Abstract
Data from lesion studies suggests that the ability to perceive speech sounds, as measured by auditory comprehension tasks, is supported by temporal lobe systems in both the left and right hemisphere. For example, patients with left temporal lobe damage and auditory comprehension deficits (i.e., Wernicke’s aphasics), nonetheless comprehend isolated words better than one would expect if their speech perception system had been largely destroyed (70–80% accuracy). Further, when comprehension fails in such patients their errors are more often semantically-based, than-phonemically based. The question addressed by the present study is whether this ability of the right hemisphere to process speech sounds is a result of plastic reorganization following chronic left hemisphere damage, or whether the ability exists in undamaged language systems. We sought to test these possibilities by studying auditory comprehension in acute left versus right hemisphere deactivation during Wada procedures. A series of 20 patients undergoing clinically indicated Wada procedures were asked to listen to an auditorily presented stimulus word, and then point to its matching picture on a card that contained the target picture, a semantic foil, a phonemic foil, and an unrelated foil. This task was performed under three conditions, baseline, during left carotid injection of sodium amytal, and during right carotid injection of sodium amytal. Overall, left hemisphere injection led to a significantly higher error rate than right hemisphere injection. However, consistent with lesion work, the majority (75%) of these errors were semantic in nature. These findings suggest that auditory comprehension deficits are predominantly semantic in nature, even following acute left hemisphere disruption. This, in turn, supports the hypothesis that the right hemisphere is capable of speech sound processing in the intact brain.
Data from lesion studies (Baker, Blumsteim, & Goodglass, 1981; Blumstein, Cooper, Zurif, & Caramazza, 1977; Gainotti, Micelli, Silveri, & Villa, 1982; Miceli, Gainotti, Caltagirone, & Masullo, 1980) suggests that the ability to perceive speech sounds (phonemes), as measured by auditory comprehension tasks, is supported by temporal lobe systems in both the left and right hemisphere(Hickok & Poeppel, 2000, 2004, 2007). For example, patients with left temporal lobe damage and auditory comprehension deficits (i.e., Wernicke’s aphasics), nonetheless comprehend isolated words with approximately 70–80% accuracy on tasks where chance is 25%. Further, when comprehension fails in such patients their errors are more often semantically-based, than-phonemically based. That is, given a stimulus word “bear” they are more likely to err by choosing a picture of a semantically similar item (moose), than a picture of a phonemically similar item (pear)(Gainotti et al., 1982).
These, and similar findings(Baker et al., 1981) have lead to the conclusion that auditory comprehension deficits following left temporal lobe lesions stem from disruptions primarily at post phonemic processing levels, either in the semantic system(Gainotti et al., 1982) or in the mapping between phonemic and semantic representations(Baker et al., 1981; Hickok & Poeppel, 2000, 2004, 2007). On this view, other brain regions must be capable of carrying out phonemic level processing. Additional lesion evidence has suggested that right hemisphere superior temporal lobe regions support such functions. For example, bilateral superior temporal lobe lesions appear to destroy the phonemic processing systems as this pattern of damage is associated with word deafness, a syndrome in which the capacity to comprehend speech is effectively nil(Buchman, Garron, Trost-Cardamone, Wichter, & Schwartz, 1986; Poeppel, 2001). In sum, the lesion data suggest that both hemispheres are capable of processing phonemic-level information during auditory comprehension.
One unresolved question, however, is whether the right hemisphere’s capacity to process phonemic information is a result of compensatory plastic reorganization following left hemisphere insult, or whether the ability exists in undamaged language systems. If the capacity results from plastic reorganization, one would expect severe disruption of phonemic processes during the acute phases of left hemisphere disruption, with a gradual recovery of function. However, if phonemic processing in auditory comprehension remains relatively spared even acutely, this would support the view that the right hemisphere is, under normal circumstances, capable of phonemic processing.
Breese and Hillis (Breese & Hillis, 2004) investigated this question in a study of 122 acute left hemisphere stroke patients (< 48h from onset) using both a four-alternative forced choice (4AFC) word-to-picture matching task with phonemic, semantic, and unrelated picture foils, as well as a picture verification task: a picture was presented along with an auditory word that either matched the picture (e.g., picture=FORK, word=fork), was a phonemic foil (e.g., word= cork), or was a semantic foil (e.g., word=spoon). Of interest is whether patients would have more trouble rejecting semantic than phonemic foils. A majority of patients (73/122) “made too few errors to allow classification” (p. 6). While a sizable fraction of the remaining patients committed primarily semantic errors, incorrectly accepting semantic foils (33%), only one patient committed primarily phonemic errors. The authors concluded that underspecified semantic representations could account for the majority of error patterns in their sample.
Here we use a different method, the Wada procedure (Wada & Rasmussen, 1960), to assess the effects of acute disruption of left or right hemisphere function on auditory comprehension. Previous reports have indicated that auditory comprehension for words and simple phrases is relatively intact following left hemisphere anesthesia(McGlone, 1984; Wada & Rasmussen, 1960), consistent with the view that the right hemisphere is capable of speech processing. However, previous studies have not used comprehension tasks with phonemic and semantic distractors, nor have they assessed the level of processing that is disrupted when errors occur. Consistent with the lesion literature, we predict relatively good overall performance with left or right hemisphere anesthesia, but that when errors occur, they will be primarily semantic in nature.
Methods
Subjects
Nineteen individuals (16 female, mean age 39.8 years, range 18–61) undergoing clinically indicated Wada procedures at NYU Epilepsy Center participated in the study. English was the first language of all participants, and all were left dominant for language function as determined by the clinical Wada testing procedure (object naming). Table 1 presents additional biographical and clinical information.
Table 1.
Subject biographical information.
| subject | age | sex | edu (yrs) | handedness | lang lateralization | indication | age of seizure onset |
|---|---|---|---|---|---|---|---|
| S01 | 37 | F | 16 | R | L | L TLE | 34 |
| S03 | 35 | F | 16 | R | L | L subinsular tumor | 34 |
| S04 | 44 | F | 14 | R | L | R TLE | 2 |
| S05 | 43 | M | 14 | R | L | R TLE | 8 |
| S06 | 34 | F | 14 | R | L | R TLE | 21 |
| S07 | 25 | F | 19 | L | L | R TLE | 12 |
| S08 | 54 | M | 16 | R | L | R TLE | 51 |
| S09 | 42 | F | 12 | R | L | R PLE | 6 |
| S10 | 61 | F | 12 | R | L | L TLE | 40 |
| S11 | 18 | F | 12 | R | L | L frontal tumor | 16 |
| S12 | 49 | M | 14 | L | L | L TLE | 25 |
| S15 | 29 | F | 17 | B (LQ=40) | L | R tuberculum sellae meningioma | n/a |
| S16 | 45 | F | 12 | R | L | R FLE | 5 |
| S18 | 40 | F | 14 | R | L | L TLE | 2 |
| S19 | 38 | F | 16 | B (LQ=56) | L | Bilateral TLE | 2 |
| S20 | 49 | F | 16 | R | L | R F-TLE | 37 |
| S23 | 46 | F | 18 | R | L | R TLE | 0 |
| S24 | 41 | F | 16 | R | L | R TLE (hippocampal tumor) | 29 |
| S26 | 51 | F | 12 | R | L | L TLE | 4 |
| S27 | 35 | F | 12 | R | L | B TLE | 8 |
R -- right, L -- left, TLE -- temporal lobe epilepsy, FLE -- frontal lobe epilepsy, PLE -- parietal lobe epilepsy, F-TLE --fronto-temporal epilepsy, LQ -- laterality quotient
Stimuli and Procedures
The study was approved by the NYU Institutional Review Board and all subjects gave written informed consent. Subjects underwent the Wada procedure as part of a comprehensive pre-surgical evaluation for medically uncontrolled epilepsy. Sodium amobarbital was injected via catheter (transfemoral approach) into each internal carotid artery (ICAs) beginning with the ICA ipsilateral to presumed side of seizure onset. The amobarbital was injected gradually over several seconds, and the dose was individualized to achieve complete contralateral arm plegia. Stimuli for the clinical procedure included 8 unique objects and 4 pictures for each side. Research stimuli were presented immediately after the clinical stimuli, or when the subject was able to perform the task. A four-alternative forced choice, word-to-picture matching procedure was used to assess comprehension. Auditorily presented target words were read aloud by the examiner in the context of a command, “Point to the X.” The three distractor pictures included a phonemically similar item (differing by one feature at word onset, e.g., +/− voicing difference in pear vs. bear), a semantically similar item (e.g., bear vs. moose), and an unrelated item. Twenty-four unique trials were presented across baseline, left-hemisphere injection, and right-hemisphere injection conditions (8 trials in each). Amobarbital effect was measured at the beginning and end of stimuli presentation by assessing hand and face weakness, and verbal comprehension when appropriate. At least 30 minutes elapsed between the right and left ICA injections. The mean amobarbital dose was 104.6mg (range 85–130), and the mean time after injection to first research stimulus exposure was 4.2 minutes and 3.1 minutes for left and right hemispheres respectively.
Results
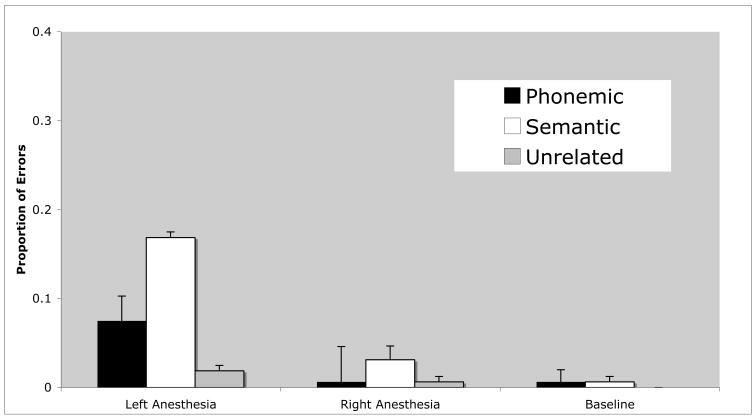
Table 2 and Figure 1 present error rates broken down by type of error (phonemic, semantic, unrelated) and condition (baseline, left injection, right injection). Overall performance was well above chance level for each condition (left injection, 77% correct; right injection, 96% correct; baseline, 98% correct; chance = 25%). Although left hemisphere injection induced the greatest number of errors, the vast majority, 75%, of these were semantic in nature.
Table 2.
Mean error rates by type for each testing condition.
| Error Type | ||||
|---|---|---|---|---|
| Phonemic | Semantic | Unrelated | Total | |
| Left Anesthesia | 0.075 | 0.169 | 0.019 | 0.263 |
| Right Anesthesia | 0.006 | 0.031 | 0.006 | 0.044 |
| Baseline | 0.006 | 0.006 | 0.000 | 0.013 |
| Total | 0.088 | 0.206 | 0.025 | |
Figure 1.
Error rate by type of error as a function of Wada testing condition.
A multivariate analysis of variance was carried out with test condition (baseline, left injection, right injection) entered as the independent variable, and error type (phonemic, semantic, unrelated) as dependent variables. This analysis confirmed a significant effect of test condition on both phonemic and semantic errors (F(2,56) = 5.69, p = .006, and F(2,56) = 12.28, p < .001, respectively). Pairwise comparisons revealed that the phonemic and semantic error rates were significantly higher with left hemisphere injection than with either right injection or during baseline testing (left errors > baseline errors: phonemic, p = .028, semantic, p < .001; left errors > right errors: phonemic, p = .009, semantic, p = .001). Paired t-tests also revealed that subjects made significantly more semantic than phonemic errors across all test conditions (t(59) = 2.27, p = .013, one-tailed), and within the left injection condition alone (t(18) = 1.96, p = .03, one-tailed). These differences also survived a sign test: semantic errors > phonemic errors across all conditions (p = .01), and semantic errors > phonemic errors within the left injection conditions alonge (p = .04). Although there are too few male participants to explore possible gender effects, the three males in the sample showed similar patterns of performance with semantic errors far outnumbering phonemic errors (average: 21% vs. 0%) and left injection leading to more errors than right injections (average: 21% vs. 1%).
The mean grip strength (average at start and end of research protocol for each subject in each hemisphere) during administration of the research procedure was 3.81 for left hemisphere injection and 4.01 for right hemisphere injection (n.s., p = .47), on a five point scale (5 = normal grip strength). However, grip strength did not predict the overall error rate (r2 = .03), nor did it predict semantic or phonemic error rates separately (r2 = .02 and .1 respectively, all n.s.).
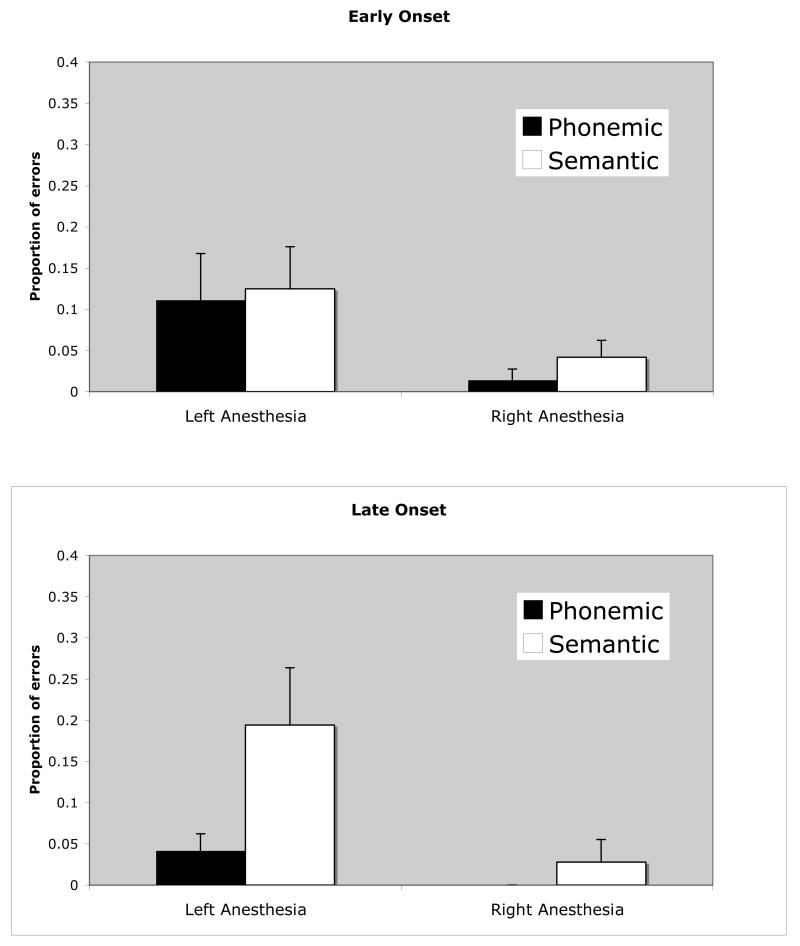
Our sample varied in terms of the onset of neurological disease, including a sizable portion of participants who has early childhood onsets (Table 1). It is possible that the reason the patients in our sample made relatively few phonemic errors is that their pre-test neurological condition caused a reorganization of function resulting in atypical bilateral organization of phonemic processes. This may be particularly true of cases with onsets during early brain development. If this were the case, we might expect patients with later disease onsets to show a greater proportion of phonemic errors than patients with earlier disease onsets. To test this possibility we divided our dataset into cases with onset between age 0 and 8 (n = 9; mean age of onset = 4.1; s.d. = 2.7)), and cases with onsets age 21 or older (n=9; mean age of onset = 33.9; s.d. = 8.9). Two intermediate cases with onsets at 12 and 16 years were excluded from this analysis. As unrelated errors were negligible, we focused on semantic and phonological errors. We found that age of disease onset had no effect on the overall error rate in either hemisphere when semantic and phonological errors were collapsed. In fact, each group had exactly the same error rate overall with left hemisphere anesthesia (11.8%), and had statistically indistinguishable error rates with right hemisphere anesthesia (early onset = 2.8%, late onset 1.4%, t(34) = .74, p = .46, 2-tailed). However, the pattern of phonemic and semantic errors was quite different between the two groups with left hemisphere anesthesia, although in an unexpected direction (Figure 2). In contrast to the predicted effects of early vs. late onset, we found that late onset subjects made roughly five times more semantic (19.4%) than phonemic (4.2 %) errors (t(8) = 2.48, p = .038, 2-tailed), whereas early onset subjects committed semantic and phonemic errors at the same rate (12.5% and 11.1% respectively, (t(8) = .18, n.s.). These results show that the late onset group is driving the effect (greater semantic than phonological errors) in the overall analysis. Thus, plastic reorganization due to early neurological disease cannot explain the relative lack of phonological disruption with left hemisphere injection.
Figure 2.
Error rate by type of error as a function of Wada testing condition for early and late onset groups.
Another variable that may influence our findings is the length of time between onset of disease and Wada assessment. Unfortunately this variable is highly correlated with age of onset in our sample. In fact, the distributions of years-from-disease-onset-to-test in the two groups did not overlap (early onset group, range: 27–47 years; late onset group, range: 1–24 years). For this reason, the separate contributions of age of onset and time between onset and test cannot be determined in this study.
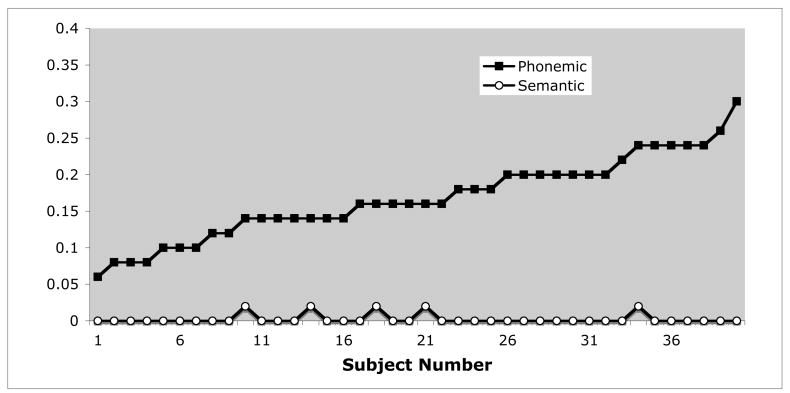
Finally, one might wonder whether an underlying phonological perception deficit might nonetheless disproportionately affect processing at higher levels. This might be the case, for example, if underspecified phonological representations fail to activate the semantic target sufficiently, leading to increased competition among semantic competitors. On this view, left hemisphere disruption may produce primarily phonemic perception deficits that become exaggerated at higher levels of processing. This possibility can be assessed by simulation a perceptual deficit in normal subjects and observing its effects on error patterns in our four-choice picture matching task. If a phonemic perceptual deficit is driving the high rate of semantic errors, normal subjects should make a similarly high number of semantic errors when the word stimuli are perceptually degraded. To assess this possibility, we tested 40 right-handed, healthy adults (27 female, mean age 22.3, range 18–27) on an expanded 50-item version of our task. A perceptual deficit was simulated by presenting target words in noise. Adding noise induced errors on our task (overall mean error rate = 17.6 %) with substantial cross subject variation (range = 6–34%, s.d. = 6.1%). Virtually all of these errors were phonemic however (95.4% of all errors; semantic = 1.4% of all errors; unrelated = 3.1% of all errors). Not surprisingly, given the dominance of phonemic errors, no level of phonemic “impairment” led to systematic increases in semantic errors. This can be seen clearly in Figure 3 which shows phonological and semantic error rates for each subject, with each subject’s data sorted in ascending order from left to right by the rate of phonological errors.
Figure 3.
Phonemic and semantic error rates on a 50-item, speech-in-noise version of the word to picture matching test for each of 40 healthy participants, with data sorted into ascending order by proportion of phonemic errors. Note that the semantic error rate is unaffected by varying degrees of induced phonemic perception difficulty.
Discussion
Acute disruption of left or right hemisphere function using the Wada procedure did not produce profound impairments in phonemic perception. In fact, even with left hemisphere anesthesia, single word comprehension performance was well above chance levels. Of the errors that were committed, the vast majority (75%) were semantic in nature. This finding is consistent with both chronic (Baker et al., 1981; Gainotti et al., 1982; Hickok & Poeppel, 2007; Miceli et al., 1980) and acute (Breese & Hillis, 2004) lesion studies, which have consistently found that word-level auditory comprehension impairments result predominantly from post-phonemic processes. Since disruption of neither the left nor the right hemisphere substantially impairs phonemic perception in auditory comprehension tasks, it follows that both hemispheres are capable of processing speech sounds reasonably well (Hickok & Poeppel, 2000, 2004, 2007). Previous work with chronic lesion patients left open the possibility that the right hemisphere’s capacity to process speech sounds for comprehension was a result of compensatory plasticity. However, even acute disruption of left hemisphere function fails to induce high phonemic error rates suggesting that the right hemisphere has the capacity to process speech sounds in the intact system.
It is possible that relatively good performance overall (77% correct) is a result of sampling comprehension during a stage of partial anesthesia as grip strength was on average approximately 78% of normal. Perhaps if function were assessed under a deeper anesthesia, more errors would have been noted (the lack of correlation between grip strength and error rate suggests this may not be true, however). While we cannot rule out this possibility, it is important to note that the critical finding is not the degree of overall impairment, but the pattern of errors that were committed. The rate of semantic versus phonological errors found in the present study is nearly identical to that found in studies of chronic aphasics (Table 3), as is the overall error rate. This provides converging evidence for the view that word-level auditory comprehension failures in unilateral disruption arise predominantly from post-phonemic processing levels.
Table 3.
Similar error patterns in a sample of unselected aphasics (ref 4) compared to Wada patients with left hemisphere anesthesia (present study).
| LH Anesthesia1 | Aphasics2 | |
|---|---|---|
| Correct Responses | 73.8% | 72% |
| Semantic Errors | 17% | 19.70% |
| Phonemic Errors | 7.50% | 5% |
| Unrelated Errors | <1% | 3% |
Present study
From Gainiotti et al. 1975
Interestingly, however, when we partitioned our dataset according to the age of onset of neurological disease, different patterns emerged. While the overall error rate was the same for both early and late onset groups, only the late onset group showed the predominance of semantic errors (and very few phonemic errors). The early onset group did not differ in their semantic versus phonological error rate. This result argues against the view that the relatively paucity of phonemic errors following left hemisphere deactivation is a consequence of plastic reorganization in our sample secondary to early, chronic neurological disease. Put differently, one could have argued that patients undergoing Wada procedures have phonemic perception systems that are more bilaterally organized than neurological healthy individuals as a result of their chronic neurological disease. This sort of reorganization would be particularly evident in subjects with seizure onsets in early childhood when the potential for plastic reorganization is greatest. If this were true, one would expect that early onset subjects would commit the fewest phonemic errors, and late onset subjects the most phonemic errors. In fact, the reverse pattern was true suggesting that bilateral organization is the typical state of the neurological healthy population. The early onset group also had the longest time from onset of seizures to Wada assessment, ruling out a similar plasticity-based argument due to the long-term effects of chronic seizures.
While evidence from a variety of sources including both acute and chronic dysfunction make a strong case for bilateral capacity for speech sound processing, this does not mean that the speech processing systems in the two hemispheres are computationally identical. In fact, there is solid evidence from neuropsychology (Robin, Tranel, & Damasio, 1990) and neuroimaging (Boemio, Fromm, Braun, & Poeppel, 2005; Liebenthal, Binder, Spitzer, Possing, & Medler, 2005) for computational asymmetries of some form (Hickok & Poeppel, 2007; Zatorre, Belin, & Penhune, 2002). The present study also found small but reliable differences in the rate of phonemic errors following left versus right hemisphere disruption providing further evidence for hemispheric asymmetries in speech sound processing. While it is agreed that asymmetries exist, the precise nature of the computational differences is still being debated (Hickok & Poeppel, 2007).
In summary, the present study found that acute functional disruption of either the left or right hemisphere did not cause severe disruptions in phonological processing during word-level auditory comprehension. This finding indicates that both hemispheres have the capacity to process speech sounds. When errors occurred, they were predominantly semantic in nature implicating a post-phonemic level of processing as the dominant source or comprehension errors in unilateral dysfunction.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Baker E, Blumsteim SE, Goodglass H. Interaction between phonological and semantic factors in auditory comprehension. Neuropsychologia. 1981;19:1–15. doi: 10.1016/0028-3932(81)90039-7. [DOI] [PubMed] [Google Scholar]
- Blumstein SE, Cooper WE, Zurif EB, Caramazza A. The perception and production of voice-onset time in aphasia. Neuropsychologia. 1977;15:371–383. doi: 10.1016/0028-3932(77)90089-6. [DOI] [PubMed] [Google Scholar]
- Boemio A, Fromm S, Braun A, Poeppel D. Hierarchical and asymmetric temporal sensitivity in human auditory cortices. Nat Neurosci. 2005;8(3):389–395. doi: 10.1038/nn1409. [DOI] [PubMed] [Google Scholar]
- Breese EL, Hillis AE. Auditory comprehension: is multiple choice really good enough? Brain Lang. 2004;89(1):3–8. doi: 10.1016/S0093-934X(03)00412-7. [DOI] [PubMed] [Google Scholar]
- Buchman AS, Garron DC, Trost-Cardamone JE, Wichter MD, Schwartz M. Word deafness: One hundred years later. Journal of Neurology, Neurosurgury, and Psychiatry. 1986;49:489–499. doi: 10.1136/jnnp.49.5.489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gainotti G, Micelli G, Silveri MC, Villa G. Some anatomo-clinical aspects of phonemic and semantic comprehension disorders in aphasia. Acta Neurologica Scandinavica. 1982;66:652–665. doi: 10.1111/j.1600-0404.1982.tb04530.x. [DOI] [PubMed] [Google Scholar]
- Hickok G, Poeppel D. Towards a functional neuroanatomy of speech perception. Trends in Cognitive Sciences. 2000;4:131–138. doi: 10.1016/s1364-6613(00)01463-7. [DOI] [PubMed] [Google Scholar]
- Hickok G, Poeppel D. Dorsal and ventral streams: A framework for understanding aspects of the functional anatomy of language. Cognition. 2004;92:67–99. doi: 10.1016/j.cognition.2003.10.011. [DOI] [PubMed] [Google Scholar]
- Hickok G, Poeppel D. The cortical organization of speech processing. Nat Rev Neurosci. 2007;8(5):393–402. doi: 10.1038/nrn2113. [DOI] [PubMed] [Google Scholar]
- Liebenthal E, Binder JR, Spitzer SM, Possing ET, Medler DA. Neural substrates of phonemic perception. Cereb Cortex. 2005;15(10):1621–1631. doi: 10.1093/cercor/bhi040. [DOI] [PubMed] [Google Scholar]
- McGlone J. Speech comprehension after unilateral injection of sodium amytal. Brain and Language. 1984;22:150–157. doi: 10.1016/0093-934x(84)90084-1. [DOI] [PubMed] [Google Scholar]
- Miceli G, Gainotti G, Caltagirone C, Masullo C. Some aspects of phonological impairment in aphasia. Brain and Language. 1980;11:159–169. doi: 10.1016/0093-934x(80)90117-0. [DOI] [PubMed] [Google Scholar]
- Poeppel D. Pure word deafness and the bilateral processing of the speech code. Cognitive Science. 2001;25:679–693. [Google Scholar]
- Robin DA, Tranel D, Damasio H. Auditory perception of temporal and spectral events in patients with focal left and right cerebral lesions. Brain and Language. 1990;39:539–555. doi: 10.1016/0093-934x(90)90161-9. [DOI] [PubMed] [Google Scholar]
- Wada J, Rasmussen T. Intracarotid injection of sodium amytal for the lateralization of cerbral speech dominance. journal of Neurosurgery. 1960;17:266–282. doi: 10.3171/jns.2007.106.6.1117. [DOI] [PubMed] [Google Scholar]
- Zatorre RJ, Belin P, Penhune VB. Structure and function of auditory cortex: music and speech. Trends in Cognitive Sciences. 2002;6:37–46. doi: 10.1016/s1364-6613(00)01816-7. [DOI] [PubMed] [Google Scholar]