Abstract
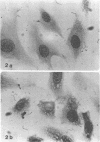
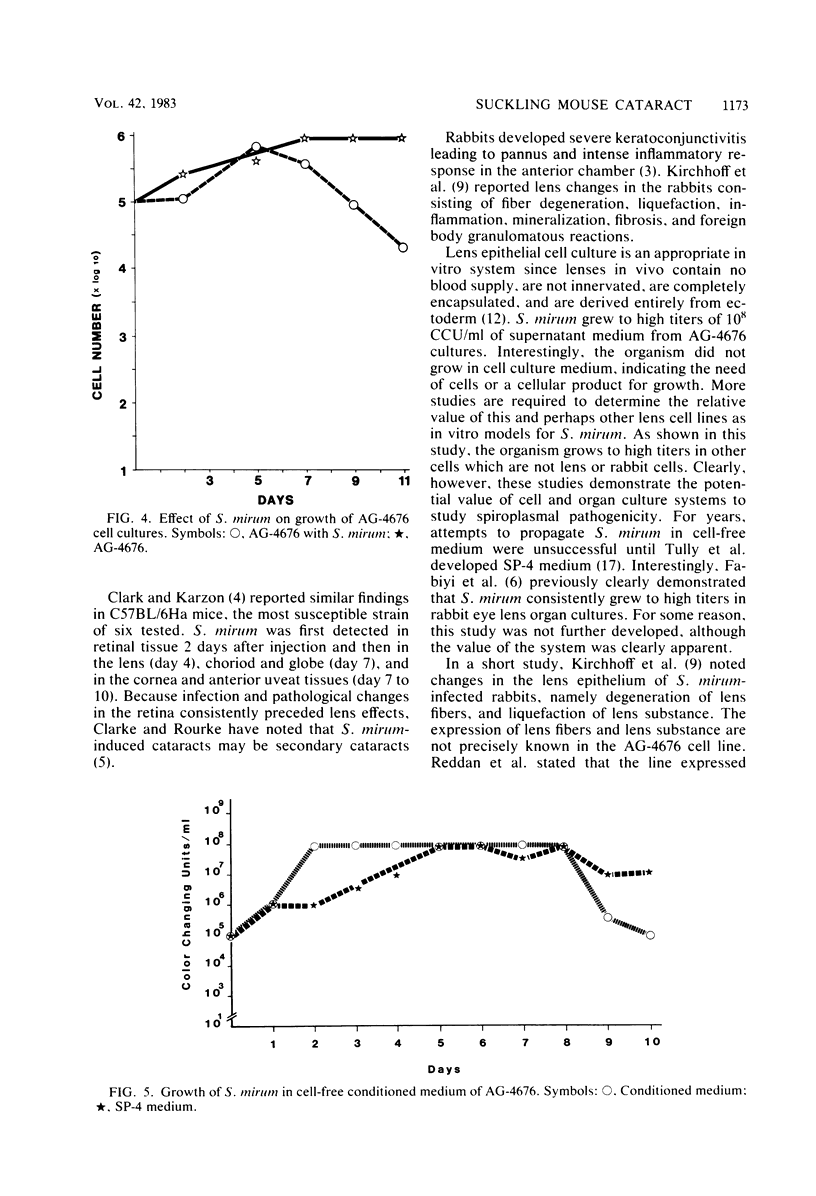
Spiroplasma mirum (suckling mouse cataract agent) was studied in an epithelial cell line AG-4676, derived from rabbit eye lens. Rabbit eye lens is a natural target tissue of S. mirum infection. The organism grew rapidly in this cell line, reaching titers of 10(7) to 10(9) color change units per ml at 7 days after infection. This is the same level as that achieved in SP-4 medium designed specifically for S. mirum. No lag period was apparent in growth in AG-4676. S. mirum did not grow in Dulbecco minimal essential medium-10% fetal bovine serum, the medium for AG-4676, indicating the need for cells or a cellular product. S. mirum-infected AG-4676 cells exhibited vacuolization and granulation and an increase in polynucleation compared with uninfected controls (36/100 versus 14/100, P less than 0.001). Infection significantly decreased the growth rate of AG-4676, especially late in the growth cycle. In a representative experiment, growth of AG-4676 at 11 days was reduced from 9 X 10(5) to 2 X 10(4) cells by S. mirum infection. S. mirum grew to high titers in conditioned medium of AG-4676, obtained from cell-free supernatants of 1- to 5-day-old AG-4676 cultures. This growth promotion was not due to osmotic conditioning of the medium. Preliminary characterization of this growth promotion substance showed it to be active after 0.22-micron filtration, heating at 56 degrees C for 30 min, freezing and thawing, and dilution at 10(-1) but not 10(-2). AG-4676-propagated S. mirum produced death or cataracts in suckling Wistar rats at the same frequency (55/60, 91.7%) as SP-4-propagated organisms (60/65, 92.3%).
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bastardo J. W., Ou D., Bussell R. H. Biological and physical properties of the suckling mouse cataract agent grown in chicken embryos. Infect Immun. 1974 Feb;9(2):444–451. doi: 10.1128/iai.9.2.444-451.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CLARK H. F. SUCKLING MOUSE CATARACT AGENT. J Infect Dis. 1964 Dec;114:476–487. doi: 10.1093/infdis/114.5.476. [DOI] [PubMed] [Google Scholar]
- Clark H. F., Karzon D. T. Growth curve studies of the suckling mouse cataract agent in individual compartments of the eye. Proc Soc Exp Biol Med. 1969 Jul;131(3):693–696. doi: 10.3181/00379727-131-33954. [DOI] [PubMed] [Google Scholar]
- Fabiyi A., Elizan T. S., Pounds J. E. Suckling mouse cataract agent (SMCA) in tissue culture. Proc Soc Exp Biol Med. 1971 Jan;136(1):88–91. doi: 10.3181/00379727-136-35200. [DOI] [PubMed] [Google Scholar]
- Friedlaender R. P., Barile M. F., Kuwabara T., Clark H. F. Ocular pathology induced by the suckling mouse cataract agent. Invest Ophthalmol. 1976 Aug;15(8):640–647. [PubMed] [Google Scholar]
- Hayashi I., Larner J., Sato G. Hormonal growth control of cells in culture. In Vitro. 1978 Jan;14(1):23–30. doi: 10.1007/BF02618171. [DOI] [PubMed] [Google Scholar]
- Kirchhoff H., Heitmann J., Trautwein G. Pathogenicity of Spiroplasma sp. strain SMCA in rabbits: clinical, microbiological, and histological aspects. Infect Immun. 1981 Jul;33(1):292–296. doi: 10.1128/iai.33.1.292-296.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirchhoff H., Kuwabara T., Barile M. F. Pathogenicity of Spiroplasma sp. strain SMCA in Syrian hamsters: clinical, microbiological, and histological aspects. Infect Immun. 1981 Jan;31(1):445–452. doi: 10.1128/iai.31.1.445-452.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGarrity G. J., Sarama J., Vanaman V. Factors influencing microbiological assay of cell-culture mycoplasma. In Vitro. 1979 Feb;15(2):73–81. doi: 10.1007/BF02618100. [DOI] [PubMed] [Google Scholar]
- Reddan J. R., Friedman T. B., Mostafapour M. K., Bondy R. L., Sutherland S. H., McGee S. J., Goldenberg E. M. Donor age influences the growth of rabbit lens epithelial cells in vitro. Vision Res. 1981;21(1):11–23. doi: 10.1016/0042-6989(81)90131-0. [DOI] [PubMed] [Google Scholar]
- Steiner T., McGarrity G. J., Phillips D. M. Cultivation and partial characterization of spiroplasmas in cell cultures. Infect Immun. 1982 Jan;35(1):296–304. doi: 10.1128/iai.35.1.296-304.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tully J. G., Rose D. L., Yunker C. E., Cory J., Whitcomb R. F., Williamson D. L. Helical mycoplasmas (spiroplasmas) from Ixodes ticks. Science. 1981 May 29;212(4498):1043–1045. doi: 10.1126/science.7233197. [DOI] [PubMed] [Google Scholar]
- Tully J. G., Whitcomb R. F., Clark H. F., Williamson D. L. Pathogenic mycoplasmas: cultivation and vertebrate pathogenicity of a new spiroplasma. Science. 1977 Mar 4;195(4281):892–894. doi: 10.1126/science.841314. [DOI] [PubMed] [Google Scholar]
- Tully J. G., Whitcomb R. F., Williamson D. L., Clark H. F. Suckling mouse cataract agent is a helical wall-free prokaryote (spiroplasma) pathogenic for vertebrates. Nature. 1976 Jan 15;259(5539):117–120. doi: 10.1038/259117a0. [DOI] [PubMed] [Google Scholar]
- Yu R. L., Aronson M. M., Nichols W. W. High-resolution bands in human fibroblast chromosomes induced by actinomycin D. Cytogenet Cell Genet. 1981;31(2):111–114. doi: 10.1159/000131634. [DOI] [PubMed] [Google Scholar]