Abstract
Mucus overproduction in inflammatory and obstructive airway diseases is associated with goblet cell (GC) metaplasia in airways. Although the mechanisms involved in GC metaplasia and mucus hypersecretion are not completely understood, association with oxidative stress and epidermal growth factor receptor (EGFR) signaling has been reported. To explore the mechanisms involved in oxidative stress–induced GC metaplasia, cultures of differentiated normal human bronchial epithelial cells grown at the air–liquid interface were exposed to reactive oxygen species (ROS) generated by xanthine/xanthine oxidase. EGFR activation and signaling was assessed by measuring EGF and transforming growth factor-α release and EGFR and 44/42MAPK phosphorylation. The GC population was evaluated by confocal microscopy. ROS-induced EGFR activation resulted in GC proliferation and increased MUC5AC gene and protein expression. Signaling was due to pro-EGF processing by tissue kallikrein (TK), which was activated by ROS-induced hyaluronan breakdown. It was inhibited by catalase, a TK inhibitor, and EGF-blocking antibodies. Exposure to recombinant TK mimicked the ROS effects, increasing the expression of MUC5AC and lactoperoxidase. In addition, ROS induced the antiapoptotic factor Bcl-2 in a TK-dependent fashion. In conclusion, ROS-induced GC metaplasia in normal human bronchial epithelial cells is associated with HA depolymerization and EGF processing by TK followed by EGFR signaling, suggesting that increases in TK activity could contribute to GC metaplasia and mucus hypersecretion in diseases such as asthma and chronic bronchitis. The data also suggest that increases in GC population could be sustained by the associated upregulation of Bcl-2 in airway epithelial cells.
Keywords: EGFR, goblet cells, hyaluronan, NHBE cells, tissue kallikrein
Mucus overproduction is associated with a variety of acute reactions to bacterial and viral infections (1, 2) and with chronic airway inflammatory diseases such as asthma (3–5), chronic bronchitis (6–8), bronchiectasis (9), or cystic fibrosis (10). Although goblet cell (GC) metaplasia with mucus hypersecretion, aimed at protecting the airway epithelium, is associated with increased morbidity and mortality in such diseases, the pathways involved in GC differentiation and therefore the mechanisms responsible for GC hyperplasia/metaplasia are not completely understood. Numerous stimuli, such as endogenous oxidants induced by neutrophil elastase (11) or exogenous oxidants produced by cigarette smoke (12, 13), diesel engine emissions (14), or neutrophils (15) and T helper-2 lymphocyte-derived cytokines IL-4, IL-9, and IL-13 (16–19), are known to induce GC metaplasia or mucin hypersecretion in animal models and in vitro studies. The antiapoptotic factor Bcl-2 appears to be crucial to sustain the increase in GC numbers (20), and its expression is upregulated in GC in the airways of patients with asthma (21) or cystic fibrosis compared with healthy individuals (20). Bcl-2 is also increased in lipopolysaccharide-induced GC metaplasia in rat airways (22). Until recently, mucin production was considered the only function of GC necessary to provide a defense “shield” between the epithelium and the environment. However, GCs have been shown to synthesize additional products important for host defense, such as lactoperoxidase (LPO) (23–25) in human and sheep and surfactant protein D (26) in rats.
Epidermal growth factor receptor (EGFR) activation (27) with subsequent signaling via EGFR tyrosine kinase and mitogen-activated protein kinase (MAPK) seems to be a convergent pathway involved in GC hyperplasia and increased MUC5AC gene expression (12, 15) induced by oxidative stress and T helper-2 cytokines. EGFR activation can be achieved by multiple EGFR ligands: EGF, transforming growth factor (TGF)-α, heparin-binding EGF-like growth factor (HB-EGF), amphiregulin, epiregulin, and betacellulin (28–31). These ligands are synthesized as transmembrane precursors and are cleaved to their active form by matrix metalloproteases of the ADAM family (32–35), with the exception of EGF, which is cleaved by the serine protease tissue kallikrein (TK) in kidney (36), in salivary and mammary glands (37, 38), and in airways submucosal gland cells (39). TK is present in the airways (40, 41), and its activity is elevated in the bronchoalveolar lavage of patients with asthma (42, 43) and in patients with chronic bronchitis (44, 45); exposure to air pollutants can also induce TK upregulation (46). Studies from this laboratory have shown that the glycosaminoglycan hyaluronan (HA) immobilizes TK at the apical pole of airway epithelium and inhibits its catalytic activity (41, 47); consequently, HA degradation results in a dramatic increase in TK activity (41). Because HA is depolymerized by reactive oxygen species (ROS) (48), increased airway TK activity is expected in diseases associated with increased airway ROS production. TK activity is increased in patients with chronic bronchitis and other diseases associated with oxidative stress and mucus hypersecretion when compared with normal subjects (42, 44) possibly due to continued degradation of HA by ROS (49–51). We have also shown that ROS-induced EGFR signaling and MUC5AC hypersecretion is associated with HA depolymerization followed by pro-EGF processing by TK in primary cultures of human submucosal gland (SMG) cells (39).
The goal of the present study was to test whether ROS–induced, EGFR-dependent GC metaplasia is initiated by HA depolymerization in polarized human airway epithelial cells. To test this hypothesis, we exposed normal human bronchial epithelial (NHBE) cells grown and differentiated at the air–liquid interface (ALI) to oxidative stress and assessed HA depolymerization, TK activation, pro-EGF processing, EGFR and 44/42MAPK phosphorylation, gene and protein expression of specific goblet cell products (MUC5AC, LPO and Bcl-2), and goblet cell population.
MATERIALS AND METHODS
Materials
All materials were purchased from Sigma Chemical Co. (St. Louis, MO) unless otherwise specified.
Primary Cultures of NHBE Cells at the ALI
Primary cultures of NHBE cells were obtained from human lung donors, whose lungs were rejected for transplantation, through the University of Miami Life Alliance Organ Recovery Agency with approval from the local Institutional Review Board. NHBE cells, harvested as described (52), were plated on collagen-coated plastic dishes, grown to confluence in bronchial epithelial growth medium yielding undifferentiated airway epithelial cells, and passaged after enzyme dissociation with trypsin (53). Cells from Passage 1 were plated onto 24-mm Transwell-clear culture inserts (Corning Costar Corporation, Cambridge, MA) coated with human placental collagen. The culture medium has been described (52, 53). It contained 50% Dulbecco's modified Eagle's medium and 50% Lechner and LaVeck (LHC) basal medium supplemented with insulin (5 μg/ml), hydrocortisone (0.072 ng/ml), epidermal growth factor (0.5 ng/ml), tri-iodothyronine (T3, 6.5 ng/ml), transferrin (10 μg/ml), epinephrine (0.6 μg/ml), phosphorylethanolamine (0.5 μM), ethanolamine (0.5 μM), bovine pituitary extract (1% vol/vol), BSA (0.5 mg/ml), CaCl2 (0.08 mM), trace elements (1×), stock 4 (1×), stock 11 (1×), penicillin/streptomycin (100 μg/ml), and retinoic acid (0.05 μM). Cells were grown in an incubator at 37°C in ambient air supplemented with 5% CO2. The apical surface was exposed to air as soon as the cells reached confluence. Cultures were used for experiments after reaching full differentiation (∼ 3 wk on air) as assessed by visual confirmation of beating cilia and mucus. The cultures used in this study had 89 ± 4% of ciliated cells (n = 10 lung donors) measured by labeling ciliated cells with antiacetylated tubulin mAb and counted with Metamorph software (Universal Imaging Corporation, Molecular Devices, Sunnyvale, CA) as described below.
Protocols
Exogenous EGF was removed from the media 48 h before the studies. Immediately before all experiments, 500 μl of PBS was applied to the apical surface of the cultures. After treatment, apical supernatants were collected, and cells were lysed with 20 mM sodium phosphate, 150 mM NaCl, 5 mM EDTA, 50 mM Hepes, 1% Triton X 100, 50 mM NaF, 1 mM sodium orthovanadate, 5 mM PMSF, 10 μg/ml leupeptin, and 10 μg/ml aprotinin at pH 7.8 (lysis buffer) for 30 min at 4°C. To remove insoluble material, cell lysates were centrifuged at 12,000 × g for 5 min at 4°C. Supernatants and cell lysates were frozen at −20°C for later analysis.
In experiments designed to test the effects of ROS on EGFR ligand processing and signaling, NHBE cells (n = 3 different lung donors, in triplicate wells for each experimental condition) were apically exposed to PBS or 0.6 mM xanthine plus 0.05 units of xanthine oxidase (X/XO) for 30 min. This time is enough to elicit EGFR activation persistent for 24 h as has been reported when the stimulus is applied apically on polarized epithelial cells (54). ROS exposure was done in the presence or absence of catalase (150 U/ml), the specific TK peptide inhibitor (PI) RPGLPVRFESPLRINIIKE-NH2 (39, 55) (100 μg/ml), or the metalloprotease inhibitor GM6001 (25 μM; Chemicon International, Temecula, CA). This method helps to differentiate the TK-processed EGF from the other EGFR ligands (TGF-α, HB-EGF, amphiregulin, and betacellulin), which are activated by metalloproteases of the ADAM family and sensitive to inhibition by GM6001. To determine if ROS effects were mediated by EGFR, cells were pretreated 15 min before X/XO with functionally blocking anti-EGFR antibodies (2 μg/ml; Calbiochem, San Diego, CA). To confirm that the effects of oxidative stress were mediated by EGF, additional cells from two lung donors were treated with PBS or X/XO in the presence or absence of neutralizing rabbit anti-human EGF antibodies (1 μg/ml; US Biological, Swampscott, MA).
In experiments designed to define changes in cell phenotype, NHBE cells (n = 3 different lung donors) were exposed once daily for 3 d to PBS or X/XO in the presence or absence of catalase, PI, GM6001, or blocking anti-EGFR antibodies in the same concentrations as described previously. At Day 4, replicate filters were used for RNA extraction, protein measurements, or immunohistochemistry. Filters from two lung donors treated with PBS, X/XO, or X/XO + catalase as before were maintained at normal ALI conditions during three additional days to determine if the phenotypic changes were maintained after the removal of oxidative insult. At Day 7, cultures were used for RNA extraction or immunohistochemistry.
Because these experiments suggested that TK and not metalloproteases were involved in processing pro-forms of EGFR ligands in these cells, as in human SMG cells (39), experiments were designed to directly test the ability of TK to induce GC metaplasia. NHBE cells were exposed daily for 3 d to PBS (control) or recombinant TK (1 μM) produced in Pichia pastoris as described (39, 56) in 500 μl PBS in the presence or absence of PI (4 μM). At Day 4, replicate filters were used for GC quantification by immunohistochemistry.
MUC5AC and Acetylated Tubulin Immunofluorescence
To visualize phenotypic changes induced by ROS on NHBE cells cultures grown at the ALI, anti-MUC5AC antibodies to label goblet cells and antiacetylated tubulin antibodies to label ciliated cells were used. Replicate cell culture inserts treated as described previously were fixed and permeabilized with cold acetone-methanol (1:1) for 1 min. After blocking 1 h with BSA 1% in PBS, 10 μg/ml of mouse anti-MUC5AC antibody (Chemicon International) was added to the luminal surface overnight. Cells were washed with PBS, and Alexa 488–labeled anti-mouse IgG antibody (0.1 μg/ml; Molecular Probes, Carlsbad, CA) was added followed by mAb to acetylated tubulin (μg/ ml) for 2 h. Cells were washed, and Alexa 555-conjugatad anti-mouse antibody (0.1 μg/ml) was incubated for 1 h. Transwell inserts were excised and mounted on slides with Gel/Mount (Biomeda, Foster City, CA) containing 4′,6-diamidine-2-phenylindole. Images were captured with a confocal laser-scanning microscope (Zeiss LSM, Thornwood, NY).
Phosphorylated and Total EGFR Immunoblotting
To test if the phenotypic change on NHBE cultures after X/XO treatment was due to EGFR signaling, EGFR activation was assessed as previously described (39). Duplicate aliquots of cell lysates (n = 3 different lung donors) containing equal amounts of protein were used for estimating total and phosphorylated EGFR (pEGFR) on parallel Western blots. EGFR was immunoprecipitated using a rabbit anti-EGFR antibody (1 μg/ml; Santa Cruz Biotechnology, Santa Cruz, CA) and protein A-agarose beads (Santa Cruz Biotechnology). Pellets from immunoprecipitation were electrophoresed on 4–15% Tris-HCl Ready Gels (BioRad, Hercules, CA) and transferred electrophoretically to polyvinylidene fluoride membranes (Millipore, Billerica, MA). The membranes were blocked with 1% gelatin in Tris-buffered saline (TBS) containing 0.05% Tween-20 (1 h) followed by antiphosphotyrosine mAb (PY99, 2 μg/ml; Santa Cruz Biotechnology) or anti-EGFR mAb (Ab-3, 1 μg/ml; Calbiochem). Secondary antibody was an alkaline phosphatase (AP)-conjugated goat anti-mouse IgG (0.5 μg/ml, Kirkegaard and Perry Laboratories [KPL]). For visualization, bromochloroindolyl phosphate/nitro blue tetrazolium was used as a substrate. Developed blots were photographed using the GelDoc XRS system (BioRad), and quantification of the pEGFR/EGFR ratio was done using Quantity One software (BioRad).
Phosphorylated and Total 44/42MAPK Immunoblotting
Aliquots of cells lysates containing equal amounts of protein (n = 3 different lung donors) were run on 4–15% Tris-HCl Ready gels and transferred to polyvinylidene fluoride membranes as described previously. Visualization of 44/42MAPK and phosphorylated MAPK (pMAPK) was achieved using the PhosphoPlus p44/p42 MAP kinase (Thr202/Thr204) Antibody kit according to the manufacturer's instructions (Cell Signaling Technology, Beverly, MA). Developed blots were photographed and pMAPK/MAPK ratios were assessed as described previously for EGFR.
Visualization of HA Depolymerization and Quantitative Analysis of HA
To confirm that X/XO induced HA depolymerization under the experimental conditions, we assessed the release of soluble HA from the apical pole of epithelial cells and analyzed molecular distribution and average molecular mass of HA as previously described (39). The HA content of the supernatants was estimated using a biotinylated HA-binding protein, ELISA-like assay as described by Bray and colleagues (57). In addition, supernatants of NHBE cell cultures, treated with PBS, X/XO, or X/XO plus catalase as described previously, were collected, pooled, and digested with proteinase K (125 μg/ml for 2 h at 60°C). Digested samples were precipitated in 85% ethanol, and pellets were used for agarose electrophoresis. One set of pellets was resuspended in water and run in 0.7% agarose-Tris-Borate-EDTA gels as described (58). For the estimation of molecular mass, HA standards (Select-HA HiLadder and LoLadder; Hyalose, Austin TX) were used. After electrophoresis, samples were transferred to a Biodyne B nylon membrane (Pall Gelman Laboratory, Ann Arbor, MI) and probed with bHABP (1 μg/ml) (Seikagaku, Tokyo, Japan) followed by streptavidin-alkaline phosphatase (BioRad). Color was developed with bromochloroindolyl phosphate/nitro blue tetrazolium.
TK Enzyme Activity
TK enzyme activity was determined using DL-Val-Leu-Arg-pNA as a substrate as described previously (41, 59). Briefly, samples (100 μl) were incubated in an ultra-low–binding, 96-well plate (Corning, Acton, MA) with 10 μl trypsin (5 μg/ml) for 15 min at 37°C to activate pro-kallikrein. After adding 40 μl soybean trypsin inhibitor (SBTI) (1 mg/ml) and 100 μl of DL-Val-Leu-Arg-pNA (2 mM; ICN, Irvine, CA) in 200 mM Tris (pH 8.2), absorbance was measured at 412 nm using a microplate reader (Molecular Devices, Sunnyvale, CA). Enzyme concentrations were calculated by interpolating activities from the recombinant TK (rTK) standard curve.
Quantitative Analysis of EGF and TGF-α
The EGFR ligands EGF and TGF-α were measured in apical media (n = 4 different lung donors) using commercially available ELISA kits according to the manufacturers' guidelines (Quantikine kit; R&D Systems, Minneapolis, MN, and Calbiochem, respectively).
RT-PCR
RNA was extracted from NHBE cells cultured at the ALI (n = 3 different lung donors) using Trisol (Invitrogen, Carlsbad, CA). cDNA was obtained using SuperScript First Strand Synthesis System for RT/PCR kit (Invitrogen). MUC5AC- and Bcl-2–specific oligonucleotide primers were designed according to Shao and colleagues (60) (sense: 5′-TCC GGC CTC ATC TTC TCC-3′; antisense: 5′-ACT TGG GCA CTG GTG CTG-3′) and Floros and colleagues (61) (sense: 5′-TTT GAG TTC GGT GGG GTC AT-3′; antisense: 5′-TGA CTT CAC TTG TGG CCC AG-3′), respectively. The expected fragments amplified by PCR were 679 bp for MUC5AC and 274 bp for Bcl-2, and their identities were confirmed by sequence analysis. As quantitative controls, primers for β-actin (sense: 5′-ATC TGG CAC CAC ACC TTC TA-3′; antisense: 5′-CGT CAT ACT CCT GCT TGC TG-3′) (61) were used. cDNA was amplified by 30 cycles at 94°C for 30 s, 57°C for 45 s, and 72°C for 45 s, followed by a 3-min elongation at 72°C. PCR products were sequenced using the ABI Prism 3100 Genetic Analyzer (Applied Biosystems, Foster City, CA).
MUC5AC ELISA
ELISA assay was performed as previously described (39). Briefly, cell lysates were incubated with bicarbonate-carbonate buffer (pH 9.2) for 1 h at 37°C in a MaxiSorp 96-well plate (Nunc, Rochester, NY) or until dry. Plates were washed three times with TBS and blocked with 2% BSA for 1 h at room temperature. Plates were washed three times with TBS and incubated with a monoclonal anti-MUC5AC antibody (10 μg/ml) (Chemicon International). After 1 h, wells were washed with TBS, and 100 μl of AP-conjugated goat anti-mouse IgG (0.2 μg/ml, KPL) was dispensed into each well. After 1 h, plates were washed with TBS. Color was developed with p-nitrophenyl phosphate and stopped with 3 N NaOH. Absorbance was read at 410 nm. Results were expressed as percent changes at A410 above the PBS control. This was necessary because purified MUC5AC is not available to calibrate the assays.
Goblet Cell Quantification
MUC5AC-positive cells labeled by immunofluorescence as described previously were counted using optical fluorescent microscopy. From every slide, 15 fields at 40× magnification were randomly selected and loaded into the Metamorph software. Five counting frames of 125 × 125 μm were randomly applied to each of the 15 selected fields; cells within regions were marked and counted, including cells intersecting the upper and left borders of each frame. The total number of cells per frame was estimated by counting the total number of nuclei within each frame. For rTK-treated cells, the apical surface of NHBE cells was gently rinsed with PBS (37°C) to remove mucus and debris. Cells were fixed with acetone-methanol (1:1) for 1 min (−20°C). After fixation, two of the inserts obtained from three lung donors were labeled with anti-MUC5AC antibody or 10 μg/ml (Chemicon International) after blocking with 1% BSA in PBS. The third piece was used as a nonimmune control. Increase of mucous cells was determined analyzing the number of MUC5AC-positive cells on 10 fields/filter in triplicate samples of each experimental condition. Digital pictures were obtained with an Olympus BH-2 microscope (Olympus America Inc., Melville, NY). Relative quantification of MUC5AC-positive cells was done using Metamorph software. Results were expressed as fold increase of the number of positive MUC5AC cells/field over the control values (PBS).
Immunohistochemical Analysis of EGFR, Bcl-2, and LPO
To test if the use of rTK, which signals downstream from ROS in the induction of GC proliferation, could mimic ROS effects and to validate the GC counts using antibodies to additional GC products (LPO and Bcl-2), inserts treated with PBS or rTK in the presence or absence of PI as described in Protocols, were fixed with acetone-methanol (1:1). Duplicate inserts obtained from three lung donors were divided into four pieces. After blocking as described previously, the cells were labeled with anti-10 μg/ml EGFR (Ab-3; Calbiochem), 20 μg/ml anti-Bcl-2 (Chemicon), or anti-LPO (1:200, generated as described) (24) antibodies. AP-conjugated goat anti-mouse IgG or goat anti-rabbit IgG antibodies (0.2 μg/ml) (KPL) were applied, and color was developed using NBT/BCIP as substrate.
EGFR Immunofluorescence
To test if X/XO induced apical expression of EGFR as suggested in Figure 8, inserts treated with PBS or X/XO in the presence or absence of catalase as described in Protocols were pretreated with 0.2% Triton X-100 in 100 mM KCl, 3 mM MgCl2, 1 mM CaCl2, 200 mM sucrose, and 10 mM Hepes (pH 7.1) before fixation with acetone-methanol (1:1). After blocking as described previously, the cells were labeled with 10 μg/ml anti-EGFR (Santa Cruz Biotechnology). Cells were then washed with PBS, and 0.1 μg/ml Alexa 488–labeled mouse anti-IgG (Molecular Probes) was added. Excised inserts were mounted on slides with Gel/Mount (Biomeda). Images from the apical pole were captured with a confocal laser-scanning microscope (Zeiss LSM).
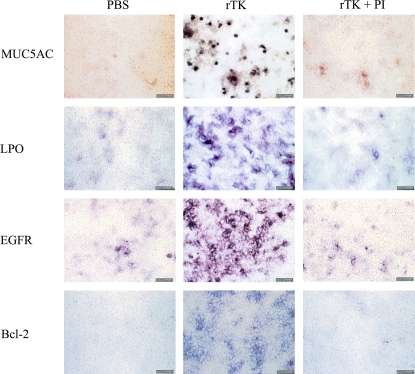
Figure 8.
rTK induce goblet cell proliferation. GC were identified in NHBE cells treated once daily during 3 d with rTK (1 μM) in the presence or absence of PI (4 μM) by immunocytochemistry using antibodies against MUC5AC and LPO. rTK increased all the markers analyzed, and its effect was inhibited by PI. EGFR and Bcl-2 expression were also induced by rTK. Bar = 100 μm.
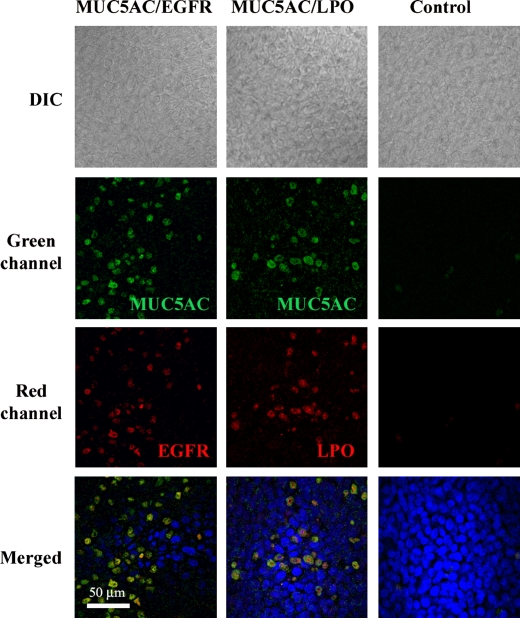
Colocalization Studies
To confirm that the GC markers used (MUC5AC, LPO) labeled the same cell population and that those cells were the ones with high expression of EGFR as reported (27), duplicate cell culture inserts treated with X/XO once daily for 3 d, as described in Protocols, were fixed and permeabilized with cold acetone-methanol (1:1) for 1 min. After blocking for 1 h with BSA 1% in PBS, 10 μg/ml of anti-MUC5AC antibody was added to the luminal surface overnight. Cells were washed with PBS, and 0.1 μg/ml Alexa 488–labeled mouse anti-IgG (Molecular Probes) was added. Colocalization with EGFR or LPO was achieved by the addition of rabbit anti-EGFR (1:100; Santa Cruz Biotechnology) or anti-LPO (1:200) antibodies for 2 h at room temperature. After washing, Alexa 555-conjugated anti-rabbit IgG (0.1 μg/ml) was incubated for 1 h at room temperature. Excised inserts were mounted on slides with Gel/Mount (Biomeda) containing 4′,6-diamidine-2-phenylindole for nuclei visualization. Images were captured with a confocal laser-scanning microscope (Zeiss LSM).
Statistical Analysis
Data were expressed as means ± SEM. Differences between multiple groups were compared using a one-way ANOVA followed by the Tukey Kramer honestly significant difference test. The Levene test was used to analyze the homogeneity of variances. Significance was accepted at P < 0.05.
RESULTS
X/XO Stimulates GC Differentiation/Proliferation on NHBE Cells
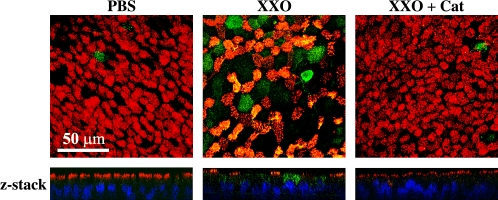
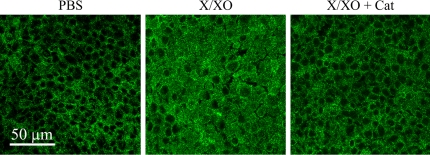
To examine if oxidative stress induced changes in the phenotype of differentiated NHBE cells grown at the ALI, antibodies to MUC5AC (GC marker) and acetylated tubulin (cilia marker) were used for double-labeling immunofluorescence as described in Materials and Methods. As observed in confocal microscopy images (Figure 1), apical treatment with once daily X/XO for 3 d induced an increase of MUC5AC-positive cells and a decrease of ciliated cells. Colocalization in X/XO treated cells was due to MUC5AC released by goblet cells and present on top of the cilia, rather than being released by the same cells, because it disappeared after cells were washed with PBS at 37°C. In those filters, MUC5AC labeling was limited to CG.
Figure 1.
ROS stimulates goblet cell metaplasia on NHBE cells. To identify ciliated epithelial cells and GC on NHBE cultures treated once daily during 3 d with PBS or X/XO in the presence or absence of catalase (Cat), cells were double-labeled with antibodies to MUC5AC (green channel) and acetylated tubulin (red channel) and scanned using confocal microscopy (original magnification: ×63) focused in the apical pole of cells. Z-axis reconstruction is shown.
These phenotypic changes were inhibited by catalase, suggesting that oxidative stress induced GC metaplasia on NHBE cultures grown at the ALI. In addition, X/XO-treated cells (Figure 1) seem larger than cells in the other panels. This finding was not consistent, and not all cultures tested (obtained from different lung donors) showed this “hyperplastic” response. A larger sample population is needed to determine if GC hyperplasia is associated with oxidative stress.
Apical X/XO Induces EGFR Activation and Signaling in NHBE Cells
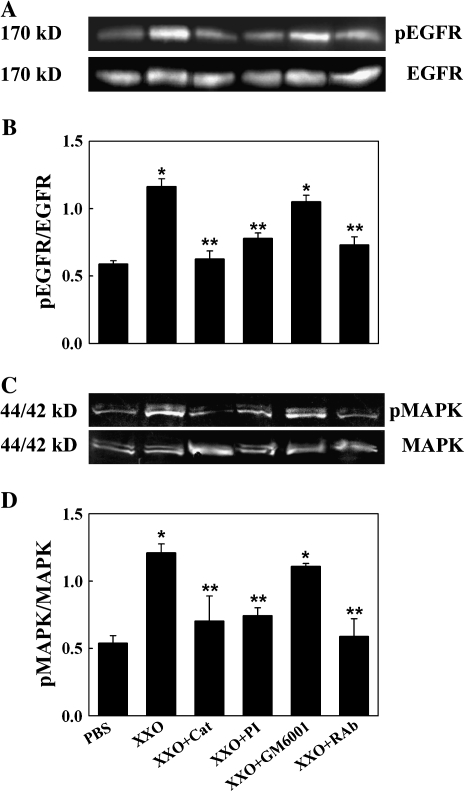
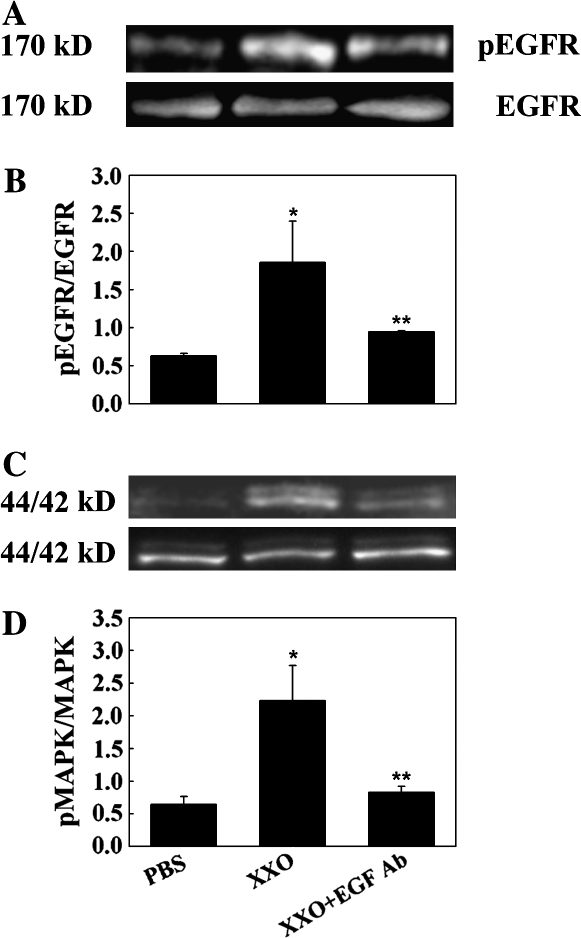
To test if the ROS-induced GC metaplasia was mediated by EGFR, EGFR phosphorylation and signaling was assessed by exposing cells apically to ROS generated by X/XO for 30 min as described in Materials and Methods. Apical X/XO treatment induced EGFR activation (measured by pEGFR/EGFR ratios) and signaling, determined by the phosphorylation of 44/42MAPK (pMAPK/MAPK ratios) (Figure 2). X/XO-induced EGFR and 44/42MAPK activation was due to ROS generation because it was inhibited by catalase. These data suggest that apical oxidative stress on NHBE cells causes EGFR activation and signaling through 44/42MAPK. Because EGFR ligands are synthesized as transmembrane precursors and are cleaved to their active form by matrix metalloproteases of the ADAM family or the serine protease TK, inhibitors of these proteases were used. The fact that TK protease inhibitors and not metalloprotease inhibitors and neutralizing anti-EGF antibodies (Figure 3) inhibited ROS-induced EGFR and MAPK activation strongly suggests that EGF was the ligand involved in EGFR signaling.
Figure 2.
ROS increase EGFR and MAPK activation in NHBE cultures. Cells were exposed for 30 min to apical PBS (control), X/XO or X/XO plus catalase (Cat), TK inhibitor (PI), metaloprotease inhibitor (GM6001), or functionally blocking anti-EGFR monoclonal antibodies (RAb). Cell lysates were analyzed by Western blot. (A) pEGFR (top); total EGFR (bottom). (B) pEGFR/EGFR ratios. (C) p44/42MAPK (top); total 44/42MAPK (bottom). (D) pMAPK/MAPK ratios. *P < 0.01 compared with PBS (control). **P < 0.01 compared with X/XO.
Figure 3.
EGF-neutralizing antibodies inhibit ROS-induced EGFR and MAPK activation in NHBE cultures. Cells were exposed for 30 min to apical PBS (control), X/XO, or X/XO plus anti-EGF antibodies. Cell lysates were analyzed by Western blot. (A) pEGFR (top); total EGFR (bottom). (B) pEGFR/EGFR ratios. (C) p44/42MAPK (top); total 44/42MAPK (bottom). (D) pMAPK/MAPK ratios. *P < 0.01 compared with PBS (control). **P < 0.01 compared with X/XO.
Apical X/XO Induces HA Depolymerization, TK Activation, and pro-EGF Processing in NHBE Cells
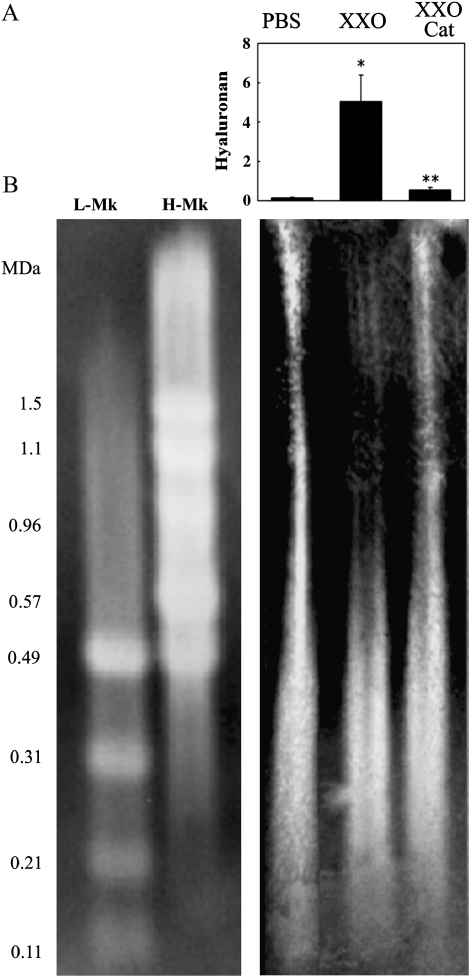
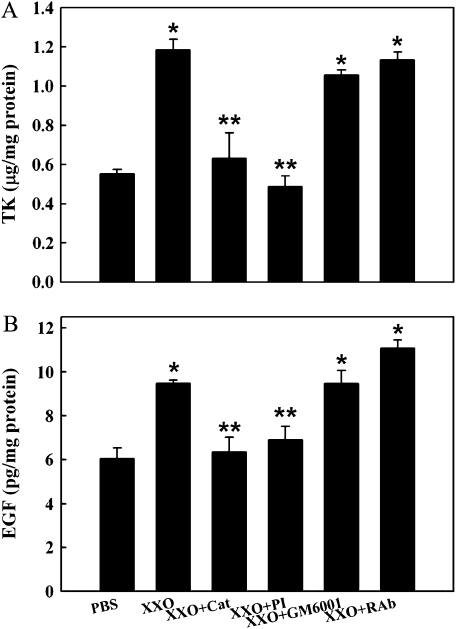
Because ROS-induced EGFR activation is mediated by pro-EGF activation due to TK release secondary to HA depolymerization in SMG cells (39), we tested if those mechanisms were operative in NHBE cells. HA levels, average molecular size, TK activity, and released EGF were assessed in apical washes of cultures treated as described in Materials and Methods. X/XO exposure for 30 min increased HA concentrations in apical washes when compared with PBS control (0.15 ± 0.03 μg/mg cell lysate protein versus 5.05 ± 1.33 μg/mg; P < 0.05) (Figure 4A). Catalase treatment prevented ROS-induced increases in HA levels (0.53 ± 0.13 μg/mg; P > 0.05 compared with X/XO). In addition, X/XO treatment resulted in a decrease in the HA average molecular size from > 1.5 MDa to ∼ 0.6 MDa (increased mobility and decrease in HA size and heterogeneity), whereas catalase treatment prevented HA breakdown (> 1.5 MDa) (Figure 4B). These results are consistent with HA breakdown and release from the epithelial cell surface. Concomitant with the HA depolymerization, TK activity and EGF levels increased from 1.18 ± 0.03 μg/mg protein versus 0.55 ± 0.05 μg/mg (P < 0.05) and 9.5 ± 0.4 pg/mg protein versus 6.1 ± 0.5 pg/mg (P < 0.05), respectively (Figure 5A). The levels of TGF-α, an EGFR ligand that is abundant in airway epithelial cells but is not processed by TK, were not affected by oxidative stress in these experiments (2.3 ± 0.1 pg/mg cell lysate protein versus 2.5 ± 0.2 pg/mg; P > 0.05). Catalase or PI treatment resulted in TK (0.63 ± 0.19 and 0.49 ± 0.10 μg/mg protein) and EGF (6.3 ± 0.7 and 6.9 ± 0.6 pg/mg protein) concentrations comparable to baseline levels (Figure 5), whereas GM6001 had no effect on TK (1.06 ± 0.03) or EGF (9.5 ± 0.6) levels. These results show that X/XO induced HA depolymerization, TK activation, and pro-EGF processing in NHBE cells grown at the ALI because these effects were inhibited by catalase and EGF release was inhibited by the specific TK peptide inhibitor.
Figure 4.
ROS induces HA depolymerization and release. (A) NHBE cells (n = 3 different lung donors) were exposed for 30 min to PBS or X/XO alone or with catalase in the apical compartment. Released HA was assessed by ELISA and normalized to the simultaneously recovered cell lysates protein (μg HA/mg protein). *P < 0.01 compared with PBS (control). **P < 0.01 compared with X/XO. (B) HA depolymerization was estimated by comparing HA average molecular size in samples and HA standards Select-HA HiLadder (H-Mk) and LoLadder (L-Mk) run in agarose and followed by transblotting using bHABP.
Figure 5.
ROS increase TK activity and EGF release into the apical compartment of human NHBE cells. Apical compartments of human NHBE cell cultures (n = 3 different lungs) were exposed to PBS or X/XO alone or with catalase (Cat), TK inhibitor (PI), metalloprotease inhibitor (GM6001), or functionally blocking anti-EGFR monoclonal antibodies (RAb) for 30 min. Apical washes were analyzed for TK with an enzyme activity assay (μg/mg cell lysate protein, A) and for EGF levels (pg/mg cell lysate protein, B) with an ELISA. *P < 0.01 compared with PBS (control). **P < 0.01 compared with X/XO.
GC Metaplasia due to Oxidative Stress Is Mediated by EGF-Induced EGFR Activation
To test if the observed EGFR activation by X/XO resulted in changes in the GC population or secretion, we assessed MUC5AC as an end-point of EGFR-induced phenotypic transformation of GC. MUC5AC gene and protein expression were measured after apical treatment once daily for 3 d as described in Materials and Methods. Exposure to X/XO induced MUC5AC gene expression, as evidenced by MUC5AC/actin mRNA ratios (2.13 ± 0.43; P < 0.05) compared with PBS control (0.21 ± 0.02). The increase in MUC5AC expression was inhibited by catalase (0.94 ± 0.12), the specific TK PI (0.65 ± 0.07), and functionally blocking anti-EGFR antibodies (0.83 ± 0.08) (all P < 0.05 compared with X/XO-treated cells). The metalloprotease inhibitor GM6001 did not significantly inhibit the X/XO effect (1.87 ± 0.22; P > 0.05), confirming that EGFR ligands processed by metalloproteases of the ADAM family (i.e., TGF-α, HB-EGF, or amphiregulin) were not involved in MUC5AC upregulation in these cells under these conditions (Figure 6A). Consistent with gene expression findings, ELISA analysis (Figure 6B) showed that X/XO exposure increased MUC5AC protein expression (32.5 ± 9.3% above PBS control; P < 0.05). This effect was inhibited by catalase (10.1 ± 3.6%), PI (1.1 ± 4.2%), and functionally blocking anti-EGFR antibodies (7.2 ± 6.0% above baseline) (all P < 0.05 versus X/XO). In contrast, no significant differences were observed with GM6001 pretreatment (18.8 ± 7.5% above baseline; P > 0.05). These results suggest that ROS-induced MUC5AC increases in NHBE cell cultures were mediated by TK processing of pro-EGF followed by EGFR activation. To determine if MUC5AC increases were associated with GC metaplasia, we assessed GC numbers as described in Materials and Methods. X/XO treatment once daily for 3 d resulted in an increase of the percentage of MUC5AC-positive cells (21.6 ± 3.4%) compared with PBS control cells (3.3 ± 1.2%). This effect was inhibited by catalase (3.8 ± 0.7), PI (3.1 ± 0.8), and RAb (3.9 ± 0.9%) but not by GM6001 (19.6 ± 3.1%). This inhibition profile is consistent with the observed gene expression (Figure 6C) and confirms that GC differentiation/proliferation was associated with increases in MUC5AC.
Figure 6.
ROS-induced MUC5AC expression and GC metaplasia is mediated by EGF. Human NHBE cells were exposed to PBS or X/XO for three consecutive days in the presence or absence of catalase, PI, GM6001, or functionally blocking anti-EGFR monoclonal antibodies (RAb). (A) MUC5AC mRNA (top); MUC5AC/actin ratios (bottom). (B) MUC5AC protein was assessed by ELISA and expressed as changes above PBS control. (C) Percentage of MUC5AC-positive cells. *P < 0.05 compared with PBS. **P < 0.05 compared with X/XO (n = 3 different lung donors).
X/XO Induces Bcl-2 Expression Human Airway Epithelia
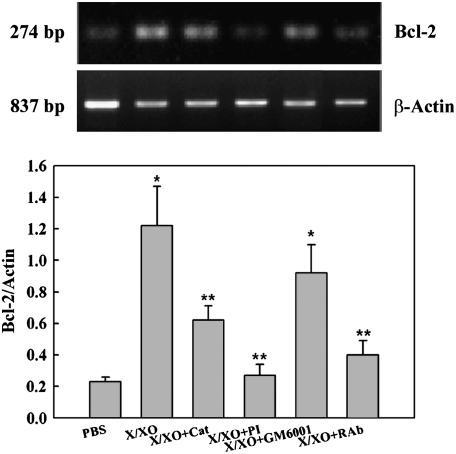
Because Bcl-2 is an anti apoptotic marker that has been reported to sustain GC metaplasia (20), we assessed Bcl-2 gene expression in the experimental conditions described previously. X/XO treatment increased the Bcl-2/Actin mRNA ratio (1.22 ± 0.25; P < 0.05) compared with control (0.23 ± 0.03) (Figure 7). The increases in the Bcl-2/Actin mRNA ratio induced by X/XO were inhibited by catalase (0.62 ± 0.90), PI (0.27 ± 0.07), and functionally blocking anti-EGFR antibodies (0.40 ± 0.09) (all P < 0.05 compared with X/XO-treated cells) but not by GM6001 (0.88 ± 0.14; P > 0.05). These findings suggest that EGFR activation resulted in an additional mechanism aimed at sustaining increased GC number by slowing the turnover rate of these cells during oxidative stress. These effects were long lasting: The number of GC remained high (18.3 ± 4.1% MUC5AC positive cells, n = 2 lung donors) after those cultures were exposed to normal ALI conditions during three additional days after the chronic treatment with X/XO (results not shown). This result agrees with an increased Bcl-2 gene expression measured as the Bcl-2/Actin ratio in X/XO treated cells (1.72 ± 0.09) versus PBS (0.48 ± 0.09; P < 0.05) or X/XO + catalase–treated cells (1.43 ± 0.05; P < 0.05) at Day 7 (after 3 d of normal conditions).
Figure 7.
ROS-induce Bcl-2 mRNA expression. Human NHBE cells were exposed to PBS or X/XO for three consecutive days in the presence or absence of catalase, PI, GM6001, or functionally blocking anti-EGFR monoclonal antibodies (RAb). Bcl-2 mRNA expression assessed by RT- PCR (top) and expressed as Bcl-2/actin ratios (bottom). X/XO increased Bcl-2 mRNA. This effect could be blocked with catalase, PI, or RAb, whereas GM6001 had no effect. Graph is representative of three different lung donors.
Goblet Cell Differentiation/Proliferation Is Induced by TK on NHBE Cells
To confirm that, as suggested in the previous experiment, GC metaplasia is due, at least in part, to TK processing of pro-EGF, we treated cultures with rTK as described in Materials and Methods and used MUC5AC immunolabeling to identify GC. After 3 d of daily treatment, quantification of MUC5AC-positive cells showed that rTK increased mucous cells (5.5 ± 0.5-fold over PBS) compared with PBS control cells (1.0 ± 0.1; P < 0.01) (Figure 8). This effect was specifically inhibited by PI (1.8 ± 0.3; P < 0.01). Figure 8 depicts photomicrographs representative of three separate experiments (n = 3 lung donors). Labeling of additional GC products (LPO, EGFR, and Bcl-2; see Figure 8) confirmed the ability of TK to mimic the ROS-induced GC proliferation/differentiation.
X/XO Induces Apical Distribution of EGFR
To confirm that EGFR is present in the apical pole in X/XO-treated cells (daily during 3 d) as suggested in Figure 8, images obtained from confocal microscopy from the apical pole of the cells fluorescently labeled with anti-EGFR antibodies as described in Materials and Methods were analyzed. In addition to the basolateral distribution of EGFR characteristic of normal airway epithelium and cultures treated with PBS, cells exposed to X/XO evidenced apical distribution of EGFR (Figure 9). Catalase pretreatment specifically prevented apical expression of the receptor, suggesting that the X/XO induces apical expression and/or translocation of EGFR.
Figure 9.
X/XO induces apical distribution of EGFR. NHBE cells treated for 3 d with X/XO were labeled using antibodies against EGFR (green) and scanned using confocal microscopy (original magnification: ×63). Picture shows the apical pole of the cultures.
MUC5AC Colocalizes with LPO and EGFR
To confirm that the assessed mucous markers induced by X/XO treatment belong to the same cell population, double labeling using MUC5AC with LPO or EGFR antibodies was performed as described in Materials and Methods. Analysis using confocal microscopy (Figure 10) showed that MUC5AC and LPO as well as MUC5AC and EGFR were visualized in the same cells, supporting the notion that EGFR was associated with GC differentiation/proliferation in airway epithelium exposed to oxidative stress. The data also validated MUC5AC as a useful tool to assess GC population in primary cultures of NHBE cells grown at the ALI.
Figure 10.
EGFR and LPO colocalize with MUC5AC. NHBE cells treated 3 d with X/XO were double labeled using antibodies against MUC5AC (green), EGFR (red), or LPO (red) and scanned using confocal microscopy (original magnification: ×63).
DISCUSSION
The data presented here support the hypothesis that oxidative stress–induced GC metaplasia of differentiated primary cultures of human airway epithelial cells is due, at least in part, to a cascade of events initiated by the breakdown of HA, the activation of TK, the release of mature EGF, and EGFR activation. We have previously shown that HA is present at the apical pole of airway epithelial cells (47, 62) and that it is bound to TK, inhibiting its catalytic activity (41); therefore, we expected that ROS-induced HA depolymerization resulted in TK activation. We did not test if, in addition to the TK release from HA–TK complexes, X/XO induced increased TK expression. If this occurs, HA depolymerization would be necessary for the release of active TK because high-molecular-weight HA present at the epithelial surface would be sufficient to control the catalytic activity of the increased levels of TK. We also anticipated that TK should be able to process pro-EGF as reported in primary cultures of human submucosal glands cells and mimic ROS effects (39). The novel findings in this study were that HA depolymerization induced by ROS resulted in phenotypic changes on differentiated primary cultures of NHBE cells using a relatively short-term exposure (once daily for 3 d) to oxidative stress. This treatment led to GC metaplasia, which was associated with EGFR activation by EGF. In the present study, we did not address the origin of the increased GC population, but the metaplasia is likely the result of proliferation from basal cells, as has been reported (63), or differentiation from nonproliferating pre-existent cells in combination with the increased half life due to upregulation of antiapoptotic factors (22, 64, 65).
Our data confirm findings by other groups who reported that EGFR signaling was shown to result in MUC5AC induction, although there are with some discrepancies regarding to the ligands responsible for EGFR signaling. We found that in NHBE cells grown at the ALI, the ligand responsible for EGFR activation under oxidative stress was EGF, processed from its transmembrane pro-form by TK. The inhibitory effect of neutralizing anti-EGF antibodies on ROS-induced EGFR and MAPK activation confirmed these findings.
In contrast, other reports have shown that oxidative stress–induced GC metaplasia or MUC5AC expression was associated with TGF-α (66, 67), amphiregulin (68, 69), or HB-EGF (13). The activation of all the transmembrane pro-ligands mentioned previously requires the activity of metalloproteases of the ADAM family. In our system, metalloproteases seem not to play a role in GC metaplasia because responses were not inhibited by the metalloprotease inhibitor GM6001. Confirming this notion, we found that EGF and not TGF-α levels increased in response to oxidative stress in our cells; these results are in contrast to the report of Shao and colleagues (67), who reported that H2O2 production induced by PMA resulted in an increase of TGF-α−mediated EGFR activation. There are several explainations for the discrepancies between the studies. First, Shao and colleagues used a much lower concentration of retinoic acid in their culture media (∼ 3 × 10−10 M versus 5 × 10−8 M in our cultures). This is likely relevant because TGF-α gene expression is induced by these low concentrations of retinoic acid (70, 71). We used the concentration that Koo and colleagues (71) found to be necessary to maintain mucous cell differentiation in NHBE cell cultures. Second, primary cells cultures, unlike cell lines, contain a heterogeneous cellular population (e.g., ciliated, secretory, basal) and show greater variability in a number of responses (72). Our studies were performed in Passage 1 cells with > 80% ciliated cells. Under these conditions, our initial number of GCs was ∼ 3%. Third, the initial culture conditions, such as the characteristics of the cultures (number of ciliated and goblet cells), the cell passage, and the media composition used by Shao and colleagues (67), were not fully detailed, and therefore a comparison with our initial conditions is not possible. Fourth, differences such as in the stimuli used to induce ROS production (PMA instead of X/XO) may be implied in activating different enzymes (TACE versus TK). Last, EGF was removed from our culture media 48 h before and during treatments (to allow us to assess the role of the endogenous, membrane-bound pro-EGF processing). The fact that those authors have used media supplemented with EGF may have affected the endogenous synthesis of pro-EGF.
Other stimuli, such as IL-13 or neutrophil elastase (73), have been shown to induce GC metaplasia (74–76) and epithelial cell proliferation (77) by metalloprotease activation (e.g., TACE) and TGF-α release on bronchial epithelial cells (76) or by enhancing MUC5AC RNA stability (73). The pathways involved in GC metaplasia and hypersecretion using those stimuli differ but do not conflict with our observations. For instance, primary bronchial or nasal epithelial cells were treated with IL-13 from the moment that they were exposed to air. Under these conditions, differentiation into GC phenotype was higher than in epithelial cells grown in the absence of IL-13 (74–76). In contrast to our ROS exposure of completely differentiated NHBE cells (∼ 21 d on air and with > 80% of ciliated cells) once daily for 3 d, the exposure to IL-13 was lengthy (14 d) starting as soon as cells were exposed to air (∼ 7 d) and during the differentiation stage. Therefore, differences in experimental conditions and stimuli used in those reports likely explain the discrepancies in the EGFR ligands involved in GC metaplasia.
In our cultures, the basolateral distribution EGFR is modified by oxidative stress, which induced EGFR expression and/or translocation to the apical surface of NHBE cells. This change in localization allows ligand/receptor interaction because EGF (and most ErbB receptor ligands) is expressed at the apical compartment of epithelial cells and is separated by tight junctions from its receptor (78), which is localized basolaterally in normal epithelium (29). This pattern has been described in the airway epithelium of smokers (79) and patients with asthma (80), suggesting that oxidative stress could mediate EGFR apical expression and/or translocation. This study also shows that EGFR is colocalized with MUC5AC in GC, as has been described in the epithelium of patients with asthma (27), suggesting that these cultures are a useful model to assess epithelial responses to oxidative stress.
In addition, we found that X/XO induced Bcl-2 gene expression and that this effect was mimicked by rTK. In Bcl-2, protein expression was not evident by immunohistochemistry in the control cells but was highly expressed after rTK treatment. These results are in agreement with Bcl-2 increases induced by EGF and p44/42 MAPK in other tissues (81, 82). Thus, EGFR activation resulted in an additional mechanism aimed at sustaining increased GC number by slowing the turnover rate of these cells during oxidative stress. These effects were long lasting: Bcl-2 gene expression and the number of GC remained high after those cultures were exposed to normal ALI conditions for three additional days after the chronic treatment with X/XO. These observations seem to be clinically relevant to hypersecretory states associated with human airway diseases. For example, an increased Bcl-2 immunoreactivity has been described in the airways of patients with asthma (21) and in mucous cells from patients with cystic fibrosis (20). The fact that inhibition of Bcl-2 expression resulted in reduction of GC metaplasia (20) suggests that a decrease in GC turnover could contribute to the observed increase in GC population in human epithelium in such conditions.
We found that another GC product, LPO (23), was induced by rTK treatment. This is consistent with the notion that GC, in addition to increased mucins in response to oxidative stress, are also capable of increasing the ROS scavenging properties of airway mucus through LPO (23, 83).
In summary, these results provide a direct mechanistic link between sustained airway ROS insult during inflammatory responses and the development of GC metaplasia and mucous hypersecretion, characteristics of asthma and COPD. Our results suggest that HA plays a key role in regulating oxidative stress–induced GC metaplasia in human airways by regulating TK activity. The role of specific oxidants from exogenous (e.g., tobacco smoke) or endogenous [i.e., H2O2 generation mediated by Duox (84)] sources in HA depolymerization and EGFR-mediated GC metaplasia needs further investigation.
Acknowledgments
The authors thank Dr. Gregory E. Conner and Matthias Salathe for critical comments and suggestions.
This work was supported by the Florida Department of Health and by grants NIH HL-68992 and HL-073156 (all R.M.F.).
Originally Published in Press as DOI: 10.1165/rcmb.2005-0386OC on January 19, 2006
Conflict of Interest Statement: None of the authors has a financial relationship with a commercial entity that has an interest in the subject of this manuscript.
References
- 1.Dohrman A, Miyata S, Gallup M, Li JD, Chapelin C, Coste A, Escudier E, Nadel J, Basbaum C. Mucin gene (MUC 2 and MUC 5AC) upregulation by Gram-positive and Gram-negative bacteria. Biochim Biophys Acta 1998;1406:251–259. [DOI] [PubMed] [Google Scholar]
- 2.Hashimoto K, Graham BS, Ho SB, Adler KB, Collins RD, Olson SJ, Zhou W, Suzutani T, Jones PW, Goleniewska K, et al. Respiratory syncytial virus in allergic lung inflammation increases Muc5ac and gob-5. Am J Respir Crit Care Med 2004;170:306–312. [DOI] [PubMed] [Google Scholar]
- 3.Dunnill MS. The pathology of asthma, with special reference to changes in the bronchial mucosa. J Clin Pathol 1960;13:27–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Aikawa T, Shimura S, Sasaki H, Ebina M, Takishima T. Marked goblet cell hyperplasia with mucus accumulation in the airways of patients who died of severe acute asthma attack. Chest 1992;101:916–921. [DOI] [PubMed] [Google Scholar]
- 5.Ordonez CL, Khashayar R, Wong HH, Ferrando R, Wu R, Hyde DM, Hotchkiss JA, Zhang Y, Novikov A, Dolganov G, et al. Mild and moderate asthma is associated with airway goblet cell hyperplasia and abnormalities in mucin gene expression. Am J Respir Crit Care Med 2001;163:517–523. [DOI] [PubMed] [Google Scholar]
- 6.Reid LM. Pathology of chronic bronchitis. Lancet 1954;266:274–278. [PubMed] [Google Scholar]
- 7.Wanner A. The role of mucus in chronic obstructive pulmonary disease. Chest 1990;97:11S–15S. [DOI] [PubMed] [Google Scholar]
- 8.Markewitz BA, Owens MW, Payne DK. The pathogenesis of chronic obstructive pulmonary disease. Am J Med Sci 1999;318:74–78. [DOI] [PubMed] [Google Scholar]
- 9.Fahy JV, Schuster A, Ueki I, Boushey HA, Nadel JA. Mucus hypersecretion in bronchiectasis: the role of neutrophil proteases. Am Rev Respir Dis 1992;146:1430–1433. [DOI] [PubMed] [Google Scholar]
- 10.Hauber HP, Tsicopoulos A, Wallaert B, Griffin S, McElvaney NG, Daigneault P, Mueller Z, Olivenstein R, Holroyd KJ, Levitt RC, et al. Expression of HCLCA1 in cystic fibrosis lungs is associated with mucus overproduction. Eur Respir J 2004;23:846–850. [DOI] [PubMed] [Google Scholar]
- 11.Fischer BM, Voynow JA. Neutrophil elastase induces MUC5AC gene expression in airway epithelium via a pathway involving reactive oxygen species. Am J Respir Cell Mol Biol 2002;26:447–452. [DOI] [PubMed] [Google Scholar]
- 12.Takeyama K, Jung B, Shim JJ, Burgel PR, Dao-Pick T, Ueki IF, Protin U, Kroschel P, Nadel JA. Activation of epidermal growth factor receptors is responsible for mucin synthesis induced by cigarette smoke. Am J Physiol Lung Cell Mol Physiol 2001;280:L165–L172. [DOI] [PubMed] [Google Scholar]
- 13.Basbaum C, Li D, Gensch E, Gallup M, Lemjabbar H. Mechanisms by which gram-positive bacteria and tobacco smoke stimulate mucin induction through the epidermal growth factor receptor (EGFR). Novartis Found Symp 2002;248:171–176; discussion 176–180, 277–282. [PubMed] [Google Scholar]
- 14.Harrod KS, Jaramillo RJ, Rosenberger CL, Wang SZ, Berger JA, McDonald JD, Reed MD. Increased susceptibility to RSV infection by exposure to inhaled diesel engine emissions. Am J Respir Cell Mol Biol 2003;28:451–463. [DOI] [PubMed] [Google Scholar]
- 15.Takeyama K, Dabbagh K, Jeong Shim J, Dao-Pick T, Ueki IF, Nadel JA. Oxidative stress causes mucin synthesis via transactivation of epidermal growth factor receptor: role of neutrophils. J Immunol 2000;164:1546–1552. [DOI] [PubMed] [Google Scholar]
- 16.Wills-Karp M, Luyimbazi J, Xu X, Schofield B, Neben TY, Karp CL, Donaldson DD. Interleukin-13: central mediator of allergic asthma. Science 1998;282:2258–2261. [DOI] [PubMed] [Google Scholar]
- 17.Dabbagh K, Takeyama K, Lee HM, Ueki IF, Lausier JA, Nadel JA. IL-4 induces mucin gene expression and goblet cell metaplasia in vitro and in vivo. J Immunol 1999;162:6233–6237. [PubMed] [Google Scholar]
- 18.Rose MC, Nickola TJ, Voynow JA. Airway mucus obstruction: mucin glycoproteins, MUC gene regulation and goblet cell hyperplasia. Am J Respir Cell Mol Biol 2001;25:533–537. [DOI] [PubMed] [Google Scholar]
- 19.Atherton HC, Jones G, Danahay H. IL-13-induced changes in the goblet cell density of human bronchial epithelial cell cultures: MAP kinase and phosphatidylinositol 3-kinase regulation. Am J Physiol Lung Cell Mol Physiol 2003;285:L730–L739. [DOI] [PubMed] [Google Scholar]
- 20.Harris JF, Fischer MJ, Hotchkiss JR, Monia BP, Randell SH, Harkema JR, Tesfaigzi Y. Bcl-2 sustains increased mucous and epithelial cell numbers in metaplastic airway epithelium. Am J Respir Crit Care Med 2005;171:764–772. [DOI] [PubMed] [Google Scholar]
- 21.Vignola AM, Chiappara G, Siena L, Bruno A, Gagliardo R, Merendino AM, Polla BS, Arrigo AP, Bonsignore G, Bousquet J, et al. Proliferation and activation of bronchial epithelial cells in corticosteroid-dependent asthma. J Allergy Clin Immunol 2001;108:738–746. [DOI] [PubMed] [Google Scholar]
- 22.Tesfaigzi Y, Harris JF, Hotchkiss JA, Harkema JR. DNA synthesis and Bcl-2 expression during development of mucous cell metaplasia in airway epithelium of rats exposed to LPS. Am J Physiol Lung Cell Mol Physiol 2004;286:L268–L274. [DOI] [PubMed] [Google Scholar]
- 23.Salathe M, Holderby M, Forteza R, Abraham WM, Wanner A, Conner GE. Isolation and characterization of a peroxidase from the airway. Am J Respir Cell Mol Biol 1997;17:97–105. [DOI] [PubMed] [Google Scholar]
- 24.Gerson C, Sabater J, Scuri M, Torbati A, Coffey R, Abraham JW, Lauredo I, Forteza R, Wanner A, Salathe M, et al. The lactoperoxidase system functions in bacterial clearance of airways. Am J Respir Cell Mol Biol 2000;22:665–671. [DOI] [PubMed] [Google Scholar]
- 25.Wijkstrom-Frei C, El-Chemaly S, Ali-Rachedi R, Gerson C, Cobas MA, Forteza R, Salathe M, Conner GE. Lactoperoxidase and human airway host defense. Am J Respir Cell Mol Biol 2003;29:206–212. [DOI] [PubMed] [Google Scholar]
- 26.Kasper M, Sims G, Koslowski R, Kuss H, Thuemmler M, Fehrenbach H, Auten RL. Increased surfactant protein D in rat airway goblet and Clara cells during ovalbumin-induced allergic airway inflammation. Clin Exp Allergy 2002;32:1251–1258. [DOI] [PubMed] [Google Scholar]
- 27.Takeyama K, Fahy JV, Nadel JA. Relationship of epidermal growth factor receptors to goblet cell production in human bronchi. Am J Respir Crit Care Med 2001;163:511–516. [DOI] [PubMed] [Google Scholar]
- 28.Hunter T, Cooper JA. Epidermal growth factor induces rapid tyrosine phosphorylation of proteins in A431 human tumor cells. Cell 1981;24:741–752. [DOI] [PubMed] [Google Scholar]
- 29.Polosa R, Prosperini G, Leir SH, Holgate ST, Lackie PM, Davies DE. Expression of c-erbB receptors and ligands in human bronchial mucosa. Am J Respir Cell Mol Biol 1999;20:914–923. [DOI] [PubMed] [Google Scholar]
- 30.Strachan L, Murison JG, Prestidge RL, Sleeman MA, Watson JD, Kumble KD. Cloning and biological activity of epigen: a novel member of the epidermal growth factor superfamily. J Biol Chem 2001;276:18265–18271. [DOI] [PubMed] [Google Scholar]
- 31.Jones JT, Akita RW, Sliwkowski MX. Binding specificities and affinities of egf domains for ErbB receptors. FEBS Lett 1999;447:227–231. [DOI] [PubMed] [Google Scholar]
- 32.Sunnarborg SW, Hinkle CL, Stevenson M, Russell WE, Raska CS, Peschon JJ, Castner BJ, Gerhart MJ, Paxton RJ, Black RA, et al. Tumor necrosis factor-alpha converting enzyme (TACE) regulates epidermal growth factor receptor ligand availability. J Biol Chem 2002;277:12838–12845. [DOI] [PubMed] [Google Scholar]
- 33.Asakura M, Kitakaze M, Takashima S, Liao Y, Ishikura F, Yoshinaka T, Ohmoto H, Node K, Yoshino K, Ishiguro H, et al. Cardiac hypertrophy is inhibited by antagonism of ADAM12 processing of HB-EGF: metalloproteinase inhibitors as a new therapy. Nat Med 2002;8:35–40. [DOI] [PubMed] [Google Scholar]
- 34.Lemjabbar H, Basbaum C. Platelet-activating factor receptor and ADAM10 mediate responses to Staphylococcus aureus in epithelial cells. Nat Med 2002;8:41–46. [DOI] [PubMed] [Google Scholar]
- 35.Izumi Y, Hirata M, Hasuwa H, Iwamoto R, Umata T, Miyado K, Tamai Y, Kurisaki T, Sehara-Fujisawa A, Ohno S, et al. A metalloprotease-disintegrin, MDC9/meltrin-gamma/ADAM9 and PKCdelta are involved in TPA-induced ectodomain shedding of membrane-anchored heparin-binding EGF-like growth factor. EMBO J 1998;17:7260–7272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jorgensen E, Nexo E, Poulsen SS. The membrane fraction of homogenized rat kidney contains an enzyme that releases epidermal growth factor from the kidney membranes. Biochim Biophys Acta 1991;1074:284–288. [DOI] [PubMed] [Google Scholar]
- 37.Jorgensen PE, Nexo E, Poulsen SS, Almendingen M, Berg T. Processing of epidermal growth factor in the rat submandibular gland by Kallikrein-like enzymes. Growth Factors 1994;11:113–123. [DOI] [PubMed] [Google Scholar]
- 38.Jahnke GD, Chao J, Walker MP, Diaugustine RP. Detection of a kallikrein in the mouse lactating mammary gland: a possible processing enzyme for the epidermal growth factor precursor. Endocrinology 1994;135:2022–2029. [DOI] [PubMed] [Google Scholar]
- 39.Casalino-Matsuda SM, Monzon ME, Conner GE, Salathe M, Forteza RM. Role of hyaluronan and reactive oxygen species in tissue kallikrein-mediated epidermal growth factor receptor activation in human airways. J Biol Chem 2004;279:21606–21616. [DOI] [PubMed] [Google Scholar]
- 40.Proud D, Vio CP. Localization of immunoreactive tissue kallikrein in human trachea. Am J Respir Cell Mol Biol 1993;8:16–19. [DOI] [PubMed] [Google Scholar]
- 41.Forteza R, Lauredo I, Abraham WM, Conner GE. Bronchial tissue kallikrein activity is regulated by hyaluronic acid binding. Am J Respir Cell Mol Biol 1999;21:666–674. [DOI] [PubMed] [Google Scholar]
- 42.Christiansen SC, Proud D, Cochrane CG. Detection of tissue kallikrein in the bronchoalveolar lavage fluid of asthmatic subjects. J Clin Invest 1987;79:188–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Christiansen SC, Proud D, Sarnoff RB, Juergens U, Cochrane CG, Zuraw BL. Elevation of tissue kallikrein and kinin in the airways of asthmatic subjects after endobronchial allergen challenge. Am Rev Respir Dis 1992;145:900–905. [DOI] [PubMed] [Google Scholar]
- 44.Zhang M, Peng B, Niehus J, Baumgarten CR, Brunnee T, Thalhofer S, Dorow P, Kunkel G. Kinin generation in acute pneumonia and chronic bronchitis. Eur Respir J 1997;10:1747–1753. [DOI] [PubMed] [Google Scholar]
- 45.O'Riordan TG, Weinstein MD, Abraham WM, Forteza R. Elevated tissue kallikrein activity in airway secretions from patients with tracheobronchitis associated with prolonged mechanical ventilation. Lung 2003;181:237–244. [DOI] [PubMed] [Google Scholar]
- 46.Lapshina IM, Surovikina MS, Mal'tseva LM, Masich TA, Iurina TM. [The kallikrein-kinin system in dust-induced bronchitis.] Sov Med 1984;11:16–21. [PubMed] [Google Scholar]
- 47.Forteza R, Lieb T, Aoki T, Savani RC, Conner GE, Salathe M. Hyaluronan serves a novel role in airway mucosal host defense. FASEB J 2001;15:2179–2186. [DOI] [PubMed] [Google Scholar]
- 48.Agren UM, Tammi RH, Tammi MI. Reactive oxygen species contribute to epidermal hyaluronan catabolism in human skin organ culture. Free Radic Biol Med 1997;23:996–1001. [DOI] [PubMed] [Google Scholar]
- 49.Balazs EA, Watson D, Duff IF, Roseman S. Hyaluronic acid in synovial fluid: I. Molecular parameters of hyaluronic acid in normal and arthritis human fluids. Arthritis Rheum 1967;10:357–376. [DOI] [PubMed] [Google Scholar]
- 50.Matsumura G, Herp A, Pigman W. Depolymerization of hyaluronic acid by autoxidants and radiatiions. Radiat Res 1966;28:735–752. [PubMed] [Google Scholar]
- 51.Halliwell B, Hoult JR, Blake DR. Oxidants, inflammation, and anti-inflammatory drugs. FASEB J 1988;2:2867–2873. [DOI] [PubMed] [Google Scholar]
- 52.Nlend MC, Bookman RJ, Conner GE, Salathe M. Regulator of G-protein signaling protein 2 modulates purinergic calcium and ciliary beat frequency responses in airway epithelia. Am J Respir Cell Mol Biol 2002;27:436–445. [DOI] [PubMed] [Google Scholar]
- 53.Bernacki SH, Nelson AL, Abdullah L, Sheehan JK, Harris A, Davis CW, Randell SH. Mucin gene expression during differentiation of human airway epithelia in vitro: Muc4 and muc5b are strongly induced. Am J Respir Cell Mol Biol 1999;20:595–604. [DOI] [PubMed] [Google Scholar]
- 54.Kuwada SK, Lund KA, Li XF, Cliften P, Amsler K, Opresko LK, Wiley HS. Differential signaling and regulation of apical vs. basolateral EGFR in polarized epithelial cells. Am J Physiol 1998;275:C1419–C1428. [DOI] [PubMed] [Google Scholar]
- 55.Cagliari CI, De Caroli FP, Nakahata AM, Araujo MS, Nakaie CR, Sampaio MU, Sampaio CA, Oliva ML. Action of Bauhinia bauhinioides synthetic peptides on serine proteinases. Biochem Biophys Res Commun 2003;311:241–245. [DOI] [PubMed] [Google Scholar]
- 56.Chan H, Springman EB, Clark JM. Expression and characterization of human tissue kallikrein variants. Protein Expr Purif 1998;12:361–370. [DOI] [PubMed] [Google Scholar]
- 57.Bray BA, Hsu W, Turino GM. Lung hyaluronan as assayed with a biotinylated hyaluronan-binding protein. Exp Lung Res 1994;20:317–330. [DOI] [PubMed] [Google Scholar]
- 58.Lee HG, Cowman MK. An agarose gel electrophoretic method for analysis of hyaluronan molecular weight distribution. Anal Biochem 1994;219:278–287. [DOI] [PubMed] [Google Scholar]
- 59.Geiger R, Miska W. Human tissue kallikrein. Methods Enzymol 1988;163:102–115. [DOI] [PubMed] [Google Scholar]
- 60.Shao MX, Ueki IF, Nadel JA. Tumor necrosis factor alpha-converting enzyme mediates MUC5AC mucin expression in cultured human airway epithelial cells. Proc Natl Acad Sci USA 2003;100:11618–11623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Floros KV, Thomadaki H, Lallas G, Katsaros N, Talieri M, Scorilas A. Cisplatin-induced apoptosis in HL-60 human promyelocytic leukemia cells: differential expression of BCL2 and novel apoptosis-related gene BCL2L12. Ann N Y Acad Sci 2003;1010:153–158. [DOI] [PubMed] [Google Scholar]
- 62.Monzon ME, Casalino-Matsuda SM, Forteza RM. Identification of glycosaminoglycans in human airway secretions. Am J Respir Cell Mol Biol 2005;34:135–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Voynow JA, Fischer BM, Roberts BC, Proia AD. Basal-like cells constitute the proliferating cell population in cystic fibrosis airways. Am J Respir Crit Care Med 2005;172:1013–1018. [DOI] [PubMed] [Google Scholar]
- 64.Rose MC, Voynow JA. Respiratory tract mucin genes and mucin glycoproteins in health and disease. Physiol Rev 2006;86:245–278. [DOI] [PubMed] [Google Scholar]
- 65.Rogers DF. The airway goblet cell. Int J Biochem Cell Biol 2003;35:1–6. [DOI] [PubMed] [Google Scholar]
- 66.Takeyama K, Dabbagh K, Lee HM, Agusti C, Lausier JA, Ueki IF, Grattan KM, Nadel JA. Epidermal growth factor system regulates mucin production in airways. Proc Natl Acad Sci USA 1999;96:3081–3086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Shao MX, Nadel JA. Dual oxidase 1-dependent MUC5AC mucin expression in cultured human airway epithelial cells. Proc Natl Acad Sci USA 2005;102:767–772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lemjabbar H, Li D, Gallup M, Sidhu S, Drori E, Basbaum C. Tobacco smoke-induced lung cell proliferation mediated by tumor necrosis factor alpha-converting enzyme and amphiregulin. J Biol Chem 2003;278:26202–26207. [DOI] [PubMed] [Google Scholar]
- 69.Blanchet S, Ramgolam K, Baulig A, Marano F, Baeza-Squiban A. Fine particulate matter induces amphiregulin secretion by bronchial epithelial cells. Am J Respir Cell Mol Biol 2004;30:421–427. [DOI] [PubMed] [Google Scholar]
- 70.Miller LA, Zhao YH, Wu R. Inhibition of TGF-alpha gene expression by vitamin A in airway epithelium. J Clin Invest 1996;97:1429–1435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Koo JS, Yoon JH, Gray T, Norford D, Jetten AM, Nettesheim P. Restoration of the mucous phenotype by retinoic acid in retinoid-deficient human bronchial cell cultures: changes in mucin gene expression. Am J Respir Cell Mol Biol 1999;20:43–52. [DOI] [PubMed] [Google Scholar]
- 72.Adler KB, Li Y. Airway epithelium and mucus: intracellular signaling pathways for gene expression and secretion. Am J Respir Cell Mol Biol 2001;25:397–400. [DOI] [PubMed] [Google Scholar]
- 73.Voynow JA, Young LR, Wang Y, Horger T, Rose MC, Fischer BM. Neutrophil elastase increases MUC5AC mRNA and protein expression in respiratory epithelial cells. Am J Physiol 1999;276:L835–L843. [DOI] [PubMed] [Google Scholar]
- 74.Laoukili J, Perret E, Willems T, Minty A, Parthoens E, Houcine O, Coste A, Jorissen M, Marano F, Caput D, et al. IL-13 alters mucociliary differentiation and ciliary beating of human respiratory epithelial cells. J Clin Invest 2001;108:1817–1824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kondo M, Tamaoki J, Takeyama K, Nakata J, Nagai A. Interleukin-13 induces goblet cell differentiation in primary cell culture from guinea pig tracheal epithelium. Am J Respir Cell Mol Biol 2002;27:536–541. [DOI] [PubMed] [Google Scholar]
- 76.Yoshisue H, Hasegawa K. Effect of MMP/ADAM inhibitors on goblet cell hyperplasia in cultured human bronchial epithelial cells. Biosci Biotechnol Biochem 2004;68:2024–2031. [DOI] [PubMed] [Google Scholar]
- 77.Booth BW, Adler KB, Bonner JC, Tournier F, Martin LD. Interleukin-13 induces proliferation of human airway epithelial cells in vitro via a mechanism mediated by transforming growth factor-alpha. Am J Respir Cell Mol Biol 2001;25:739–743. [DOI] [PubMed] [Google Scholar]
- 78.Vermeer PD, Einwalter LA, Moninger TO, Rokhlina T, Kern JA, Zabner J, Welsh MJ. Segregation of receptor and ligand regulates activation of epithelial growth factor receptor. Nature 2003;422:322–326. [DOI] [PubMed] [Google Scholar]
- 79.O'Donnell RA, Richter A, Ward J, Angco G, Mehta A, Rousseau K, Swallow DM, Holgate ST, Djukanovic R, Davies DE, et al. Expression of ErbB receptors and mucins in the airways of long term current smokers. Thorax 2004;59:1032–1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Polosa R, Puddicombe SM, Krishna MT, Tuck AB, Howarth PH, Holgate gs ST, Davies DE. Expression of c-erbB receptors and ligands in the bronchial epithelium of asthmatic subjects. J Allergy Clin Immunol 2002;109:75–81. [DOI] [PubMed] [Google Scholar]
- 81.Clark JA, Lane RH, Maclennan NK, Holubec H, Dvorakova K, Halpern MD, Williams CS, Payne CM, Dvorak B. Epidermal growth factor reduces intestinal apoptosis in an experimental model of necrotizing enterocolitis. Am J Physiol Gastrointest Liver Physiol 2005;288:G755–G762. [DOI] [PubMed] [Google Scholar]
- 82.Liu YZ, Boxer LM, Latchman DS. Activation of the Bcl-2 promoter by nerve growth factor is mediated by the p42/p44 MAPK cascade. Nucleic Acids Res 1999;27:2086–2090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.El-Chemaly S, Salathe M, Baier S, Conner GE, Forteza R. Hydrogen peroxide-scavenging properties of normal human airway secretions. Am J Respir Crit Care Med 2003;167:425–430. [DOI] [PubMed] [Google Scholar]
- 84.Forteza R, Salathe M, Miot F, Conner GE. Regulated hydrogen peroxide production by Duox in human airway epithelial cells. Am J Respir Cell Mol Biol 2005;32:462–469. [DOI] [PubMed] [Google Scholar]