asbestos-Induced Mitochondrial Dysfunction and Apoptosis
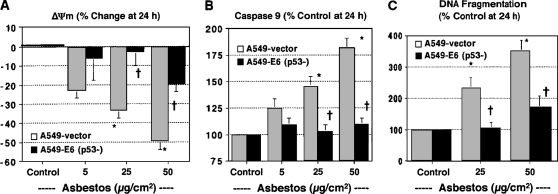
To further study the role of p53 in mediating asbestos-induced A549 cell mitochondrial dysfunction and apoptosis, we used A549-E6 cells that were transfected with a plasmid expressing the human papillomavirus (HPV) type 16 E6 protein (A549-E6 cells), which blocks p53 function by targeting it for ubiquitin-dependent proteolytic degradation (24). A549-E6 cells have a functionally inactive p53 gene product and lose the G1 checkpoint control necessary for radiation-induced cell cycle arrest (24). As expected, A549-empty vector control cells exposed to asbestos resulted in dose-dependent mitochondrial dysfunction (Figures 2A and 2B) and apoptosis (Figure 2C). In contrast, A549-E6 cells nearly completely blocked asbestos-induced alterations in mitochondrial function and apoptosis (Figure 2). These findings in A549-E6 cells along with the pifithrin data noted previously support a role for p53-dependent transcription in mediating AEC mitochondria-regulated apoptosis after asbestos exposure.
Figure 2.
A549-E6 cells are protected against asbestos-induced ΔΨm, caspase 9 activation, and apoptosis. A549 empty vector (gray bars) or A549-E6 transfected cells (solid bars) were exposed to various doses of asbestos (0–50 μg/cm2) for 24 h. ΔΨm (A), caspase 9 activity (B), and DNA fragmentation (C) were determined as described in Materials and Methods. Data are expressed as mean ± SEM. *P < 0.05 versus control; †P < 0.05 versus A549-vector (n = 6).
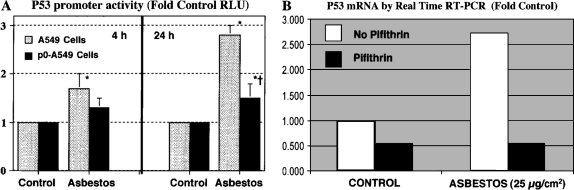
Asbestos Increases A549 Cell p53 Promoter Activity and mRNA Expression: Inhibition by Pifithrin, Iron Chelators, a Free Radical Scavenger, A549-E6 Cells, or ρ0-A549 Cells
To determine whether asbestos augments p53 promoter activity, we used a luciferase reporter plasmid assay. As compared with controls, asbestos increased p53 promoter activity by ∼ 160% as early as a 1 h and persisted at 24 h (∼ 190% increase) (Table 2). The levels noted at 24 h with asbestos were comparable to our positive control, doxorubicin (189% increase; P < 0.05 versus control). To determine whether iron-derived ROS mediate asbestos-induced p53 promoter activity, the cells were treated for 25 min with an iron chelator (phytic acid [500 μM] or deferoxamine [1 mM]) or sodium benzoate (100 μM), a free radical scavenging agent, and exposed to asbestos for 24 h. We previously showed that each of these agents inhibits asbestos-induced ROS generation, AEC mitochondrial dysfunction, and apoptosis (4–6). Each of the inhibitors caused negligible changes in baseline p53 promoter activity as compared with control (RLU [n = 4]: control 7,062 ± 511; deferoxamine 6,785 ± 612; sodium benzoate 9,793 ± 187; phytic acid 5,923 ± 500). Consistent with our earlier studies, each agent nearly completely prevented p53 promoter activity after 1, 4, and 24 h (Table 2). Furthermore, asbestos-induced A549 cell p53 promoter activity at 4 h was not detectable in A549-E6 cells (A549: 346 ± 58% versus A549-E6: 102 ± 3% control levels; P < 0.05; n = 6) or in doxorubicin-exposed A549-E6 cells (data not shown). Using real-time RT-PCR, we noted that asbestos increased p53 mRNA levels more than 2.5-fold after 24 h and that pifithrin completely abolished this (Figure 3B).
TABLE 2.
ASBESTOS-INDUCED P53 PROMOTER ACTIVITY IS ABOLISHED BY AN IRON CHELATOR (PHYTIC ACID AND DEFEROXAMINE) OR FREE RADICAL SCAVENGER (SODIUM BENZOATE)
| Exposure
|
|||
|---|---|---|---|
| Condition | 1 h | 4 h | 24 h |
| Control | 100 ± 0* | 100 ± 0 | 100 ± 0 |
| Asbestos (25 μg/cm2) | 160 ± 10† | 172 ± 6† | 190 ± 5† |
| + Phytic acid (500 μM) | 120 ± 8‡ | 100 ± 1‡ | 125 ± 5‡ |
| + Deferoxamine (1 mM) | 105 ± 2‡ | 100 ± 1‡ | 105 ± 1‡ |
| + Na-benzoate (100 μM) | 135 ± 22 | 100 ± 1‡ | 109 ± 4‡ |
Data expressed as mean ± SEM % control Luciferase RLU after various exposure periods (1, 4, and 24 h); n = 6.
P < 0.05 versus control.
P < 0.05 versus asbestos.
Figure 3.
Asbestos augments A549 cell p53 promoter activity and mRNA expression, and these effects are attenuated in ρ0-A549 cells and by pifithrin. (A) A549 (gray bars) or ρ0-A549 cells (solid bars) were exposed to asbestos (25 μg/cm2) for 4 h (left graph) or 24 h (right graph). p53 promoter activity was determined as described in Materials and Methods. Data are expressed as mean ± SEM. *P < 0.05 versus control; †P < 0.05 versus A549-asbestos (n = 6). (B) A549 (gray bars) or ρ0-A549 cells (solid bars) were treated with pifithrin (Pif) (30 μM × 24 h) and exposed to asbestos (25 μg/cm2) for 24 h. mRNA was obtained for real-time RT-PCR as described in Materials and Methods. Data are expressed as the fold-control p53 mRNA levels corrected for 18s mRNA (n = 3).
To assess whether mitochondria-derived ROS mediate asbestos-induced A549 cell p53 promoter activity, we used ρ0-A549 cells that lack mitochondrial DNA and a functional mitochondrial electron transport chain for oxidative phosphorylation that we have previously characterized in detail as incapable of mitochondrial-derived ROS production (6). Asbestos-induced p53 promoter activity was significantly reduced in ρ0-A549 cells (Figure 3A). With the results of the inhibitor studies described previously, these data suggest that asbestos-induced p53 promoter activity is triggered, at least in part, by iron-derived ROS originating from the mitochondria.
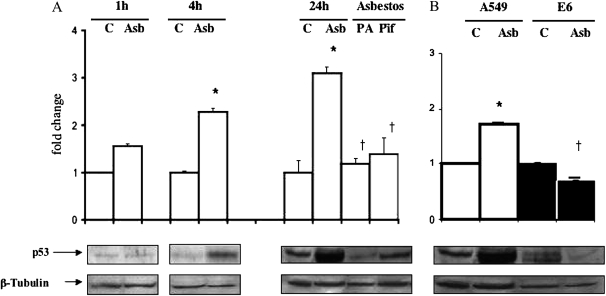
Asbestos Increases p53 Protein Levels and Mitochondria-Targeted Bax Protein Deposition
Consistent with the work of others (17), we confirmed that amosite asbestos stimulates p53 protein expression in A549 cells (Figure 4). Although negligible changes in asbestos-induced p53 protein levels were evident after 1 h, a time-point at which we observed significant p53 promoter activity (Table 2), significant increases in p53 protein levels were seen at 4 h (2.5-fold increase versus control; P < 0.05) and persisted at 24 h (3-fold increase versus control; P < 0.05). p53-Dependent transcription inhibitors (pifithrin and HPV-E6) nearly completely blocked asbestos-induced p53 protein levels at 24 h (Figures 4A and 4B). Similar to our earlier studies showing that phytic acid blocks asbestos-induced ROS production, mitochondrial dysfunction, apoptosis (4–6), and p53 promoter activity (Table 2), phytic acid also prevents asbestos-induced p53 protein expression (Figure 4A). These data demonstrate that asbestos induces A549 cell p53 protein expression and that iron-derived ROS have an important role in mediating this effect.
Figure 4.
Asbestos induces A549 cell p53 protein expression and phytic acid, or pifithrin blocks this effect. (A) A549 cells were exposed to asbestos (25 μg/cm2) for variable periods (1–24 h). p53 proteins levels were determined by Western analysis. For the 24-h time point, some cells were treated with phytic acid (PA) (500 μM × 30 min) or pifithrin (PIF) (30 μM × 24 h) before being exposed to asbestos. Data expressed as mean ± SEM of control value. *P < 0.05 versus control; †P < 0.05 versus A549-asbestos (n = 6). (B) A549 (open bars) or A549-E6 (solid bars) cells were exposed to asbestos (25 μg/cm2) for 24 h, and p53 proteins levels were determined by Western analysis as described previously. Asbestos increased p53 protein levels in A549 cells; this was completely blocked in A549-E6 cells. The levels of beta tubulin were used to confirm comparable protein loading. Data are expressed as mean ± SEM of control value. *P < 0.05 versus control; †P < 0.05 versus asbestos (n = 6).
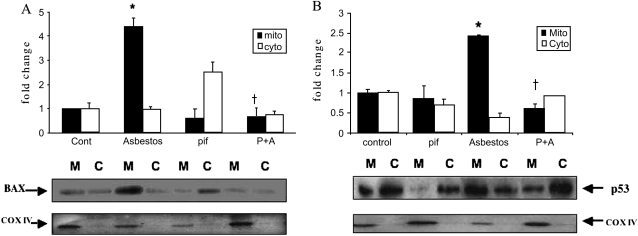
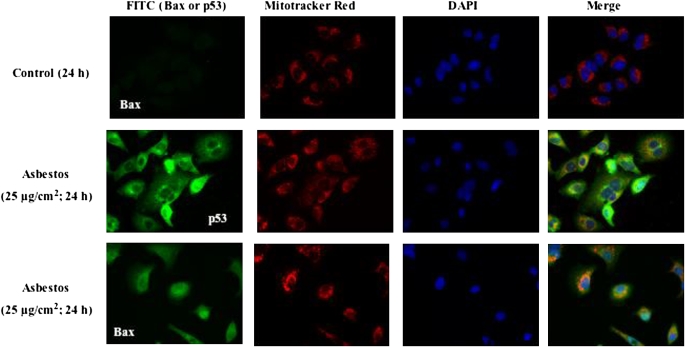
One mechanism by which p53 activates the intrinsic apoptotic death pathway is by promoting Bax and p53 mitochondrial translocation (8–10). To explore this possibility, A549 cells were treated with asbestos for 24 h; then subcellular fractions of protein from the mitochondria and cytosol were assessed by Western analysis. Asbestos increased mitochondria-targeted Bax and p53 protein levels (4.0- and 2.3-fold increases, respectively, compared with controls) (Figure 5). Pifithrin completely blocked asbestos-induced mitochondrial translocation of Bax and p53 (Figure 5). Using confocal microscopy, we corroborated that asbestos, unlike controls, activates the expression of Bax and p53 and that mitochondrial translocation of both is evident in the merged images (Figure 6).
Figure 5.
Asbestos-induced A549 cell mitochondrial Bax (A) and p53 (B) translocation is reduced by pifithrin. A549 were exposed to asbestos (25 μg/cm2) in the presence or absence of pifithrin (pif) for 24 h. Mitochondrial and cytosolic protein were obtained and assessed for Bax (A) and p53 (B) protein by Western analysis. The differences observed in the levels of Bax and p53 from three experiments are shown in a densitometric analysis of mitochondrial (M; solid bars) and cytosolic (C; open bars) proteins. The levels of cytochrome oxidase IV (COXIV) were used to confirm the presence of mitochondrial protein and comparable loading. Data are expressed as mean ± SEM of control value. *P < 0.05 versus control; †P < 0.05 versus asbestos. P+A: pifithrin and asbestos.
Figure 6.
Unlike controls, asbestos induces mitochondrial colocalization of p53 and Bax. A549 cells were exposed for 24 h to control medium (first row) or asbestos (25 μg/cm2; second and third rows), and mitochondrial accumulation of p53 (second row) and Bax (third row) were assessed by confocal immunofluorescence. After exposure, the cells were collected and triple-stained for mitochondria (Mito-tracker Red), nuclei (DAPI), and pro-apoptotic molecule (p53 or Bax; FITC-PoAb). Asbestos-induced mitochondrial localization of p53 and Bax are evident as the yellow-orange punctate cytoplasmic/perinuclear staining in the merged samples (fourth column). There is also evidence of p53 and PUMA nuclear staining in some cells.
Amosite Asbestos Induces p53 Expression in Cells at the Bronchoalveolar Duct Regions in Rat Lungs, and Phytic Acid Attenuates This
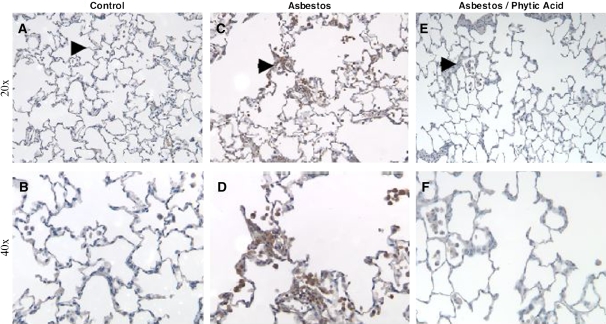
Previous studies have established that asbestos induces p53 expression in AEC and macrophages at the bronchoalveolar duct regions in rodent lungs, but it is unknown whether an iron chelator affects p53 expression (15). We reported that a single intratracheal instillation of amosite asbestos (5 mg) into rat lungs induces pulmonary fibrosis after 2 wk and that phytic acid is protective (26). To confirm whether asbestos induces p53 activation to cells in the bronchoalveolar duct regions, we used archived paraffin-embedded rat lungs from this same rat model of asbestosis that were freshly sectioned and assessed for p53 expression by IHC. Asbestos increased p53 expression in cells at the bronchoalveolar duct region as compared with saline-treated control rat lungs (Figure 7). Although cell-specific identification studies were not performed, p53 expression seems to be evident in AEC and macrophages of asbestos-exposed rats, as previously shown by others (15). The new information in the present study is that phytic acid significantly reduced p53 expression in all cells at the bronchoalveolar duct region (Figure 7).
Figure 7.
Asbestos-induced p53 immunohistochemical staining of cells at the bronchoalveolar duct junctions and alveoli is reduced by phytic acid. Rats were exposed to a single intratracheal instillation of PBS (1 ml), amosite asbestos (5 mg in 1 ml PBS), or asbestos (5 mg) treated with phytic acid (500 μM in 1 ml PBS × 24 h and then instilled together). Two weeks after exposure, the lungs were obtained and p53 IHC assessed as described in Materials and Methods at 20× (A, C, E) and 40 (B, D, F) magnification. Under control conditions (A, B), only rare p53 immunostaining was noted at the bronchoalveolar duct junctions (arrowhead). In contrast, asbestos induced significant p53 immunostaining in cells at the bronchoalveolar duct junctions, alveoli, and alveolar macrophages (C, D). Phytic acid significantly reduced p53 immunostaining (E, F).
DISCUSSION
Accumulating evidence suggests that one mechanism by which asbestos causes pulmonary toxicity involves iron-derived ROS from the mitochondria that cause mitochondrial dysfunction, DNA damage, and apoptosis to important target cells, such as AEC (1, 2, 4–6, 23, 29). Although p53 is a critically important molecule for regulating the cellular response to DNA damage, it is unknown whether p53 regulates asbestos-induced AEC through the mitochondria-regulated death pathway. The major finding of this study is that inhibitors of p53-dependent transcription (pifithrin and E6 protein) block asbestos-induced AEC mitochondrial dysfunction and apoptosis. A key role for mitochondria-derived ROS is suggested by our finding that p53 promoter activity is significantly reduced in asbestos-exposed ρ0-A549 cells that are incapable of ROS production from the mitochondria. We also demonstrate that asbestos induces Bax and p53 translocation to the mitochondria and that this is blocked by pifithrin. Finally, we provide in vivo evidence showing that asbestos augments p53 expression in cells at the bronchoalveolar duct junctions and that phytic acid inhibits this. Collectively, these data suggest that p53-dependent transcriptional mechanisms mediate asbestos-induced AEC mitochondrial dysfunction and apoptosis in part due to mitochondrial-derived ROS production and mitochondrial translocation of Bax and p53.
Although p53 triggers apoptosis by complex mechanisms involving the mitochondrial and death receptor pathways, we focused on the mitochondria because we and others have established that asbestos fibers, unlike inert particulates (e.g., glass beads or titanium dioxide), cause apoptosis by the mitochondria-regulated death pathway (4–6, 29). Further, we detected negligible levels of caspase 8 (the death receptor activated caspase) in our model (5). One of the key findings of this study is that inhibitors of p53-dependent transcriptional activity (pifithrin and E6 protein) block asbestos-induced mitochondrial dysfunction, as assessed by ΔΨm and caspase 9 activation (Figures 1 and 2; Table 1), and apoptosis, as assessed by TUNEL staining and DNA fragmentation (Figures 1 and 3; Table 1). We observed comparable effects in A549 cells, a malignant line of bronchoalveolar cells with AT-2–like features and a wild-type p53 function, and primary isolated rat AT2 cells (Figure 1 and Table 1). These findings are similar to our previous studies showing that asbestos causes DNA strand breaks, mitochondrial dysfunction, and apoptosis in both cell types (4, 5, 23). The protective effects noted in A549-E6 cells exposed to asbestos in the current study are comparable to the beneficial effects observed against radiation-induced cytotoxicity (24). Our data are consistent with a study showing that crocidolite asbestos induces p53 expression and A549 cell apoptosis and extend these findings by suggesting that there is a mechanistic interaction between p53 and the mitochondria-regulated death pathway (19).
In this study, we showed that asbestos stimulates A549 cell p53 promoter activity and mRNA and protein expression. Using a p53 luciferase reporter assay, we noted that asbestos induces p53 promoter activity as early as 1 h after exposure and that this persists over 24 h (Table 2 and Figure 3). We also found that asbestos increases p53 mRNA levels, as assessed by real-time RT-PCR (Figure 3). Asbestos-induced p53 protein expression was first evident at 4 h and remained elevated at 24 h (Figure 4). These findings are consistent with the work of others showing that asbestos induces A549 and mesothelial cell p53 expression, upregulates p21 (a downstream gene activated by p53), inhibits cellular proliferation, and promotes apoptosis (17–19). Similar to these studies, we have observed that amosite asbestos causes dose-dependent inhibition of A549 cell proliferation as assessed by a colony forming unit assay (unpublished observation). Evidence presented herein extends our understanding of the pathophysiology of asbestos by demonstrating that inhibitors of p53-dependent transcription (pifithrin and E6 protein) block asbestos-induced A549 cell p53 promoter activity and mRNA and protein expression. These inhibitors also prevented subsequent asbestos-induced AEC mitochondrial dysfunction and apoptosis (Figures 1 and 2).
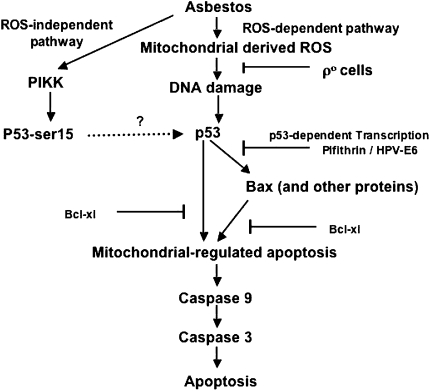
Accumulating evidence implicates redox mechanisms in regulating p53 expression, but it is unknown whether asbestos-induced p53 expression is similarly affected (11–13). Several lines of evidence, including data presented in the current study, firmly implicate iron-derived ROS from the mitochondria in mediating p53 expression and subsequent mitochondria-regulated apoptosis. First, previous studies by others and by our group using iron chelators, antioxidants, and ρ0-A549 cells incapable of mitochondrial ROS generation have established that one mechanism by which asbestos causes apoptosis is by generating iron-derived ROS from the mitochondria (4–6, 30). Second, in the present study we showed that an iron chelator (phytic acid or deferoxamine) or a free radical scavenger (sodium benzoate) blocked asbestos-induced p53 promoter activity (Table 2) and protein expression (Figure 4). Our findings with asbestos-exposed AEC are in agreement with studies by others demonstrating that catalase blocks p53 expression in HepG2 cells exposed to DNA damaging agents (31) and that iron chelators and free radical scavengers prevent Cr(VI)-induced A549 cell p53 activation by inhibiting hydroxyl radical formation (13). Finally, our data demonstrating that asbestos-induced p53 promoter activity is significantly reduced in ρ0-A549 cells as compared with A549 cells (Figure 3) concurs with our previous study showing that asbestos induces significantly less oxidative stress, mitochondrial dysfunction, and apoptosis in ρ0-A549 cells (6). These findings also agree with the observation that p0 human fibroblasts are incapable of oxidant-induced increases in p53 levels caused by hypoxia (32). Collectively, these data suggest a novel mechanism involving mitochondrial-derived ROS in mediating amosite asbestos-induced p53 expression and subsequent AEC apoptosis by the intrinsic pathway. In contrast to the findings presented here, Matsuoka and colleagues (33) showed that chrysotile asbestos increases p53 protein accumulation in A549 cells due to phosphorylation of serine 15 by phosphatidylinositol 3-kinase related kinase family member, such as ataxia-telangiectasia mutated kinase. Further, they found that this was not blocked by catalase, deferoxamine, or N-acetylcysteine, suggesting that p53 stabilization by phosphorylation of serine 15 can occur by mechanisms that are independent of ROS production. Although ataxia-telangiectasia mutated kinase is implicated in mediating mitochondria-regulated apoptosis by radiation (34), the role of phosphorylation of serine 15 on p53 in mediating apoptosis was not examined in this study (33). A hypothetical model depicting the oxidant-dependent and oxidant-independent mechanisms by which asbestos affects p53 expression and apoptosis is shown in Figure 8. Intracellular p53 stabilization is a highly complex process that involves post-translational modification of multiple sites within p53 by phosphorylation and acetylation (35, 36). Further studies exploring the signaling mechanisms by which asbestos induces p53 stabilization and how this affects apoptosis are nedded.
Figure 8.
Hypothetical model of asbestos-induced alveolar epithelial cell apoptosis. After asbestos internalization into AEC, asbestos induces p53 expression though ROS-dependent and ROS-independent pathways. The ROS-dependent pathway involves mitochondrial-derived ROS, and the ROS-independent pathway involves the phosphorylation of serine 15 (p53-ser15) by phosphatidylinositol 3-kinase related kinases. Asbestos-induced AEC apoptosis by the mitochondria-regulated death pathway is triggered by p53-dependent transcription of pro-apoptotic family members and by a direct effect of p53 on the mitochondria.
The mechanisms by which p53 modulates apoptosis are complex and incompletely understood, but one established pathway involves affects on the Bcl-2 family of anti- and pro-apoptotic proteins via p53-dependent transcription and p53 transcription-independent mechanisms (8–10). Using Western analysis and confocal microscopy, we showed that asbestos induces mitochondrial translocation of Bax and p53 (Figures 5 and 6). Moreover, a mechanistic explanation for our findings that inhibitors of p53-dependent transcription prevent asbestos-induced AEC mitochondrial dysfunction and apoptosis was suggested by the observation that pifithrin prevents mitochondrial translocation of Bax and p53 (Figure 5). Our findings with asbestos are consistent with studies demonstrating that p53 induces Bax translocation to the mitochondria after exposure to DNA damaging agents (8–10). p53 mutants that fail to induce apoptosis also are incapable of activating Bax translocation (9, 37). Bax/Bak double knockout cells block the capacity of DNA-damaging agents to induce apoptosis by the mitochondria-regulated death pathway (38). Because the death effector, Bax, is one of several p53-dependent transcriptional targets that have been implicated in promoting apoptosis by the intrinsic pathway (e.g., Bax, Bak, Puma, and others), it is possible that additional pro-apoptotic pathways may be activated in our model (8–10). In addition to acting as a BH3-only–like protein, p53 represses the transcription of anti-apoptotic Bcl2 family members, such as Bcl2 and Bcl-xl (9, 10). We previously reported that A549 cells over-expressing Bcl-xl are protected against asbestos-induced, mitochondria-regulated apoptosis (5). Although our data suggest a crucial role for p53-dependent transcription in mediating asbestos-induced, mitochondria-regulated AEC apoptosis, p53 transcription-independent mechanisms may also be important given that asbestos induces p53 promoter activity within 1 h and protein levels by 4 h. As previously suggested by others (10), detailed studies exploring the complex relationship between p53-dependent transcription and transcription-independent pathways will be of interest.
The in vivo relevance of our in vitro findings was investigated by assessing p53 expression in cells at the bronchoalveolar duct junctions in a rat model of asbestosis that we have previously described (26). In this model, a single intratracheal instillation of amosite asbestos induces histologic and biochemical evidence of pulmonary fibrosis after 2 wk, and each of these effects is blocked by phytic acid. Similar to the work of Misra and coworkers (15), we confirmed that asbestos increased p53 expression as assessed by IHC using freshly sectioned lung samples from archived paraffin-embedded lung tissue from this earlier study (Figure 7). p53 staining was evident in cells along the bronchoalveolar duct junctions, the alveoli, and alveolar macrophages. The novel finding in this study is that phytic acid, which we previously showed inhibits asbestos-induced pulmonary fibrosis (26), attenuated asbestos-induced p53 expression (Figure 7). These data add to the accumulating in vivo evidence that ROS have an important role in mediating pulmonary fibrosis, including that caused by asbestos exposure, and extend our understanding of the pathogenesis by implicating a p53–mitochondria interaction (26, 39). There is some in vivo evidence that compromising AEC p53 function prevents recovery from fibrogenic stimuli. Pulmonary fibrosis is augmented in a mouse transgene with a dominant-negative mutant form of p53 expressed from the surfactant protein C promoter after exposure to asbestos or bleomycin (40, 41). Because this approach targets primarily AT2 cells, it is unknown whether alveolar type 1 cell p53 expression is important. Our findings implicating p53 in mediating asbestos-induced AEC apoptosis are consistent with a recent study showing that silica failed to induce apoptosis in lung cells of p53 knockout animals but did so in p53 wild-type mice (42). Given the importance of AEC apoptosis in mediating pulmonary fibrosis (3) and p53 in regulating cellular life (e.g., growth arrest/DNA repair) and death decisions in response to asbestos-induced DNA damage that are cell type specific (43, 44), our findings suggest that approaches aimed at specifically blocking AEC p53 apoptotic function may limit the fibrogenic effects of asbestos.
An important consideration in the present study is the use of various pharmacologic inhibitors. Although pifithrin was isolated for its ability to specifically suppress p53-mediated transactivation and as a specific inhibitor of p53-dependent transcription (27), more recent evidence shows that it can also act by inhibiting heat shock and glucocorticoid signaling pathways (45). We reasoned that the A549 cells transfected with the HPV-E6 gene, which have a functionally inactive p53 gene product by targeting p53 for ubiquitination, provided a molecular approach for generating p53-deficient A549 cells; others have shown that A549-E6 cells are resistant to G1 checkpoint control, which is necessary for radiation-induced cell cycle arrest (24). Furthermore, the concordance of our findings with pifithrin and A549-E6 cells in multiple assays assessing asbestos-induced p53 promoter activity and mRNA and protein expression supports the important regulatory role of p53 in our model. Pharmacologic inhibitors may also block asbestos uptake into AEC, a critical initial event that leads to uncontrolled iron mobilization, DNA damage, and apoptosis (46, 47). However, genetic approaches using overexpression of anti-apoptotic molecules, such as bcl-xl (5), and E6- or p0-A549 cells demonstrate that iron-derived ROS released from the mitochondria, p53 activation, and the mitochondria-regulated death pathway have important regulatory roles in mediating asbestos-induced AEC apoptosis. Also, deferoxamine and the combination of superoxide dismutase and catalase block asbestos-induced mesothelial cell apoptosis independent of fiber uptake (30). A specific role for iron binding by iron chelators is supported by our observations that iron-loaded phytic acid is unable to prevent AEC DNA damage and apoptosis (4, 5, 23). Thus, fiber uptake seems necessary but not sufficient for inducing cellular dysfunction and apoptosis.
In summary, we have demonstrated that p53-dependent transcription mediates asbestos-induced AEC mitochondrial dysfunction and apoptosis. Our data implicate that iron-derived ROS from the mitochondria and Bax and p53 mitochondrial translocation in part cause these effects. We also found that phytic acid, which is an iron chelator that attenuates asbestos-induced pulmonary fibrosis in rats (26), also inhibits p53 expression in cells at the bronchoalveolar duct region. We speculate that the interactive effects between p53 and the mitochondria have a crucial role in regulating AEC survival and/or malignant transformation after asbestos exposure. Overabundant apoptosis may promote fibrosis, whereas insufficient apoptotic mechanisms may facilitate the formation of a malignant clone of cells harboring mutated DNA (3, 43). Given the recent observation that p53 augments the incorporation step of base excision repair in the mitochondria, the mitochondria DNA may be an especially important target (48). Mitochondrial DNA, as compared with nuclear DNA, is more susceptible to oxidative DNA damage, including that caused by asbestos, and acquires mutations at a 10-fold higher rate (29, 49). We reason that strategies aimed at reducing asbestos-induced mitochondrial ROS production and mitochondrial DNA damage should preserve the barrier function of the alveolar epithelium and thereby prevent pulmonary fibrosis and malignant transformation.
Acknowledgments
The authors thank Dr. K. J. Russell (University of Washington) for the kind gift of A549-E6 and A549-empty vector control cells.
This work was supported by a Merit Review grant from the Department of Veterans Affairs (D.W.K.) and by the National Institutes of Health grants GM-60472 (N.C.) and PO1-HL-071643 (N.C. and D.W.K.).
Originally Published in Press as DOI: 10.1165/rcmb.2005-0352OC on December 15, 2005
Conflict of Interest Statement: None of the authors has a financial relationship with a commercial entity that has an interest in the subject of this manuscript.
References
- 1.Mossman BT, Churg A. Mechanisms in the pathogenesis of asbestosis and silicosis. Am J Respir Crit Care Med 1998;157:1666–1680. [DOI] [PubMed] [Google Scholar]
- 2.Kamp DW, Weitzman SA. The molecular basis of asbestos induced lung injury. Thorax 1999;54:638–652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lee CG, Cho SJ, Kang MJ, Chapoval SP, Lee PJ, Noble PW, Yehualaeshet T, Lu B, Flavell RA, Milbrandt J, et al. Early growth response gene 1-mediated apoptosis is essential for transforming growth factor B1-induced pulmonary fibrosis. J Exp Med 2004;200:377–389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Aljandali A, Pollack H, Yeldandi A, Li Y, Weitzman SA, Kamp DW. Asbestos causes apoptosis in alveolar epithelial cells: role of iron-induced free radicals. J Lab Clin Med 2001;137:330–339. [DOI] [PubMed] [Google Scholar]
- 5.Panduri V, Weitzman SA, Chandel N, Kamp DW. The mitochondria-regulated death pathway mediates asbestos-induced alveolar epithelial cell apoptosis. Am J Respir Cell Mol Biol 2003;28:241–248. [DOI] [PubMed] [Google Scholar]
- 6.Panduri V, Weitzman SA, Chandel N, Kamp DW. Mitochondria-derived free radicals mediate asbestos-induced alveolar epithelial cell apoptosis. Am J Physiol Lung Cell Mol Physiol 2004;286:L1220–L1227. [DOI] [PubMed] [Google Scholar]
- 7.Weitzman SA, Graceffa P. Asbestos catalyzes hydroxyl radical and superoxide generation from hydrogen peroxide. Arch Biochem Biophys 1984;228:373–376. [DOI] [PubMed] [Google Scholar]
- 8.Green DR, Kroemer G. The pathophysiology of mitochondrial death. Science 2004;305:626–629. [DOI] [PubMed] [Google Scholar]
- 9.Oren M. Decision making by p53: life, death and cancer. Cell Death Differ 2003;10:431–442. [DOI] [PubMed] [Google Scholar]
- 10.Schuler M, Green DR. Transcription, apoptosis and p53: Catch 22. Trends Genet 2005;21:182–187. [DOI] [PubMed] [Google Scholar]
- 11.Macip S, Igarashi M, Berggren P, Yu J, Lee SW, Aaroson SA. Influence of reactive oxygen species in p53-mediated cell fate decisions. Mol Cell Biol 2003;23:8576–8585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hainaut P, Milner J. Redox modulation of p53 conformation and sequence specific DNA binding in vitro. Cancer Res 1993;53:4469–4473. [PubMed] [Google Scholar]
- 13.Wang S, Leonard SS, Ye J, Ding M, Shi Y. The role of hydroxyl radical as a messenger in Cr(VI)-induced p53 activation. Am J Physiol Cell Physiol 2000;279:C868–C875. [DOI] [PubMed] [Google Scholar]
- 14.Kuwano K, Kunitake R, Kawasaki M, Nomoto Y, Hagimoto N, Nakanishi Y, Hara N. p21Waf1/Cip1/Sdi1 and p53 expression in association with DNA strand breaks in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med 1996;154:477–483. [DOI] [PubMed] [Google Scholar]
- 15.Mishra A, Liu J-Y, Brody AR, Morris GF. Inhaled asbestos fibers induce p53 expression in the rat lung. Am J Respir Cell Mol Biol 1997;16:479–485. [DOI] [PubMed] [Google Scholar]
- 16.Plataki M, Koutsopoulos AV, Darivianaki K, Delides G, Siafakas NM, Bouros D. Expression of apoptotic and antiapoptotic markers in epithelial cells in idiopathic pulmonary fibrosis. Chest 2005;127:266–274. [DOI] [PubMed] [Google Scholar]
- 17.Johnson NF, Jaramillo RJ. p53, Cip1, and Gadd153 expression following treatment of A549 cells with natural man-made vitreous fibers. Environ Health Perspect 1997;105:1143–1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Levresse V, Renier A, Fleury-Feith J, Levy F, Moritz S, Vivo C, Pilatte Y, Jaurand MC. Analysis of cell cycle disruptions in cultures of rat pleural mesothelial cells exposed to fibers. Am J Respir Cell Mol Biol 1997;17:660–671. [DOI] [PubMed] [Google Scholar]
- 19.Paakko P, Ramet M, Vahakangas K, Korpela N, Soini U, Turunen S, Jaworska M, Gillissen A. Crocidolite asbestos causes an induction of p53 and apoptosis in cultured A549 lung carcinoma cells. Apoptosis 1998;3:203–212. [DOI] [PubMed] [Google Scholar]
- 20.Lin F, Liu Y, Liu Y, Keshava N, Li S. Crocidolite induces cell transformation and p53 gene mutation in BALB/c-3T3 cells. Teratog Carcinog Mutagen 2000;20:273–281. [DOI] [PubMed] [Google Scholar]
- 21.Nuorva K, Makitaro R, Huhti E, Kamel D, Vahakangas K, Bloigu R, Soini Y, Paakko P. p53 protein accumulation in lung carcinomas of patients exposed to asbestos and tobacco smoke. Am J Respir Crit Care Med 1994;150:528–533. [DOI] [PubMed] [Google Scholar]
- 22.Husgafvel-Oursiainen K, Kannio A, Oksa P, Suitiala T, Koskinen H, Partanen R, Hemminki K, Smith S, Rosenstock-leibu R, Brandt-Rauf P. Mutations, tissue accumulation and serum levels of p53 in patients with occupational cancers from asbestos and silica exposure. Environ Mol Mutagen 1997;30:224–230. [PubMed] [Google Scholar]
- 23.Kamp DW, Israbian VA, Preusen SE, Zhang CX, Weitzman SA. Asbestos causes DNA strand breaks in cultured pulmonary epithelial cells: role of iron-catalyzed free radicals. Am J Physiol 1995;268:L471–L480. [DOI] [PubMed] [Google Scholar]
- 24.Russell KJ, Wiens LW, Demers GW, Galloway DA, Plon SE, Groudine M. Abrogation of the G2 checkpoint results in differential radiosensitization of G1 checkpoint-deficient and G1 checkpoint-competent cells. Cancer Res 1995;55:1639–1642. [PubMed] [Google Scholar]
- 25.Giulietti A, Overbergh L, Valckx D, Decallonne B, Bouillon R, Mathieu C. An overview of real-time quantitative PCR: application to quantify cytokine gene expression. Methods 2001;25:386–401. [DOI] [PubMed] [Google Scholar]
- 26.Kamp DW, Israbian VA, Yeldandi AV, Panos RJ, Graceffa P, Weitzman SA. Phytic acid, an iron chelator, attenuates pulmonary inflammation and fibrosis in rats after intratracheal instillation of asbestos. Toxicol Pathol 1995;23:689–695. [DOI] [PubMed] [Google Scholar]
- 27.Komarov PG, Komarova EA, Kondratov RV, Christov-Tselkov K, Coon JS, Chernov MV, Gudkov AV. A chemical inhibitor of p53 that protects mice from side effects of cancer therapy. Science 1999;285:1733–1737. [DOI] [PubMed] [Google Scholar]
- 28.Lorenzo E, Ruiz-Ruiz C, Quesada AJ, Hernandez G, Rodriguez A, Lopez-Rivas A, Redondo JM. Doxorubicin induces apoptosis and CD95 gene expression in human primary endothelial cells through a p53-dependent mechanism. J Biol Chem 2002;277:10883–10892. [DOI] [PubMed] [Google Scholar]
- 29.Shukula A, Jung M, Stern M, Fukagawa NK, Taatjes DJ, Sawyer D, Van Houten B, Mossman BT. Asbestos induces mitochondrial DNA damage and dysfunction linked to the development of apoptosis. Am J Physiol Lung Cell Mol Physiol 2003;285:L1018–L1025. [DOI] [PubMed] [Google Scholar]
- 30.Broaddus VC, Yang L, Scavo LM, Ernst JD, Boylan AM. Asbestos induces apoptosis of human and rabbit pleural mesothelial cells via reactive oxygen species. J Clin Invest 1996;98:2050–2059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bai J, Cederbaum AI. Catalase protects HepG2 cells from apoptosis induced by DNA damaging agents by accelerating the degradation of p53. J Biol Chem 2003;278:4660–4667. [DOI] [PubMed] [Google Scholar]
- 32.Chandel NS, Vander Heiden MG, Thompson CB, Schumacker PT. Redox regulation of p53 during hypoxia. Oncogene 2000;19:3840–3848. [DOI] [PubMed] [Google Scholar]
- 33.Matsuoka M, Igisu H, Morimoto Y. Phosphorylation of p53 protein in A549 human pulmonary epithelial cells exposed to asbestos fibers. Environ Health Perspect 2003;111:509–512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Borges HL, Xu Y, Wang JY. Radiation-induced apoptosis in developing mouse retina exhibits dose-dependent requirement for ATM phosphorylation of p53. Cell Death Differ 2004;11:494–502. [DOI] [PubMed] [Google Scholar]
- 35.Bartek J, Lukas C, Lukas J. Checking on DNA damage in S phase. Nat Rev Mol Cell Biol 2004;5:792–804. [DOI] [PubMed] [Google Scholar]
- 36.Shiloh Y. ATM and related protein kinases: safeguarding genome integrity. Nat Rev Cancer 2003;3:155–168. [DOI] [PubMed] [Google Scholar]
- 37.Friedlander P, Haupt Y, Prives C, Oren M. A mutant p53 that discriminates between p53-responsive genes cannot induce apoptosis. Mol Cell Biol 1996;16:4961–4971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wei MC, Zong WX, Cheng EH, Lindsten T, Panoutsakopoulou V, Ross AJ, Roth KA, MacGregor GR, Thompson CB, Korsmeyer SJ. Proapoptotic BAX and BAK: a requisite gateway to mitochondrial dysfunction and death. Science 2001;292:727–730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mossman BT, Marsh JP, Sesko A, Hill S, Shatos MA, Doherty J, Petruska J, Adler KB, Hemenway D, Mickey R, et al. Inhibition of lung injury, inflammation, and interstitial pulmonary fibrosis by polyethylene glycol-conjugated catalase in a rapid inhalation model of asbestosis. Am Rev Respir Dis 1990;141:1266–1271. [DOI] [PubMed] [Google Scholar]
- 40.Nelson A, Mendoza T, Hoyle GW, Brody AR, Fermin C, Morris GF. Enhancement of fibrogenesis by the p53 tumor suppressor protein in asbestos-exposed rodents. Chest 2001;120:33S–34S. [DOI] [PubMed] [Google Scholar]
- 41.Ghosh S, Mendoza T, Ortiz LA, Hoyle GW, Fermin CD, Brody AR, Friedman M, Morris GF. Bleomycin sensitivity of mice expressing dominant-negative p53 in the lung epithelium. Am J Respir Crit Care Med 2002;166:890–897. [DOI] [PubMed] [Google Scholar]
- 42.Wang L, Bowman L, Lu Y, Rojanasakul Y, Mercer RR. Castranova essential role of p53 in silica-induced apoptosis. Am J Physiol Lung Cell Mol Physiol 2005;288:L488–L496. [DOI] [PubMed] [Google Scholar]
- 43.Morris GF, Notwick AR, David O, Fermin C, Brody AR, Friedman M. Development of lung tumors in mutant p53-expressing mice after inhalation exposure to asbestos. Chest 2004;125:85S–86S. [DOI] [PubMed] [Google Scholar]
- 44.Kopnin PB, Kravchenko IV, Furalyov VA, Pylev LN, Kopnin BP. Cell type-specific effects of asbestos on intracellular ROS levels, DNA oxidation and G1 cell cycle checkpoint. Oncogene 2004;23:8834–8840. [DOI] [PubMed] [Google Scholar]
- 45.Komarova EA, Neznanov N, Komarov PG, Chernov MV, Wang KH, Gudkov AV. p53 inhibitor pifithrin alpha can suppress heat shock and glucocorticoid signaling pathways. J Biol Chem 2003;278:15465–15468. [DOI] [PubMed] [Google Scholar]
- 46.Chao CC, Lund LG, Zinn KR, Aust AE. Iron mobilization from crocidolite asbestos by human lung carcinoma cells. Arch Biochem Biophys 1994;314:384–391. [DOI] [PubMed] [Google Scholar]
- 47.Liu W, Ernst JD, Broaddus VC. Phagocytosis of crocidolite asbestos induces oxidative stress, DNA damage, and apoptosis in mesothelial cells. Am J Respir Cell Mol Biol 2000;23:371–378. [DOI] [PubMed] [Google Scholar]
- 48.Souza-Pinto NC, Harris CC, Bohr VA. p53 functions in the incorporation step in DNA base excision repair in mouse live mitochondria. Oncogene 2004;23:6559–6568. [DOI] [PubMed] [Google Scholar]
- 49.Fliss MS, Usadel H, Cabellero OL, Wu L, Buta MR, Eleff SM, Jen J, Sidransky D. Facile detection of mitochondrial DNA mutation in tumors and bodily fluids. Science 2000;287:2017–2019. [DOI] [PubMed] [Google Scholar]