Abstract
Peroxiredoxin 6 (Prdx6) is a novel peroxidase enzyme that is expressed at a high level in the lung. We tested the hypothesis that transgenic (Tg) mice overexpressing Prdx6 would exhibit increased resistance to hyperoxia-induced lung injury. Wild-type and Tg mice were exposed to 100% O2 and evaluated for survival, lung histopathology, total protein, and nucleated cells in bronchoalveolar lavage fluid (BALF), and oxidation of lung protein and lipids. Prdx6 protein expression and enzyme activity were ∼ 3-fold higher in Tg lungs compared with wild-type. Tg mice survived longer during exposure to 100% O2 (LT50 104 ± 2.8 h in Tg versus 88.9 ± 1.1 h for wild-type). Lung wet/dry weight ratio and total protein and nucleated cell count in lung lavage fluid were significantly greater in wild-type mice at 72 and 96 h of hyperoxia compared with Tg mice. At 96 h of hyperoxia, Tg mice had less epithelial cell necrosis, perivascular edema, and inflammatory cell recruitment by light microscopy, and lower TBARS and protein carbonyls in lung homogenate (P < 0.05). These results show that Tg mice have increased defense against lung injury in hyperoxia, providing evidence that Prdx6 functions as a lung antioxidant enzyme.
Keywords: 1-cys peroxiredoxin, lipid peroxidation, lung inflammation, oxidative stress, protein carbonyls, TBARS
Hyperoxic lung injury is characterized by epithelial and capillary endothelial cell injury resulting in increased pulmonary capillary permeability, inflammation, and respiratory failure (1–3). Hyperoxia results in the increased production of reactive oxygen species (ROS) within cells; the resultant lung damage is caused by an imbalance between the rates of ROS generation and ROS scavenging by antioxidant defenses (4–6). Thus, enhancing antioxidant defenses should accelerate the detoxification of ROS and decrease their damaging effects (7, 8). However, studies of the classical antioxidant enzymes, including superoxide dismutases (SOD), GSH peroxidases (GPx), and catalase, have provided ambiguous results for their role in defense against oxygen toxicity. Recently, a possible antioxidant role has been suggested for the enzymes known as peroxiredoxins.
The Prdxs are a superfamily of nonseleno-peroxidases that catalyze the reduction of a broad spectrum of peroxides. Five of the six mammalian members of this family contain two reactive cysteines and use thioredoxin as the reductant (9). Peroxiredoxin 6 (Prdx6) has a single redox-active cysteine and uses GSH to catalyze the reduction of H2O2, fatty acid hydroperoxides and phospholipid hydroperoxides (PLOOH) (10). This protein has been recognized as a novel antioxidant enzyme that may play a critical role in defense against lung oxidative damage (8, 11, 12). Prdx6 is highly enriched in lung compared with other organs, and is expressed at especially high levels in Clara and alveolar epithelial type II cells (13). Overexpression of Prdx6 protected glutamine synthetase in NIH 3T3 cells against H2O2-mediated inactivation (7) and inhibited membrane phospholipid peroxidation and apoptosis in lung epithelial NCI-H441 cells subjected to ·OH stress by Cu2+/ascorbate treatment (14). Antisense-mediated decrease in expression of Prdx6 in rat L2 cells, another lung epithelial cell line, resulted in increased oxidant sensitivity and cell death (15). With in vivo studies, adenovirus-mediated overexpression of the Prdx6 gene in mouse lung increased survival in hyperoxia and protected against lung injury (8), while deletion of Prdx6 gene in knockout mice increased sensitivity to high oxygen exposure (12) and paraquat-induced oxidative stresses (16). Therefore, we hypothesize that overexpression of the Prdx6 gene in transgenic (Tg) mice will be protective against lung damage in hyperoxia. If so, this will be further evidence that Prdx6 can play an important role in protecting lungs against oxidative injury.
MATERIALS AND METHODS
Animals
The use of animals for these studies was approved by the University of Pennsylvania Animal Care and Use Committee (IACUC). Two experimental groups of mice were studied: the Prdx6 Tg group (n = 37) and a C57BL/6 wild-type (control) group (n = 45). All were male weighing 24–28 g (age 8–11 wk) at the time of exposure. Wild-type mice, free of specific pathogens, were obtained from the Jackson Laboratory (Bar Harbor, ME). Tg mice were provided by Dr. Beverly Paigen (Jackson Labs) and subsequently bred at the University of Pennsylvania animal facility. The generation and genotyping of Tg mice has been described previously (17). The transgene consists of a genomic clone containing the entire Prdx6 gene, which was injected into oocytes from C57BL/6J female mice. Prdx6 Tg mice grow and reproduce normally with no obvious phenotypic difference from the wild-type.
Hyperoxic Animal Model
Mice were exposed to O2 (medical grade) at 1 atmosphere absolute in a Plexiglas chamber (Braintree Scientific Inc., Braintree, MA) as described previously (8). Oxygen was passed through a bubble humidifier and introduced into the sealed chamber at 6–8 liters/min to provide ∼ 5 gas exchanges per hour. The chamber oxygen concentration was measured continuously with an oxygen analyzer (Pacifitech, Temecula, CA) and exceeded 98% for an input gas of 100% O2. For convenience, exposure under these conditions is termed 100% O2. Chamber CO2 was absorbed with a soda lime filter and was maintained at < 0.2%. Relative humidity in the chamber was 45–50%. Mice were allowed food and water ad libitum and maintained on a 12-h dark:light cycle. Cages were opened daily for ∼ 5 min for change of water, food, and bedding and for removal of dead mice. For the study of survival, mice were observed initially at 2- to 4-h intervals, and after 50% mortality at ∼ 12-h intervals.
Harvesting of Lung Tissue
After induction of anesthesia (pentobarbital, 50 mg/kg, intraperitoneally) at the end of exposure, a midline laparotomy/thoracotomy was performed. The trachea was cannulated for continuous ventilation and mice were exsanguinated by laceration of the left renal artery and vein. In some experiments, the whole lung was processed for evaluation of tissue histology. In most experiments, the hilum of the right upper and middle lobes was ligated, and those lobes were removed for measurement of the wet-to-dry weight ratio. The remaining lung lobes were lavaged three times by instillation and aspiration of 0.5 ml PBS containing 0.5 mM EDTA, pH 8.0. The bronchoalveolar lavage fluid (BALF) was placed on ice for cell counting and analysis of protein concentration. The pulmonary vasculature then was flushed with PBS by cannulation of the pulmonary artery via the right ventricle, followed by en bloc removal of the heart and lungs. The heart and large airways were dissected away from the lungs and discarded. The perfused lung was then rapidly frozen in liquid nitrogen and stored at −80°C for measurement of thiobarbituric acid–reactive substance (TBARS) and protein carbonyls.
Expression of Prdx6 Protein and Antioxidant Enzymes
Frozen lung tissue was thawed in 0.05% Tween 20-TBS buffer containing 10 mM Tris-HCl (pH 7.5) and 150 mM NaCl, and homogenized using a Potter-Elvehjem homogenizer at 4°C in the presence of Complete Protease Inhibitor Cocktail (Boehringer Mannheim, Indianapolis, IN). Tissue extracts were then sonicated, centrifuged at 10,000 × g for 20 min, and protein concentration was determined. Protein samples (10 μg) were subjected to 12% SDS-PAGE gel electrophoresis (Invitrogen, Carlsbad, CA) and then were transferred to a polyvinylidene fluoride (PVDF) membrane (Millipore, Bedford, MA). Membranes were incubated in blocking solution (0.1% Tween 20–TBS buffer containing 10% nonfat dry milk) for 1 h and were probed with a polyclonal antibody to Prdx6 peptide (1:2,000 dilution) followed by peroxidase-conjugated secondary antibody (1:2,000 dilution) as described previously (8, 15). Additional immunoblots were probed for catalase, CuZn SOD, and Mn SOD using antibodies as described previously (12) and a polyclonal Ab to GSH peroxidase 1 (GPx1) (Lab Frontier, Seoul, Korea). The reaction was detected by enhanced chemiluminescence (ECL; NEN Life Science, Boston, MA) and quantified by densitometric scanning of X-ray film using the FluorS multi-imager (BioRad, Hercules, CA). For some blots, the reaction was detected by fluorescence using an Odyssey scanner (Li-Cor Biosciences, Lincoln, NE) according to the directions of the manufacturer.
Immunocytochemistry and Histology
Immunohistochemical staining was used to localize and to compare the distribution of Prdx6 expression in Tg mice with that in wild-type mice. Fresh whole lungs were excised and paraffin embedded after inflation and overnight fixation. Longitudinal sections were cut at a thickness of 6 μm. Endogenous peroxidase activity was blocked with 2.25% H2O2, and nonspecific binding sites were blocked with nonimmune goat serum. Sections were incubated with Prdx6 primary polyclonal antibody (1:500) followed by biotinylated secondary antibodies, avidin–biotin–horseradish peroxidase complexes, and diaminobenzidine (DAB) as a substrate. Sections were counterstained with hematoxylin (17). For immunofluorescence evaluation, sections were stained with FITC–avidin after incubation with the biotinylated antibodies.
For histolologic analysis, fresh whole lungs were excised, washed repeatedly with ice-cold PBS, and inflation fixed for 30 min at 4°C by instilling 1 ml of 0.2% glutaraldehyde plus 2% formaldehyde in PBS. The fixed tissues were dehydrated in a graded ethanol series followed by xylene and embedded in paraffin. Longitudinal sections were cut at a thickness of 6 μm and stained with hematoxylin and eosin (12). Fifteen randomly selected lung sections from three control mice and the same number from three oxygen-exposed (96 h) mice were examined by three “blinded” observers who had experience in the evaluation of lung histology and were not associated with this project. Lungs were scored from 1 (slight) to 5 (severe) with reference to alveolar and interstitial edema, infiltration with polymorphonuclear neutrophils (PMN), hemorrhage, and epithelial/endothelial necrosis. The results for each lung from the three observers were averaged to obtain a mean value for each parameter.
Wet:Dry Weight Ratio and BALF Analysis
Wet:dry weight and BALF analyses were performed immediately after sample collection. To measure the ratio of wet to dry lung weight, specimens were lightly blotted, weighed immediately, and then placed in a heated vacuum chamber (Scientific Glass Apparatus, Bloomfield, NJ) until repeated weighing demonstrated no change in weight (∼ 3 d). Total nucleated cells in an aliquot of BALF were counted with a Coulter Counter (Coulter Electronics, Luton, UK). The remaining BALF then was centrifuged for 20 min at 1,000 rpm and 4°C for determination of protein concentration of the supernatant.
Biochemical Measurements
Prdx6 peroxidase activity was determined by a coupled NADPH/GSH reductase assay with 0.66 mM GSH and 8 μM 1-palmitoyl-2-linolenoyl phosphatidylcholine hydroperoxide (PLPCOOH) as substrate (10). For analysis of tissue TBARS, an aliquot of frozen lung (1:10) was homogenized under N2 in PBS containing 0.01% butylated hydroxytoluene, and the homogenate was reacted with thiobarbituric acid and assayed at 535 nm as described previously (8, 18, 19). For analysis of tissue protein carbonyls, frozen lung was homogenized under N2 in buffer containing complete Protease Inhibitor Cocktail (Boehringer Mannheim, Indianapolis, IN) and centrifuged at 100,000 × g for 15 min; the supernatant was reacted with dinitrophenylhydrazine and assayed at 360 nm as previously described (8, 18, 19). For assay of GSH, the frozen lung homogenate was extracted with ice-cold 5% metaphosphoric acid, and analyzed by HPLC (Waters Corp., Medford, MA) with a reverse-phase Nova-Pack 3.9 × 300 mm HR C18 (60 Å pore size and 6 μm particle size) column and Alliance 2695 separation unit. Samples were eluted isocratically (0.5 ml/min) with 23 mM NH4 formate (pH 5)/MeOH (9:1, vol/vol) (14). Eluate absorbance was measured at 220 nm with a PDA 996 UV-VIS detector and data were processed with MILLENIUM software (Waters). Protein in BALF and lung homogenate was measured by Coomassie blue dye binding with bovine γ globulin as the standard (BioRad).
Statistical Analysis
Data are expressed as mean ± SEM. Statistical significance was assessed with SigmaStat software (Jandel Scientific, San Jose, CA). Group differences were evaluated by one-way ANOVA or by Student's t test as appropriate. Survival curves were compared using a log-rank test. Differences between mean values were considered statistically significant at P < 0.05.
RESULTS
Prdx6 and Antioxidant Enzyme Expression
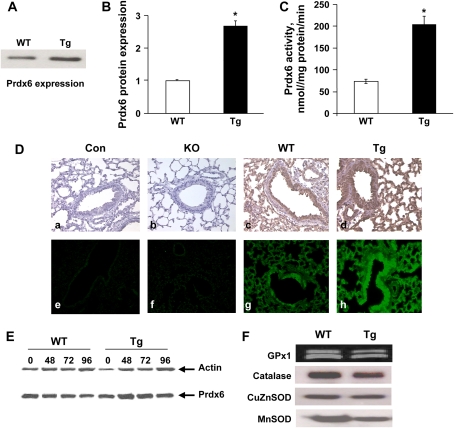
It has previously been shown that the Tg mice expressed significantly elevated levels of Prdx6 mRNA and protein in liver, aorta, and peritoneal macrophages (17). By immunoblot analysis, Prdx6 expression in the lung of Tg mice was 3-fold increased compared with wild-type mice (Figures 1A and 1B). This matched a 300% increase in Prdx6 enzyme activity in lung homogenate from Tg mice using PLPCOOH as a substrate (Figure 1C). Prdx6 was expressed widely throughout the wild-type lung, except for vascular and airway smooth muscle, with high levels of expression in cells of the airway epithelium and alveolar septum as shown using either DAB or FITC-avidin detection (Figure 1D). Prdx6 distribution in the lungs of Tg mice was similar to that in the wild-type mice (Figure 1). Exposure to hyperoxia resulted in a slight increase in Prdx6 expression in both wild-type and Tg lungs (Figure 1E) although the changes for n = 3 (not shown) were not statistically significant. This contrasts with our previous report that showed a significant although modest (50%) increase in Prdx6 protein expression with 63–72 h exposure of this strain of wild-type mice to 100% O2 (11). The expression of other antioxidant enzymes, GPx1, Mn SOD, CuZn SOD, and catalase, was not appreciably altered in Tg as compared to wild-type mice (Figure 1F). For GPx1, the Odyssey scanner indicated a doublet for GPx1 (Figure 1F) which was not detected by enhanced chemiluminescence (not shown).
Figure 1.
Prdx6 and antioxidant enzyme expression. (A) Representative immunoblot for Prdx6 in lung homogenate from wild-type (WT) and transgenic (Tg) mice under control conditions. Lung homogenate protein was 10 μg per lane. (B) Quantitation by densitometry of Prdx6 expression in control wild-type and transgenic lungs for n = 3. *P < 0.05 vs. wild type. (C) Prdx6 enzymatic (peroxidase) activity of lung homogenate with 8 μM 1-palmitoyl-2-linolenoyl-sn-glycero-3-phosphorylcholine hydroperoxide (PLPCOOH) substrate in the presence of 0.66 mM GSH. Values are means ± SE for n = 3. *P < 0.05 compared with wild-type. (D) Prdx6 protein expression in lung tissue of Tg, Prdx6 null (KO), and wild-type mice detected by immunohistochemical staining using a polyclonal antibody to Prdx6 peptide. (a–d) Detected with diaminobenzidine (DAB) (brown). (e–h) Detected with FITC florescence (green). The control (con) is the absence of primary antibody. Original magnification 200x. (E) Expression of Prdx6 and actin by Western blot of wild-type and transgenic mouse lung homogenates under control conditions (zero time) or after exposure to 100% oxygen for 48, 72, or 96 h. (F) Western blots of lung homogenate from non-exposed wild-type and transgenic mice using pAbs for GPx1, catalase, CuZn SOD, and Mn SOD. Blots were evaluated with the Odyssey system for GPx1 and by densitometry for the other proteins. Protein loaded was 10 μg for GPx1 and Mn SOD, 30 μg for catalase, and 5 μg for CuZn SOD.
General Response and Mortality in Hyperoxia
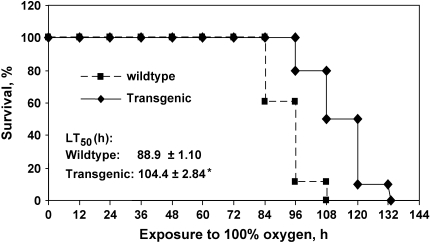
Mice were exposed to 100% oxygen continuously to study survival. Wild-type mice showed decreased response to stimulation by 24 h of hyperoxia and lethargy by 48 h. The general response of Tg mice was similar to that of wild-type mice but was delayed by ∼ 12–24 h. The survival for the wild-type and Tg groups was significantly different (Figure 2A). The initial death in wild-type mice exposed to 100% oxygen occurred at 84 h, whereas no Tg mice died until 96 h. The time to 50% lethality (LT50) for the Tg group was 15.5 h (17.4%) longer than that in the wild-type group (P < 0.01) (Figure 2).
Figure 2.
Survival of Prdx6 Tg mice and wild-type mice in hyperoxia. (A) The survival times of age-matched (10 wk) wild-type (n = 18), and Prdx6 Tg (n = 10) mice from several litters were measured during exposure to 100% oxygen. *P < 0.01 compared with wild-type mice.
Lung Histology, Wet:Dry Ratio, and BALF Analysis
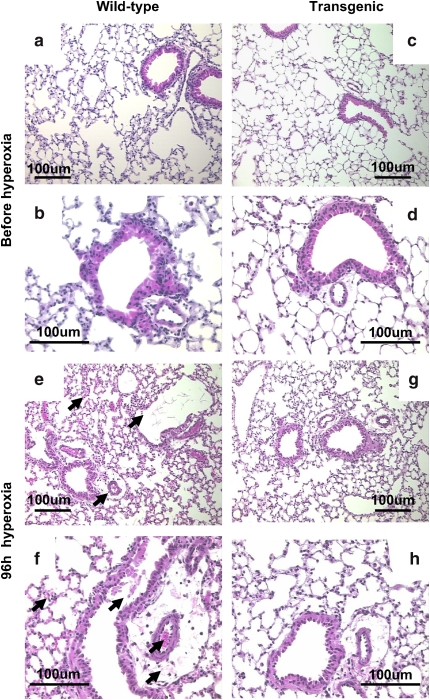
Exposure to 100% oxygen for 96 h caused histologic changes in lungs of both wild-type and Tg mice, but the changes were less severe in the lungs of Tg mice; lungs from wild-type mice had more necrosis of bronchiolar epithelium, more perivascular and alveolar edema, and an increased number of inflammatory cells in the airspaces (Figure 3). Evaluation on a semiquantitative scale (n = 3 mice in each group) by a panel of observers confirmed the consistently greater lung injury at 96 h of O2 in the wild-type mice (Table 1).
Figure 3.
Lung morphology with hyperoxia. Lungs were fixed, sectioned, and stained with hematoxylin and eosin. Lungs of Prdx6 Tg (c, d) and age-matched wild-type mice (a, b) showed similar histologic appearance before oxygen exposure. With 96 h exposure to hyperoxia, wild-type mice (e, f) compared with Prdx6 Tg mice (g, h) showed greater degree of tissue injury (arrows) with perivascular edema, hemorrhage, cellular necrosis, and cellular infiltration. Photomicrographs are representative of n = 3 in each group. The scale bar is 100 μm.
TABLE 1.
HISTOLOGIC EVALUATION OF LUNG INJURY AT 96 H OF OXYGEN EXPOSURE
| Wild-Type | Transgenic | |
|---|---|---|
| Edema | 4.4 ± 0.2 | 2.1 ± 0.8 |
| PMN infiltration | 4.5 ± 0.1 | 2.1 ± 0.4 |
| Hemorrhage | 4.6 ± 0.2 | 0.6 ± 0.7 |
| Cellular necrosis | 4.4 ± 0.3 | 0.7 ± 0.4 |
Indices were graded from 1 (slight) to 5 (severe) in a “blinded” evaluation by three observers. Values are mean ± SE for n = 3 lungs in each group.
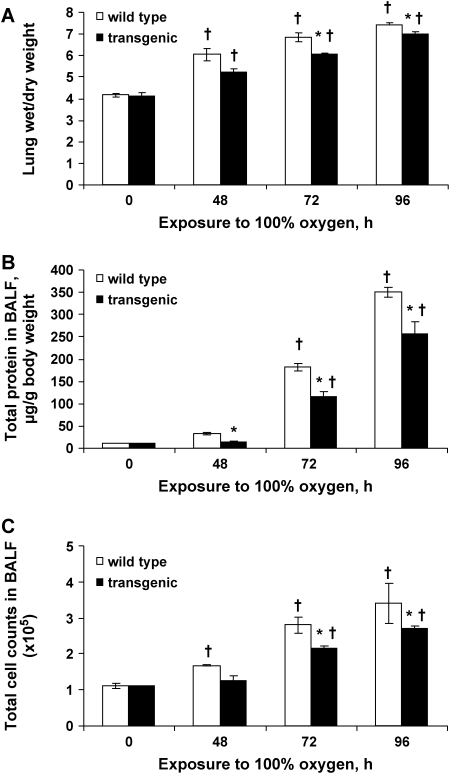
Hyperoxic exposure resulted in a time-dependent increase in lung wet:dry ratio in both wild-type and Tg mice. The increased ratio was significantly less in Tg lungs compared with wild-type lungs at 72 and 96 h (Figure 4A). Protein content and nucleated cell counts in BALF from wild-type mice showed incremental increases at each of the three time points of hyperoxia (Figures 4B and 4C). The incremental increase in these indices of injury for Tg mice was significantly less at each time point compared with wild-type mice (Figures 4B and 4C). At 96 h, BALF protein in lungs from wild-type mice was increased 37-fold and nucleated cells 3.5-fold compared with the unexposed control mice; in Tg mice, the increases were 28-fold for BALF protein and 2.6-fold for BALF nucleated cells.
Figure 4.
Lung wet:dry weight ratio and bronchoalveolar lavage fluid (BALF) analysis. Lungs were analyzed before and at 48, 72, and 96 h of exposure to 100% O2. (A) Ratio of wet to dry weight of mouse lung. (B) Protein content in BALF. (C) Nucleated cells in BALF. Results are mean ± SE (n = 4 for each time point) for Prdx6 Tg and wild-type mice at indicated times of hyperoxia. *P < 0.05 for Prdx6 Tg versus wild-type at the same time of exposure. †P < 0.05 versus the corresponding zero time control.
Lung TBARS, Protein Carbonyls, and GSH
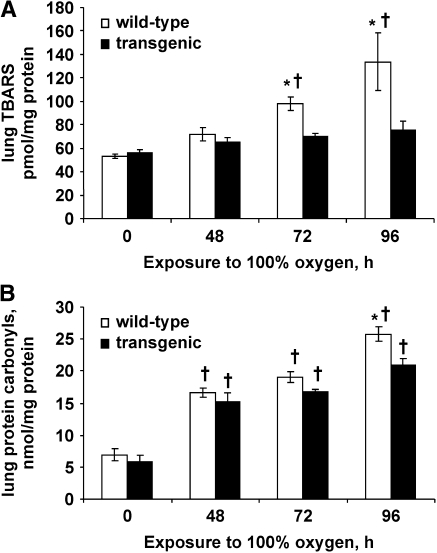
Before oxygen exposure, wild-type and Tg mice had similar levels of lung TBARS and protein carbonyls (Figure 5). The content of TBARS in control lungs was ∼ 50 pmol/mg protein; this value (and the increase with hyperoxia) is different from our previously published results in mouse lungs (8, 12) because of a methodologic difference related to lung extraction. The lung content of both TBARS and protein carbonyls was increased significantly in wild-type mice after 48 h and showed further increases at 72 and 96 h of hyperoxia. The levels of both markers of oxidant stress were lower in lung homogenate from Tg mice compared with wild-type mice; the difference between the two groups was significant at 72 and 96 h of oxygen exposure for TBARS and at 96 h for protein carbonyls (Figures 5A and 5B). At 96 h, TBARS were increased 270% and protein carbonyls 370% in wild-type mice compared with unexposed control mice; in the Tg mice, these increases were 200% for TBARS and 320% for protein carbonyls.
Figure 5.
Lung TBARS and protein carbonyls. TBARS (A) and protein carbonyls (B) in mouse lung homogenate were measured before and at 48, 72, and 96 h of hyperoxia. Values are means ± SE (n = 4 for each time point). *P < 0.05 versus transgenic at the same time of exposure. †P < 0.05 versus the corresponding zero time control.
Total GSH (oxidized plus reduced) in the lung homogenate at zero time and at 72 h of hyperoxia was slightly greater for Tg compared to wild-type mice; the mean value for total glutathione decreased after 72 h O2 exposure (Table 2). However, none of these changes was statistically significant (P > 0.05). The GSH to GSSG ratio indicates that approximately 99% of lung tissue glutathione was in the reduced form in both wild-type and Tg mice and was not significantly altered by O2 exposure (Table 2).
TABLE 2.
TOTAL GLUTATHIONE IN THE LUNG HOMOGENATE AND RATIO OF REDUCED (GSH) TO OXIDIZED (GSSG) GLUTATHIONE
| GSH + GSSG
|
GSH/GSSG
|
|||
|---|---|---|---|---|
| control | hyperoxia | control | hyperoxia | |
| Wild-Type | 11.5 ± 1.0 | 9.4 ± 1.7 | 104 ± 10.6 | 98 ± 12.1 |
| Transgenic | 14.1 ± 3.0 | 12.4 ± 2.0 | 93 ± 9.8 | 85 ± 3.0 |
GSH is expressed as nmol/mg lung protein. Control is pre-exposure and hyperoxia is 100% O2 for 72 h. Values are mean ± SE for n = 3.
DISCUSSION
Continuous exposure of mice to hyperoxia causes progressive lung injury with alveolar capillary leak, lung inflammation, oxidation of tissue components, and death (1, 2, 12). The mechanism for injury is thought to be secondary to increased generation of ROS. ROS, through their effects on cellular constituents, can lead to alterations of normal cellular metabolism, signaling, and function (5, 6, 20). To minimize damage, cells depend on the scavenging of ROS by enzymatic and nonenzymatic antioxidant defenses. Thus, overexpression of antioxidant enzymes could protect lung cells from hyperoxia-induced lung injury (1, 8, 21, 22), while decreased enzyme expression should potentiate injury. This concept has been tested using altered expression in mice of several antioxidant enzymes. Mice deficient in cytosolic CuZnSOD did not exhibit hypersensitivity to hyperoxic exposure (1), and its overexpression had mixed effects on survival under hyperoxia that varied with age and sex of the mice (22). Likewise, deletion of catalase or cytosolic GPx (GPx1) in mice had no effect on sensitivity to the toxic effect of oxygen (23, 24). The latter enzyme is the ubiquitous GPx that is widely distributed in most cell types. Thus, CuZuSOD, catalase, and GPx1 expression appear to have relatively little affect on hyperoxic lung injury. On the other hand, deficiency of MnSOD in homozygous null mice increased sensitivity to oxygen in neonates and its overexpression in Tg mice gave partial protection in hyperoxia (25, 26). Extracellular (EC)-SOD null mice also were more susceptible to hyperoxia, and EC-SOD Tg mice had reduced hyperoxia-induced neutrophilic inflammation (27, 28). These results from studies of the classical antioxidant enzymes suggest that MnSOD and EC-SOD have a role in defense against oxygen toxicity, but leave open the possibility that other enzymes are also critically important.
Prdx6 is a novel antioxidant enzyme, highly expressed in lung, which uses GSH to catalyze the reduction of H2O2 and phospholipid hydroperoxides (PLOOH) (10). In cell culture models, this enzyme protects cell membranes from oxidant-induced lipid peroxidation and apoptotic cell death (14, 15). Prdx6 null mice are more sensitive to oxygen (12) and paraquat-induced oxidative stresses (16) while adenovirus-mediated overexpression of Prdx6 in lung increased mouse survival with hyperoxia (8). However, interpretation of the results with the latter overexpression study is complicated by lung inflammation induced by the adenovirus (8). In the present study, Tg Prdx6-overexpressing mice showed longer survival than wild-type mice on exposure to 100% oxygen. Tg mice had less lung epithelial cell necrosis, decreased perivascular edema and inflammatory cell recruitment, less of an increase in lung wet/dry weight ratio, lower total protein and fewer nucleated cells in lung lavage fluid, and smaller increases in lung tissue TBARS and protein carbonyls. These results indicate that overexpression of the Prdx6 gene can reduce airway inflammation and parenchymal lung injury associated with exposure to hyperoxia.
The GSH-dependent phospholipid hydroperoxide peroxidase activity was ∼ 3-fold higher in lung homogenate from Tg mice as compared with wild-type mice. Using PLPCOOH as a substrate for GSH-dependent peroxidase assay reflects either Prdx6 or glutathione peroxidase type 4 (GPx4) activity in the lung, since phospholipid hydroperoxides are not a substrate for GPx1 (10, 12). We have reported previously that peroxidase activity with PLPCOOH in lung homogenate was reduced by 96% in Prdx6 knockout mice, thus indicating that Prdx6 is mainly responsible for this activity and that GPx4 has a relatively minor role. There was no effect of Prdx6 knockout on GSH-mediated H2O2 reduction (12) consistent with the more important role of GPx1 (and catalase) in reduction of this substrate. Thus, enhanced oxygen sensitivity in Prdx6 null mice was not related to GSH-dependent H2O2 reduction. Further, deletion of the GPx1 gene and the resultant marked decrease in peroxidase activity for H2O2 had no effect on oxygen sensitivity (24).
Based on these results, we postulate that the role of Prdx6 in hyperoxia may be in the reversal of cell membrane lipid peroxidation through reduction of phospholipid hydroperoxides. Evidence for this effect is provided by analysis of lung lipid peroxidation with hyperoxia as reflected by measurement of lung TBARS in lung homogenate showing decreased levels in Prdx6 overexpressing mice (Figure 5A) and increased levels in Prdx6 knockout mice (12) compared with wild-type. Peroxidation of membrane phospholipids would be expected to have a major effect on cellular function and viability. Since GPx1 cannot reduce phospholipid hydroperoxides, it does not participate in this aspect of antioxidant defense. The alternative pathway to repair peroxidized membrane phospholipids is through a deacylation step involving phospholipase A2, reduction of the liberated peroxidized fatty acid by a GPx, and reacylation of the lysophospholipid with an acyl transferase. This alternate pathway is significantly less efficient than the direct reduction of peroxidized phospholipid (29).
The results of the present study indicate protection against hyperoxic stress by overexpression of Prdx6, but the magnitude of protection was relatively modest compared to the 2.5–3-fold increase in lung Prdx6 content. The failure of Prdx6 to protect to a greater extent was not due to depletion of the enzyme or the reductant (GSH) with hyperoxia. Further, distribution of the enzyme in wild-type and Tg lungs was similar at the organ (Figure 1) and cellular (unpublished data) levels. These observations suggest that peroxidized membrane phospholipids represent only part of the pathophysiology of oxygen toxicity and that other mechanisms also play important roles. Overexpression of Prdx6 was sufficient to prevent the increase in lung TBARS (lipid peroxidation) but had a lesser (but nonetheless significant) effect on protein carbonyls (protein oxidation) and other indices of lung injury.
In summary, the results indicate that overexpression of Prdx6 in Tg mice increased defense against lung injury with hyperoxia, supporting prior evidence that Prdx6 functions as an important lung antioxidant enzyme.
Acknowledgments
The authors thank Dr. Beverly Paigen for supplying the transgenic mice; Dr. Peter Bell of the Cell Morphology Core of the University of Pennsylvania Gene Therapy Program for assistance with histology slide preparation; Drs. Bruno Schremmer, Sandra Bates, and Tatyana Milovanova for scoring of histology studies; and Ms. Jennifer Rossi for typing the manuscript.
Support was provided by HL-65543 from the National Institutes of Health and CFFS886 from the Cystic Fibrosis Foundation.
Originally Published in Press as DOI: 10.1165/rcmb.2005-0333OC on January 6, 2006
Conflict of Interest Statement: None of the authors has a financial relationship with a commercial entity that has an interest in the subject of this manuscript.
References
- 1.Ho YS. Transgenic and knockout models for studying the role of lung antioxidant enzymes in defense against hyperoxia. Am J Respir Crit Care Med 2002;166:S51–S56. [DOI] [PubMed] [Google Scholar]
- 2.Fisher AB, Forman HJ, Glass M. Mechanisms of pulmonary oxygen toxicity. Lung 1984;162:255–259. [DOI] [PubMed] [Google Scholar]
- 3.Mantell LL, Horowitz S, Davis JM, Kazzaz JA. Hyperoxia-induced cell death in the lung–the correlation of apoptosis, necrosis, and inflammation. Ann N Y Acad Sci 1999;887:171–180. [DOI] [PubMed] [Google Scholar]
- 4.Freeman BA, Crapo JD. Hyperoxia increases oxygen radical production in rat lungs and lung mitochondria. J Biol Chem 1981;256:10986–10992. [PubMed] [Google Scholar]
- 5.Aerts C, Wallaert B, Voisin C. In vitro effects of hyperoxia on alveolar type II pneumocytes: inhibition of glutathione synthesis increases hyperoxic cell injury. Exp Lung Res 1992;18:845–861. [DOI] [PubMed] [Google Scholar]
- 6.Ho YS, Dey MS, Crapo JD. Antioxidant enzyme expression in rat lungs during hyperoxia. Am J Physiol 1996;270:L810–L818. [DOI] [PubMed] [Google Scholar]
- 7.Kang SW, Baines IC, Rhee SG. Characterization of a mammalian peroxiredoxin that contains one conserved cysteine. J Biol Chem 1998;273:6303–6311. [DOI] [PubMed] [Google Scholar]
- 8.Wang Y, Manevich Y, Feinstein SI, Fisher AB. Adenovirus-mediated transfer of the 1-cys peroxiredoxin gene to mouse lung protects against hyperoxic injury. Am J Physiol Lung Cell Mol Physiol 2004;286:L1188–L1193. [DOI] [PubMed] [Google Scholar]
- 9.Rhee SG, Kang SW, Netto LE, Seo MS, Stadtman ER. A family of novel peroxidases, peroxiredoxins. Biofactors 1999;10:207–209. [DOI] [PubMed] [Google Scholar]
- 10.Fisher AB, Dodia C, Manevich Y, Chen JW, Feinstein SI. Phospholipid hydroperoxides are substrates for non-selenium glutathione peroxidase. J Biol Chem 1999;274:21326–21334. [DOI] [PubMed] [Google Scholar]
- 11.Kim HS, Manevich Y, Feinstein SI, Pak JH, Ho YS, Fisher AB. Induction of 1-cys peroxiredoxin expression by oxidative stress in lung epithelial cells. Am J Physiol Lung Cell Mol Physiol 2003;285:L363–L369. [DOI] [PubMed] [Google Scholar]
- 12.Wang Y, Feinstein SI, Manevich Y, Ho YS, Fisher AB. Lung injury and mortality with hyperoxia are increased in peroxiredoxin 6 gene-targeted mice. Free Radic Biol Med 2004;37:1736–1743. [DOI] [PubMed] [Google Scholar]
- 13.Kim TS, Dodia C, Chen X, Hennigan BB, Jain M, Feinstein SI, Fisher AB. Cloning and expression of rat lung acidic Ca(2+)-independent PLA2 and its organ distribution. Am J Physiol 1998;274:L750–L761. [DOI] [PubMed] [Google Scholar]
- 14.Manevich Y, Sweitzer T, Pak JH, Feinstein SI, Muzykantov V, Fisher AB. 1-Cys peroxiredoxin overexpression protects cells against phospholipid peroxidation-mediated membrane damage. Proc Natl Acad Sci USA 2002;99:11599–11604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pak JH, Manevich Y, Kim HS, Feinstein SI, Fisher AB. An antisense oligonucleotide to 1-cys peroxiredoxin causes lipid peroxidation and apoptosis in lung epithelial cells. J Biol Chem 2002;277:49927–49934. [DOI] [PubMed] [Google Scholar]
- 16.Wang X, Phelan SA, Forsman-Semb K, Taylor EF, Petros C, Brown A, Lerner CP, Paigen B. Mice with targeted mutation of peroxiredoxin 6 develop normally but are susceptible to oxidative stress. J Biol Chem 2003;278:25179–25190. [DOI] [PubMed] [Google Scholar]
- 17.Phelan SA, Wang X, Wallbrandt P, Forsman-Semb K, Paigen B. Overexpression of Prdx6 reduces H2O2 but does not prevent diet-induced atherosclerosis in the aortic root. Free Radic Biol Med 2003;35:1110–1120. [DOI] [PubMed] [Google Scholar]
- 18.Ayene IS, Dodia C, Fisher AB. Role of oxygen in oxidation of lipid and protein during ischemia/reperfusion in isolated perfused rat lung. Arch Biochem Biophys 1992;296:183–189. [DOI] [PubMed] [Google Scholar]
- 19.Zhao G, al-Mehdi AB, Fisher AB. Anoxia-reoxygenation versus ischemia in isolated rat lungs. Am J Physiol 1997;273:L1112–L1117. [DOI] [PubMed] [Google Scholar]
- 20.Ward NS, Waxman AB, Homer RJ, Mantell LL, Einarsson O, Du Y, Elias JA. Interleukin-6-induced protection in hyperoxic acute lung injury. Am J Respir Cell Mol Biol 2000;22:535–542. [DOI] [PubMed] [Google Scholar]
- 21.Danel C, Erzurum SC, Prayssac P, Eissa NT, Crystal RG, Herve P, Baudet B, Mazmanian M, Lemarchand P. Gene therapy for oxidant injury-related diseases: adenovirus-mediated transfer of superoxide dismutase and catalase cDNAs protects against hyperoxia but not against ischemia-reperfusion lung injury. Hum Gene Ther 1998;9:1487–1496. [DOI] [PubMed] [Google Scholar]
- 22.White CW, Avraham KB, Shanley PF, Groner Y. Transgenic mice with expression of elevated levels of copper-zinc superoxide dismutase in the lungs are resistant to pulmonary oxygen toxicity. J Clin Invest 1991;87:2162–2168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ho YS, Xiong Y, Ma W, Spector A, Ho DS. Mice lacking catalase develop normally but show differential sensitivity to oxidant tissue injury. J Biol Chem 2004;279:32804–32812. [DOI] [PubMed] [Google Scholar]
- 24.Ho YS, Magnenat JL, Bronson RT, Cao J, Gargano M, Sugawara M, Funk CD. Mice deficient in cellular glutathione peroxidase develop normally and show no increased sensitivity to hyperoxia. J Biol Chem 1997;272:16644–16651. [DOI] [PubMed] [Google Scholar]
- 25.Asikainen TM, Huang TT, Taskinen E, Levonen AL, Carlson E, Lapatto R, Epstein CJ, Raivio KO. Increased sensitivity of homozygous Sod2 mutant mice to oxygen toxicity. Free Radic Biol Med 2002;32:175–186. [DOI] [PubMed] [Google Scholar]
- 26.Ho YS, Vincent R, Dey MS, Slot JW, Crapo JD. Transgenic models for the study of lung antioxidant defense: enhanced manganese-containing superoxide dismutase activity gives partial protection to B6C3 hybrid mice exposed to hyperoxia. Am J Respir Cell Mol Biol 1998;18:538–547. [DOI] [PubMed] [Google Scholar]
- 27.Folz RJ, Abushamaa AM, Suliman HB. Extracellular superoxide dismutase in the airways of transgenic mice reduces inflammation and attenuates lung toxicity following hyperoxia. J Clin Invest 1999;103:1055–1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Oury TD, Schaefer LM, Fattman CL, Choi A, Weck KE, Watkins SC. Depletion of pulmonary EC-SOD after exposure to hyperoxia. Am J Physiol Lung Cell Mol Physiol 2002;283:L777–L784. [DOI] [PubMed] [Google Scholar]
- 29.Zhao L, Wang HP, Zhang HJ, Weydert CJ, Oberley LW, Buettner GR. L-PhGPx expression can be suppressed by antisense oligodeoxynucleotides. Arch Biochem Biophys 2003;417:212–218. [DOI] [PubMed] [Google Scholar]