Abstract
Oxidants in cigarette smoke and generated from asbestos fibers activate mitogen-activated protein kinase (MAPK) signaling cascades in lung epithelial cells in vitro and in vivo. These signaling pathways lead to the enhanced ability of Jun and Fos family members (i.e., components of the activator protein [AP]-1 transcription factor) to activate transcription of a number of AP-1–dependent target genes involved in cell proliferation or death, differentiation, and inflammation. Research by the Basbaum laboratory has been critical in showing that mucin transcription in response to cigarette smoke and gram-positive bacteria is mediated through activation of the epidermal growth factor receptor and MAPK cascades. Work from our laboratories supports the concept that MAPK signaling and AP-1 transactivation by cigarette smoke and asbestos may synergize in lung epithelial cell injury, compensatory proliferation of lung epithelial cells, and carcinogenesis, supporting a mechanistic framework for the striking increases in lung cancer incidence in asbestos workers who smoke. Targeting of MAPKs and inter-related signaling cascades may be critical to the prevention of lung cancers and control of mucin overproduction in a number of lung diseases including asthma, cystic fibrosis, chronic bronchitis, and chronic obstructive pulmonary disease.
Keywords: activator protein-1, asbestos, cigarette smoke, epidermal growth factor receptor, extracellular signal regulated kinases
Reactive oxygen species (ROS) and reactive nitrogen species are mediators of cell signaling in airway and pulmonary epithelial cells by a variety of toxic agents, including asbestos and silica, cigarette smoke, airborne particulate matter, diesel exhaust, and ozone. These signaling cascades are under intense investigation by a number of laboratories because of their links to exacerbation of epithelial cell injury and proliferation, cell survival and chemoresistance, altered differentiation, and production of chemokines or cytokines mediating inflammation.
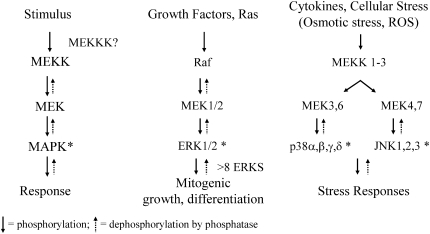
The mitogen-activated protein kinase (MAPK) cascades consist of serine threonine kinases that are sequentially phosphorylated by upstream kinases (MAPKKK, MAPKK) and subdivided into three major pathways: extracellular signal-regulated kinases (ERKs), c-Jun-NH2-terminal kinases (JNKs 1, 2, and 3) (also referred to as stress-activated protein kinases), and p38 kinases (1–3) (Figure 1). MAPK cascades can be initiated by activation of receptor tyrosine kinases such as the epidermal growth factor receptor (EGFR) or other factors stimulating phosphorylation of upstream MAPKKK and MAPKK. Because the MAPK cascades involve sequential phosphorylation of protein kinases, factors inhibiting the phosphatases that normally check these pathways also cause net increases in the phosphorylation (i.e., activation) of MAPK proteins.
Figure 1.
The MAPK cascades and cell outcomes.
Specific MAPKs control the activation of fos and jun family protooncogenes and their protein products (c-Jun, Jun B, Jun D, c-Fos, Fos B, Fra-1, and Fra-2) that are also known as AP-1 family members. These “early response protooncogene” products dimerize to form the AP-1 transcription factor, a converging point that regulates the expression of genes involved in cell proliferation, differentiation, transformation, inflammation, pulmonary defense, and autoregulation of AP-1 gene transcription (4). For example, several genes encoding antioxidant enzymes, surfactant proteins, extracellular matrix metalloproteinases (MMPs), surfactant proteins, and growth factors and receptors contain bona fide site(s) of AP-1 in their promoter and/or enhancer regions (5). For these reasons, oxidant-induced signaling events initiating MAPK pathways, activation of AP-1 family members, and transactivation of AP-1–dependent gene expression are thought to be critical to deciphering mechanisms of toxic injury by cigarette smoke and a variety of toxic oxidants affecting epithelial cells of the respiratory tract. The fact that there is interplay between MAPK activation and other signaling pathways, such as the nuclear factor-κB cascade, makes dissection of these signaling events difficult (6).
Signaling Pathways Elicited by Cigarette Smoke Stimulate Transcription of Mucin Genes through the EGFR
With its long-standing history of studying mucin regulation and production in differentiated human airway epithelial cells, the Basbaum laboratory was poised to investigate the mechanisms of mucin synthesis and overproduction by components of cigarette smoke. They first showed that increased mucin transcription by cigarette smoke was mediated through activation of the EGFR via cleavage of amphiregulin by the MMP ADAM 17 (7). In contrast, EGFR activation by gram-positive bacteria occurred through cleavage of the transmembrane ligand HBEGF by ADAM 10. Subsequently, they reported that synthesis of the predominant airway mucin MUC5AC was transcriptionally upregulated by cigarette smoke and was mediated by an AP-1–containing response element binding JunD and Fra-1 (8). These events required activation of the ERK and JNK pathways with EGFR activation critical to ERK but not JNK activation, which was Src dependent. Studies using scavengers of ROS showed that EGFR and JNK cascades were independently initiated pathways by ROS in cigarette smoke.
MAPK Signaling Pathways Are Linked to Squamous Differentiation of Airway Epithelial Cells and Cigarette Smoke–Induced Fra-1 Expression via the EGFR
In support of signaling work by the Basbaum laboratory showing the importance of MAPK pathways induced by cigarette smoke in mucus production by airway epithelial cells, studies in the Reddy laboratory linked JNK1 and AP-1 signaling to phorbol 13-myristate 12 acetate (PMA)-induced squamous epithelial cell differentiation in Clara-like H441 cells (9). PMA is a classical tumor promoter in skin and lung models of carcinogenesis and is known to generate ROS and inflammation, which are linked to cell injury and chronic epithelial cell proliferation. In these experiments, Reddy and colleagues showed that PMA induced markers (small proline-rich proteins [SPRRs]) of squamous cell differentiation, which suggested different roles of fra1 and fra2 in human SPRR1B expression. Moreover, a catalytically inactive JNK1 mutant significantly inhibited PMA-inducible SPRR1B promoter activity in H441 cells, indicating a critical role of JNK1 and selective activation of individual Fra proteins in the regulation of squamous cell differentiation. These studies may be relevant to the mechanisms of development of squamous metaplasia, a lesion induced by cigarette smoke and asbestos in human and rodent lungs that can progress to squamous cell carcinoma (10).
Recently, the effects of cigarette smoke on Jun and Fos family member expression and regulation was documented in the 1HAEo nonmalignant human bronchial epithelial cell line, showing that upregulation of c-Jun, c-Fos, and Fra-1 occurred (11). Because of data linking Fra-1 to malignant transformation of mesothelial cells by asbestos fibers (12, 13) and in skin carcinogenesis (14), mechanisms of cigarette smoke–induced Fra-1 expression were carefully dissected in this study. Results revealed that (1) cigarette smoke stimulated fra-1 induction at the transcriptional level; this effect was suppressed with the use of the EGFR phosphorylation inhibitor AG1478. (2) AG1478 inhibited ERK, JNK, and p38 kinase signaling pathways stimulated by cigarette smoke. (3) Inhibition of all three MAPK pathways markedly suppressed fra-1 induction. Because shedding of EGF and EGF-like ligands by MMPs has been implicated in the initiation of EGFR signaling in a variety of cell types, the use of a compound (GM6001) inhibiting MMP activity was also tested. These studies showed an obligatory role of a MMP-initiated signaling pathway in EGFR-MAPK-fra-1 transcription by cigarette smoke. Most importantly, they provide one mechanistic explanation for why MMPs are causally linked to smoke-induced diseases such as emphysema (15).
A causal role for ERK MAPK signaling in the induction of MMP-1 by cigarette smoke has been shown in pulmonary epithelial cells (16). Consistent with this result, an enhanced level of ERK activity was detected in the lung tissues from patients with emphysema as compared with control subjects. Moreover, the facts that Fra-1 regulates high-level constitutive expression of MMP-1 (17) and that diesel exhaust particles induce Fra-1 binding to the MMP-9 promoter, a process leading to enhanced transcription (18), strongly suggest the existence of an autoregulatory loop between AP-1 family member activation and selective MMP expression after toxicant exposures.
ERK1/2 Signaling Is Linked to Asbestos-Induced Fra-1 Expression in Lung Epithelial Cells and Mesothelial Cells
Asbestos is a group of carcinogenic and fibrogenic fibers associated with the development of lung cancers, mesothelioma, and pulmonary/pleural fibrosis (19). Asbestos fibers generate ROS or reactive nitrogen species after inhalation via direct interactions with epithelial cells or alveolar macrophages (i.e., frustrated phagocytosis of fibers accompanied by an oxidative burst and by indirect mechanisms involving inflammation). In addition, amphibole types of asbestos fibers have available iron on the fiber surface that catalyzes the formation of ROS (20).
In rodent pleural mesothelial cells, asbestos fibers preferentially activate the ERK1/2 pathway, in contrast to the JNK1/2 and p38 MAPK pathways (21), via phosphorylation of the EGFR (22). A number of other nonpathogenic particles and synthetic fibers did not activate ERK1/2. Treatment of cells with AG1478 prevented the induction of c-fos and apoptosis by asbestos, which also induced increases in EGFR message and protein levels (23). Phosphorylation of ERKs 1 and 2 by asbestos were inhibited by the addition of catalase (21), suggesting that this is an oxidant-dependent process. Immunofluorescence approaches to map association of asbestos fibers with the external domain of the EGFR in human mesothelial cells suggest that long asbestos fibers interact physically with the EGFR and cause dimerization (24) but do not exclude the possibility that ROS generation at these sites of fiber contact may be critical in modulating MAPK and other redox-associated cell signaling cascades, such as nuclear factor-κB (25).
The functional ramifications of EGFR/ERK1/2 signaling by asbestos include the initiation of cell cycling in a murine alveolar type II epithelial cell line (C10), suggesting that EGFR-initiated ERK1/2 signaling may play a role in aberrant proliferation induced by asbestos in lung epithelial cells in vitro and after inhalation (26, 27). ERK5 is also induced in C10 cells by asbestos fibers through an oxidant-dependent process that is not dependent on EGFR activation (28). ERK1/2 and ERK5 activation by asbestos is Src-dependent, and activation of all three signaling events is essential for the initiation of asbestos-induced epithelial cell proliferation (28).
We have known for over a decade that asbestos fibers activate the early response proto-oncogenes c-fos and c-jun in rodent mesothelial and tracheal epithelial cells in vitro (29), but fra-1 has also emerged as an intriguing fos/jun family member that is unique to asbestos-induced transformation of mesothelial cells (12, 13). ERK1/2 dependent Fra-1 expression is a critical player in tumor promotion in skin carcinogenesis (14). In asbestos-associated transformation of rodent mesothelial cells, the use of dominant negative ERK1 or Fra-1 constructs reverses the phenotype of mesothelioma cells to that of normal mesothelial cells (12). Moreover, Fra-1 expression is increased in human mesotheliomas and in other tumor types. We have used siRNA approaches to show that Fra-1 expression is critical to the expression of genes, such as cd44 and c-met, that are critical to migration and autocrine growth factor production in transformation and invasion of mesotheliomas (13).
Interplay between Particulates, Oxidative Stress, and Signaling Pathways in Lung
Most recently, we have linked the regulation of asbestos-induced EGFR activation, fra-1 transactivation, the expression of AP-1 family members, and AP-1 to DNA binding in C10 cells to intracellular levels of glutathione and γ-glutamylcysteine synthetase levels (30), suggesting again the importance of redox signaling by particle-induced oxidative stress. The recent observation that diesel exhaust, a known source of particles and other agents that induce oxidative stress, activates redox-sensitive transcription factors and kinases in human airways (31) confirms observations in vitro and in animal models and suggests that these signaling pathways may initiate human airway diseases by ROS-inducing particulates. Using gene profiling, we have determined time-dependent expression of more than 38 signal transduction genes and oxidative-stress genes in mouse lungs after inhalation of chrysotile asbestos over a 40-d period (32). These include the AP-1–regulated gene heme oxygenase, a protein we reported several years ago to be upregulated in human mesothelial cells after exposure to crocidolite asbestos in vitro (33). This protein has since been causally linked to defense against lung injury produced by oxidants (34).
A Mechanistic Model for Cigarette Smoke and Asbestos in MAPK Cell Signaling
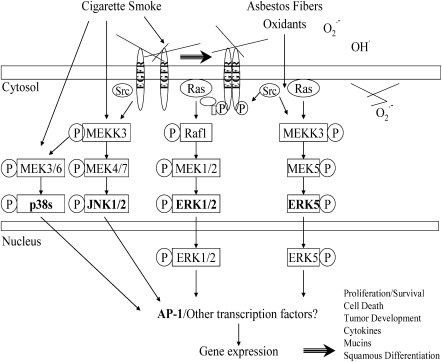
Cigarette smoke and asbestos act synergistically in the development of lung cancers and possibly pulmonary fibrosis. The data summarized in this article provide a mechanistic framework for oxidant-induced cell signaling by cigarette smoke and asbestos (Figure 2). In this model, the generation of oxidants by these toxicants seems to be the primary stimulus for activation of MAPK cascades and AP-1 in lung epithelium. Phosphorylation and/or dimerization of the EGFR is linked to activation of the JNK 1/2 cascade by cigarette smoke and ERK 1/2 and ERK 5 cascades by asbestos fibers. The fact that cigarette smoke is associated with the activation of all three MAPK pathways in lung epithelial cells is unsurprising given the multiplicity of particulates, gases, and chemicals in smoke. AP-1 activation and cross-talk with other transcription factors likely determine gene expression governing epithelial outcomes, such as proliferation versus cell death.
Figure 2.
Mechanisms of activation of MAPK and AP-1 by cigarette smoke or asbestos. This model is based upon data from studies evaluating these agents individually in lung epithelial cells.
In support of our observations on lung epithelial cells in vitro, imaging of phosphorylated ERK1/2 by immunohistochemistry has shown that activated ERKs are expressed preferentially in distal bronchiolar epithelium and epithelial cells in the alveolar duct region, sites of asbestos fiber impaction after inhalation (27). In vitro, phosphorylated ERK1/2 is translocated to the nucleus of C10 alveolar epithelial cells after addition of asbestos fibers, and the time frame of this process determines cell fate (35). At low, proliferative concentrations of asbestos fibers, there is an initial nuclear accumulation of phosphorylated ERK1/2 that diminishes over time and culminates in increased expression of cyclin D1 and in the entry of cells into S phase. In contrast, when higher concentrations of fibers are added, phosphorylated ERK1/2 accumulates in the nucleus where apoptosis-inducing factor is detected, preceding apoptosis.
Presently, we are examining patterns of activation of EGFR and MAPK signaling pathways using immunohistochemistry and Western blot analyses on mouse lungs after acute inhalation of cigarette smoke and asbestos fibers alone and in combination. In addition, we will be examining critical AP-1–regulated gene expression, which may be linked to phenotypic and functional endpoints in epithelial cells after dual exposures to these toxicants. These studies should shed light on possible mechanisms of synergy by cigarette smoke and asbestos on the development of lung cancers and pulmonary fibrosis and should identify critical targets for prevention and therapy of these and other associated lung diseases.
Acknowledgments
The authors thank Trisha Barrett for illustrations and Jennifer Díaz for formatting this manuscript.
This research was supported by a Program Project grant from NHLBI (HL69004) (B.T.M.) and grant NIEHS ES11863 (S.P.R.).
Originally Published in Press as DOI: 10.1165/rcmb.2006-0047SF on February 16, 2006
Conflict of Interest Statement: None of the authors has a financial relationship with a commercial entity that has an interest in the subject of this manuscript.
References
- 1.Davis RJ. The mitogen-activated protein kinase signal transduction pathway. J Biol Chem 1993;268:14553–14556. [PubMed] [Google Scholar]
- 2.Karin M. The regulation of AP-1 activity by mitogen-activated protein kinases. J Biol Chem 1995;270:16483–16486. [DOI] [PubMed] [Google Scholar]
- 3.Karin M, Gallagher E. From JNK to pay dirt: jun kinases, their biochemistry, physiology and clinical importance. IUBMB Life 2005;57:283–295. [DOI] [PubMed] [Google Scholar]
- 4.Shaulian E, Karin M. AP-1 in cell proliferation and survival. Oncogene 2001;20:2390–2400. [DOI] [PubMed] [Google Scholar]
- 5.Reddy SP, Mossman BT. Role and regulation of activator protein-1 in toxicant-induced responses of the lung. Am J Physiol Lung Cell Mol Physiol 2002;283:L1161–L1178. [DOI] [PubMed] [Google Scholar]
- 6.Tang G, Minemoto Y, Dibling B, Purcell NH, Li Z, Karin M, Lin A. Inhibition of JNK activation through NF-kappaB target genes. Nature 2001;414:313–317. [DOI] [PubMed] [Google Scholar]
- 7.Basbaum C, Li D, Gensch E, Gallup M, Lemjabbar H. Mechanisms by which gram-positive bacteria and tobacco smoke stimulate mucin induction through the epidermal growth factor receptor (EGFR). Novartis Found Symp 2002;248:171–176 (Discussion: 176–180, 277–182). [PubMed] [Google Scholar]
- 8.Gensch E, Gallup M, Sucher A, Li D, Gebremichael A, Lemjabbar H, Mengistab A, Dasari V, Hotchkiss J, Harkema J, et al. Tobacco smoke control of mucin production in lung cells requires oxygen radicals AP-1 and JNK. J Biol Chem 2004;279:39085–39093. [DOI] [PubMed] [Google Scholar]
- 9.Vuong H, Patterson T, Adiseshaiah P, Shapiro P, Kalvakolanu DV, Reddy SP. JNK1 and AP-1 regulate PMA-inducible squamous differentiation marker expression in Clara-like H441 cells. Am J Physiol Lung Cell Mol Physiol 2002;282:L215–L225. [DOI] [PubMed] [Google Scholar]
- 10.Mossman BT, Craighead JE, MacPherson BV. Asbestos-induced epithelial changes in organ cultures of hamster trachea: inhibition by retinyl methyl ether. Science 1980;207:311–313. [DOI] [PubMed] [Google Scholar]
- 11.Zhang Q, Adiseshaiah P, Reddy SP. Matrix metalloproteinase/epidermal growth factor receptor/mitogen-activated protein kinase signaling regulate fra-1 induction by cigarette smoke in lung epithelial cells. Am J Respir Cell Mol Biol 2005;32:72–81. [DOI] [PubMed] [Google Scholar]
- 12.Ramos-Nino ME, Timblin CR, Mossman BT. Mesothelial cell transformation requires increased AP-1 binding activity and ERK-dependent Fra-1 expression. Cancer Res 2002;62:6065–6069. [PubMed] [Google Scholar]
- 13.Ramos-Nino ME, Scapoli L, Martinelli M, Land S, Mossman BT. Microarray analysis and RNA silencing link fra-1 to cd44 and c-met expression in mesothelioma. Cancer Res 2003;63:3539–3545. [PubMed] [Google Scholar]
- 14.Young MR, Li JJ, Rincon M, Flavell RA, Sathyanarayana BK, Hunziker R, Colburn N. Transgenic mice demonstrate AP-1 (activator protein-1) transactivation is required for tumor promotion. Proc Natl Acad Sci USA 1999;96:9827–9832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hautamaki RD, Kobayashi DK, Senior RM, Shapiro SD. Requirement for macrophage elastase for cigarette smoke-induced emphysema in mice. Science 1997;277:2002–2004. [DOI] [PubMed] [Google Scholar]
- 16.Mercer BA, Kolesnikova N, Sonett J, D'Armiento J. Extracellular regulated kinase/mitogen activated protein kinase is up-regulated in pulmonary emphysema and mediates matrix metalloproteinase-1 induction by cigarette smoke. J Biol Chem 2004;279:17690–17696. [DOI] [PubMed] [Google Scholar]
- 17.Tower GB, Coon CI, Belguise K, Chalbos D, Brinckerhoff CE. Fra-1 targets the AP-1 site/2G single nucleotide polymorphism (ETS site) in the MMP-1 promoter. Eur J Biochem 2003;270:4216–4225. [DOI] [PubMed] [Google Scholar]
- 18.Zhang Q, Kleeberger SR, Reddy SP. DEP-induced fra-1 expression correlates with a distinct activation of AP-1-dependent gene transcription in the lung. Am J Physiol Lung Cell Mol Physiol 2004;286:L427–L436. [DOI] [PubMed] [Google Scholar]
- 19.Mossman B, Bignon J, Corn M, Seaton A, Gee J. Asbestos: scientific developments and implications for public policy. Science 1990;247:294–301. [DOI] [PubMed] [Google Scholar]
- 20.Shukla A, Gulumian M, Hei TK, Kamp D, Rahman Q, Mossman BT. Multiple roles of oxidants in the pathogenesis of asbestos-induced diseases. Free Radic Biol Med 2003;34:1117–1129. [DOI] [PubMed] [Google Scholar]
- 21.Jimenez L, Zanella C, Fung H, Janssen Y, Vacek P, Charland C, Goldberg J, Mossman B. Role of extracellular signal-regulated protein kinases in apoptosis by asbestos and H2O2. Am J Physiol Lung Cell Mol Physiol 1997;273:L1029–L1035. [DOI] [PubMed] [Google Scholar]
- 22.Zanella C, Posada J, Tritton T, Mossman B. Asbestos causes stimulation of the ERK-1 mitogen-activated protein kinase cascade after phosphorylation of the epidermal growth factor receptor. Cancer Res 1996;56:5334–5338. [PubMed] [Google Scholar]
- 23.Zanella C, Timblin C, Cummins A, Jung M, Goldberg J, Raabe R, Tritton T, Mossman BT. Asbestos-induced phosphorylation of epidermal growth factor receptor is linked to c-fos expression and apoptosis. Am J Physiol Lung Cell Mol Physiol 1999;277:L684–L693. [DOI] [PubMed] [Google Scholar]
- 24.Pache J, Janssen Y, Walsh E, Quinlan T, Zanella C, Low R, Taatjes D, Mossman B. Increased epidermal growth factor-receptor (EGF-R) protein in a human mesothelial cell line in response to long asbestos fibers. Am J Pathol 1998;152:333–340. [PMC free article] [PubMed] [Google Scholar]
- 25.Janssen Y, Barchowsky A, Treadwell M, Driscoll K, Mossman B. Asbestos induces nuclear factor kappa B (NF-kappa B) DNA-binding activity and NF-kappa B-dependent gene expression in tracheal epithelial cells. Proc Natl Acad Sci USA 1995;92:8458–8462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Buder-Hoffmann S, Palmer C, Vacek P, Taatjes D, Mossman B. Different accumulation of activated extracellular signal-regulated kinases (ERK 1/2) and role in cell-cycle alterations by epidermal growth factor, hydrogen peroxide, or asbestos in pulmonary epithelial cells. Am J Respir Cell Mol Biol 2001;24:405–413. [DOI] [PubMed] [Google Scholar]
- 27.Cummins A, Palmer C, Mossman B, Taatjes D. Persistent localization of activated extracellular signal-regulated kinases (ERK1/2) is epithelial cell-specific in an inhalation model of asbestosis. Am J Pathol 2003;162:713–720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Scapoli L, Ramos-Nino M, Martinelli M, Mossman B. Src-dependent ERK5 and Src/EGFR-dependent ERK1/2 activation is required for cell proliferation by asbestos. Oncogene 2004;23:805–813. [DOI] [PubMed] [Google Scholar]
- 29.Heintz N, Janssen Y, Mossman B. Persistent induction of c-fos and c-jun expression by asbestos. Proc Natl Acad Sci USA 1993;90:3299–3303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shukla A, Flanders T, Lounsbury KM, Mossman BT. The gamma-glutamylcysteine synthetase and glutathione regulate asbestos-induced expression of activator protein-1 family members and activity. Cancer Res 2004;64:7780–7786. [DOI] [PubMed] [Google Scholar]
- 31.Pourazar J, Mudway IS, Samet JM, Helleday R, Blomberg A, Wilson SJ, Frew AJ, Kelly FJ, Sandstrom T. Diesel exhaust activates redox-sensitive transcription factors and kinases in human airways. Am J Physiol Lung Cell Mol Physiol 2005;289:L724–L730. [DOI] [PubMed] [Google Scholar]
- 32.Sabo-Attwood T, Ramos-Nino M, Bond J, Butnor KJ, Heintz N, Gruber AD, Steele C, Taatjes DJ, Vacek P, Mossman BT. Gene expression profiles reveal increased mClca3 (Gob5) expression and mucin production in a murine model of asbestos-induced fibrogenesis. Am J Pathol 2005;167:1243–1256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Janssen YM, Marsh JP, Absher MP, Gabrielson E, Borm PJ, Driscoll K, Mossman BT. Oxidant stress responses in human pleural mesothelial cells exposed to asbestos. Am J Respir Crit Care Med 1994;149:795–802. [DOI] [PubMed] [Google Scholar]
- 34.Lee PJ, Alam J, Wiegand GW, Choi AM. Overexpression of heme oxygenase-1 in human pulmonary epithelial cells results in cell growth arrest and increased resistance to hyperoxia. Proc Natl Acad Sci USA 1996;93:10393–10398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yuan Z, Taatjes DJ, Mossman BT, Heintz NH. The duration of nuclear extracellular signal-regulated kinase 1 and 2 signaling during cell cycle reentry distinguishes proliferation from apoptosis in response to asbestos. Cancer Res 2004;64:6530–6536. [DOI] [PubMed] [Google Scholar]