Abstract
Bacterial flagellin can interact with both Toll-like receptor 5 (TLR5) and the cell surface glycolipid, asialoGM1, to activate an innate immune response. The induction of mucin by flagellin in human lung epithelial cells (NCIH292) is dependent on asialoGM1 ligation, ATP receptor signaling, Ca2+ mobilization, and Erk1/2 activation. Conversely, the activation of NF-κB by flagellin is dependent on signaling through TLR5. These results prompted us to ask whether the flagellin-induced TLR5 signaling pathway was intersecting with or mutually independent of the nucleotide receptor pathway activated downstream of asialoGM1. Herein, we demonstrate that the release of ATP induced by flagellin is dependent on a Toll signaling cascade. Although Toll was able to activate NF-κB in the absence of extracellular ATP, Toll required ATP to activate Erk1/2. These results suggest interdependence between the asialoGM1 and TLR5 pathways and reveal a previously unsuspected role for autocrine extracellular ATP signaling in TLR signaling.
Keywords: flagellin, ATP, Erk1/2, TLR5
Vertebrate hosts detect and respond to bacterial flagellin. However, mechanisms remain poorly understood. Two types of flagellin receptors have been identified: (1) the glycolipids/gangliosides asialoGM1, GM1, and GD1a (1); and (2) the transmembrane protein, Toll-like receptor 5 (TLR5) (2).
TLR5 signaling occurs via a classical Toll pathway beginning with the binding of a pathogen molecule to a transmembrane TLR and recruitment of the cytosolic adapter, MyD88, that interacts directly with the Toll–IL-1 receptor domain. MyD88 then recruits the serine/threonine kinase IRAK (IL-1 receptor–associated kinase). Via phosphorylation, IRAK activates TNF-α receptor–associated factor (TRAF6), which in turn activates TRAF-associated kinase (TAK). TAK links TRAF6 to the IKK complex that phosphorylates IκBα. Upon phosphorylation, IκBα is degraded, thereby liberating NF-κB transcription factors to which it is tethered in resting cells. The activation of NF-κB permits transactivation of many of the host defense genes induced by Toll signaling (3–6).
However, NF-κB's target genes are not the only ones induced by TLR signaling. The upregulation of many key cytokines, such as IL-8 and MIP3α, and innate defense proteins such as defensins occur via the activation of other transcription factors such as AP-1, NF-IL6, and Elk-1 (7–10). Some of these require activation through extracellular signal–regulated kinase (Erk) 1/2 (11, 12), an event potentially independent of IRAK, TRAF, and TAK (7, 12, 13).
Previous studies have shown flagellin-induced activation of Erk1/2 downstream of asialoGM1 (ASGM1) ligation (14, 15). Earlier we reported that the induction of mucin by flagellin in human lung epithelial cells was dependent on asialoGM1 ligation, ATP receptor signaling, Ca2+ mobilization, and Erk1/2 activation. The possibility that ASGM1 and TLR5 might interact as co-receptors was suggested by data showing that both receptors co-localize with flagellin (15).
In the present studies, we examined the possibility of a functional interaction. Our data suggest that ASGM1 and TLR5 cooperate to activate an ATP receptor. More generally, these findings provide the first evidence that certain aspects of Toll receptor function are dependent on ATP receptor signaling.
MATERIALS AND METHODS
Reagents
All tissue culture media and antibiotics were obtained from Invitrogen (Carlsbad, CA). All chemical inhibitors were purchased from CalBiochem EMD Biosciences (La Jolla, CA), except Reactive Blue 2, which came from Sigma-Aldrich (St. Louis, MO). Anti-asialoGM1 antibody (α-ASGM1, rabbit polyclonal) was purchased from Wako Chemicals USA (Richmond, VA). Phospho- and nonphospho-Erk1/2 antibodies were from Cell Signaling Technology Inc. (Beverly, MA). Rabbit IgG for control experiments was purchased from Zymed (South San Francisco, CA). All other antibodies and Protein A agarose were purchased from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA). Fugene 6 was purchased from Roche Applied Science (Indianapolis, IN). Purified flagellin was purchased from InvivoGen (San Diego, CA). All other reagents were purchased from Sigma.
Cell Culture and Transfection
NCIH292 cells transiently transfected with an NF-κB promoter–driven luciferase reporter gene (pNFκB-TA-Luc; BD Biosciences Clontech, Mountain View, CA) or Chinese hamster ovary (CHO-K1) cells transiently transfected with full-length human TLR5 (A. Gewirtz, Emory University, Atlanta, GA) and P2Y2 (J. Boyer, University of North Carolina, Chapel Hill, NC) were maintained in RPMI medium supplemented with 10% FBS, penicillin, and streptomycin (100 μg/ml). Unless otherwise noted, cells were grown in 48-well plates until they reached 60–70% confluence and then transfected overnight with Fugene 6 reagent according to the manufacturer's protocol. After transfection, the medium was changed to serum-free RPMI for 24 h, and cells were incubated with either the appropriate stimulus (i.e., purified flagellin or anti-asialoGM1 antibody) or serum-free medium control for 4–6 h. Cells were lysed using 100 μl Reporter Lysis Buffer (Promega, Madison, WI), and relative light units of luciferase activity read using 150 μl luciferase substrate (Roche/BMB).
MyD88 dominant-negative mutant (MyDC) was the gift of R. Medzhitov (Yale University School of Medicine, New Haven, CT) (16). TLR5 deletion mutant BsrG1 (Δ695–858) acted as a dominant-negative mutant for TLR5 as previously described (17). For co-transfections using dominant-negative mutants (DNMs), 0.1 μg of both NFκB and the DNM were used per well. Cells were transfected overnight with Fugene 6 and lysed after 44 h. Empty expression vector was added when necessary to equalize the total amount of DNA transfected.
Immunoprecipitation and Immunoblots
Cell lysates were prepared by adding 500 μl of lysis buffer (20 mM Tris HCl, 150 mM NaCl, 0.5% Triton X 100, 0.1%SDS, 1 mM EDTA, 1 mM sodium orthovanadate, and a protease inhibitor cocktail containing pepstatin A, E-64, bestatin, leupeptin, aprotinin, and AEBSF), sonicating on ice, and pre-clearing by centrifugation at 10,000 rpm for 10 min at 4°C. Equal amounts of lysates were immunoprecipitated overnight at 4°C using α-ASGM1 antibody and Protein A agarose beads. The lysate–antibody–bead complex was spun down and washed three times with lysis buffer. After the final wash, 40 μl of SDS gel-loading buffer was added, the mixture was heated at 100°C for 3 min, and proteins were resolved by SDS-PAGE. For immunoblot analysis, proteins were transferred to nitrocellulose membranes using the Bio-Rad Mini Trans-Blot Electrophoretic Transfer Cell. Membranes were blocked in PBS containing 0.1% Tween (PBS/Tween) and supplemented with 5% nonfat dry milk. After 1 h at room temperature, blots were washed with PBS/Tween and incubated with the anti-TLR5 antibody (Santa Cruz Biotechnology, Inc.) overnight at 4°C. After removing primary antibody with several washes of PBS/Tween, the blot was placed in the appropriate HRP-conjugated secondary antibody for 45 min After several washes, the antibody-antigen complexes were visualized using the ECL chemiluminescence detection system (Amersham Life Sciences, Piscataway, NJ). Baseline levels of TLR5 in the lysates were visualized by immunoprecipitation and immunostaining with anti-TLR5 antibody (rabbit polyclonal).
Immunoblots with anti–phospho-Erk1/2 and anti–phospho-IκBα antibodies were performed on cells grown in 6-well plates and lysed in 125 μl Laemmlli sample buffer supplemented with β-mercaptoethanol (Bio-Rad Laboratories, Hercules, CA). Proteins were separated by SDS-PAGE, transferred to nitrocellulose, and blocked as described above. Blots were incubated with anti–phospho-Erk1/2 (1:2,000) or anti–phospho-IκBα (1:2,000) overnight at 4°C, washed with PBS/Tween, and exposed to the appropriate HRP-conjugated secondary for 45 min at room temperature. After washing, antibody–antigen complexes were detected using ECL. Blots were stripped and reprobed with either anti-Erk1/2 (1:1,000) or anti-actin (1:1,000) to quantify baseline levels of total protein.
Immunofluorescence and Confocal Imaging
NCIH292 cells plated 3 × 104 cells/cm2 on Lab-Tek II slide wells (Nalge-Nunc International, Rochester, NY) were incubated on ice in serum-free RPMI medium containing 600 nM Bodipy GM1 (Molecular Probes, Inc., Eugene, OR) for 30 min before the addition of flagellin.
Cells were maintained at either 4°C or warmed to 37°C for 10 min and then exposed to flagellin (10 μg/ml) for 10 min. Cells were washed once with PBS, fixed with 3% paraformaldehyde for 20 min at room temperature, and blocked with 10% normal goat serum for 1 h at room temperature. Immunostaining for 2 h at room temperature using anti-TLR5 rabbit polyclonal antibody (1:200) or an equivalent amount of isotype control was followed by four washes with PBS and a 1-h incubation with anti-rabbit cy3 secondary antibody (1:2,000). Nuclei were stained blue with DAPI using Vectashield Mounting medium (Vector Laboratories, Inc., Burlingame, CA). Cells were analyzed using the Zeiss Confocal Laser Scanning Microscope, LSM 510 (Zeiss, Thornwood, NY).
Calcium Imaging
For real-time recording of [Ca2+]i, cells were grown in a monolayer on 10 mm2 polyornithine-coated chambered glass coverslips (Applied Scientific Instrumentation, Eugene, OR). NCIH292 cells transfected with MyD88 DNM or vector control were loaded with Fura-2 (30 min at 37°C) in Calcium Imaging Buffer (CIB) containing (in mM) 130 NaCl, 3 KCl, 2.5 CaCl2, 0.6 MgCl2, 1.2 NaHCO3, 10 glucose, 10 HEPES, pH 7.45, with 1 μM Fura-2 acetoxymethyl ester and 0.01% pleuronic acid (Molecular Probes), then rinsed twice with CIB. Images were collected using a Nikon Diaphot fluorescence microscope equipped with a variable filter wheel (Sutter Instruments, Novato, CA) and an intensified CCD camera (Hamamatsu, Bridgewater, NJ). Dual images (340 and 380 nm excitation, 510 nm emission) were collected and pseudocolor ratiometric images monitored during the experiment (Metafluor software; Universal Imaging, Molecular Devices Corp., Downingtown, PA). Cells were initially imaged in 100 μl of CIB, after which 100 μl CIB containing a 1:50 dilution of α-ASGM1 antibody or 10 μg/ml flagellin was added. The number of MyD88 DNM-transfected cells that mobilized calcium in response to ASGM1 ligation was quantified in a minimum of three wells and compared with cells transfected with empty vector. Experiments were repeated a minimum of three times.
ATP Bioluminescence Assay
ATP release into the extracellular medium was monitored using the luciferin/luciferase-based Enlighten ATP assay system (Promega). Direct measurement of ATP release was performed by growing NCIH292 cells to confluence in 12-well culture plates at 37°C, and then placing them in serum-free medium (SFM) overnight. The next day, cells were treated with flagellin or α-ASGM1 antibody in a total of 1 ml of medium per well. At indicated times after the addition of bacterial stimulus, 100-μl volumes were carefully removed from the surface without perturbing the cells and added to 100 μl luciferin/luciferase reagent before reading in a Monolight luminometer. SFM containing the luciferase reagent alone was placed in the control wells.
RESULTS
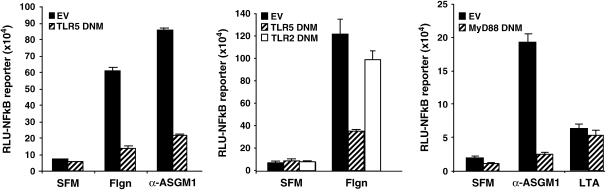
In an earlier report, we showed that ASGM1 ligation causes the release of ATP, the activation of an ATP receptor, and downstream events including the activation of PLC, the mobilization of intracellular Ca2+, Erk1/2 phosphorylation, and mucin transcription (14). Subsequently, it was reported that flagellin's effects on mammalian cells were mediated by TLR5 (2, 17). This raises the question of whether the observed responses are triggered by direct binding of flagellin to TLR5, asialoGM1, or both (18, 19). One possibility is that both molecules are used as coreceptors. If ASGM1 is a co-receptor for TLR5 then ligation of ASGM1 using either flagellin or an agonist antibody that binds to and activates ASGM1 (α-ASGM1) would activate the TLR5 signaling pathway (20, 21). One of the major downstream effects of Toll receptor signaling is the activation of NF-κB. Both flagellin and the agonist antibody, α-ASGM1 stimulated an NF-κB–luciferase reporter (Figure 1). Stimulation by both agents was inhibited by DNM forms of either TLR5 (Figure 1, left) or its downstream adaptor MyD88 (Figure 1, right), but not by a DNM directed against TLR2 (Figure 1, middle). Specific involvement of TLR5 in the flagellin response is indicated by intact NF-κB activation in the presence of a TLR2 DNM. Moreover, the MyD88 DNM was ineffective in blocking NF-κB activation by lipoteichoic acid (LTA), indicating specificity as previously described (22). DNM inhibition of the response to the agonist antibody α-ASGM1 indicated that ASGM1 requires functional interaction with TLRs to affect downstream signaling.
Figure 1.
NF-κB activation by flagellin is Toll dependent. NCIH292 cells were co-transfected with an NF-κB TA-luciferase reporter (BD Biosciences) and either empty vector (EV, solid black bar) or DNM (hatched bar) constructs for TLR5 (left panel), TLR2 (middle panel), or MyD88 (right panel). Thirty-six hours after transfection, cells were stimulated with flagellin (Flgn, 10 μg/ml), an agonist antibody directed against the flagellin receptor, asialoGM1 (α-ASGM1, 1:100 dilution), or lipoteichoic acid (LTA, 50 μg/ml) for 6 h before cell lysis. Both flagellin and α-ASGM1 induced NF-κB luciferase activity. The induction by both agents was abolished by DNMs directed against TLR5 and MyD88. To indicate specificity, flagellin-induced NF-κB activation was unaffected by the TLR2 DNM and NF-κB activation by LTA was unaffected by the MyD88 DNM (22).
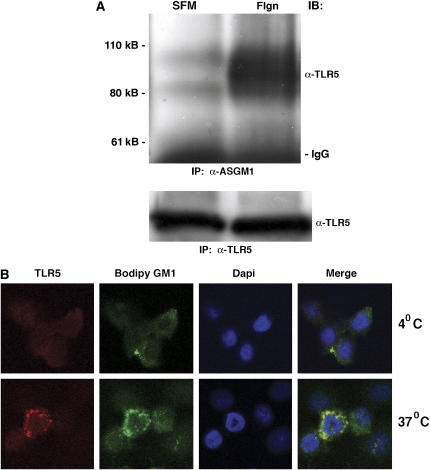
If ASGM1 and TLR5 act as co-receptors then it should be possible to demonstrate their interaction. As seen in Figure 2A, an antibody directed against ASGM1 was able to co-immunoprecipitate TLR5. Notably, the amount of TLR5 interacting with ASGM1 increased greatly in the presence of flagellin (top panel), although flagellin did not increase the amount of TLR5 available for direct immunoprecipitation (bottom panel). Similar results were found with immunolocalization studies shown in Figure 2B. Lung epithelial cells grown on slide wells were loaded with Bodipy GM1 (FITC-green) and fluorescently labeled with TLR5 (cy3-red). Upon exposure to flagellin, colocalization of TLR5 and GM1 was apparent in cells maintained at 37°C (Figure 2B, bottom panel, far right) but not in cells maintained at 4°C (Figure 2B, top panel, far right) or in cells stained with isotype control rabbit IgG (data not shown).
Figure 2.
Flagellin induces co-localization of asialoGM1 and GM1 with TLR5. (A) NCIH292 cells exposed to flagellin (Flgn, 10 μg/ml, 30 min) were lysed and immunoprecipitated with either α-ASGM1 antibody (top panel) or α-TLR5 antibody (bottom panel) and then immunoblotted with α-TLR5 antibody. Increased association between ASGM1 and TLR5 occurred in the presence of flagellin (A, top panel), while expression of TLR5 in the same lysates remained equivalent (A, bottom panel). (B) NCIH292 cells plated on Lab-Tek II slide wells were incubated on ice in serum-free RPMI medium containing 600nM Bodipy GM1 (mimicking ASGM1; Molecular Probes) for 30 min before the addition of flagellin. Cells were maintained at either 4°C (B, top panel) or 37°C (B, bottom panel) for 15 min and then exposed to flagellin (10 μg/ml, 10 min). Cells were fixed with 3% paraformaldehyde for 20 min at room temperature and immunostained using rabbit polyclonal α-TLR5 antibody (1:200) with an α-rabbit cy3 secondary antibody (1:2,000). Nuclei were stained blue using Dapi in Vectashield Mounting medium (Vector Laboratories, Inc.). Co-localization of TLR5 and GM1 (far right panel) is apparent in cells maintained at 37°C but not those maintained at 4°C. Staining with isotype control rabbit IgG was negative (not shown).
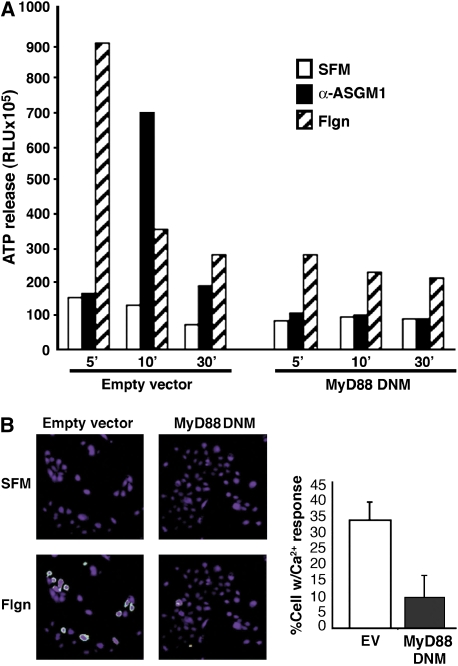
Based on the above data, we wanted to determine the significance of the ASGM1/TLR5 interaction. To assess the potential Toll dependence at each step in the ASGM1-triggered pathway leading to the autocrine release of ATP and mobilization of intracellular Ca2+ (14), we blocked Toll signaling using DNM MyD88. Data in Figure 3A show that ATP release was strongly stimulated by either flagellin or α-ASGM1 antibody, although time-courses were different. The release of ATP extracellularly is a prerequisite for ATP receptor activation. Independent of the stimulus, ATP release was inhibited in cells transfected with the MyD88 mutant. This showed that Toll receptors were involved upstream of ASGM1-activated ATP release. This effect on ATP release predicted that signaling downstream of ATP receptors would also be suppressed by DNM MyD88.
Figure 3.
Flagellin-induced ATP release and calcium mobilization are Toll dependent. NCIH292 cells transfected with empty vector (EV) or MyD88 DNM were exposed to α-ASGM1 antibody (1:100) or flagellin (Flgn, 10 μg/ml) for times indicated. (A) ATP release into the extracellular medium was monitored using a luciferin/luciferase assay. ATP release was inhibited by DNM MyD88. (B) Ratiometric Ca2+ imaging of islands of NCIH292 cells loaded with Fura-2/AM show increases in the 340/380-nm excitation fluorescence ratio (510 nm emission) ∼ 20 s after exposure to flagellin (B, bottom left panel). This increase in Ca2+ mobilization is reduced in the presence of DNM MyD88 (B, bottom right panel). Quantification of results from three separate experiments is shown in the histogram.
ATP receptors (of the P2Y subtype) are abundant on respiratory epithelial cells (23) and signal to downstream effectors via phospholipase C (PLC), which cleaves phosphatidylinositol biphosphate (PIP2) to form inositol triphosphate (IP3) and diacylglycerol (DAG). IP3 can bind to receptors on the endoplasmic reticulum, thereby releasing endoplasmic reticulum–stored Ca2+ into the cytoplasm. Consistent with its ability to inhibit ATP release (Figure 3A), DNM MyD88 also blocked mobilization of Ca2+ (Figure 3B).
The results obtained with DNM MyD88 strongly suggested that the ASGM1/ATP-dependent effects we described earlier (14) are controlled by Toll signaling. This raises the question as to whether ATP receptors might therefore constitute a previously unrecognized effector system by which Toll receptors mediate innate immunity.
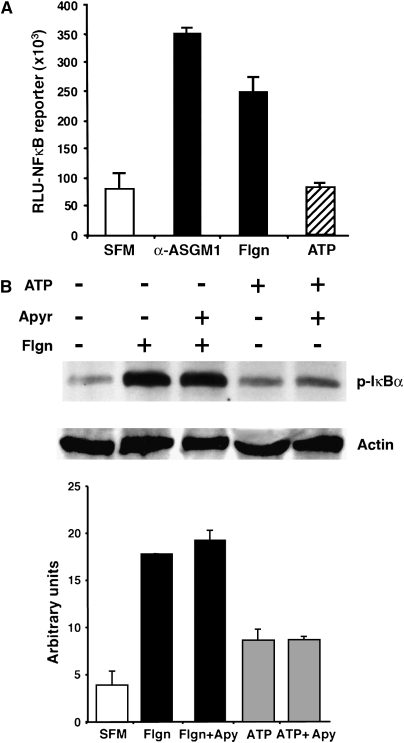
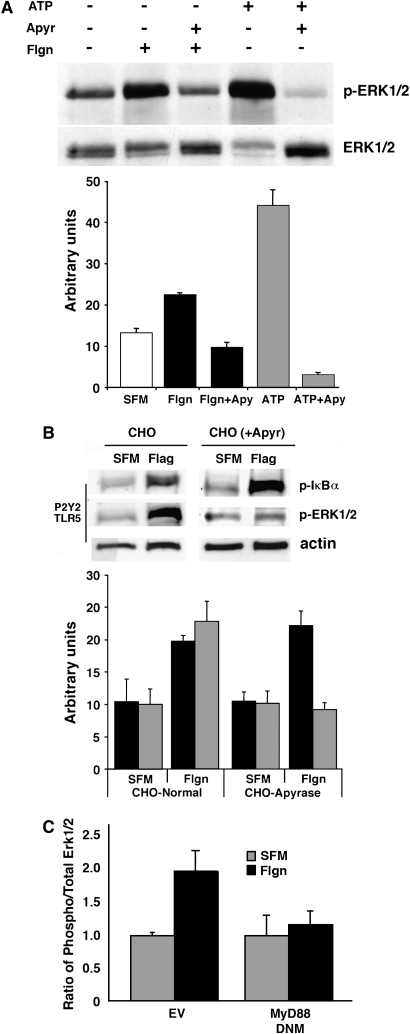
To examine a potential role for ATP in classical Toll signaling, we monitored NF-κB reporter activity and IκBα phosphorylation in response to the exogenous addition of extracellular ATP. Our experiments showed that ATP was neither necessary nor sufficient for the activation of IκB/NFκB (Figures 4A and 4B). Interestingly, though, extracellular ATP was indispensable for the activation of Erk1/2 (Figures 5A and 5B). Specifically, for experiments shown in Figures 4 and 5, we used the ATP-hydrolyzing enzyme apyrase to test the importance of extracellular ATP to both the flagellin-induced NF-κB and Erk1/2 pathways. Results showed that flagellin-induced IκBα phosphorylation proceeded normally in the presence of the ATP-degrading enzyme (Figure 4B). Consistent with this, the administration of exogenous ATP had minimal effect on IκBα phosphorylation (Figure 4B).
Figure 4.
ATP does not activate NF-κB. (A) NCIH292 cells transfected with an NF-κB–TA luciferase reporter showed increased reporter activity in response to α-ASGM1 (1:100) and flagellin (Flgn, 10 μg/ml), but not to ATP (100 μM). All incubations were for 6 h. (B) IκBα is phosphorylated in response to flagellin in presence or absence of the ATP ectonucleotidase, apyrase (1.5 U/ml). Administration of exogenous ATP has little effect. Quantification of IκBα phosphorylation by densitometry is shown in the bottom panel.
Figure 5.
ATP release in response to flagellin activates Erk1/2. Cell lysates prepared from (A) NCIH292 cells and (B) CHO cells were analyzed by Western blot using antibodies directed against phospho-Erk1/2. Membranes were then stripped and reprobed using an antibody against total Erk1/2 (A) or actin (B). In A, exogenous addition of ATP (100 μM) or flagellin (Flgn, 10μg/ml) for 10 min induced Erk1/2 phosphorylation that was inhibited in the presence of the ATP ectonucleotidase, apyrase (1.5 U/ml). Results from three separate experiments are quantified (mean area ± SEM) by densitometry. (B) Flagellin induced Erk1/2 phosphorylation in CHO cells transiently transfected with TLR5 and the nucleotide receptor, P2Y2. Phosphorylation did not occur in CHO cells overexpressing apyrase (CHO(+Apyr)). In contrast, flagellin-induced IκBα phosphorylation occurred normally in both wild type CHO and CHO(+Apyr) cells. Densitometry was performed to summarize the results (mean area ± SEM) from three separate experiments. IκBα phosphorylation is denoted using solid black bars, while Erk1/2 phosphorylation is denoted by shaded bars. (C) Densitometry data summarizing phospho-Erk/total Erk ratios for flagellin stimulated and DNM MyD88-inhibited NCIH292 cells (mean area ± SEM) shows Erk phosphorylation is Toll like receptor dependent.
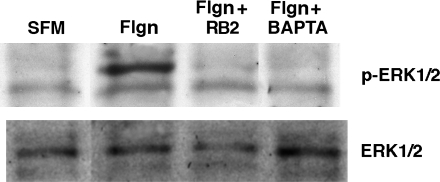
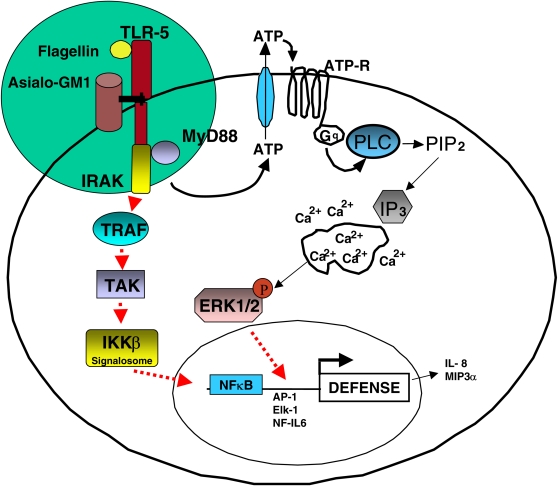
In contrast, data in Figure 5A show that Erk1/2, which (like IκBα) is phosphorylated in response to flagellin, is highly sensitive to apyrase, and thus to the presence of extracellular ATP. Indeed, the administration of exogenous ATP was sufficient to stimulate Erk phosphorylation in cells that had not seen flagellin (Figure 5A). Further validation of these results in CHO-K1 cells stably overexpressing apyrase (CHO-K1-[Apyr] cells, gift of Dr. Jose Boyer, University of North Carolina School of Medicine, Chapel Hill, and Inspire Pharmaceuticals Inc., Durham, North Carolina [24]) confirmed that flagellin-induced phosphorylation of IκBα remained intact and Erk1/2 phosphorylation was inhibited in the presence of extracellular ATP-hydrolysis (Figure 5B). The results in Figures 4 and 5 demonstrate that flagellin activates IκB/NFκB and Erk by mechanisms differing in their dependence on ATP, although both are dependent on Toll receptors (Figures 1 and 5C). Of particular interest is the fact that Erk phosphorylation by flagellin is dependent on both Toll receptors and extracellular ATP (Figures 5A–5C). Taken together with data in Figure 3A showing that flagellin-induced ATP release is Toll dependent, results in Figure 5 suggest for the first time that ATP autocrine signaling is a mechanism engaged by TLRs to launch antibacterial responses. In agreement with the operation of such a mechanism, both Reactive Blue 2, a nucleotide receptor antagonist, and BAPTA AM, a Ca2+ signaling inhibitor, blocked flagellin-induced Erk1/2 phosphorylation (Figure 6). These results suggest that flagellin increases the association between flagellin receptors ASGM1 and TLR5 to stimulate the release of ATP. ATP binds and activates a G protein–coupled nucleotide receptor on the cell surface, leading to Ca2+ mobilization and Erk1/2 phosphorylation. Conversely, flagellin-induced NF-κB activation, although Toll dependent, occurs independent of ATP (Figure 7).
Figure 6.
Erk1/2 phosphorylation in response to flagellin is nucleotide receptor– and calcium-dependent. Cell lysates prepared from NCIH292 cells exposed to flagellin (Flgn, 10 μg/ml, 10 min) were analyzed by Western blot using antibodies directed against phospho-Erk1/2. Membranes were then stripped and reprobed using an antibody against total Erk1/2. The P2Y nucleotide receptor inhibitor Reactive Blue 2 (RB2, 100 μM) and the Ca2+ chelator (BAPTA AM, 15 μM) were administered 30 min before and during the 10-min flagellin exposure. Control cells exposed to inhibitor alone were negative for Erk1/2 phosphorylation (not shown).
Figure 7.
Cartoon depicting events in flagellin-triggered host cell signaling. Although Toll is able to activate NF-κB in the absence of extracellular ATP, Toll-signaling requires ATP to activate Erk1/2. Flagellin causes an increased association between ASGM1 and TLR5 to mediate the autocrine release of ATP. Once released from the cell, ATP binds and activates a G protein–coupled nucleotide receptor on the cell surface, leading to Ca2+ mobilization and Erk1/2 phosphorylation.
DISCUSSION
Polymers of bacterial flagellin are organized into flagella, organelles whose whip-like action enhances bacterial motility. As part of their life cycle, bacteria degrade and renew flagellin; in doing so, they release flagellin near host cell membranes. In these studies, we have established a functional link between two previously described flagellin receptors, ASGM1 and TLR5. Having previously identified the distal effector mechanisms of ASGM1 (14), we show here that they are Toll dependent. A surprising implication of this is that TLRs represent upstream effectors of a nucleotide receptor signaling network, specifically, one lying upstream of MAP kinase Erk1/2 activation.
Erk is a member of the mitogen-activated protein kinase (MAPK) family consisting of Erk, Jnk, and p38. Erk 1 and 2 are isoenzymes with molecular weights of 42 and 44 kD, respectively (25). Upon phosphorylation, Erk translocates to the nucleus of the stimulated cell, where it can phosphorylate nuclear substrates, thereby activating them as transcription factors (e.g., c-fos, Elk-1, Ets, RSK) (26–29). Erk's ability to accumulate in the nucleus depends on its phosphorylation state (30). The classical upstream effectors of Erk1/2 are Ras, Raf, and Mek. The mechanism by which Toll receptors communicate with Erk is unclear (7, 15). Tolls generally communicate with distal effector molecules through a pathway involving IRAK and TRAF6. Interestingly, TRAF6-induced activation of Erk (but not NF-κB) has been found to be Ras independent (31), thereby showing that Toll receptors activate NF-κB and Erk via somewhat different mechanisms, consistent with our data in Figures 4 and 5. Moreover, recent work indicates that flagellin-induced IL-8 and MIP3α gene expression occurs via a TRAF6-mediated MEK-dependent (but NF-κB–independent) pathway (7). Interestingly, this pathway appears to be independent of the evolutionarily conserved signaling intermediate in Toll pathways (ECSIT), which interacts with MEKK1 and thereby links TRAF6 to Erk1/2 signaling (7, 32). In sum, the available data are controversial and do not specify the mechanism by which Toll receptors activate Erk.
The Toll–Erk signaling pathway is of great significance to innate immune responses involving cytokine induction (7, 15). Cytokines are needed for the recruitment of inflammatory cells to infection sites and also to facilitate cell–cell communication at these sites, thereby optimizing pathogen clearance. It has been suggested that the induction of cytokines IL-8 and MIP3α by flagellin relies on an NF-κB–independent signaling pathway that requires Erk1/2 (7). Our data indicate that whatever the precise mechanism mediating Erk activation by flagellin, it is dependent on both Toll receptors and extracellular ATP (Figures 5A–5C).
ATP is released from cells as a result of multiple stressors, including: ischemia, injury, and inflammation. The mechanism of ATP release from intact cells has received considerable attention but remains poorly understood (reviewed in Refs. 33 and 34). Upon release, extracellular ATP has been shown to act through specific cell surface receptors to regulate a variety of responses, including: platelet aggregation, smooth muscle contractility, neurotransmitter function, induction of cell death, mucociliary clearance, and mitogenic stimulation (reviewed in Refs. 35–37). These effects are mediated via two different subfamilies of extracellular nucleotide-binding P2 receptors, P2X and P2Y. P2Y receptors are metabotropic receptors coupled to G proteins and P2X receptors are ligand-gated ion channels. Both our previous work and the current study suggest an important role for G protein–coupled P2Y receptor activation in flagellin-induced Erk1/2 phosphorylation (14). It is important to consider, however, that P2X receptors may be equally involved along with P2Y receptors in this response. In both salivary acinar cells and astrocytes, Erk1/2 is activated via a PKC-dependent signaling pathway initiated by P2X7 (38, 39). Human T cells and mast cells also exhibit ATP-induced activation of Erk1/2 that is mediated through P2X7 (40, 41), whereas P2X4 invokes neuropathic pain via MAPK-induced production of IL-1β, IL-6, and TNF-α (42). It is worth noting that ATP-initiated P2X/MAPK signaling in T cells leads to the activation of transcription factor AP-1, while NF-κB activation is reduced. This agrees with our current observation that flagellin-mediated Erk1/2 phosphorylation occurs via an ATP-dependent pathway that does not involve NF-κB. Moreover, because P2X/Erk activation of AP-1 is fully dependent on the influx of extracellular Ca2+, it suggests that the release of calcium from both intracellular stores (mediated by P2Y receptors) and extracellular stores (mediated by P2X receptors) may be involved in the TLR signaling pathway activated by flagellin. Further studies will be necessary to define the ATP release mechanisms initiated by these receptors, as well as the specific molecular sequence of events that mediates MAPK activation in response to bacterial pathogens.
Earlier work clearly demonstrated that TLR5 recognizes a conserved site on flagellin required for protofilament formation and motility (18). Interestingly, efforts to define the affinity constant for flagellin binding to TLR5 have been hampered by interactions of flagellin with lipid bilayers (19). Precedent for a membrane lipid coreceptor can be found for LPS signaling via TLR4 and the GPI-linked membrane protein CD14, which functions to concentrate LPS at the host cell surface (43). Gangliosides have also been shown to act as coreceptors with TLR5 for flagellin in the induction of hBD-2 (44). This work suggests that the interaction of gangliosides such as GD1a, GD1b, and GT1b with flagellin and/or TLR5 promotes hBD-2 expression via an MAPK signaling pathway that involves AP-1. Moreover, both ganglioside and TLR5 expression are necessary for full hBD-2 upregulation in response to flagellin (44). Thus, bacterial ligand may be captured by high-affinity interactions with lipid-anchored receptors, but signal transduction requires their interaction with Toll-like receptors. In our own studies, TLR5-deficient CHO cells overexpressing ASGM1 showed little if any NF-κB activation in response to flagellin (data not shown). This supports the notion that signaling downstream of ganglioside receptors is Toll dependent, and is consistent with a recent study showing that the inhibitory effect of gangliosides on flagellin signaling in macrophage-like cells occurs in the absence of an effect on flagellin binding to TLR5 (45).
An important implication of Toll-dependent signaling downstream of ASGM1 is that TLRs represent upstream effectors of a nucleotide receptor signaling network. Direct examination of the relationship between TLRs and P2Y receptors has been complicated by difficulties in identifying the specific nucleotide receptor involved in flagellin signaling. Rank order efficiency testing in lung epithelial cells has not been consistent with the profile of any currently recognized nucleotide receptors, and a yet-unidentified P2Y receptor that mediates flagellin signaling cannot be ruled out. This possibility is the subject of further investigation as is the potential involvement of P2X receptors in TLR/ATP signaling.
In summary, our experiments show that host responses triggered at the flagellin receptor ASGM1 are dependent on the intact functioning of Toll-like receptors, specifically TLR5. The point of intersection of the two receptor pathways is at or above the level of ATP release (14). This further reveals for the first time that certain aspects of TLR signaling are dependent on an autocrine ATP receptor loop in epithelial cells.
Acknowledgments
The authors thank Jose Boyer for providing the apyrase-overexpressing CHO cells and Zena Werb for valuable discussions and critical review of this manuscript.
This work was supported by grants from the National Institutes of Health (K23 EY00371 [to N.M.], R01 HL073309 [to C.B.], K08 EY014473 [to I.M.]).
Originally Published in Press as DOI: 10.1165/rcmb.2005-0441OC on January 26, 2006
Conflict of Interest Statement: None of the authors has a financial relationship with a commercial entity that has an interest in the subject of this manuscript.
References
- 1.Feldman M, Bryan R, Rajan S, Scheffler L, Brunnert S, Tang H, Prince A. Role of flagella in pathogenesis of Pseudomonas aeruginosa pulmonary infection. Infect Immun 1998;66:43–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hayashi F, Smith KD, Ozinsky A, Hawn TR, Yi EC, Goodlett DR, Eng JK, Akira S, Underhill DM, Aderem A. The innate immune response to bacterial flagellin is mediated by Toll-like receptor 5. Nature 2001;410:1099–1103. [DOI] [PubMed] [Google Scholar]
- 3.Shuto T, Xu H, Wang B, Han J, Kai H, Gu XX, Murphy TF, Lim DJ, Li JD. Activation of NF-kappa B by nontypeable Hemophilus influenzae is mediated by toll-like receptor 2–TAK1-dependent NIK-IKK alpha /beta-I kappa B alpha and MKK3/6-p38 MAP kinase signaling pathways in epithelial cells. Proc Natl Acad Sci USA 2001;98:8774–8779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Opitz B, Schroder NW, Spreitzer I, Michelsen KS, Kirschning CJ, Hallatschek W, Zahringer U, Hartung T, Gobel UB, Schumann RR. Toll-like receptor-2 mediates Treponema glycolipid and lipoteichoic acid-induced NF-kappaB translocation. J Biol Chem 2001;276:22041–22047. [DOI] [PubMed] [Google Scholar]
- 5.Asehnoune K, Strassheim D, Mitra S, Kim JY, Abraham E. Involvement of reactive oxygen species in Toll-like receptor 4-dependent activation of NF-kappa B. J Immunol 2004;172:2522–2529. [DOI] [PubMed] [Google Scholar]
- 6.Barton GM, Medzhitov R. Toll-like receptor signaling pathways. Science 2003;300:1524–1525. [DOI] [PubMed] [Google Scholar]
- 7.Rhee SH, Keates AC, Moyer MP, Pothoulakis C. MEK is a key modulator for TLR5-induced interleukin-8 and MIP3alpha gene expression in non-transformed human colonic epithelial cells. J Biol Chem 2004;279:25179–25188. [DOI] [PubMed] [Google Scholar]
- 8.Roebuck KA. Regulation of interleukin-8 gene expression. J Interferon Cytokine Res 1999;19:429–438. [DOI] [PubMed] [Google Scholar]
- 9.Hobbie S, Chen LM, Davis RJ, Galan JE. Involvement of mitogen-activated protein kinase pathways in the nuclear responses and cytokine production induced by Salmonella typhimurium in cultured intestinal epithelial cells. J Immunol 1997;159:5550–5559. [PubMed] [Google Scholar]
- 10.Krisanaprakornkit S, Kimball JR, Dale BA. Regulation of human beta-defensin-2 in gingival epithelial cells: the involvement of mitogen-activated protein kinase pathways, but not the NF-kappaB transcription factor family. J Immunol 2002;168:316–324. [DOI] [PubMed] [Google Scholar]
- 11.Mitsuno Y, Yoshida H, Maeda S, Ogura K, Hirata Y, Kawabe T, Shiratori Y, Omata M. Helicobacter pylori induced transactivation of SRE and AP-1 through the ERK signalling pathway in gastric cancer cells. Gut 2001;49:18–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Guha M, O'Connell MA, Pawlinski R, Hollis A, McGovern P, Yan SF, Stern D, Mackman N. Lipopolysaccharide activation of the MEK-ERK1/2 pathway in human monocytic cells mediates tissue factor and tumor necrosis factor alpha expression by inducing Elk-1 phosphorylation and Egr-1 expression. Blood 2001;98:1429–1439. [DOI] [PubMed] [Google Scholar]
- 13.Akhtar M, Watson JL, Nazli A, McKay DM. Bacterial DNA evokes epithelial IL-8 production by a MAPK-dependent, NF-kappaB-independent pathway. FASEB J 2003;17:1319–1321. [DOI] [PubMed] [Google Scholar]
- 14.McNamara N, Khong A, McKemy D, Caterina M, Boyer J, Julius D, Basbaum C. ATP transduces signals from ASGM1, a glycolipid that functions as a bacterial receptor. Proc Natl Acad Sci USA 2001;98:9086–9091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Adamo R, Sokol S, Soong G, Gomez MI, Prince A. Pseudomonas aeruginosa flagella activate airway epithelial cells through asialoGM1 and toll-like receptor 2 as well as toll-like receptor 5. Am J Respir Cell Mol Biol 2004;30:627–634. [DOI] [PubMed] [Google Scholar]
- 16.Medzhitov R, Preston-Hurlburt P, Kopp E, Stadlen A, Chen C, Ghosh S, Janeway CA Jr. MyD88 is an adaptor protein in the hToll/IL-1 receptor family signaling pathways. Mol Cell 1998;2:253–258. [DOI] [PubMed] [Google Scholar]
- 17.Gewirtz AT, Navas TA, Lyons S, Godowski PJ, Madara JL. Cutting edge: bacterial flagellin activates basolaterally expressed TLR5 to induce epithelial proinflammatory gene expression. J Immunol 2001;167:1882–1885. [DOI] [PubMed] [Google Scholar]
- 18.Smith KD, Andersen-Nissen E, Hayashi F, Strobe K, Bergman MA, Barrett SL, Cookson BT, Aderem A. Toll-like receptor 5 recognizes a conserved site on flagellin required for protofilament formation and bacterial motility. Nat Immunol 2003;4:1247–1253. [DOI] [PubMed] [Google Scholar]
- 19.Mizel SB, West AP, Hantgan RR. Identification of a sequence in human toll-like receptor 5 required for the binding of Gram-negative flagellin. J Biol Chem 2003;278:23624–23629. [DOI] [PubMed] [Google Scholar]
- 20.DiMango E, Zar HJ, Bryan R, Prince A. Diverse Pseudomonas aeruginosa gene products stimulate respiratory epithelial cells to produce interleukin-8. J Clin Invest 1995;96:2204–2210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Davies J, Dewar A, Bush A, Pitt T, Gruenert D, Geddes DM, Alton EW. Reduction in the adherence of Pseudomonas aeruginosa to native cystic fibrosis epithelium with anti-asialoGM1 antibody and neuraminidase inhibition. Eur Respir J 1999;13:565–570. [DOI] [PubMed] [Google Scholar]
- 22.Lemjabbar H, Basbaum C. Platelet-activating factor receptor and ADAM10 mediate responses to Staphylococcus aureus in epithelial cells. Nat Med 2002;8:41–46. [DOI] [PubMed] [Google Scholar]
- 23.Cressman VL, Lazarowski E, Homolya L, Boucher RC, Koller BH, Grubb BR. Effect of loss of P2Y(2) receptor gene expression on nucleotide regulation of murine epithelial Cl(-) transport. J Biol Chem 1999;274:26461–26468. [DOI] [PubMed] [Google Scholar]
- 24.Alvarado-Castillo C, Harden TK, Boyer JL. Regulation of P2Y1 receptor-mediated signaling by the ectonucleoside triphosphate diphosphohydrolase isozymes NTPDase1 and NTPDase2. Mol Pharmacol 2005;67:114–122. [DOI] [PubMed] [Google Scholar]
- 25.Boulton TG, Nye SH, Robbins DJ, Ip NY, Radziejewska E, Morgenbesser SD, DePinho RA, Panayotatos N, Cobb MH, Yancopoulos GD. ERKs: a family of protein-serine/threonine kinases that are activated and tyrosine phosphorylated in response to insulin and NGF. Cell 1991;65:663–675. [DOI] [PubMed] [Google Scholar]
- 26.Janknecht R, Monte D, Baert JL, de Launoit Y. The ETS-related transcription factor ERM is a nuclear target of signaling cascades involving MAPK and PKA. Oncogene 1996;13:1745–1754. [PubMed] [Google Scholar]
- 27.Wang Y, Prywes R. Activation of the c-fos enhancer by the erk MAP kinase pathway through two sequence elements: the c-fos AP-1 and p62TCF sites. Oncogene 2000;19:1379–1385. [DOI] [PubMed] [Google Scholar]
- 28.Marais R, Wynne J, Treisman R. The SRF accessory protein Elk-1 contains a growth factor-regulated transcriptional activation domain. Cell 1993;73:381–393. [DOI] [PubMed] [Google Scholar]
- 29.Xing J, Ginty DD, Greenberg ME. Coupling of the RAS-MAPK pathway to gene activation by RSK2, a growth factor-regulated CREB kinase. Science 1996;273:959–963. [DOI] [PubMed] [Google Scholar]
- 30.Khokhlatchev AV, Canagarajah B, Wilsbacher J, Robinson M, Atkinson M, Goldsmith E, Cobb MH. Phosphorylation of the MAP kinase ERK2 promotes its homodimerization and nuclear translocation. Cell 1998;93:605–615. [DOI] [PubMed] [Google Scholar]
- 31.Kashiwada M, Shirakata Y, Inoue JI, Nakano H, Okazaki K, Okumura K, Yamamoto T, Nagaoka H, Takemori T. Tumor necrosis factor receptor-associated factor 6 (TRAF6) stimulates extracellular signal-regulated kinase (ERK) activity in CD40 signaling along a ras- independent pathway. J Exp Med 1998;187:237–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kopp E, Medzhitov R, Carothers J, Xiao C, Douglas I, Janeway CA, Ghosh S. ECSIT is an evolutionarily conserved intermediate in the Toll/IL-1 signal transduction pathway. Genes Dev 1999;13:2059–2071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schwiebert EM, Zsembery A. Extracellular ATP as a signaling molecule for epithelial cells. Biochim Biophys Acta 2003;1615:7–32. [DOI] [PubMed] [Google Scholar]
- 34.Lazarowski ER, Boucher RC, Harden TK. Mechanisms of release of nucleotides and integration of their action as P2X- and P2Y-receptor activating molecules. Mol Pharmacol 2003;64:785–795. [DOI] [PubMed] [Google Scholar]
- 35.Di Virgilio F, Chiozzi P, Ferrari D, Falzoni S, Sanz JM, Morelli A, Torboli M, Bolognesi G, Baricordi OR. Nucleotide receptors: an emerging family of regulatory molecules in blood cells. Blood 2001;97:587–600. [DOI] [PubMed] [Google Scholar]
- 36.Apasov S, Koshiba M, Redegeld F, Sitkovsky MV. Role of extracellular ATP and P1 and P2 classes of purinergic receptors in T-cell development and cytotoxic T lymphocyte effector functions. Immunol Rev 1995;146:5–19. [DOI] [PubMed] [Google Scholar]
- 37.Brake AJ, Julius D. Signaling by extracellular nucleotides. Annu Rev Cell Dev Biol 1996;12:519–541. [DOI] [PubMed] [Google Scholar]
- 38.Bradford MD, Soltoff SP. P2X7 receptors activate protein kinase D and p42/p44 mitogen-activated protein kinase (MAPK) downstream of protein kinase C. Biochem J 2002;366:745–755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sun P, Enslen H, Myung PS, Maurer RA. Differential activation of CREB by Ca2+/calmodulin-dependent protein kinases type II and type IV involves phosphorylation of a site that negatively regulates activity. Genes Dev 1994;8:2527–2539. [DOI] [PubMed] [Google Scholar]
- 40.Budagian V, Bulanova E, Brovko L, Orinska Z, Fayad R, Paus R, Bulfone-Paus S. Signaling through P2X7 receptor in human T cells involves p56lck, MAP kinases, and transcription factors AP-1 and NF-kappa B. J Biol Chem 2003;278:1549–1560. [DOI] [PubMed] [Google Scholar]
- 41.Bulanova E, Budagian V, Orinska Z, Hein M, Petersen F, Thon L, Adam D, Bulfone-Paus S. Extracellular ATP induces cytokine expression and apoptosis through P2X7 receptor in murine mast cells. J Immunol 2005;174:3880–3890. [DOI] [PubMed] [Google Scholar]
- 42.Inoue K. The function of microglia through purinergic receptors: neuropathic pain and cytokine release. Pharmacol Ther 2006;109:210–226. [DOI] [PubMed] [Google Scholar]
- 43.Jiang Q, Akashi S, Miyake K, Petty HR. Lipopolysaccharide induces physical proximity between CD14 and toll-like receptor 4 (TLR4) prior to nuclear translocation of NF-kappa B. J Immunol 2000;165:3541–3544. [DOI] [PubMed] [Google Scholar]
- 44.Ogushi K, Wada A, Niidome T, Okuda T, Llanes R, Nakayama M, Nishi Y, Kurazono H, Smith KD, Aderem A, et al. Gangliosides act as co-receptors for Salmonella enteritidis FliC and promote FliC induction of human beta-defensin-2 expression in Caco-2 cells. J Biol Chem 2004;279:12213–12219. [DOI] [PubMed] [Google Scholar]
- 45.West AP, Dancho BA, Mizel SB. Gangliosides inhibit flagellin signaling in the absence of an effect on flagellin binding to toll-like receptor 5. J Biol Chem 2005;280:9482–9488. [DOI] [PubMed] [Google Scholar]