Abstract
The mechanical properties of the lung periphery are major determinants of overall lung function, and can change dramatically in disease. In this review we examine the various experimental techniques that have provided data pertaining to the mechanical properties of the lung periphery, together with the mathematical models that have been used to interpret these data. These models seek to make a clear distinction between the central and peripheral compartments of the lung by encapsulating functional differences between the conducing airways, the terminal airways and the parenchyma. Such a distinction becomes problematic in disease, however, because of the inevitable onset of regional variations in mechanical behavior throughout the lung. Accordingly, lung models are used both in the inverse sense as vehicles for extracting physiological insight from experimental data, and in the forward sense as virtual laboratories for the testing of specific hypothesis about mechanisms such as the effects of regional heterogeneities. Pathologies such as asthma, acute lung injury and emphysema can alter the mechanical properties of the lung periphery through the direct alteration of intrinsic tissue mechanics, the development of regional heterogeneities in mechanical function, and the complete derecruitment of airspaces due to airway closure and alveolar collapse. We are now beginning to decipher the relative contributions of these various factors to pathological alterations in peripheral lung mechanics, which may eventually lead to the development and assessment of novel therapies.
Keywords: lung impedance, inverse and forward modeling, asthma, emphysema, acute lung injury
1. Introduction
The structure of the lung has evolved to be uniquely suited for gas exchange, which of course is its primary function. A particular feature of the lung’s specialized structure is the enormous surface area of the blood-gas barrier, necessary because the transfer of oxygen and carbon dioxide between blood and alveolus takes place by passive diffusion. Blood and air are delivered to this huge diffusive interface via a system of branching conduits that begin with single conduits, the pulmonary artery and trachea respectively, each having a cross-sectional area of only a few square centimeters in humans. The sequential branching of the vascular and airway trees, however, eventually results in a set of parallel conduits numerous enough to have a combined area of many square meters (West, 2004). In the case of the airway tree, this extreme change in cross-section is associated with a change in the dominant physical processes involved in gas transport, from convective to diffusive as one moves distally into the lung. There is also a change in physiological function; the majority of the airway tree is involved only in the bulk transport of gas, while gas exchange with the blood occurs only at the very end of the tree. These various features make it natural, and often useful, to consider the lung as having two distinct regions, central and peripheral.
Of course, a little thought shows that subdividing a very complicated organ such as the lung into two disparate regions can be problematic. The concept of central and peripheral regions might mesh well with the commonly invoked model of the lung as a single compartment model served by a single airway, but reality is not nearly so simple. A key question is where to place the border between the central and distal regions. Should this border be defined anatomically or functionally? Is there even a definable border at all, given that the progression from conducting airway to respiratory zone (a region bordered by alveoli) is gradual? These issues become even more confusing in disease when the lung is likely to develop substantial regional differences in its structure and mechanical properties, invoking the concept of a multi-compartment system consisting of a collection of parallel balloon-and-pipe models connecting in parallel to a common central airway. Under these conditions, the distinction between the central and peripheral lung becomes blurred to the point that it becomes useful only to think of the periphery as being some region subtended by a sufficiently distal branch of the airway tree, yet it remains an important issue because all major lung diseases, such as asthma and emphysema, involve the lung parenchyma and small airways.
Our understanding of peripheral lung mechanics is thus not solely determined by the development of novel experimental techniques to study pressure-flow relationships in individual segments of the lung. As noted in a previous review (Bates and Lutchen, 2005), interpreting the mechanical data provided by these techniques has required the development of mathematical/computational models based on anatomical information from 3-dimensional imaging modalities. In this review we examine the history of these methods and how they have brought us to our current understanding of lung peripheral mechanics.
2. Probing Peripheral Lung Mechanics Directly
2.1 The retrograde catheter
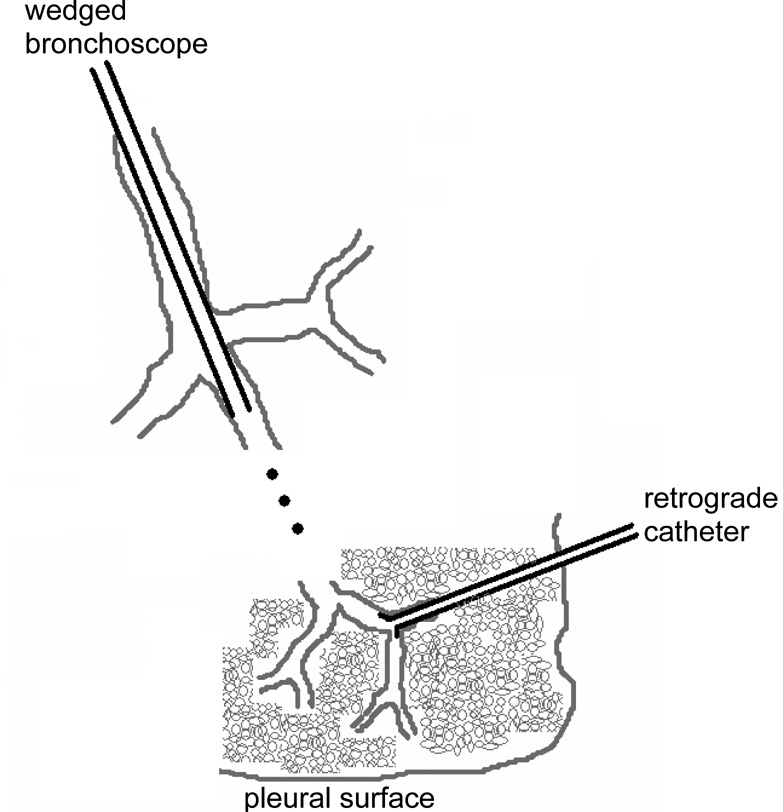
The first direct investigations of the lung periphery were provided by the retrograde catheter, introduced by Macklem and Mead in 1967 (Macklem and Mead, 1967). These investigators pulled a thin flexible catheter along the airways and out through a hole in the parenchyma until its flared end became wedged in a small airway (Fig. 1). By measuring the pressure at the other end of the catheter during ventilation of the lung, they were able to determine that most of the flow resistance of the normal airway tree was located proximally, thus establishing the notion that the periphery of the lung constitutes a silent zone. Subsequent calculations based on the dimensions of the various branches of the tree also indicated that most of its flow resistance normally occurs in the first 10 or so airway generations (Pedley et al., 1970). The retrograde catheter technique, however, was restricted to use in isolated lungs, and it also suffered from the frequency-response problems inherent in measuring pressure through a long thin conduit. Nevertheless, this technique played a seminal role in the science of pulmonary physiology by giving rise to the concept of the peripheral airways as constituting a “silent zone”; the combined cross-section of the peripheral airways is so great in a normal lung as to make only a small contribution to total airway resistance, so that significant narrowing of these airways has to occur before the results are manifest in terms of a change in overall lung function.
Figure 1.
Configurations of the retrograde and wedged catheter.
2.2 The alveolar capsule
The frequency response limitation of the retrograde catheter was overcome with the development of the alveolar capsule method, which also allowed a much smaller portion of the peripheral lung to be studied. Originally used by Sasaki et al. (Sasaki et al., 1977), this technique was subsequently perfected by Fredberg et al. (Fredberg et al., 1984). The alveolar capsule consists of a small conduit with a flanged end that is glued to the pleural surface of the lung with cyanoacrylate, thereby isolating a small section of pleura a few mm across. Careful puncturing of the pleura within the capsule then brings the sub-pleural alveoli into direct communication with the capsule chamber. A miniature piezoresistive pressure transducer lodged into the capsule provides a continuous recording of alveolar pressure. Most studies with the alveolar capsule have been applied to the lungs after the chest is opened and widely retracted, for obvious practical reasons. Consequently, the measurements of alveolar pressure obtained are not influenced by the mechanical properties of the chest wall, although with special surgical preparation the technique has been be applied in closed-chest dogs (Bates et al., 1989).
Since its introduction, the alveolar capsule technique has been used extensively in many species including dogs (Bates et al., 1988; Ludwig et al., 1990; Hantos et al., 1992b), rabbits (Tepper et al., 1992), rats (Shikanai-Yasuda et al., 1997), and mice (Tomioka et al., 2002), and has increased our understanding of pulmonary mechanics substantially. In particular, this technique has shown that sub-pleural alveolar pressures at different locations throughout the normal lung are almost identical when ventilation occurs within the frequency range of normal breathing. Of course, the lung is a complicated structure so some degree of regional heterogeneity is to be expected, and indeed can be detected in terms of both ventilation and perfusion with the appropriate investigative techniques (Altemeier et al., 2000; Kreck et al., 2001). Nevertheless, from a mechanical point of view the normal lung, despite its structural complexity, behaves in a remarkably homogeneous fashion at the frequencies of normal breathing. Furthermore, a substantial fraction of the lung resistance determined at these frequencies consists of a component due to viscous dissipation of energy in the lung tissues (Ludwig et al., 1987; Bates et al., 1988). Tissue resistance decreases with frequency, however, to the extent that by about 5 Hz lung resistance is approximately equivalent to the resistance of the airway tree alone. Multiple alveolar capsules installed on the same lung simultaneously have demonstrated that regional heterogeneities in alveolar pressure develop as frequency increases (Fredberg et al., 1984), and as bronchoconstriction develops (Fredberg et al., 1985; Ludwig et al., 1990). These studies highlight the fact that pathology invariably afflicts the lung in a heterogeneous manner, so airway narrowing affects some regions of the lung more than others.
2.3 The alveolar capsule oscillator
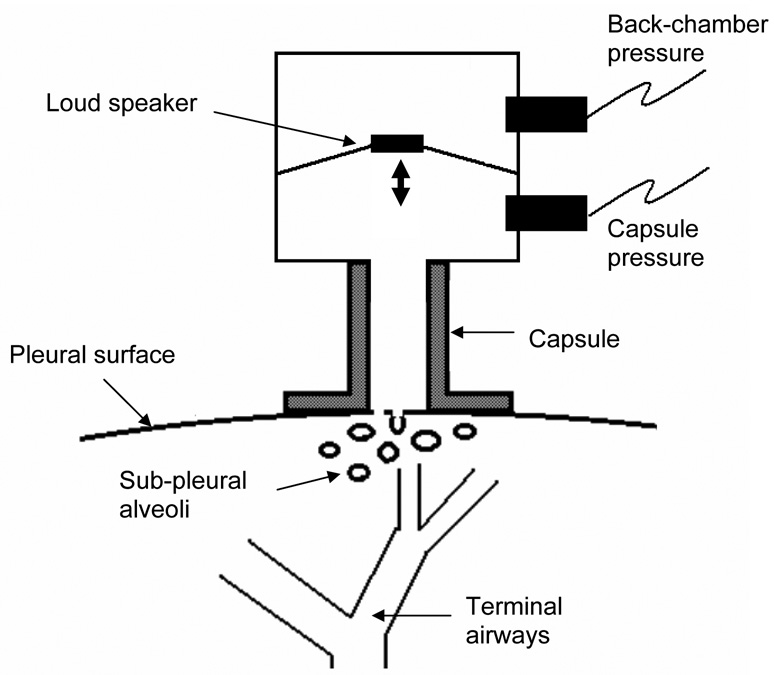
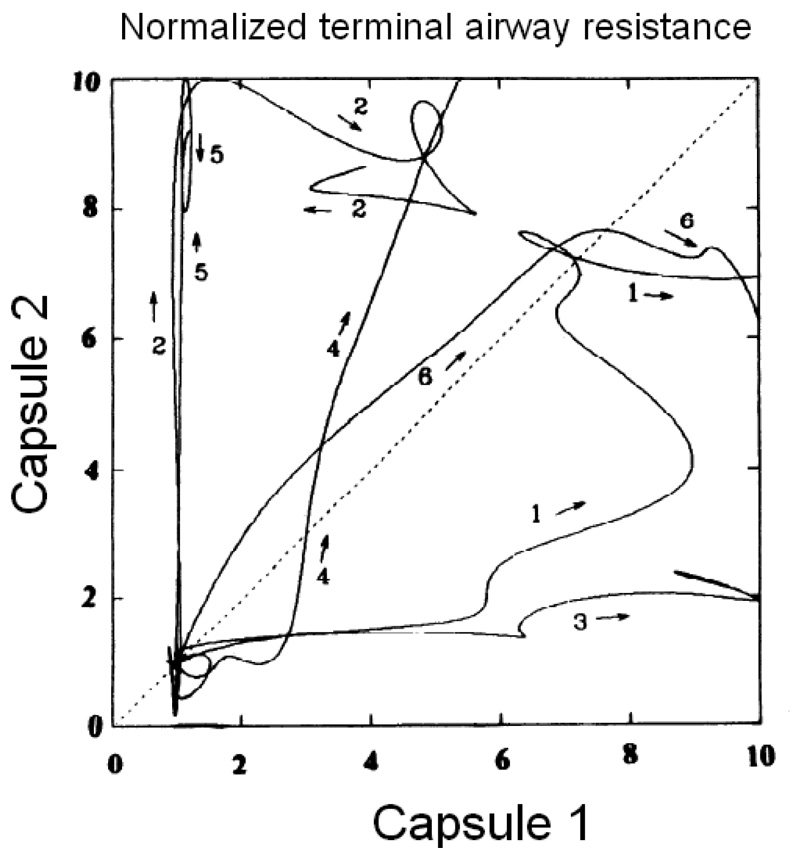
The alveolar capsule technique has its limitations, however, particularly in terms of its ability to resolve the nature of a regional abnormality in lung function. That is, while this technique makes differences in regional alveolar pressures clearly apparent, it does not allow one to decide if the differences are due to changes in local airway resistance or local tissue stiffness. In fact, if the airway tree constricts heterogeneously, the alveolar capsule no longer allows total lung resistance to be partitioned cleanly between its airway and tissue components, but instead tends to overestimate the contribution from the tissue (Lutchen et al., 1996b). To overcome this deficiency, one needs to know how the flow entering the airway tree is apportioned between the various regions whose pressures are being monitored, and direct measurement of such flows has so far eluded us. Davey and Bates (Davey and Bates, 1993) solved this problem by applying known oscillatory flows into the lung via the alveolar capsule while simultaneously measuring capsule pressure. Using a device called the alveolar capsule oscillator (Fig. 2), they identified an alveolar input impedance (ZA) that increased with bronchial challenge and which was interpreted in terms of a simple lumped-parameter model consisting of a local acinar compliance connecting to the rest of the lung through a terminal airway. At low frequencies of flow oscillation, ZA is determined primarily by the flow resistance of the terminal airway. Conversely, as frequency approaches 200 Hz, ZA becomes determined mainly by the stiffness of the tissues immediately surrounding the capsule, thereby providing a measure of acinar tissue elastance. The alveolar capsule oscillator was used to show that bronchoconstriction in dogs results in extensively heterogeneous responses in both local resistance and elastance (Mishima et al., 1994; Balassy et al., 1995; Mishima et al., 1996). These heterogeneities are both spatial and temporal in nature (Fig. 3). The same principle was also used by Suki et al. (Suki et al., 1994a) to show that the airways of a completely degassed dog lung open during slow inflation in a series of avalanches whose magnitudes follow a power-law probability distribution function.
Figure 2.
Schematic representation of the alveolar capsule oscillator.
Figure 3.
Plots of capsule oscillator measurements of terminal airway resistance obtained from two different capsule sites on the lung of 6 dogs during the acute development of bronchoconstriction to methacholine (acinar elastance was similarly heterogeneous). Each curve has been normalized to have a baseline (pre-methacholine) value of 0 and a maximum value of 10. A homogeneously responding lung would produce diagonal plots. The experimental data thus show that bronchoconstriction is highly heterogeneous both spatially and temporally (reproduced with permission from (Mishima et al., 1994).
Nevertheless, both the alveolar capsule technique and its oscillatory extension suffer from a fundamental sampling limitation in that they only garner information from alveoli just under the pleural surface, and only from a small number of them. It is not difficult to install half a dozen independent capsules on the lung of a dog, but this still represents a gross under-sampling of the tens of thousands of ascini within the entire lung. In a smaller animal such as a mouse, only a single capsule has been used (Tomioka et al., 2002).
2.4 The wedged catheter
A regional investigative technique that can be applied to the lung in intact animals and even human subjects is the wedged catheter, which is the converse of the retrograde catheter (Fig. 1). Here, a catheter is passed into the lungs via the trachea until its distal tip becomes firmly wedged in an airway. This technique was pioneered several decades ago (Woolcock and Macklem, 1971) but, unlike the retrograde catheter, has continued to be used in more recent studies to reveal the details of regional lung mechanics (Hantos et al., 1997). Conventional bronchoscopes have also been used, and typically wedge in about the 5th or 6th airway generation. Flow of gas can then be directed through the instrument channel of the scope into the isolated segment of lung. The wedged bronchoscope has been used with steady flow to assess the resistance of the collateral channels of the lung (Ludwig et al., 1988; Kaminsky et al., 2000). Kaminsky et al. (Kaminsky et al., 2004) oscillated flow through a wedged bronchoscope in human subjects to identify the input impedance of a segment approximately 1/30 the volume of the entire lung. The major practical difficulty with this technique was being able to resolve the impedance of the lung segment from that of the bronchoscope itself using measurements of flow and pressure made at the proximal end of the scope. Nevertheless, by using a bronchoscope with an especially wide-bore instrument channel, Kaminsky et al. were able to observe how segmental impedance changed following challenge with methacholine and albuterol.
2.5 In vivo microscopy
At a smaller scale, the technique of in vivo microscopy has been used to directly observe how alveoli behave when subjected to controlled inflation pressures. Here, a section of the pleural surface in a living animal is stabilized against a glass cover slip through which a low-power microscope is used to view individual alveoli during mechanical ventilation (Schiller et al., 2001; Allen et al., 2005). As with the alveolar capsule technique, in vivo microscopy suffers from sampling limitations because it is restricted to the study of only those alveoli that can be seen immediately under the pleural membrane. Nevertheless, this technique has produced a large amount of data related to alveolar collapse and reopening in the edematous condition known as acute lung injury in which the alveoli may become filled with fluid, especially at low lung volumes.
2.6 Lung explants
Small peripheral airway segments, and their response to chemical stimulation, can be observed directly using a technique known as lung explantation in which a slice of lung tissue is maintained alive under physiological conditions in an organ bath. Analysis of video images of the explant surface yields recordings of airway and blood vessel lumen areas as functions of time following challenge with various mediators of smooth muscle tone. Although not strictly a measurement of mechanics (because it has not yet been possible to obtain associated measurements of stress), this technique has provided a great deal of information about the pharmaco-mechanics of the airway wall (Bergner and Sanderson, 2003) as well as the way that the lung periphery may contribute to the overall mechanical properties of the lung in asthma (Adler et al., 1998).
3. Probing peripheral lung mechanics indirectly
As is evident from the above discussion, most of the techniques developed for studying peripheral lung mechanics are highly invasive, and many suffer from severe sampling limitations. For these reasons, and because of the need to develop methods for probing the global mechanical properties of the lung periphery that can be applied in human patients, recent investigations have resorted to the analysis of pressure and flow signals measured at the airway opening. In principle, such signals can be obtained without invasive surgery, but they represent the weighted influence of all lung regions. Consequently, extracting mechanical information about the lung periphery from these signals is not straightforward. The general approach to extracting such information is known as inverse modeling, or system identification, and relies on the use of mathematical and computational modeling. Specifically, the overall mechanical impedance of the lung (ZL) is measured over a range of frequencies (f), and then interpreted with the aid of a suitable inverse model comprised of components representing distinct central and peripheral elements in the lung (Hantos et al., 1987; Hantos et al., 1992a; Hantos et al., 1992b; Hirai et al., 1999; Tgavalekos et al., 2003). Ideally, the parameters of the inverse model correspond to physiologically important quantities (such as the flow resistance of the airway tree), and are evaluated by fitting the model to the measured ZL.
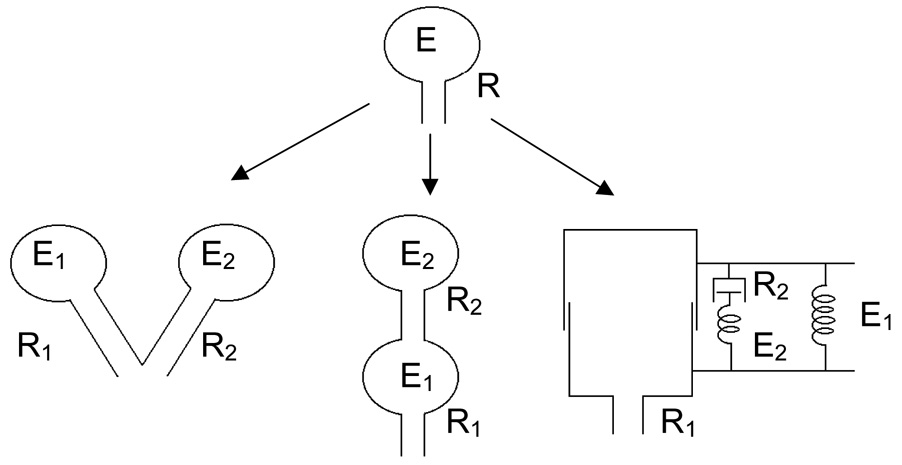
3.1 The single-compartment model
The simplest and still most widely invoked anatomically-based model of pulmonary mechanics is that consisting of a single homogeneously ventilated alveolar compartment served by a single conduit (Fig. 4, top). The equation of motion of this model is obtained by assigning an elastic constant (E) to the alveolar compartment and a flow-resistive constant (R) to the conduit so that the total transpulmonary pressure (Ptp) across the system is
| (1) |
where P0 is the pressure across the lungs at functional residual capacity (FRC). Values for RL and EL are readily obtained by fitting Eq. 1 to measured records of Ptp, V̇ and V using multiple linear regression.
Figure 4.
The single-compartment linear model (top) and its three two-compartment extensions: the parallel two-compartment model (lower left), the series two-compartment model (lower middle) and the viscoelastic model (lower right). The various flow resistive elements, resistance Ri, and elastic compartments, elastance Ei (i = 1,2), are indicated in the figures (reproduced with permission from (Bates et al., 2005).
3.2 Two-compartment models
While the single-compartment model embodies the obvious representation of the lungs in terms of its proximal conducting airways (the conduit) and its distal parenchymal tissue (the compartment), it is clearly a gross oversimplification of reality. In 1956, Otis et al (Otis et al., 1956) supposed that different regions of the lung might not all be ventilated to the same degree, and proposed a two-compartment model consisting of two independent versions of the single-compartment model (Fig. 4, lower left). They showed that when the time-constants of the two compartments are different, their respective shares of the total ventilation change with ventilation frequency. This gives the model an overall compliance that decreases as frequency increases. Mead (Mead, 1969) subsequently pointed out that a serial arrangement of compartments (Fig. 4, lower middle) can, in principle, produce the same frequency-dependent effects on compliance as the parallel arrangement. Here, the proximal compartment represents distensible central airways, while the distal compartment represents a uniformly ventilated lung periphery. Ventilation heterogeneity in the lungs can thus be compartmentalized in either a parallel or a serial fashion. However, only one model configuration, that of Mead, acknowledges the distinct existence of proximal and peripheral lung regions.
To complicate matters further, another two-compartment model that pre-dates even that of Otis et al. (Otis et al., 1956) was proposed by Mount in 1955 (Mount, 1955). This model posits that the frequency-dependent natures of compliance and resistance have nothing to do with ventilation heterogeneity, either parallel or serial, but rather are due to the viscoelastic nature of the tissues comprising the walls of the alveolar compartment. This means that the tension generated in lung tissue following sudden stretch will adapt slowly over time (stress adaptation), or equivalently that a sudden change in tension will be followed by a gradual response in length (creep).
The various two-compartment models discussed above (Fig. 4) are all physiologically plausible, yet they all obey a differential equation of exactly the same form so it is not possible to decide among them simply on the basis of pressure-flow relationships measured at the airway opening (Similowski and Bates, 1991). Deciding between these models requires additional physiological information. This information was eventually provided by the alveolar capsule technique when it was shown that normal lungs ventilated at normal breathing frequencies are best described by the model originally proposed by Mount (Mount, 1955), namely a uniformly ventilated alveolar compartment surrounded by viscoelastic tissue (Bates et al., 1988; Ludwig et al., 1990).
3.3 The constant-phase model
The viscoelasticity in the Mount model is embodied in a 3-element Kelvin body comprising a spring in parallel with a series spring-resistor combination (Fig. 4, lower right), and predicts that the stress adaptation to a sudden change in length will follow an exponential time course. Experiments on strips of lung tissue, however, show that stress adaptation is more accurately described by a power function of time. Equivalently, the magnitude of tissue impedance decreases with the inverse of frequency raised to some power close to unity (Bates et al., 1994). This led Hantos et al. (Hantos et al., 1987; Hantos et al., 1992b) to propose, on the basis of much prior work by Hildebrandt (Hildebrandt, 1970), a model in which the dissipative and elastic components of tissue impedance bear a ratio with respect to each other that is independent of frequency. This has come to be called the constant-phase model, with impedance given by
| (2) |
where RN is a Newtonian flow resistance closely approximating that of the airway tree (Tomioka et al., 2002), I is an inertance mostly due to the mass of gas in the central airways, G is a term reflecting viscous energy dissipation within the tissues, and H reflects energy storage in the tissues. G and H are linked via the parameter α through the equation
| (3) |
Exactly why lung tissue should behave in the manner embodied by the last term in Eq. 2 is still something of a mystery. Interestingly, many relaxation systems in nature seem to follow this type of behavior (Bates et al., 1994), for reasons which may have to do with the occurrence of avalanches in which many discrete yield events take place in a sequential chain (Bates, 2007).
Constant-phase tissue mechanics may also be explicable on the basis of polymer physics, and interestingly can be shown to arise from a differential equation containing a fractional derivative (Suki et al., 1994b). In any case, a single alveolar compartment of constant-phase tissue served by a single airway accounts for experimental observations of ZL with remarkable accuracy in terms of only 4 parameters, RN, I, G and H. The units of RN and I are those conventionally associated with resistance and inertance, but the units of G and H in Eq. 2 involve the parameter α and are thus rather cumbersome. Ito et al. (Ito et al., 2004) noted that normalizing Eq. 2 to the frequency f0 = 1/2π causes the parameters G and H to retain their same numerical values while simplifying their units to pressure.time.volume−1.
The parameters of the constant-phase model (particularly RN, G and H) have been used in numerous studies to characterize lung impedance up to frequencies of about 20 Hz in both humans (Kaczka et al., 1999; Henderson et al., 2003) and animals (Hantos et al., 1992a; Hantos et al., 1992b; Hirai et al., 1999; Gomes et al., 2000; Wagers et al., 2004), and allow a convenient partitioning of lung mechanics into a central airway component (RN and I) and a peripheral tissue component (G and H). It must be noted, however, that accurate evaluation of these parameters requires that all spontaneous breathing activity be suspended while forced oscillations in flow are applied to the airway opening. In conscious humans this necessitates voluntary apnea for periods of at least several seconds, while animals must be deeply anesthetized and possibly paralyzed. Also, when challenged with bronchial agonists, the lungs invariably become markedly heterogeneous, resulting in alveolar pressures that may vary greatly from one region to another even during regular ventilation (Fredberg et al., 1985; Ludwig et al., 1990). This significantly complicates the question of deciding which structures constitute the central compartment of the lung and where the periphery begins, even when the constant-phase model (Eq. 2) continues to provide a good fit to ZL (Hantos et al., 1992b; Tomioka et al., 2002; Wagers et al., 2004). Not surprisingly, RN increases during bronchoconstriction, reflecting decreased calibers of the conducting airways. However, G and H also invariably increase even when it is only the airways that are actually affected by a bronchial agonist. There are several reasons why bronchoconstriction appears to effect the lung periphery, some real and some only apparent. For example, it is possible for airway narrowing to have a real affect on the mechanical properties of the tissues through the mechanism of airway-parenchymal interdependence whereby the parenchymal attachments to the airways walls become distorted and stiffened as the airway walls constrict (Lauzon et al., 1992; Mitzner et al., 1992). Ventilation heterogeneity, by contrast, leads to apparent changes in tissue mechanics (and hence G and H) through the mechanism originally identified by Otis et al. (Otis et al., 1956). Also, bronchoconstriction can cause some of the smaller airways to actually close altogether through the mechanism of liquid bridging (Wagers et al., 2004; Lundblad et al., 2007), which leads to proportionate increases in both G and H.
With all these mechanisms likely occurring simultaneously, the possibility of deciding between any of them on the basis of measurements of ZL might seem hopeless. Fortunately, however, some headway can be made by considering the ratio G/H, known as hysteresivity (Fredberg and Stamenovic, 1989). Lutchen et al. showed, using data simulated by an anatomically-based lung model (Lutchen et al., 1996a), as well as in rats ventilated with gases of different viscosities during bronchoconstriction (Lutchen et al., 1996b), that much of the increase in G reflects the development of airway constriction heterogeneity rather than a real increase in the resistive attributes of the tissues. Bates and Allen (Bates and Allen, 2006) also established the link between increases in hysteresivity and heterogeneity analytically, and showed that this suggests that airways do not narrow indefinitely but rather snap completely shut once they reach a certain minimum radius, probably as a result of the formation of liquid bridges across the airway lumen. In any case, the constant-phase model does not contain a distinct mechanism to account for heterogeneity, so if the dependence of ZL on frequency increases because of heterogeneity, the constant-phase model will account for it through an increase in G relative to H.
Suki and coworkers have developed a heterogeneous form of the constant-phase model in which a parallel distribution of units, each described by Eq. 2, are allowed to have different values of either RN or H. The model with variable RN gave improved fits to impedance measurements obtained in bronchoconstricted dogs (Suki et al., 1997), while the variable H model reflected increased variability in tissue properties in mice treated with elastase (Ito et al., 2004). More recently, various distribution functions for H have also been implemented in the model and fitted to ZL data from dogs with acute lung injury (Kaczka et al., 2005). For most applications, however, the basic constant-phase model expressed by Eq. 2 and Eq. 3 is probably sufficient to convey, through the behavior of its parameters RN, I, G and H, how the central airways and the lung periphery are differentially affected by an experimental intervention. It is important to understand, however, that mechanical changes in the lung “periphery” in this context include the effects of both heterogeneous constriction of very small airways and alterations in intrinsic tissue properties, and it is difficult to distinguish one from the other on the basis of ZL alone.
3.4 Models of impedance at intermediate and high frequencies
The impedance spectrum of the lung at intermediate frequencies, typically from about 4 to 30 Hz, received a great deal of attention several decades ago when the forced oscillation approach to assessing lung mechanics was originally being developed. ZL over this frequency range can be interpreted using a 6-element model that partitions the lung into a central airway component and a distal tissue component (Peslin et al., 1985). However, computational power had become sufficient in the late 1970’s to allow a few researchers to probe the lung at frequencies into the hundreds or even thousands of Hz using the forced oscillation technique. This resulted in detailed modeling of the conducting airway tree, beginning with Fredberg and Hoenig (Fredberg and Hoenig, 1978) who introduced an efficient computational method based on the asymmetrical airway tree model of Horsfield et al. (Horsfield et al., 1971) to simulate ZL up to 10,000 Hz. Further efforts in this direction, however, did not come until Suki et al. (Suki et al., 1993) measured wave propagation in isolated trachea and derived equations to link the physical properties of the airway wall tissue (e.g. circumferential Young’s modulus, viscosity and density) to the volumetric resistance, elastance and inertance values that are used in transmission line equations describing airway mechanics. A number of subsequent studies have used these relations both for low and high frequency modeling (Habib et al., 1994; Lutchen et al., 1996a; Gillis and Lutchen, 1999) to compute lung impedance over a wide range of frequencies. While this approach has provided accurate model fits to experimental data, the increasing complexity of ZL with increasing frequency has hindered its physiological interpretation above a few hundred Hz. Also, the decrease of wavelength with frequency means that forced oscillatory pressure waves become less able to probe the lung periphery as frequency increases.
3.5 The role of forward modeling
As the above discussion shows, the precise physiological interpretation of inverse model parameters is rarely straightforward, particularly in situations that depart from normality, because the model is always much simpler than the real system under study. Thus, behavior attributed to the actions of a single mechanism in the model may actually be the result of many disparate mechanisms in the real system. Determining if this is the case experimentally is frequently impossible, so an invaluable alternative approach is to apply the inverse model to simulated data generated using a forward model in which the mechanisms under consideration are incorporated. Here, pressures and flows are simulated using a (usually much more complicated) model of the lung in which the parameters are assigned specific values on the basis of prior knowledge. The simulated data are then subjected to the inverse model to test hypotheses about specific mechanisms that might explain the experimental data. With modern computers, there is virtually no practical limit to the complexity that can be incorporated into a forward model, but of course its utility rests on the accuracy and relevance of the information it incorporates. Nevertheless, an accurate forward model can serve as a virtual laboratory in which data are generated under precisely controlled and known conditions, and the results on the parameters of the inverse model assessed.
Forward models of the lung have now reached a high degree of morphological and anatomical accuracy thanks to high-resolution three-dimensional imaging modalities such a computed tomography (CT) and magnetic resonance imaging (MRI) (Tgavalekos et al., 2003), and can be used effectively to interpret the results of inverse modeling. Lutchen and co-workers have exploited this approach extensively in the study of bronchoconstriction in asthmatic subjects to show that the frequency dependence of ZL exhibits distinct patterns characteristic of the spatial distribution of airway narrowing (Lutchen and Gillis, 1997; Gillis et al., 1999; Lutchen et al., 2001; Henderson et al., 2003) . The forward-inverse modeling approach has also been used to elucidate the mechanisms responsible for bronchial responsiveness in animal models of lung disease. For example, Mishima et al. (Mishima et al., 1996) used the Horsfield asymmetric branching model of the dog lung to simulate ZA. Comparison of these calculations to experimental measurements provided by the alveolar capsule oscillator showed that ZA reflects the mechanics of a lung region equal in size to approximately a single acinus. Thorpe and Bates (Thorpe and Bates, 1997) applied a similar approach to the entire dog lung to interpret experimental measurements of the time-course of impedance following bronchial challenge. Lutchen and co-workers have also recently developed an approach known as image functional modeling (Tgavalekos et al., 2003; Tgavalekos et al., 2005) in which detailed 3-dimensional images of the lung are used to inform an anatomically based computational model (Howatson Tawhai et al., 2000) that is then matched functionally to impedance data from a particular patient in order to provide a personalized description of the link between structure and function in the lung of a given subject (Tgavalekos et al., 2003). This approach allows the relative roles of central versus peripheral airway mechanics to be deduced. during asthmatic constriction (Bellardine Black et al., 2007).
4. Mechanical pathophysiology of the lung periphery
4.1 Asthma
Asthma is a complex disease defined on the basis of its symptoms, and which affects a large fraction of the population in developed countries (Masoli et al., 2004). A hallmark feature of asthma is airways hyperresponsiveness, defined as an abnormally pronounced decrement in lung function elicited by challenge with a standard dose of a smooth muscle agonist such as methacholine. This bronchoconstrictor response is also reversible by administration of a bronchodilator such as albuterol. Despite decades of intensive research into the pathophysiology of asthma, there is still confusion about what the dominant causes of airways hyperresponsiveness might be, but it seems clear that the lung periphery is involved in a major way. For example, using a forward model based on the Horsfield asymmetrical airway tree structure, Lutchen and co-workers (Lutchen et al., 1997; Gillis et al., 1999; Lutchen et al., 2001; Henderson et al., 2003) predicted that both RL and EL are profoundly elevated when only about 10% of the peripheral airways are severely narrowed. Subsequent experimental measurements in asthmatics showed very similar characteristics, leading to the conclusion that heterogeneous airway closures in the lung periphery had occurred in these subjects. Wagers et al. (Wagers et al., 2004) used a computational model to show that allergically inflamed mice are hyperresponsive to methacholine as a result of a increased closure of small peripheral airways caused by a thickened airway mucosa. This causes a greatly increased fraction of the lung to become derecruited during bronchoconstriction even when the degree of airway smooth muscle shortening is normal, and shows that a diagnosis of asthma does not necessarily imply that the underlying pathology is limited to abnormalities in the smooth muscle. Indeed, recent evidence suggests that severe asthma may involve a synergistic interaction between conducting airway constriction and peripheral airway closure (Bates et al., 2007). The image functional modeling studies of Lutchen et al. (Tgavalekos et al., 2005) show that a preponderance of severe airway constriction frequently occurs in the smaller airways of asthmatic individuals. Direct confirmation of the role of the lung periphery in asthma has been made by Kaminsky et al. (Kaminsky et al., 2004) by applying forced oscillations in flow through a wedged bronchoscope. They showed that the impedance of a peripheral lung segment is elevated in asthmatics compared to controls (Fig. 5).
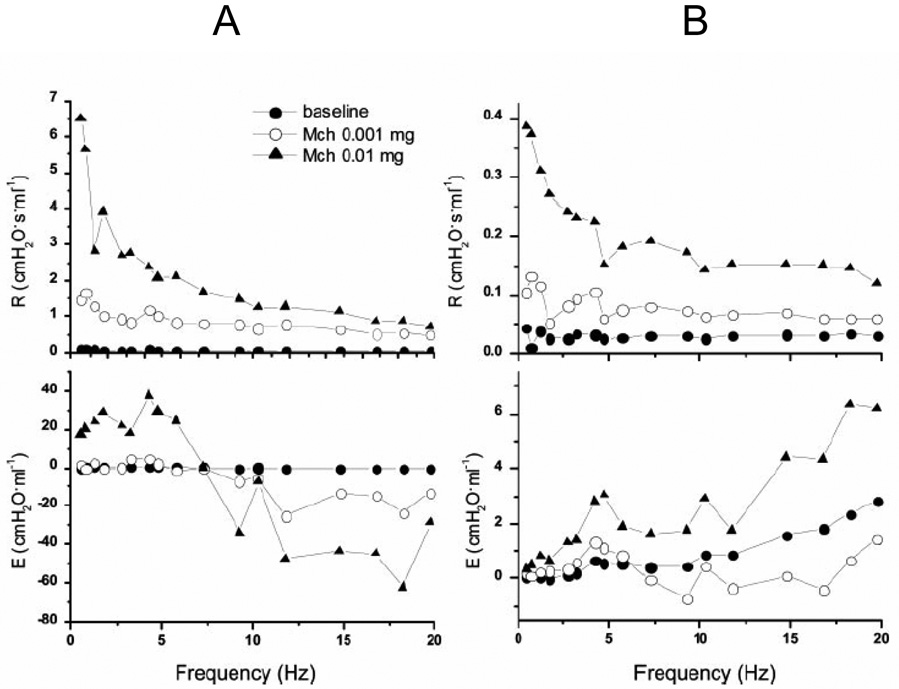
Figure 5.
Representative impedance spectra from two asthmatic subjects measured through a wedged bronchoscope at baseline and following two different doses of methacholine administration through the instrument channel of the bronchoscope. Note how bronchoconstriction greatly increases the negative frequency dependence of the real part of impedance (R) in both subjects, indicative of an increase in the heterogeneity of regional time-constants throughout the lung. Note, also, how the product of angular frequency with the imaginary part of impedance (E) varies between the two subjects. Subject A has a well defined resonant frequency (E = 0) indicating that the compliance of the airways did not play a major role in the way the lung responded to challenge. Subject B, in contrast, has an E that increases progressively with frequency indicative of central airway shunting, possibly due to the airway narrowing in this subject being concentrated in peripheral airways that prevented the applied oscillations in flow from moving beyond the airways and into the parenchymal regions of the lung (reproduced with permission from (Kaminsky et al., 2004).
4.2 Acute Lung Injury
Acute lung injury is a condition that can develop following severe trauma or a systemic bacterial infection, and is characterized by a sudden massive pulmonary edema that is often fatal (Dreyfuss and Saumon, 1998). Edematous fluid leaking into the distal airspaces of the lung can interfere with the surface-tension-lowering properties of pulmonary surfactant, and even result in complete alveolar filling. Not surprisingly, alterations in peripheral lung mechanics are a major feature of acute lung injury, manifest as a marked decrease in overall lung compliance, one of the key features of the condition. The major cause of the decrease in compliance is thought to be derecruitment of lung units; the remaining open units become over-inflated while accommodating the entire tidal volume delivered during mechanical ventilation, with commensurately increased inflation pressures. This interpretation of altered global lung mechanics in acute lung injury has been corroborated by Allen et al. (Allen et al., 2005) who used in vivo microscopy to show that abnormalities in global lung stiffness in injured rat lungs can be explained by the amount of alveolar collapse (Fig. 6). Regional heterogeneities of peripheral lung function also play a major role in the altered mechanics of acute lung injury (Kaczka et al., 2005)
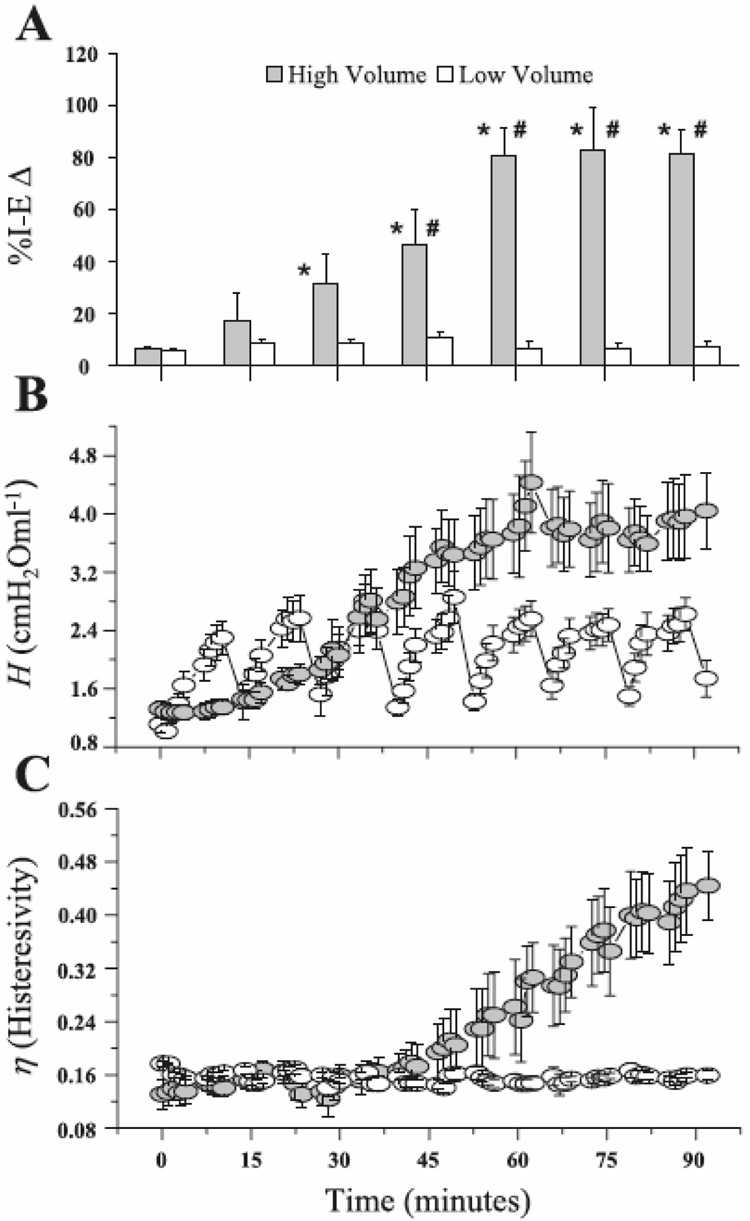
Figure 6.
A) Percent change in open alveoli between the end of inspiration and the end of expiration (%I-EΔ) observed by in vivo microscopy in rats receiving either high volume (injurious) or low volume (protective) mechanical ventilation. %I-EΔ in the high-volume group increases progressively with time indicating an increase in the fraction of alveoli that collapse with each expiration, while %I-EΔ does not change in the low-volume group indicating no accrual of lung tissue damage. B) Corresponding measurements of the parameter H (Eq. 2), an index of lung stiffness. C) Corresponding measurements of the ratio of G to H, termed hysteresivity. In the low-volume rats, even thought H increases transiently until reversed by periodic deep lung inflations, hysteresivity does not change with time, indicating that gradual but reversible derecruitment of alveoli was responsible for the changes in H. By contrast, hysteresivity increased progressively and irreversibly in the high-volume rats, indicating that their lung tissues were becoming increasingly damaged with time (reproduced with permission from (Allen et al., 2005).
Re-opening (recruiting) collapsed lung units through the application of elevated inflation pressures during mechanical ventilation is the main principle around which acute lung injury is currently managed in intensive care units. Although the conventional view has been that the successful recruitment of collapsed lung units is merely a matter of applying a sufficiently high inflation pressure, recent work makes it clear that there is a dynamic component to both recruitment and derecruitment (Allen and Bates, 2004; Allen et al., 2005). The dynamics of recruitment and derecruitment, which has been captured in an empirical computational model (Bates and Irvin, 2002), may also explain why varying tidal volume cycle-by-cycle (called variable ventilation) seems to be more efficacious than conventional ventilation in keeping the injured lung open (Bellardine et al., 2006).
4.3 Emphysema
Emphysema is a debilitating type of chronic obstructive disease with no known cure. There have been several mechanisms proposed to account for its pathogenesis, including a protease-antiprotease imbalance, chronic inflammation and oxidative stress (Barnes, 2000), all of which are likely to result in the remodeling and tensile weakening of the elastic and collagenous fibers in the alveolar walls. Accordingly, Suki et al. (Suki et al., 2003) argued that the progressive nature of the disease may in part reflect compounding mechanical failure caused by the continual stress produced by breath-to-breath variations in transpulmonary pressure. A computational network model of this process shows that the morphology of emphysematous lung tissue is greatly dependent on whether the destruction of alveolar walls is correlated or random. Specifically, when the destruction is directed preferentially to alveolar walls under the greatest stress, terminal airspaces coalesce and form progressively larger holes in the parenchyma, leading to a significant reduction in surface area available for gas exchange (Fig. 7). The functional mechanical consequences of these alterations include increased lung volumes and increased work of breathing due to dynamic airway collapse caused by increased lung compliance. If the tissue destruction progresses to include small airways, peripheral structure and function become even more heterogeneous resulting in a lung with a wide distribution of time-constants and an increased frequency dependence of resistance and elastance.
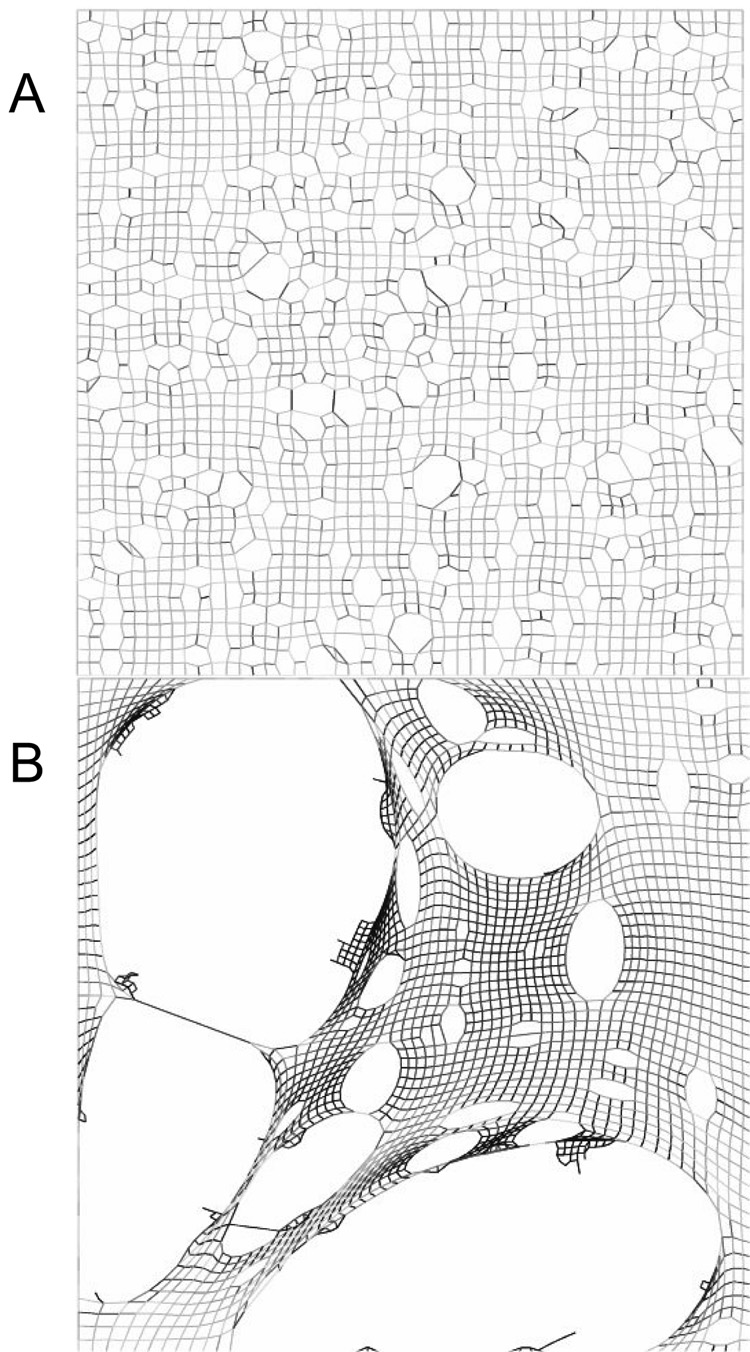
Figure 7.
Network modeling simulations of the development of emphysema occurring (A) through random destruction of alveolar walls, and (B) through the preferential destruction of walls under the greatest stress. The latter has an appearance much closer to real emphysematous lung tissue (adapted with permission from (Suki et al., 2003).
To try and account for this heterogeneity explicitly, Ito et al. (Ito et al., 2004) developed an inverse model consisting of multiple constant-phase tissue units in parallel, characterized by a distribution of values for the parameter H in Eq. 2. They fit the model to impedance spectra measured in mice treated with elastase, an enzyme known to cause lung tissue destruction similar to that seen in human emphysema. The number of parameters in this distributed model is only one larger than that in Eq. 2, yet it is able to provide significantly improved descriptions of ZL data from heterogeneous lungs, making it useful as a means of characterizing an important feature of lung pathology. Ito et al. (Ito et al., 2006) recently applied this inverse model to ZL data from pallid mice and found that it predicted collagen-related molecular abnormalities at the early age of 7 weeks, which is remarkable considering that pallid mice were previously thought to develop adult emphysema only by about one year of age (Martorana et al., 1993). Kaczka et al. (Kaczka et al., 2007) further studied the utility of this model by generating ZL data from a heterogeneous model and establishing that reasonable estimates of the distribution of H can be recovered.
5. Conclusions
It is clear that the mechanical properties of the lung periphery are major determinants of overall lung function, and can change dramatically in disease. Understanding the precise role of the lung periphery, however, must be undertaken in the context of mathematical models of the lung that encapsulate functional differences between the conducing airways, the terminal airways and the parenchyma, because disease is invariably accompanied by the onset of regional variations in mechanical behavior that blur the anatomic distinction between central and peripheral lung regions. Our understanding of the role of the lung periphery thus proceeds through the continual interplay between advances in mathematical/computational modeling and the development of novel experimental methods, each supporting and extending the other. In particular, models are used both in the inverse sense as vehicles for extracting physiological insight from experimental data, and in the forward sense as virtual laboratories for the testing of specific hypothesis about mechanism. Pathologies such as asthma, acute lung injury and emphysema can have a spectrum of effects that alter the mechanical properties of the lung periphery in ways that are, broadly speaking, divided into three categories. These include direct alterations in the intrinsic mechanical properties of the lung parenchymal tissue, the development of regional heterogeneities in mechanical function distributed topographically throughout the lung, and the complete derecruitment of airspaces due to airway closure and alveolar collapse. The combined use of direct physiological measurement, imaging, and inverse modeling are now allowing us to decipher the relative contributions of these various factors to pathological alterations in peripheral lung mechanics. This is beginning to shed light on how microscopic alterations in the periphery of the lung lead to the overall changes in function that affect breathing sensation and quality of life.
Acknowledgements
This work was supported by the National Heart, Lung, and Blood Institute Grants HL-59215, HL-67273, HL-75593 and HL-87788 and the Centers of Biomedical Research Excellence Grant P20 RR15557 from the National Center for Research Resources.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adler A, Cowley EA, Bates JHT, Eidelman DH. Airway-parenchymal interdependence after airway contraction in rat lung explants. J Appl Physiol. 1998;85:231–237. doi: 10.1152/jappl.1998.85.1.231. [DOI] [PubMed] [Google Scholar]
- Allen G, Bates JHT. Dynamic mechanical consequences of deep inflation in mice depend on type and degree of lung injury. J Appl Physiol. 2004;96:293–300. doi: 10.1152/japplphysiol.00270.2003. [DOI] [PubMed] [Google Scholar]
- Allen GB, Pavone LA, DiRocco JD, Bates JHT, Nieman GF. Pulmonary impedance and alveolar instability during injurious ventilation in rats. J Appl Physiol. 2005;99:723–730. doi: 10.1152/japplphysiol.01339.2004. [DOI] [PubMed] [Google Scholar]
- Altemeier WA, McKinney S, Glenny RW. Fractal nature of regional ventilation distribution. J Appl Physiol. 2000;88:1551–1557. doi: 10.1152/jappl.2000.88.5.1551. [DOI] [PubMed] [Google Scholar]
- Balassy Z, Mishima M, Bates JHT. Changes in regional lung impedance after intravenous histamine bolus in dogs: effects of lung volume. J Appl Physiol. 1995;78:875–880. doi: 10.1152/jappl.1995.78.3.875. [DOI] [PubMed] [Google Scholar]
- Barnes PJ. Chronic obstructive pulmonary disease. N Engl J Med. 2000;343:269–280. doi: 10.1056/NEJM200007273430407. [DOI] [PubMed] [Google Scholar]
- Bates JHT. A recruitment model of quasi-linear power-law stress adaptation in lung tissue. Ann Biomed Eng. 2007;35:1165–1174. doi: 10.1007/s10439-007-9291-0. [DOI] [PubMed] [Google Scholar]
- Bates JHT, Abe T, Romero PV, Sato J. Measurement of alveolar pressure in closed-chest dogs during flow interruption. J Appl Physiol. 1989;67:488–492. doi: 10.1152/jappl.1989.67.1.488. [DOI] [PubMed] [Google Scholar]
- Bates JHT, Allen GB. The estimation of lung mechanics parameters in the presence of pathology: a theoretical analysis. Ann Biomed Eng. 2006;34:384–392. doi: 10.1007/s10439-005-9056-6. [DOI] [PubMed] [Google Scholar]
- Bates JHT, Cojocaru A, Haverkamp HC, Rinaldi LM, Irvin CG. The Synergistic Interactions of Allergic Lung Inflammation and Intra-tracheal Cationic Protein. Am J Respir Crit Care Med. 2007 doi: 10.1164/rccm.200706-832OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bates JHT, Irvin CG. Time dependence of recruitment and derecruitment in the lung: a theoretical model. J Appl Physiol. 2002;93:705–713. doi: 10.1152/japplphysiol.01274.2001. [DOI] [PubMed] [Google Scholar]
- Bates JHT, Ludwig MS, Sly PD, Brown K, Martin JG, Fredberg JJ. Interrupter resistance elucidated by alveolar pressure measurement in open-chest normal dogs. J Appl Physiol. 1988;65:408–414. doi: 10.1152/jappl.1988.65.1.408. [DOI] [PubMed] [Google Scholar]
- Bates JHT, Lutchen KR. The interface between measurement and modeling of peripheral lung mechanics. Respir Physiol Neurobiol. 2005;148:153–164. doi: 10.1016/j.resp.2005.04.021. [DOI] [PubMed] [Google Scholar]
- Bates JHT, Maksym GN, Navajas D, Suki B. Lung tissue rheology and 1/f noise. Ann Biomed Eng. 1994;22:674–681. doi: 10.1007/BF02368292. [DOI] [PubMed] [Google Scholar]
- Bellardine Black CL, Hoffman AM, Tsai LW, Ingenito EP, Suki B, Kaczka DW, Simon BA, Lutchen KR. Relationship between dynamic respiratory mechanics and disease heterogeneity in sheep lavage injury. Crit Care Med. 2007;35:870–878. doi: 10.1097/01.CCM.0000257331.42485.94. [DOI] [PubMed] [Google Scholar]
- Bellardine CL, Hoffman AM, Tsai L, Ingenito EP, Arold SP, Lutchen KR, Suki B. Comparison of variable and conventional ventilation in a sheep saline lavage lung injury model. Crit Care Med. 2006;34:439–445. doi: 10.1097/01.ccm.0000196208.01682.87. [DOI] [PubMed] [Google Scholar]
- Bergner A, Sanderson MJ. Airway contractility and smooth muscle Ca(2+) signaling in lung slices from different mouse strains. J Appl Physiol. 2003;95:1325–1332. doi: 10.1152/japplphysiol.00272.2003. discussion 1314. [DOI] [PubMed] [Google Scholar]
- Davey BL, Bates JHT. Regional lung impedance from forced oscillations through alveolar capsules. Respir Physiol. 1993;91:165–182. doi: 10.1016/0034-5687(93)90097-t. [DOI] [PubMed] [Google Scholar]
- Dreyfuss D, Saumon G. Ventilator-induced lung injury: lessons from experimental studies. Am J Respir Crit Care Med. 1998;157:294–323. doi: 10.1164/ajrccm.157.1.9604014. [DOI] [PubMed] [Google Scholar]
- Fredberg J, Hoenig A. Mechanical response of the lungs at high frequencies. J Biomech Eng. 1978;100:57–66. [Google Scholar]
- Fredberg JJ, Ingram RH, Jr, Castile RG, Glass GM, Drazen JM. Nonhomogeneity of lung response to inhaled histamine assessed with alveolar capsules. J Appl Physiol. 1985;58:1914–1922. doi: 10.1152/jappl.1985.58.6.1914. [DOI] [PubMed] [Google Scholar]
- Fredberg JJ, Keefe DH, Glass GM, Glass GM, Frantz ID., 3rd Alveolar pressure nonhomogeneity during small-amplitude high-frequency oscillation. J Appl Physiol. 1984;57:788–800. doi: 10.1152/jappl.1984.57.3.788. [DOI] [PubMed] [Google Scholar]
- Fredberg JJ, Stamenovic D. On the imperfect elasticity of lung tissue. J Appl Physiol. 1989;67:2408–2419. doi: 10.1152/jappl.1989.67.6.2408. [DOI] [PubMed] [Google Scholar]
- Gillis HL, Lutchen KR. Airway remodeling in asthma amplifies heterogeneities in smooth muscle shortening causing hyperresponsiveness. J Appl Physiol. 1999;86:2001–2012. doi: 10.1152/jappl.1999.86.6.2001. [DOI] [PubMed] [Google Scholar]
- Gomes RF, Shen X, Ramchandani R, Tepper RS, Bates JHT. Comparative respiratory system mechanics in rodents. J Appl Physiol. 2000;89:908–916. doi: 10.1152/jappl.2000.89.3.908. [DOI] [PubMed] [Google Scholar]
- Habib RH, Suki B, Bates JHT, Jackson AC. Serial distribution of airway mechanical properties in dogs: effects of histamine. J Appl Physiol. 1994;77:554–566. doi: 10.1152/jappl.1994.77.2.554. [DOI] [PubMed] [Google Scholar]
- Hantos Z, Adamicza A, Govaerts E, Daroczy B. Mechanical impedances of lungs and chest wall in the cat. J Appl Physiol. 1992a;73:427–433. doi: 10.1152/jappl.1992.73.2.427. [DOI] [PubMed] [Google Scholar]
- Hantos Z, Daroczy B, Suki B, Nagy S, Fredberg JJ. Input impedance and peripheral inhomogeneity of dog lungs. J Appl Physiol. 1992b;72:168–178. doi: 10.1152/jappl.1992.72.1.168. [DOI] [PubMed] [Google Scholar]
- Hantos Z, Petak F, Adamicza A, Asztalos T, Tolnai J, Fredberg JJ. Mechanical impedance of the lung periphery. J Appl Physiol. 1997;83:1595–1601. doi: 10.1152/jappl.1997.83.5.1595. [DOI] [PubMed] [Google Scholar]
- Hantos Z, Suki B, Csendes T, Daróczy B. Constant-phase modelling of pulmonary tissue impedance. Bull. Eur. Physiopath. Respir. 1987;23 suppl. 12:326s. [Google Scholar]
- Henderson AC, Ingenito EP, Atileh H, Israel E, Suki B, Lutchen KR. How does airway inflammation modulate asthmatic airway constriction? An antigen challenge study. J Appl Physiol. 2003;95:873–882. doi: 10.1152/japplphysiol.00075.2003. discussion 863. [DOI] [PubMed] [Google Scholar]
- Hildebrandt J. Pressure-volume data of cat lung interpreted by a plastoelastic, linear viscoelastic model. J Appl Physiol. 1970;28:365–372. doi: 10.1152/jappl.1970.28.3.365. [DOI] [PubMed] [Google Scholar]
- Hirai T, McKeown KA, Gomes RF, Bates JHT. Effects of lung volume on lung and chest wall mechanics in rats. J Appl Physiol. 1999;86:16–21. doi: 10.1152/jappl.1999.86.1.16. [DOI] [PubMed] [Google Scholar]
- Horsfield K, Dart G, Olson DE, Filley GF, Cumming G. Models of the human bronchial tree. J Appl Physiol. 1971;31:207–217. doi: 10.1152/jappl.1971.31.2.207. [DOI] [PubMed] [Google Scholar]
- Howatson Tawhai M, Pullan AJ, Hunter PJ. Generation of an anatomically based three-dimensional model of the conducting airways. Ann Biomed Eng. 2000;28:793–802. doi: 10.1114/1.1289457. [DOI] [PubMed] [Google Scholar]
- Ito S, Bartolak-Suki E, Shipley JM, Parameswaran H, Majumdar A, Suki B. Early emphysema in the tight skin and pallid mice: roles of microfibril-associated glycoproteins, collagen, and mechanical forces. Am J Respir Cell Mol Biol. 2006;34:688–694. doi: 10.1165/rcmb.2006-0002OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito S, Ingenito EP, Arold SP, Parameswaran H, Tgavalekos NT, Lutchen KR, Suki B. Tissue heterogeneity in the mouse lung: effects of elastase treatment. J Appl Physiol. 2004;97:204–212. doi: 10.1152/japplphysiol.01246.2003. [DOI] [PubMed] [Google Scholar]
- Kaczka DW, Hager DN, Hawley ML, Simon BA. Quantifying mechanical heterogeneity in canine acute lung injury: impact of mean airway pressure. Anesthesiology. 2005;103:306–317. doi: 10.1097/00000542-200508000-00014. [DOI] [PubMed] [Google Scholar]
- Kaczka DW, Ingenito EP, Israel E, Lutchen KR. Airway and lung tissue mechanics in asthma. Effects of albuterol. Am J Respir Crit Care Med. 1999;159:169–178. doi: 10.1164/ajrccm.159.1.9709109. [DOI] [PubMed] [Google Scholar]
- Kaczka DW, Massa CB, Simon BA. Reliability of estimating stochastic lung tissue heterogeneity from pulmonary impedance spectra: a forward-inverse modeling study. Ann Biomed Eng. 2007;35:1722–1738. doi: 10.1007/s10439-007-9339-1. [DOI] [PubMed] [Google Scholar]
- Kaminsky DA, Bates JHT, Irvin CG. Effects of cool, dry air stimulation on peripheral lung mechanics in asthma. Am J Respir Crit Care Med. 2000;162:179–186. doi: 10.1164/ajrccm.162.1.9806079. [DOI] [PubMed] [Google Scholar]
- Kaminsky DA, Irvin CG, Lundblad L, Moriya HT, Lang S, Allen J, Viola T, Lynn M, Bates JHT. Oscillation mechanics of the human lung periphery in asthma. J Appl Physiol. 2004;97:1849–1858. doi: 10.1152/japplphysiol.00300.2004. [DOI] [PubMed] [Google Scholar]
- Kreck TC, Krueger MA, Altemeier WA, Sinclair SE, Robertson HT, Shade ED, Hildebrandt J, Lamm WJ, Frazer DA, Polissar NL, Hlastala MP. Determination of regional ventilation and perfusion in the lung using xenon and computed tomography. J Appl Physiol. 2001;91:1741–1749. doi: 10.1152/jappl.2001.91.4.1741. [DOI] [PubMed] [Google Scholar]
- Lauzon AM, Dechman G, Bates JHT. Time course of respiratory mechanics during histamine challenge in the dog. J Appl Physiol. 1992;73:2643–2647. doi: 10.1152/jappl.1992.73.6.2643. [DOI] [PubMed] [Google Scholar]
- Ludwig MS, Dreshaj I, Solway J, Munoz A, Ingram RH., Jr. Partitioning of pulmonary resistance during constriction in the dog: effects of volume history. J Appl Physiol. 1987;62:807–815. doi: 10.1152/jappl.1987.62.2.807. [DOI] [PubMed] [Google Scholar]
- Ludwig MS, Romero PV, Sly PD, Fredberg JJ, Bates JH. Interpretation of interrupter resistance after histamine-induced constriction in the dog. J Appl Physiol. 1990;68:1651–1656. doi: 10.1152/jappl.1990.68.4.1651. [DOI] [PubMed] [Google Scholar]
- Ludwig MS, Shore SA, Anderson K, Drazen JM. Temporal and regional variability of collateral resistance response to histamine. J Appl Physiol. 1988;64:2142–2149. doi: 10.1152/jappl.1988.64.5.2142. [DOI] [PubMed] [Google Scholar]
- Lundblad LK, Thompson-Figueroa J, Allen GB, Rinaldi L, Norton RJ, Irvin CG, Bates JHT. Airway hyperresponsiveness in allergically inflamed mice: the role of airway closure. Am J Respir Crit Care Med. 2007;175:768–774. doi: 10.1164/rccm.200610-1410OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lutchen KR, Gillis H. Relationship between heterogeneous changes in airway morphometry and lung resistance and elastance. J Appl Physiol. 1997;83:1192–1201. doi: 10.1152/jappl.1997.83.4.1192. [DOI] [PubMed] [Google Scholar]
- Lutchen KR, Greenstein JL, Suki B. How inhomogeneities and airway walls affect frequency dependence and separation of airway and tissue properties. J Appl Physiol. 1996a;80:1696–1707. doi: 10.1152/jappl.1996.80.5.1696. [DOI] [PubMed] [Google Scholar]
- Lutchen KR, Hantos Z, Petak F, Adamicza A, Suki B. Airway inhomogeneities contribute to apparent lung tissue mechanics during constriction. J Appl Physiol. 1996b;80:1841–1849. doi: 10.1152/jappl.1996.80.5.1841. [DOI] [PubMed] [Google Scholar]
- Lutchen KR, Jensen A, Atileh H, Kaczka DW, Israel E, Suki B, Ingenito EP. Airway constriction pattern is a central component of asthma severity: the role of deep inspirations. Am J Respir Crit Care Med. 2001;164:207–215. doi: 10.1164/ajrccm.164.2.2008119. [DOI] [PubMed] [Google Scholar]
- Macklem PT, Mead J. Resistance of central and peripheral airways measured by a retrograde catheter. J Appl Physiol. 1967;22:395–401. doi: 10.1152/jappl.1967.22.3.395. [DOI] [PubMed] [Google Scholar]
- Martorana PA, Brand T, Gardi C, van Even P, de Santi MM, Calzoni P, Marcolongo P, Lungarella G. The pallid mouse. A model of genetic alpha 1-antitrypsin deficiency. Lab Invest. 1993;68:233–241. [PubMed] [Google Scholar]
- Masoli M, Fabian D, Holt S, Beasley R. The global burden of asthma: executive summary of the GINA Dissemination Committee report. Allergy. 2004;59:469–478. doi: 10.1111/j.1398-9995.2004.00526.x. [DOI] [PubMed] [Google Scholar]
- Mead J. Contribution of compliance of airways to frequency-dependent behavior of lungs. J Appl Physiol. 1969;26:670–673. doi: 10.1152/jappl.1969.26.5.670. [DOI] [PubMed] [Google Scholar]
- Mishima M, Balassy Z, Bates JH. Acute pulmonary response to intravenous histamine using forced oscillations through alveolar capsules in dogs. J Appl Physiol. 1994;77:2140–2148. doi: 10.1152/jappl.1994.77.5.2140. [DOI] [PubMed] [Google Scholar]
- Mishima M, Balassy Z, Bates JHT. Assessment of local lung impedance by the alveolar capsule oscillator in dogs: a model analysis. J Appl Physiol. 1996;80:1165–1172. doi: 10.1152/jappl.1996.80.4.1165. [DOI] [PubMed] [Google Scholar]
- Mitzner W, Blosser S, Yager D, Wagner E. Effect of bronchial smooth muscle contraction on lung compliance. J Appl Physiol. 1992;72:158–167. doi: 10.1152/jappl.1992.72.1.158. [DOI] [PubMed] [Google Scholar]
- Mount L. The ventilation flow-resistance and compliance of rat lungs. J Physiol (Lond) 1955;127:157–167. doi: 10.1113/jphysiol.1955.sp005246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otis AB, McKerrow CB, Bartlett RA, Mead J, McIlroy MB, Selver-Stone NJ, Radford EP., Jr. Mechanical factors in distribution of pulmonary ventilation. J Appl Physiol. 1956;8:427–443. doi: 10.1152/jappl.1956.8.4.427. [DOI] [PubMed] [Google Scholar]
- Pedley TJ, Schroter RC, Sudlow MF. The prediction of pressure drop and variation of resistance within the human bronchial airways. Respir Physiol. 1970;9:387–405. doi: 10.1016/0034-5687(70)90094-0. [DOI] [PubMed] [Google Scholar]
- Peslin R, Duvivier C, Gallina C. Total respiratory input and transfer impedances in humans. J Appl Physiol. 1985;59:492–501. doi: 10.1152/jappl.1985.59.2.492. [DOI] [PubMed] [Google Scholar]
- Sasaki H, Takishima T, Sasaki T. Influence of lung parenchyma on dynamic bronchial collapsibility of excised dog lungs. J Appl Physiol. 1977;42:699–705. doi: 10.1152/jappl.1977.42.5.699. [DOI] [PubMed] [Google Scholar]
- Schiller HJ, McCann UG, 2nd, Carney DE, Gatto LA, Steinberg JM, Nieman GF. Altered alveolar mechanics in the acutely injured lung. Crit Care Med. 2001;29:1049–1055. doi: 10.1097/00003246-200105000-00036. [DOI] [PubMed] [Google Scholar]
- Shikanai-Yasuda MA, Pereira PM, Yamashiro-Kanashiro E, Duarte MI, Assis CM, Geraldes EA, Saldiva PH. Lung tissue mechanics in the early stages of induced paracoccidioidomycosis in rats. Braz J Med Biol Res. 1997;30:1175–1179. doi: 10.1590/s0100-879x1997001000006. [DOI] [PubMed] [Google Scholar]
- Similowski T, Bates JHT. Two-compartment modelling of respiratory system mechanics at low frequencies: gas redistribution or tissue rheology? Eur Respir J. 1991;4:353–358. [PubMed] [Google Scholar]
- Suki B, Barabasi AL, Hantos Z, Petak F, Stanley HE. Avalanches and power-law behaviour in lung inflation. Nature. 1994a;368:615–618. doi: 10.1038/368615a0. [DOI] [PubMed] [Google Scholar]
- Suki B, Barabasi AL, Lutchen KR. Lung tissue viscoelasticity: a mathematical framework and its molecular basis. J Appl Physiol. 1994b;76:2749–2759. doi: 10.1152/jappl.1994.76.6.2749. [DOI] [PubMed] [Google Scholar]
- Suki B, Habib RH, Jackson AC. Wave propagation, input impedance, and wall mechanics of the calf trachea from 16 to 1,600 Hz. J Appl Physiol. 1993;75:2755–2766. doi: 10.1152/jappl.1993.75.6.2755. [DOI] [PubMed] [Google Scholar]
- Suki B, Lutchen KR, Ingenito EP. On the progressive nature of emphysema: roles of proteases, inflammation, and mechanical forces. Am J Respir Crit Care Med. 2003;168:516–521. doi: 10.1164/rccm.200208-908PP. [DOI] [PubMed] [Google Scholar]
- Suki B, Yuan H, Zhang Q, Lutchen KR. Partitioning of lung tissue response and inhomogeneous airway constriction at the airway opening. J Appl Physiol. 1997;82:1349–1359. doi: 10.1152/jappl.1997.82.4.1349. [DOI] [PubMed] [Google Scholar]
- Tepper R, Sato J, Suki B, Martin JG, Bates JHT. Low-frequency pulmonary impedance in rabbits and its response to inhaled methacholine. J Appl Physiol. 1992;73:290–295. doi: 10.1152/jappl.1992.73.1.290. [DOI] [PubMed] [Google Scholar]
- Tgavalekos NT, Tawhai M, Harris RS, Musch G, Vidal-Melo M, Venegas JG, Lutchen KR. Identifying airways responsible for heterogeneous ventilation and mechanical dysfunction in asthma: an image functional modeling approach. J Appl Physiol. 2005;99:2388–2397. doi: 10.1152/japplphysiol.00391.2005. [DOI] [PubMed] [Google Scholar]
- Tgavalekos NT, Venegas JG, Suki B, Lutchen KR. Relation between structure, function, and imaging in a three-dimensional model of the lung. Ann Biomed Eng. 2003;31:363–373. doi: 10.1114/1.1557972. [DOI] [PubMed] [Google Scholar]
- Thorpe CW, Bates JHT. Effect of stochastic heterogeneity on lung impedance during acute bronchoconstriction: a model analysis. J Appl Physiol. 1997;82:1616–1625. doi: 10.1152/jappl.1997.82.5.1616. [DOI] [PubMed] [Google Scholar]
- Tomioka S, Bates JHT, Irvin CG. Airway and tissue mechanics in a murine model of asthma: alveolar capsule vs. forced oscillations. J Appl Physiol. 2002;93:263–270. doi: 10.1152/japplphysiol.01129.2001. [DOI] [PubMed] [Google Scholar]
- Wagers S, Lundblad LK, Ekman M, Irvin CG, Bates JHT. The allergic mouse model of asthma: normal smooth muscle in an abnormal lung? J Appl Physiol. 2004;96:2019–2027. doi: 10.1152/japplphysiol.00924.2003. [DOI] [PubMed] [Google Scholar]
- West JB. The Essentials. Philadelphia: Lippincott Williams & Wilkins; 2004. Respiratory Physiology. [Google Scholar]
- Woolcock AJ, Macklem PT. Mechanical factors influencing collateral ventilation in human, dog, and pig lungs. J Appl Physiol. 1971;30:99–115. doi: 10.1152/jappl.1971.30.1.99. [DOI] [PubMed] [Google Scholar]