Abstract
Alveolar epithelial type II cells secrete lung surfactant via exocytosis. Soluble N-ethylmaleimide–sensitive factor attachment protein receptors (SNARE) are implicated in this process. Lipid rafts, the cholesterol- and sphingolipid-rich microdomains, may offer a platform for protein organization on the cell membrane. We tested the hypothesis that lipid rafts organize exocytotic proteins in type II cells and are essential for the fusion of lamellar bodies, the secretory granules of type II cells, with the plasma membrane. The lipid rafts, isolated from type II cells using 1% Triton X-100 and a sucrose gradient centrifugation, contained the lipid raft markers, flotillin-1 and -2, whereas they excluded the nonraft marker, Na+-K+ ATPase. SNAP-23, syntaxin 2, and VAMP-2 were enriched in lipid rafts. When type II cells were depleted of cholesterol, the association of SNAREs with the lipid rafts was disrupted and the formation of fusion pore was inhibited. Furthermore, the cholesterol-depleted plasma membrane had less ability to fuse with lamellar bodies, a process mediated by annexin A2. The secretagogue-stimulated secretion of lung surfactant from type II cells was also reduced by methyl-β-cyclodextrin. When the raft-associated cell surface protein, CD44, was cross-linked using anti-CD44 antibodies, the CD44 clusters were observed. Syntaxin 2, SNAP-23, and annexin A2 co-localized with the CD44 clusters, which were cholesterol dependent. Our results suggested that lipid rafts may form a functional platform for surfactant secretion in alveolar type II cells, and raft integrity was essential for the fusion between lamellar bodies with the plasma membrane.
Keywords: alveolar type II cells, lipid rafts, membrane fusion, SNARE proteins, surfactant secretion
Lung alveolar epithelium consists of two different types of cells, the cuboidal type II cells and squamous type I cells. Type II cells synthesize, store, and secrete a surface-active lipid-rich substance, the lung surfactant. The released surfactant lines the alveolar epithelium, lowers the surface tension, and thus prevents the collapse of alveoli at end-expiration. Lung surfactant deficiency causes respiratory distress syndrome (RDS) in infants. Type II cells are also involved in defense, injury and repair, and trans-differentiation into type I cells.
Lung surfactant, stored in lamellar bodies, is released upon its fusion with the plasma membrane via exocytosis. The formation of fusion pore precedes the release of lamellar body contents. The SNARE (soluble N-ethylmaleimide–sensitive factor attachment protein receptors) hypothesis was proposed to elucidate the mechanisms of membrane fusion during exocytosis. During fusion, the proteins on the plasma membrane (target or t-SNAREs) and vesicles (vesicular or v-SNARE) form a highly stable, hetero-tetrameric SNARE complex. The two coiled-coil domains are contributed by soluble N-ethylmaleimide–sensitive factor attachment protein (SNAP)-25/23 and one each from syntaxin and VAMP (vesicle-associated membrane protein) (1). NSF (N-ethylmaleimide–sensitive factor), an ATPase, binds to its ligand, α-SNAP, and then to the SNARE complex to dissociate it and, thus, recycling the interacting components for subsequent cycles. In vitro studies have revealed that SNARE complex formation suffices membrane fusion (2), but in vivo fusion is a much faster and more complex phenomenon due to the existence of numerous SNARE regulatory proteins. The transmembrane regions of syntaxin appear to line the fusion pore (3). Previous studies from our laboratory have indicated that syntaxin 2, SNAP-23, α-SNAP, and NSF were required for surfactant secretion (4, 5).
Annexins are a family of highly conserved proteins known for their Ca2+--dependent association with the negatively charged phospholipids. Some of the members mediated aggregation, as well as the fusion of liposomes. Annexin A2 tetramer had an exceptionally low Ca2+ requirement for inducing the membrane fusion. Annexin A2 tetramer has been reported not only to promote the fusion of lamellar bodies with liposomes or the plasma membrane at μM Ca2+ concentrations, but also to reconstitute surfactant secretion in permeabilized type II cells (6, 7). The silencing of annexin A2 by RNA interference in primary cultures of alveolar type II cells significantly reduced the surfactant secretion (8).
The studies during the past decade have revolutionized the understanding of membrane organization. According to the newly proposed raft hypotheses (9, 10), membrane lipids exist in two phases, ordered phase rendered by saturated lipids and disordered phase by unsaturated lipids. The ordered phase lipids form lipid microdomains or lipid rafts, which are rich in cholesterol, sphingolipids, and gangliosides, and are reported to sequester and segregate a number of specific proteins. Thus, they are ideally suited for various processes, including membrane traffic, signal transduction, and apical protein sorting (9). Their involvements in other functions are being reported regularly. The resident raft proteins include GPI-anchored proteins on the exoplasmic leaflet and doubly acylated proteins, and palmitate-anchored proteins on the cytoplasmic leaflet. With their unique property of clustering, these microdomains provide an interacting platform for the various proteins to bring about the ultimate cellular response.
SNARE proteins have been shown to be associated with the lipid rafts in several cell types (11–14). However, in vitro, syntaxin 1A and synaptobrevin 2 preferred disordered domains when constituted into giant unilamellar vesicles (15). There are no reports of SNARE protein organization in type II cells. Furthermore, whether lipid rafts participate in the formation of fusion pore and thus membrane fusion was unclear. We hypothesized that lipid rafts organize SNARE proteins on the plasma membrane of alveolar type II cells and are essential for the fusion of lamellar bodies and the plasma membrane during exocytosis. To this end, we isolated lipid rafts from type II cells and determined the association of SNARE proteins with the lipid rafts. We further determined whether the disruption of lipid rafts by the depletion of cholesterol affected fusion pore formation, membrane fusion, and surfactant secretion. In addition, a raft-associated cell surface transmembrane glycoprotein, CD44, was cross-linked to form raft clusters using anti-CD44 antibodies; we then determined whether the raft clusters contained exocytotic proteins.
MATERIALS AND METHODS
Reagents and Antibodies
All chemicals and reagents were of analytical grade and purchased from Sigma (St. Louis, MO), unless otherwise stated. Elastase was purchased from Worthington Chemicals (Lakewood, NJ). Fetal bovine serum (FBS) was from Gibco (Grand Island, NY). Minimal essential medium (MEM) was from ICN Biomedicals (Aurora, OH). Nitrocellulose membrane was from Schleicher and Schuell (Keene, NH). Horseradish peroxidase (HRP)-conjugated goat anti-rabbit antibody, and protein molecular mass markers, were from Bio-Rad (Hercules, CA). Cy3-conjugated anti-mouse IgG, HRP-conjugated goat anti-mouse antibodies, and bovine serum albumin (BSA) were from Jackson Immunologicals (West Grove, PA). Paraformaldehyde was from Electron Microscopy Services (Ft. Washington, PA). Monoclonal anti-flotillin–1 and –2, anti–α-SNAP, anti-NSF, and anti-CD44 antibodies were from BD Transduction Laboratories (Lexington, KY). Monoclonal anti-Na+-K+ ATPase antibodies were from Upstate Biotechnology (Lake Placid, NY). Monoclonal anti-LB180 antibodies were from Covance Research products (Richmond, CA). Polyclonal rabbit anti-syntaxin 2 and anti–SNAP-23 antibodies were from Synaptic Systems (Gottingen, Germany). Polyclonal rabbit anti–VAMP-2 antibodies were from Stressgen (Victoria, BC, Canada). Polyclonal goat anti–SP-C antibodies were from Santa Cruz Biotechnology (Santa Cruz, CA). Amphiphilic dye, N-(3-triethylammoniumpropyl)-4-(4-[dibutylamino]styryl) pyridinium dibromide (FM 1–43), octadecyl rhodamine B chloride (R18), all Alexa-conjugated secondary antibodies, and Amplex Red Cholesterol Assay Kit were purchased from Molecular Probes (Eugene, OR). Enhanced chemiluminescence (ECL) detection system was from Amersham Biotech (Piscataway, NJ). Polyclonal anti-annnexin A2 antibodies were raised in rabbits against isolated bovine annexin A2 and were affinity-purified. TRI reagents were from Molecular Research Center (Cincinnati, OH).
Isolation and Culturing of Alveolar Type II Cells
Alveolar type II cells were isolated from male Sprague-Dawley rats (175–200 g) as previously described (16). The perfused lungs were lavaged, digested with elastase, chopped, and filtered. The cells were subjected to IgG panning to remove contaminating macrophages. The unattached cells were pelleted, resuspended in MEM, counted for number, and assayed for viability using trypan-blue exclusion assay. The cell viability ranged from 95–97%. Purity of the cells was > 90%, as ascertained by Papanicolou's staining. For the RT-PCR experiment, > 96% pure freshly isolated type II cells were obtained by our recently modified protocol (17), in which an additional step, using anti-leukocyte common antigen antibodies and magnetic beads, was included to remove the residual macrophages.
For studying the differential expression of flotillins, isolated type II cells were plated on 35 mm2 plastic dishes at a density of 1.5 × 106 per dish. The cells were cultured for 3 or 7 d in MEM supplemented with 10% FBS. Media was changed after overnight culture and then every other day. In this culture system, type II cells loose their phenotypes and convert to the type I–like cells with some characteristics of alveolar type I cells.
RT-PCR
Total RNA was isolated from the lung by homogenizing the tissue in TRI reagent or from freshly isolated type II cells by dissolving them in the reagent. The mRNA (1 μg) was reverse-transcribed to cDNA using M-MLV reverse transcriptase and random hexamer primers and PCR-amplified using gene specific primers against caveolin-1α (forward: 5′-AAATTGATCTGGTCAACCGC-3′; reverse: 5′-ATCTCTTCCTGCGTGCTGAT-3′), caveolin-1β (forward: 5′-ATTGGTTTTAC CGCTTGCTG-3′; reverse: 5′-ATCTCTTCCTGCGTGCTGAT-3′), caveolin-2 (forward: 5′-CTTCATTGCGGGTATCCTGT-3′; reverse: 5′-CAGTTGTGGCTCAGTTGCAT-3′), caveolin-3 (forward: 5′-GG ACATTGTGAAGGTGGATTT-3′; reverse: 5′-GCACTGGATCT CAATCAGGTA-3′), flotillin-1 (forward: 5′-GCTGAAGAAAGC CACCTACG-3′; reverse: 5′-CTCAGCTTCAGCTTCTGCCT-3′), flotillin-2 (forward: 5′-GGGTACAAGGGTTCTGCGTA-3′; reverse: 5′-TCTGTGCCTCTATGGTGCAG-3′). 18S rRNA was amplified using classic 18S RNA primer pairs (Ambion, Austin, TX). The conditions for PCR amplification were: 10 mM Tris-HCl (pH 9.0), 50 mM KCl and 0.1% Triton X-100, 1.6 mM MgCl2, 0.16 mM dNTP mix, 1.6 μM of each primer, 1 U DNA Taq polymerase, and 20 ng of cDNA in a final reaction volume of 25 μl. The thermal conditions were 94°C for 2 min, 35 cycles of 94°C 30 s, 55°C 40 s, 72°C 1 min, followed by 72°C for 8 min The PCR products were electrophoretically separated on agarose gel for studying the expression pattern of the raft marker proteins.
Western Blot
Type II cells, freshly isolated or cultured on plastic dishes for 3 and 7 d, MLE-12 cells, and L2 cells were lysed in the lysis buffer (50 mM Tris-HCl, pH 7.4, 150 mM NaCl, 1% Triton X-10, 5 mM EGTA, 1 mM PMSF, 10 μg/ml aprotonin, 10 μg/ml leupeptin, and 1 mM benzamidine). Lung tissue was homogenized in the lysis buffer. Lamellar bodies (LB) and plasma membrane (PM) were directly dissolved in the SDS sample buffer. Proteins were separated on SDS-PAGE, transferred onto nitrocellulose membranes, and immunoblotted with specific antibodies. The primary antibodies were used at the following dilutions: 1:250 for flotillin-1 and VAMP-2; 1:1,000 for syntaxin 2, annexin A2, SNAP-23, Na+-K+ ATPase, and NSF; and 1:5,000 for flotillin-2 and α-SNAP. HRP-conjugated secondary antibodies were used at a 1:2,500 dilution in all the cases. The protein bands were visualized with the ECL reagents. The protein bands were quantified using a Bio-Rad densitometric scanner.
Immunohistochemistry
The lungs from male Sprague-Dawley rats were perfused with 50 mM PBS (pH 7.4) and then lavaged four times with 5 ml of normal saline. Lungs were fixed by infusing 5 ml of 4% paraformaldehyde into the lungs and kept immersed in the same solution at room temperature for overnight. Paraffin-embedded lungs were sectioned (2 μm) and placed on glass slides (Fisher Scientific, Pittsburgh, PA). The slides were deparaffinized with xylene and rehydrated with graded alcohol and PBS. Antigen retrieval was done by boiling the slides with citrate buffer (10 mM disodium citrate, pH 6.0, and 0.05% Tween-20) for 20 min. Immunohistochemistry was performed as previously described (18). Goat anti–SP-C antibodies were used at a dilution of 1:50, whereas mouse anti-flotillin–1 and –2 were used at a dilution of 1:100. Alexa 546-conjugated anti-goat and Alexa 488-conjugated anti-mouse antibodies were used at 1:250 dilutions.
Isolation of Lipid Rafts
Lipid rafts were isolated from alveolar type II cells according to the previously reported method (12). In brief, freshly isolated type II cells (25–30 × 106) were washed twice with MEM and once with MBS buffer (25 mM MES and 150 mM NaCl, pH 6.5). The cell pellet was lysed in 700 μl of ice-cold lysis buffer (MBS with 1% Triton X-100, 5 mM EGTA; 1 mM PMSF, 10 μg/ml aprotonin, 10 μg/ml leupeptin, and 1 mM benzamidine) for 45 min on ice. Six hundred microliters of lysate was carefully mixed with an equal volume of 80% sucrose (wt/vol) and gently laid at the bottom of an ultracentrifuge tube. Later, 1,200 μl each of 30% and 5% sucrose were laid over the 40% sucrose gradient and centrifuged at 200,000 × g for 16 h. Seven fractions were collected from top to bottom as follows: first two fractions of 600 μl each, followed by three fractions of 400 μl each and two fractions of 600 μl each. Pellet was dissolved in 600 μl of lysis buffer and labeled as the eighth fraction. Equal volumes of each fraction were immunoblotted for various raft and nonraft marker proteins. For studying the effects of cholesterol depletion on lipid raft association of SNARE proteins, type II cells were solubilized in MBS containing saponin (0.5% wt/vol), 0.5% TX-100, and a cocktail of protease inhibitors. A similar procedure was used in PC12 cells (12).
Fusion Pore Formation
Alveolar type II cells were grown on coverslips overnight. After removal of the unattached cells and equilibration with fresh MEM for 30 min, amphiphilic dye FM1–43 (4 μM) was added and incubated for 10 min (19). The cells were stimulated with a combination of secretagogues (0.1 μM phorbol 12-myristate 13-acetate, 100 μM ATP, and 20 μM terbutaline) for indicated periods of time in the presence of the dye. The cells were rinsed with 50 mM ice-cold PBS and fixed with 4% paraformaldehyde. Under these conditions, the signals were not lost, although extensive washing resulted in the loss of the signals. Excess fixative was quenched with 50 mM ammonium chloride solution in PBS for 5 min. The cells were later washed three times with PBS and mounted onto the slides using an in-house mounting solution (1.5% wt/vol n-propyl gallate and 60% vol/vol glycerol in PBS). The positively stained lamellar bodies were counted in 50 cells selected at random under oil immersion (×100). Cholesterol depletion from alveolar type II cells was done by incubating the cells with media containing 3 mM methyl β-cyclodextrin (MCD) for ∼ 30 min at 37°C. The control cells were treated the same with MCD–cholesterol complex (40 μg cholesterol and 0.5% or 3.78 mM MCD). The cells were washed to remove MCD and incubated with fresh media for further studies. The extent of cholesterol depletion was assessed by cholesterol oxidase method (Amplex Red Cholesterol Assay Kit), as per the instructions of the manufacturer.
To determine if cholesterol depletion affected lamellar body staining, overnight cultured type II cells were used. After fixation, permeabilization, and blocking for nonspecific proteins, the cells were incubated with LB-180 antibodies (1:1,000) for overnight. Later, they were incubated with Cy3-conjugated anti-mouse antibodies (1:250). Florescence was observed with Nikon Eclipse E600 Microscope (Nikon, Lewisville, TX).
Membrane Fusion
Lamellar bodies and plasma membrane were isolated from perfused rat lungs according to our previous protocols (7). The fusion of lamellar bodies with the plasma membrane was determined by the de-quenching of R18 as previously described (7). R18-labeled plasma membrane (5 μg protein) was incubated with lamellar bodies (10 μg protein) in 1 ml of Ca2+-EGTA buffer (40 mM Hepes, pH 7.0, 100 mM KCl, 1 mM EGTA, 1 mM Ca2+ free Ca2+) at 37°C for 2 min The purified annexin A2 tetramer was added to initiate the fusion. Fluorescence was monitored at Ex = 560 nm and Em = 590 nm. Fusion was expressed as a percentage of the maximal fluorescence (in the presence of 0.1% Triton X-100).
Surfactant Secretion from Type II Cells
Isolated alveolar type II cells were pre-labeled with 0.6 μCi of [3H] choline and grown in 35 mm2 cell culture dishes. After overnight culture, unattached cells were removed by washing cells with warm MEM and then incubated in fresh media for 30 min The cells were stimulated with 0.1 μM phorbol 12-myristate 13-acetate, 100 μM ATP, and 20 μM terbutaline for 2 h. Surfactant secretion assay was done as previously described (6). For cholesterol depletion, cells were incubated with 3 mM MCD for 30 min before the stimulation.
Raft Clustering
CD44 cross-linking was done as described (20). In brief, freshly isolated type II cells were washed three times with MEM. The cells were equilibrated in MEM, supplemented with 1 mM MgCl2 and 1 mM CaCl2, at 37°C for 30 min For cross-linking, the cells were incubated with mouse anti-CD44 antibodies (20 μg/ml) for 60 min, followed by incubation with Alexa 488–conjugated anti-mouse antibodies (20 μg/ml) for another 60 min. The reaction was stopped by adding ice-cold PBS. The cells were fixed with 4% paraformaldehyde and cytospun onto glass slides. The control type II cells were incubated with mouse anti-CD44 antibodies and then fixed, followed by the incubation with the secondary antibodies (see below). For cholesterol depletion, the cells were treated with 3 mM MCD for 30 min, washed three times with MEM, and cross-linked as described above.
For the double-labeling with CD44 and SNARE or annexin A2 antibodies, the slides above were washed with PBS and then permeabilized with 0.5% Triton X-100 for 30 min. The fixed cells were incubated with 10% FBS and 1% BSA for blocking nonspecific binding. The cells were incubated with rabbit anti–annexin A2, syntaxin 2, and SNAP-23 antibodies at 1:100, 1:50, and 1:100 dilutions, respectively at 4°C overnight. The slides were washed and incubated with Alexa 488–conjugated anti-mouse antibodies (20 μg/ml, for control non–cross-linking cells only) and Alexa 546–conjugated anti-rabbit antibodies at 1:250 dilutions. The cells were examined with a Leica confocal laser scanning fluorescent microscope (Leica, Bannockburn, IL).
Protein Concentration Assay
Total protein concentration in cell lysates was determined by the Dc method (Bio-Rad), and the protein concentration in each fraction of sucrose gradients was measured by the Bradford assay (Bio-Rad).
MTT Assay
Overnight grown type II cells (1.5 ×106) in 35 mm dishes were used for the assay. The cells were treated with different concentrations of MCD for 30 min at 37°C. Next, cells were washed with 50 mM PBS and incubated with 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT, 0.5 mg/ml) for 2 h at 37°C. Later, formazan crystals were dissolved in 2 ml of dimethyl sulfoxide by shaking the dishes for 10 min. Absorbance was measured at 570 nm. The results were expressed as a percentage of the untreated control cells.
RESULTS
Identification of Raft Marker Proteins
Previous studies have reported the presence of lipid raft marker proteins in lung tissue (21, 22). However, it is unclear whether these markers exist in alveolar type II cells. RT-PCR was performed to assess the presence of mRNA of various lipid raft markers in these cells, including caveolin-1α, -1β, -2, and -3, and flotillin-1 and -2. Our results indicated that flotillin-1 and -2 mRNAs were expressed in both type II cells and lung tissue, whereas caveolin-1α, -1β, -2, and -3 mRNAs were only expressed in lung tissue, but not in type II cells (Figure 1A). Western blotting revealed that flotillin-1 and -2 proteins were expressed in freshly isolated type II cells (Figure 1B). However, flotillin-1 and -2 protein expression decreased when type II cells were cultured on plastic dishes, in which type II cells were known to trans-differentiate into the cells with some type I cell characteristics. Furthermore, both flotillin-1 and -2 were highly enriched in lamellar bodies and plasma membrane. They were also expressed in two lung epithelial cell lines: MLE-12 (mouse) and L2 (rat). Double-labeling with anti–SP-C (alveolar type II cell marker) and anti–flotillin-1 and -2 on rat lung tissue revealed that both flotillins were specifically localized in alveolar type II cells, but not in type I cells. The controls without primary antibodies did not show labeling (data not shown).
Figure 1.
Identification of lipid raft markers in alveolar type II cells. (A) RT-PCR. The mRNAs extracted from freshly isolated alveolar type II cells (> 96% purity, T2) and whole lung tissue (L) were reverse-transcribed and amplified for 35 cycles with gene specific primers against caveolin (Cav) 1α, 1β, 2, and 3, flotillin (Flot) 1 and 2, and 18S rRNA. PCR products were electrophoretically separated on an agarose gel. MW: 100-bp DNA ladder. (B) Western blot. Freshly isolated type II cells (D0), 3- or 7-d cultured type II cells on plastic dishes (D3 and D7), lung epithelial cell lines, MLE-12 and L2 cells, along with lung homogenate (LH), purified plasma membrane (PM), and lamellar bodies (LB), were either lysed and/or solubilized in SDS sample buffer. Equal amounts of total protein (20 μg) were separated by SDS-PAGE and immunoblotted using anti–flotillin-1 and -2 antibodies. (C) Immunohistochemistry. Lung tissue sections were permeabilized and blocked before being incubated with goat anti–SP-C and mouse anti–flotillin-1 and -2 antibodies. They were then incubated with donkey anti-goat Alexa 546–conjugated and rabbit anti-mouse Alexa 488–conjugated antibodies. The slides were observed for immunoflorescence. Upper panels: double labeling with anti–flotillin-1 and SP-C antibodies; lower panels: double labeling with anti–flotillin-2 and SP-C antibodies. Shown in the inset is the magnified image (×100) of a single type II cell. Scale bar: 50 μm.
SNARE Protein Association with Lipid Rafts
Lipid rafts are characterized by their resistance to nonionic detergent solubilization and floatation when subjected for a discontinuous sucrose gradient centrifugation. In addition, they are highly enriched in cholesterol. We solubilized type II cells in ice-cold 1% Triton X-100 and subjected the lysate to a gradient centrifugation. A white flocculent band was visible at the interface of 5 and 30% sucrose gradients. Seven fractions, collected from top to bottom (fractions 1–7), and the pellet (fraction 8), dissolved in lysis buffer, were analyzed for the contents of total proteins and cholesterol. Fraction 3 contained only 0.79 ± 0.15% of total protein, but 22.30 ± 4.76% of total cholesterol (Figures 2A and 2B). In contrast, soluble fractions (fractions 6 and 7) contained significant amount of total proteins (29.44 ± 2.74 and 38.94 ± 1.79%, respectively), but a relatively small amount of cholesterol (21.43 ± 1.1 and 12.56 ± 2.23% of total cholesterol, respectively). Each of the fractions was also screened for the presence of the lipid raft markers, flotillin-1 and –2, and the non–lipid raft marker, Na+-K+ ATPase. The results showed that fraction 3 was enriched in flotillin-1 and -2, whereas Na+-K+ ATPase was confined to the soluble fractions 6 and 7 (Figure 2C). Thus, fraction 3 was confirmed to be the detergent insoluble and cholesterol-rich raft fraction. We further quantified the data by calculating the enrichment. A ratio of the flotillin band density and total protein in fraction 3 was first obtained. Similarly, a ratio of flotillin band densities in all the fractions and total protein in all the fractions was obtained. The first value was divided with the second to arrive at enrichment for flotillin. The data revealed that flotillin-1 and -2 were enriched by 42.66- ± 2.00- and 28.76- ± 9.09-fold (n = 3) in the raft fraction. To study the effect of cholesterol sequestration on the lipid raft integrity, we depleted cholesterol from type II cells using saponin (0.5% wt/vol) and 0.5% TX-100 in lysis buffer according to a reported procedure (12). In this case, cholesterol content in fraction 3 was reduced by ∼ 75%. A major portion of flotillin-1 and -2 shifted from raft (fraction 3) to non-raft fraction (Figure 2C).
Figure 2.
Effect of cholesterol depletion on SNARE protein association with lipid rafts. (A, B) Isolation of lipid rafts from alveolar type II cells. Lipid rafts were isolated from freshly isolated type II cells based on their detergent insolubility in 1% Triton X-100 floatation on sucrose gradients. Seven fractions (1–7) were collected from top to bottom and the pellet solubilized in lysis buffer (fraction 8). Protein (A) and cholesterol (B) were measured in each of the fractions before and after cholesterol depletion (0.5% Triton X-100 and 0.5%, wt/vol saponin). The results were expressed as a percent of total protein and cholesterol, respectively. Data shown are means ± SE (n = 3). Diamonds, 1% Triton X-100; circles, 0.5% Triton X-100 and 0.5% saponin. (C) SNARE association with lipid rafts. Type II cells were lysed in either 1% Triton X-100 (control) or 0.5% Triton X-100 and 0.5% saponin (cholesterol depletion) and subjected to raft isolation. Seven fractions were collected from top to bottom. P, pellet. Percentages (5, 30, and 40%) represent sucrose gradient. Each fraction was examined by Western blot for the presence of the lipid raft marker proteins flotillin-1 and -2, the non–lipid raft protein marker Na+-K+ ATPase, various SNARE proteins, SNAP-23, syntaxin 2, and VAMP-2. The immunublots shown are representative of three independent experiments.
To examine the association of SNARE proteins with the lipid rafts of type II cells, an equal volume of each fraction was immunoblotted for the presence of major SNARE isoforms in type II cells: SNAP-23, syntaxin 2, and VAMP-2. Both t-SNAREs were present in the rafts, but SNAP-23 was highly enriched (24.12- ± 4.80-fold, n = 3) in lipid rafts in comparison with syntaxin 2 (11.80- ± 3.73-fold, n = 3) (Figure 2C). On the other hand, VAMP-2, a v-SNARE, was moderately enriched (17.73- ± 4.45-fold, n = 3) in lipid rafts. Other two SNARE regulatory proteins: α-SNAP and NSF were not found in the raft fraction of type II cells (data not shown). When type II cells were solubilized in 0.5% saponin and 0.5% TX-100, the amount of SNARE proteins associated with the raft fraction was markedly decreased. The results indicated a genuine association of SNARE proteins with the lipid rafts.
Effect of Cholesterol Depletion on Fusion Pore Formation
Having confirmed the association of SNARE proteins with the lipid rafts, we then questioned whether it has functional relevance to surfactant exocytosis in type II cells. To maintain the integrity of cell membrane and viability of the cells, MCD was used to deplete membrane cholesterol from type II cells. Cyclodextrins have been reported to specifically deplete membrane cholesterol (23). Incubation of type II cells with MCD for 30 min at 37°C resulted in a dose-dependent decrease of cholesterol in type II cells (Figure 3A). 3 mM MCD depleted 57 ± 6% of cholesterol without significantly affecting the cell viability (> 90%).
Figure 3.
Cholesterol depletion inhibits the fusion pore formation in type II cells. (A) Cholesterol concentration and viability: Overnight cultured type II cells were treated with different concentrations of methyl-β-cyclodextrin (MCD) and the total cholesterol concentration and cell viability were measured using the Amplex red cholesterol assay kit and MTT assay, respectively. The results were expressed as percentage of control. Data shown are means ± SE from three independent cell preparations. Squares, cholesterol; diamonds, cell viability. (B) Fusion pore formation. The overnight cultured cells were treated with 3 mM MCD or MCD-cholesterol complex (MCD-chol) and stimulated (sti) with secretagogues (0.1 mM ATP, 0.1 μM PMA, and 20 μM terbutaline) for 30 min. The fusion pore formation was monitored by FM1-43 fluorescence. Upper panels: FM1–43 staining; lower panels: phase contrast. (C) Quantification. The fusion pore formation was expressed as the number of the FM1–43–positive lamellar bodies per 50 cells. The results shown are means ± SE (n = 3 independent cell preparations). (D) Effect of cholesterol depletion on the quality of lamellar bodies. Overnight cultured type II cells were treated with 3 mM MCD. The number of lamellar bodies was monitored by staining the cells with antibodies against a lamellar body membrane protein, LB-180.
During exocytosis, surfactant-laden lamellar bodies fuse with the plasma membrane and release their contents via the fusion pore. The dynamics of surfactant release can be monitored for the formation of fusion pore using an amphiphilic dye, FM 1–43 (19, 24). The amphiphilic dye gains access to the lipids in the lamellar bodies through the fusion pore and stains the vesicular contents green. We sought to determine whether cholesterol depletion affects the fusion pore formation. Type II cells were pre-loaded for 10 min with FM1–43 (4 μM) and stimulated with a mixture of secretagogues. The formation of fusion pore was monitored by counting positively stained lamellar bodies. When the cells were cholesterol-depleted with 3 mM MCD, the fusion pore formation was completely inhibited. When the cells were treated with cholesterol-MCD complex, the fusion pore formation was unaffected (Figures 3B and 3C). The effect of cholesterol depletion on the fusion pore formation is not due to the decrease in the number of lamellar bodies caused by MCD, because MCD does not affect the number of lamellar bodies as determined by staining type II cells with antibodies against a lamellar body membrane protein, LB-180 (Figure 3D). The number of positively stained lamellar bodies in the control and cholesterol-depleted cells was 25.92 ± 1.42 and 24.56 ± 1.37 per cell, respectively.
Effect of Cholesterol Depletion on Membrane Fusion
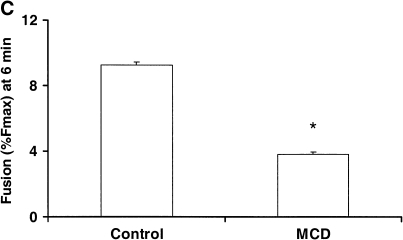
We have previously shown that annexin A2 promotes the fusion of lamellar bodies with the plasma membrane using an in vitro assay (7). Using this assay, we determined whether the depletion of cholesterol affects the membrane fusion. The incubation of the isolated plasma membrane with MCD resulted in a dose-dependent reduction of cholesterol in the plasma membrane preparation (Figure 4A). Three millimolars of MCD depleted ∼ 50% of cholesterol from the plasma membrane. Under this condition, annexin A2–mediated fusion of lamellar bodies with the cholesterol-depleted plasma membrane was significantly decreased (Figures 4B and 4C). The controls without the addition of annexin A2 did not show fusion for the untreated or cholesterol-depleted plasma membrane.
Figure 4.
Cholesterol depletion alters membrane fusion activity. (A) Plasma membranes isolated from lung tissue were treated with different concentrations of MCD for 30 min at 37°C. Cholesterol content was assayed and the results expressed as a percentage of control. Data shown are means ± SE (n = 3). (B, C) Five micrograms per milliliter of control or cholesterol-depleted plasma membranes (3 mM MCD) were mixed with 10 μg/ml of lamellar bodies in 1 ml of 1 mM Ca2+-EGTA buffer, and membrane fusion was initiated by addition of 10 μg annexin A2 tetramer. The basal fusion was without the addition of annexin A2 teramer. B illustrates fusion curve as a function of time and C the fusion content at 6 min after the addition of annexin A2 (means ± SE, n = 3). *P < 0.05 versus control.
Effect of Cholesterol Depletion on Surfactant Secretion in Type II Cells
We also examined the effects of cholesterol depletion on surfactant secretion. As shown in Figure 5, the secretion of lung surfactant was stimulated 4-fold by secretagogues. Three millimolars of MCD reduced the stimulated surfactant secretion by 69%. MCD also reduced the basal secretion, but did not reach a significant level.
Figure 5.
Effect of cholesterol depletion on surfactant secretion. Overnight cultured type II cells were treated with 3 mM MCD for 30 min and then incubated without (basal) and with 0.1 mM ATP, 0.1 μM PMA, and 20 μM terbutaline for 2 h (stimulated). Surfactant secretion was assayed. *P < 0.05 versus no MCD treatment (n = 3) Open bars, -MCD; shaded bars, +MCD.
Co-Localization of SNARE Proteins with Raft Clusters
The characteristic feature of rafts is their clustering upon cross-linking the raft-associated proteins. The clusters thus aggregate the different proteins associated with them and might represent the sites for protein interactions. CD44 is a transmembrane cell surface glycoprotein expressed by type II cells. We cross-linked CD44 proteins using anti-CD44 and secondary antibodies to determine whether SNARE proteins were recruited to raft clusters. Both control and cross-linked cells were counterstained with anti–syntaxin 2 or SNAP-23. In the control cells, CD44, syntaxin 2, and SNAP-23 stained the plasma membrane, and cholesterol depletion did not change the distribution of these proteins (Figures 6A and 6B). However, in the cross-linking cells, CD44 forms clusters or patches. Syntaxin 2 and SNAP-23 co-localized with the CD44 clusters, suggesting that syntaxin 2 and SNAP-23 may reside in the same lipid rafts. When type II cells were depleted of cholesterol with 3 mM MCD, CD44 cluster formation was decreased, indicating that it was a cholesterol-dependent process. Incomplete reduction in cluster formation was observed owing to a 50–60% decrease in cholesterol content under our conditions.
Figure 6.
SNARE proteins co-localize with raft clusters: Freshly isolated type II cells were depleted of cholesterol with 3 mM MCD. The control and depleted cells were incubated with anti-CD44 antibodies or anti-CD44 plus anti-mouse antibodies for 60 min, followed by fixation with 4% paraformaldehyde. Cells were double-labeled with anti-CD44 and anti-syntaxin 2 (A) or anti–SNAP-23 (B) antibodies. Images were taken with a confocal fluorescence microscope.
Annexin A2 and Raft Clustering
Our previous reports have indicated that annexin A2 plays an important role in surfactant secretion cells (6, 7). We investigated whether annexin A2 associated with the lipid rafts of type II cells. Since annexin A2 binds to phospholipids in a Ca2+-dependent manner, we included 200 μM Ca2+ in the lysis buffer and subjected the type II cell lystate to the raft isolation. We found that annexin A2 was enriched in the raft fraction by 38-fold in the presence of Ca2+ (Figure 7A). There was no association of annexin A2 with lipid raft in the absence of Ca2+. We further cross-linked CD44 proteins to see whether annexin A2 was recruited to rafts clusters. In the control cells, annexin A2 stained the plasma membrane (Figure 7B). Cholesterol depletion eliminated annexin A2 staining. In the cross-linking cells, a co-localization of annexin A2 with CD44 in the patches was evident, suggesting that annexin A2 was recruited to the raft clusters at the cytosolic side. The clusters were reduced by the treatment of the cells with MCD.
Figure 7.
Annexin A2 co-localizes with the lipid raft clusters. (A) Association of annexin A2 with the lipid rafts. Freshly isolated type II cells were lysed with 1% Triton X-100 in the presence or absence of 200 μM Ca2+, and separated on sucrose gradients. Seven fractions were collected from top to bottom. P, pellet. Percentages (5, 30, and 40%) represent sucrose gradient. Each fraction was immunoblotted for annexin A2. (B) Raft clustering. Freshly isolated type II cells were incubated with anti-CD44 antibodies or anti-CD44 plus anti-mouse antibodies for 60 min, followed by fixation with 4% formaldehyde. Cells were double-labeled with anti-CD44 and anti-annexin A2 antibodies. Images were taken with a confocal fluorescence microscope.
DISCUSSION
Exocytosis is an orderly process involving the interaction of highly conserved and diverse classes of proteins, resulting in the fusion between two opposing membranes. Lung surfactant secretion by type II cells is a highly regulated. SNAREs and annexin A2 have been implicated in surfactant secretion. Our present studies demonstrated that SNARE proteins were associated with the lipid rafts of type II cells and that lipid raft integrity was essential for this association as well as fusion pore formation, membrane fusion, and lung surfactant secretion. The cross-linking of a transmembrane glycoprotein, CD44, using antibodies caused raft clustering and the recruitment of SNAREs and annexin A2 into these raft clusters in a cholesterol-dependent manner. These results suggested that lipid rafts may organize the exocytotic proteins on the membrane, and that raft clusters may provide the docking and fusion sites for exocytosis.
Earlier studies have shown that caveolin and flotillins were highly expressed in lung tissue (21, 22, 25, 26). Caveolin-1 protein was detected in both alveolar epithelial type I and type II cells, but distinct plasma membrane invaginations were not observed in type II cells (25–27). However, we were not able to detect caveolins-1 to -4 in the freshly isolated type II cells by RT-PCR, although their mRNA expressions were observed in lung tissue under the same conditions. The reasons for the contrary results are not clear. Both mRNA and protein expression of flotillin-1 and -2 were present in type II cells. Immunohistochemistry confirmed their specific localization in type II cells, but not type I cells. Flotillin-1 and -2 protein expression in the type II cells cultured on plastic dishes was decreased. A similar expression pattern for flotillin was reported when PC12 cells were cultured in vitro (28). Expression pattern of flotillins and caveolins in type I and II cells might reflect the specific roles of these proteins in different cells, although there are a number of cells expressing both caveolins and flotillins, such as skate erythrocytes (29) and neonatal rat cardiomyocytes (30). In addition, flotillin-1 and -2 were enriched on the plasma membrane and lamellar bodies, indicating their possible roles in membrane trafficking.
Lipid rafts offer an interacting platform for many proteins involved in signal transduction and membrane trafficking, apart from being involved in protein sorting in a number of cells. Based on cellular fractionation and biochemical analysis, SNARE proteins were observed to be enriched in the lipid rafts of alveolar type II cells. This is also supported by the co-localization of SNAP-23 and syntaxin 2 with raft clusters upon cross-linking of a raft-associated cell surface protein CD44. The extent of enrichment for SNAP-23 was higher than its cognate t-SNARE partner, syntaxin 2, and v-SNARE protein, VAMP-2. Similar results were observed in other cells (12, 14). The enrichment of SNAP-23 might be attributed to the palmitoylation at multiple cysteine residues. Most recently, it has been reported that SNAP-23 was enriched to a higher extent than SNAP-25 in PC12 cells, due to the substitution of phenylalanine residue in SNAP-25 with a cysteine residue in SNAP-23 (31). However, the mechanism for the association of syntaxin 2/VAMP-2 with lipid rafts of type II cells is unknown. Most syntaxin isoforms were associated with lipid rafts to various degrees depending on cell type and isoform (12, 32, 33). However, one report showed that syntaxin 2 was excluded from the lipid rafts in mast cells (14). When reconstituted into simple artificial membrane (liposome)-containing phospholipids, cholesterol and spingomyelin, syntaxin 1A and VAMP-2 preferred liquid-disordered domains (non–raft phase) (15), even though syntaxin 1A was able to bind to cholesterol (13). Although role of other lipid factors, such as phosphatidylinositol-4,5-bisphosphate in the targeting of syntaxin to lipid rafts cannot be ruled out (34), it appeared that additional intrinsic factors and/or protein–protein interactions were required for the association of syntaxin and VAMP with lipid rafts in biological membranes. For example, SNAP-23 might bring syntaxin 2 into the rafts via the formation of a heterodimer, since syntaxin and SNAP-23/25 were observed to form dimers, which may act as a docking site for VAMP-2, ultimately leading to the formation of a ternary SNARE complex (35). Also, the cleavage of SNAP-25 by botulinum neurotoxin E disrupted syntaxin 1-SNAP-25 dimer and its co-localization in defined clusters (36). There was a significant amount of SNAREs present in nonraft fraction. Since lipid rafts are dynamic structures, it is likely that SNAREs can move in and out of lipid rafts, depending on the cell status and the physiologic response.
When type II cells were treated with MCD, cellular cholesterol content was reduced to ∼ 50–60%. Under the depleted conditions, the secretagogue-stimulated surfactant secretion decreased by ∼ 70%. Since MCD cannot enter the cells (23), it might deplete cholesterol on the plasma membrane without affecting intracellular membrane cholesterol. Thus, the actual reduction of cholesterol on the plasma membrane may be higher than 50%, which may account for a higher inhibition of surfactant secretion. The reduced secretion was probably not due to the decreased surfactant synthesis owing to the inability of MCD to permeate the cells or due to the depletion of lamellar bodies, because MCD does not affect the number of lamellar bodies. Since SNARE proteins are enriched in lipid rafts and the lipid raft integrity depends on cholesterol, the reduced surfactant secretion by MCD is likely due to the disruption of lipid raft integrity and thus disassembly of interacting platforms, provided by lipid rafts that are necessary for the SNARE complex formation.
The size of lipid rafts is small (< 70 nm). Although cross-linking and fluorescence energy transfer (FRET) revealed their existence in living cells (37, 38), lipid rafts are too small to function as docking and fusion sites. Individual rafts contain only a few numbers of proteins for protein–protein interactions to occur. However, lipid rafts might aggregate or form clusters and act as the possible sites of exocytosis. Indeed, we observed that syntaxin 2 and SNAP-23 were co-localized with CD44 clusters. The mechanisms of lipid raft cluster formation in the cells are, however, unclear. One of the candidates that might play a role in this process is annexin A2. It was associated with lipid rafts in type II cells (Figure 7) and other cells (39) in a Ca2+-dependent manner. The N-terminus of annexin A2 is required for the association (40). The Ca2+-independent binding of annexin II to the membrane has also been reported (41), and this binding appears to depend on cholesterol (42, 43). Similar to syntaxin 2 and SNAP-23, annexin A2 was recruited to raft clusters upon the cross-linking of CD44 (Figure 7), suggesting role of annexin A2 in raft clustering and, thus, in the creation of docking and fusion sites in secretory cells. This conclusion is further supported by the following observations: (1) the infection of HeLa cells with enteropathogenic Escherichia coli induced actin-rich pedestals, clustering of raft clusters, and recruitment of annexin A2 to such clusters (44); (2) a trans-dominant mutant of annexin A2 comprising the N-terminal domain of annexin A2 and the N-terminus of p11 lead to the formation of large annexin A2 aggregates and co-clustering of the raft-associated protein, CD44, with the aggregates (20); and (3) annexin A2 was found to be associated with nicotine-induced lipid rafts in chromaffin cells (45).
In summary, lipid rafts in type II cells may organize the SNARE protein machinery, and their integrity is crucial for the fusion of lamellar bodies with the plasma membrane.
Acknowledgments
The authors thank Dr. Nick Cross for stimulating discussion and Ms. Candice Marsh for secretarial assistance.
This work was supported by NIH R01 HL-052146 and R01 HL-071628 (L.L.).
Originally Published in Press as DOI: 10.1165/rcmb.2005-0418OC on January 26, 2006
Conflict of Interest Statement: None of the authors has a financial relationship with a commercial entity that has an interest in the subject of this manuscript.
References
- 1.Sutton RB, Fasshauer D, Jahn R, Brunger AT. Crystal structure of a SNARE complex involved in synaptic exocytosis at 2.4 A resolution. Nature 1998;395:347–353. [DOI] [PubMed] [Google Scholar]
- 2.Weber T, Zemelman BV, McNew JA, Westermann B, Gmachl M, Parlati F, Sollner TH, Rothman JE. SNAREpins: minimal machinery for membrane fusion. Cell 1998;92:759–772. [DOI] [PubMed] [Google Scholar]
- 3.Han X, Wang CT, Bai J, Chapman ER, Jackson MB. Transmembrane segments of syntaxin line the fusion pore of Ca2+-triggered exocytosis. Science 2004;304:289–292. [DOI] [PubMed] [Google Scholar]
- 4.Abonyo BO, Wang P, Narasaraju TA, Rowan WH III, McMillan DH, Zimmerman UJ, Liu L. Characterization of alpha-soluble N-ethylmaleimide-sensitive fusion attachment protein in alveolar type II cells: implications in lung surfactant secretion. Am J Respir Cell Mol Biol 2003;29:273–282. [DOI] [PubMed] [Google Scholar]
- 5.Abonyo BO, Gou D, Wang P, Narasaraju T, Wang Z, Liu L. Syntaxin 2 and SNAP-23 are required for regulated surfactant secretion. Biochemistry 2004;43:3499–3506. [DOI] [PubMed] [Google Scholar]
- 6.Liu L, Wang M, Fisher AB, Zimmerman UJP. Involvement of annexin II in exocytosis of lamellar bodies from alveolar epithelial type II cells. Am J Physiol 1996;270:L668–L676. [DOI] [PubMed] [Google Scholar]
- 7.Chattopadhyay S, Sun P, Wang P, Abonyo B, Cross NL, Liu L. Fusion of lamellar body with plasma membrane is driven by the dual action of annexin II tetramer and arachidonic acid. J Biol Chem 2003;278:39675–39683. [DOI] [PubMed] [Google Scholar]
- 8.Gou D, Wang P, Jin N, Liu L. Silencing of annexin II in primary culture of alveolar epithelial type II cells by RNA interference. Annexins 2004;1:31–38. [Google Scholar]
- 9.Rajendran L, Simons K. Lipid rafts and membrane dynamics. J Cell Sci 2005;118:1099–1102. [DOI] [PubMed] [Google Scholar]
- 10.Brown DA, London E. Functions of lipid rafts in biological membranes. Annu Rev Cell Dev Biol 1998;14:111–136. [DOI] [PubMed] [Google Scholar]
- 11.Lafont F, Verkade P, Galli T, Wimmer C, Louvard D, Simons K. Raft association of SNAP receptors acting in apical trafficking in Madin-Darby canine kidney cells. Proc Natl Acad Sci USA 1999;96:3734–3738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chamberlain LH, Burgoyne RD, Gould GW. SNARE proteins are highly enriched in lipid rafts in PC12 cells: implications for the spatial control of exocytosis. Proc Natl Acad Sci USA 2001;98:5619–5624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lang T, Bruns D, Wenzel D, Riedel D, Holroyd P, Thiele C, Jahn R. SNAREs are concentrated in cholesterol-dependent clusters that define docking and fusion sites for exocytosis. EMBO J 2001;20:2202–2213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pombo I, Rivera J, Blank U. Munc18–2/syntaxin3 complexes are spatially separated from syntaxin3-containing SNARE complexes. FEBS Lett 2003;550:144–148. [DOI] [PubMed] [Google Scholar]
- 15.Bacia K, Schuette CG, Kahya N, Jahn R, Schwille P. SNAREs prefer liquid-disordered over “raft” (liquid-ordered) domains when reconstituted into giant unilamellar vesicles. J Biol Chem 2004;279:37951–37955. [DOI] [PubMed] [Google Scholar]
- 16.Dobbs LG, Gonzalez R, Williams MC. An improved method for isolating type II cells in high yield and purity. Am Rev Respir Dis 1986;134:141–145. [DOI] [PubMed] [Google Scholar]
- 17.Chen JW, Chen Z, Narasaraju T, Jin N, Liu L. Isolation of hightly pure alveolar epithelial type I and type II cells from rat lungs. Lab Invest 2004;84:727–735. [DOI] [PubMed] [Google Scholar]
- 18.Narasaraju TA, Jin N, Narendranath CR, Chen Z, Gou D, Liu L. Protein nitration in rat lungs during hyperoxia exposure: a possible role of myeloperoxidase. Am J Physiol Lung Cell Mol Physiol 2003;285:L1037–L1045. [DOI] [PubMed] [Google Scholar]
- 19.Bates SR, Tao JQ, Notarfrancesco K, DeBolt K, Shuman H, Fisher AB. Effect of surfactant protein-A on granular pneumocyte surfactant secretion in vitro. Am J Physiol Lung Cell Mol Physiol 2003;285:L1055–L1065. [DOI] [PubMed] [Google Scholar]
- 20.Oliferenko S, Paiha K, Harder T, Gerke V, Schwarzler C, Schwarz H, Beug H, Gunthert U, Huber LA. Analysis of CD44-containing lipid rafts: Recruitment of annexin II and stabilization by the actin cytoskeleton. J Cell Biol 1999;146:843–854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nanjundan M, Possmayer F. Pulmonary lipid phosphate phosphohydrolase in plasma membrane signalling platforms. Biochem J 2001;358:637–646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Palestini P, Calvi C, Conforti E, Daffara R, Botto L, Miserocchi G. Compositional changes in lipid microdomains of air-blood barrier plasma membranes in pulmonary interstitial edema. J Appl Physiol 2003;95:1446–1452. [DOI] [PubMed] [Google Scholar]
- 23.Neufeld EB, Cooney AM, Pitha J, Dawidowicz EA, Dwyer NK, Pentchev PG, Blanchette-Mackie EJ. Intracellular trafficking of cholesterol monitored with a cyclodextrin. J Biol Chem 1996;271:21604–21613. [DOI] [PubMed] [Google Scholar]
- 24.Haller T, Ortmayr J, Friedrich F, Volkl H, Dietl P. Dynamics of surfactant release in alveolar type II cells. Proc Natl Acad Sci USA 1998;95:1579–1584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Campbell L, Hollins AJ, Al-Eid A, Newman GR, von Ruhland C, Gumbleton M. Caveolin-1 expression and caveolae biogenesis during cell transdifferentiation in lung alveolar epithelial primary cultures. Biochem Biophys Res Commun 1999;262:744–751. [DOI] [PubMed] [Google Scholar]
- 26.Newman GR, Campbell L, von Ruhland C, Jasani B, Gumbleton M. Caveolin and its cellular and subcellular immunolocalisation in lung alveolar epithelium: implications for alveolar epithelial type I cell function. Cell Tissue Res 1999;295:111–120. [DOI] [PubMed] [Google Scholar]
- 27.Kolleck I, Guthmann F, Ladhoff AM, Tandon NN, Schlame M, Rustow B. Cellular cholesterol stimulates acute uptake of palmitate by redistribution of fatty acid translocase in type II pneumocytes. Biochemistry 2002;41:6369–6375. [DOI] [PubMed] [Google Scholar]
- 28.Volonte D, Galbiati F, Li S, Nishiyama K, Okamoto T, Lisanti MP. Flotillins/cavatellins are differentially expressed in cells and tissues and form a hetero-oligomeric complex with caveolins in vivo. Characterization and epitope-mapping of a novel flotillin-1 monoclonal antibody probe. J Biol Chem 1999;274:12702–12709. [DOI] [PubMed] [Google Scholar]
- 29.Musch MW, Koomoa DL, Goldstein L. Hypotonicity-induced exocytosis of the skate anion exchanger skAE1: role of lipid raft regions. J Biol Chem 2004;279:39447–39453. [DOI] [PubMed] [Google Scholar]
- 30.Rybin VO, Grabham PW, Elouardighi H, Steinberg SF. Caveolae-associated proteins in cardiomyocytes: caveolin-2 expression and interactions with caveolin-3. Am J Physiol Heart Circ Physiol 2003;285:H325–H332. [DOI] [PubMed] [Google Scholar]
- 31.Salaun C, Gould GW, Chamberlain LH. The SNARE proteins SNAP-25 and SNAP-23 display different affinities for lipid rafts in PC12 cells: regulation by distinct cysteine-rich domains. J Biol Chem 2005;280:1236–1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gil C, Soler-Jover A, Blasi J, Aguilera J. Synaptic proteins and SNARE complexes are localized in lipid rafts from rat brain synaptosomes. Biochem Biophys Res Commun 2005;329:117–124. [DOI] [PubMed] [Google Scholar]
- 33.Xia F, Gao X, Kwan E, Lam PP, Chan L, Sy K, Sheu L, Wheeler MB, Gaisano HY, Tsushima RG. Disruption of pancreatic beta-cell lipid rafts modifies Kv2.1 channel gating and insulin exocytosis. J Biol Chem 2004;279:24685–24691. [DOI] [PubMed] [Google Scholar]
- 34.Aoyagi K, Sugaya T, Umeda M, Yamamoto S, Terakawa S, Takahashi M. The activation of exocytotic sites by the formation of phosphatidylinositol-4,5-bisphosphate microdomains at syntaxin clusters. J Biol Chem 2005;280:17346–17352. [DOI] [PubMed] [Google Scholar]
- 35.Hu K, Carroll J, Fedorovich S, Rickman C, Sukhodub A, Davletov B. Vesicular restriction of synaptobrevin suggests a role for calcium in membrane fusion. Nature 2002;415:646–650. [DOI] [PubMed] [Google Scholar]
- 36.Rickman C, Meunier FA, Binz T, Davletov B. High affinity interaction of syntaxin and SNAP-25 on the plasma membrane is abolished by botulinum toxin E. J Biol Chem 2004;279:644–651. [DOI] [PubMed] [Google Scholar]
- 37.Friedrichson T, Kurzchalia TV. Microdomains of GPI-anchored proteins in living cells revealed by crosslinking. Nature 1998;394:802–805. [DOI] [PubMed] [Google Scholar]
- 38.Varma R, Mayor S. GPI-anchored proteins are organized in submicron domains at the cell surface. Nature 1998;394:798–801. [DOI] [PubMed] [Google Scholar]
- 39.Babiychuk EB, Draeger A. Annexins in cell membrane dynamics. Ca(2+)-regulated association of lipid microdomains. J Cell Biol 2000;150:1113–1124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Babiychuk EB, Monastyrskaya K, Burkhard FC, Wray S, Draeger A. Modulating signaling events in smooth muscle: cleavage of annexin 2 abolishes its binding to lipid rafts. FASEB J 2002;16:1177–1184. [DOI] [PubMed] [Google Scholar]
- 41.Liu L, Tao JQ, Zimmerman UJP. Annexin II binds to the membrane of A549 cells in a calcium-dependent and calcium-independent manner. Cell Signal 1997;9:299–364. [DOI] [PubMed] [Google Scholar]
- 42.Harder T, Kellner R, Parton RG, Gruenberg J. Specific release of membrane-bound annexin II and cortical cytoskeletal elements by sequestration of membrane cholesterol. Mol Biol Cell 1997;8:533–545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ayala-Sanmartin J, Henry JP, Pradel LA. Cholesterol regulates membrane binding and aggregation by annexin 2 at submicromolar Ca(2+) concentration. Biochim Biophys Acta 2001;1510:18–28. [DOI] [PubMed] [Google Scholar]
- 44.Zobiack N, Rescher U, Laarmann S, Michgehl S, Schmidt MA, Gerke V. Cell-surface attachment of pedestal-forming enteropathogenic E. coli induces a clustering of raft components and a recruitment of annexin 2. J Cell Sci 2002;115:91–98. [DOI] [PubMed] [Google Scholar]
- 45.Chasserot-Golaz S, Vitale N, Umbrecht-Jenck E, Knight D, Gerke V, Bader MF. Annexin 2 promotes the formation of lipid microdomains required for calcium-regulated exocytosis of dense-core vesicles. Mol Biol Cell 2005;16:1108–1119. [DOI] [PMC free article] [PubMed] [Google Scholar]