Abstract
Severe impairment of mucociliary transport (MCT) is a hallmark of cystic fibrosis (CF) lung disease. Recent studies demonstrate that pharmacologic inhibition of anion and liquid secretion in pig tracheas models the MCT defect in CF through depletion of the periciliary fluid component of airway surface liquid. In the present study, the effectiveness of various aqueous instillates on rehydration of the airway surface and restoration of MCT was assessed in this model. Excised porcine tracheas were mounted in a chamber that permitted simultaneous measurement of MCT and adventitial exposure of the airways to Krebs solution. When anion and liquid secretion were inhibited by treatment with bumetanide and dimethylamiloride, MCT was greatly reduced. Luminal instillation of aqueous solutions containing surface-active substances (1% Tween80 or calfactant) transiently restored MCT to high rates in nearly all tissues. Mucosal treatment with only Krebs solution or hypertonic saline restored MCT in only one half of the tracheas. We conclude that aqueous salt solutions alone can hydrate airway surfaces and restore MCT in some tissues, but surface-active substances may provide additional benefit in restoring MCT in this model of mucociliary stasis. We speculate that administration of surface-active substances, by aerosol or lavage, might help to restore MCT in the airways of patients with CF.
Keywords: airway surface liquid, cystic fibrosis, mucociliary transport, pigs, surfactants
Cystic fibrosis (CF) is one of the most prevalent lethal genetic diseases that afflict Caucasians (1). It is caused by mutations in the gene that codes for the cystic fibrosis transmembrane conductance regulator (CFTR), which normally functions as a cAMP-activated anion channel (2, 3). Although this disease disrupts exocrine functions of many organ systems, mortality in CF typically results from complications of the lung disease, which include production of viscid mucus, impaired mucociliary transport, chronic airway infections, and bronchiectasis. Progress in the treatment of CF lung disease in recent decades has arisen largely from improvements in antibiotic and physical therapeutic treatments. Unfortunately, current antimicrobial regimens are rarely adequate to eliminate these pathogenic bacteria, most commonly Pseudomonas aeruginosa, from the airways. Although the complex factors that predispose the airways to colonization by these microbiologic pathogens remain to be fully defined, severe impairments in cough and mucociliary clearance are thought to contribute greatly to the inability of CF airways to adequately expel these organisms from the lung.
CFTR, which is normally expressed in the apical membranes of surface ciliated epithelium and serous cells of the submucosal glands (4), is thought to serve as an important regulator of airway surface liquid volume. In normal airways, this anion channel potentially controls surface liquid volume in two ways: (1) by serving as a critical conduction pathway for Cl− and 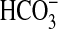 across the apical membrane to support liquid secretion across airway glandular and surface epithelia (5, 6) and (2) by attenuation of airway liquid absorption across the surface epithelium through inhibition of epithelial sodium channels in the apical membrane of these cells (7). Consequently, in CF airways, where CFTR is dysfunctional, these CFTR-dependent pathways are disrupted, leading to the depletion of periciliary fluid through hyperabsorption across the surface epithelium, as demonstrated by Matsui and coworkers (8), and/or hyposecretion from glands, as shown by Joo and colleagues (9). Recently, Trout and coworkers (10) showed that CF-like depletion of periciliary liquid could be duplicated in the isolated, perfused pig lung by using inhibitors of Cl− and
across the apical membrane to support liquid secretion across airway glandular and surface epithelia (5, 6) and (2) by attenuation of airway liquid absorption across the surface epithelium through inhibition of epithelial sodium channels in the apical membrane of these cells (7). Consequently, in CF airways, where CFTR is dysfunctional, these CFTR-dependent pathways are disrupted, leading to the depletion of periciliary fluid through hyperabsorption across the surface epithelium, as demonstrated by Matsui and coworkers (8), and/or hyposecretion from glands, as shown by Joo and colleagues (9). Recently, Trout and coworkers (10) showed that CF-like depletion of periciliary liquid could be duplicated in the isolated, perfused pig lung by using inhibitors of Cl− and 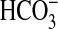 secretion to block airway fluid secretion. These same liquid secretion inhibitors inhibited mucociliary transport (11), a hallmark finding in CF airway disease. In the present study, we hypothesized that simple exposure of airway surfaces to aqueous solutions might replenish periciliary liquid and restore mucociliary transport in the fluid-depleted porcine trachea. We examined the effectiveness of physiologic salt solution and surfactant-supplemented instillates in the restoration of mucociliary transport function in this model.
secretion to block airway fluid secretion. These same liquid secretion inhibitors inhibited mucociliary transport (11), a hallmark finding in CF airway disease. In the present study, we hypothesized that simple exposure of airway surfaces to aqueous solutions might replenish periciliary liquid and restore mucociliary transport in the fluid-depleted porcine trachea. We examined the effectiveness of physiologic salt solution and surfactant-supplemented instillates in the restoration of mucociliary transport function in this model.
MATERIALS AND METHODS
Young pigs (∼ 10–15 kg) were procured from local vendors and treated twice daily for five consecutive days with an oral suspension of sulfamethoxazole and trimethoprim as a precaution against bacterial infection. After at least 3 d following completion of the antibacterial regimen, animals were sedated with intramuscular ketamine and xylazine and killed by lethal intravenous injection of pentobarbital. Full-length tracheas were rapidly excised, immersed in Krebs Ringer bicarbonate solution (KRB), and gradually warmed to 37°C. In most experiments, the tracheas were exposed to 100 μM bumetanide and 100 μM dimethylamiloride (DMA) for 45 min to inhibit Cl− and 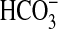 secretion, respectively. This pretreatment regimen has been shown to inhibit submucosal gland liquid secretion (5), deplete periciliary liquid volume (10), and impair mucociliary transport (11), thus modeling the anion and fluid secretion defect in CF. These inhibitors are not overtly toxic in that they have no direct effect on ciliary motility in porcine surface epithelial cells (11). The tracheas were removed from the KRB bath, and the trachealis muscle was resected along its entire length. The open ends of the tracheas were tied with suture onto cylindrical cannulae that were mounted in a rack that held the trachea in a static, horizontal position such that the slit formed by removal of the trachealis muscle was facing upward (see Figures E1 and E2 in the online supplement). The rack that was holding the trachea was placed in a polycarbonate box that was filled with warm KRB that also contained bumetanide and DMA. The level of KRB within the box was high enough to bathe most of the adventitial surface of the tracheas without spilling over into the mucosal lumen through the open slit. The KRB within the box was constantly bubbled with O2 containing 5% CO2 to maintain solution oxygenation and pH. The box was covered with a glass plate that permitted the ventral mucosal surface of the tracheas to be observed from above the box through the slit in the airways. The glass lid was warmed with adhesive heating strips to prevent water condensation on the inner surface of the lid. To maintain the box and its contents at 37°C, the box was weighted to the bottom of a heated water bath. The atmosphere within the box was at physiologic temperature and close to water saturation.
secretion, respectively. This pretreatment regimen has been shown to inhibit submucosal gland liquid secretion (5), deplete periciliary liquid volume (10), and impair mucociliary transport (11), thus modeling the anion and fluid secretion defect in CF. These inhibitors are not overtly toxic in that they have no direct effect on ciliary motility in porcine surface epithelial cells (11). The tracheas were removed from the KRB bath, and the trachealis muscle was resected along its entire length. The open ends of the tracheas were tied with suture onto cylindrical cannulae that were mounted in a rack that held the trachea in a static, horizontal position such that the slit formed by removal of the trachealis muscle was facing upward (see Figures E1 and E2 in the online supplement). The rack that was holding the trachea was placed in a polycarbonate box that was filled with warm KRB that also contained bumetanide and DMA. The level of KRB within the box was high enough to bathe most of the adventitial surface of the tracheas without spilling over into the mucosal lumen through the open slit. The KRB within the box was constantly bubbled with O2 containing 5% CO2 to maintain solution oxygenation and pH. The box was covered with a glass plate that permitted the ventral mucosal surface of the tracheas to be observed from above the box through the slit in the airways. The glass lid was warmed with adhesive heating strips to prevent water condensation on the inner surface of the lid. To maintain the box and its contents at 37°C, the box was weighted to the bottom of a heated water bath. The atmosphere within the box was at physiologic temperature and close to water saturation.
The tracheas were allowed to stabilize in this configuration for 45 min. During this stabilization period, each trachea was closely observed for evidence of accumulation of luminal mucus liquid at the cranial end and gradual drying of the mucosal surface. We deemed these characteristics to be evidence that the tissue was capable of mucociliary transport. Airways that did not exhibit these characteristics were omitted from the study. Then, 100 μM acetylcholine was added to the bath to induce mucus secretion from submucosal glands. When glandular liquid secretion is blocked with inhibitors of Cl− and 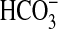 secretion, acetylcholine induces secretion of a low-volume, thick mucus (12). After another 45-min stabilization period, measurement of mucociliary transport was begun. A few small flakes of dried India ink were sprinkled on the mucosal surface at the caudal end of the trachea. A millimeter scale was placed next to the tracheas within the box to provide an index for particle movement. A video camera, located above the box, recorded the movements of the ink flakes with a video tape recorder as these particles were swept in the cranial direction by mucociliary transport. Mucociliary transport was measured in six consecutive 30-min periods. The first three periods established baseline rates of mucociliary transport. Then, the mucosal lumen of the tracheas was slowly filled with one of four aqueous solutions. When instilling these solutions, care was taken to minimally disrupt the mucus layer on the mucosal surface. Once the airway lumen was filled, the solutions were immediately drained as completely as possible. The effects of the instillates on mucociliary transport were assessed in three additional 30-min periods. The effects of four different aqueous instillates were evaluated: normal KRB, hypertonic saline (300 mM NaCl), 1% Tween80 in KRB, and calfactant. Tween80 is a polysorbate nonionic surfactant that is commonly used as an emulsifying food additive. Calfactant (Infasurf) is a natural surfactant product obtained from calf lung lavage that contains endogenous surfactant phospholipids and surfactant-associated proteins (SP-B, SP-C, and SP-D) in buffered saline. It is used in the treatment of neonatal respiratory distress syndrome (13). A graphic summary of the basic protocol is shown in supplement E3.
secretion, acetylcholine induces secretion of a low-volume, thick mucus (12). After another 45-min stabilization period, measurement of mucociliary transport was begun. A few small flakes of dried India ink were sprinkled on the mucosal surface at the caudal end of the trachea. A millimeter scale was placed next to the tracheas within the box to provide an index for particle movement. A video camera, located above the box, recorded the movements of the ink flakes with a video tape recorder as these particles were swept in the cranial direction by mucociliary transport. Mucociliary transport was measured in six consecutive 30-min periods. The first three periods established baseline rates of mucociliary transport. Then, the mucosal lumen of the tracheas was slowly filled with one of four aqueous solutions. When instilling these solutions, care was taken to minimally disrupt the mucus layer on the mucosal surface. Once the airway lumen was filled, the solutions were immediately drained as completely as possible. The effects of the instillates on mucociliary transport were assessed in three additional 30-min periods. The effects of four different aqueous instillates were evaluated: normal KRB, hypertonic saline (300 mM NaCl), 1% Tween80 in KRB, and calfactant. Tween80 is a polysorbate nonionic surfactant that is commonly used as an emulsifying food additive. Calfactant (Infasurf) is a natural surfactant product obtained from calf lung lavage that contains endogenous surfactant phospholipids and surfactant-associated proteins (SP-B, SP-C, and SP-D) in buffered saline. It is used in the treatment of neonatal respiratory distress syndrome (13). A graphic summary of the basic protocol is shown in supplement E3.
KRB contained 112.0 mM NaCl, 4.7 mM KCl, 2.5 mM CaCl2, 2.4 mM MgSO4, 1.2 mM KH2PO4, 25.0 mM NaHCO3, and 11.6 mM glucose. The pH of the KRB was maintained at 7.4 by constant gassing with 95% O2-5% CO2. Stock solutions of bumetanide and DMA were prepared with DMSO. Calfactant (Infasurf) was purchased from Forest Pharmaceuticals, Inc. (St. Louis, MO). All other drugs and chemicals were purchased from Sigma Chemical Co. (St. Louis, MO).
The effect of the instillates on mucociliary transport was evaluated by Student's paired t test. P < 0.050 was considered significant. Aggregate data are reported as means ± SEM.
RESULTS
In the presence of acetylcholine only, mucociliary transport rates over the 3-h protocol period were variable but generally fell in the range previously reported for porcine tracheas of ∼ 40 cm/h (11) (Figure 1A). However, when tracheas were first treated with bumetanide and DMA, to inhibit liquid secretion by respectively blocking Cl− and 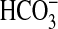 secretion, mucociliary transport was reduced to very low rates as previously demonstrated (Figure 1B) (11). These results indicate that the mucociliary stasis induced by these inhibitors persists for the duration of the 3-h protocol.
secretion, mucociliary transport was reduced to very low rates as previously demonstrated (Figure 1B) (11). These results indicate that the mucociliary stasis induced by these inhibitors persists for the duration of the 3-h protocol.
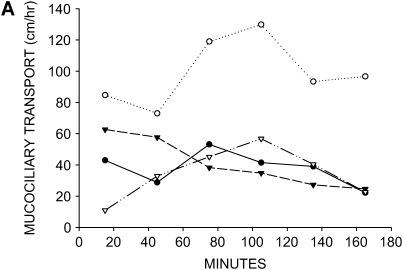
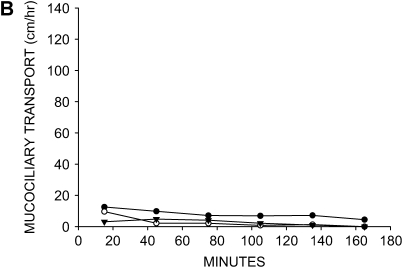
Figure 1.
Effect of anion and liquid-secretion inhibitors on mucociliary transport. (A) Mucociliary transport rates in four tracheas that were treated with 100 μM acetylcholine only (no luminal exposure, no inhibitors). (B) Mucociliary transport rates in three tracheas that were pretreated with 100 μM bumetanide and 100 μM DMA to inhibit anion and liquid secretion before addition of 100 μM acetylcholine (no luminal exposure). Mucociliary transport is essentially abolished by treatment with these inhibitors.
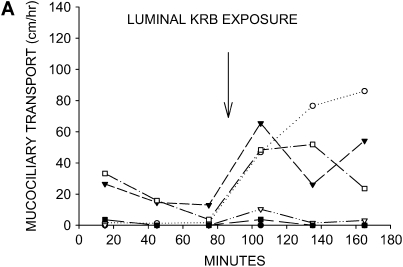
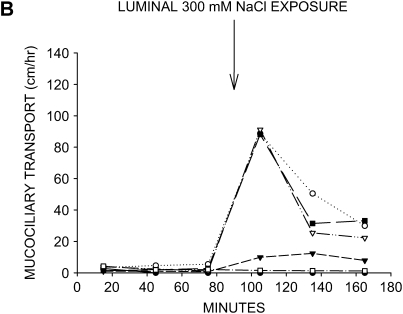
When the tracheal lumens were briefly exposed to KRB, mucociliary transport was restored to substantial rates in three of the six tracheas (Figure 2A). This finding indicates that isotonic physiologic salt solution is capable of reversing the impairment of mucociliary transport in this model, but only 50% of the tissues respond to this maneuver. Hypertonic saline, which potentially augments mucociliary transport by altering the salt content of the mucus and/or creating an osmotic force for drawing water across the airway surface epithelium, has been suggested as a therapeutic treatment for restoring mucociliary transport in patients with CF (14). When 300 mM NaCl solution was used as the tracheal instillate, the response was similar to KRB, with mucociliary transport being restored in three of the six tissues (Figure 2B). The increase in mucociliary transport seen with hypertonic saline was also accompanied by a noticeable fall in transport rate by the end of the experimental period.
Figure 2.
Effects of aqueous salt solutions on mucociliary transport in liquid-depleted tracheas. All tracheas were treated with bumetanide and DMA to inhibit anion and liquid secretion. (A) Mucociliary transport rates in six tracheas exposed to KRB (physiologic salt solution). (B) Mucociliary transport rates in six tracheas exposed to hypertonic saline (300 mM NaCl). Substantial rates of mucociliary transport were achieved in approximately one half of the airways with both instillates.
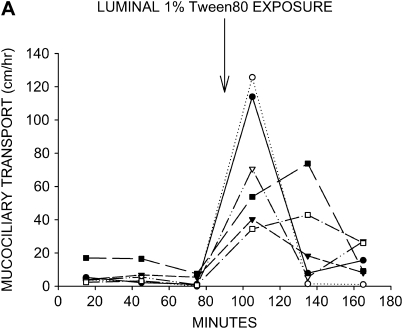
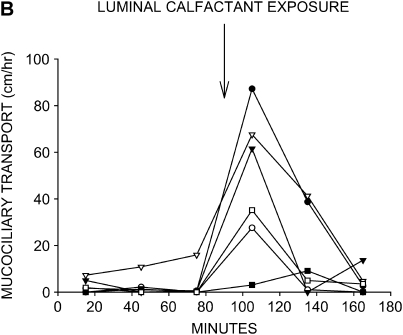
Surface-active substances have been shown to have a stimulatory effect on mucociliary transport in model systems (15) and to improve pulmonary function in a limited clinical trial of chronic bronchitics (16). When we used a 1% Tween80 solution as the instillate, mucociliary transport was restored to high rates in all six tissues (Figure 3A). Calfactant also stimulated mucociliary transport to high rates in five of the six tracheas studied (Figure 3B). As seen with hypertonic saline, the instillate-induced increases in mucociliary transport rate fell to near baseline inhibitor rates by the end of the protocol in both Tween80- and calfactant-treated tracheas.
Figure 3.
Effects of surface-active substances on mucociliary transport in liquid-depleted tracheas. All tracheas were treated with bumetanide and DMA to inhibit anion and liquid secretion. (A) Mucociliary transport rates in seven tracheas exposed to 1% Tween80 in KRB. (B) Mucociliary transport rates in six tracheas exposed to calfactant bovine surfactant. Mucociliary transport rates substantially increased in nearly all tracheas treated with either of these surface-active substances.
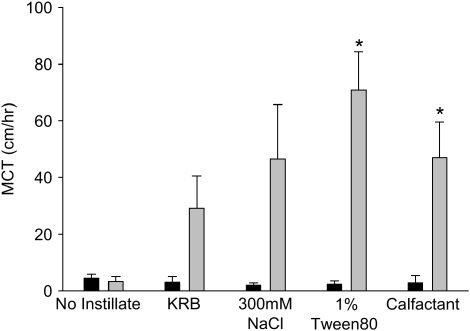
A summary of the peak effects of instillate treatment is shown in Figure 4. This figure contrasts the rates of mucociliary transport in the periods immediately before and after instillation of the various solutions. Even though KRB and hypertonic saline substantially increased mucociliary transport in approximately one-half of the airways, these effects were statistically insignificant (P = 0.050 and P = 0.063, respectively), albeit by narrow margins. In contrast, 1% Tween80 and calfactant increased mucociliary transport significantly (P = 0.003 and P = 0.013, respectively).
Figure 4.
Peak mucociliary transport responses to airway instillates. Mucociliary transport rates for the periods immediately before and after application of the instillates are shown for each of the treatments. Tracheas that received no instillate are shown for comparison. Asterisks indicate that mucociliary transport rates were significantly greater (P < 0.05) in the period after solution instillation.
DISCUSSION
Surface-active substances have been previously shown to stimulate mucociliary transport in in vitro and in vivo systems. In the frog palate model, mucociliary transport of airway sputum was significantly increased when the surface of the palate was sprayed with physiologic surfactant compared with a saline spray (17). Intrabronchial administration of Curosurf (a porcine lung isolate containing alveolar phospholipids and apoproteins including SP-B and SP-C but not SP-A) in healthy anesthetized dogs increased mucociliary transport by ∼ 5-fold compared with saline administration (15). Consistent with these findings, we observed in the present study that two different surface-active substances, Tween80 and calfactant, induced substantial increases in mucociliary transport in our in vitro model of the fluid-depleted porcine trachea. Although we observed that isotonic and hypertonic saline were capable of restoring mucociliary transport in some tissues, it seemed that application of surface-active substances provided additional benefit. Because Tween80 was at least the equal of calfactant at restoring mucociliary transport, the beneficial effects could not have been due to the presence of surfactant-associated proteins, which were present only in calfactant.
In our model of the fluid-depleted pig trachea, mucociliary transport is impaired by depletion of the periciliary “sol” fluid layer, which normally surrounds the cilia, providing a low-viscosity medium through which the cilia can freely move (10, 11). The depletion of this layer is caused by the pharmacologic inhibition of Cl− and 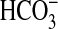 secretion, which blocks secretion of liquid by the submucosal glands (11) and perhaps by the surface epithelium. When liquid secretion is inhibited, the periciliary fluid is likely to be quickly absorbed by ongoing Na+-dependent absorptive processes. Because a similar impairment of anion and fluid secretion is likely to occur in CF, we speculate that this preparation serves as a useful model of this pathologic consequence of CF disease. Transmission electron micrographs of the airway surface of tissues treated with these inhibitors show that a thin layer of dense mucus compresses the cilia between the mucus layer and the apices of the epithelial cells (10) resembling that seen in scanning electron micrographs of CF airways (18).
secretion, which blocks secretion of liquid by the submucosal glands (11) and perhaps by the surface epithelium. When liquid secretion is inhibited, the periciliary fluid is likely to be quickly absorbed by ongoing Na+-dependent absorptive processes. Because a similar impairment of anion and fluid secretion is likely to occur in CF, we speculate that this preparation serves as a useful model of this pathologic consequence of CF disease. Transmission electron micrographs of the airway surface of tissues treated with these inhibitors show that a thin layer of dense mucus compresses the cilia between the mucus layer and the apices of the epithelial cells (10) resembling that seen in scanning electron micrographs of CF airways (18).
We reasoned that simple addition of physiologic salt solution to the airway mucosa might replenish this periciliary liquid and thus restore mucociliary transport. Our results indicate that restoration of mucociliary transport occurs in some tissues after such treatment. We found that the addition of hypertonic saline solution to the lumen also proved to be effective in some tissues. Hypertonic saline treatment of CF sputum in vitro has been shown to reduce its viscosity and elasticity and increase its transportability when placed on bovine tracheas (14). Inhaled aerosols of hypertonic saline have also been shown to increase mucociliary clearance from the lungs of patients with CF (19). It was not apparent in our study, however, that hypertonic saline provided additional benefit to instillation of isotonic physiologic salt solution.
It is unclear how surfactants aid in the restoration of mucociliary transport in the present study. In our model, the mucus gel layer seems to become adherent to the surface epithelial cells (10). Breaking these mucus plaques free of the epithelial surface may be the critical step in the recovery of mucociliary transport activity. The adhesivity property of mucus, which is assessed by measuring the contact angle of a mucus droplet on a surface, has been shown to be increased in CF mucus (20). Surfactant application reduces the adhesivity of CF mucus (21); thus, these surface-active substances may play the important role of reducing mucus adhesion to the epithelial surface and may allow greater access of free liquid to the periciliary space. Surfactants are also capable of altering the viscoelastic properties of mucus in ways that favor stimulation of mucociliary transport. Martin and coworkers (22) showed that sodium deoxycholate, sodium taurodeoxycholate, sodium glycocholate, and lysophosphatidylcholine decreased the viscosity and elasticity of bronchial mucus and suggested that this effect was due to structural breakdown of the mucins. However, De Sanctis and associates (15) demonstrated that in vivo administration of Curosurf to normal dog airways substantially increased mucociliary transport without significantly altering the viscoelastic properties of airway mucus. Surfactants may aid in solubilizing lipids or reducing the hydrophobicity of nonpolar domains in airway mucus. This may be of particular benefit to patients with CF because the airway mucus in CF has a higher lipid content than normal mucus (23), accounting for nearly 40% of its dry weight (24). This higher lipid content of CF mucus seems to be related to degree of infection and is sufficient to significantly increase the viscosity of this material (25). An additional benefit is that surfactants have been shown to have a stimulatory effect on ciliary beat frequency (15, 26), an effect that could partially account for the stimulation of mucociliary transport with these substances. We cannot completely discount the possibility that the observed stimulation of mucociliary transport by the instillates was a toxic response. We believe that this is unlikely with KRB, which is a buffered physiologic salt solution, or calfactant, which is an isolate of bovine lung surfactant. Hypertonic saline could induce osmotic cell shrinkage of the mucosal epithelium, but this effect should be transient and well tolerated. Of the four instillate solutions, Tween80 is the most likely to have toxicity issues because it is a nonionic detergent. However, hypertonic saline and Tween80 induced nearly identical responses to KRB and calfactant, respectively; these treatments have minimal expectations for toxicity.
Severe impairment of mucociliary transport is considered by many to be a hallmark of CF lung disease, yet literature reports of clearance rates in patients with CF are widely variable, with some studies showing similar and even increased clearance relative to normal subjects (27). Differences in experimental design and methodology, such as patient breathing patterns, particle deposition patterns, tracers, cough, posture, time-frame for clearance, and whole lung versus regional clearance, contribute to this variability. A recent retrospective study of patients with CF whose airway function ranged from normal to severe obstruction carefully controlled for many of these variables and demonstrated that a consistent reduction in clearance of ∼ 50% was evident in CF whole, central, and intermediate lung regions (28). Clearance in this study seemed to be independent of disease severity (28). Similar magnitude reductions of mucociliary transport are seen in CF nasal passages (29), which are less likely to be influenced by obstructive disease and asymmetric tracer distribution than the intrapulmonary airways. These studies suggest that a defect in mucociliary clearance exists in CF airways even though baseline levels of transport seem to be higher (4.5–5.5 mm/min for the nose) than we report in our volume-depleted trachea model (0.3–0.5 mm/min). Thus, our model of mucociliary stasis may be more severe than the defect in CF airways in vivo. This could be explained if (1) the anion secretion inhibitors used in the present study block significant quantities of CFTR-independent anion and liquid secretion or (2) the inflammatory milieu of the CF airway induces passive fluid leakage into the airway lumen sufficient to support a minimal level of mucociliary transport. More study is needed to resolve this issue.
In the present study, we observed that the high rates of mucociliary transport achieved with surface-active substances were short lived. Within the 90-min period of exposure, mucociliary transport rates in most tissues returned to the low rates of the initial control periods. We expect that this occurred because the high rates of stimulated transport rapidly swept the replenished airway liquid to the cranial end of the trachea. Because the secretion inhibitors were present in the bath, the tracheas quickly returned to their initial state of periciliary fluid depletion. This observation raises an important point—The beneficial effects of surfactants on restoring mucociliary transport in a disease such as CF depends upon delivery of liquid volume to the airway surface. This would not be a problem with a short-term interventional procedure, such as bronchoalveolar lavage, where large volumes of liquid are instilled into the lung. However, accomplishing this task in ambulatory patients would be much more difficult. Because the capability of CF airways to secrete liquid by normal physiologic means is likely to be pathologically limited, the problem remains as to how quantities of liquid adequate to support mucociliary transport can be safely delivered to airway surfaces over long periods of time. A successful solution to this problem will hopefully arise from numerous ongoing studies investigating the use of isosmotic and hyperosmotic aerosols, inhibitors of Na+ and liquid absorption, and stimulators of CFTR-independent pathways for airway anion and liquid secretion.
Supplementary Material
Acknowledgments
The authors acknowledge the excellent technical assistance of Mita Patel, Charlyn Partridge, and Jennifer Garrison.
This work was supported by NIH grant HL-63302.
This work was previously published in abstract form (30).
This article has an online supplement, which is accessible from this issue's table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1165/rcmb.2005-0214OC on December 15, 2005
Conflict of Interest Statement: S.T.B. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. J.C.P. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. C.R.H. received $177,000 in research grants from 1992–1998 from Ony, Inc. for participation in multi-center trials.
References
- 1.Colten HR. Cystic fibrosis. In: Wilson JD, Braunwald E, Isselbacher KJ, Petersdorf RG, Martin JB, Fauci AS, Root RK, editors. Harrison's principles of internal medicine, 12th ed. New York: McGraw-Hill; 1991. pp. 1072–1074.
- 2.Riordan JR, Rommens JM, Kerem B-S, Alon N, Rozmahel R, Grzelczak Z, Zielenski J, Lok S, Plavsic N, Chou J-L, et al. Identification of the cystic fibrosis gene: cloning and characterization of complementary DNA. Science 1989;245:1066–1072. [DOI] [PubMed] [Google Scholar]
- 3.Anderson MP, Gregory RJ, Thompson S, Sousa DW, Paul S, Mulligan RC, Smith AE, Welsh MJ. Demonstration that CFTR is a chloride channel by alteration of its anion selectivity. Science 1991;253:202–205. [DOI] [PubMed] [Google Scholar]
- 4.Engelhardt JF, Yankaskas JR, Ernst ST, Yang Y, Marino CR, Boucher RC, Cohn JA, Wilson JM. Submucosal glands are the predominant site of CFTR expression in the human bronchus. Nat Genet 1992;2:240–248. [DOI] [PubMed] [Google Scholar]
- 5.Ballard ST, Trout L, Bebök Z, Sorscher EJ, Crews A. CFTR involvement in chloride, bicarbonate and liquid secretion by airway submucosal glands. Am J Physiol 1999;277:L694–L699. [DOI] [PubMed] [Google Scholar]
- 6.Tarran R, Grubb BR, Gatzy JT, Davis CW, Boucher RC. The relative roles of passive surface forces and active ion transport in the modulation of airway surface liquid volume and composition. J Gen Physiol 2001;118:223–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stutts MJ, Canessa CM, Olsen JC, Hamrick M, Cohn JA, Rossier BC, Boucher RC. CFTR as a cAMP-dependent regulator of sodium channels. Science 1995;269:847–850. [DOI] [PubMed] [Google Scholar]
- 8.Matsui H, Grubb BR, Tarran R, Randell SH, Gatzy JT, Davis CW, Boucher RC. Evidence for periciliary liquid layer depletion, not abnormal ion composition, in the pathogenesis of cystic fibrosis airways disease. Cell 1998;95:1005–1015. [DOI] [PubMed] [Google Scholar]
- 9.Joo NS, Irokawa T, Wu JV, Robbins RC, Whyte RI, Wine JJ. Absent secretion to vasoactive intestinal peptide in cystic fibrosis airway glands. J Biol Chem 2002;277:50710–50715. [DOI] [PubMed] [Google Scholar]
- 10.Trout L, Townsley MI, Bowden A, Ballard ST. Disruptive effects of anion secretion inhibitors on airway mucus morphology in isolated perfused pig lungs. J Physiol 2003;549:845–853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ballard ST, Trout L, Mehta A, Inglis SK. Liquid secretion inhibitors reduce mucociliary transport in glandular airways. Am J Physiol 2002; 283:L329–L335. [DOI] [PubMed] [Google Scholar]
- 12.Trout L, King M, Feng W, Inglis SK, Ballard ST. Inhibition of airway liquid secretion and its effects on the physical properties of airway mucus. Am J Physiol 1998;274:L258–L263. [DOI] [PubMed] [Google Scholar]
- 13.Onrust SV, Dooley M, Goa KL. Calfactant: a review of its use in neonatal respiratory distress syndrome. Paediatr Drugs 1999;1:219–243. [DOI] [PubMed] [Google Scholar]
- 14.Wills PJ, Hall RL, Chan W-M, Cole PJ. Sodium chloride increases the ciliary transportability of cystic fibrosis and bronchiectasis sputum on the mucus-depleted bovine trachea. J Clin Invest 1997;99:9–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.De Sanctis GT, Tomkiewicz RP, Rubin BK, Schück S, King M. Exogenous surfactant enhances mucociliary clearance in the anesthetized dog. Eur Respir J 1994;7:1616–1621. [DOI] [PubMed] [Google Scholar]
- 16.Anzueto A, Jubran A, Ohar JA, Piquette CA, Rennard SI, Colice G, Pattishall EN, Barrett J, Engle M, Perret KA, et al. Effects of aerosolized surfactant in patients with stable chronic bronchitis. JAMA 1997;278:1426–1431. [PubMed] [Google Scholar]
- 17.Allegra L, Bossi R, Braga P. Influence of surfactant on mucociliary transport. Eur J Respir Dis 1985;142(Suppl):71–76. [PubMed] [Google Scholar]
- 18.Simel DL, Mastin JP, Pratt PC, Wisseman CL, Shelburne JD, Spock A, Ingram P. Scanning electron microscopic study of the airways in normal children and in patients with cystic fibrosis and other lung diseases. Pediatr Pathol 1984;2:47–64. [DOI] [PubMed] [Google Scholar]
- 19.Robinson M, Hemming AL, Regnis JA, Wong AG, Bailey DL, Bautovich GJ, King M, Bye PT. Effect of increasing doses of hypertonic saline on mucociliary clearance in patients with cystic fibrosis. Thorax 1997;52:900–903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Girod de Bentzmann S, Pierrot D, Fuchey C, Zahm J-M, Morançais J-L, Puchelle E. Distearoyl phosphatidylglycerol liposomes improve surface and transport properties of CF mucus. Eur Respir J 1993;6:1156–1161. [PubMed] [Google Scholar]
- 21.Chung C, Van Hoof L, Policova Z, Beharry S, Sherman PM, Neumann AW, Durie P. Surface hydrophobicity is increased in the ileum and proximal colon of cystic fibrosis mice. Pediatr Res 1999;46:174–178. [DOI] [PubMed] [Google Scholar]
- 22.Martin GP, Marriot C, Kellaway IW. Direct effect of bile salts and phospholipids on the physical properties of mucus. Gut 1978;19:103–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Slomiany A, Murty VLN, Aono M, Snyder CE, Herp A, Slomiany BL. Lipid composition of tracheaobronchial secretions from normal individuals and patients with cystic fibrosis. Biochim Biophys Acta 1982;710:106–111. [DOI] [PubMed] [Google Scholar]
- 24.Sahu S, Lynn WS. Lipid composition of airway secretions from patients with asthma and patients with cystic fibrosis. Am Rev Respir Dis 1977; 115:233–239. [DOI] [PubMed] [Google Scholar]
- 25.Galabert C, Jacquot J, Zahm JM, Puchelle E. Relationships between the lipid content and the rheological properties of airway secretions in cystic fibrosis. Clin Chim Acta 1987;164:139–149. [DOI] [PubMed] [Google Scholar]
- 26.Kakuta Y, Sasaki H, Takishima T. Effect of artificial surfactant on ciliary beat frequency in guinea pig trachea. Respir Physiol 1991;83:313–322. [DOI] [PubMed] [Google Scholar]
- 27.Robinson M, Bye PTB. Mucociliary clearance in cystic fibrosis. Pediatr Pulmonol 2002;33:293–306. [DOI] [PubMed] [Google Scholar]
- 28.Robinson M, Eberl S, Tomlinson C, Daviskas E, Regnis JA, Bailey DL, Torzillo PJ, Menache M, Bye PT. Regional mucociliary clearance in patients with cystic fibrosis. J Aerosol Med 2000;13:73–86. [DOI] [PubMed] [Google Scholar]
- 29.Armengot M, Escribano A, Carda C, Sanchez C, Romero C, Basterra J. Nasal mucociliary transport and ciliary ultrastructure in cystic fibrosis: a comparative study with healthy volunteers. Int J Pediatr Otorhinolaryngol 1997;40:27–34. [DOI] [PubMed] [Google Scholar]
- 30.Ballard ST, Hamm CR, Partridge C. Effects of surface active substances on restoration of mucociliary transport in fluid-depleted porcine tracheas. Pediatr Pulmonol 2003;209–210.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.