Abstract
Granulocyte macrophage colony-stimulating factor (GM-CSF) stimulates survival, proliferation, differentiation, and function of myeloid cells. Recently, GM-CSF has been shown to be important for normal pulmonary homeostasis. We report that GM-CSF is induced in lung leukocytes during infection with Gram-negative bacteria. Therefore, we postulated that deficiencies in GM-CSF would increase susceptibility to Gram-negative infection in vivo. After an intratracheal inoculum with Pseudomonas aeruginosa, GM-CSF−/− mice show decreased survival compared with wild-type mice. GM-CSF−/− mice show increased lung, spleen, and blood bacterial CFU. GM-CSF−/− mice are defective in the production of cysteinyl leukotrienes, prostaglandin E2, macrophage inflammatory protein, and keratinocyte-derived chemokine in lung leukocytes postinfection. Despite these defects, inflammatory cell recruitment is not diminished at 6 or 24 h postinfection, and the functional activity of polymorphonuclear leukocytes from the lung and peritoneum against P. aeruginosa is enhanced in GM-CSF−/− mice. In contrast, alveolar macrophage (AM) phagocytosis, killing, and H2O2 production are defective in GM-CSF−/− mice. Although the absence of GM-CSF has profound effects on AMs, peritoneal macrophages seem to have normal bactericidal activities in GM-CSF−/− mice. Defects in AM function may be related to diminished levels of IFN-γ and TNF-α postinfection. Thus, GM-CSF−/− mice are more susceptible to lung infection with P. aeruginosa as a result of impaired AM function.
Keywords: innate immunity, macrophage, neutrophil, bacterial infection
Pseudomonas aeruginosa is a versatile Gram-negative opportunistic pathogen that can be found in soil, water, and vegetation or isolated from the skin, throat, and stool of healthy persons (1). P. aeruginosa is found in 4–12% of healthy individuals, whereas 40–50% of hospitalized patients may be carriers (2). This bacterium is commonly spread from patient to patient via contact with fomites or by ingestion of contaminated food and water. There are two groups of patients that are susceptible to P. aeruginosa infections. The first group includes patients with functional immune defects, such as those who have recently undergone bone marrow transplant (3), HIV-infected persons (4), or patients with cancer (5). The second group includes patients with structural defects or impaired local defenses, such as cystic fibrosis (6) or burns (7). P. aeruginosa infections are responsible for significant numbers of nosocomial and community-acquired episodes of bacteremia with high morbidity and mortality rates (4).
For a host to clear P. aeruginosa from the lung, a functional innate immune system, particularly macrophages and polymorphonuclear leukocytes (PMNs), must be present (8, 9). Granulocyte macrophage colony-stimulating factor (GM-CSF) is a hematopoietic growth factor that is able to stimulate in vitro survival, proliferation, differentiation, and function of myeloid cells and their precursors, particularly PMNs, eosinophils, granulocytes, and monocyte/macrophages (10). GM-CSF is expressed by a variety of pulmonary cells, including activated T cells, macrophages, fibroblasts, and epithelial cells (11, 12). GM-CSF plays a critical role in surfactant homeostasis (13) and in stimulating the terminal differentiation of alveolar macrophages (AMs) through induction of the transcription factor PU.1 (14, 15). The function of GM-CSF in vivo has been examined through the use of gene knock-out mice. GM-CSF−/− mice have increased numbers of leukocytes in the lung (16, 17); however, these mice are more susceptible to Plasmodium chabaud AS infections [blood-stage malaria (18)], Group B Streptococcus (17), and Pneumocystis carinii (11). AMs from GM-CSF−/− mice demonstrate defective uptake of FITC-labeled microbeads (16) and Escherichia coli in vitro (14). GM-CSF−/− mice have been documented to produce decreased levels of TNF-α (3, 16) and less IFN-γ (15). Additionally, GM-CSF−/− mice display dysregulated eicosanoid synthesis, which may influence host defense function (19–21). The phenotype of GM-CSF−/− mice in response to Gram-negative infections in vivo has not been examined. Furthermore, PMN function in GM-CSF−/− mice is unexplored. The current study was undertaken to explore the role of GM-CSF in regulating the innate immune response to P. aeruginosa in vivo.
MATERIALS AND METHODS
Animals
GM-CSF−/− mice were generated by Dranoff and colleagues (22) and back-crossed eight generations against C57BL/6 mice. All of the GM-CSF−/− mice were used by 6 mo of age, before developing noticeable pathology associated with pulmonary alveolar proteinosis (22). Bitransgenic mice, in which GM-CSF is expressed exclusively in the lung, were generated in GM-CSF−/− mice by transgenic expression of a chimeric gene containing GM-CSF under the control of the surfactant protein C (SPC) promoter (SPC-GM mice) (23). The specificity of the SPC promoter results in targeted expression of GM-CSF by type II alveolar epithelial cells in these SPC-GM mice. The SPC-GM mice were a generous gift from Jeff Whitsett. The mice were bred in the University Laboratory Animal Medicine facilities under specific pathogen-free conditions at the University of Michigan. C57BL/6 mice (wild type [WT]) were obtained from Jackson Laboratory (Bar Harbor, ME). The University of Michigan Committee on the Use and Care of Animals approved these experiments.
Intratracheal Infection with P. aeruginosa
P. aeruginosa PAO1 stock was grown as previously described (3). Briefly, a 1:1,000 dilution of P. aeruginosa PAO1 stock was grown in tryptic soy broth (TSB) (DIFCO, Detroit, MI) for 18 h at 37°C. Bacterial concentration was determined by measuring absorbance at 600 nm compared with a predetermined standard curve. Bacteria were diluted to the desired concentration for inoculation. Animals were anesthetized by intraperitoneal administration of 200 μl of a sterile solution of ketamine (1 ml of a 100 mg/ml solution), xylazine (0.33 ml of a 20 mg/ml solution), and saline (8.67 ml). The trachea was exposed in sterile fashion, and 30 μl of P. aeruginosa inoculum (or saline as a control) was administered intratracheally using a sterile 26-gauge needle. The skin edges were carefully re-apposed with surgical glue (Nexaband; Abbot Laboratories, Chicago, IL). The P. aeruginosa inoculum was plated on blood agar plates in serial 10-fold dilutions and incubated at 37°C. Bacterial colonies were counted after 24 h to confirm the actual dose administered.
Quantification of Bacterial Burden in Lung and Blood
After an intratracheal inoculum with P. aeruginosa, mice were killed after 6 or 24 h. The thoracic cavity was exposed, and blood was collected by puncture of the right ventricle using syringes pre-lubricated with 50 μl of 1,000 U/ml heparin. After blood collection, the pulmonary circulation was perfused with saline before the lungs and the spleen were excised. The lungs and spleen were suspended in 1 ml normal saline and homogenized. Then, 10 μl of each specimen (lung, spleen, and blood) was plated on blood agar plates using serial 10-fold dilutions and incubated at 37°C. Bacterial colonies were counted at 24 h after intratracheal inoculum as CFU/ml of blood or CFU/whole lung or spleen.
Pulmonary Leukocyte Isolation
Six or 24 h after intratracheal inoculation of P. aeruginosa or saline, mice were killed, and the lung leukocytes were obtained as previously described (3). Briefly, the pulmonary circulation was perfused with PBS. Lungs were harvested and minced to a slurry, and each specimen was suspended in a digest solution containing collagenase (15 mg), DNase I (0.25 ml or 250 KU units), and complete media (CM) (Dulbecco's modified Eagle's medium [DMEM], 1% penicillin-streptomycin, 1% L-glutamine, 10% fetal calf serum, and 0.1% Fungizone). The suspension was incubated on a rocker for 30 min at 37°C. The cells were dispersed in solution by repetitive suction and centrifuged at 500 × g for 10 min. After the supernatant was decanted, each pellet was briefly resuspended in cold NH4Cl red blood cell lysing solution, neutralized by adding 10 ml of CM, and centrifuged. Cell pellets were resuspended in 5 ml of plain media (DMEM, 1% penicillin-streptomycin, 1% L-glutamine). The samples were dispersed in similar fashion as described previously and passed through a NITEX nylon screen (#103–100/32; Sefar America, Kansas City, MO), which was rinsed with an additional 5 ml CM to obtain a single-cell suspension. Ten milliliters of 40% percoll (Sigma, St. Louis, MO) was added to the cell suspension, and the solution was centrifuged at 2,000 × g for 20 min. The supernatant was gently decanted, and the soft pellet of cells were resuspended in 3 ml of CM. Cells were counted on a hemocytometer.
Differential Staining
Cytospins of lung leukocytes were made by centrifuging 50,000 cells onto microscope slides using a Shandon Cytospin 3 (Astmoore, England). The slides were allowed to air dry and were stained using a modified Wright-Giemsa (WG) stain. For WG staining, the slides were fixed/pre-stained for 2 min with a one-step, methanol-based WG stain (Harleco; EM Diagnostics, Gibbstown, NJ). Slides were rinsed in water and dipped eight times in Diff-Quick Solution I followed by five dips in Diff-Quick Solution II (Baxter Scientific, Miami, FL). Slides were rinsed in water and allowed to air dry. A total of 200 cells were counted from randomly chosen high-power microscope fields for each sample. The differential percentage was multiplied by the total leukocyte number to derive the absolute number of monocyte/macrophages, PMNs, lymphocytes, and eosinophils per sample.
Harvesting AMs and Lung PMNs
Resident AMs from mice were obtained via ex vivo lung lavage following a previously described protocol (24). These cells were collected in lavage fluid (containing CM and 5 μM EDTA), spun at 1,000 × g for 10 min, and resuspended in serum-free media (SFM). The cells were counted on a hemocytometer. AMs were plated in 96-well plates and allowed to adhere in SFM for 1 h. The SFM was removed, and CM was added. AMs were cultured in CM at 37°C overnight before being assayed. Purity was > 95% by differential analysis.
Elicited lung PMNs were obtained by bronchoalveolar lavage (BAL) 20 h after intratracheal injection with 25 μg of LPS derived from P. aeruginosa (Sigma). At this point, the percentage of PMNs in the lavage ranged from 88–94% as determined by differential staining. PMNs were collected by centrifugation, washed twice, plated in 96-well plates, allowed to adhere for 30 min in SFM, and immediately used for analysis.
Elicitation of Peritoneal Macrophages and PMNs
For elicited peritoneal macrophages (PMs), the mice were subjected to peritoneal lavage 4 d after injection with 2 ml of 4% glycogen (Sigma) dissolved in PBS. Purity of the PM population ranged from 80–89% monocyte/macrophages at this point in separate experiments. PMs were counted on a hemocytometer. PMs were plated in 96-well plates and allowed to adhere in SFM for 1 h. The SFM was removed, and CM was added. PMs were cultured in CM at 37°C overnight before being assayed.
To isolate peritoneal PMNs, mice were given an intraperitoneal injection of 2.5 ml of 4% glycogen. Five hours later, the peritoneal cavity was lavaged twice with 7–9 ml of PBS, and cells were collected by centrifugation. The percentage of PMNs in the lavage was determined by differential staining analysis (the percentage ranged from 70–80% in separate experiments). PMNs were plated in 96-well plates and allowed to adhere for 30 min in SFM and immediately used for analysis.
In Vitro Phagocytosis Assay
AMs and PMNs were harvested as previously described, and the ability of these cells to phagocytose unopsonized bacteria was examined using fluorescently labeled E. coli bioparticles (Vybrant phagocytosis assay; Molecular Probes, Eugene, OR), which was modified slightly from a previously described method (3). Briefly, AMs or PMNs were plated at a density of 2 × 105 cells/well in a half-area, black, 96-well plate. At the time of assay, the supernatant was removed, and 50 μl of FITC-labeled E. coli was added to each well (which corresponds to a ratio of bacteria to phagocytes of 60:1) and incubated for 2 h at 37°C. After the incubation, fluorescence of the extracellular FITC-labeled E. coli was quenched by the addition of 50 μl of trypan blue. Intracellular bacteria were quantitated by fluorometry (3).
Tetrazolium Dye Reduction Assay of Bacterial Killing
The ability of AMs and PMNs from WT and GM-CSF−/− mice to kill P. aeruginosa was quantified using a tetrazolium dye reduction assay as described elsewhere (25, 26). Briefly, 2 × 105 AMs or PMNs from WT and GM-CSF−/− mice were aliquoted into duplicate 96-well plates: an experimental (37°C) plate and a control (4°C) plate. To maximize ingestion of bacteria for the killing assay in macrophages, P. aeruginosa was opsonized with 4% anti–P. aeruginosa mouse-derived immune serum as previously described (27). Cells from both plates were infected with 100 μl of opsonized P. aeruginosa (2 × 108 CFU/ml; multiplicity of infection, 50:1) for 30 min at 37°C to allow phagocytosis to occur. The bacterial killing protocol was assessed as described previously (26). Briefly, the cells on the experimental plate were washed with PBS and incubated at 37°C for 90 min. At the same time, the cells on the control plate were washed with PBS, lysed with TSB and 0.5% saponin (Sigma), and stored at 4°C. After 90 min, the cells from experimental plate were lysed with TSB and 0.5% saponin. Both plates were incubated at 37°C for 2.5 h to allow unkilled intracellular bacteria to expand. MTT (3-(4, 5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) (5 mg/ml) (Sigma) was added to each plate and incubated for 30 min at 37°C. The amount of formazan product produced in the reaction is proportional to the number of bacteria present. Solubilization solution (isopropanol/0.1N HCL + 1% triton X-100) was added to dissolve formazan salts, and the absorbance was read at 595 nm. Results were expressed as percentage of survival of ingested bacteria, where the survival of ingested bacteria = (A595 control plate/A595 experimental plate) × 100%. Intracellular lactate dehydrogenase (LDH) levels were used to normalize for differences in the adherence of cells from WT and GM-CSF−/− mice to tissue culture plates (see below).
H2O2 Detection Assay
Cellular H2O2 secretion from AMs and PMNs was measured colormetrically from WT and GM-CSF−/− mice as previously described (25) using Amplex Red reagent according to the manufacturer's instructions (Molecular Probes). Briefly, cells were plated in 96-well plates, and 100 μl of Amplex Red solution (50 μM of Amplex Red reagent and 10 U/ml HRP into PBS) was added to each well for 45 min at 37°C. The H2O2 production was measured by taking the absorbance at A560 and comparing it with a standard curve with known H2O2 concentrations. To account for differences in cellular adhesion to tissue culture plates between WT and GM-CSF−/− mice, data were normalized to total intracellular LDH levels (see below).
LDH Assay to Normalize for Cellular Adherence
We have observed differences in the adherence of leukocytes from GM-CSF−/− and WT mice to tissue culture plates that could potentially confound experimental results (16). To correct for such differences, we quantified total intracellular levels of the housekeeping protein LDH using a commercially available assay (Roche, Indianapolis, IN). Assays were preformed in parallel on AMs and PMNs in each experiment, and results were used for normalization purposes as described previously. Briefly, cells were plated as in each experiment, incubated as appropriate, and washed twice with PBS and lysed with lysis solution (Triton X-100 in phenol red free DMEM; Promega, Madison, WI). Cellular LDH content was measured according to manufacturer's instructions. We confirmed that intracellular levels of LDH were not different between strains when equal numbers of AMs or PMNs from WT or GM-CSF−/− mice were lysed immediately without adherence and analyzed.
ELISA Measurements in Supernatants of Isolated Cells
Lung leukocytes were cultured for 24 h from saline or P. aeruginosa–infected mice. After 24 h, cell-free supernatants were collected and analyzed by specific ELISA (cytokines: Opti-EIA, BD PharMingen San Diego, California; chemokines: R&D systems, Minneapolis, MN; PGs/LTs: Cayman Chemical, Ann Arbor, MI) according to the manufacturers' instructions.
Statistical Analysis
Statistical significance was analyzed using Prism 3.0 statistical program (GraphPad Software, San Diego, CA). Comparisons between two experimental groups were preformed with Student's t test. Comparisons among three or more experimental groups were performed with ANOVA with a post hoc Bonferroni test to determine significance. A value of P < 0.05 was considered significant.
RESULTS
Lung Leukocytes Produced Increased GM-CSF in Response to P. aeruginosa
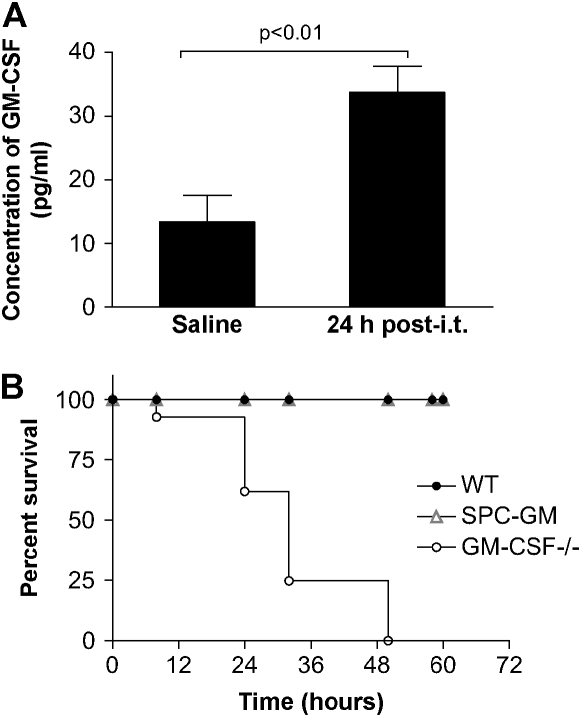
WT and GM-CSF−/− mice were injected with saline or P. aeruginosa (6.8 CFU log10 scale), and lung leukocytes were harvested 24 h after intratracheal inoculum. Lung leukocytes from GM-CSF−/− mice were unable to make GM-CSF in response to saline or infection, confirming the genetic knock-out (data not shown). In contrast, the amount of GM-CSF produced by lung leukocytes was elevated in response to a P. aeruginosa infection in WT mice (Figure 1A, P < 0.01). These results suggest that GM-CSF might play a role in the normal innate immune response to P. aeruginosa in the lung.
Figure 1.
GM-CSF is an important regulator of the innate immune response during a P. aeruginosa infection. (A) GM-CSF−/− and WT mice were inoculated intratracheally with saline or P. aeruginosa (6.8 CFU log10 scale), and lung leukocytes were isolated. We verified that GM-CSF−/− mice were not able to produce GM-CSF even when infected with P. aeruginosa, compared with an increase in the production of GM-CSF post-challenge by the WT lung leukocytes (closed bars). Data represent n = 4 per group and are representative of three similar experiments. (B) GM-CSF−/−, SPC-GM, and WT mice were infected intratracheally with P. aeruginosa at a dose of 6.0 CFU log10 scale, and survival was monitored for 72 h. At 48 h after intratracheal inoculation, the GM-CSF−/− mice (open circles) have 100% mortality compared with 100% survival of SPC-GM (gray triangles) and WT mice (closed circles). The GM-CSF−/− mice have increased mortality to P. aeruginosa infections (P = 0.0001). Data represent n = 7 mice per group and are representative of two similar experiments.
GM-CSF−/−, but Not SPC-GM, Mice Had an Increased Susceptibility to P. aeruginosa Infections Compared with WT Mice
We inoculated WT and GM-CSF−/− mice with a dose of 6.0 CFU log10 scale of P. aeruginosa and analyzed survival over a 72-h period. After 48 h, there was 100% mortality in the GM-CSF−/− mice compared with no deaths in the WT mice (Figure 1B). To determine whether GM-CSF expression was important systemically or locally in the lung, we examined the survival of SPC-GM mice in response to the same dose of P. aeruginosa. Local lung expression of GM-CSF was sufficient to restore host defense functions in these mice as indicated by their ability to survive Gram-negative infection in vivo (Figure 1B).
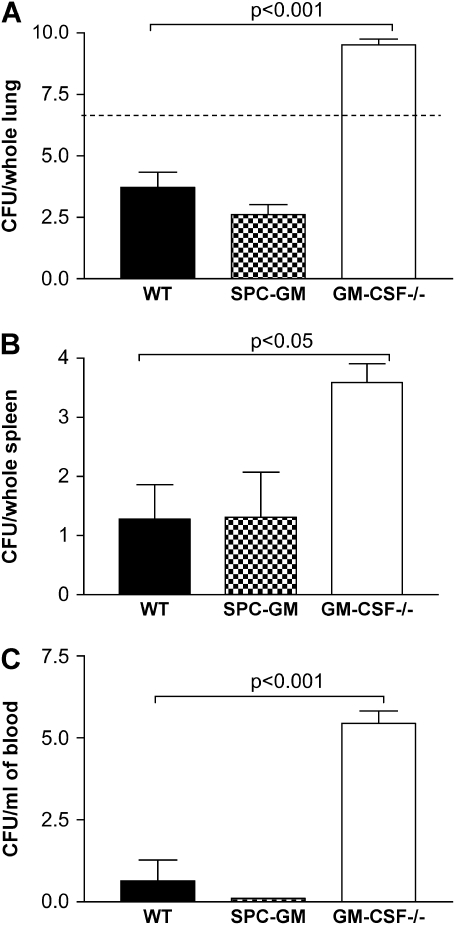
GM-CSF−/− Mice Had Increased Bacterial Burden and Bacterial Dissemination Compared with WT and SPC-GM Mice
Mice of all three genotypes were infected intratracheally with P. aeruginosa (6.7 CFU log10 scale), and the bacterial burden and amount of dissemination were analyzed. These results showed that GM-CSF−/− mice had an increase in bacterial burden; however, the restoration of GM-CSF to the lung restored bacterial clearance to WT levels (Figure 2A). The inability of the GM-CSF−/− mice to control the P. aeruginosa infection in the lung also resulted in increased bacterial dissemination to the spleen (Figure 2B) and the blood (Figure 2C). There was no difference in dissemination in SPC-GM mice compared with WT mice, providing further support for the conclusion that the lung is the critical site for GM-CSF expression within the setting of pneumonia.
Figure 2.
GM-CSF−/− mice have increased bacterial burden in the lungs and increased bacterial dissemination into the blood and spleen compared with SPC-GM and WT mice. Lungs, spleen, and blood were harvested 24 h after intratracheal infection with P. aeruginosa (6.7 CFU log10 scale) from GM-CSF−/−, SPC-GM, and WT mice and plated for CFU analysis. (A) There were increased CFU in the lungs of GM-CSF−/− mice (open bar) compared with the WT (closed bar) and SPC-GM mice (checkered bar) 24 h postinfection (P < 0.001). GM-CSF−/− mice (open bar) have increased bacterial dissemination as shown by higher CFU found in the spleen (B) and blood (C) compared with WT (closed bars) and SPC-GM (checkered bars) mice. Data represent n = 5 mice per group and are representative of two similar experiments. The dotted line represents the original inoculum dose (6.7 CFU log10 scale). The GM-CSF−/− mice had increased bacterial burden in their lung (P < 0.001) and increased dissemination into the spleen (P < 0.05) and blood (P < 0.001).
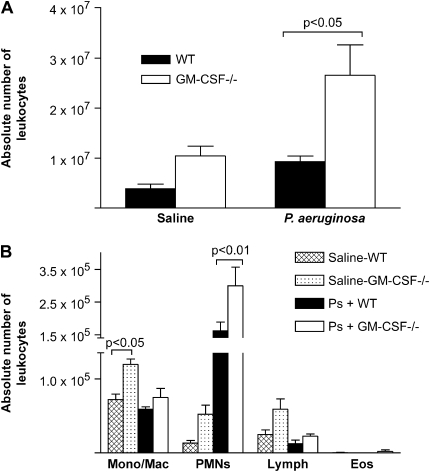
GM-CSF−/− Mice Showed Enhanced Accumulation of Leukocytes in the Lung 24 h Postinfection
To determine whether GM-CSF−/− mice displayed defects in cellular recruitment or accumulation in the lung, GM-CSF−/− and WT mice were given an intratracheal inoculation of 6.5 CFU log10 scale P. aeruginosa or saline, and lung leukocytes were harvested 24 h post-infection and enumerated (Figure 3A). After saline instillation, GM-CSF−/− mice tended to have more lung leukocytes than their WT counterparts, confirming previous observations (16, 17). This did not reach statistical significance in all experiments. After saline challenge, GM-CSF−/− mice had significantly elevated numbers of monocyte/macrophages (Figure 3B); however, the increase in PMNs was not significant. In response to P. aeruginosa infection, the GM-CSF−/− mice recruited significantly more (P < 0.05) lung leukocytes than did WT mice at 24 h postinfection. PMNs were the predominant cell type recruited to the lung in response to P. aeruginosa in both genotypes of mice.
Figure 3.
GM-CSF−/− mice show no defect in cellular accumulation at 24 h after P. aeruginosa. WT mice and GM-CSF−/− mice were given an intratracheal infection with P. aeruginosa at a dose of 6.5 CFU in log10 scale. Total primary lung leukocytes and inflammatory subsets were enumerated 24 h after intratracheal inoculation. (A) Total leukocytes accumulated in response to a saline treatment were not statistically different in GM-CSF−/− mice (open bars) versus WT mice (closed bars), although the trend was for the GM-CSF−/− mice to have more cells. There was a significant increase in the number of lung leukocytes in the GM-CSF−/− mice (open bars) compared with WT mice (closed bars) after P. aeruginosa (P < 0.05). (B) After saline treatment, there were more monocyte/macrophages in the GM-CSF−/− mice (dotted bars) compared with the WT mice (checked bars) (P < 0.05). In response to P. aeruginosa, PMNs are the predominant cell type in WT (closed bars) and GM-CSF−/− mice (open bars), and GM-CSF−/− mice recruit more PMNs (P < 0.01). Data represent n = 4 mice per condition and are representative of two experiments.
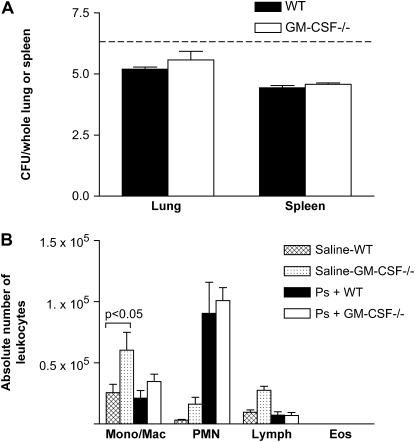
GM-CSF−/− Mice Were not Deficient in Their Early Response against P. aeruginosa
To determine whether the defective clearance in the GM-CSF−/− mice reflected delayed early responses, GM-CSF−/− and WT mice were inoculated intratracheally with P. aeruginosa (6.6 CFU log10 scale per animal) and examined 6 h later. Bacterial burden and dissemination were measured by CFU analysis. At 6 h, there was no difference in the bacterial burden found in the lungs of WT and GM-CSF−/− mice (Figure 4A). Similarly, there was no difference in bacterial dissemination to the spleen at 6 h postinfection between WT and GM-CSF−/− mice (Figure 4A). We next examined leukocyte accumulation at 6 h after P. aeruginosa or saline instillation (Figure 4B). Overall, fewer cells were recovered from the lungs at 6 h postchallenge compared with 24 h postchallenge. However, the types of cells that accumulated and their absolute numbers were similar at 6 h between WT and GM-CSF−/− mice in response to P. aeruginosa. We also examined mice at 2 and 4 h postchallenge. PMN recruitment was not evident in either genotype at 2 h postinfection. However, by 4 h, PMN recruitment was evident, and results were similar to those presented for the 6-h time point in Figure 4.
Figure 4.
GM-CSF−/− mice had an intact early response against P. aeruginosa compared with WT mice. GM-CSF−/− and WT mice were infected with P. aeruginosa (6.6 CFU in log10 scale) and examined 6 h after infection. (A) The bacterial burden in the lungs and spleen of WT (closed bars) and GM-CSF−/− (open bars) mice was examined by CFU analysis. Six hours postinfection, we show no significant increase in the amount of bacterial burden in the lung or in the amount of bacterial dissemination in the spleen in GM-CSF−/− mice compared with WT mice. The dotted line represents the original inoculum dose. (B) There is no defect in the ability of GM-CSF−/− mice to recruit inflammatory subsets to the site of infection. The absolute number of monocytes/macrophages and PMNs in the GM-CSF−/− mice (open bars) compared with the WT mice (closed bars) 6 h after P. aeruginosa infection are not different. Data represent n = 5 mice per condition and are representative of two experiments.
Lung Leukoctyes from GM-CSF−/− Mice Were Defective in Their Ability to Generate CXC Chemokines in Response to P. aeruginosa
Our results indicate that GM-CSF−/− mice recruited excessive numbers of PMNs to the lung at 24 h after P. aeruginosa infection. To determine whether this increase corresponded with elevated expression of chemokines known to be chemotactic for PMNs, we measured KC and MIP-2 levels in lung leukocyte cultures from mice given saline or P. aeruginosa. For KC, the levels in saline and P. aeruginosa–infected WT mice, respectively, were 310 ± 60 and 570 ± 40 pg/ml. In GM-CSF−/− mice, the values for KC were 160 ± 20 and 170 ± 20 pg/ml, respectively. For MIP-2, the values in WT mice were 2.67 ± 1.19 and 7.5 ± 0.75 ng/ml for saline and P. aeruginosa–infected mice, respectively. In GM-CSF−/− mice, the values were 1.98 ± 0.98 and 0.70 ± 0.35 ng/ml, respectively. We observed no significant difference in the amount of KC (P = 0.08) or MIP-2 (P = 0.70) produced by lung leukocytes from saline-treated mice from both genotypes. Twenty-four hours after P. aeruginosa infection, there was a significant increase in the amount of KC (P = 0.009) and MIP-2 (P = 0.01) that was produced by the WT lung leukocytes. There was no increase in the amount of KC or MIP-2 after P. aeruginosa infection in the GM-CSF−/− mice.
Lung Leukocytes from GM-CSF−/− Mice Demonstrated Impaired Eicosanoid Production 24 h after P. aeruginosa Infection
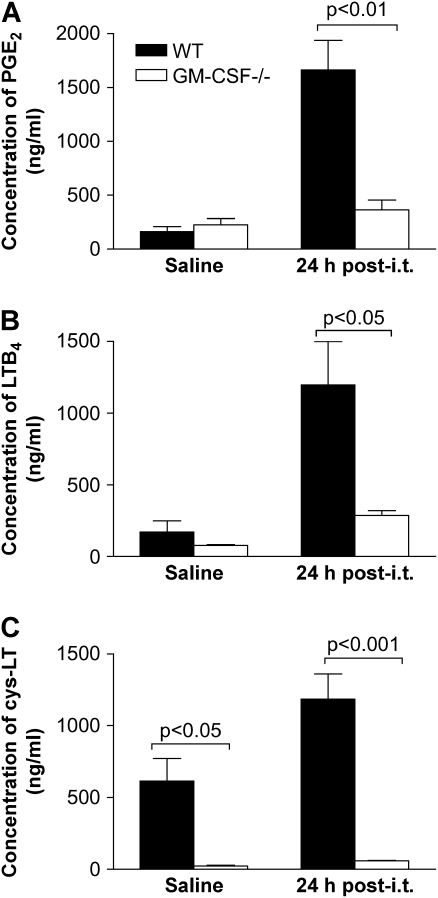
The ability of equal numbers of lung leukocytes from GM-CSF−/− and WT mice to produce lipid mediators was examined after P. aeruginosa infection. There were similar levels of PGE2 produced post-saline by cells from GM-CSF−/− and WT mice (Figure 5A). However, 24 h after infection, lung leukocytes isolated from WT mice produced 4-fold more PGE2 than GM-CSF−/− lung leukocytes. There were also significant decreases in the amount of leukotrienes (LTs) produced by lung leukocytes from GM-CSF−/− mice compared with WT mice, as shown by decreased levels of LTB4 (Figure 5B) and cys-LTs (Figure 5C) pre- and postinfection.
Figure 5.
GM-CSF−/− lung leukocytes were not able to produce eicosanoids in response to a 24 h P. aeruginosa infection. GM-CSF−/− and WT mice were inoculated with saline or P. aeruginosa (6.5 CFU in log10 scale), and 24 h later lung leukocytes were collected and incubated overnight. (A) GM-CSF−/− lung leukocytes (open bars) produced less PGE2 compared with WT (closed bars) cells. (B and C) Lung leukocytes from GM-CSF−/− mice (open bars) were unable to produce LTB4 (B) or cys-LT (C) in response to P. aeruginosa infection compared with WT lung leukocytes (closed bars). Data represent n = 5 mice per condition and are representative of two experiments.
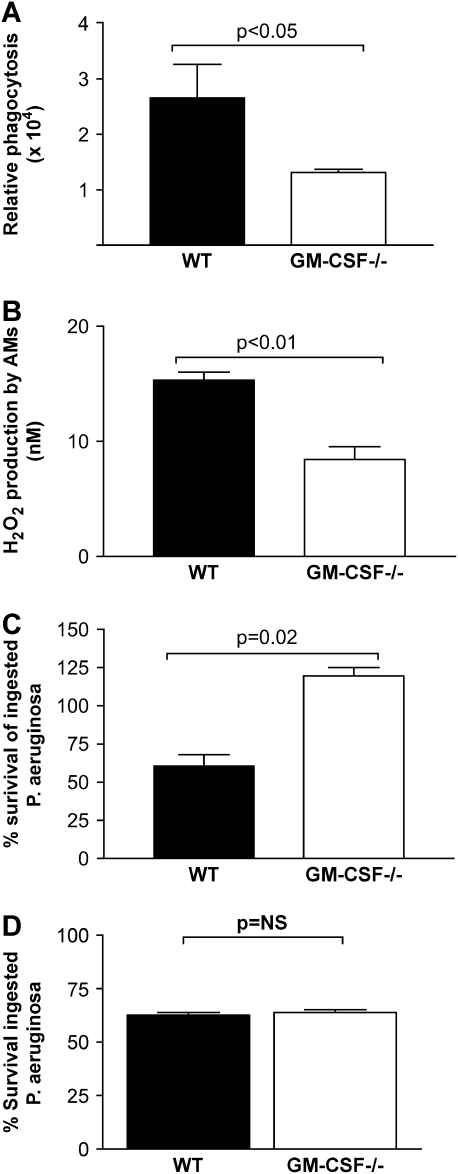
GM-CSF−/− AMs Showed Defective In Vitro Phagocytosis and Bacterial Killing
We tested AMs from WT and GM-CSF−/− mice for their ability to phagocytose un-opsonized bacteria using an in vitro phagocytosis assay. We observed that AMs from GM-CSF−/− mice were unable to phagocytose un-opsonized Gram-negative bacteria in vitro as effectively as WT AMs (Figure 6A, P < 0.05). We next examined the ability of AMs from GM-CSF−/− mice to generate H2O2. There was a significant decrease (P < 0.01) in the GM-CSF−/− AMs' ability to produce H2O2 (Figure 6B) compared with WT AMs. To determine whether this difference could contribute to differences in bacterial killing between WT and GM-CSF−/− mice, we tested the ability of AMs from GM-CSF−/− mice to kill P. aeruginosa directly. Our results indicated a significant increase in survival of ingested bacteria in GM-CSF−/− AMs compared with WT AMs (Figure 6C).
Figure 6.
GM-CSF−/− AMs, but not PMs, show defective phagocytosis and killing of Gram-negative bacteria in vitro. AMs were tested from WT or GM-CSF−/− mice and assayed for their ability to phagocytose or kill Gram-negative bacteria in vitro. (A) The AMs from GM-CSF−/− mice (open bars) were deficient in their ability to phagocytose FITC-labeled, Gram-negative E. coli in vitro compared with AMs from WT mice (closed bars) (P < 0.05). This assay measures only intracellular bacteria because extracellular bacterial fluorescence was quenched by trypan blue. (B) There was a significant (P < 0.01) decrease in the ability of AMs from GM-CSF−/− mice (open bars) to produce H2O2 compared with AMs from WT mice (closed bars). (C) AMs from GM-CSF−/− mice (open bars) show a significant increase (P = 0.02) in the survival of ingested P. aeruginosa compared with survival seen in WT mice (closed bars). These data represent n = 4–5 mice per condition and are representative of two experiments. (D) PMs were collected via peritoneal lavage from WT or GM-CSF−/− mice on Day 4 after glycogen elicitation. PMs were tested for their ability to kill ingested bacteria using the tetrazolium dye reduction assay. There was no statistical difference in the ability of PMs from either strain to kill ingested P. aeruginosa (n = 6).
Macrophage Defects in GM-CSF−/− Mice Are Confined to the Alveolar Compartment
To determine whether the bactericidal defects seen in AMs were present in macrophages from a different anatomic site, glycogen-elicited PMs were evaluated for their ability to kill ingested P. aeruginosa. Bactericidal activity was similar in PMs elicited from WT and GM-CSF−/− mice (Figure 6D). These results suggest that GM-CSF is specifically required for AM, but not PM, antibactericidal activity.
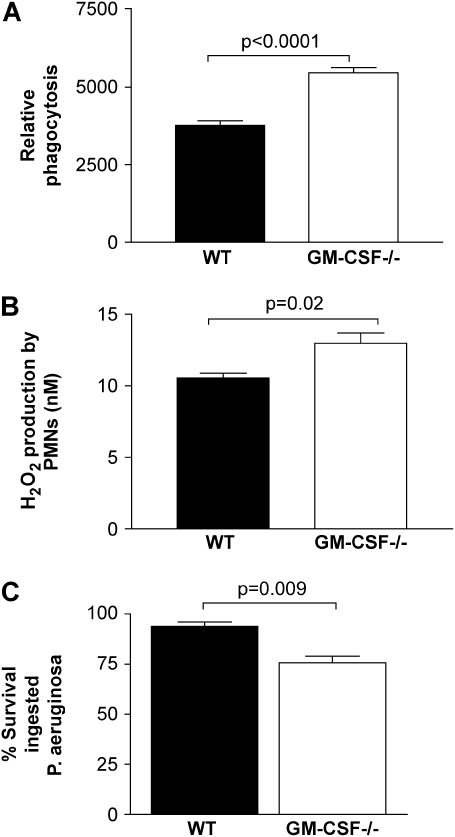
PMNs in GM-CSF−/− Mice Show Enhanced Bactericidal Activity
We assessed whether GM-CSF deficiency resulted in host defense defects in PMNs. PMNs elicited to the peritoneal cavity were tested for phagocytic activity against P. aeruginosa. Cells from GM-CSF−/− mice were better at phagocytosis (Figure 7A, P < 0.0001) and produced more H2O2 (Figure 7B, P = 0.02) than did PMNs elicited to the peritoneal cavity of WT mice. Figure 7C demonstrates that PMNs recruited to the lung in response to P. aeruginosa–derived LPS are better able to kill ingested P. aeruginosa than are PMNs recruited to the lungs of WT mice (P = 0.009). Thus, PMNs recruited to the lung and peritoneal cavity display enhanced antibacterial host defense functions in the absence of GM-CSF.
Figure 7.
PMN host defense functions are enhanced in GM-CSF−/− compared with WT mice. (A) PMNs elicited to the peritoneal cavity 5 h after glycogen elicitation from GM-CSF−/− mice (open bars) show a significant increase (P < 0.001, n = 6) in the ability to phagocytose Gram-negative bacteria in vitro compared with PMNs from WT mice (closed bars). (B) Similarly, H2O2 production by peritoneal PMNs was greater in GM-CSF−/− mice than in WT mice (P = 0.02, n = 5). Data are representative of two experiments. (C) PMNs were collected by lavage from lungs of WT (closed bars) or GM-CSF−/− mice (open bars) 24 h after intratracheal instillation of P. aeruginosa–derived LPS. PMNs were adhered for 30 min in SFM before being analyzed for the ability to kill ingested P. aeruginosa using the tetrazolium dye reduction assay. PMNs isolated from the lungs of GM-CSF−/− mice were significantly better at limiting intracellular bacterial growth compared with PMNs from the lungs of WT mice (P = 0.009, n = 6).
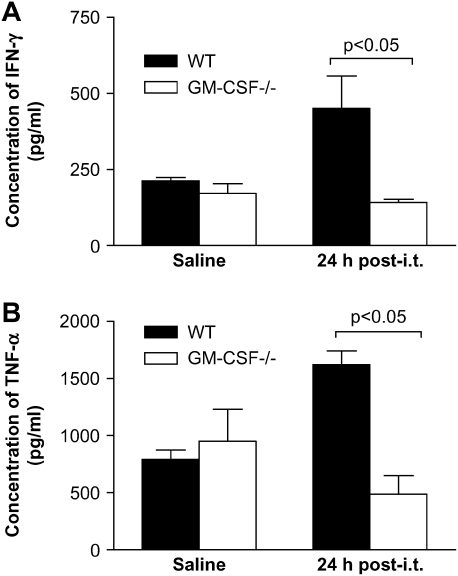
Decreased Production of Activating Cytokines after P. aeruginosa Infection in Lung Leukocytes from GM-CSF−/− Compared with WT Mice
We tested the ability of lung leukocytes from WT and GM-CSF−/− mice to produce activating cytokines after saline or P. aeruginosa infection. Lung leukocytes from GM-CSF−/− and WT mice produced similar levels of IFN-γ (Figure 8A) and TNF-α (Figure 8B) post-saline. However, post-infection, significant increases in the amount of TNF-α and INF-γ were observed in WT, but not GM-CSF−/−, mice (Figure 8). Thus, GM-CSF−/− lung leukocytes were unable to upregulate the production of activating cytokines after P. aeruginosa infection.
Figure 8.
Lung leukocytes from GM-CSF−/− were inefficient at producing activating cytokines in response to a 24-h P. aeruginosa infection. GM-CSF−/− and WT mice were inoculated with saline or P. aeruginosa (6.8 CFU in log10 scale), and lung leukocytes were collected. GM-CSF−/− mice (open bars) were unable to stimulate production of activating cytokines IFN-γ (A) and TNF-α (B) in response to P. aeruginosa infection compared with WT mice (closed bars). Data represent n = 4 mice per condition and are representative of two experiments.
DISCUSSION
We have examined the role of GM-CSF in the innate immune response to the important Gram-negative pathogen P. aeruginosa. We show that GM-CSF expressed by the lung leukocytes is elevated in normal mice in response to P. aeruginosa infection. GM-CSF has a key role in host defense because GM-CSF−/− mice have increased susceptibility to intratracheal P. aeruginosa infection at 24 h postinfection. This is the first description of the in vivo response of GM-CSF−/− mice to infection by a Gram-negative pathogen. GM-CSF−/− mice, but not SPC-GM mice, are more susceptible to P. aeruginosa infections as shown by decreased survival and increased bacterial burden and dissemination compared with WT mice. This demonstrates that local, not systemic, activity of GM-CSF was sufficient for the normal response to this pathogen. The increased susceptibility of the GM-CSF−/− mice is not due to defects in cellular recruitment or delayed early responses despite decreased production of lipid mediators and CXC chemokines. Rather, our data indicate that GM-CSF−/− mice are more susceptible to P. aeruginosa infection as a result of dysfunctional AM phagocytosis and killing. The defect in macrophage host defense functions in GM-CSF−/− mice seems to be confined to the alveolar space because PM function was normal in these mice. Furthermore, the defect seems to be restricted to AMs only because PMNs from the lung and peritoneal cavity showed enhanced function in GM-CSF−/− mice. The lack of activating cytokines by lung leukocytes may contribute to the increased susceptibility to P. aeruginosa that is seen in GM-CSF−/− mice.
GM-CSF is important for in vivo host defense against Gram-negative infection. When WT mice are infected with P. aeruginosa, there is an increase in the amount of GM-CSF produced by lung leukocytes. GM-CSF−/− mice are more susceptible to pulmonary infection with P. aeruginosa. Not only is the lung bacterial burden higher in GM-CSF−/− mice, but bacteria are able to disseminate from the lungs to the blood and spleen, indicating systemic spread. Thus, we believe that the impaired survival of the GM-CSF−/− mice is related to an inability to control bacterial growth and dissemination. Restoration of GM-CSF to the lungs by expression exclusively in type II alveolar epithelial cells (in SPC-GM mice) restores clearance to WT levels. Given that the host defense defects noted in these mice are confined to AMs, it is perhaps not surprising that lung-restricted expression of GM-CSF can fully restore the antibacterial defenses of GM-CSF−/− mice. AMs from GM-CSF−/− mice, but not SPC-GM mice, have an immature phenotype with altered β-integrin expression, showing decreased CD11a and CD11c, but not CD11b, expression (16). Collectively, these data suggest that paracrine expression of GM-CSF (likely from the lung alveolar epithelial cells) supports the maturation and activation of AMs.
One possible explanation for the increased susceptibility of GM-CSF−/− mice to P. aeruginosa could have been a defect in lung leukocyte recruitment. Previous studies have shown that GM-CSF−/− mice have increased numbers of lung leukocytes at baseline (16, 17, 28). The current studies support these earlier reports. GM-CSF−/− mice have increased numbers of monocytes/macrophages at baseline. No previous studies have examined the recruitment of leukocytes in response to Gram-negative infections in these mice. In response to P. aeruginosa, lung leukocytes are recruited in WT and GM-CSF−/− mice. The predominant cell type recruited in response to P. aeruginosa is PMNs. Our kinetic studies demonstrate that GM-CSF−/− mice are able to recruit PMNs to the lung in response to P. aeruginosa at early time points. At 6 h, the absolute numbers of PMNs in the lungs of WT and GM-CSF−/− mice are not different. The bacterial burden within the lungs of WT and GM-CSF−/− mice is also similar at 6 h postinfection. PMNs were recruited to the lungs of WT and GM-CSF−/− mice in similar numbers as early as 4 h postinfection. By 24 h postinfection, the numbers of PMNs in GM-CSF−/− mice are significantly higher than the number found in WT mice. This likely reflects the fact that the bacterial burden in the lungs of GM-CSF−/− mice is also significantly greater at 24 h.
It is not clear what signals direct the influx of PMNs to the lungs of GM-CSF−/− mice. Previous studies with Gram-positive infection suggested that MIP-2 was responsible for the recruitment of PMNs to the lungs of GM-CSF−/− mice (17). We observed increases in MIP-2, KC, and LTB4 in response to P. aeruginosa infection in lung leukocyte cultures from WT mice. All of these factors are known PMN recruitment molecules. However, we did not observe an increase in any of these mediators in the lung leukocyte cultures from GM-CSF−/− mice. Thus, it seems that alternative mechanisms may be responsible for PMN recruitment to Gram-negative infections in the absence of GM-CSF. Several potential mechanisms could explain these results. One possibility is that compensatory growth factor expression may be responsible for the increased production of monocytes/macrophages and PMNs in GM-CSF−/− mice under basal and stimulated conditions. Previous work has shown there to be increased MCP-1 expression in BAL fluid from GM-CSF−/− mice compared with WT mice (16). Other possible compensatory molecules could include growth factors such as IL-3, granulocyte colony-stimulating factor, or monocyte colony-stimulating factor, as has been suggested previously (29). A second possibility is that GM-CSF−/− leukocytes display increased levels of CD11b, which is the CR3 complement receptor (16). This could allow for increased complement-mediated recruitment of leukocytes to the lung. Finally, the fact that IFN-γ and TNF-α expressing lung leukocytes are reduced in GM-CSF−/− mice could inhibit normal PMN apoptosis, leading to PMN accumulation (30).
Arachidonic acid (AA) is the main precursor for the eicosanoid pathway. Through cyclooxygenase or lipoxygenase, AA is converted to PGs or LTs, respectively. LTs are important mediators of innate immunity by enhancing host defenses, such as AM phagocytosis and killing of Klebsiella pneumoniae (20, 25) and PMN killing of Cryptococcus neoformans (31). PGE2 is an important regulator of phagocytosis and has been shown to inhibit AM function via E prostanoid receptor 2 stimulation and cAMP elevation (21). Macrophages from GM-CSF−/− mice produce fewer LTs (16) and less PGE2 (28) when compared with WT mice post-saline. This may not be surprising given the fact that GM-CSF can increase the amount of the eicosanoid precursor, AA, via actions on cytosolic phospholipase A2 (32). During a P. aeruginosa infection, the decrease in the amount of PGs and LTs produced persists. Thus, the defective macrophage phagocytosis is not due to an overproduction of PGs. However, the decreased production of LTB4 and cys-LTs could contribute to the defective phagocytosis we have observed in AMs.
AMs and PMNs have been shown to be important for the clearance of P. aeruginosa (3, 9, 33, 34). Previous studies have suggested that PMNs may be the predominant cell type responsible for P. aeruginosa clearance. Mice that cannot recruit PMNs as a result of CXCR2 gene deletion are more susceptible to P. aeruginosa (34). Mice that had AMs depleted with clodronate liposomes also showed increased susceptibility to P. aeruginosa, but this was associated with defects in PMN recruitment (33). Thus, it was thought that AMs may have a predominant role as PMN recruiters. Our studies suggest that the recruitment and function of PMNs in GM-CSF−/− mice is intact. In fact, PMNs from the lungs of GM-CSF−/− mice were significantly better at killing ingested P. aeruginosa compared with PMNs from WT mice. Similarly, PMNs purified from another anatomic location, the peritoneal cavity, displayed enhanced phagocytosis and H2O2 production in GM-CSF−/− mice. These studies are the first to report on PMN function in GM-CSF−/− mice. Although diminished PMN recruitment can be associated with increased susceptibility to P. aeruginosa (34), our results suggest that defects in the AMs alone (or possibly in all lung macrophages) are sufficient to cause enhanced susceptibility.
We demonstrate that AMs from GM-CSF−/− mice are defective in phagocytosis and killing of Gram-negative bacteria in vitro. Previous studies have also noted defects in AM phagocytosis from GM-CSF−/− mice. AMs from GM-CSF−/− mice are unable to phagocytose FITC-labeled microbeads (16) or E. coli (14) in vitro as efficiently as AMs from WT mice. Our work extends these observations and demonstrates that killing of P. aeruginosa by AMs from GM-CSF−/− mice is impaired. The addition of PU.1 to AMs from GM-CSF−/− mice is able to restore phagocytosis and killing of E. coli and group B Streptococcus in vitro (14). Our data suggest that GM-CSF is uniquely required for AM function but may be less critical for the function of macrophages from other anatomic sites. It is also possible that GM-CSF is crucial for interstitial lung macrophage function, but this was not addressed in our experiments. We show that AMs, but not PMs, from GM-CSF−/− mice display bactericidal defects. In that context, it is interesting to note that the lung environment is highly enriched for GM-CSF and that it is produced in large quantities by alveolar epithelial cells (35). Furthermore, GM-CSF is a known mitogen for AMs (36). One caveat to our interpretations is that the PMs were studied after glycogen elicitation, whereas AMs were studied from unmanipulated mice. Thus, activation or recruitment signals may be able to overcome inherent macrophage defects in the absence of GM-CSF.
Cytokines are important signaling molecules that activate cells of the host immune system at the site of infection. Previous work has shown that decreased levels of TNF-α in cells isolated from BAL and in the BAL fluid correlate with increased susceptibility to P. aeruginosa infection (37). We show a significant decrease in the ability of lung leukocytes from GM-CSF−/− mice to produce TNF-α and IFN-γ 24 h after P. aeruginosa infection (Figure 8). This is consistent with previous work showing that GM-CSF−/− mice have decreased proinflammatory cytokines produced postinfection. AMs from GM-CSF−/−, but not SPC-GM, mice are unable to produce TNF-α in response to P. carinii infection (11) or to LPS (16) unless rescued with PU.1 expression (14). There are also decreases in the amount of IFN-γ produced by BAL cells from GM-CSF−/− mice during an adenoviral insult (15). Our results concur with the published work in that the increased susceptibility of the GM-CSF−/− mice to P. aeruginosa correlates with an inability to produce proinflammatory cytokines.
In summary, our results demonstrate that GM-CSF is a critical cytokine regulating the host defense against P. aeruginosa. GM-CSF−/− mice do not have defects in the early recruitment of leukocytes to the site of infection. However, AMs from GM-CSF−/− mice display profound defects in the ability to phagocytose and kill ingested P. aeruginosa. This is likely related to the defective production of H2O2 by these cells. Defective killing by GM-CSF−/− AMs may also be secondary to defects in the production of activating lipids (cys-LTs) and cytokines (TNF-α and IFN-γ). Macrophage defects in GM-CSF−/− mice seem to be confined to the lung because PM function is intact. Furthermore, PMN function in the lung and the peritoneal cavity is enhanced in GM-CSF−/− mice. We conclude that GM-CSF is critical for the regulation of AM function.
This work was supported by NIH grants HL071586 (B.B.M.), HL078727 (D.M.A.), HL51082 (G.B.T.), P50HL56402 (B.B.M. and G.B.T.), P50HL60289 (R.P. and T.J.S.), and T32-AI-07413 (M.N.B.). M.N.B. is also funded by the Herman and Dorothy Miller Fund. R.P. and G.B.T. are funded by Merit Review Awards from the Medical Research Service of the Department of Veterans Affairs.
Originally Published in Press as DOI: 10.1165/rcmb.2005-0246OC on February 10, 2006
Conflict of Interest Statement: None of the authors has a financial relationship with a commercial entity that has an interest in the subject of this manuscript.
References
- 1.Holder IA. Pseudomonas immunotherapy: a historical overview. Vaccine 2004;22:831–839. [DOI] [PubMed] [Google Scholar]
- 2.Rolston KV, Bodey GP. Pseudomonas aeruginosa infection in cancer patients. Cancer Invest 1992;10:43–59. [DOI] [PubMed] [Google Scholar]
- 3.Ojielo CI, Cooke K, Mancuso P, Standiford TJ, Olkiewicz KM, Clouthier S, Corrion L, Ballinger MN, Toews GB, Paine R III, et al. Defective phagocytosis and clearance of Pseudomonas aeruginosa in the lung following bone marrow transplantation. J Immunol 2003;171:4416–4424. [DOI] [PubMed] [Google Scholar]
- 4.Vidal F, Mensa J, Martinez JA, Almela M, Marco F, Gatell JM, Richart C, Soriano E, Jimenez de Anta MT. Pseudomonas aeruginosa bacteremia in patients infected with human immunodeficiency virus type 1. Eur J Clin Microbiol Infect Dis 1999;18:473–477. [DOI] [PubMed] [Google Scholar]
- 5.Maschmeyer G, Braveny I. Review of the incidence and prognosis of Pseudomonas aeruginosa infections in cancer patients in the 1990s. Eur J Clin Microbiol Infect Dis 2000;19:915–925. [DOI] [PubMed] [Google Scholar]
- 6.Hoiby N, Krogh Johansen H, Moser C, Song Z, Ciofu O, Kharazmi A. Pseudomonas aeruginosa and the in vitro and in vivo biofilm mode of growth. Microbes Infect 2001;3:23–35. [DOI] [PubMed] [Google Scholar]
- 7.Lyczak JB, Cannon CL, Pier GB. Establishment of Pseudomonas aeruginosa infection: lessons from a versatile opportunist. Microbes Infect 2000;2:1051–1060. [DOI] [PubMed] [Google Scholar]
- 8.Knapp S, Leemans JC, Florquin S, Branger J, Maris NA, Pater J, van Rooijen N, van der Poll T. Alveolar macrophages have a protective antiinflammatory role during murine pneumococcal pneumonia. Am J Respir Crit Care Med 2003;167:171–179. [DOI] [PubMed] [Google Scholar]
- 9.Welsh DA, Mason CM. Host defense in respiratory infections. Med Clin North Am 2001;85:1329–1347. [DOI] [PubMed] [Google Scholar]
- 10.Stanley E, Lieschke GJ, Grail D, Metcalf D, Hodgson G, Gall JA, Maher DW, Cebon J, Sinickas V, Dunn AR. Granulocyte/macrophage colony-stimulating factor-deficient mice show no major perturbation of hematopoiesis but develop a characteristic pulmonary pathology. Proc Natl Acad Sci USA 1994;91:5592–5596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Paine R III, Preston AM, Wilcoxen S, Jin H, Siu BB, Morris SB, Reed JA, Ross G, Whitsett JA, Beck JM. Granulocyte-macrophage colony-stimulating factor in the innate immune response to Pneumocystis carinii pneumonia in mice. J Immunol 2000;164:2602–2609. [DOI] [PubMed] [Google Scholar]
- 12.Charbeneau RP, Christensen PJ, Chrisman CJ, Paine R III, Toews GB, Peters-Golden M, Moore BB. Impaired synthesis of prostaglandin E2 by lung fibroblasts and alveolar epithelial cells from GM-CSF−/− mice: implications for fibroproliferation. Am J Physiol Lung Cell Mol Physiol 2003;284:L1103–L1111. [DOI] [PubMed] [Google Scholar]
- 13.Trapnell BC, Whitsett JA, Nakata K. Pulmonary alveolar proteinosis. N Engl J Med 2003;349:2527–2539. [DOI] [PubMed] [Google Scholar]
- 14.Shibata Y, Berclaz PY, Chroneos ZC, Yoshida M, Whitsett JA, Trapnell BC. GM-CSF regulates alveolar macrophage differentiation and innate immunity in the lung through PU.1. Immunity 2001;15:557–567. [DOI] [PubMed] [Google Scholar]
- 15.Berclaz PY, Shibata Y, Whitsett JA, Trapnell BC. GM-CSF, via PU.1, regulates alveolar macrophage Fcgamma R-mediated phagocytosis and the IL-18/IFN-gamma -mediated molecular connection between innate and adaptive immunity in the lung. Blood 2002;100:4193–4200. [DOI] [PubMed] [Google Scholar]
- 16.Paine R III, Morris SB, Jin H, Wilcoxen SE, Phare SM, Moore BB, Coffey MJ, Toews GB. Impaired functional activity of alveolar macrophages from GM-CSF-deficient mice. Am J Physiol Lung Cell Mol Physiol 2001;281:L1210–L1218. [DOI] [PubMed] [Google Scholar]
- 17.LeVine AM, Reed JA, Kurak KE, Cianciolo E, Whitsett JA. GM-CSF-deficient mice are susceptible to pulmonary group B streptococcal infection. J Clin Invest 1999;103:563–569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Riopel J, Tam M, Mohan K, Marino MW, Stevenson MM. Granulocyte-macrophage colony-stimulating factor-deficient mice have impaired resistance to blood-stage malaria. Infect Immun 2001;69:129–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Peters-Golden M, Canetti C, Mancuso P, Coffey MJ. Leukotrienes: underappreciated mediators of innate immune responses. J Immunol 2005;174:589–594. [DOI] [PubMed] [Google Scholar]
- 20.Bailie MB, Standiford TJ, Laichalk LL, Coffey MJ, Strieter R, Peters-Golden M. Leukotriene-deficient mice manifest enhanced lethality from Klebsiella pneumonia in association with decreased alveolar macrophage phagocytic and bactericidal activities. J Immunol 1996;157:5221–5224. [PubMed] [Google Scholar]
- 21.Aronoff DM, Canetti C, Peters-Golden M. Prostaglandin E2 inhibits alveolar macrophage phagocytosis through an E-prostanoid 2 receptor-mediated increase in intracellular cyclic AMP. J Immunol 2004;173:559–565. [DOI] [PubMed] [Google Scholar]
- 22.Dranoff G, Crawford AD, Sadelain M, Ream B, Rashid A, Bronson RT, Dickersin GR, Bachurski CJ, Mark EL, Whitsett JA, et al. Involvement of granulocyte-macrophage colony-stimulating factor in pulmonary homeostasis. Science 1994;264:713–716. [DOI] [PubMed] [Google Scholar]
- 23.Huffman JA, Hull WM, Dranoff G, Mulligan RC, Whitsett JA. Pulmonary epithelial cell expression of GM-CSF corrects the alveolar proteinosis in GM-CSF-deficient mice. J Clin Invest 1996;97:649–655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Peters-Golden M, Thebert P. Inhibition by methylprednisolone of zymosan-induced leukotriene synthesis in alveolar macrophages. Am Rev Respir Dis 1987;135:1020–1026. [DOI] [PubMed] [Google Scholar]
- 25.Serezani CH, Aronoff DM, Jancar S, Mancuso P, Peters-Golden M. Leukotrienes enhance the bactericidal activity of alveolar macrophages against Klebsiella pneumoniae through the activation of NADPH oxidase. Blood 2005;106:1067–1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Peck R. A one-plate assay for macrophage bactericidal activity. J Immunol Methods 1985;82:131–140. [DOI] [PubMed] [Google Scholar]
- 27.Mancuso P, Standiford TJ, Marshall T, Peters-Golden M. 5-Lipoxygenase reaction products modulate alveolar macrophage phagocytosis of Klebsiella pneumoniae. Infect Immun 1998;66:5140–5146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Moore BB, Coffey MJ, Christensen P, Sitterding S, Ngan R, Wilke CA, McDonald R, Phare SM, Peters-Golden M, Paine R III, et al. GM-CSF regulates bleomycin-induced pulmonary fibrosis via a prostaglandin-dependent mechanism. J Immunol 2000;165:4032–4039. [DOI] [PubMed] [Google Scholar]
- 29.Lieschke GJ, Stanley E, Grail D, Hodgson G, Sinickas V, Gall JA, Sinclair RA, Dunn AR. Mice lacking both macrophage- and granulocyte-macrophage colony-stimulating factor have macrophages and coexistent osteopetrosis and severe lung disease. Blood 1994;84:27–35. [PubMed] [Google Scholar]
- 30.Chen GH, McDonald RA, Wells JC, Huffnagle GB, Lukacs NW, Toews GB. The gamma interferon receptor is required for the protective pulmonary inflammatory response to Cryptococcus neoformans. Infect Immun 2005;73:1788–1796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Coffey MJ, Phare SM, George S, Peters-Golden M, Kazanjian PH. Granulocyte colony-stimulating factor administration to HIV-infected subjects augments reduced leukotriene synthesis and anticryptococcal activity in neutrophils. J Clin Invest 1998;102:663–670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Brock TG, McNish RW, Coffey MJ, Ojo TC, Phare SM, Peters-Golden M. Effects of granulocyte-macrophage colony-stimulating factor on eicosanoid production by mononuclear phagocytes. J Immunol 1996;156:2522–2527. [PubMed] [Google Scholar]
- 33.Kooguchi K, Hashimoto S, Kobayashi A, Kitamura Y, Kudoh I, Wiener-Kronish J, Sawa T. Role of alveolar macrophages in initiation and regulation of inflammation in Pseudomonas aeruginosa pneumonia. Infect Immun 1998;66:3164–3169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tsai WC, Strieter RM, Mehrad B, Newstead MW, Zeng X, Standiford TJ. CXC chemokine receptor CXCR2 is essential for protective innate host response in murine Pseudomonas aeruginosa pneumonia. Infect Immun 2000;68:4289–4296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Christensen PJ, Bailie MB, Goodman RE, O'Brien AD, Toews GB, Paine R III. Role of diminished epithelial GM-CSF in the pathogenesis of bleomycin-induced pulmonary fibrosis. Am J Physiol Lung Cell Mol Physiol 2000;279:L487–L495. [DOI] [PubMed] [Google Scholar]
- 36.Chen GH, Curtis JL, Mody CH, Christensen PJ, Armstrong LR, Toews GB. Effect of granulocyte-macrophage colony-stimulating factor on rat alveolar macrophage anticryptococcal activity in vitro. J Immunol 1994;152:724–734. [PubMed] [Google Scholar]
- 37.Morissette C, Francoeur C, Darmond-Zwaig C, Gervais F. Lung phagocyte bactericidal function in strains of mice resistant and susceptible to Pseudomonas aeruginosa. Infect Immun 1996;64:4984– 4992. [DOI] [PMC free article] [PubMed] [Google Scholar]