Abstract
Visceral adiposity is a strong determinant of growth hormone (GH) secretion, and states of GH deficiency are associated with increased visceral adiposity and decreased lean body mass. The purpose of our study was to determine the sensitivity and specificity of different methods of assessing body composition [anthropometry, dual-energy X-ray absorptiometry (DXA), and computed tomography (CT)] to predict GH deficiency in premenopausal women and threshold values for each technique to predict GH deficiency, using receiver operator characteristic (ROC) curve analysis. We studied a group of 45 healthy lean, overweight, and obese premenopausal women who underwent anthropometric measurements (body mass index, waist and hip circumferences, skin fold thickness), DXA, CT, and a GH-releasing hormone-arginine stimulation test. ROC curve analysis was used to determine cutoff values for each method to identify GH deficiency. Visceral adiposity measured by CT showed the highest sensitivity and specificity for identifying subjects with GH deficiency with a cutoff of >9,962 mm2 [area under the curve (AUC), 0.95; sensitivity, 100%; specificity, 77.8%; P = 0.0001]. Largest waist circumference showed high sensitivity and specificity with a cutoff of >101.7 cm (AUC, 0.89; sensitivity, 88.9%; specificity, 75%; P = 0.0001). When the ROC curves of visceral fat measured by CT and largest waist circumference were compared, the difference between the two methods was not statistically significant (P = 0.36). Our study showed that the largest waist circumference predicts the presence of GH deficiency in healthy premenopausal women with high sensitivity and specificity and nearly as well as CT measurement of visceral adiposity. It can be used to identify women in whom GH deficiency is likely and therefore in whom formal GH stimulation testing might be indicated.
Keywords: obesity, body composition, growth hormone deficiency, waist circumference, computed tomography, dual-energy X-ray absorptiometry, receiver operator characteristic curve analysis
obesity is highly prevalent in the Western world, and visceral adiposity is an independent predictor of metabolic complications such as dyslipidemia, type 2 diabetes, and cardiovascular disease (25, 35). Prior studies have established that visceral adiposity is a strong determinant of growth hormone (GH) secretion (11, 31, 32) and that GH plays a role in modulating body composition. States of GH deficiency are associated with increased body fat, including visceral adiposity, and decreased lean body mass (10, 14), whereas states of GH excess are associated with decreased body fat and increased lean body mass (4).
The GH-releasing hormone (GHRH)-arginine stimulation test is a sensitive and specific test for diagnosing GH deficiency, and a cutoff limit of 5 ng/ml has been used to diagnose GH deficiency in adults (6). However, this test is invasive, time consuming, and expensive. Should GH deficiency be determined to be a treatable condition in young women with visceral adiposity, it would be useful to be able to perform a simple body composition measurement to identify subjects who might be at risk for GH deficiency and for whom formal GH simulation testing might be more likely to yield a positive result. Computed tomography (CT) can quantify visceral and subcutaneous fat depots (7) and is the gold standard for measuring visceral fat. However, it is expensive and involves radiation exposure. Several clinical methods, including anthropometry and dual-energy X-ray absorptiometry (DXA), have been used as surrogates for estimating body fat (8, 15, 19, 20, 27, 28, 34), but these measurements do not allow for the evaluation of visceral fat content. The purpose of our study was to compare sensitivity and specificity of simple, less invasive measures of body composition, such as anthropometry, with CT and DXA to predict GH deficiency in a group of lean, overweight, and obese premenopausal women. In addition, we wanted to determine threshold values for each technique to predict GH deficiency by using receiver operator characteristic (ROC) curve analysis.
MATERIALS AND METHODS
The study was approved by the institutional review board of Partners Healthcare and was Health Insurance Portability and Accountability Act compliant. Written informed consent was obtained from all subjects before the study.
Subjects.
The study group comprised 45 healthy premenopausal women who were recruited from the community through advertisements. Exclusion criteria included hypothalamic or pituitary disorders, diabetes mellitus or other chronic illnesses, estrogen or glucocorticoid use, and weight >280 lb. (due to the limitations of the DXA and CT scanners). Participants were admitted to the General Clinical Research Center at the Massachusetts General Hospital, where testing was performed. Each participant underwent anthropometric measurements, DXA, and CT, as detailed below, and a GHRH-arginine stimulation test. For the GHRH-arginine stimulation test, GHRH (1 μg/kg iv) plus arginine (0.5 g/kg iv; maximum 30 g) were administered, and GH levels were drawn at baseline and every 30 min for 2 h (6). GH deficiency was based on standard criteria used to diagnose adults with hypopituitarism (peak GH after stimulation with GHRH and arginine <5 ng/ml) (6). Clinical characteristics, peak GH after GHRH-arginine stimulation, and fat mass, measured by DXA, have been previously published (29, 30).
Biochemical analyses.
Serum samples were collected and stored at −80°C. Serum GH was measured using an immunoradiometric assay kit, with a minimum detection limit of 0.01 ng/ml, an intra-assay coefficient of variation of 3.1–5.4%, and an interassay coefficient of variation of 5.9–11.5%.
Anthropometry.
Body weight was measured at a standard balance beam scale to the nearest 0.1 kg in triplicate and averaged. Height was measured with subjects barefoot to the nearest 0.1 cm in triplicate and averaged. Body mass index (BMI) was calculated as weight divided by height squared (kg/m2).
Skin fold thickness of the triceps, biceps, subscapular, and suprailiac areas was measured using metal calipers in triplicate and averaged. Skin fold measurements were used to estimate percent body fat.
Body circumferences were measured at the waist at the smallest circumference between the lowest rib and iliac crest, at the level of the umbilicus, the midpoint between the lowest rib and iliac crest, the iliac crest, and at the hip using a metal tape to the nearest 0.1 cm in triplicate and averaged. The largest circumference represents the largest value obtained from the above measures. Iliac waist-to-hip and largest hip-to-waist ratios were determined from the circumferential measurements of the waist at the level of the umbilicus and the hips at the level of the iliac crest taken with the patient in a standing position.
All measurements were performed by research bionutritionists who had been previously trained and certified to perform these procedures.
Dual X-ray absorptiometry.
DXA measurements of body composition were performed using a Hologic QDR 4500 scanner (Waltham, MA). The following parameters were obtained: percent fat of the right and left upper and lower extremities, percent trunk fat, and total percent body fat. In addition, the amount of fat (in g) of the upper and lower extremities, trunk, and total body was obtained. Coefficients of variation of DXA have been reported as 3% for body fat mass (21).
Computed tomography.
Each subject underwent cross-sectional CT scan of the abdomen at the level of L4. Assessment of visceral and subcutaneous abdominal fat compartments by single-slice CT image of the abdomen was performed. Patients were placed supine, feet first in the scanner, and with flexion of the knees to minimize lumbar lordosis. A lateral scout image was obtained to identify the level of L4, which served as the landmark for the single-slice image. Scan parameters for each image were standardized (144 table height, 80 kV, 70 mA, 2 s, 1-cm slice thickness, 48 field of view). Fat attenuation coefficients were set at −50 to −250 Hounsfield units (HU) as described by Borkan et al. (7). Total abdominal cross-sectional area was computed by outlining the outer contour of the abdomen. A second outline of the back and abdominal wall musculature (inner contour) was used to define the subcutaneous fat area. Visceral abdominal fat was defined as the area within the inner contour comprising all pixels with attenuation coefficients between −50 and −250 HU. The total fat area was calculated as the sum of subcutaneous fat and visceral abdominal fat. These values were used to calculate the area of subcutaneous adipose tissue, visceral adipose tissue, and total adipose tissue.
Statistical analysis.
JMP Statistical Discoveries (version 4.0.2; SAS Institute, Cary, NC) and MedCalc (version 9.2.1.0; Mariakerke, Belgium) were used for statistical analysis. The means and SD were calculated, and groups were compared using the Student's t-test. ROC curve analysis of different methods of body composition measurements was performed to determine sensitivity and specificity, area under the ROC curve, and confidence intervals (CI), as well as cutoff values for each method to detect GH deficiency.
Since this was an exploratory study, we did not perform a validation study. However, we performed a cross-validated error estimate to determine the error estimate for each measure.
Power calculation.
The t-test power was used to approximate the power of the ROC curve test. With proposed sample sizes of 9 for the GH-deficient and 36 for the GH-sufficient groups, the study will have a power of 82.3% to yield a statistically significant result (that the area under the ROC curve is greater than 0.5). This computation assumes that the mean difference is 1.1 (corresponding to means of 1.1 vs. 0.0) and the common within-group standard deviation is 1.0.
RESULTS
Clinical characteristics of study subjects.
Subject characteristics are shown in Table 1. Age of study participants ranged from 19 to 45 yr, mean 33 ± 8.3 yr (SD). Study participants ranged in BMI from 19.2 to 43.6 kg/m2, mean 30.9 ± 6.5 kg/m2 (SD), and were categorized as lean (n = 10) if BMI was <25 kg/m2, overweight (n = 12) if BMI was ≥25 and <30 kg/m2, and obese (n = 23) if BMI was ≥30 kg/m2, based on World Health Organization definitions (1). Nine patients had GH deficiency as determined by the GHRH-arginine stimulation test, and 36 subjects were GH sufficient. Subjects with GH deficiency were slightly older and had higher weight, BMI, and total, subcutaneous, and visceral fat, as determined by CT, compared with the GH-sufficient subjects. Clinical characteristics of the two groups are shown in Table 2.
Table 1.
Clinical characteristics of all subjects
| Variable | All Subjects |
|---|---|
| n | 45 |
| Age, yr | 33±8.3 |
| Weight, kg | 82±18.3 |
| BMI, kg/m2 | 30.9±6.5 |
| GH stimulation peak, ng/ml | 14.8±11 |
Values are means and SD. BMI, body mass index; GH, growth hormone.
Table 2.
Clinical characteristics of GH-deficient and GH-sufficient subjects
| Variable | GH Peak 5 ng/ml | GH Peak ≥5 ng/ml | P Value |
|---|---|---|---|
| n | 9 | 36 | |
| Age, yr | 38.4±6.2 | 31.7±8.2 | 0.03 |
| Weight, kg | 101.3±17.3 | 78.1±15.6 | 0.0003 |
| BMI, kg/m2 | 37±5.2 | 29.4±6 | 0.001 |
| Total abdominal fat, mm2 | 82,078±21,911 | 48,026±22,625 | 0.0002 |
| Subcutaneous fat, mm2 | 51,895±16,320 | 33,693±14,474 | 0.002 |
| Visceral fat, mm2 | 16,852±5,349 | 7,127±3,868 | <0.0001 |
| GH stimulation peak, ng/ml | 3.4±1.2 | 17.7±10.5 | 0.0002 |
Body composition determinants of GH deficiency.
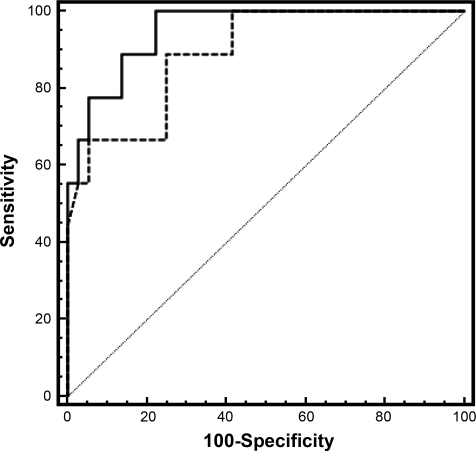
Results of ROC curve analyses are summarized in Table 3. On the basis of ROC curves, visceral adiposity measured by CT showed the highest sensitivity and specificity for identifying subjects with GH deficiency. The area under the curve (AUC) was 0.95 with a cutoff value of >9,962 mm2, sensitivity was 100%, and specificity was 77.8% (P = 0.0001). Sensitivity and specificity of the cross-validated error estimate were 89 and 75%, respectively. The largest waist circumference was the umbilical waist circumference in 75% of patients. Largest waist circumference showed high sensitivity and specificity when a cutoff value of >101.7 cm was used (AUC, 0.89; sensitivity, 88.9%; specificity, 75%; P = 0.0001). Sensitivity and specificity of the cross-validated error estimate were 67 and 72%, respectively. With the use of a cutoff value of >80 cm for largest waist circumference, as used to diagnose metabolic syndrome by the International Diabetes Federation (3), sensitivity was 100%, but specificity was only 9%. With the use of a cutoff value of >88 cm for the largest waist circumference, as proposed by Lean et al. (18) to determine visceral adiposity, sensitivity was 100%, but specificity dropped to 25%. Comparing ROC curves of visceral fat measured by CT and largest waist circumference (cutoff value of >101.7 cm), the difference between the two methods was not statistically significant (P = 0.36) (Fig. 1).
Table 3.
ROC curve analysis of different body composition methods
| Method | Threshold | Sensitivity | Specificity | ROC AUC | 95% Confidence Interval | P Value |
|---|---|---|---|---|---|---|
| BMI, kg/m2 | >36.4 | 66.7 | 88.9 | 0.83 | 0.69–0.92 | 0.0002 |
| Weight, kg | >79.8 | 100 | 63.9 | 0.85 | 0.71–0.94 | 0.0001 |
| Waist circumference-largest, cm | >101.7 | 88.9 | 75 | 0.89 | 0.76–0.96 | 0.0001 |
| Waist circumference-umbilicus, cm | >101.7 | 88.9 | 74.3 | 0.89 | 0.75–0.96 | 0.0001 |
| Waist circumference-iliac, cm | >99 | 100 | 66.7 | 0.84 | 0.7–0.93 | 0.0001 |
| Waist circumference-mid, cm | >98.7 | 88.9 | 73.5 | 0.85 | 0.71–0.94 | 0.0001 |
| Waist circumference-smallest, cm | >88.7 | 100 | 60 | 0.86 | 0.72–0.94 | 0.0001 |
| Hip circumference, cm | >109 | 100 | 50 | 0.8 | 0.66–0.91 | 0.0009 |
| Iliac waist-hip ratio | >0.85 | 77.8 | 58.3 | 0.7 | 0.54–0.82 | 0.057 |
| Largest waist-hip ratio | >0.87 | 100 | 66.6 | 0.85 | 0.72–0.94 | 0.0001 |
| Skin body fat, % | >36.4 | 88.9 | 68.6 | 0.79 | 0.64–0.89 | 0.002 |
| Skin biceps, mm | >10.7 | 100 | 52.8 | 0.73 | 0.57–0.85 | 0.025 |
| Skin subscapular, mm | >18.5 | 100 | 33.3 | 0.64 | 0.48–0.78 | 0.18 |
| Skin suprailiac, mm | >22.3 | 100 | 47.2 | 0.71 | 0.55–0.83 | 0.043 |
| Skin triceps, mm | >22.7 | 100 | 52.8 | 0.76 | 0.61–0.87 | 0.008 |
| Abdomenal CT total area, mm2 | >66800 | 88.9 | 80.6 | 0.85 | 0.71–0.94 | 0.0001 |
| Abdomenal CT subcutaneous fat, mm2 | >54700 | 66.7 | 91.7 | 0.8 | 0.66–0.91 | 0.0009 |
| Abdpmenal CT visceral fat, mm2 | >9962 | 100 | 77.8 | 0.95 | 0.84–0.99 | 0.0001 |
| DXA fat left arm, % | >43.8 | 100 | 61.1 | 0.8 | 0.65–0.9 | 0.0012 |
| DXA fat right arm, % | >42.1 | 100 | 63.9 | 0.83 | 0.69–0.92 | 0.0002 |
| DXA fat left leg, % | >45 | 77.8 | 75 | 0.77 | 0.62–0.88 | 0.0043 |
| DXA fat right leg, % | >44.6 | 77.8 | 77.8 | 0.76 | 0.61–0.87 | 0.0078 |
| DXA fat trunk, % | >39.9 | 100 | 66.7 | 0.85 | 0.71–0.94 | 0.0001 |
| DXA %fat total | >39.1 | 100 | 63.9 | 0.83 | 0.69–0.93 | 0.0001 |
| DXA fat trunk, g | >16246 | 100 | 69.4 | 0.87 | 0.74–0.95 | 0.0001 |
| DXA fat total, g | >31677 | 100 | 66.7 | 0.88 | 0.74–0.95 | 0.0001 |
| DXA lean total, g | >45637 | 100 | 36.1 | 0.7 | 0.55–0.83 | 0.05 |
| DXA lean trunk, g | >23323 | 100 | 61.1 | 0.77 | 0.62–0.88 | 0.004 |
ROC, receiver operator characteristic; CT, computed tomography; DXA, dual-energy X-ray absorptiometry.
Fig. 1.
Receiver operator characteristic (ROC) curve of visceral fat measured by computed tomography (CT; solid line) and largest waist circumference (dashed line) to detect growth hormone (GH) deficiency. Although the area under the curve (AUC) is larger for the CT measurement, the difference is not significant (AUC for CT, 0.95; AUC for largest waist circumference, 0.89; P = 0.36).
Determination of total and trunk fat content as measured by DXA showed an AUC of 0.87 and 0.88 with sensitivity of 100% and specificity of 69.4 and 66.7%, respectively, with the use of a cutoff value of >1,6246 and >3,1677 g, respectively (P = 0.0001). Sensitivity and specificity of the cross-validated error estimate were 67 and 64%, respectively, for total fat and 67 and 67%, respectively, for trunk fat. Comparing ROC curves of visceral fat measured by CT and trunk fat (g) measured by DXA, the difference between the two methods was not statistically significant (P = 0.36). The largest waist-to-hip ratio demonstrated an AUC of 0.85, sensitivity was 100%, and specificity was 66.6% when a cutoff value of >0.85 was used (P = 0.0001). Sensitivity and specificity of the cross-validated error estimate were 67 and 57%, respectively. Comparing the ROC curves of visceral fat as measured by CT and largest hip-to-waist ratio, the difference between the two methods was not statistically significant (P = 0.3).
DISCUSSION
Our study showed that the largest waist circumference can predict GH deficiency in premenopausal women and that this measurement is almost as sensitive and specific for predicting GH deficiency as visceral fat measured by abdominal CT. These data may be of importance if further research confirms the association of GH deficiency with increased cardiovascular risk.
An increased prevalence of visceral adiposity and cardiovascular events has been established in women with GH deficiency due to hypopituitarism. Studies have shown that decreased GH secretion is an independent risk factor for visceral obesity and cardiovascular disease in this patient population. Higher cardiovascular mortality in female GH-deficient patients than in males has been found (9, 24). This may reflect a relatively more severe state of GH deficiency in women compared with men, since GH secretion is nearly twice as high in young, healthy women as in men (11). Therefore, we focused our study on healthy overweight and obese women and the relationship between visceral adiposity and GH deficiency in this patient population.
Multiple studies have demonstrated decreases in visceral adiposity, without a change in overall weight or BMI in GH-deficient patients during physiological GH administration (5, 15). Although not approved by the Food and Drug Administration (FDA) for clinical use, GH replacement has been studied in subjects with visceral adiposity without pituitary or hypothalamic disease and may improve insulin sensitivity over time, potentially due to adipose reduction. In a study by Johannsson et al. (17), administration of low-dose GH to obese men resulted in decreased visceral fat mass, suggesting a possible therapeutic role for GH in patients with visceral obesity. In a study evaluating GH administration in obese postmenopausal women (12), 12 mo of GH administration reduced the amount of visceral fat and increased thigh muscle mass, whereas no change in subcutaneous adipose tissue was observed. Therefore, if further research confirms these effects, it might be useful to develop diagnostic tools to identify subjects with visceral adiposity who might be at risk for being GH deficient and in whom formal GH stimulation testing should be performed.
Sophisticated imaging modalities such as CT or MRI are able to distinguish visceral from subcutaneous fat with a high level of precision, but these methods are expensive and time consuming, and CT involves radiation exposure (2, 26). Simple anthropometric variables such as waist circumference and waist-to-hip ratio have been used to estimate visceral adipose tissue. Several studies have indicated that the waist circumference is strongly related to health risks associated with obesity and that it correlates with visceral fat measured by CT (13, 16). Our study has shown that the largest waist circumference is an easy and reliable method that can predict GH deficiency in premenopausal women. Waist circumferences can be measured at several locations. In our study, the waist circumference measured at the umbilicus corresponded in 75% of study participants to the largest waist circumference. Visceral adiposity measured with CT showed a higher sensitivity and specificity in detecting GH deficiency than largest waist circumference. However, the difference between the two methods was not statistically significant. Based on ROC curves, the most sensitive and specific cutoff was >102 cm in women for largest waist circumference. Current guidelines suggest a cutoff of >88 cm in women based on detection of many metabolic risk factors (16). In our study, the specificity of detecting GH deficiency dropped from 75 to 25% when 88 cm was used as a cutoff. The International Diabetes Federation suggests a cutoff value for largest waist circumference of >80 cm to diagnose metabolic syndrome. With the use of this cutoff value, the specificity in our study dropped to 9%. In a study by Wahrenberg et al. (33), a cutoff value of >100 cm was sensitive and specific for predicting insulin resistance in men and women. Based on our data, a waist circumference of >102 cm in premenopausal women provides a useful reference value to identify obese women who may be at risk for GH deficiency and who should undergo formal GH stimulation testing.
In our study, BMI and iliac waist-to-hip ratio measurements showed low sensitivity and specificity in predicting GH deficiency. Trunk and total fat as determined by DXA showed high sensitivity and specificity in detecting GH deficiency. However, sensitivity and specificity were higher for the largest waist circumference. In addition, DXA requires radiation exposure. As expected, skin fold measurements did not predict GH deficiency in our population. We performed skin fold thickness measurements of the triceps, biceps, subscapular, and suprailiac areas to present the complete spectrum of anthropometric measurements.
Our study had several limitations. First is the relatively small number of subjects who were GH deficient (n = 9) compared with those who were GH sufficient (n = 36). Second, we only studied premenopausal women. There are sex- and age-related differences in the relation of waist measurement to accumulation of visceral adipose tissue (22, 23). Thus it is likely that different cutoff values for waist circumference as predictors of GH deficiency would be found in pre- and postmenopausal women and in men. We also did not perform a validation study to test the proposed cutoff values derived in our study in a different population. However, we performed a cross-validated error estimate that confirmed our cutoff values. Without a larger sample size and without appropriate cross-validation data, our cutoff values for waist circumference should be viewed with caution until results of larger studies have become available. Since there were only nine cases in our sample, estimates of sensitivity would have a standard error of 13% (calculated at 80% sensitivity). A measured sensitivity of 100% would have a 95% lower confidence bound of 72%. For many clinical applications, this is not adequate, and further validation studies in larger patient populations should be performed.
In conclusion, the largest waist circumference predicts the presence of GH deficiency in premenopausal women without hypothalamic or pituitary disease with high sensitivity and specificity and nearly as well as CT measurement of visceral adiposity. This provides further evidence of the importance of visceral fat mass as a predictor and possible mechanism for GH deficiency in young healthy women. GH replacement has not been established to be a safe and effective treatment for young overweight or obese women and is not FDA approved. However, should GH deficiency be established to have important cardiovascular risk or metabolic consequences in the future, we raise the possibility that a simple test that can be performed in any office with a tape measure might be able to identify women in whom GH deficiency is likely and therefore in whom formal GH stimulation testing might be indicated.
GRANTS
This work was supported in part by National Institutes of Health Grants HL-077674, M01 RR-01066, and K23 RR-23090.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Obesity: preventing and managing the global epidemic. Report of a WHO consultation. World Health Organ Tech Rep Ser 894: i–xii, 1–253, 2000. [PubMed] [Google Scholar]
- 2.Abate N, Burns D, Peshock RM, Garg A, Grundy SM. Estimation of adipose tissue mass by magnetic resonance imaging: validation against dissection in human cadavers. J Lipid Res 35: 1490–1496, 1994. [PubMed] [Google Scholar]
- 3.Alberti KG, Zimmet P, Shaw J. The metabolic syndrome—a new worldwide definition. Lancet 366: 1059–1062, 2005. [DOI] [PubMed] [Google Scholar]
- 4.Bengtsson BA, Brummer RJ, Eden S, Bosaeus I. Body composition in acromegaly. Clin Endocrinol (Oxf) 30: 121–130, 1989. [DOI] [PubMed] [Google Scholar]
- 5.Bengtsson BA, Eden S, Lonn L, Kvist H, Stokland A, Lindstedt G, Bosaeus I, Tolli J, Sjostrom L, Isaksson OG. Treatment of adults with growth hormone (GH) deficiency with recombinant human GH. J Clin Endocrinol Metab 76: 309–317, 1993. [DOI] [PubMed] [Google Scholar]
- 6.Biller BM, Samuels MH, Zagar A, Cook DM, Arafah BM, Bonert V, Stavrou S, Kleinberg DL, Chipman JJ, Hartman ML. Sensitivity and specificity of six tests for the diagnosis of adult GH deficiency. J Clin Endocrinol Metab 87: 2067–2079, 2002. [DOI] [PubMed] [Google Scholar]
- 7.Borkan GA, Gerzof SG, Robbins AH, Hults DE, Silbert CK, Silbert JE. Assessment of abdominal fat content by computed tomography. Am J Clin Nutr 36: 172–177, 1982. [DOI] [PubMed] [Google Scholar]
- 8.Bosaeus I, Johannsson G, Rosen T, Hallgren P, Tolli J, Sjostrom L, Bengtsson BA. Comparison of methods to estimate body fat in growth hormone deficient adults. Clin Endocrinol (Oxf) 44: 395–402, 1996. [DOI] [PubMed] [Google Scholar]
- 9.Bulow B, Hagmar L, Mikoczy Z, Nordstrom CH, Erfurth EM. Increased cerebrovascular mortality in patients with hypopituitarism. Clin Endocrinol (Oxf) 46: 75–81, 1997. [DOI] [PubMed] [Google Scholar]
- 10.Carrel AL, Allen DB. Effects of growth hormone on adipose tissue. J Pediatr Endocrinol Metab 13, Suppl 2: 1003–1009, 2000. [PubMed] [Google Scholar]
- 11.Clasey JL, Weltman A, Patrie J, Weltman JY, Pezzoli S, Bouchard C, Thorner MO, Hartman ML. Abdominal visceral fat and fasting insulin are important predictors of 24-hour GH release independent of age, gender, and other physiological factors. J Clin Endocrinol Metab 86: 3845–3852, 2001. [DOI] [PubMed] [Google Scholar]
- 12.Franco C, Brandberg J, Lonn L, Andersson B, Bengtsson BA, Johannsson G. Growth hormone treatment reduces abdominal visceral fat in postmenopausal women with abdominal obesity: a 12-month placebo-controlled trial. J Clin Endocrinol Metab 90: 1466–1474, 2005. [DOI] [PubMed] [Google Scholar]
- 13.Freiberg MS, Pencina MJ, D'Agostino RB, Lanier K, Wilson PW, Vasan RS. BMI vs. waist circumference for identifying vascular risk. Obesity (Silver Spring) 16: 463–469, 2008. [DOI] [PubMed] [Google Scholar]
- 14.Gerver WJ, De Bruin R, Delemarre v d Waal HA, Aldewereld B, Theunissen P, Westerterp KR. Effects of discontinuation of growth hormone treatment on body composition and metabolism. Horm Res 53: 215–220, 2000. [DOI] [PubMed] [Google Scholar]
- 15.Han TS, Lean ME. Bioelectrical impedance analysis in nutritional research. Nutrition 14: 707–708, 1998. [DOI] [PubMed] [Google Scholar]
- 16.Han TS, van Leer EM, Seidell JC, Lean ME. Waist circumference action levels in the identification of cardiovascular risk factors: prevalence study in a random sample. BMJ 311: 1401–1405, 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Johannsson G, Marin P, Lonn L, Ottosson M, Stenlof K, Bjorntorp P, Sjostrom L, Bengtsson BA. Growth hormone treatment of abdominally obese men reduces abdominal fat mass, improves glucose and lipoprotein metabolism, and reduces diastolic blood pressure. J Clin Endocrinol Metab 82: 727–734, 1997. [DOI] [PubMed] [Google Scholar]
- 18.Lean ME, Han TS, Morrison CE. Waist circumference as a measure for indicating need for weight management. BMJ 311: 158–161, 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lukaski HC Methods for the assessment of human body composition: traditional and new. Am J Clin Nutr 46: 537–556, 1987. [DOI] [PubMed] [Google Scholar]
- 20.Mattsson S, Thomas BJ. Development of methods for body composition studies. Phys Med Biol 51: R203–R228, 2006. [DOI] [PubMed] [Google Scholar]
- 21.Mazess RB, Barden HS, Bisek JP, Hanson J. Dual-energy x-ray absorptiometry for total-body and regional bone-mineral and soft-tissue composition. Am J Clin Nutr 51: 1106–1112, 1990. [DOI] [PubMed] [Google Scholar]
- 22.Misra A, Wasir JS, Vikram NK. Waist circumference criteria for the diagnosis of abdominal obesity are not applicable uniformly to all populations and ethnic groups. Nutrition 21: 969–976, 2005. [DOI] [PubMed] [Google Scholar]
- 23.Molarius A, Seidell JC, Visscher TL, Hofman A. Misclassification of high-risk older subjects using waist action levels established for young and middle-aged adults—results from the Rotterdam Study. J Am Geriatr Soc 48: 1638–1645, 2000. [DOI] [PubMed] [Google Scholar]
- 24.Nilsson B, Gustavasson-Kadaka E, Bengtsson BA, Jonsson B. Pituitary adenomas in Sweden between 1958 and 1991: incidence, survival, and mortality. J Clin Endocrinol Metab 85: 1420–1425, 2000. [DOI] [PubMed] [Google Scholar]
- 25.Rexrode KM, Carey VJ, Hennekens CH, Walters EE, Colditz GA, Stampfer MJ, Willett WC, Manson JE. Abdominal adiposity and coronary heart disease in women. JAMA 280: 1843–1848, 1998. [DOI] [PubMed] [Google Scholar]
- 26.Rossner S, Bo WJ, Hiltbrandt E, Hinson W, Karstaedt N, Santago P, Sobol WT, Crouse JR. Adipose tissue determinations in cadavers–a comparison between cross-sectional planimetry and computed tomography. Int J Obes 14: 893–902, 1990. [PubMed] [Google Scholar]
- 27.Smith SR, Lovejoy JC, Greenway F, Ryan D, deJonge L, de la Bretonne J, Volafova J, Bray GA. Contributions of total body fat, abdominal subcutaneous adipose tissue compartments, and visceral adipose tissue to the metabolic complications of obesity. Metabolism 50: 425–435, 2001. [DOI] [PubMed] [Google Scholar]
- 28.Snijder MB, Visser M, Dekker JM, Seidell JC, Fuerst T, Tylavsky F, Cauley J, Lang T, Nevitt M, Harris TB. The prediction of visceral fat by dual-energy X-ray absorptiometry in the elderly: a comparison with computed tomography and anthropometry. Int J Obes Relat Metab Disord 26: 984–993, 2002. [DOI] [PubMed] [Google Scholar]
- 29.Utz A, Yamamoto A, Sluss P, Breu J, Miller KK. Androgens may mediate a relative preservation of IGF-1 levels in overweight and obese women despite reduced growth hormone secretion. J Clin Endocrinol Metab 93: 4033–4040, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Utz AL, Yamamoto A, Hemphill L, Miller KK. Growth hormone deficiency by growth hormone releasing hormone-arginine testing criteria predicts increased cardiovascular risk markers in normal young overweight and obese women. J Clin Endocrinol Metab 93: 2507–2514, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vahl N, Jorgensen JO, Jurik AG, Christiansen JS. Abdominal adiposity and physical fitness are major determinants of the age associated decline in stimulated GH secretion in healthy adults. J Clin Endocrinol Metab 81: 2209–2215, 1996. [DOI] [PubMed] [Google Scholar]
- 32.Vahl N, Jorgensen JO, Skjaerbaek C, Veldhuis JD, Orskov H, Christiansen JS. Abdominal adiposity rather than age and sex predicts mass and regularity of GH secretion in healthy adults. Am J Physiol Endocrinol Metab 272: E1108–E1116, 1997. [DOI] [PubMed] [Google Scholar]
- 33.Wahrenberg H, Hertel K, Leijonhufvud BM, Persson LG, Toft E, Arner P. Use of waist circumference to predict insulin resistance: retrospective study. BMJ 330: 1363–1364, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wajchenberg BL Subcutaneous and visceral adipose tissue: their relation to the metabolic syndrome. Endocr Rev 21: 697–738, 2000. [DOI] [PubMed] [Google Scholar]
- 35.Zhang C, Rexrode KM, van Dam RM, Li TY, Hu FB. Abdominal obesity and the risk of all-cause, cardiovascular, and cancer mortality: sixteen years of follow-up in US women. Circulation 117: 1658–1667, 2008. [DOI] [PubMed] [Google Scholar]