Abstract
Leukocytes synthesize a variety of inflammatory mediators that are packaged and stored in the cytoplasm within membrane-bound granules. Upon stimulation, the cells secrete the granule contents via an exocytotic process whereby the granules translocate to the cell periphery, the granule membranes fuse with the plasma membrane, and the granule contents are released extracellularly. We have reported previously that another exocytotic process, release of mucin by secretory cells of the airway epithelium, is regulated by the myristoylated alanine-rich C kinase substrate (MARCKS) (Li Y, Martin LD, Spizz G, Adler KB. MARCKS protein is a key molecule regulating mucin secretion by human airway epithelial cells in vitro. J Biol Chem 2001;276:40982–40990; Singer M, Martin LD, Vargaftig BB, Park J, Gruber AD, Li Y, Adler KB. A MARCKS-related peptide blocks mucus hypersecretion in a mouse model of asthma. Nat Med 2004;10:193–196). In those studies, mucin secretion in vitro and in vivo was attenuated by a synthetic peptide identical to the N-terminus of MARCKS, named the MANS peptide (Li and colleagues, 2001). In this study, we used the MANS peptide to investigate possible involvement of MARCKS in secretion of leukocyte granule proteins. In neutrophils isolated from human blood, phorbol 12-myristate 13-acetate–induced myeloperoxidase release was attenuated in a concentration-dependent manner by MANS but not by equal concentrations of a missense control peptide. In additional studies using human leukocyte cell lines, secretion of eosinophil peroxidase from the eosinophil-like cell line HL-60 clone 15, lysozyme from the monocytic leukemia cell line U937, and granzyme from the lymphocyte natural killer cell line NK-92 were attenuated by preincubation of the cells with MANS but not with the missense control peptide. The results indicate that MARCKS protein may play an important role in the secretion of membrane-bound granules from different leukocytes. MARCKS may be an important component of secretory pathways associated with release of granules by different cell types.
Keywords: MARCKS protein, leukocytes, degranulation
Leukocytes synthesize a number of inflammatory mediators that are packaged and stored in cytoplasmic membrane-bound granules. These mediators include myeloperoxidase (MPO) in neutrophils (1), eosinophil peroxidase (EPO) and major basic protein in eosinophils (2), lysozyme in monocytes/macrophages (3, 4), and granzyme in natural killer (NK) cells and cytotoxic lymphocytes (5–8). These mediators are released at sites of injury and contribute to inflammation and repair in the lung and elsewhere. Leukocytes release these granules via an exocytotic mechanism (9, 10), but the regulatory molecules and specific pathways involved in the exocytotic process have not been fully described.
Several exogenous stimuli can provoke degranulation of leukocytes via a pathway that involves activation of protein kinase C (PKC) and subsequent phosphorylation events (9–13). MARCKS (myristoylated alanine-rich C kinase substrate), a ubiquitous phosphorylation target of PKC, is highly expressed in leukocytes (14–16). We have previously demonstrated that MARCKS protein is involved in exocytotic secretion of mucin by goblet cells that line the respiratory airways (17, 18). In airway epithelial cells, the N-terminus of MARCKS seems to be integral to the secretory process. The mechanism seems to involve the binding of MARCKS to membranes of intracellular mucin granules because a peptide against the N-terminus of MARCKS blocked mucin secretion and binding of MARCKS to mucin granule membranes in these cells (18). Because MARCKS is a prominent protein in leukocytes, we investigated whether or not MARCKS, and specifically its N-terminus, could play a role in leukocyte degranulation.
In these studies, we used four different leukocyte types or models that secrete specific granule contents in response to phorbol ester–induced activation of PKC. First, neutrophils were isolated from human blood, and the in vitro release of MPO by these cells was assessed. Due to difficulties in isolating sufficient amounts of other leukocyte types from blood, we investigated the release of membrane-bound inflammatory mediators from commercially available human leukocyte cell lines. The human promyelocytic cell line HL-60 clone 15 was used to assess secretion of EPO (19–22), the monocytic leukemia cell line U937 was used to assess secretion of lysozyme (3, 4, 23), and the lymphocyte NK cell line NK-92 was used to assess the release of granzyme (6–8). In all cases, the cells were preincubated with a range of concentrations of a synthetic peptide identical to the 24-amino-acid MARCKS N-terminus (myristoylated N-terminal sequence peptide [MANS]), or a missense control peptide (random N-terminal sequence peptide [RNS]), which consists of the same 24 amino acids arranged in random order. In each of the cell types, we found that MANS, but not RNS, attenuated the release of inflammatory mediators in a concentration-dependent manner over 0.5–3.0 h. The results suggest that MARCKS protein—specifically its N-terminal region—could be involved in intracellular pathways leading to leukocyte degranulation.
MATERIALS AND METHODS
Peptides
The MANS and RNS peptides were synthesized by Genemed Synthesis, Inc. (San Francisco, CA). The MANS peptide consists of a sequence identical to the first 24 amino acids of MARCKS; MA-GAQFSK TAAKGEAAAERPGEAAVA (where MA = N-terminal myristate chain). The RNS peptide contains the same amino acid composition as MANS but arranged in random order: MA-GTAPAAEGAGAEV KRASAEAKQAF. The peptides have been described previously (17).
Human Neutrophil Isolation
These studies were approved by the NCSU human studies Institutional Review Board. Human neutrophils were isolated as previously described (24) with slight modifications. Briefly, heparinized venous blood was obtained from normal healthy volunteers, diluted with RPMI-1640 (Cellgro; Mediatech, Inc., Herndon, VA) at a ratio of 1:1, layered onto a Histopaque (density, 1.077 g/ml) (Sigma-Aldrich Co., St. Louis, MO), and centrifuged at 400 × g for 20 min at 4°C. The supernatant and mononuclear cells at the interface were carefully removed, and erythrocytes in the sediment were lysed in chilled distilled water. Isolated granulocytes were washed twice with Hanks' balanced salts solution (HBSS) and resuspended in HBSS on ice. The neutrophils used for the experiments were of > 98% purity with < 2% contamination by eosinophils, and the viability was > 99% as determined by Trypan blue dye exclusion.
Measurement of Released Neutrophil MPO Activity
For measurement of MPO release, purified human neutrophils suspended in HBSS were aliquoted at 4 × 106 cells/ml in 15-ml tubes and preincubated with 50 or 100 μM of MANS or RNS peptide for 10 min at 37°C. The cells were stimulated with 100 nM phorbol 12-myristate 13-acetate (PMA) for up to 3 h. The reaction was terminated by placing the tubes on ice and centrifugation at 400 × g for 5 min at 4°C.
MPO activity in the cell supernatant was assayed using tetramethylbenzidine (TMB) based on a previously established technique (25). Briefly, 100 μl of TMB substrate solution was added to 50 μl of cell supernatants or standard human MPO (EMD Biosciences Inc., San Diego, CA) in a 96-well microplate followed by incubation at room temperature for 15 min. The reaction was terminated by the addition of 50 μl of 1M H2SO4, and absorbance was read at 450 nm in a spectrophotometric microplate reader (VERSA max; Molecular Devices, Sunnyvale, CA).
Leukocyte Culture
Three types of human leukocyte cell lines (the promyelocytic cell line HL-60 clone 15, the monocytic cell line U937, and the lymphocyte natural killer cell line NK-92) were purchased from American Type Culture Collection (ATCC, Rockville, MD). HL-60 clone 15 cells (ATCC CRL-1964) were maintained in medium consisting of RPMI 1640 with L-glutamine supplemented with 10% heat-inactivated FBS (Gibco; Invitrogen Co., Carlsbad, CA), 50 IU/ml penicillin, 50 μg/ml streptomycin, and 25 mM HEPES buffer (pH 7.8) at 37°C in an atmosphere containing 5% CO2. Final differentiation to an eosinophil-like phenotype was initiated by culturing cells at 5 × 105 cells/ml in the same medium containing 0.5 mM butyric acid (Sigma-Aldrich) for 5 d as previously described (21, 26). U937 cells (ATCC CRL-1593.2) were grown at 37°C in an atmosphere of 5% CO2 in complete medium consisting of RPMI 1640 with L-glutamine supplemented with 10% FBS, 50 IU/ml penicillin, and 50 μg/ml streptomycin. NK-92 cells (ATCC CRL-2407) were maintained in α-MEM medium (Sigma-Aldrich Co.) supplemented with 20% FBS, 100 U/ml of IL-2 (Chemicon International, Inc., Temecula, CA), 5 × 10−5 M of 2-mercaptoethanol, 50 IU/ml penicillin, and 50 μg/ml streptomycin at 37°C in an atmosphere containing 5% CO2. Cell morphology was judged by assessment of Wright-Giemsa–stained cells. Viability of cells harvested for experiments was assessed by trypan blue exclusion, and populations of cells with viability > 95% were used.
Incubation of Cells for Degranulation Assays
HL-60 clone 15, U937, and NK-92 cells were washed and resuspended at 2.5 × 106 cells/ml in phenol red-free RPMI-1640 (Cellgro; Mediatech, Inc.) for all degranulation assays. Aliquots of cells in 15-ml tubes were preincubated with indicated concentrations of MANS or RNS peptide for 10 min at 37°C. The cells were stimulated with PMA for up to 2 h. The reaction was terminated by placing tubes on ice and centrifuging cells at 400 × g for 5 min at 4°C.
For measurements of released MPO from neutrophils and released lysozyme from U937 cells, we were able to quantify secretion by using as standards human MPO and egg white ovalbumin, respectively. However, for released EPO from HL-60 clone 15 cells and released granzyme from NK-92 cells, there were no available standards to use for quantification. Hence, we measured released and intracellular (from lysed cells) levels of EPO and granzyme and expressed the released EPO and granzyme as a percentage of total (intracellular and released) for each. To measure intracellular EPO in HL-60 clone 15 cells and intracellular granzyme in NK-92 cells, appropriate aliquots of 0.1% triton X-100–lysed cells were taken for quantification of intracellular granule proteins (see below). All treatments are expressed as percentage of control to minimize variability between cultures.
Measurement of Hl-60 EPO Release
EPO activity released by HL-60 clone 15 cells was assayed using TMB according to a previously established technique (27). Briefly, 100 μl of TMB substrate solution was added to 50 μl of sample in a 96-well microplate and incubated at room temperature for 15 min. The reaction was terminated by addition of 50 μl of 1.0M H2SO4, and absorbance was read at 450 nm in a spectrophotometric microplate reader. The amount of secreted EPO was expressed as percentage of total content, using the amount obtained in the same number of triton X-100–lysed cells.
Measurement of Monocyte Lysozyme Secretion
Lysozyme secreted by U937 cells was measured using a spectrophotometric assay as described previously (4) with slight modification. Briefly, 100 μl of sample was mixed with 100 μl of a Micrococcus lysodeikticus (Sigma-Aldrich Co.) suspension (0.3 mg/ml in 0.1 M sodium phosphate buffer, pH 7.0) in a 96-well microplate. The decrease in absorbance at 450 nm was measured at room temperature. A calibration curve was constructed using chicken egg white lysozyme (EMD Biosciences) as a standard.
Measurement of NK Cell Granzyme Secretion
Granzyme secreted from NK-92 cells was assayed by measuring hydrolysis of Nα-benzyloxycarbonyl-L-lysine thiobenzyl ester (BLT) essentially as described previously (8). Fifty microliters of supernatant was transferred to a 96-well plate, and 150 μl of BLT solution (0.2 mM BLT; EMD Biosciences, Inc., and 0.22 mM DTNB; Sigma-Aldrich Co.) in PBS (pH 7.2) was added to the supernatant. Absorbance at 410 nm was read after incubation for 30 min at room temperature. Results were expressed as percentage of total cellular enzyme content, using the amount obtained in the same number of triton X-100–lysed cells.
Statistical Analysis
Statistical significance of the differences between various treatment groups was assessed with one-way ANOVA. P values of < 0.05 were taken as significant.
RESULTS
Neutrophil MPO Release
As shown in Figure 1, 100 nM PMA increased human neutrophil MPO release by approximately 3-fold at 30 min, increasing to ∼ 5- to 6-fold after 3 h. The MANS peptide had no effect at 30 min, but by 1 h the higher concentration of MANS (100 μM) had a significant inhibitory effect. By 2 h and persisting at 3 h, the MANS peptide significantly attenuated MPO activity in a concentration-dependent manner. The RNS peptide did not affect PMA-induced MPO release at any of the time points or concentrations tested.
Figure 1.
MPO release by human neutrophils. Human neutrophils were preincubated with the indicated peptide for 10 min and exposed to PMA (100 nM) + peptide for (A) 30 min, (B) 1 h, (C) 2 h, and (D) 3 h. MPO activity in the supernatant was assayed using TMB substrate solution. The results are shown as mean ± SE (n = 5 at each point). #Significantly different from medium control (P < 0.01). *Significantly different from PMA stimulation (P < 0.05). **Significantly different from PMA stimulation (P < 0.01). Results are presented as percentage of control. The actual amounts of control MPO were (A) 30 min: 147.1 ± 58.2 mU/ml; (B) 1 h: 154.6 ± 52.0 mU/ml; (C) 2 h: 170.8 ± 59.4 mU/ml; and (D) 3 h: 184.3 ± 48.1 mU/ml.
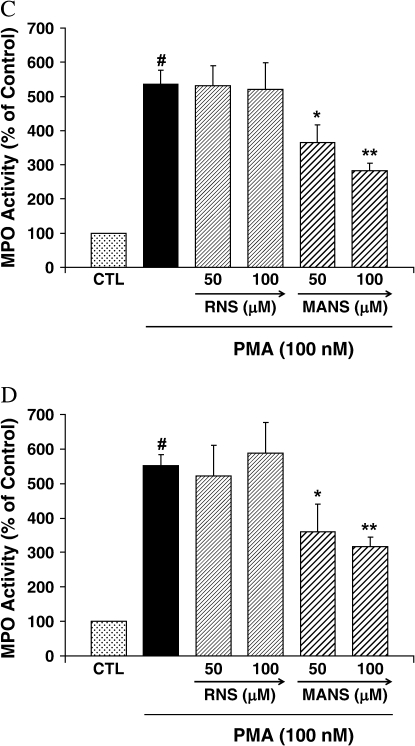
HL-60 EPO Release
EPO activity in the supernatant of HL-60 clone 15 cells was significantly enhanced at 1 and 2 h after PMA stimulation (Figure 2). At 1 and 2 h, MANS at 50 or 100 μM significantly attenuated EPO release. The RNS peptide did not affect PMA-enhanced EPO release at any of the time points or concentrations tested.
Figure 2.
EPO release by HL-60 clone 15 cells. Cells were preincubated with the indicated peptide for 10 min and exposed to PMA (100 nM) + peptide for (A) 1 h and (B) 2 h. EPO activity in the supernatant was assayed using TMB substrate solution. The results are shown as mean ± SE (n = 5 at each point). #Significantly different from medium control (P < 0.01). *Significantly different from PMA stimulation (P < 0.05). **Significantly different from PMA stimulation (P < 0.01). Results are presented as percentage of control. The actual amounts of control EPO were (A) 1 h: 6.9 ± 0.3% of total EPO and (B) 2 h: 7.5 ± 0.6% of total EPO.
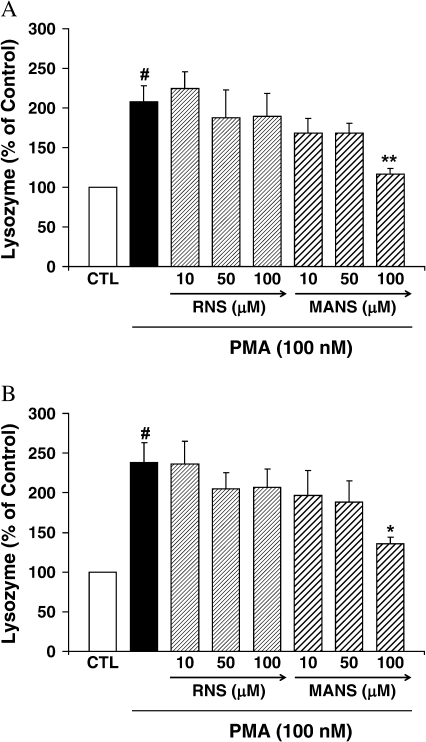
U937 Lysozyme Release
Lysozyme secretion by U937 cells was increased by PMA stimulation by 1 h after incubation and increased at 2 h (Figure 3). Lysozyme secretion was significantly attenuated at 1 and 2 h post-stimulation by 100 μM but not 50 or 10 μM of MANS. The RNS peptide did not affect PMA-enhanced lysozyme secretion at any of the time points or concentrations tested.
Figure 3.
Lysozyme secretion by U937 cells. Cells were preincubated with the indicated peptide for 10 min and exposed to PMA (100 nM) + peptide for (A) 1 h and (B) 2 h. Lysozyme secretion was assayed by measuring the reaction of lysozyme with Micrococcus lysodeikticus suspension. The results are shown as mean ± SE (n = 5 at each point). #Significantly different from medium control (P < 0.01). *Significantly different from PMA stimulation (P < 0.05). **Significantly different from PMA stimulation (P < 0.01). Results are presented as percentage of control. The actual amounts of control lysozyme were (A) 1 h: 256.1 ± 36.8 ng/ml and (B) 2 h: 348.9 ± 47.6 ng/ml.
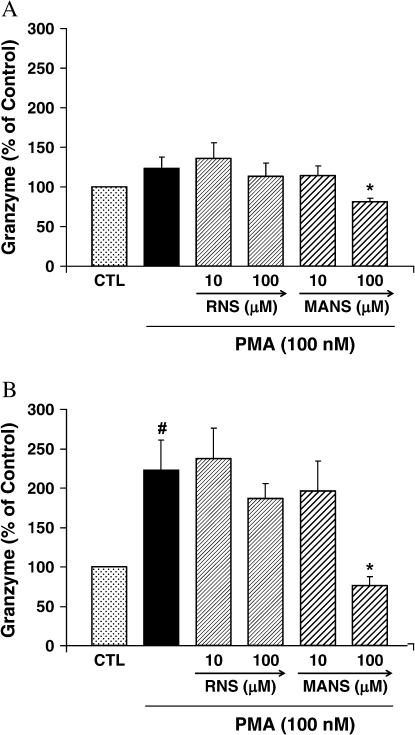
NK Cell Granzyme Release
Granzyme secretion by NK-92 cells was not significantly increased by PMA at 1 h but increased over 2-fold at 2 h (Figure 4). At 1 and 2 h after incubation, 100 μM but not 10 μM of MANS attenuated granzyme secretion. The RNS peptide did not affect PMA-enhanced granzyme secretion at any of the time points or concentrations tested.
Figure 4.
Granzyme secretion by NK92 cells. Cells were preincubated with the indicated peptide for 10 min and exposed to PMA (100 nM) + peptide for (A) 1 h and (B) 2 h. Granzyme secretion was assayed by measuring hydrolysis of BLT. The results are shown as mean ± SE (n = 5 at each point). #Significantly different from medium control (P < 0.01). *Significantly different from PMA stimulation (P < 0.05). Results are presented as percentage of control. The actual amounts of control granzyme were (A) 1 h: 13.5 ± 1.4% of total granzyme and (B) 2 h: 14.9 ± 1.6% of total granzyme.
Cytotoxicity
None of the treatments generated a toxic response in the cells, as assessed by LDH retention/release (data not shown) (28).
DISCUSSION
In the studies reported here, degranulation of isolated human neutrophils and cell lines representative of human eosinophils, monocytes/macrophages, and lymphocytes in response to PKC activation was attenuated by pre- and coincubation with a peptide identical to the N-terminal region of MARCKS protein, whereas a missense peptide had no effect. Although the time courses and concentrations varied somewhat among the cell types, in all cases the MANS peptide attenuated PKC-induced degranulation. This is similar to the inhibitory effect of similar concentrations of MANS on secretion of mucin by airway epithelial cells in vitro (17) and in vivo (18).
We used four different types of leukocytes to investigate a role for MARCKS protein in release of inflammatory mediators. Neutrophils isolated directly from human volunteers were used for the initial studies. In neutrophils, inflammatory mediators are stored in primary (azurophil), secondary (specific), and tertiary (gelatinase) granules and in secretory vesicles. Primary granules contain MPO, lysozyme, elastase, and proteinase 3 (16). Although mechanisms regulating exocytotic secretion of these granules are only partially understood, several key molecules in the process have been identified, including intracellular Ca2+ transients (29, 30), G proteins, tyrosine and protein kinases (especially PKC) (11–13), Rac2 (25, 31), and various SNAPs (soluble NSF [N-ethylmaleimide-sensitive factor] attachment protein), SNAREs (SNAP receptors), and VAMPs (vesicle-associated membrance proteins) (32, 33).
Eosinophils contain crystalloid granules that contain major basic protein, eosinophil cationic protein, EPO, and eosinophil-derived neurotoxin (2). In this study, the human promyelocytic cell line HL-60 clone 15 was used to examine secretion of EPO. This cell line was established from a clone of HL-60 that had been grown at an elevated pH for 2 mo (19) and treated with butyric acid to allow the cells to differentiate so as to exhibit many of the characteristics of peripheral blood eosinophils, including expression of eosinophil-specific granule proteins (20–22).
Granule-bound enzymes of monocytes/macrophages include lysozyme, acid phosphatase, and β-glucuronidase. We examined lysozyme secretion from U937 cells, which is a cell line derived from a human histiocytic lymphoma and widely used as a monocytic cell line that can be activated by a variety of agonists, such as PMA (3, 4, 23).
NK cells and cytotoxic lymphocytes contain potent cytotoxic granules including perforin, which is a pore-forming protein, and granzymes, which are lymphocyte-specific serine proteases. The NK-92 cell line is an IL-2–dependent human line established from a patient with rapidly progressive non-Hodgkin's lymphoma (6). NK-92 cells express high levels of molecules involved in the perforin-granzyme cytolytic pathway that targets a wide range of malignant cells (6, 7).
A potential role for MARCKS in the degranulation pathway in these cells was investigated for two reasons. First, we have shown previously that exocytotic release of another product stored in cytoplasmic membrane-bound granules, mucin in airway secretory cells, is regulated by MARCKS protein (17, 18), so the possibility of a similar mechanism controlling release of leukocyte granules was explored. Second, it has been shown that neutrophils and macrophages, upon priming by such proinflammatory agents as TNF-α, dramatically increase their synthesis of MARCKS protein: As much as 90% of the new protein formed by neutrophils in response to TNF-α or lipopolysaccharide is MARCKS (34). This suggests that MARCKS could have an important role in the release of inflammatory mediators when these cells are stimulated by agonists, especially those that work by activating PKC (9–13).
MARCKS, a protein of ∼ 82 kD, has three evolutionarily conserved regions (14–16, 35): the N-terminus, the phosphorylation site domain, and the multiple homology 2 domain. The N-terminus, a 24-amino-acid stretch with a myristic acid moiety attached to a terminal glycine residue, is involved in the binding of MARCKS to membranes (35) and possibly to calmodulin (36). In previous studies, we have shown that the MANS peptide, which is identical to the N-terminus of MARCKS and therefore might compete with native MARCKS in cells for membrane binding, inhibited the release of mucin from human airway epithelial cells in vitro (17) and from airways of mice with allergic inflammation (18). This implicates the N-terminus in the secretory response and, indeed, we have shown that MARCKS binds to membranes of mucin granules within airway epithelium, and that preincubation of cells with the MANS peptide inhibits this binding (18). Although these findings present a different paradigm for the mechanism of secretion, they do not preclude a potential action of MARCKS in binding to cytoskeletal elements around the cell periphery to regulate granule release, as has been suggested in other cell types (37, 38).
Involvement of MARCKS protein in release of inflammatory mediators by infiltrating leukocytes is relevant to inflammation in all tissues and organs. Although this includes lung diseases characterized by airway inflammation (e.g., asthma, chronic obstructive pulmonary disease, and cystic fibrosis) and a MARCKS-related mechanism might be similar for mucus secretion and leukocyte degranulation, inflammation and mucus secretion in the airways are two separate and independent processes. Mucus production and secretion can be provoked by a number of factors, including mediators released by inflammatory cells, but there is no known direct link by which excess mucus can cause inflammation.
In summary, the results of this study suggest that MARCKS (and presumably its N-terminal region) plays a role in leukocyte degranulation. These findings, combined with our previous observations in airway epithelial cells, suggest that there may exist a common pathway involving MARCKS that regulates exocytotic secretion in different cell types. Given the complexity of the pathway that has been proposed previously for MARCKS-related secretion (17), it is unlikely that such a highly evolved multi-step pathway would be limited to a single cell type. Further studies should be performed to elucidate the mechanism(s) involved in leukocyte granule secretion and the exact role of MARCKS in this process. The results of this study suggest the possibility of new therapeutic targets for anti-inflammatory therapy.
Acknowledgments
The authors thank Nancy Akley for her assistance and Drs. Yoshio Okubo and Kenji Ishihara for helpful discussions.
This work was supported by grant R37 HL36982 from the National Institutes of Health and grant 2003-CFG-8006 from the North Carolina Biotechnology Center.
Originally Published in Press as DOI: 10.1165/rcmb.2006-0030RC on March 16, 2006
Conflict of Interest Statement: S.T. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. J.P. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. S.F. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. S.K. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. I.P. is an employee of BioMarck Pharmaceuticals. K.B.A. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript.
References
- 1.Borregaard N, Cowland JB. Granules of the human neutrophilic polymorphonuclear leukocyte. Blood 1997;89:3503–3521. [PubMed] [Google Scholar]
- 2.Gleich GJ. Mechanisms of eosinophil-associated inflammation. J Allergy Clin Immunol 2000;105:651–663. [DOI] [PubMed] [Google Scholar]
- 3.Hoff T, Spencker T, Emmendoerffer A, Goppelt-Struebe M. Effects of glucocorticoids on the TPA-induced monocytic differentiation. J Leukoc Biol 1992;52:173–182. [DOI] [PubMed] [Google Scholar]
- 4.Balboa MA, Saez Y, Balsinde J. Calcium-independent phospholipase A2 is required for lysozyme secretion in U937 promonocytes. J Immunol 2003;170:5276–5280. [DOI] [PubMed] [Google Scholar]
- 5.Bochan MR, Goebel WS, Brahmi Z. Stably transfected antisense granzyme B and perforin constructs inhibit human granule-mediated lytic ability. Cell Immunol 1995;164:234–239. [DOI] [PubMed] [Google Scholar]
- 6.Gong JH, Maki G, Klingemann HG. Characterization of a human cell line (NK-92) with phenotypical and functional characteristics of activated natural killer cells. Leukemia 1994;8:652–658. [PubMed] [Google Scholar]
- 7.Maki G, Klingemann HG, Martinson JA, Tam YK. Factors regulating the cytotoxic activity of the human natural killer cell line, NK-92. J Hematother Stem Cell Res 2001;10:369–383. [DOI] [PubMed] [Google Scholar]
- 8.Takayama H, Trenn G, Sitkovsky MV. A novel cytotoxic T lymphocyte activation assay. J Immunol Methods 1987;104:183–190. [DOI] [PubMed] [Google Scholar]
- 9.Burgoyne RD, Morgan A. Secretory granule exocytosis. Physiol Rev 2003;83:581–632. [DOI] [PubMed] [Google Scholar]
- 10.Logan MR, Odemuyiwa SO, Moqbel R. Understanding exocytosis in immune and inflammatory cells: the molecular basis of mediator secretion. J Allergy Clin Immunol 2003;111:923–932. [PubMed] [Google Scholar]
- 11.Smolen JE, Sandborg RR. Ca2+-induced secretion by electropermeabilized human neutrophils: the roles of Ca2+, nucleotides and protein kinase C. Biochim Biophys Acta 1990;1052:133–142. [DOI] [PubMed] [Google Scholar]
- 12.Niessen HW, Verhoeven AJ. Role of protein phosphorylation in the degranulation of electropermeabilized human neutrophils. Biochim Biophys Acta 1994;1223:267–273. [DOI] [PubMed] [Google Scholar]
- 13.Naucler C, Grinstein S, Sundler R, Tapper H. Signaling to localized degranulation in neutrophils adherent to immune complexes. J Leukoc Biol 2002;71:701–710. [PubMed] [Google Scholar]
- 14.Aderem AA, Albert KA, Keum MM, Wang JK, Greengard P, Cohn ZA. Stimulus-dependent myristoylation of a major substrate for protein kinase C. Nature 1988;332:362–364. [DOI] [PubMed] [Google Scholar]
- 15.Thelen M, Rosen A, Nairn AC, Aderem A. Regulation by phosphorylation of reversible association of a myristoylated protein kinase C substrate with the plasma membrane. Nature 1991;351:320–322. [DOI] [PubMed] [Google Scholar]
- 16.Hartwig JH, Thelen M, Rosen A, Janmey PA, Nairn AC, Aderem A. MARCKS is an actin filament crosslinking protein regulated by protein kinase C and calcium-calmodulin. Nature 1992;356:618–622. [DOI] [PubMed] [Google Scholar]
- 17.Li Y, Martin LD, Spizz G, Adler KB. MARCKS protein is a key molecule regulating mucin secretion by human airway epithelial cells in vitro. J Biol Chem 2001;276:40982–40990. [DOI] [PubMed] [Google Scholar]
- 18.Singer M, Martin LD, Vargaftig BB, Park J, Gruber AD, Li Y, Adler KB. A MARCKS-related peptide blocks mucus hypersecretion in a mouse model of asthma. Nat Med 2004;10:193–196. [DOI] [PubMed] [Google Scholar]
- 19.Fischkoff SA. Graded increase in probability of eosinophilic differentiation of HL-60 promyelocytic leukemia cells induced by culture under alkaline conditions. Leuk Res 1988;12:679–686. [DOI] [PubMed] [Google Scholar]
- 20.Rosenberg HF, Ackerman SJ, Tenen DG. Human eosinophil cationic protein: molecular cloning of a cytotoxin and helminthotoxin with ribonuclease activity. J Exp Med 1989;170:163–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tiffany HL, Li F, Rosenberg HF. Hyperglycosylation of eosinophil ribonucleases in a promyelocytic leukemia cell line and in differentiated peripheral blood progenitor cells. J Leukoc Biol 1995;58:49–54. [DOI] [PubMed] [Google Scholar]
- 22.Badewa AP, Hudson CE, Heiman AS. Regulatory effects of eotaxin, eotaxin-2, and eotaxin-3 on eosinophil degranulation and superoxide anion generation. Exp Biol Med 2002;227:645–651. [DOI] [PubMed] [Google Scholar]
- 23.Sundstrom C, Nilsson K. Establishment and characterization of a human histiocytic lymphoma cell line (U-937). Int J Cancer 1976;17:565–577. [DOI] [PubMed] [Google Scholar]
- 24.Takashi S, Okubo Y, Horie S. Contribution of CD54 to human eosinophil and neutrophil superoxide production. J Appl Physiol 2001;91:613–622. [DOI] [PubMed] [Google Scholar]
- 25.Abdel-Latif D, Steward M, Macdonald DL, Francis GA, Dinauer MC, Lacy P. Rac2 is critical for neutrophil primary granule exocytosis. Blood 2004;104:832–839. [DOI] [PubMed] [Google Scholar]
- 26.Tiffany HL, Alkhatib G, Combadiere C, Berger EA, Murphy PM. CC chemokine receptors 1 and 3 are differentially regulated by IL-5 during maturation of eosinophilic HL-60 cells. J Immunol 1998;160:1385–1392. [PubMed] [Google Scholar]
- 27.Lacy P, Mahmudi-Azer S, Bablitz B, Hagen SC, Velazquez JR, Man SF, Moqbel R. Rapid mobilization of intracellularly stored RANTES in response to interferon-gamma in human eosinophils. Blood 1999;94:23–32. [PubMed] [Google Scholar]
- 28.Park J-A, He F, Martin LD, Li Y, Adler KB. Human neutrophil elastase induces hypersecretion of mucin from human bronchial epithelial cells in vitro via a PKCδ-mediated mechanism. Am J Pathol 2005;167:651–661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Richter J, Ng-Sikorski J, Olsson I, Andersson T. Tumor necrosis factor-induced degranulation in adherent human neutrophils is dependent on CD11b/CD18-integrin-triggered oscillations of cytosolic free Ca2+. Proc Natl Acad Sci USA 1990;87:9472–9476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Blackwood RA, Ernst JD. Characterization of Ca2(+)-dependent phospholipid, binding, vesicle aggregation and membrane fusion by annexins. Biochem J 1990;266:195–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lacy P, Abdel-Latif D, Steward M, Musat-Marcu S, Man SF, Moqbel R. Divergence of mechanisms regulating respiratory burst in blood and sputum eosinophils and neutrophils from atopic subjects. J Immunol 2003;170:2670–2679. [DOI] [PubMed] [Google Scholar]
- 32.Sollner T, Whiteheart SW, Brunner M, Erdjument-Bromage H, Geromanos S, Tempst P, Rothman JE. SNAP receptors implicated in vesicle targeting and fusion. Nature 1993;362:318–324. [DOI] [PubMed] [Google Scholar]
- 33.Lacy P. The role of Rho GTPases and SNAREs in mediator release from granulocytes. Pharmacol Ther 2005;107:358–376. [DOI] [PubMed] [Google Scholar]
- 34.Thelen M, Rosen A, Nairn AC, Aderem A. Tumor necrosis factor alpha modifies agonist-dependent responses in human neutrophils by inducing the synthesis and myristoylation of a specific protein kinase C substrate. Proc Natl Acad Sci USA 1990;87:5603–5607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Seykora JT, Myatt MM, Allen LAH, Ravetch JV, Aderem A. Molecular determinants of the myristoyl-electrostatic switch of MARCKS. J Biol Chem 1996;271:18797–18802. [DOI] [PubMed] [Google Scholar]
- 36.Matsubara M, Titani K, Taniguchi H, Hayashi N. Direct involvement of protein myristoylation in myristorylated alanine-rich C kinase substrate (MARCKS)-calmodulin interaction. J Biol Chem 2003;278:48898–48902. [DOI] [PubMed] [Google Scholar]
- 37.Elzagallaai A, Rose SD, Trifaro JM. Platelet secretion induced by phorbol ester stimulation is mediated through phosphorylation of MARCKS: a MARCKS-derived peptide blocks MARCKS phosphorylation and serotonin release without affecting pleckstrin phosphorylation. Blood 2000;95:894–902. [PubMed] [Google Scholar]
- 38.Trifaro J, Rose SD, Lejen T, Elzagallaai A. Two pathways control chromaffin cell cortical F-actin dynamics during exocytosis. Biochimie 2000;82:339–352. [DOI] [PubMed] [Google Scholar]