Abstract
Background & Aims
Recent studies of C. difficile infection (CDI) indicate a dramatic increase in metronidazole failure. The aims of this study were to compare current and historical rates of metronidazole failure and identify risk factors for metronidazole failure.
Methods
89 patients with CDI in 2004–2006 were followed for 60 days. Data were compared to a historical cohort of 63 CDI patients studied prospectively in 1998. Metronidazole failure was defined as persistent diarrhea after 10 days of therapy or a change of therapy to vancomycin. Stool samples were analyzed for the presence of NAP-1 strain.
Results
Metronidazole failure rates were 35% in both the 1998 and 2004–2006 cohorts. There was no difference in the median time to resolution of diarrhea (8 vs. 5 days, p = 0.52) or the proportion with more than 10 days of diarrhea (35% vs. 29%, p = 0.51). Risk factors for metronidazole failure included recent cephalosporin use (OR 32, 95% CI 5–219), CDI on admission (OR 23, 95% CI 3–156), and transfer from another hospital (OR 11, 95% CI 2–72). The frequency of NAP-1 infection in patients with and without metronidazole failure was similar (26% vs. 21%, p = 0.67).
Conclusions
We found no difference in metronidazole failure rates in 1998 and 2004–2006. Patients with recent cephalosporin use, CDI on admission, and transfer from another hospital were more likely to fail metronidazole and may benefit from early aggressive therapy. Infection with the epidemic NAP-1 strain was not associated with metronidazole failure in endemic CDI.
Keywords: anaerobic bacterium, antibiotic, colitis, hospital-acquired infection, infection control, infectious diarrhea, megacolon, nosocomial infection, pseudomembranous colitis, spore, toxic megacolon, toxin
Introduction
Clostridium difficile is the leading known cause of nosocomial infectious diarrhea in the developed world and is associated with substantial morbidity and mortality 1. Since the first description of antibiotic-associated pseudomembranous colitis in 1978, the incidence of C. difficile infection (CDI) has increased substantially and now afflicts approximately 7 per 1,000 hospitalized patients (range 0.1–30 per 1,000) in non-epidemic settings 1. Recent outbreaks of CDI in North America and Europe have been characterized by more severe and refractory disease 2–5. A unique and virulent strain of C. difficile, designated North American pulsed-field gel electrophoresis type-1 (NAP-1), has been implicated in these outbreaks 2, 3. This epidemic NAP-1 strain is characterized by fluoroquinolone resistance, binary toxin production, mutation of the tcdC gene, and increased in vitro toxin production 2, 3, 6.
Guidelines for CDI therapy recommend oral metronidazole as the first-line agent 7. Oral vancomycin is reserved for patients who have severe or fulminant disease, failure of metronidazole treatment, or other contraindications or intolerances 7. Studies published between 1978 and 1996 reported average failure rates of 2% for both metronidazole (range 0–6%) and vancomycin (range 0–14%) 8, 9. However, studies published between 2004 and 2007 report an average failure rate of 19% for metronidazole (range 7–38%) compared to only 4% for vancomycin (range 3–6%) 4, 5, 8, 10–12. A randomized controlled trial showed that metronidazole was inferior to vancomycin in severe CDI but equivalent in mild disease 12. Despite these indications of deterioration in response to metronidazole therapy, no study has directly compared metronidazole failure rates in the pre- and post-NAP-1 epidemic eras or explored the role of NAP-1 in metronidazole failure in endemic nosocomial CDI. Only two previous studies purposely examined risk factors associated with metronidazole failure in CDI and found low albumin, intensive care unit (ICU) stay, and continued antibiotic use during CDI treatment as independent predictors 10, 11. However, these studies were retrospective in nature or small in size.
To address these questions, we conducted a prospective study of hospitalized patients with CDI at our institution and compared the data to a previously studied prospective cohort in the pre-NAP-1 epidemic era. This analysis allowed us to compare metronidazole failure rates before and after NAP-1 became prevalent, identify risk factors associated with metronidazole failure, and examine the role of NAP-1 in metronidazole failure.
Materials & Methods (additional methods described in the supplement)
2004–2006 CDI Cohort and Definitions
All adult patients with CDI hospitalized at Beth Israel Deaconess Medical Center, Boston, Massachusetts between December 2004 and May 2006 were eligible for study entry. Diarrhea was defined as a change in bowel habit with 3 or more unformed bowel movements a day for at least 2 days. CDI was defined as diarrhea coupled with a positive stool C. difficile toxin assay and not attributed to any other cause. Failure of metronidazole therapy was defined as persistence of diarrhea for more than 10 days after starting metronidazole or a clinical decision by the subject’s physician to start vancomycin prior to the 10-day endpoint.
Data Collection
Case identification and subject data collection are described in details in the supplement 4, 5, 10–15. All patients were assessed within one day of a positive stool C. difficile toxin assay. Baseline patient characteristics and clinical information were recorded. Resolution of CDI was monitored on a daily basis until discharge. Patients were followed for a total of 60 days.
C. difficile Culture and NAP-1 Strain Typing
Stool samples were collected from a subset of patients enrolled between December 2004 and December 2005 for NAP-1 strain typing. C. difficile culture and strain typing are described in details in the supplement 2, 16, 17.
1998 CDI Cohort
In a previous investigation, hospitalized patients with CDI between January and May 1998 were studied prospectively under a protocol almost identical to that used in the current study with the main exception that NAP-1 strain typing was not performed. Results on antibody responses to C. difficile toxins, mortality, health care costs, and risk factors for CDI in this historical cohort have been published 14, 15, 18, 19.
Statistical Analyses
Statistical methods are described in details in the supplement 5, 10, 12, 14, 15. Patients in the 2004–2006 cohort were analyzed for risk factors associated with metronidazole failure. The association between metronidazole failure and potential predictors was examined using simple logistic regression analysis, from which a multivariable logistic regression model with stepwise selection was built. All statistical analyses were performed using the SAS software system, version 9.1 (SAS Institute Inc., Carey, NC, U.S.A.).
Results
Baseline Patient Characteristics
We prospectively identified 276 patients with CDI between December 2004 and May 2006, of whom 89 (32%) were enrolled. Among the 187 patients not enrolled, 34 were discharged or died before the initial assessment, 33 were incompetent and had no available health care proxies, and 120 declined to participate for various reasons including 49 that were critically ill. Conversely, the enrollment rate was 100% in the historical cohort where all 63 patients with known CDI between January and May 1998 were studied 15.
The baseline characteristics of patients in the 2004–2006 and 1998 cohorts are summarized in Table 1. When compared to the 1998 cohort, the 2004–2006 cohort contained fewer patients 85 years or older (30% vs. 16%, p = 0.03), admitted from nursing homes and rehabilitation centers (35% vs. 22%, p = 0.09), or with serious underlying illnesses (Horn’s index severe 40% vs. 25%, p = 0.05; fulminant 22% vs. 1%, p < 0.0001). Conversely, more patients in the 2004–2006 cohort were immunosuppressed (19% vs. 42%, p = 0.003).
Table 1.
Baseline Characteristics of Patients in the 1998 and 2004–2006 Cohorts
| Variable | 1998 cohort (n = 63) | 2004–2006 cohort (n = 89) | P value |
|---|---|---|---|
| Age (year), median (range) | 74 (19–97) | 70 (22–94) | 0.32 |
| ≥65, n (%) | 41 (65%) | 56 (63%) | 0.79 |
| ≥85, n (%) | 19 (30%) | 14 (16%) | 0.03 |
|
| |||
| Male sex, n (%) | 29 (46%) | 44 (49%) | 0.68 |
|
| |||
| White race, n (%) | 53 (85%) (n = 62) | 73 (82%) | 0.57 |
| Residence before admission, n (%) | |||
| Home | 33 (52%) | 53 (60%) | 0.38 |
| Nursing home or rehabilitation center | 22 (35%) | 20 (22%) | 0.09 |
| Other hospital | 8 (13%) | 16 (18%) | 0.38 |
|
| |||
| Prior CDI, n (%) | 4 (6%) | 15 (17%) | 0.05 |
|
| |||
| Number of recently used antibiotics, median (range) | 2 (1–7) | 3 (0–9) | 0.04 |
| Clindamycin, n (%) | 13 (21%) | 9 (10%) | 0.07 |
| Metronidazole, n (%) | 8 (13%) | 15 (17%) | 0.48 |
| Penicillin, n (%) | 16 (25%) | 17 (19%) | 0.35 |
| Cephalosporin, n (%) | 42 (67%) | 35 (39%) | 0.0009 |
| Fluoroquinolone, n (%) | 16 (25%) | 47 (53%) | 0.0007 |
| Aminoglycoside, n (%) | 21 (33%) | 4 (4%) | <.0001 |
| Sulfonomide, n (%) | 7 (11%) | 12 (13%) | 0.66 |
|
| |||
| Acid anti-secretory medication, n (%) | 40 (63%) | 70 (79%) | 0.04 |
| H-2RA | 24 (38%) | 11 (12%) | 0.0002 |
| PPI | 16 (25%) | 66 (74%) | <.0001 |
|
| |||
| TPN, n (%) | 7 (11%) | 5 (6%) | 0.24 |
|
| |||
| Tube feeding, n (%) | 13 (21%) | 7 (8%) | 0.02 |
|
| |||
| Charlson score, median (range) | 4 (0–13) | 3 (0–11) | 0.98 |
|
| |||
| Horn’s index, n (%) | |||
| Mild | 8 (13%) | 2 (2%) | 0.02 |
| Moderate | 16 (25%) | 64 (72%) | <.0001 |
| Severe | 25 (40%) | 22 (25%) | 0.05 |
| Fulminant | 14 (22%) | 1 (1%) | <.0001 |
|
| |||
| Immunosuppression, n (%) | 12 (19%) | 37 (42%) | 0.003 |
|
| |||
| ICU admission, n (%) | 10 (16%) | 31 (35%) | 0.01 |
|
| |||
| Inpatient death, n (%) | 9 (14%) | 9 (10%) | 0.43 |
|
| |||
| Length of stay (day), median (range) | 12 (1–56) | 9 (1–69) | 0.20 |
|
| |||
| CDI on admission, n (%) | 24 (38%) | 43 (48%) | 0.21 |
|
| |||
| Severe CDI, n (%) | 20 (32%) | 34 (38%) | 0.41 |
|
| |||
| Treatment of CDI, n (%) | (n = 62) | ||
| Supportive | 3 (5%) | 0 | 0.07 |
| Metronidazole | 58 (94%) | 66 (74%) | 0.002 |
| Vancomycin | 1 (2%) | 4 (4%) | 0.65 |
| Metronidazole and vancomycin | 0 | 19 (21%) | <.0001 |
|
| |||
| Duration of treatment (day), median (range) | |||
| Metronidazole | 10 (1–22) (n = 58) | 14 (2–42) (n = 84) | <.0001 |
| Vancomycin | 13 (n = 1) | 15 (4–188) (n = 23) | 0.56 |
|
| |||
| Duration of CDI (day), median (range) | 9 (1–39) | 10 (2–56) (n = 86) | 0.42 |
|
| |||
| Metronidazole failure, n (%) | 18 (35%) (n = 52) | 28 (35%) (n = 79) | 0.92 |
| Change to vancomycin | 0 | 14 (18%) | 0.001 |
| Duration of diarrhea >10 days | 18 (35%) | 14 (18%) | 0.03 |
Evolution in the practice of medicine is clearly reflected in the two cohorts. Over time, there has been a significant increase in the use of fluoroquinolones (25% vs. 53%, p = 0.0007) along with a decrease in the use of cephalosporins (67% vs. 39%, p = 0.0009), aminoglycosides (33% vs. 4%, p < 0.0001), and clindamycin (21% vs. 10%, p = 0.07). There has also been a dramatic increase in PPI use (25% vs. 74%, p < 0.0001) and a lesser decrease in H-2RA use (38% vs. 12%, p = 0.0002). Overall, the use of antibiotics (median 2 vs. 3, p = 0.04) and acid anti-secretory medications (63% vs. 79%, p = 0.04) has increased.
Compared to the 1998 cohort, more patients in the 2004–2006 cohort had prior CDI (6% vs. 17%, p = 0.05), but the proportion of patients with CDI on hospital admission or severe CDI was similar. The management of CDI has changed significantly over time with a shift from supportive care (5% vs. 0, p = 0.07) and metronidazole monotherapy (94% vs. 74%, p = 0.002) to combination therapy with metronidazole and vancomycin given sequentially or concurrently (0 vs. 21%, p < 0.0001). The duration of metronidazole treatment has also increased (median 10 vs. 14 days, p < 0.0001). Despite theses changes, there was no difference in the duration of CDI between the two cohorts. Other clinical outcomes such as length of hospital stay and inpatient mortality were also similar, although more patients in the 2004–2006 cohort required ICU admissions (16% vs. 35%, p = 0.01).
Comparison of Metronidazole Failure Rates
Of the 63 patients in the 1998 cohort, metronidazole failure could not be determined in 11 – 6 died within 10 days of starting metronidazole, 1 was treated with vancomycin alone, 3 received supportive care, and therapy was unknown in 1. Of the 89 patients in the 2004–2006 cohort, 10 could not be assessed for metronidazole failure – 2 were lost to follow-up, 2 died within 10 days after starting metronidazole, and 6 received vancomycin alone or in combination with metronidazole initially.
Metronidazole failure rates were identical at 35% in both cohorts – 18/52 in the 1998 cohort and 28/79 in the 2004–2006 cohort. All 18 metronidazole failures in the 1998 cohort had persistent diarrhea after 10 days of treatment; none was switched to vancomycin. In contrast, 14 of the 28 metronidazole failures in the 2004–2006 cohort had more than 10 days of diarrhea after treatment; the other 14 were switched to vancomycin after having received metronidazole for a median duration of 3 days (range 1–10 days). Indications for change of therapy included persistent diarrhea (8/14), severe abdominal pain (2/14), sepsis (2/14), rash (1/14), and nausea (1/14).
Figure 1 illustrates the time to resolution of CDI after treatment in the 1998 and 2004–2006 cohorts. The median time to resolution of diarrhea was 8 days (range 0–34 days) in the 1998 cohort and 5 days (range 1–56 days) in the 2004–2006 cohort (p = 0.52). The proportion of patients with more than 10 days of diarrhea was 35% (18/52) in the 1998 cohort and 29% (23/79) in the 2004–2006 cohort (p = 0.51).
Figure 1. Time to resolution of CDI after treatment for patients with available data on metronidazole failure in the 1998 and 2004–2006 cohorts.
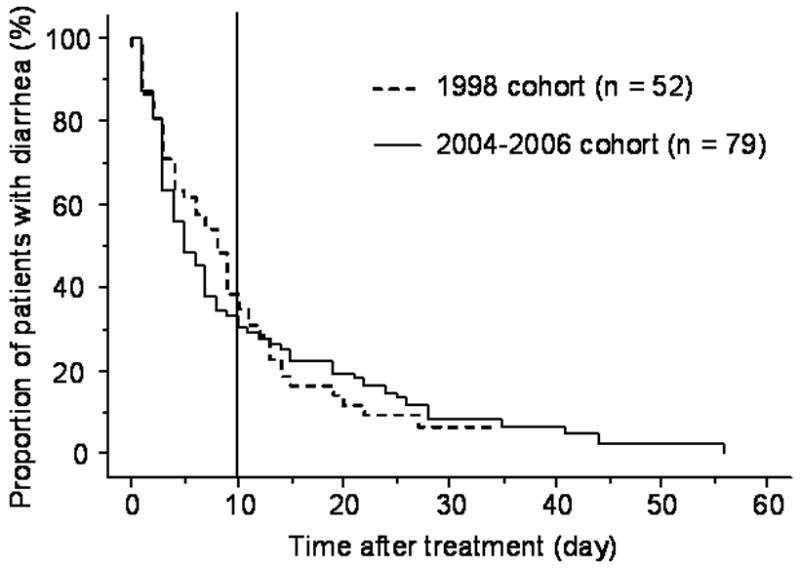
The median time to resolution of diarrhea was 8 days (range 0–34 days) in the 1998 cohort and 5 days (range 1–56 days) in the 2004–2006 cohort (p = 0.52). The proportion of patients with more than 10 days of diarrhea was 35% (18/52) in the 1998 cohort and 29% (23/79) in the 2004–2006 cohort (p = 0.51).
We further divided the 2004–2006 cohort into patients who were switched to vancomycin (14/79) versus patients who remained on metronidazole (65/79), and Figure 2 illustrates the time to resolution of CDI after treatment in these two groups. The median time to resolution of diarrhea was 13 days (range 2–28 days) for those switched to vancomycin compared to only 4 days (range 1–56 days) for those remaining on metronidazole (p = 0.15). Similarly, the proportion of patients with more than 10 days of diarrhea was 64% (9/14) among those switched to vancomycin compared to 22% (14/65) among those remaining on metronidazole (p = 0.003).
Figure 2. Time to resolution of CDI after treatment among patients switched to vancomycin and patients remaining on metronidazole in the 2004–2006 cohort.
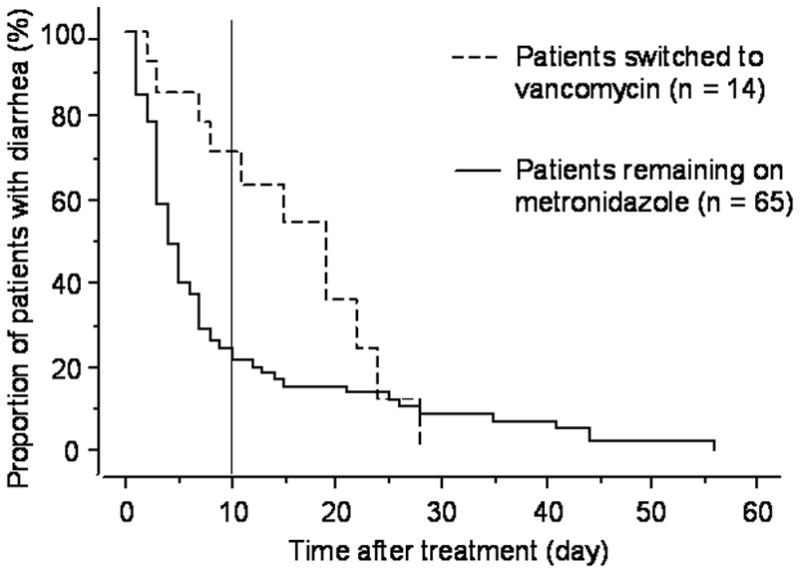
The median time to resolution of diarrhea was 13 days (range 2–28 days) for patients switched to vancomycin compared to 4 days (range 1–56 days) for patients remaining on metronidazole (p = 0.15). The proportion of patients with more than 10 days of diarrhea was 64% (9/14) among patients switched to vancomycin compared to 22% (14/65) among patients remaining on metronidazole (p = 0.003).
Risk Factors for Metronidazole Failure
We analyzed patients in the 2004–2006 cohort for risk factors associated with metronidazole failure, and Table 2 summarizes the results of the unadjusted univariate analysis. Recent cephalosporin use was strongly associated, whereas increasing age, white race, admission from another health care facility, presence of CDI on hospital admission, low albumin, high WBC, increasing number of recently used antibiotics, macrolide use, serious underlying illness (Horn’s index severe to fulminant), and ileus all showed trends toward being more frequent in metronidazole failure.
Table 2.
Univariate Analysis of Risk Factors Associated with Metronidazole Failure in the 2004–2006 Cohort
| Variable | Change to vancomycin (n = 14) | Duration of diarrhea >10 days (n = 14) | Total metronidazole failure (n = 28) | No metronidazole failure (n = 51) | P value* |
|---|---|---|---|---|---|
| Age (year), median (range) | 83 (22–93) | 74 (48–94) | 76 (22–94) | 68 (29–91) | 0.09 |
| ≥65, n (%) | 10 (71%) | 11 (79%) | 21 (75%) | 30 (58%) | 0.15 |
| ≥85, n (%) | 5 (36%) | 2 (14%) | 7 (25%) | 6 (12%) | 0.14 |
|
| |||||
| Male sex, n (%) | 7 (50%) | 8 (57%) | 15 (54%) | 22 (42%) | 0.29 |
|
| |||||
| White race, n (%) | 13 (93%) | 13 (93%) | 26 (93%) | 39 (75%) | 0.06 |
|
| |||||
| Residence before admission, n (%) | |||||
| Home | 6 (43%) | 7 (50%) | 13 (46%) | 34 (67%) | 0.08 |
| Nursing home or rehabilitation center | 5 (36%) | 3 (21%) | 8 (29%) | 11 (22%) | 0.49 |
| Other hospital | 3 (21%) | 4 (29%) | 7 (25%) | 6 (12%) | 0.14 |
|
| |||||
| Prior CDI, n (%) | 1 (7%) | 2 (14%) | 3 (11%) | 7 (14%) | 0.70 |
|
| |||||
| Number of recently used antibiotics, median (range) | 3 (1–9) | 3 (1–6) | 3 (1–9) | 2 (0–7) | 0.20 |
| Clindamycin, n (%) | 2 (14%) | 0 | 2 (7%) | 7 (14%) | 0.39 |
| Metronidazole, n (%) | 2 (14%) | 2 (14%) | 4 (14%) | 9 (18%) | 0.70 |
| Vancomycin, n (%) | 6 (43%) | 6 (43%) | 12 (43%) | 21 (41%) | 0.88 |
| Penicillin, n (%) | 2 (14%) | 2 (14%) | 4 (14%) | 11 (22%) | 0.43 |
| Cephalosporin, n (%) | 8 (57%) | 9 (64%) | 17 (61%) | 15 (29%) | 0.008 |
| Carbopenem, n (%) | 4 (29%) | 1 (7%) | 5 (18%) | 6 (12%) | 0.46 |
| Macrolide, n (%) | 2 (14%) | 4 (29%) | 6 (21%) | 5 (10%) | 0.16 |
| Fluoroquinolone, n (%) | 10 (71%) | 8 (57%) | 18 (64%) | 26 (51%) | 0.26 |
| Aminoglycoside, n (%) | 0 | 0 | 0 | 3 (6%) | 0.98 |
| Sulfonomide, n (%) | 3 (21%) | 0 | 3 (11%) | 6 (12%) | 0.89 |
|
| |||||
| Acid anti-secretory medication, n (%) | 12 (86%) | 11 (79%) | 23 (82%) | 38 (75%) | 0.44 |
| H-2RA | 3 (21%) | 1 (7%) | 4 (14%) | 7 (14%) | 0.95 |
| PPI | 11 (79%) | 10 (71%) | 21 (75%) | 36 (71%) | 0.68 |
|
| |||||
| TPN, n (%) | 0 | 1 (7%) | 1 (4%) | 4 (8%) | 0.47 |
|
| |||||
| Tube feeding, n (%) | 1 (7%) | 0 | 1 (4%) | 3 (6%) | 0.66 |
|
| |||||
| Charlson score, median (range) | 5 (1–11) | 3 (0–10) | 4 (0–11) | 3 (0–10) | 0.32 |
|
| |||||
| Horn’s index severe to fulminant, n (%) | 5 (36%) | 5 (36%) | 10 (36%) | 10 (20%) | 0.12 |
|
| |||||
| Immunosuppression, n (%) | 8 (57%) | 5 (36%) | 13 (46%) | 18 (35%) | 0.35 |
|
| |||||
| ICU admission before or at time of CDI diagnosis, n (%) | 3 (21%) | 5 (36%) | 8 (29%) | 14 (27%) | 0.92 |
|
| |||||
| Length of stay before CDI diagnosis, median (range) | 1, 0–11 | 2, 0–29 | 1 (0–29) | 2 (0–27) | 0.89 |
|
| |||||
| CDI on admission, n (%) | 10 (71%) | 8 (57%) | 18 (64%) | 22 (43%) | 0.08 |
|
| |||||
| Severe CDI, n (%) | 10 (71%) | 1 (7%) | 11 (39%) | 18 (35%) | 0.72 |
|
| |||||
| Albumin (g/dl), median (range) | 2.6 (2.1–3.4) (n = 9) | 2.9 (2.5–3.8) (n = 10) | 2.8 (2.1–3.8) (n = 19) | 3.1 (2–4.3) (n = 31) | 0.08 |
| ≤2.5, n (%) | 4 (44%) | 1 (10%) | 5 (26%) | 6 (19%) | 0.57 |
|
| |||||
| WBC (/μl), median (range) | 18 (0.4–41.2) | 14.3 (0.2–32.5) | 16 (0.2–41.2) | 12.3 (0.2–40.6) | 0.15 |
| ≥15,000, n (%) | 9 (64%) | 6 (43%) | 15 (54%) | 16 (31%) | 0.06 |
| ≥20,000, n (%) | 6 (43%) | 1 (7%) | 7 (25%) | 9 (18%) | 0.44 |
|
| |||||
| Cr (mg/dl), median (range) | 1.7 (0.7–11.5) | 1.1 (0.6–5.7) | 1.3 (0.6–11.5) | 1.3 (0.4–9.9) (n = 50) | 0.74 |
| ≥2.3, n (%) | 5 (36%) | 2 (14%) | 7 (25%) | 12 (24%) | 0.92 |
|
| |||||
| Ileus, n (%) | 2 (17%) (n = 12) | 1 (25%) (n = 4) | 3 (19%) (n = 16) | 1 (4%) (n = 23) | 0.18 |
|
| |||||
| Colitis, n (%) | 9 (75%) (n = 12) | 0 (n = 2) | 9 (64%) (n = 14) | 10 (48%) (n = 21) | 0.34 |
|
| |||||
| Duration of diarrhea before CDI treatment (day), median (range) | 2, 0–28 | 1, 0–14 | 2 (0–28) | 2 (0–21) | 0.85 |
|
| |||||
| Concurrent antibiotic use, n (%) | 14 (100%) | 9 (64%) | 23 (82%) | 37 (73%) | 0.34 |
|
| |||||
| NAP-1, n (%) | 3 (43%) (n = 7) | 2 (17%) (n = 12) | 5 (26%) (n= 19) | 7 (21%) (n = 33) | 0.67 |
P value for total metronidazole failure vs. no metronidazole failure
Compared to patients with persistent diarrhea after 10 days of metronidazole treatment, patients switched to vancomycin were more likely to have severe CDI (7% vs. 71%, p = 0.004), WBC greater than 20,000/μl (7% vs. 43%, p = 0.05), and concurrent antibiotic use during CDI treatment (64% vs. 100%, p = 0.04). These findings suggest that the clinician’s decision to change therapy was guided by the severity of the patient’s CDI and the requirement for concurrent antibiotics almost certainly reflected a sicker population.
We assessed the severity of CDI by a combination of parameters, including creatinine, WBC greater than 15,000 or 20,000/μl, colitis, CDI-related ICU admission, and severe CDI as defined a priori, but none of these correlated with metronidazole failure. We also examined variables that might influence the clinical response to metronidazole therapy, including prior CDI, length of hospital stay before C. difficile diagnosis, duration of diarrhea before CDI treatment, use of acid anti-secretory medications, tube feeding, TPN, comorbidities (Charlson score), immunosuppression, ICU admission before or at the time of C. difficile diagnosis, and concurrent antibiotic use during CDI treatment, but none showed any correlation with metronidazole failure either. We further compared the incidence of NAP-1 infection between patients with and without metronidazole failure and found no statistically significant difference (26% vs. 21%, p = 0.67).
We compared clinical outcomes between failures and responders of metronidazole therapy and found no difference in ICU admission or inpatient mortality. Patients who failed metronidazole had a trend for longer hospital stays (median 11 vs. 8 days, p = 0.11), but this difference was independently accounted for by their serious underlying illnesses (Horn’s index severe to fulminant) rather than by metronidazole failure.
Table 3 summarizes the results of multivariate analysis on risk factors associated with metronidazole failure in the 2004–2006 cohort. Increasing age (OR 1.1, 95% CI 1.0–1.1), transfer from another hospital (OR 11, 95% CI 2–72), presence of CDI on hospital admission (OR 23, 95% CI 3–156), and recent cephalosporin use (OR 32, 95% CI 5–219) were all significant predictors, although the correlation between age and metronidazole failure was weak. A total of 18 patients with metronidazole failure had CDI on admission, of whom 13 (72%) were admitted because of CDI. Conversely, 7 patients with metronidazole failure were transferred from other hospitals, of whom only 2 (29%) were transferred for CDI. No confounders of metronidazole failure were identified.
Table 3.
Multivariate Analysis of Risk Factors Associated with Metronidzole Failure in the 2004–2006 Cohort
| Variable | OR (95% CI) | P value |
|---|---|---|
| Age | 1.1 (1.0–1.1) | 0.01 |
| Transfer from another hospital | 11 (2–72) | 0.009 |
| CDI on admission | 23 (3–156) | 0.001 |
| Recent cephalosporin use | 32 (5–219) | 0.0004 |
Discussion
Previous studies of CDI therapy reported equally low failure rates of 2% for both metronidazole and vancomycin 8, 9. However, recent studies report a much higher metronidazole failure rate of 19% compared to only 4% for vancomycin 4, 5, 8, 10–12. These data suggest a dramatic decline in the efficacy of metronidazole therapy for CDI, possibly linked to the emergence of more virulent strains of C. difficile including the epidemic NAP-1 strain. Our data draw both of these broadly held opinions into question.
In our prospective study, we found the rate of metronidazole failure between 2004 and 2006 to be 35%. Somewhat unexpectedly, this rate was the same as that observed in a previously studied cohort in 1998. While within the range of failure rates found by other analyses conducted after 2000 (22–38%), this rate is far greater than those reported by studies before 2000 (0–16%) 4, 5, 8–12. One explanation for this higher-than-expected metronidazole failure rate may be the poor health condition of the 1998 cohort, which contained many patients 85 years or older, admitted from nursing homes and rehabilitation centers, and with severe or fulminant underlying illnesses. In contrast, the 2004–2006 cohort contained significantly fewer critically ill patients. Another explanation for the relatively high failure rate is that our a priori definition of treatment success was rigorous, and our follow-up included careful evaluation of the frequency and consistency of bowel movements. Thus, unlike earlier studies, patients who merely had improvement but not complete resolution of their diarrhea were classified as metronidazole failures.
A second unexpected finding in our study was the lack of association between NAP-1 infection and failure of metronidazole therapy. Although a high occurrence of NAP-1 might be expected in the background of high fluoroquinolone use, the prevalence at our institution was 23% (12/52), much lower than that reported at other North American institutions during the peak of CDI outbreaks (51–82%) 2, 3. In addition, NAP-1 was equally prevalent in patients with and without metronidazole failure (26% vs. 21%, p = 0.67). This result contrasts with reports that imply more frequent metronidazole failure in connection with NAP-1 infection in epidemic settings 2–5. Our analysis was limited by the number of stool samples available for strain typing (52/79), thereby reducing the power of the study to detect smaller true differences. A larger study designed to examine the relationship between NAP-1 and various clinical outcomes of endemic CDI is currently in progress. This non-correlation between NAP-1 and metronidazole failure suggests that a complex interaction of bacterial, host, and environmental factors may be responsible for poor outcomes in recent CDI outbreaks 2, 14, 15, 18.
Interestingly, half of the patients in the 2004–2006 cohort (14/28) were considered metronidazole failures because their therapy was changed to vancomycin whereas all patients in the 1998 cohort (18/18) remained on metronidazole. The median time to resolution of diarrhea for those switched to vancomycin was 13 days, and a majority (9/14) had persistent diarrhea after 10 days of treatment. These finding support the contention that the clinical decision to change therapy was largely correct, as patients destined to suffer prolonged diarrhea were preferentially selected. Alternatively, the implication may be that vancomycin did not improve clinical outcome, as patients still had prolonged diarrhea despite the switch.
Unlike previous studies that identified low albumin, intensive care unit stay, and continuation of antibiotics during CDI treatment as risk factors for metronidazole failure, we found recent cephalosporin use (OR 32, 95% CI 5–219) to be the strongest predictor 10, 11. Although the use of cephalosporins has decreased over time, they are well-known high-risk antibiotics in inducing CDI and resistance has been shown in all clinical C. difficile strains 1, 8. Interestingly, a previous study also found an association between cephalosporin use and poor metronidazole response 4. The mechanism whereby cephalosporins are linked to an increased risk of CDI treatment failure is unclear, but their use was thought to serve as a marker for ill patients 4.
Presence of CDI on hospital admission (OR 23, 95% CI 3–156) and transfer from another hospital (OR 11, 95% CI 2–72) were two other strong predictors of metronidazole failure in our study. Since a majority of patients with CDI on admission were admitted for this condition, a reasonable inference would be that the severity of their disease was beyond the scope of outpatient treatment. The same could not be said for patients transferred from other hospitals, as a majority of these were transferred for reasons other than CDI. However, the need for transfer to a tertiary care hospital indicates a sick population – 54% (7/13) of patients transferred from other hospitals in the 2004–2006 cohort had serious underlying illnesses (Horn’s index severe to fulminant) compared to 20% (13/66) of those admitted from home or nursing homes and rehabilitation centers (p = 0.01).
In summary, our study found metronidazole failure rates to be stable in the pre- and post-NAP-1 epidemic eras. Recent cephalosporin use, presence of CDI on hospital admission, and transfer from another hospital were significant risk factors for metronidazole failure. Infection with the epidemic NAP-1 strain did not result in an increased risk of metronidazole failure in this study of endemic CDI.
Our study has implications for the management of CDI. By identifying patients at high risk for metronidazole failure, we may be able to treat them with more aggressive therapy such as earlier use of oral vancomycin. They may also be candidates for novel therapies such as C. difficile toxin-binding agents and passive or active immunization against C. difficile toxins 20–24. Our finding that the epidemic NAP-1 strain is not associated with more frequent metronidazole failure in endemic CDI indicates that the dramatically increased risk of therapy failure, morbidity, and mortality in recent CDI outbreaks maybe multifactorial and not solely caused by NAP-1 virulence.
Supplementary Material
Acknowledgments
This work was supported by grants from the National Institutes of Health (RO-1 AI053069 to Ciarán P. Kelly; K30-HL04095 to the Scholars in Clinical Science Program at Harvard Medical School, in which Mary Y. Hu was enrolled; and T32-DK0776 to Seema Maroo) and the Irish Health Research Board (RP/2005/72 to Lorraine Kyne). We also acknowledge the generous contributions of the subjects who participated in this study.
Grant Support
This work was supported by grants from the National Institutes of Health (RO-1 AI053069 to Ciarán P. Kelly; K30-HL04095 to the Scholars in Clinical Science Program at Harvard Medical School, in which Mary Y. Hu was enrolled; and T32-DK0776 to Seema Maroo) and the Irish Health Research Board (RP/2005/72 to Lorraine Kyne).
Footnotes
Financial Disclosures
During the past two years, Ciarán P. Kelly has acted as a scientific consultant for (Acambis Inc., Actelion Inc., Oscient Pharmaceuticals, Salix Pharmaceuticals, and ViroPharm Inc.) and has received research grant funding from (Actelion Inc., Genzyme Inc., Massachusetts Biologics Laboratories, Medarex Inc., and Salix Pharmaceuticals) companies that are producing or developing treatments for C. difficile-associated disease. No potential conflicts of interest exist for other authors.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Kelly CP, Pothoulakis C, LaMont JT. Clostridium difficile colitis. N Engl J Med. 1994;330:257–62. doi: 10.1056/NEJM199401273300406. [DOI] [PubMed] [Google Scholar]
- 2.McDonald LC, Killgore GE, Thompson A, Owens RC, Jr, Kazakova SV, Sambol SP, Johnson S, Gerding DN. An epidemic, toxin gene-variant strain of Clostridium difficile. N Engl J Med. 2005;353:2433–41. doi: 10.1056/NEJMoa051590. [DOI] [PubMed] [Google Scholar]
- 3.Loo VG, Poirier L, Miller MA, Oughton M, Libman MD, Michaud S, Bourgault AM, Nguyen T, Frenette C, Kelly M, Vibien A, Brassard P, Fenn S, Dewar K, Hudson TJ, Horn R, Rene P, Monczak Y, Dascal A. A predominantly clonal multi-institutional outbreak of Clostridium difficile-associated diarrhea with high morbidity and mortality. N Engl J Med. 2005;353:2442–9. doi: 10.1056/NEJMoa051639. [DOI] [PubMed] [Google Scholar]
- 4.Musher DM, Aslam S, Logan N, Nallacheru S, Bhaila I, Borchert F, Hamill RJ. Relatively poor outcome after treatment of Clostridium difficile colitis with metronidazole. Clin Infect Dis. 2005;40:1586–90. doi: 10.1086/430311. [DOI] [PubMed] [Google Scholar]
- 5.Pepin J, Valiquette L, Gagnon S, Routhier S, Brazeau I. Outcomes of Clostridium difficile-Associated Disease Treated With Metronidazole or Vancomycin Before and After the Emergence of NAP1/027. Am J Gastroenterol. 2007 doi: 10.1111/j.1572-0241.2007.01539.x. [DOI] [PubMed] [Google Scholar]
- 6.Warny M, Pepin J, Fang A, Killgore G, Thompson A, Brazier J, Frost E, McDonald LC. Toxin production by an emerging strain of Clostridium difficile associated with outbreaks of severe disease in North America and Europe. Lancet. 2005;366:1079–84. doi: 10.1016/S0140-6736(05)67420-X. [DOI] [PubMed] [Google Scholar]
- 7.Fekety R. Guidelines for the diagnosis and management of Clostridium difficile-associated diarrhea and colitis. American College of Gastroenterology, Practice Parameters Committee. Am J Gastroenterol. 1997;92:739–50. [PubMed] [Google Scholar]
- 8.Aslam S, Hamill RJ, Musher DM. Treatment of Clostridium difficile-associated disease: old therapies and new strategies. Lancet Infect Dis. 2005;5:549–57. doi: 10.1016/S1473-3099(05)70215-2. [DOI] [PubMed] [Google Scholar]
- 9.Bricker E, Garg R, Nelson R, Loza A, Novak T, Hansen J. Antibiotic treatment for Clostridium difficile-associated diarrhea in adults. Cochrane Database Syst Rev. 2005:CD004610. doi: 10.1002/14651858.CD004610.pub2. [DOI] [PubMed] [Google Scholar]
- 10.Fernandez A, Anand G, Friedenberg F. Factors associated with failure of metronidazole in Clostridium difficile-associated disease. J Clin Gastroenterol. 2004;38:414–8. doi: 10.1097/00004836-200405000-00005. [DOI] [PubMed] [Google Scholar]
- 11.Modena S, Gollamudi S, Friedenberg F. Continuation of antibiotics is associated with failure of metronidazole for Clostridium difficile-associated diarrhea. J Clin Gastroenterol. 2006;40:49–54. doi: 10.1097/01.mcg.0000190761.80615.0f. [DOI] [PubMed] [Google Scholar]
- 12.Zar FA, Bakkanagari SR, Moorthi KM, Davis MB. A comparison of vancomycin and metronidazole for the treatment of Clostridium difficile-associated diarrhea, stratified by disease severity. Clin Infect Dis. 2007;45:302–7. doi: 10.1086/519265. [DOI] [PubMed] [Google Scholar]
- 13.Nair S, Yadav D, Corpuz M, Pitchumoni CS. Clostridium difficile colitis: factors influencing treatment failure and relapse--a prospective evaluation. Am J Gastroenterol. 1998;93:1873–6. doi: 10.1111/j.1572-0241.1998.00541.x. [DOI] [PubMed] [Google Scholar]
- 14.Kyne L, Warny M, Qamar A, Kelly CP. Asymptomatic carriage of Clostridium difficile and serum levels of IgG antibody against toxin A. N Engl J Med. 2000;342:390–7. doi: 10.1056/NEJM200002103420604. [DOI] [PubMed] [Google Scholar]
- 15.Kyne L, Warny M, Qamar A, Kelly CP. Association between antibody response to toxin A and protection against recurrent Clostridium difficile diarrhoea. Lancet. 2001;357:189–93. doi: 10.1016/S0140-6736(00)03592-3. [DOI] [PubMed] [Google Scholar]
- 16.Klaassen CH, van Haren HA, Horrevorts AM. Molecular fingerprinting of Clostridium difficile isolates: pulsed-field gel electrophoresis versus amplified fragment length polymorphism. J Clin Microbiol. 2002;40:101–4. doi: 10.1128/JCM.40.1.101-104.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Killgore G, Thompson A, Johnson S, Brazier J, Kuijper E, Pepin J, Frost EH, Savelkoul P, Nicholson B, van den Berg RJ, Kato H, Sambol SP, Zukowski W, Woods C, Limbago B, Gerding DN, McDonald LC. Comparison of seven techniques for typing international epidemic strains of Clostridium difficile: restriction endonuclease analysis, pulsed-field gel electrophoresis, PCR-ribotyping, multilocus sequence typing, multilocus variable-number tandem-repeat analysis, amplified fragment length polymorphism, and surface layer protein A gene sequence typing. J Clin Microbiol. 2008;46:431–7. doi: 10.1128/JCM.01484-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kyne L, Sougioultzis S, McFarland LV, Kelly CP. Underlying disease severity as a major risk factor for nosocomial Clostridium difficile diarrhea. Infect Control Hosp Epidemiol. 2002;23:653–9. doi: 10.1086/501989. [DOI] [PubMed] [Google Scholar]
- 19.Kyne L, Hamel MB, Polavaram R, Kelly CP. Health care costs and mortality associated with nosocomial diarrhea due to Clostridium difficile. Clin Infect Dis. 2002;34:346–53. doi: 10.1086/338260. [DOI] [PubMed] [Google Scholar]
- 20.Louie TJ, Peppe J, Watt CK, Johnson D, Mohammed R, Dow G, Weiss K, Simon S, John JF, Jr, Garber G, Chasan-Taber S, Davidson DM. Tolevamer, a novel nonantibiotic polymer, compared with vancomycin in the treatment of mild to moderately severe Clostridium difficile-associated diarrhea. Clin Infect Dis. 2006;43:411–20. doi: 10.1086/506349. [DOI] [PubMed] [Google Scholar]
- 21.Leung DY, Kelly CP, Boguniewicz M, Pothoulakis C, LaMont JT, Flores A. Treatment with intravenously administered gamma globulin of chronic relapsing colitis induced by Clostridium difficile toxin. J Pediatr. 1991;118:633–7. doi: 10.1016/s0022-3476(05)83393-1. [DOI] [PubMed] [Google Scholar]
- 22.Salcedo J, Keates S, Pothoulakis C, Warny M, Castagliuolo I, LaMont JT, Kelly CP. Intravenous immunoglobulin therapy for severe Clostridium difficile colitis. Gut. 1997;41:366–70. doi: 10.1136/gut.41.3.366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wilcox MH. Descriptive study of intravenous immunoglobulin for the treatment of recurrent Clostridium difficile diarrhoea. J Antimicrob Chemother. 2004;53:882–4. doi: 10.1093/jac/dkh176. [DOI] [PubMed] [Google Scholar]
- 24.Sougioultzis S, Kyne L, Drudy D, Keates S, Maroo S, Pothoulakis C, Giannasca PJ, Lee CK, Warny M, Monath TP, Kelly CP. Clostridium difficile toxoid vaccine in recurrent C. difficile-associated diarrhea. Gastroenterology. 2005;128:764–70. doi: 10.1053/j.gastro.2004.11.004. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


