Abstract
5-HT3 receptor antagonists (e.g. tropisetron) combined with dexamethasone are effective for the acute phase of cisplatin (CIS)-induced emesis. This study determined the possible additive or synergistic antiemetic efficacy of Δ9-THC when combined with tropisetron or dexamethasone (DEX). Δ9-THC (0 – 10 mg/kg i.p.) was injected in combination with tropisetron (0 – 5 mg/kg i.p.) or dexamethasone (0 – 20 mg/kg i.p.) prior to CIS (20 mg/kg i.p.) in the least shrew, and the induced emesis was recorded for 60 minutes. CIS-induced vomiting was dose-dependently and significantly attenuated by individual administration of Δ9-THC (59–97% reductions) and tropisetron (79–100% attenuation), but not dexamethasone (26–40%), although a trend (p < .1) towards reduced vomiting frequency following DEX was noted. Low doses of Δ9-THC (0.25 or 0.5 mg/kg) when combined with low doses of tropisetron (0.025, 0.1, or 0.25 mg/kg) were more efficacious in reducing emesis frequency than when given individually, but Δ9-THC had no antiemetic interactions with DEX. However, no tested combination provided a significantly greater effect on the number of animals vomiting than their individually-administered counterparts. The modest interaction of Δ9-THC with tropisetron suggests they activate overlapping antiemetic mechanisms, while the lack of interaction with dexamethasone needs further clarification.
Keywords: Cisplatin, Emesis, Antiemetic, Delta-9-THC, Tropisetron, Dexamethasone
1. Introduction
Nausea and vomiting are the most distressing side effects in cancer patients receiving chemotherapeutic agents, especially cisplatin (CIS; (Coates et al., 1983)). This discomfort is prolonged in the case of CIS and its congeners because they induce a biphasic pattern of vomiting. In chemotherapy-induced vomiting (CIV), an initial phase, dubbed the acute phase, occurs up to 24 hrs after administration of CIS, and is followed by a quiescent, non-emetic period of one to several days. Finally, after the quiescent period, the patient suffers a second set of bouts (about 2–7 days after CIS administration), the delayed phase (Hesketh et al., 2003b). With regard to CIV, the aim of antiemetic therapy is to abolish both phases, and thus improve patient quality of life and ensure that patients continue effective antitumor therapy.
The emetic reflex arc is a highly complex system whose mechanism is only partially characterized. Emetogens can act directly, in the gastrointestinal tract (GIT), and/or indirectly, by activating central nervous system (CNS) nuclei through stimulation of vagal afferents whose somata are in the nodose ganglion, and whose terminals are in the area postrema (AP), the nucleus of the solitary tract (NTS), and the dorsal motor nucleus of the vagus nerve (DMNX). This cluster of nuclei is collectively described as the dorsal vagal complex (DVC). Although the DVC is isolated from the periphery by the blood-brain barrier (BBB), the AP is a circumventricular organ which allows some emetogens to bypass the BBB and directly stimulate the area (Hornby, 2001). CIS-induced vomiting appears to involve stimulation of the entire DVC, and possibly other areas, by the aforementioned vagal afferents. This initial activation produces the giant retroperistaltic contraction that forces intestinal contents back to the stomach. Further stimulation of the NTS by AP and vagal afferents then inhibits DMNX motor neuron activity. This relaxes the lower esophageal sphincter (LES) muscle, and allows stomach and upper intestinal muscles to contract and expel the irritating contents (Aylwin et al., 1998; Browning and Travagli, 1999; Hornby and Abrahams, 2000; Onishi et al., 2007). Depending on the emetogen, different neurochemical systems are involved, although several key neurotransmitters are stimulated in common. CIV is not produced via a single specific neurotransmitter, but by the release of various emetogenic signals including serotonin, substance P (SP), dopamine, and prostaglandins (Goto et al., 1998; Kasabdji et al., 1996; Saito et al., 1999; Veyrat-Follet et al., 1997).
Because CIS induces release of multiple emetogenic stimuli, no currently available antiemetic administered alone can provide complete efficacy against CIV. Thus, different classes of antiemetics have been developed, such as the serotonin 5-HT3 receptor antagonists tropisetron and ondansetron, which are highly effective against the acute phase of CIV, but poorly effective against the delayed phase (for reviews, see (de Bruijn, 1992; Milne and Heel, 1991)). Recently, stimulation of NK1 neurokinin receptors was shown to be strongly emetogenic, and to play a key role in modulating the delayed phase of CIV (Darmani et al., 2008). Indeed, NK1 antagonists are more efficacious antiemetics against delayed-phase CIV than 5-HT3 antagonists (Hesketh et al., 2003a; Pendergrass et al., 2004), although they are significantly less effective against acute phase CIV. To boost the efficacy of either 5-HT3 or NK1 antagonist antiemetics, anti-inflammatory steroids such as dexamethasone (DEX) are frequently used in combination with them (Darmani, 2002; Hesketh et al., 2006). Clinical trials have demonstrated the effectiveness of DEX as an adjunct therapy in combination with a number of different antiemetics, in patients receiving a wide range of chemotherapeutic regimens (for review, see (Grunberg, 2007)). However, the mechanism of action of DEX is still unknown, and results in animal models have been highly variable. DEX administered alone has been found reasonably effective in the acute phase in most, but not all animal species, but only modestly effective in the delayed phase in most animals, and even ineffective in the house musk shrew model (Fukunaka et al., 1998; Malik et al., 2007; Rudd et al., 2000; Sam et al., 2003; Tanihata et al., 2004). Because these model animals had different emetic responses to equivalent doses of CIS, the doses of antiemetics needed in the cited studies varied significantly, thus muddying the question of how much species differences and/or dosing differences contributed to the responses to DEX and the other tested antiemetics.
Research also points to Δ9-tetrahydrocannabinol (Δ9-THC), one of the psychoactive constituents of marijuana, as an antiemetic therapy against CIV. Δ9-THC inhibits CIV via stimulation of brainstem, and possibly GIT, CB1 cannabinoid receptors (Darmani, 2001b; Van Sickle et al., 2003). The exact locus and mechanism of their antiemetic effect are still unclear, but cannabinoid receptors are known to be coupled to G-proteins, and frequently inhibit synaptic transmission when stimulated. Thus, their activation may reduce emetogen-induced stimulation of the DVC (Ray et al., 2008; Van Sickle et al., 2003). Cannabinoids show promise as antiemetics against both phases of CIV in animal models (Darmani, 2001b; Ray et al., 2008; Van Sickle et al., 2003) as well as in limited clinical use (Abrahamov et al., 1995; Darmani, 2005).
Clinical studies suggest that in blocking acute-phase CIV, a combination of a 5-HT3 receptor antagonist and DEX is more efficacious relative to either compound tested alone (Hesketh et al., 2006). Early clinical data regarding Δ9-THC were similar to those regarding DEX, in that Δ9-THC in combination with other antiemetics (e.g. prochlorperizine) enhances the antiemetic efficacy of treatment relative to either compound administered alone (Garb, 1981; Lane et al., 1990). More recently published data specifically confirm the enhanced antiemetic efficacy of 5-HT3 receptor antagonists when paired with Δ9-THC (Kwiatkowska et al., 2004), although this work was limited by only testing single doses of each compound in combination. Thus, to better compare the combined antiemetic efficacy of Δ9-THC with a 5-HT3 antagonist or DEX, we studied the dose-response efficacy (whether additive, synergistic, or ineffective) of Δ9-THC with either the 5-HT3 receptor antagonist tropisetron, or the anti-inflammatory steroid DEX, on the acute phase of CIS-induced emesis in the least shrew (Cryptotis parva) model species. Our lab has documented in detail that the emetic responses of this shrew species to CIS (acute and delayed phases), Δ9-THC, tropisetron, and DEX are very similar to those seen in humans (Darmani, 2001a, 1998; Darmani et al., 2005; Ray et al., 2008).
2. Methods
2.1. Animals and drugs
Both male and female least shrews (Cryptotis parva, 4–6 g, 35–60 days old) were used in the study. The feeding and maintenance of shrews have been described previously (Darmani, 1998; Darmani et al., 1999). Animal care was provided and monitored according to the “Guide for the Care and Use of Laboratory Animals”, DHSS Publication, revised, 1985, and all procedures were approved by the Institutional Animal Care and Use Committee of the Western University of Health Sciences.
Tropisetron was bought from Research Biochemicals Inc., Natick, MA, and all other drugs used were purchased from Sigma/RBI (St. Louis, MO). Δ9-THC, provided through NIDA (Rockville, MD), and dexamethasone (DEX), were initially dissolved to twice the stated concentrations in a 1:1:18 solution of ethanol/Emulphor™/0.9% saline, and then further diluted by the addition of an equal volume of saline. Cis-diammineplatinum (II) dichloride (cisplatin) and tropisetron were dissolved in distilled water.
2.2. Generation of dose-response curves
The present protocols were based upon our published findings that tropisetron dose-dependently attenuated cisplatin-induced vomiting (Darmani, 1998), and upon the basic and clinical studies examining combinations of Δ9-THC, prochlorperizine, and dexamethasone (Garb, 1981; Hesketh et al., 2006; Kwiatkowska et al., 2004; Lane et al., 1990). Briefly, all animals were randomly divided into two groups. On the test day, shrews were habituated to the laboratory environment for at least 1 hour prior to experimentation. Each animal was transferred to a 20×18×21 cm clean, clear plastic cage and offered four mealworms (Tenebrio sp.) 30 minutes prior to CIS injection (20 mg/kg, intraperitoneal). In the first group, 10 minutes prior to CIS administration, shrews were injected with a dose of either: a) tropisetron alone (0, 0.025, 0.1, 0.25, 1 or 5 mg/kg, i.p., n=8–12), or b) tropisetron in combination with a dose of Δ9-THC (0, 0.1, 0.25, 0.5, 1 or 5 mg/kg, i.p.). In the second group of shrews, 10 minutes prior to CIS administration the animals were injected i.p. with either a) varying doses of DEX alone (0, 0.25, 1, 5, 10, or 20 mg/kg, n=11–14), or b) varying doses of DEX in combination with different doses of Δ9-THC (0, 0.1, 0.25, 0.5, 1, 2.5, 5 or 10 mg/kg, i.p.). All drugs were administered at a volume of 0.1 ml/10g body weight. Immediately following the second injection, each shrew was placed in the observation cage, and both the number of animals vomiting per group and the frequency of vomiting (oral ejections of food or liquid) were recorded for the next 60 minutes.
2.3. Statistical analysis
Poisson regression and logistic regression analyses were employed for two-way analysis of vomiting frequency and protection from emesis, respectively. Because the results did not converge, a one-factor analysis for various doses of one drug in the presence of a given dose of the second drug was employed. Data regarding the frequency of emesis were analyzed by the Kruskal–Wallis nonparametric one-way analysis of variance (ANOVA), and post hoc analysis by Dunn’s multiple comparisons test. The incidence of emesis (number of animals vomiting) was analyzed by Fisher’s exact test to determine whether there were differences between groups, and when appropriate, pairwise comparisons were also made. A p value of < 0.05 was necessary to achieve statistical significance.
3. Results
3.1. Effect of tropisetron and Δ9-THC alone or in combination on the frequency of cisplatin-induced emesis
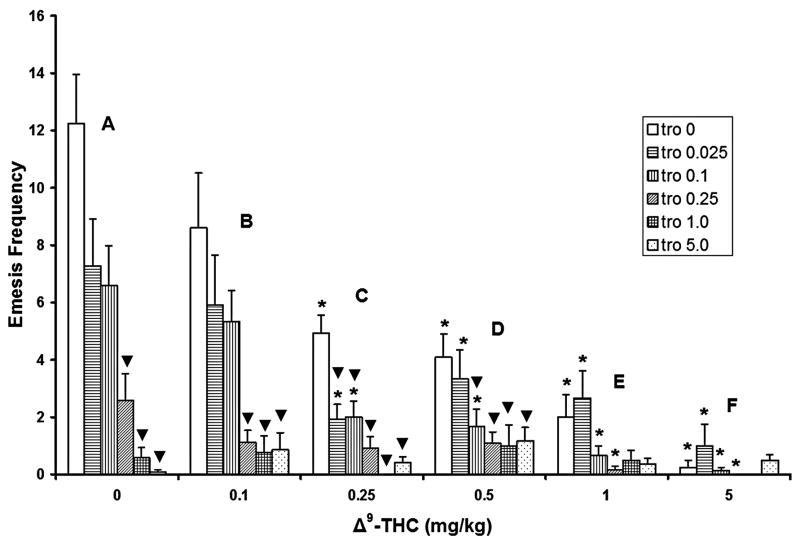
When administered without Δ9-THC (figure 1, group A), intraperitoneal administration of tropisetron dose-dependently reduced the frequency of cisplatin-induced emesis relative to its corresponding 0 mg/kg control group, with statistically significant reductions occurring at the 0.25 (79% reduction), 1 (94%), and 5 (99.8%) mg/kg doses. When pretreated with 0.1 mg/kg Δ9-THC (fig. 1, group B), the 0.25, 1, and 5 mg/kg doses of tropisetron also caused significant reductions in vomiting frequency (88%, 90.6%, and 89.4% respectively) relative to their corresponding 0 mg/kg control group. At the 0.25 mg/kg Δ9-THC dose, significant reductions in vomit frequency occurred at all tested doses of tropisetron (60%, 59.8%, 84%, 100%, and 93% respectively) relative to their corresponding control group (fig. 1, group C). At the 0.5 mg/kg Δ9-THC dose, tropisetron produced significant reductions in the frequency of emesis at the 0.1 (57.5% reduction), 0.25 (70%), 1 (70.1%), and 5 (69.8%) mg/kg doses, relative to its 0 mg/kg corresponding control group (fig. 1, group D). However, none of the cited doses of tropisetron had a significant effect on the frequency of CIS-induced vomiting (fig. 1, groups E and F) when combined with the 1 or 5 mg/kg doses of Δ9-THC.
Figure 1.
Both tropisetron (0, 0.025, 0.1, 0.25, 1, and 5 mg/kg) and Δ9-THC (0, 0.1, 0.25, 0.5, 1 and 5 mg/kg) by themselves reduced the frequency of cisplatin-induced (20 mg/kg) emesis in a dose-dependent manner. Only low doses of Δ9-THC (0.25 and 0.5 mg/kg) in combination with low doses of tropisetron (0.025 and 0.1 mg/kg) had significantly greater antiemetic efficacy in reducing the frequency of emesis relative to corresponding vehicle-treated tropisetron control groups. *Significantly different from 0 mg/kg Δ9-THC control group for the corresponding doses of tropisetron, p<0.05. ▼ Significantly different from the corresponding tropisetron vehicle control group, p<0.05.
When administered without tropisetron, Δ9-THC dose-dependently attenuated CIS-induced vomiting frequency, relative to its corresponding 0 mg/kg vehicle control, with statistically significant reductions noted at the 0.25 (59.3% reduction), 0.5 (66.7%), 1 (83.7%) and 5 (96.7%) mg/kg doses of Δ9-THC (fig. 1, open bars, groups C–F). When co-administered with 0.025 mg/kg of tropisetron, Δ9-THC caused significant reductions in vomiting frequency at doses of 0.25 (72.6% reduction), 0.5 (56.1%), 1 (61.6%), or 5 (86.3%) mg/kg (fig. 1, horizontally striped bars, groups C–F). A dose of 0.1 mg/kg tropisetron combined with 0.25 – 5 mg/kg Δ9-THC (fig. 1, vertically striped bars, groups C–F) also caused significantly reduced vomiting frequency (reductions of 69.7%, 74.6%, 88%, and 97.7%, respectively). When combined with 0.25 mg/kg tropisetron, Δ9-THC significantly reduced vomiting frequency only at doses of 1 (91% reduction) and 5 (100%) mg/kg (fig. 1, diagonally striped bar, groups E and F). When combined with doses of 1 or 5 mg/kg tropisetron, Δ9-THC failed to produce significant reductions in the frequency of emesis (fig. 1, crosshatched and stippled bars).
Of the doses of tropisetron and Δ9-THC tested in combination, only low doses of Δ9-THC (0.25 and 0.5 mg/kg) combined with low doses of tropisetron (0.025 and 0.1 mg/kg) had significantly greater additive efficacy in reducing the frequency of emesis (fig. 1 groups C and D).
3.2. Effects of tropisetron and Δ9-THC alone or in combination on the percentage of animals vomiting in response to cisplatin
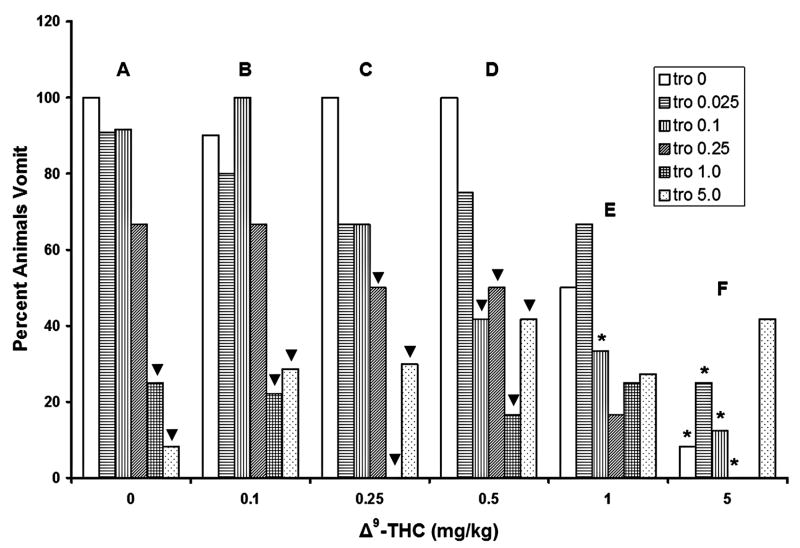
In addition to attenuating emetic frequency, tropisetron administered alone dose-dependently reduced the percentage of shrews vomiting in response to CIS, relative to its 0 mg/kg control group, with statistically significant reductions induced at the 1 (75% reduction) and 5 mg/kg (92%) doses (fig. 2, group A). Similarly, when combined with the 0.1 mg/kg dose of Δ9-THC, tropisetron significantly reduced the percentage of shrews vomiting at 1 (78%) and 5 mg/kg (71%) doses (fig. 2, group B). When co-administered with 0.25 mg/kg of Δ9-THC (fig. 2, group C), tropisetron significantly reduced the percentage of vomiting shrews at doses of 0.25 (50%), 1 (100%), and 5 mg/kg (70%). When co-administered with 0.5 mg/kg of Δ9-THC, tropisetron significantly reduced the number of animals vomiting at the 0.1 (58% reduction), 0.25 (50%), 1 (83%), and 5 mg/kg (58%) doses (fig. 2, group D). However, in combination with the 1 and 5 mg/kg doses of Δ9-THC, no dose of tropisetron significantly altered the percentage of shrews vomiting (fig. 2, groups E and F).
Figure 2.
Tropisetron dose-dependently and completely protected shrews from cisplatin-induced (20 mg/kg) emesis. Δ9-THC significantly reduced the number of shrews vomiting up to 92% in a dose-dependent manner. Δ9-THC had no significant synergistic efficacy with tropisetron in reducing the percentage of animal vomiting. *Significantly different from 0 mg/kg Δ9-THC control group for the corresponding doses of tropisetron, p<0.05. ▼ Significantly different from the corresponding tropisetron vehicle control group, p<0.05.
Post hoc analysis of the effects of Δ9-THC on the percentage of shrews vomiting revealed that when pretreated with the tested doses of tropisetron, the 5 mg/kg dose of Δ9-THC had a statistically significant effect when combined with the 0 (92% reduction), 0.025 (75%), 0.1 (88%), and 0.25 (100%) mg/kg doses of tropisetron (fig. 2, group F, asterisks), relative to the 0 mg/kg Δ9-THC control groups. Furthermore, when combined with a dose of 0.1 mg/kg tropisetron, 1 mg/kg Δ9-THC significantly reduced the number of shrews vomiting (77%), relative to its corresponding vehicle-injected control group, (fig. 2, group E, asterisk).
When based on the percentage of animals vomiting in response to CIS, no additive or synergistic antiemetic effects resulted from the tested combinations of Δ9-THC and tropisetron (fig. 2).
3.3. Effects of dexamethasone and Δ9-THC alone or in combination on the frequency of cisplatin-induced emesis
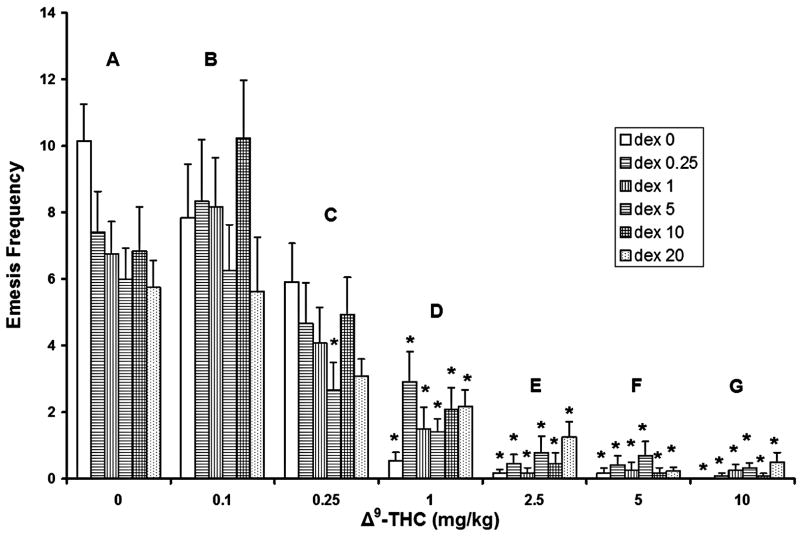
When administered without Δ9-THC, no tested doses of dexamethasone significantly attenuated the frequency of CIS-induced vomiting. While not statistically significant, there appears to be a trend towards a modest reduction in the frequency of emesis (0.05<p<0.1, fig. 3, group A).
Figure 3.
Dexamethasone (0, 0.25, 1, 5, 10, and 20 mg/kg) by itself failed to significantly attenuate the frequency of cisplatin-induced (20 mg/kg) emesis. Δ9-THC by itself significantly reduced the frequency of emesis produced by cisplatin in a dose-dependent manner. At the doses tested, Δ9-THC had no significant additive or synergistic antiemetic activity with dexamethasone. *Significantly different from 0 mg/kg Δ9-THC control group for the corresponding dexamethasone doses, p<0.05.
Δ9-THC administered alone replicated the results noted in figure 1, in that it dose-dependently reduced the frequency of emesis produced by CIS relative to its corresponding 0 mg/kg (vehicle) control group (fig. 3, groups D–G). When combined with DEX at doses of 1, 5, 10, or 20 mg/kg, Δ9-THC caused significant reductions (60.8% – 100%) in vomiting frequency at all tested doses (fig. 3, groups D–G, asterisks). Additionally, co-administration of 5 mg/kg DEX with the 0.25 mg/kg dose of Δ9-THC also significantly reduced (55.5%) the frequency of vomiting (fig. 3, group C).
At all tested doses, no combination of Δ9-THC and DEX had significant additive or synergistic antiemetic activity with regard to reducing the frequency of vomiting, relative to their corresponding vehicle-treated control groups.
3.4. Effects of dexamethasone and Δ9-THC alone or in combination on the percentage of animals vomiting in response to cisplatin
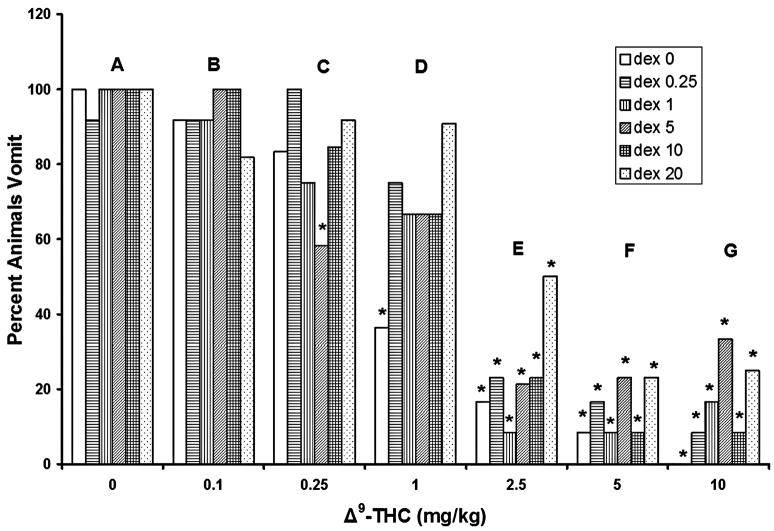
Based on analysis with Fisher’s exact test, no dose of DEX administered alone significantly reduced the number of shrews vomiting in response to CIS (figure 4, group A). Furthermore, even when combined with any of the tested doses of Δ9-THC, no dose of DEX was able to significantly reduce the number of shrews vomiting (relative to the 0 mg/kg DEX control groups, figure 4).
Figure 4.
Dexamethasone (0, 0.25, 1, 5, 10, and 20 mg/kg) failed to significantly reduce the number of shrews vomiting in response to cisplatin (20 mg/kg) exposure. Δ9-THC completely protected shrews from cisplatin-induced emesis in a dose-dependent manner. Δ9-THC had no significant additive activity with dexamethasone in reducing the emesis percentage of animals. *Significantly different from 0 mg/kg Δ9-THC control group for the corresponding dexamethasone doses, p<0.05.
The tested doses of Δ9-THC did, however, reduce the number of shrews expressing CIS-induced vomiting in an essentially dose-dependent manner. When administered without DEX, Δ9-THC at 1 mg/kg (fig. 4, group D) or greater (fig. 4, open bars, groups E–G), induced significant reductions (64%, 83.3%, 91.6%, and 100%, respectively) in the percentage of shrews vomiting, relative to their vehicle-injected control group (fig. 4, group A). The 2.5 mg/kg dose of Δ9-THC (fig. 4, group E) significantly reduced the percentage of shrews vomiting when co-administered with any of the tested doses of DEX (83%, 77%, 92%, 79%, 77%, and 50%, respectively), relative to the 0 mg/kg Δ9-THC control group. In addition, coadministration of 5 mg/kg of Δ9-THC and DEX (fig. 4, group F) significantly reduced the percentage of animals vomiting (92%, 83%, 92%, 77%, 92%, and 77%, respectively). The 10 mg/kg dose of Δ9-THC (fig. 4, group G) also significantly reduced the percentage of shrews vomiting whether administered without (100% reduction) or with DEX (92%, 83%, 67%, 92%, and 75%, respectively). Finally, in shrews pretreated with 5 mg/kg DEX, the 0.25 mg/kg dose of Δ9-THC induced a 42% reduction in the percentage of shrews vomiting relative to its vehicle-injected control group (fig. 4, diagonally striped bar, group C).
Overall, at the doses tested, Δ9-THC had no significant additive or synergistic antiemetic activity when combined with DEX.
4. Discussion
Cisplatin is clinically effective against a variety of tumors (Rosenberg, 1985), but is also one of the most emetogenic antitumor therapies currently in use (Laszlo et al., 1985). Because the discomfort from excessive vomiting can result in chemotherapy patient drop-out or poor antitumor therapy outcomes, adjunct antiemetic therapy is almost a necessity. Thus, it is important to understand the mechanisms behind CIV, both to improve the efficacy of the next generation of antiemetics and to indirectly improve antitumor therapy outcomes as well. The different classes of antiemetics currently in use, including the serotonin 5-HT3 receptor antagonists, anti-inflammatory steroids, and the cannabinoid CB1 receptor agonists, have been studied extensively and found to be varyingly effective, either alone or in combination (Bountra et al., 1996; Cubeddu et al., 1992; Darmani, 2002; Hesketh et al., 2006; Kudo et al., 2001; Kwiatkowska et al., 2004; Lane et al., 1990). Indeed, a significant body of literature has described several distinct combinations which are superior in terms of antiemetic potency: a) a 5-HT3 receptor antagonist (e.g. tropisetron or ondansetron) and DEX; b) Δ9-THC with dopamine D2 receptor antagonists; or c) Δ9-THC with a 5-HT3 receptor antagonist (Garb, 1981; Hesketh et al., 2006; Kwiatkowska et al., 2004; Lane et al., 1990). While they are mostly effective against the acute phase of CIV, none have proven particularly efficacious against the delayed phase. Further hampering progress is the limited variety of doses tested in the previously mentioned literature – in most cases only a single dose of either compound was tested in combination. The work presented here was done to more thoroughly describe the differences in, and the nature of (additive or synergistic), the enhanced antiemetic efficacy (if any) produced by combinations of different classes of antiemetics. We focused on combinations which included Δ9-THC, in part because combination therapies including Δ9-THC seem to be clinically effective, but are poorly understood mechanistically.
The current work focused on the 5-HT3 antagonist tropisetron, the CB1 agonist Δ9-THC, and the anti-inflammatory dexamethasone, and their potential antiemetic interactions. DEX is a synthetic steroid related to cortisol, and is used to treat many inflammatory and autoimmune conditions. It is also given to cancer patients to counteract CIV and certain other side effects. Clinical trials have found that DEX is effective as monotherapy, and also in combination with numerous other antiemetic agents, as a relatively broad-spectrum antiemetic (Ahn et al., 1994; Grunberg, 2007; Malik et al., 2007). However, in this study DEX administration alone (0.25 – 20 mg/kg) failed to either reduce the frequency of vomiting, or to protect shrews from CIS-induced vomiting. The mechanism and locus of activity of DEX-induced antiemetic activity are still unclear, making the analysis of this anomaly difficult. One possible cause of this anomaly is the species differences between humans and shrews. Clinically, DEX and a 5-HT3 antagonist together have demonstrated the ability to block the vast majority of acute vomiting due to CIS (Drechsler et al., 1997; Sorbe et al., 1994), but were ineffective even in combination when administered to the house musk shrew (Suncus murinus; Sam et al., 2003). In contrast to Suncus, however, to date the data related to emesis in the least shrew have been very similar to those collected for people (Darmani, 1998; Darmani et al., 2008; Darmani et al., 1999; Ray et al., 2008; and unpublished observations), and our previously published single-dose data regarding DEX administered alone have indeed shown reduced emetic activity (Darmani et al., 2005). A more likely explanation relates to the relatively high dose of CIS used in this study (20 mg/kg). DEX, like other antiemetics, is not 100% effective against CIV in humans, and the dosing regimen of CIS in chemotherapy is typically a lower dose, infused over a longer time period (Hesketh et al., 2003b; Vera et al., 2006). The high dose and intraperitoneal route of administration of CIS used here, which together can produce a very short infusion time, rapid systemic absorption, and a high initial spike in concentration, could have created a situation where the antiemetic ability of DEX was effectively overwhelmed. This would be especially true for the acute phase of CIV, due to its temporal proximity to the injection of CIS. The large dose of CIS was necessary to prevent a potential floor effect – while 10 mg/kg CIS is sufficient to produce emesis in the vast majority of least shrews, at this dose it was entirely possible that either drug administered alone could reduce vomiting to the extent that an enhanced interaction would be masked by the efficacy of the first drug. Thus, an attempt was made to strike a balance between the dose of CIS and obtaining a measurable interaction for the drug combinations.
Another key compound in this study, Δ9-THC appears to possess antiemetic efficacy in both cancer patients receiving chemotherapy, and in animal models of CIV, an effect most likely mediated by presynaptic inhibition of vagal afferents (or other terminals) via cannabinoid CB1 receptors (Darmani and Crim, 2005; Darmani and Johnson, 2004; Feigenbaum et al., 1989; Kwiatkowska et al., 2004; Van Sickle et al., 2003). However, recent evidence suggests some cannabinoids can also bind to vanilloid TRPV1 receptors (Coutts and Izzo, 2004; Sharkey et al., 2007), and certain endocannabinoids (e.g. 2-arachidonoylglycerol, 2-AG) produce constituents of the arachidonic acid cascade (e.g. prostaglandins), activating inflammatory pathways and inducing emesis (Coutts and Izzo, 2004; Cross-Mellor et al., 2007; Darmani, 2005). Thus, these two mechanisms may also play modulatory roles in the regulation of emesis by cannabinoids despite their contradictory natures. In the present study, Δ9-THC by itself attenuated both the vomiting frequency and the percentage of shrews vomiting, in a dose-dependent manner. Furthermore, low doses of Δ9-THC (0.25 and 0.5 mg/kg) in combination with low doses of tropisetron (0.025 and 0.1 mg/kg) demonstrated greater efficacy than their individually-administered counterparts in reducing the frequency of vomiting. These data are in agreement with published animal studies (Kwiatkowska et al., 2004) and clinical findings (Hesketh et al., 2003a; Meiri et al., 2007; Slatkin, 2007; Triozzi and Laszlo, 1987).
Although generally disappointing, the lack of a significant additive or synergistic antiemetic action is not surprising. There is likely to be a large overlap in the mechanisms with which these drugs block emesis, which would prevent the hoped-for enhanced antiemetic effect. For example, the mechanism of CB1 receptor antiemetic agonists, as stated above, likely relies on presynaptic inhibition. This CB1-mediated inhibition (e.g., in the DVC or GI nerve plexi) could reduce activity generated by postsynaptic, tropisetron-sensitive 5-HT3 receptor-containing neurons, or by presynaptic terminals which might colocalize these 5-HT3 receptors (Fink and Gothert, 2007). In fact, there is also evidence that cannabinoids can directly modulate 5-HT3 receptors allosterically (Barann et al., 2002; Xiong et al., 2007). If this direct crosstalk is also part of the mechanism of cannabinoid-mediated antiemesis, any potential additive effect may be dampened by interference from 5-HT3 antagonist binding. The slight enhancement of antiemetic ability by low doses of Δ9-THC in combination with low doses of tropisetron would result from incomplete receptor occupancy by either or both drugs, or possibly by incomplete anatomical overlap of cannabinoid and 5-HT3 receptors. In the case of DEX, emetic behavior would be mediated “downstream” from the presynaptic events modulated by CB1 receptors. Postsynaptic second-messenger systems, including the prostanoid-producing arachidonic acid metabolic pathways, would provide an interface through which DEX- and cannabinoid-mediated systems would overlap. The net effect in this case would be cannabinoid-mediated inhibition, or lack of stimulation, of neurons whose downstream antiemetic effector mechanisms were already inhibited, preventing the proposed enhancement of antiemetic activity by the combined drug regimen. On the other hand, the mechanisms through which tropisetron and DEX inhibit activation of emesis-initiating (or propagating) neurons would be less likely to interact with each other directly. The putative interactions among the substrates for serotonergic, cannabinergic, and inflammatory signaling are also relevant to the clinic, in that they provide a possible explanation for the relatively greater clinical effectiveness of 5-HT3 antagonists when paired with DEX (Ahn et al., 1994; Eberhart et al., 2006) versus the much less impressive results pairing Δ9-THC and 5-HT3 antagonists (Meiri et al., 2007; Slatkin, 2007). Further studies are necessary to dissect the anatomical and pharmacological substrates of emesis more thoroughly, since the underlying mechanisms of the emetic reflex are still unclear, and this understanding will be necessary for further development of more effective (i.e. broader-spectrum and/or biphasic activity) antiemetics.
Acknowledgments
This work was supported by NIH Grant #R01CA115331 to Dr. Darmani.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abrahamov A, Abrahamov A, Mechoulam R. An efficient new cannabinoid antiemetic in pediatric oncology. Life Sci. 1995;56:2097–102. doi: 10.1016/0024-3205(95)00194-b. [DOI] [PubMed] [Google Scholar]
- Ahn MJ, Lee JS, Lee KH, Suh C, Choi SS, Kim SH. A randomized double-blind trial of ondansetron alone versus in combination with dexamethasone versus in combination with dexamethasone and lorazepam in the prevention of emesis due to cisplatin-based chemotherapy. Am J Clin Oncol. 1994;17:150–6. doi: 10.1097/00000421-199404000-00012. [DOI] [PubMed] [Google Scholar]
- Aylwin ML, Horowitz JM, Bonham AC. Non-NMDA and NMDA receptors in the synaptic pathway between area postrema and nucleus tractus solitarius. Am J Physiol. 1998;275:H1236–46. doi: 10.1152/ajpheart.1998.275.4.H1236. [DOI] [PubMed] [Google Scholar]
- Barann M, Molderings G, Bruss M, Bonisch H, Urban BW, Gothert M. Direct inhibition by cannabinoids of human 5-HT3A receptors: probable involvement of an allosteric modulatory site. Br J Pharmacol. 2002;137:589–96. doi: 10.1038/sj.bjp.0704829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bountra C, Gale JD, Gardner CJ, Jordan CC, Kilpatrick GJ, Twissell DJ, Ward P. Towards understanding the aetiology and pathophysiology of the emetic reflex: novel approaches to antiemetic drugs. Oncology. 1996;53 (Suppl 1):102–9. doi: 10.1159/000227649. [DOI] [PubMed] [Google Scholar]
- Browning KN, Travagli RA. Characterization of the in vitro effects of 5-hydroxytryptamine (5-HT) on identified neurones of the rat dorsal motor nucleus of the vagus (DMV) Br J Pharmacol. 1999;128:1307–15. doi: 10.1038/sj.bjp.0702908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coates A, Abraham S, Kaye SB, Sowerbutts T, Frewin C, Fox RM, Tattersall MH. On the receiving end--patient perception of the side-effects of cancer chemotherapy. Eur J Cancer Clin Oncol. 1983;19:203–8. doi: 10.1016/0277-5379(83)90418-2. [DOI] [PubMed] [Google Scholar]
- Coutts AA, Izzo AA. The gastrointestinal pharmacology of cannabinoids: an update. Curr Opin Pharmacol. 2004;4:572–9. doi: 10.1016/j.coph.2004.05.007. [DOI] [PubMed] [Google Scholar]
- Cross-Mellor SK, Ossenkopp KP, Piomelli D, Parker LA. Effects of the FAAH inhibitor, URB597, and anandamide on lithium-induced taste reactivity responses: a measure of nausea in the rat. Psychopharmacology (Berl) 2007;190:135–43. doi: 10.1007/s00213-006-0589-7. [DOI] [PubMed] [Google Scholar]
- Cubeddu LX, Hoffmann IS, Fuenmayor NT, Malave JJ. Changes in serotonin metabolism in cancer patients: its relationship to nausea and vomiting induced by chemotherapeutic drugs. Br J Cancer. 1992;66:198–203. doi: 10.1038/bjc.1992.242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darmani NA. Antiemetic action of Δ9-tetrahydrocannabinol and synthetic cannabinoids in chemotherapy-induced nausea and vomiting. In: Onaivi ES, editor. Biology of Marijuana: From Gene to Behavior. London: Taylor and Francis; 2002. pp. 356–89. [Google Scholar]
- Darmani NA. Delta-9-tetrahydrocannabinol differentially suppresses cisplatin-induced emesis and indices of motor function via cannabinoid CB(1) receptors in the least shrew. Pharmacol Biochem Behav. 2001a;69:239–49. doi: 10.1016/s0091-3057(01)00531-7. [DOI] [PubMed] [Google Scholar]
- Darmani NA. Delta(9)-tetrahydrocannabinol and synthetic cannabinoids prevent emesis produced by the cannabinoid CB(1) receptor antagonist/inverse agonist SR 141716A. Neuropsychopharmacology. 2001b;24:198–203. doi: 10.1016/S0893-133X(00)00197-4. [DOI] [PubMed] [Google Scholar]
- Darmani NA. Endocannabinoids and gastrointestinal function. In: Onaivi ES, Sugiura T, Di Marzo V, editors. The Brain and Body’s Marijuana and Beyond. Boca Raton: CRC; 2005. pp. 393–418. [Google Scholar]
- Darmani NA. Serotonin 5-HT3 receptor antagonists prevent cisplatin-induced emesis in Cryptotis parva: a new experimental model of emesis. J Neural Transm. 1998;105:1143–54. doi: 10.1007/s007020050118. [DOI] [PubMed] [Google Scholar]
- Darmani NA, Crim JL. Delta-9-tetrahydrocannabinol differentially suppresses emesis versus enhanced locomotor activity produced by chemically diverse dopamine D2/D3 receptor agonists in the least shrew (Cryptotis parva) Pharmacol Biochem Behav. 2005;80:35–44. doi: 10.1016/j.pbb.2004.10.019. [DOI] [PubMed] [Google Scholar]
- Darmani NA, Johnson JC. Central and peripheral mechanisms contribute to the antiemetic actions of delta-9-tetrahydrocannabinol against 5-hydroxytryptophan-induced emesis. Eur J Pharmacol. 2004;488:201–12. doi: 10.1016/j.ejphar.2004.02.018. [DOI] [PubMed] [Google Scholar]
- Darmani NA, McClanahan BA, Trinh C, Petrosino S, Valenti M, Di Marzo V. Cisplatin increases brain 2-arachidonoylglycerol (2-AG) and concomitantly reduces intestinal 2-AG and anandamide levels in the least shrew. Neuropharmacology. 2005;49:502–13. doi: 10.1016/j.neuropharm.2005.04.007. [DOI] [PubMed] [Google Scholar]
- Darmani NA, Wang Y, Abad J, Ray AP, Thrush GR, Ramirez J. Utilization of the least shrew as a rapid and selective screening model for the antiemetic potential and brain penetration of substance P NK1 receptor antagonists. Brain Research. 2008;1214:58–72. doi: 10.1016/j.brainres.2008.03.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darmani NA, Zhao W, Ahmad B. The role of D2 and D3 dopamine receptors in the mediation of emesis in Cryptotis parva (the least shrew) Journal of Neural Transmission. 1999;106:1045–61. doi: 10.1007/s007020050222. [DOI] [PubMed] [Google Scholar]
- de Bruijn KM. Tropisetron. A review of the clinical experience. Drugs. 1992;43 (Suppl 3):11–22. doi: 10.2165/00003495-199200433-00005. [DOI] [PubMed] [Google Scholar]
- Drechsler S, Bruntsch U, Eggert J, Grote-Kiehn J, Gosse H, Bangerter M, Ukena D, Oehm C, Mezger J, Faerber L, Imhoff W, Untch M, Gallmeier WM. Comparison of three tropisetron-containing antiemetic regimens in the prophylaxis of acute and delayed chemotherapy-induced emesis and nausea. Support Care Cancer. 1997;5:387–95. doi: 10.1007/s005200050097. [DOI] [PubMed] [Google Scholar]
- Eberhart LH, Buning EK, Folz B, Maybauer DM, Kastner M, Kalder M, Koch T, Kranke P, Wulf H. Anti-emetic prophylaxis with oral tropisetron and/or dexamethasone. Eur J Clin Invest. 2006;36:580–7. doi: 10.1111/j.1365-2362.2006.01671.x. [DOI] [PubMed] [Google Scholar]
- Feigenbaum JJ, Richmond SA, Weissman Y, Mechoulam R. Inhibition of cisplatin-induced emesis in the pigeon by a non-psychotropic synthetic cannabinoid. Eur J Pharmacol. 1989;169:159–65. doi: 10.1016/0014-2999(89)90828-5. [DOI] [PubMed] [Google Scholar]
- Fink KB, Gothert M. 5-HT Receptor Regulation of Neurotransmitter Release. Pharmacol Rev. 2007;59:360–417. doi: 10.1124/pr.107.07103. [DOI] [PubMed] [Google Scholar]
- Fukunaka N, Sagae S, Kudo R, Endo T, Hirafuji M, Minami M. Effects of granisetron and its combination with dexamethasone on cisplatin-induced delayed emesis in the ferret. Gen Pharmacol. 1998;31:775–81. doi: 10.1016/s0306-3623(98)00102-5. [DOI] [PubMed] [Google Scholar]
- Garb S. Cannabinoids in the management of severe nausea and vomiting from cancer chemotherapy. Some additional considerations. J Clin Pharmacol. 1981;21:57S–59S. doi: 10.1002/j.1552-4604.1981.tb02574.x. [DOI] [PubMed] [Google Scholar]
- Goto H, Tachi K, Arisawa T, Niwa Y, Hayakawa T, Sugiyama S. Effects of gamma-glutamylcysteine ethyl ester in cisplatin-induced changes in prostanoid concentrations in rat gastric and colonic mucosa. Cancer Detect Prev. 1998;22:153–60. doi: 10.1046/j.1525-1500.1998.cdoa16.x. [DOI] [PubMed] [Google Scholar]
- Grunberg SM. Antiemetic activity of corticosteroids in patients receiving cancer chemotherapy: dosing, efficacy, and tolerability analysis. Ann Oncol. 2007;18:233–40. doi: 10.1093/annonc/mdl347. [DOI] [PubMed] [Google Scholar]
- Hesketh PJ, Grunberg SM, Gralla RJ, Warr DG, Roila F, de Wit R, Chawla SP, Carides AD, Ianus J, Elmer ME, Evans JK, Beck K, Reines S, Horgan KJ. The oral neurokinin-1 antagonist aprepitant for the prevention of chemotherapy-induced nausea and vomiting: a multinational, randomized, double-blind, placebo-controlled trial in patients receiving high-dose cisplatin--the Aprepitant Protocol 052 Study Group. J Clin Oncol. 2003a;21:4112–9. doi: 10.1200/JCO.2003.01.095. [DOI] [PubMed] [Google Scholar]
- Hesketh PJ, Grunberg SM, Herrstedt J, de Wit R, Gralla RJ, Carides AD, Taylor A, Evans JK, Horgan KJ. Combined data from two phase III trials of the NK1 antagonist aprepitant plus a 5HT 3 antagonist and a corticosteroid for prevention of chemotherapy-induced nausea and vomiting: effect of gender on treatment response. Support Care Cancer. 2006;14:354–60. doi: 10.1007/s00520-005-0914-4. [DOI] [PubMed] [Google Scholar]
- Hesketh PJ, Van Belle S, Aapro M, Tattersall FD, Naylor RJ, Hargreaves R, Carides AD, Evans JK, Horgan KJ. Differential involvement of neurotransmitters through the time course of cisplatin-induced emesis as revealed by therapy with specific receptor antagonists. European Journal of Cancer. 2003b;39:1074–80. doi: 10.1016/s0959-8049(02)00674-3. [DOI] [PubMed] [Google Scholar]
- Hornby PJ. Central neurocircuitry associated with emesis. Am J Med. 2001;111(Suppl 8A):106S–12S. doi: 10.1016/s0002-9343(01)00849-x. [DOI] [PubMed] [Google Scholar]
- Hornby PJ, Abrahams TP. Central control of lower esophageal sphincter relaxation. Am J Med. 2000;108(Suppl 4a):90S–98S. doi: 10.1016/s0002-9343(99)00345-9. [DOI] [PubMed] [Google Scholar]
- Kasabdji D, Shanmugam V, Rathinavelu A. Effect of cisplatin on dopamine release from PC12 cells. Life Sci. 1996;59:1793–801. doi: 10.1016/0024-3205(96)00522-x. [DOI] [PubMed] [Google Scholar]
- Kudo C, Minami M, Hirafuji M, Endo T, Hamaue N, Akita K, Murakami T, Kawaguchi H. The effects of nabumetone, a cyclooxygenase-2 inhibitor, on cisplatin-induced 5-hydroxytryptamine release from the isolated rat ileum. Res Commun Mol Pathol Pharmacol. 2001;110:117–32. [PubMed] [Google Scholar]
- Kwiatkowska M, Parker LA, Burton P, Mechoulam R. A comparative analysis of the potential of cannabinoids and ondansetron to suppress cisplatin-induced emesis in the Suncus murinus (house musk shrew) Psychopharmacology (Berl) 2004;174:254–9. doi: 10.1007/s00213-003-1739-9. [DOI] [PubMed] [Google Scholar]
- Lane M, Smith FE, Sullivan RA, Plasse TF. Dronabinol and prochlorperazine alone and in combination as antiemetic agents for cancer chemotherapy. Am J Clin Oncol. 1990;13:480–4. doi: 10.1097/00000421-199012000-00006. [DOI] [PubMed] [Google Scholar]
- Laszlo J, Clark RA, Hanson DC, Tyson L, Crumpler L, Gralla R. Lorazepam in cancer patients treated with cisplatin: a drug having antiemetic, amnesic, and anxiolytic effects. J Clin Oncol. 1985;3:864–9. doi: 10.1200/JCO.1985.3.6.864. [DOI] [PubMed] [Google Scholar]
- Malik NM, Liu YL, Cole N, Sanger GJ, Andrews PL. Differential effects of dexamethasone, ondansetron and a tachykinin NK1 receptor antagonist ( GR205171) on cisplatin-induced changes in behaviour, food intake, pica and gastric function in rats. Eur J Pharmacol. 2007;555:164–73. doi: 10.1016/j.ejphar.2006.10.043. [DOI] [PubMed] [Google Scholar]
- Meiri E, Jhangiani H, Vredenburgh JJ, Barbato LM, Carter FJ, Yang HM, Baranowski V. Efficacy of dronabinol alone and in combination with ondansetron versus ondansetron alone for delayed chemotherapy-induced nausea and vomiting. Curr Med Res Opin. 2007;23:533–43. doi: 10.1185/030079907x167525. [DOI] [PubMed] [Google Scholar]
- Milne RJ, Heel RC. Ondansetron. Therapeutic use as an antiemetic Drugs. 1991;41:574–95. doi: 10.2165/00003495-199141040-00006. [DOI] [PubMed] [Google Scholar]
- Onishi T, Mori T, Yanagihara M, Furukawa N, Fukuda H. Similarities of the neuronal circuit for the induction of fictive vomiting between ferrets and dogs. Auton Neurosci. 2007;136:20–30. doi: 10.1016/j.autneu.2007.03.002. [DOI] [PubMed] [Google Scholar]
- Pendergrass K, Hargreaves R, Petty KJ, Carides AD, Evans JK, Horgan KJ. Aprepitant: an oral NK1 antagonist for the prevention of nausea and vomiting induced by highly emetogenic chemotherapy. Drugs Today (Barc) 2004;40:853–63. doi: 10.1358/dot.2004.40.10.863745. [DOI] [PubMed] [Google Scholar]
- Ray A, Griggs L, Darmani NA. C-fos expression related to cisplatin-induced vomiting is blocked following delta-9-tetrahydrocannabinol injection in the least shrew. Behavioural Brain Research. 2008 doi: 10.1016/j.bbr.2008.07.028. Manuscript Submitted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg B. Fundamental studies with cisplatin. Cancer. 1985;55:2303–l6. doi: 10.1002/1097-0142(19850515)55:10<2303::aid-cncr2820551002>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- Rudd JA, Tse JYH, Wai MK. Cisplatin-induced emesis in the cat: effect of granisetron and dexamethasone. European Journal of Pharmacology. 2000;391:145–50. doi: 10.1016/s0014-2999(00)00061-3. [DOI] [PubMed] [Google Scholar]
- Saito R, Ariumi H, Kubota H, Nago S, Honda K, Takano Y, Kamiya H. [The role of tachykinin NK-1 receptors in emetic action in the area postrema of ferrets] Nippon Yakurigaku Zasshi. 1999;114 (Suppl 1):209P–14P. doi: 10.1254/fpj.114.supplement_209. [DOI] [PubMed] [Google Scholar]
- Sam TSW, Cheng JTY, Johnston KD, Kan KKW, Ngan MP, Rudd JA, Wai MK, Yeung JHK. Action of 5-HT3 receptor antagonists and dexamethasone to modify cisplatin-induced emesis in Suncus murinus (house musk shrew) European Journal of Pharmacology. 2003;472:135–45. doi: 10.1016/s0014-2999(03)01863-6. [DOI] [PubMed] [Google Scholar]
- Sharkey KA, Cristino L, Oland LD, Van Sickle MD, Starowicz K, Pittman QJ, Guglielmotti V, Davison JS, Di Marzo V. Arvanil, anandamide and N-arachidonoyl-dopamine (NADA) inhibit emesis through cannabinoid CB1 and vanilloid TRPV1 receptors in the ferret. Eur J Neurosci. 2007;25:2773–82. doi: 10.1111/j.1460-9568.2007.05521.x. [DOI] [PubMed] [Google Scholar]
- Slatkin NE. Cannabinoids in the treatment of chemotherapy-induced nausea and vomiting: beyond prevention of acute emesis. J Support Oncol. 2007;5:1–9. [PubMed] [Google Scholar]
- Sorbe B, Hogberg T, Himmelmann A, Schmidt M, Raisanen I, Stockmeyer M, de Bruijn KM. Efficacy and tolerability of tropisetron in comparison with a combination of tropisetron and dexamethasone in the control of nausea and vomiting induced by cisplatin-containing chemotherapy. Eur J Cancer. 1994;30A:629–34. doi: 10.1016/0959-8049(94)90534-7. [DOI] [PubMed] [Google Scholar]
- Tanihata S, Oda S, Nakai S, Uchiyama T. Antiemetic effect of dexamethasone on cisplatin-induced early and delayed emesis in the pigeon. European Journal of Pharmacology. 2004;484:311–21. doi: 10.1016/j.ejphar.2003.11.033. [DOI] [PubMed] [Google Scholar]
- Triozzi PL, Laszlo J. Optimum management of nausea and vomiting in cancer chemotherapy. Drugs. 1987;34:136–49. doi: 10.2165/00003495-198734010-00005. [DOI] [PubMed] [Google Scholar]
- Van Sickle MD, Oland LD, Mackie K, Davison JS, Sharkey KA. Delta9-tetrahydrocannabinol selectively acts on CB1 receptors in specific regions of dorsal vagal complex to inhibit emesis in ferrets. Am J Physiol Gastrointest Liver Physiol. 2003;285:G566–76. doi: 10.1152/ajpgi.00113.2003. [DOI] [PubMed] [Google Scholar]
- Vera G, Chiarlone A, Martin MI, Abalo R. Altered feeding behaviour induced by long-term cisplatin in rats. Auton Neurosci. 2006;126–127:81–92. doi: 10.1016/j.autneu.2006.02.011. [DOI] [PubMed] [Google Scholar]
- Veyrat-Follet C, Farinotti R, Palmer JL. Physiology of chemotherapy-induced emesis and antiemetic therapy. Predictive models for evaluation of new compounds. Drugs. 1997;53:206–34. doi: 10.2165/00003495-199753020-00003. [DOI] [PubMed] [Google Scholar]
- Xiong W, Hosoi M, Koo B, Zhang L. Anandamide inhibition of 5-HT3A receptors varies with receptor density and desensitization. Mol Pharmacol. 2007 doi: 10.1124/mol.107.039149. [DOI] [PubMed] [Google Scholar]