Abstract
The doxycycline-inducible, gene regulatory system allows tight control of transgene expression for the study of organ development and disease pathogenesis. Multiple recent reports have employed this model to investigate various lung diseases including emphysema. For our study, we used this transgenic system to test whether prolonged, lung-specific, overexpression of the serine protease urokinase plasminogen activator (uPA) would result in alveolar wall destruction. Double transgenic mice were generated that possessed: (1) the rat Clara cell secretory protein promoter controlling the reverse tetracycline transactivator gene (CCSP:rtTA) and (2) the tetracycline operator controlling the murine uPA cDNA (tet[O]:muPA). Mice were treated with doxycycline beginning at age 6 wk to initiate uPA overexpression. Single transgenic and wild-type animals served as controls. A second group of double transgenic and control animals were maintained off of doxycycline. At ages 10, 18, and 30 wk, the mice underwent measurements of alveolar size, lung compliance, and total lung capacity. We found that, although the uPA overexpressing mice demonstrated an emphysema phenotype, similar abnormalities occurred in the CCSP-rtTA control animals. These CCSP-rtTA–related alterations occurred even without doxycycline exposure. Evaluation of a second transgenic line possessing the human surfactant protein C promoter controlling rtTA expression also exhibited lung abnormalities consistent with emphysema. These findings indicate that pulmonary epithelial expression of rtTA alone causes an emphysema phenotype in mice. Therefore, when using this system to study emphysema pathogenesis, the inclusion of proper controls is essential for accurate data interpretation.
Keywords: alveolar enlargement, human surfactant protein C promoter, rat Clara cell secretory protein promoter, reverse tetracycline transactivator, urokinase
The doxycycline-inducible, gene regulatory system has been developed to allow tight control of gene expression during different stages of organ development and disease pathogenesis (1). The primary constituents of this system include the tetracycline transactivator (tTA) driven by a promoter of choice and the polymeric tetracycline operator-cytomegalovirus (CMV) minimal promoter (tet[O]) driving expression of the desired transgene. tTA is a fusion protein consisting of the Escherichia coli–derived repressor of the Tn10 TC-resistance operon and the c-terminal portion of the herpes simplex virion protein 16 (1, 2). This molecule binds, in the absence of tetracycline or doxycycline, to the tetracycline operator sequence and suppresses gene expression. When the fusion protein interacts with doxycycline, it undergoes a conformational change and releases from its DNA recognition sequence, allowing gene transcription to proceed. tTA has been mutated to generate a promoter molecule, designated the reverse tetracycline transactivator (rtTA), that binds to the tet(O) sequence and induces gene expression when doxycycline is present. This doxycycline-inducible transgenic system can regulate organ-specific gene expression by driving the production of tTA or rtTA off of a tissue-specific promoter (1). In the lung, the rat Clara cell 10-kD secretory protein promoter (CCSP) and the human surfactant protein C promoter (SPC) have been employed, and multiple investigators have demonstrated a tight regulation of gene expression after doxycycline administration using this approach (3). In mice, the rat CCSP promoter has activity in type II pneumocytes as well as in epithelial cells within the trachea, bronchi, and larger bronchioles. The human SPC promoter, on the other hand, induces expression in epithelial cells within the small bronchiolar airways in addition to type II pneumocytes (3, 4).
Doxycycline-inducible gene regulation has been used to drive lung-specific overexpression of a growing number of cytokines and growth factors including IL-11, IFN-γ, IL-13, FGF-7, TGF-α, TGF-β, TNF-α, and most recently, IL-1β to investigate their roles in lung development and disease pathogenesis (3, 5–11). The induced expression of a subset of cytokines and growth factors including IL-11, TGF-α, and TGF-β has been shown to disrupt alveolar development, resulting in significant alveolar enlargement (5, 8, 9). Interestingly, within the fully developed lung, the prolonged overexpression of a number of cytokines and growth factor genes including IFN-γ, IL-13, TGF-β, TNF-α, and IL-1β causes the histopathologic appearance of marked alveolar enlargement, a hallmark feature of emphysema (6, 7, 11–15). The increased production of IFN-γ, IL-13, TNF-α, and IL-1β has also been found to induce physiologic features of emphysema including abnormalities in lung volume and, in some cases, lung compliance (6, 7, 10, 11).
Emphysema is the end product of alveolar wall destruction. This disorder is believed to occur in large part from an imbalance between proteases and their inhibitors (16). This is most evident in patients with α1-antitrypsin deficiency who have inadequate levels of a serine protease inhibitor and, as a result, experience unopposed neutrophil elastase–mediated proteolysis of their alveolar walls. Because this genetic deficiency accounts for < 1% of the clinical cases of emphysema, investigators have sought other protease/antiprotease imbalances that might contribute to the pathogenesis of this disease and that might help explain individual variation in susceptibility to tobacco smoke (12, 17–19). Accordingly, we set out to investigate whether overexpression of the serine protease, urokinase plasminogen activator (uPA), would generate emphysema in adult mice. We employed the doxycycline-inducible gene regulatory system to generate increased uPA levels specifically in the lung. We report here that, although uPA overexpression was associated with an emphysema phenotype, a similar increase in lung compliance, total lung capacity, and alveolar size occurred in control mice possessing only the rtTA construct. These rtTA-related alterations in lung physiology and histology occurred in the presence or absence of doxycycline exposure.
MATERIALS AND METHODS
Generation of Transgenic Mice
uPA-overexpressing mice were generated as previously described (20). Briefly, the muPA cDNA was cloned into a tetracycline operator expression cassette. This expression cassette consisted of seven tetracycline operator (tetO) repeats, a CMV minimal promoter, and a bovine growth hormone polyadenylation sequence (a gift from Dr. J. Whitsett and Dr. J. Tichelaar, Cincinnati, OH). The resultant muPA-containing expression cassette was then excised from its plasmid backbone and microinjected into (C57BL/6 × SJL) F2 mouse eggs. These eggs were implanted into pseudo-pregnant mothers, and the resultant offspring that possessed the transgenic construct (founders) were bred with 6- to 8-wk-old C57BL/6 partners. The transgene-positive mice (F1) from this cross were then bred with either a transgenic line containing the 2.3-kb rat CCSP promoter, the 1.0-kb rtTA coding sequence, and a 2.0-kb fragment from the human growth hormone containing introns and a polyadenylation sequence or a transgenic line possessing the 3.7-kb human SPC promoter, the 1.0-kb rtTA coding sequence, and the 0.45-kb SV40 polyadenylation signal (both rtTA lines were a gift from Dr. J. Whitsett and Dr. J Tichelaar). Transgenic offspring, which possessed both the tetO-muPA and CCSP-rtTA (or the tetO-muPA and SPC-rtTA) transgenes, were then backcrossed to C57BL/6 mice for 7–9 generations. The littermates generated from this backcross possessed one of four possible genotypes: tetO-muPA:CCSP-rtTA (or tetO-muPA:SPC-rtTA) double transgenic, tetO-muPA single transgenic, CCSP-rtTA (or SPC-rtTA) single transgenic, and nontransgenic wild-type (WT). Groups of female mice with each of the four genotypes were included in the subsequent experiments. The measurements were restricted to the female littermates to avoid the confounding effect sex might have on lung volume and compliance.
Assessment of Mouse Genotypes
The genotypes of offspring from the various crossings were identified at the time of weaning as previously described (20). Briefly, DNA was isolated from tail biopsies. To determine if the tetO-muPA construct was present in the genomic DNA of an individual mouse, a PCR reaction was performed with primers that distinguished this transgene from the native uPA gene. The tetO-muPA primer had the following sequences: 5′ primer, 5′-CTCTGCAACAGAGTCGTCAAATGGAGG-3′; 3′ primer, 5′-CG GGGGAGGGGCAAACAACAGATGGCTGGC-3′ (product size 324 bp). To determine if the CCSP-rtTA construct was present in the genomic DNA of an individual mouse, a PCR reaction was performed with the following primers: 5′ primer, 5′-ACTGCCCATTGCCCAAA CAC-3′; 3′ primer, 5′-AAAATCTTGCCAGCTTTCCCC-3′ (product size 525 bp). To determine if the SPC-rtTA construct was present in the genomic DNA of an individual mouse, a PCR reaction was performed with the following primers: 5′ primer, 5′-GACACATATAA GACCCTGGTCA-3′; 3′ primer, 5′-AAAATCTTGCCAGCTTTC CCC-3′ (product size 300 bp). The PCR conditions were the same for each of the primer pairs: 90°C for 1 min, followed by annealing at 62°C for 2 min, followed by elongation at 72°C for 2 min. This temperature sequence was repeated for 30 cycles. The genotype of each experimental animal was confirmed by isolating DNA from a second tail biopsy performed immediately before the pulmonary function testing.
Doxycycline Administration
tetO-muPA:CCSP-rtTA, tetO-muPA, CCSP-rtTA, and WT female offspring were generated from a cross between tetO-muPA:CCSP-rtTA and C57BL/6 parents. These mice were maintained on regular drinking water until they reached 6 wk of age. At this time, a minimum of five animals from each genotype underwent lung volume, compliance, and morphometric analyses to establish baseline values. Separate groups of animals from each genotype were randomly assigned to continue on regular drinking water or to receive doxycycline-treated water for 4, 12, or 24 wk. The doxycycline-treated water was generated by adding the antibiotic (Sigma Chemical, St. Louis, MO) to the animals' drinking water at a concentration of 0.5 mg/ml in 1% ethanol. The solution was kept in amber bottles to prevent light exposure and was changed every Monday, Wednesday, and Friday.
In a separate experiment, female rtTA-positive offspring (i.e., possessing the tetO-muPA:SPC-rtTA and SPC-rtTA genotypes) and female rtTA-negative mice (i.e., possessing the tetO-muPA and WT genotypes) were generated from a cross between tetO-muPA:SPC-rtTA and C57BL/6 parents. These mice were maintained on regular drinking water until they were 6 wk of age. At this time, separate groups of rtTA-positive and rtTA-negative animals were continued on regular drinking water or were given doxycycline-treated water for an additional 24 wk. The antibiotic water was generated and handled in identical manner as detailed above.
Plasminogen Activator Activity Assay
Double transgenic (tetO-muPA:rtTA-CCSP) mice were exposed to doxycycline for 12 h, 24 h, 48 h, 72 h, and 1 wk. An additional group of double transgenic (tetO-muPA:rtTA-CCSP) mice received no doxycycline to evaluate for non–doxycycline-induced “leaky” gene expression. Bronchoalveolar lavage (BAL) fluid was collected by instilling 1.0 ml of sterile PBS intratracheally, aspirating the fluid after 10 s, and then centrifuging the samples for 10 min at 1,500 × g at 4°C. The supernatant was collected and then stored at −70°C until further analysis. Plasminogen activator (PA) activity in the BAL fluid samples was assessed with casein zymography according to the method of Powell-Jones with modifications as described previously (21). Samples were separated by SDS-PAGE on 10% gels containing α-casein (7 mg/ml) and human glu-plasminogen (20 μg/ml). After electrophoretic separation, the gels were washed in 1% Tween 80 for 1 h at 37°C and then incubated in PBS containing 0.1% Tween 80 overnight at room temperature. Finally, the gels were stained with Coomassie blue dye and then destained in a solution of 10% acetic acid and 50% methanol. The molecular weights of the lytic bands were calculated by comparison to human uPA and human tPA standards.
Lung Volume and Lung Compliance Measurements
The lung volume and compliance of each animal was measured with a whole body plethysmograph as part of the Pulmonary Maneuvers System (Buxco Electronics, Wilmington, NC). The mice were initially anesthetized with an intraperitoneal injection of ketamine/xylazine. A tracheostomy was then performed, and a 19-gauge metal tracheostomy tube was inserted and secured with a nylon suture. The mouse was attached via the tracheostomy tube to a mechanical ventilator in a sealed plethysmograph. Functional residual capacity (FRC) was determined by occluding the airway while the mouse attempted to breathe. The pressure that the animal exerted and the change in the animal's thoracic volume were measured and these values permitted the calculation of FRC using Boyle's law. From the FRC, the animal's total lung capacity (TLC) was calculated. Next, a quasi-static pressure/volume maneuver was used to measure the lung compliance. For this measurement, the ventilator delivered a breath to TLC (30 mm H2O) and then maintained the pressure for a short period of time before allowing slow expiration to residual volume (RV). Pressure and volume were recorded during this slow exhalation. Compliance is reported as the Cchord, which represents the slope of the compliance curve between 0 and 10 cm H2O pressure. Data for all maneuvers were analyzed using Biosystem XA software (Buxco Electronics, Inc., Wilmington, NC). The total time of mechanical ventilation was less than 10 min.
Morphometry
After the pulmonary function measurements, each animal was killed with an intraperitoneal injection of pentobarbital sodium. Each animal's lungs were then inflated to 25 cm H2O static pressure with an intratracheal instillation of 10% formalin in PBS. The lungs were then stored in 10% formalin in PBS for 24 h. Thereafter, the left lung was removed and sectioned into 3-mm slices along the long axis. A random number was generated to determine which two sections were chosen for morphometric analysis. These sections were then placed into a cassette, embedded in paraffin, and processed. Six-micron sections were fixed to glass slides and stained with hematoxylin and eosin. Morphometry was then performed by a modification of methods previously reported (22). Computer Assisted Stereology Toolbox (C.A.S.T; Olympus, Melville, NY) software was used to impose a line grid onto video images of the histologic samples for the purpose of making efficient and unbiased estimates of alveolar intercepts. Lines that were superimposed on airways and blood vessels were excluded from the measurement. The data generated from the morphometric analysis was then used to calculate the mean linear intercept.
Statistical Analysis and Data Presentation
Values are expressed as means ± SEM. Groups were compared using a two-way ANOVA with Bonferroni post-test comparisons using Prism software for Microsoft unless otherwise specified.
RESULTS
uPA Expression after Doxycycline Treatment
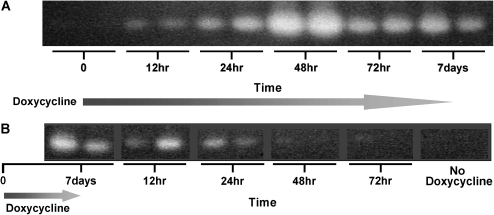
We have previously demonstrated that the tetO-muPA:CCSP-rtTA mice generate increased levels of uPA in their lung after 7 and 14 d of doxycycline treatment (20). To ensure the fidelity of this transgenic system to regulate uPA expression after 7–9 generations of backcrossing into a C57BL/6 background, we treated a group of tetO-muPA:CCSP-rtTA with doxycycline for 12 h, 24 h, 48 h, 72 h, and 7 d. At each time point, the BAL fluids from two mice were collected for uPA activity analysis using casein zymography. A separate group of tetO-muPA: CCSP-rtTA mice were maintained on regular drinking water and served as a time 0 control. As demonstrated in Figure 1A, the BAL fluid from the double transgenic mice contained uPA activity as early as 12 h after the initiation of antibiotic treatment. The level of uPA expression increased through 48 h and then remained significantly elevated at 72 h and 7 d. The tetO-muPA:CCSP-rtTA animals that were maintained on regular drinking water had undetectable uPA activity on casein zymography (Figure 1A). Prior studies have demonstrated that uPA is normally present in the alveolar space of wild-type mice. Under the conditions used for these zymographic experiments, the amount of uPA in the BAL fluid of wild-type animals was below the level of detection (data not shown).
Figure 1.
Kinetics of doxycycline-induced uPA expression. TetO-muPA:CCSP-rtTA mice were maintained on regular drinking water until 6–8 wk of age and then switched to doxycycline treatment (time 0). After the indicated time intervals, BAL fluid was collected and uPA activity was assessed using casein zymography, as shown in A. In B, tetO-muPA:CCSP-rtTA mice were maintained on regular drinking water until 6–8 wk of age and then switched to doxycycline treatment for 7 d. Thereafter the mice were returned to regular drinking water and BAL fluid was collected for casein zymography from two animals after the indicated time intervals.
We determined the rate at which the induced lung plasminogen activator activity would disappear from the lung after stopping doxycycline by treating a group of tetO-muPA:CCSP-rtTA mice for 7 d with antibiotic. Thereafter, the animals were returned to normal drinking water. Lung lavage fluids were then collected at 12, 24, 48, and 72 h after the cessation of doxycycline from two double transgenic mice. As demonstrated in Figure 1B, the level of uPA activity appeared to diminish 12 h after antibiotic cessation and was significantly decreased by 24 h. Within 48 h of stopping the doxycycline treatment, the level of uPA activity in the lavage fluid had returned to baseline.
TLC and Compliance after Doxycycline Treatment
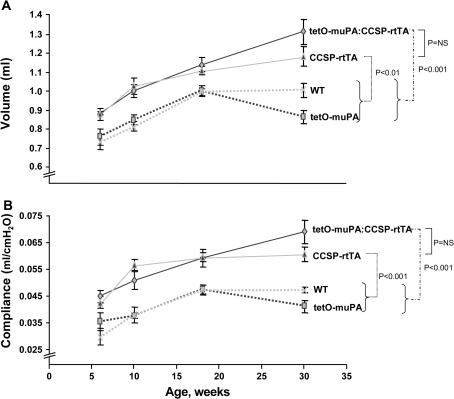
Emphysema is associated with an increase in both lung compliance and lung volume. To determine if prolonged uPA overexpression would recapitulate these abnormalities of lung physiology, female tetO-muPA:CCSP-rtTA mice were started on doxycycline treatment at 6 wk of age. To serve as controls, female tetO-muPA, CCSP-rtTA, and WT littermates were also initiated on antibiotic treatment when they reached 6 wk of age. After 4, 12, and 24 wk of doxycycline exposure, a subset of mice from each of the four genotypes underwent measurements of TLC and lung compliance. In addition, baseline TLC and compliance were obtained on a subset of 6-wk-old animals before the initiation of antibiotic treatment. To our surprise, both the uPA-overexpressing, tetO-muPA:CCSP-rtTA mice as well as the single transgenic CCSP-rtTA animals had significant increases in their total lung capacity compared with the tetO-muPA and WT groups (Figure 2A). The increase in TLC of the CCSP-rtTA animals was not statistically different from the uPA-overexpressing (tetO-muPA:CCSP-rtTA) mice at baseline or at any time point after the initiation of doxycycline treatment. At 30 wk of age, after 24 wk of doxycycline exposure, the TLC of the tetO-muPA:CCSP-rtTA group was increased by 52% and 31% compared with the tetO-muPA and WT control mice, respectively.
Figure 2.
The effect of uPA overexpression on lung compliance and TLC. Double transgenic tetO-muPA:CCSP-rtTA, single transgenic tetO-muPA and CCSP-rtTA, and nontransgenic wild-type (WT) mice were maintained on regular water until 6 wk of age. They were then given doxycycline-treated drinking water for various time intervals. Upon completion of the antibiotic treatment, TLC (A) and compliance measurements (B) were measured using a whole body plethysmograph. The data are presented as means ± SEM (n = 3–8 mice/genotype/time point).
The lung compliance curves of the four different genotypes mirrored the changes in TLC (Figure 2B). Again, both the tetO-muPA:CCSP-rtTA and the CCSP-rtTA mice demonstrated marked increases in this physiologic parameter as compared with the tetO-muPA and WT animals. After 24 wk of doxycycline treatment, the increase in compliance of the tetO-muPA:CCSP-rtTA mice was 68% and 46% greater than the tetO-muPA and WT groups, respectively. As was the case for TLC, the tetO-muPA:CCSP-rtTA and CCSP-rtTA mice were not different with respect to their lung compliance at any time point of the study. The lack of difference in TLC and compliance between these two groups of mice indicates that the emphysema phenotype is a manifestation of the CCSP-rtTA transgene and not the prolonged overexpression of uPA.
TLC and Compliance of Untreated Mice
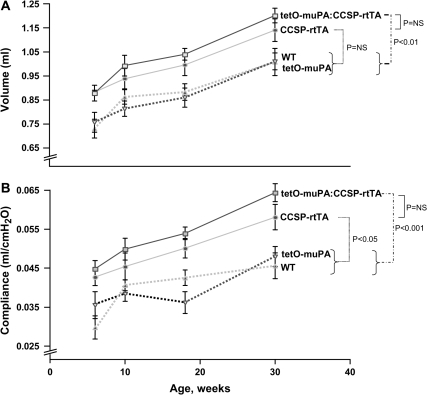
To determine if the presence of the CCSP-rtTA construct would result in an emphysema phenotype even in the absence of antibiotic treatment, we maintained groups of female tetO-muPA:CCSP-rtTA, tetO-muPA, CCSP-rtTA, and WT on regular water from birth. We then performed pulmonary function measurements at 6, 10, 18, and 30 wk of age. At these latter three time points, the mice were identical in age to the animals that had received doxycycline for 4, 12, and 24 wk, respectively. As demonstrated in Figure 3A, the tetO-muPA:CCSP-rtTA and CCSP-rtTA mice had a significant increase in their TLC compared with the other groups, even in the absence of antibiotic exposure. The tetO-muPA:CCSP-rtTA group had a 19% increase in their TLC compared with the tetO-muPA and WT controls, respectively.
Figure 3.
The effect of CCSP:rtTA on lung compliance and TLC in the absence of doxycycline treatment. CCSP-rtTA–positive and CCSP-rtTA–negative mice were maintained on regular water throughout the course of the study. At the indicated times, the animals underwent measurement of TLC and compliance using a whole body plethysmograph. The data are presented as means ± SEM (n = 3–14 mice/genotype/time point).
The presence of the CCSP-rtTA transgene, in the absence of doxycycline, also caused an increase in lung compliance (Figure 3B). The difference in compliance between the CCSP-rtTA–positive and the CCSP-rtTA-negative groups was evident at 6 wk of age and was similarly increased over the entire time course of the study. At 30 wk of age, the compliance of the tetO-muPA:CCSP-rtTA group was 33% and 39% greater than that of the tetO-muPA and WT mice, respectively.
Effect of Doxycycline on TLC and Compliance of tetO-muPA:CCSP-rtTA and CCSP-rtTA Mice
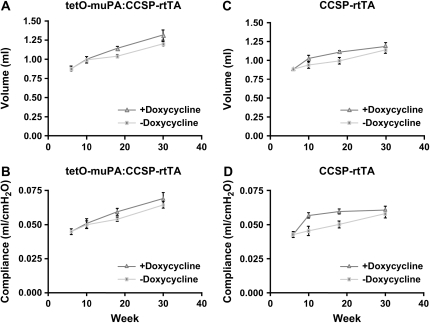
The aforementioned results demonstrate that mice possessing the CCSP-rtTA transgene develop physiologic abnormalities consistent with emphysema in the presence or absence of doxycycline treatment. To determine if doxycycline exposure worsened the emphysematous changes, we compared the TLC and lung compliance of the tetO-muPA:CCSP-rtTA group at age 10, 18, and 30 wk on and off antibiotic treatment. By recombining the data that comprise Figures 2 and 3, we found antibiotic exposure resulted in minor increases in both the mean lung volume and lung compliance measurements of the double transgenic animals (Figures 4A and 4B). Only the change in TLC reached statistical significance (P < 0.05).
Figure 4.
The effect of doxycycline on lung compliance and TLC of CCSP-rtTA–positive mice. At 6 wk of age, subgroups of animals were maintained on regular drinking water or were switched to doxycycline-containing water. The TLC (A and C) and lung compliance (B and D) of the tetO-muPA:CCSP-rtTA and CCSP-rtTA groups were measured at various time intervals. The data are presented as means ± SEM (n = 3–14 mice/genotype/time point).
We also assessed whether antibiotic treatment had an effect on the lung physiology of the CCSP-rtTA mice. Again we compared the TLC and lung compliance of this group at age 10, 18, and 30 wk on and off doxycycline treatment. As was true for the tetO-muPA:CCSP-rtTA group, antibiotic exposure had only a small additional effect on the emphysema phenotype in this group of mice that was evident at the 10 and 18 wk time points but disappeared at 30 wk (Figures 4C and 4D).
To determine if antibiotic exposure had an influence on the TLC and compliance of rtTA-negative groups, we compared these parameters in WT and tetO-muPA mice on and off doxycycline therapy at age 10, 18, and 30 wk. In these genotypes, doxycycline treatment had no effect on lung physiology at any time point (data not shown).
Effect of Genotype on Animal Weight
Our results demonstrate that animals possessing the CCSP-rtTA transgenic construct develop lung physiologic abnormalities that are consistent with emphysema. To ensure that these alterations in TLC and compliance were not simply the result of differences in animal size, we measured the body weights of the tetO-muPA:CCSP-rtTA, tetO-muPA, CCSP-rtTA, and wild-type mice at 6, 10, 18, and 30 wk of age, just before the performance of pulmonary function measurements. We found that the animals from each genotype gained weight throughout the study, and the rate of increase was greatest between 6 and 18 wk (data not shown). We also determined that there was no difference in the weights of the four groups of mice at any time point of the experiment. This was the case whether the mice were treated with antibiotic or whether they were maintained on regular drinking water.
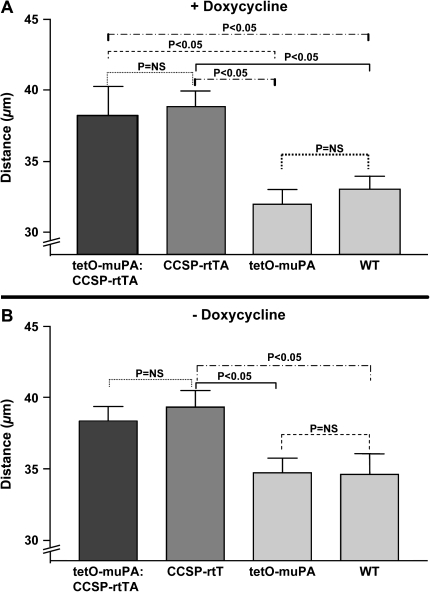
Mean Linear Intercept Values with and without Doxycycline Treatment
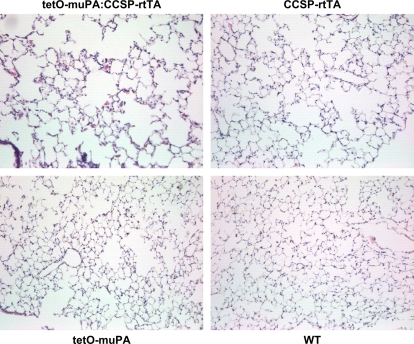
Distal airspace enlargement on histopathologic examination is a hallmark feature of emphysema. To determine if the presence of the CCSP-rtTA construct was associated not only with a change in lung physiology, but also with an increase in alveolar size, we generated H&E-stained histologic sections from 30-wk-old, tetO-muPA:CCSP-rtTA, tetO-muPA, CCSP-rtTA, and wild-type mice after 24 wk of doxycycline treatment. These specimens were produced from the same animals that had undergone the pulmonary function measurements detailed above. On visual inspection of the tissue sections, the lungs from the mouse groups that possessed the rtTA gene had obvious enlargement of the distal airspaces (Figure 5). To quantify alveolar size, we used these histologic samples to measure the mean linear intercept. As demonstrated in Figure 6A, the tetO-muPA:CCSP-rtTA and CCSP-rtTA groups were not significantly different with regard to this parameter. On the other hand, as was the case for TLC and lung compliance measurements, the tetO-muPA:CCSP-rtTA and CCSP-rtTA mice had significantly larger mean linear intercepts than the other two genotypes after 24 wk of doxycycline exposure. Together, the CCSP-rtTA–positive mice had an 18% increase in their mean linear intercept value compared with the CCSP-rtTA–negative controls.
Figure 5.
The effect of the CCSP-rtTA transgene and doxycycline treatment on alveolar histology. Double transgenic tetO-muPA:CCSP-rtTA, single transgenic CCSP-rtTA, and nontransgenic wild-type mice were maintained on regular water until 6 wk of age. Thereafter, the mice were placed on doxycycline treatment for 24 wk. At 30 wk of age, the lungs were removed, inflation fixed at 25 cm H2O pressure, sectioned, stained with hematoxylin and eosin, and photographed at ×40 magnification. Representative photomicrographs from each genotype have been included.
Figure 6.
The effect of the CCSP-rtTA transgene and doxycycline treatment on mean linear intercept. Double transgenic tetO-muPA:CCSP-rtTA, single transgenic tetO-muPA and CCSP-rtTA, and nontransgenic wild-type mice were maintained on regular water until 6 wk of age. Thereafter, a group of mice from each genotype were placed on doxycycline treatment while the remaining animals continued on regular water. At 30 wk of age, the lungs were removed, inflation fixed at 25 cm H2O pressure, sectioned, and stained with hematoxylin and eosin. The mean linear intercept was calculated from the analysis of 15–20 random ×40 microscopic fields. A represents the mean linear intercept of 30-wk-old animals that had received 24 wk of antibiotic treatment. B represents the mean linear intercept of 30-wk-old animals in received no antibiotic treatement. The values recorded are means + SEM (n = 5–8 mice/genotype).
To determine if doxycycline was required for the development of distal airspaces enlargement, we compared the mean linear intercepts of 30-wk-old mice from the four genotypes in the absence of antibiotic exposure. The mice possessing the CCSP-rtTA construct again had enlarged alveoli as compared with the other two genotypes (Figure 5B).
TLC and Compliance of SPC-rtTA–Positive and SPC-rtTA–Negative Mice
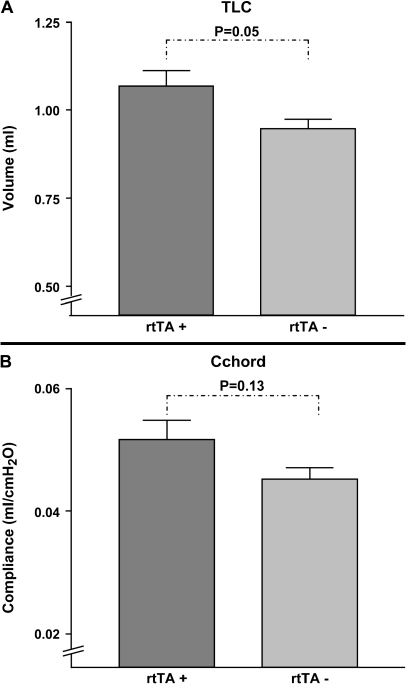
To assess whether the emphysematous changes seen in the CCSP-rtTA–positive mice were specific to this particular transgenic line, we assessed whether a different rtTA-expressing mouse would also demonstrate alterations in lung volume and compliance. To accomplish this, mice that contained the rtTA transgene driven by the human surfactant protein C promoter (SPC-rtTA) were compared with littermates that were negative for this construct. Female pups comprising the two genotypes were given regular water from birth until they reached 6 wk of age. Thereafter, the two groups were maintained on doxycycline-treated water for 24 wk. At 30 wk of age, the mice underwent measurements of TLC and compliance. As was true for the CCSP-rtTA–positive animals, the mice that contained the SPC-rtTA transgenic construct demonstrated enlargements in both their lung volume and compliance measurements (Figure 7). The degree of abnormality in these parameters was less severe than occurred in the CCSP-rtTA–positive mice. Specifically, compared with the SPC-rtTA–negative group, the SPC-rtTA–positive mice had a 13% increase in their TLC (P = 0.05) and a 14% increase in their lung compliance (P = 0.13).
Figure 7.
The effect of SPC:rtTA on lung compliance and TLC after doxycycline treatment. SPC-rtTA–positive and SPC-rtTA–negative mice were maintained on regular water until 6 wk of age. They were then placed on doxycycline treatment for 24 wk. Upon completion of the antibiotic treatment, total lung capacity (A) and compliance measurements (B) were measured using a whole body plethysmograph. The data are presented as means ± SEM (n = 6–8 mice/genotype).
DISCUSSION
In this report, we employed the doxycycline-inducible, lung-specific gene regulatory system to explore whether the prolonged overexpression of the serine protease uPA would lead to the development of emphysema in mice. In this transgenic model, only doxycycline-treated mice that possess both the tetO-muPA and the CCSP-rtTA constructs generate increased levels of uPA in their lungs. Therefore, if uPA overexpression is capable of facilitating alveolar wall destruction, only the double transgenic (tetO-muPA:CCSP-rtTA) mice should be at risk for developing emphysema after antibiotic exposure. To our surprise, our results demonstrate that the presence of the CCSP:rtTA transgene (or the SPC:rtTA construct) was the critical determinant in the generation of an emphysema phenotype and not the increase in lung-specific uPA expression. Specifically, CCSP:rtTA-positive mice manifested an increase in their lung compliance, an increase in their total lung capacity and an enlargement of their distal airspaces compared with the control groups. These CCSP:rtTA-induced alterations in lung histopathology and physiology occurred in the presence or absence of antibiotic exposure, and uPA overexpression contributed little additional effect.
The doxycycline-inducible gene regulatory system has been used to study the role of a variety of cytokines and growth factors including IFN-γ, IL-11, IL-13, TGF-β, TNF-α, and most recently IL-1β in the pathogenesis of emphysema (5–7, 10, 11, 15). Interestingly, in several of these reports, the non–antibiotic-exposed rtTA-positive transgenic animals have demonstrated airspace enlargement and compliance changes similar to what we have observed (6, 7, 10). For example, the mean compliance and chord length values of IL-13– and IFN-γ–overexpressing mice were increased compared with the control group even in the absence of doxycycline exposure (6, 7). In addition, the average mean linear intercept measurement of the untreated TNF-α transgenic animals was increased compared with the wild-type group (10). Although these baseline abnormalities in lung physiology and histopathology have been attributed to low levels of “leaky” cytokine production, as was suggested in the case of the TNF-α overexpressors, our results provide an additional or an alternative explanation that the tetracycline gene regulatory system can directly contribute to the emphysema phenotype. This is further supported by our observation that a second transgenic line with rtTA expression driven by the human SPC promoter also demonstrates physiologic changes consistent with emphysema. In addition, a similar effect of the CCSP-rtTA transgene on alveolar enlargement has been noted by Dr. Jeffery Whitsett in more than one of his founder lines (personal communication).
In our study, we found that the degree of physiologic alteration in the CCSP-rtTA and the SPC-rtTA mice differed. In two other reports that employed the doxycycline-inducible transgenic system to overexpress either IL-11 or IL-1β, the adult rtTA-positive mice demonstrated no abnormalities in lung volume or compliance without antibiotic treatment (5, 10). It remains unclear why the rtTA construct may induce varying degrees of emphysema-like changes that range from substantial to mild to minimal. We speculate that the copy number of the transgene or the level of rtTA expression might influence the resultant phenotype. It is also possible that genetic background of the mice influences the phenotypic expression of the rtTA construct.
A direct comparison of alveolar size, lung volume, and compliance between our study and others using the rtTA system is difficult because different techniques were employed to measure these parameters. However, the severity of abnormalities in our CCSP-rtTA mice appears to be milder than what has been reported when this transgenic system is used to drive the overexpression of a proinflammatory cytokine such as IL-11, IFN-γ, IL-13, or IL-1β (5–7, 11). Of note, the extent of emphysema in our studies is similar to what Hautamaki and colleagues reported in mice after 6 mo of cigarette smoke inhalation (17). Based on the observation that a growing number of diverse cytokines have been associated with an emphysema phenotype in this transgenic model, we are concerned that expression of rtTA might sensitize the lung to develop emphysema in the setting of specific stimuli that otherwise might not cause disease pathogenesis if present in isolation.
Based on our current results, the mechanism by which the rtTA transgene causes increases in alveolar size, lung volume, and lung compliance remains unclear. It is possible that the construct impacts the development, maintenance, or destruction of the alveolar septae. Both mice and humans are born with premature lungs that consist of large alveolar saccules (23). In mice, during the first 2 wk of life septation leads to lung maturation, with changes in alveolar size and number that are characteristic of adult lungs (24, 25). Our observation that the CCSP:rtTA-positive groups have alterations in their lung physiology as early as 6 wk of age suggest that the transgene has an early effect and could potentially interfere with the alveolarization process. In the non–doxycycline-treated CCSP:rtTA-positive and CCSP:rtTA- negative mice, there is a strikingly parallel increases in lung volume and compliance beyond 6 wk of age. This observation further implies that CCSP:rtTA exerts its influence on lung structure and function before the animals reach full maturity. This is similar to what has been reported for TNF-α transgenic mice, where no further increases in mean linear intercept occurred beyond the initial measurement at 3–4 mo of age despite continued antibiotic exposure (10). If the alveolar enlargement were due to destruction of previously formed septae, as occurs in emphysema, one might expect the measurements of lung physiology and morphometry in the CCSP:rtTA-positive and CCSP:rtTA-negative groups to diverge over time. On the other hand, it is possible that the influence of CCSP:rtTA on alveolar enlargement after 6 wk of age is counterbalanced by simultaneous reparative processes. The observation that TGF-β1–overexpressing mice have no histologic abnormalities in their lungs in the absence of doxycycline at age 14 d indicates, at least in this transgenic line, that the rtTA construct does not always impede normal alveolarization (9).
How the CCSP:rtTA construct might alter alveolar development, maintenance, or destruction requires further study. Based on current information, several potential mechanisms are plausible. First, rtTA is a fusion protein consisting of the mutated, E. coli–derived, repressor of the Tn10 TC-resistance operon and the c-terminal portion of the herpes simplex virion protein 16. Although this promoter molecule should specifically bind only to the tet-operon and not to eukaryotic promoter sequences, it is possible that rtTA induces or suppresses the expression of a murine gene (or genes) such as cytokines or matrix metalloproteinases that, in turn, interferes with alveolar development or accelerates alveolar destruction. Second, the expression of rtTA may result in injury to the alveolar epithelium either directly or by stimulating lung inflammation. Damage to the alveolar epithelium could then impair alveolar septation or promote alveolar loss. Finally, the rtTA transgenic construct, as the result of its site of integration into the murine genome, may alter the expression of a gene that is critical to the formation of alveoli or may contribute to emphysema pathogenesis. Of these proposed mechanisms, this latter explanation appears to be the least plausible. We have found that a different rtTA-positive transgenic line (i.e., SPC-rtTA) also demonstrates physiologic features of emphysema. Furthermore, as mentioned above, Dr. Jeffrey Whitsett has informed us that he too has seen rtTA-dependent alveolar enlargement in several different CCSP:rtTA founder lines (personal communication). These postulated mechanisms for induction of emphysema by rtTA may also sensitize the lung to further damage by stimuli that would have milder or absent effects by themselves.
In this study, we originally set out to determine whether prolonged uPA expression in the lung would induce emphysema. Although we found the CCSP-rtTA transgene to have the dominant effect on the physiologic alterations, we also detected a minor but statistically significant effect of uPA overproduction on the total lung capacity. The mean lung compliance of the 30-wk-old tetO-muPA:CCSP-rtTA was also increased compared with the other groups after antibiotic exposure, although this difference did not reach statistical significance. On the other hand, when we examined the effect of prolonged uPA overexpression on alveolar size, we found that the tetO-muPA:CCSP-rtTA mice were not different from the CCSP-rtTA control group. In light of the fact that the morphometric analysis does not corroborate the uPA-associated changes in physiology, additional studies are required to confirm the participation of the plasminogen system in the development of emphysema.
In summary, we have found that the CCSP-rtTA construct commonly used in the doxycycline-inducible, lung-specific gene regulatory system is associated with abnormalities in lung physiology and morphometry. Specifically, the CCSP-rtTA transgene induced increases in lung compliance, lung volume, and alveolar size—perturbations that are typical features of emphysema. In addition, the SPC-rtTA transgene produced abnormalities in lung physiology that were similar to, although less severe than, the CCSP-rtTA construct. Based on these observations, we conclude that the influence of rtTA on alveolar structure and function needs to be accounted for when designing and interpreting studies using this transgenic system to probe the effects of different transgenes on lung development and disease pathogenesis. Furthermore, our results indicate the importance of incorporating proper controls into the experimental design when this transgenic system is used to study emphysema. Without appropriate controls, it is difficult to assess what contribution rtTA alone is having on the disease pathogenesis.
Acknowledgments
The authors thank Drs. Jeffrey Whitsett and Jay Tichelaar for supplying us with the CCSP:rtTA mice.
This work was supported by NIH, NHLBI, K08 HL04434, and by the American Lung Association.
Originally Published in Press as DOI: 10.1165/rcmb.2005-0378OC on January 13, 2006
Conflict of Interest Statement: None of the authors has a financial relationship with a commercial entity that has an interest in the subject of this manuscript.
References
- 1.Kistner A, Gossen M, Zimmermann F, Jerecic J, Ullmer C, Lubbert H, Bujard H. Doxycycline-mediated quantitative and tissue-specific control of gene expression in transgenic mice. Proc Natl Acad Sci USA 1996;93:10933–10938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lamartina S, Roscilli G, Rinaudo CD, Sporeno E, Silvi L, Hillen W, Bujard H, Cortese R, Ciliberto G, Toniatti C. Stringent control of gene expression in vivo by using novel doxycycline-dependent trans-activators. Hum Gene Ther 2002;13:199–210. [DOI] [PubMed] [Google Scholar]
- 3.Tichelaar JW, Lu W, Whitsett JA. Conditional expression of fibroblast growth factor-7 in the developing and mature lung. J Biol Chem 2000;275:11858–11864. [DOI] [PubMed] [Google Scholar]
- 4.Stripp BR, Sawaya PL, Luse DS, Wikenheiser KA, Wert SE, Huffman JA, Lattier DL, Singh G, Katyal SL, Whitsett JA. cis-acting elements that confer lung epithelial cell expression of the CC10 gene. J Biol Chem 1992;267:14703–14712. [PubMed] [Google Scholar]
- 5.Ray P, Tang W, Wang P, Homer R, Kuhn C III, Flavell RA, Elias JA. Regulated overexpression of interleukin 11 in the lung: use to dissociate development-dependent and -independent phenotypes. J Clin Invest 1997;100:2501–2511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang Z, Zheng T, Zhu Z, Homer RJ, Riese RJ, Chapman HA Jr, Shapiro SD, Elias JA. Interferon gamma induction of pulmonary emphysema in the adult murine lung. J Exp Med 2000;192:1587–1600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zheng T, Zhu Z, Wang Z, Homer RJ, Ma B, Riese RJ Jr, Chapman HA Jr, Shapiro SD, Elias JA. Inducible targeting of IL-13 to the adult lung causes matrix metalloproteinase- and cathepsin-dependent emphysema. J Clin Invest 2000;106:1081–1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Le Cras TD, Hardie WD, Deutsch GH, Albertine KH, Ikegami M, Whitsett JA, Korfhagen TR. Transient induction of TGF-alpha disrupts lung morphogenesis, causing pulmonary disease in adulthood. Am J Physiol Lung Cell Mol Physiol 2004;287:L718–L729. [DOI] [PubMed] [Google Scholar]
- 9.Vicencio AG, Lee CG, Cho SJ, Eickelberg O, Chuu Y, Haddad GG, Elias JA. Conditional overexpression of bioactive transforming growth factor-beta1 in neonatal mouse lung: a new model for bronchopulmonary dysplasia? Am J Respir Cell Mol Biol 2004;31:650–656. [DOI] [PubMed] [Google Scholar]
- 10.Vuillemenot BR, Rodriguez JF, Hoyle GW. Lymphoid tissue and emphysema in the lungs of transgenic mice inducibly expressing tumor necrosis factor-alpha. Am J Respir Cell Mol Biol 2004;30:438–448. [DOI] [PubMed] [Google Scholar]
- 11.Lappalainen U, Whitsett JA, Wert SE, Tichelaar JW, Bry K. Interleukin-1beta causes pulmonary inflammation, emphysema, and airway remodeling in the adult murine lung. Am J Respir Cell Mol Biol 2005;32:311–318. [DOI] [PubMed] [Google Scholar]
- 12.Lanone S, Zheng T, Zhu Z, Liu W, Lee CG, Ma B, Chen Q, Homer RJ, Wang J, Rabach LA, et al. Overlapping and enzyme-specific contributions of matrix metalloproteinases-9 and -12 in IL-13-induced inflammation and remodeling. J Clin Invest 2002;110:463–474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Blackburn MR, Lee CG, Young HW, Zhu Z, Chunn JL, Kang MJ, Banerjee SK, Elias JA. Adenosine mediates IL-13-induced inflammation and remodeling in the lung and interacts in an IL-13-adenosine amplification pathway. J Clin Invest 2003;112:332–344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ma B, Zhu Z, Homer RJ, Gerard C, Strieter R, Elias JA. The C10/CCL6 chemokine and CCR1 play critical roles in the pathogenesis of IL-13-induced inflammation and remodeling. J Immunol 2004;172:1872–1881. [DOI] [PubMed] [Google Scholar]
- 15.Lee CG, Cho SJ, Kang MJ, Chapoval SP, Lee PJ, Noble PW, Yehualaeshet T, Lu B, Flavell RA, Milbrandt J, et al. Early growth response gene 1-mediated apoptosis is essential for transforming growth factor beta1-induced pulmonary fibrosis. J Exp Med 2004;200:377–389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Churg A, Wright JL. Proteases and emphysema. Curr Opin Pulm Med 2005;11:153–159. [DOI] [PubMed] [Google Scholar]
- 17.Hautamaki RD, Kobayashi DK, Senior RM, Shapiro SD. Requirement for macrophage elastase for cigarette smoke-induced emphysema in mice. Science 1997;277:2002–2004. [DOI] [PubMed] [Google Scholar]
- 18.Foronjy RF, Okada Y, Cole R, D'Armiento J. Progressive adult-onset emphysema in transgenic mice expressing human MMP-1 in the lung. Am J Physiol Lung Cell Mol Physiol 2003;284:L727–L737. [DOI] [PubMed] [Google Scholar]
- 19.Selman M, Cisneros-Lira J, Gaxiola M, Ramirez R, Kudlacz EM, Mitchell PG, Pardo A. Matrix metalloproteinases inhibition attenuates tobacco smoke-induced emphysema in Guinea pigs. Chest 2003;123:1633–1641. [DOI] [PubMed] [Google Scholar]
- 20.Sisson TH, Hanson KE, Subbotina N, Patwardhan A, Hattori N, Simon RH. Inducible lung-specific urokinase expression reduces fibrosis and mortality after lung injury in mice. Am J Physiol Lung Cell Mol Physiol 2002;283:L1023–L1032. [DOI] [PubMed] [Google Scholar]
- 21.Hattori N, Sisson TH, Xu Y, Desai TJ, Simon RH. Participation of urokinase-type plasminogen activator receptor in the clearance of fibrin from the lung. Am J Physiol 1999;277:L573–L579. [DOI] [PubMed] [Google Scholar]
- 22.Dunnill MS. Evaluation of a simple method of sampling the lung for quantitative histological analysis. Thorax 1964;19:443–448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Massaro D, Massaro GD. Critical period for alveologenesis and early determinants of adult pulmonary disease. Am J Physiol Lung Cell Mol Physiol 2004;287:L715–L717. [DOI] [PubMed] [Google Scholar]
- 24.Massaro D, Massaro GD. Invited Review: pulmonary alveoli: formation, the “call for oxygen,” and other regulators. Am J Physiol Lung Cell Mol Physiol 2002;282:L345–L358. [DOI] [PubMed] [Google Scholar]
- 25.Massaro D, Massaro GD. Retinoids, alveolus formation, and alveolar deficiency: clinical implications. Am J Respir Cell Mol Biol 2003;28:271–274. [DOI] [PubMed] [Google Scholar]