Abstract
Although there is consensus that the central nervous system mediates the increases in maximal voluntary force (maximal voluntary contraction, MVC) produced by resistance exercise, the involvement of the primary motor cortex (M1) in these processes remains controversial. We hypothesized that 1-Hz repetitive transcranial magnetic stimulation (rTMS) of M1 during resistance training would diminish strength gains. Forty subjects were divided equally into five groups. Subjects voluntarily (Vol) abducted the first dorsal interosseus (FDI) (5 bouts × 10 repetitions, 10 sessions, 4 wk) at 70–80% MVC. Another group also exercised but in the 1-min-long interbout rest intervals they received rTMS [Vol+rTMS, 1 Hz, FDI motor area, 300 pulses/session, 120% of the resting motor threshold (rMT)]. The third group also exercised and received sham rTMS (Vol+Sham). The fourth group received only rTMS (rTMS_only). The 37.5% and 33.3% gains in MVC in Vol and Vol+Sham groups, respectively, were greater (P = 0.001) than the 18.9% gain in Vol+rTMS, 1.9% in rTMS_only, and 2.6% in unexercised control subjects who received no stimulation. Acutely, within sessions 5 and 10, single-pulse TMS revealed that motor-evoked potential size and recruitment curve slopes were reduced in Vol+rTMS and rTMS_only groups and accumulated to chronic reductions by session 10. There were no changes in rMT, maximum compound action potential amplitude (Mmax), and peripherally evoked twitch forces in the trained FDI and the untrained abductor digiti minimi. Although contributions from spinal sources cannot be excluded, the data suggest that M1 may play a role in mediating neural adaptations to strength training.
Keywords: muscle, transcranial magnetic stimulation, cortical excitability
it is well established that neural mechanisms mediate the initial gains in maximal voluntary strength in response to chronic resistance exercise (9, 16, 19, 20, 39). However, there is disagreement concerning the magnitude and nature of involvement of specific structures of the central nervous system in neuronal adaptations to chronic exercise. A handful of studies used transcranial magnetic stimulation (TMS) to determine whether the primary motor cortex (M1) contributes to neuronal adaptations to resistance training-induced increases in voluntary force (9, 25, 30). Carroll et al. (9) found that resistance training did not modify the size of the TMS-produced motor-evoked potentials (MEPs) at rest, but the force of the first dorsal interosseus muscle (FDI) at which maximum MEP amplitude occurred significantly shifted from ∼50% maximal voluntary force (maximal voluntary contraction, MVC) before training to ∼35% MVC after training. Coupled with data produced by transcranial electrical brain stimulation that excites subcortical structures, Carroll et al. (9) concluded that “…resistance training changes the functional properties of spinal cord circuitry in humans, but does not substantially affect the organisation of the motor cortex.” Another study reported that the maximal MEP amplitude produced by TMS at rest and during a weak contraction of the elbow flexors increased after motor learning but not after resistance training, suggesting a minimal role for M1 in the adaptations to resistance training (30). In contrast, MEP amplitude produced by TMS during weak contractions of the tibialis anterior muscle increased 32% after resistance training (25). Therefore, under some experimental conditions and in specific muscles resistance training may increase (25), decrease (30), or produce no changes in (9) corticospinal excitability.
To address the possible role of M1 in the responses to chronic resistance exercise, we used a different approach and applied repetitive magnetic brain stimulation (rTMS) to inhibit M1 during training and potentially interfere with increases in MVC. rTMS has been used extensively to manipulate the excitability of motor cortical structures, including M1. High-frequency (>1 Hz) rTMS tends to increase corticospinal excitability, and low-frequency rTMS (≤1 Hz) administered below, at, and above resting motor threshold (rMT) tends to decrease corticospinal excitability that can last for up to an hour after the stimulation is stopped (26, 45). Even high-frequency magnetic brain stimulation in the form of continuous magnetic theta-burst stimulation, when administered after voluntary contractions, can depress corticospinal excitability (21). Several studies demonstrated that the reduction in corticospinal excitability was not accompanied by a reduction in motor output (11, 40, 43, 58). However, a few studies did report impairments in motor output, including reaction time and finger tracking performance, after bouts of 1-Hz rTMS (7, 51). Prior motor training also reduced the response to a subsequent facilitatory rTMS protocol, suggesting that rTMS influences functionally important circuits (53). Therefore, rTMS could interfere with the processes that contribute to natural behaviors. In addition, voluntary activation tested with TMS was deficient in an intrinsic hand muscle, suggesting that there is some supraspinal capacity rTMS can act upon (28). We thus examined the possibility that, akin to homeostatic-like effects, voluntary muscle contractions, which raise corticospinal excitability, would potentiate the inhibitory effects of subsequently administered 1-Hz rTMS (41, 45, 56). This enhanced inhibitory effect would in turn reduce corticospinal excitability and interfere with the processes involved in generating voluntary force. The purpose of the present study was to compare the effects of resistance exercise training with and without 1-Hz rTMS on MVC and motor cortical excitability in healthy young human volunteers. We hypothesized that 1-Hz rTMS of M1 during resistance exercise training would diminish strength gains compared with exercise training alone.
METHODS
Participants and Study Design
Forty adults (10 women, 30 men) volunteered for the study. Participants gave their informed consent to the experimental procedures, which were approved by the Institutional Review Board of the National Institute of Neurological Disorders and Stroke (NINDS). The study was performed in accordance with the Declaration of Helsinki. Participants were interviewed about their general health on the telephone and were included only if they were right-handed based on the Oldfield handedness questionnaire (42) and had no history of neurological disorders, including no head or hand injuries. After the phone interview, a neurologist conducted a neurological examination of the prospective participant to verify that he/she met inclusion criteria. Next, volunteers visited the laboratory and were familiarized with the equipment and testing and training procedures. Participants' mean age, height, and body mass were 30.3 yr (±SE 1.2), 1.76 m (±0.02), and 73.4 kg (±2.7), respectively.
Participants were randomly assigned to one of five interventions (each n = 8: 6 men, 2 women). A group of subjects strength-trained the right FDI with voluntary contractions (Vol); another group strength-trained the right FDI and received rTMS; the third group strength-trained the right FDI and received sham rTMS; the fourth group did not strength-train and received rTMS only; and participants in the control group did not exercise, did not receive rTMS, and performed testing only. The groups were similar in age, height, and body mass. Participants attended ten 20-min-long training sessions on nonconsecutive days over a 4-wk period. Training sessions 1, 5, and 10 were longer, ∼1.5 h, than the other sessions because MVC of the right FDI, the target muscle, and the right abductor digiti minimi (ADM), a control muscle, was measured and peripheral nerve stimulation and TMS experiments were conducted before and after the training bouts. ADM was used to determine the spatial specificity of strength gains. We did not use a thumb muscle as a control because in our experimental arrangement the right thumb was strongly abducted and its prime movers stretched, and passive stretch is known to change contractile properties and also provides sensory feedback (22). In all experiments subjects were comfortably seated in a reclining padded chair, equipped with an armrest on each side and an adjustable head support.
Testing Procedures
Voluntary force.
A custom-designed hand dynamometer was used for strength training and testing of the right FDI and was reconfigured for the testing of the right ADM. The dynamometer's plexi base was affixed to the chair's armrest, supporting the forearm. The participant's right hand rested on the plexi base with the palm flat. The index finger was isolated with the thumb extended and abducted and the third, fourth, and fifth fingers extended and, with a Velcro strap, abducted. The center of the proximal interphalangeal joint of the extended index finger was aligned with the center of the load cell (model 31, Honeywell-Sensotec, Columbus, OH). The wrist was stabilized with a Velcro strap wrapped around the chair's armrest and the dynamometer's plexi base. The optimal position of the load cell was determined for each subject and adjusted individually. Such a setup ensured that the sole source of force production was index finger abduction. In separate measurements with the same dynamometer reconfigured, the right little finger was also isolated from the fourth, third, and second fingers and its proximal interphalangeal joint aligned with the center of the load cell, and the abduction force was measured. MVC was determined in the right FDI and ADM. Subjects were familiarized with the procedure by performing two trials of 5-s-long force production at 50%, 75%, and 90% MVC, respectively. Subjects were then instructed to perform two trials of 5-s-long MVC with 1 min of intertrial rest, and the force was recorded from these trials on the computer. Participants received biofeedback of their force production from an oscilloscope (model TDS220, Tektronix, Richardson, TX). The order of testing of the right FDI and ADM was systematically rotated between subjects.
Electromyography.
Electromyography (EMG) activity of the right FDI and ADM was recorded with silver-silver chloride surface EMG electrodes (2-cm center-to-center interelectrode distance) placed over these muscles in a belly-tendon montage. EMG signals were amplified with a Nicolet Viking electromyography system (Skovlunde, Denmark) and band pass filtered between 10 and 2,000 Hz. Signals were digitized at a frequency of 5 kHz and fed into a laboratory computer for further off-line analysis. EMG activity was recorded during cortical and peripheral nerve stimulation. In addition, EMG activity was also recorded from the right ADM during rTMS interventions to monitor its potential spread of excitation to muscles not previously activated by rTMS as a warning sign for a seizure.
Transcranial magnetic stimulation and peripheral nerve stimulation.
TMS was delivered to the optimal scalp position for activation of the right FDI overlying left M1. Before TMS, a cap (Electro Caps International, Eaton, OH) was placed on the subject's head. For each subject we marked individual anatomic landmarks on the cap that allowed us to place the cap on the head in the same position within and across sessions. The optimal coil position was also marked in a coordinate system drawn on the surface of the cap. Thus the coil was placed on the surface of the cap in the same position within and across sessions. These procedures made it possible to stimulate the same area of the cortex within and across sessions. The intersection of the coil was placed tangentially to the scalp with the handle pointing backward and laterally at a 45° angle away from the midline over the hot spot for the FDI muscle. The direction of the current flow was posterior to anterior across M1. The coil was secured with a coil holder to ensure that the same area of the cortex was stimulated within and across sessions. Single-pulse stimuli were delivered at an interstimulus interval of 5 s. During experiments, MEPs were displayed on the monitor of the data collection computer, visually inspected, and stored on a computer for offline analysis. The coil was moved in 0.5-cm steps over M1 to identify the hot spot for activation of the FDI. MEPs were elicited by TMS delivered from a Magstim 200 stimulator (Magstim, Dyfed, UK) through a figure-eight coil (external loop diameter 8 cm; type number SP15602). Measures of cortical excitability included rMT (see below) and amplitude of MEPs in the form of a recruitment curve in sessions 1, 5, and 10 before and after an intervention. rMT was determined with 1% increment in stimulator output as the minimal stimulus intensity required to produce MEPs of at least >50 μV in peak-to-peak amplitude in at least 5 of 10 consecutive trials (48). For the recruitment curve, stimulation started at 10% below rMT and increased in 10% steps of maximal stimulator output until the MEP amplitude did not experience additional increases or the maximal stimulator output was reached. The stimulation intensities were administered in a pseudorandom order, with the highest intensity presented last. There were seven trials to determine MEP amplitudes at each stimulation intensity.
MEP amplitudes were measured peak to peak, averaged off-line, and expressed as a percentage of the maximal motor response (Mmax). To determine Mmax, the ulnar nerve was stimulated (1-ms rectangular pulse; model Viking IV, Nicolet Biomedical, Madison, WI) with supramaximal intensity by bipolar surface electrodes placed at the wrist with the subject's hand in the dynamometer. The intensity of stimulation was increased from a subliminal level until there was no further increase in the peak-to-peak amplitude of the M wave with increasing stimulation intensity. At this final stimulation intensity, we recorded the twitch-evoked force in three trials. We normalized each participant's TMS data to the individual Mmax, making it possible to compare MEP amplitudes between different test sessions. TMS trials with prior background EMG were excluded from the analysis. Stimulation intensity was expressed as a percentage of each participant's rMT. For this purpose, the mean baseline activity for each recruitment curve was calculated and values ±1 SD around the baseline were fitted with one straight-line regression formula. A second straight line (y = a + bx) was fitted to the values forming the steepest part of the curve. rMT was calculated as the intercept of the two regression lines. For the sake of group comparisons, all stimulus intensities were normalized to rMT of a stimulus-response curve determined before an intervention during the first training session. Such a normalization process makes it possible to reliably determine shifts in recruitment curves due to a specific intervention (8).
Testing Protocol
In all subjects, in sessions 1, 5, and 10 EMG electrodes were placed over the right FDI. MVC of the right FDI and ADM was measured, and Mmax evoked in the right FDI, rMT, and a recruitment curve were measured in the right FDI. Participants then completed the randomly assigned intervention. After the intervention, the measurements of the rMT and recruitment curve were repeated. Sessions 1, 5, and 10 lasted ∼1.5 h. In sessions 2–4 and 7–9 only the training protocol was administered, and these sessions lasted ∼20 min.
Interventions
Participants who received the Vol intervention exercised the right FDI with isometric contraction at an intensity of 70–80% of MVC. They performed 5 blocks of 10 contractions to a target zone set on the oscilloscope. The duration of each contraction and the intercontraction rest interval were both 5 s. Participants reached the target zone by gradually increasing force in 0.5 s, and after 4.5 s they reduced force production in 0.5 s. The interblock rest period was 60 s long. We selected the 70–80% zone instead of 100% of MVC as the exercise intensity to avoid fatigue (47) and also to eliminate a skill component (30) from the training program by having the participants aim at a single target line. If the training program had induced fatigue, it would have not been possible to determine whether rTMS had any acute, potentially diminishing effects on force production. In addition, muscle fatigue modulates the excitability of M1 (20); hence fatigue could have interacted with the effects of rTMS. At the start of each exercise session, subjects performed four contractions at 50% of MVC as a warm-up and then performed one contraction at 100% of MVC to assess strength gains and use this value as a base to compute the 70–80% zone of training intensity. Thus all intervention groups exercised in the same intensity zone of relative forces throughout the study. To determine whether the interventions modified MVC acutely within a session, subjects performed one MVC 1 min after the end of the intervention.
Participants who received the Vol+rTMS intervention followed the Vol protocol but also received rTMS during the four 60-s-long interblock periods and after the last (fifth) block of exercise. rTMS was performed in accordance with current safety recommendations (58). A figure-eight-shaped stimulation coil (external loop diameter 9 cm; type no. SP15560), optimally positioned on the scalp over the left M1, was connected to a rapid-rate magnetic stimulator (Magstim, Whitland, Dyfed, UK). The rate of stimulation was 1 Hz at an intensity of 120% of rMT. The coil was secured over the participant's head during each session, and immediately after the completion of the 10th contraction of each block rTMS was started and 60 pulses delivered. Thus there were 5 × 60 for a total of 300 pulses per session and 3,000 pulses total in 10 sessions. Participants who were assigned to the Vol+Sham intervention followed the Vol and Vol+rTMS protocols but received sham rTMS. Sham rTMS was achieved by securing the coil over the participant's head so that the plane of the coil was rotated 90° to the right with the coil wing's edge contacting the scalp. These subjects received sham rTMS at the same time intervals as did the subjects in the real rTMS groups, with the stimulator set at 120% of rMT. Sham rTMS under these conditions did not produce MEPs. Finally, members of the control group did not exercise or receive rTMS and participated in testing only. They came to the laboratory, placed their right hand in the dynamometer, and quietly sat or read a newspaper. During interventions, subjects placed the right index finger in the dynamometer with the proximal interphalangeal joint contacting the load cell interface to eliminate movement-related sensory feedback, which was especially important in the rTMS groups (45).
Statistical Analyses
All data are presented as means ± SE. Changes in muscle strength of the right FDI and ADM were, respectively, analyzed with a group (5) by session (10) and group (5) by session (3) analysis of variance (ANOVA) with repeated measures on session. Mmax and twitch-evoked force, respectively, were analyzed with a group (5) by session (3) ANOVA. Acute changes in rMT within sessions 1, 5, and 10, respectively, were analyzed with a group (5) by time (before, after intervention) ANOVA with repeated measures on time. Acute changes within sessions 1, 5, and 10 in recruitment curves were analyzed with a group (5) by time (2) by session (3) ANOVA with repeated measures on time and session. Acute changes within sessions 1–10 in MVC were analyzed with a group (4) by session (10) ANOVA with repeated measures on session.
Chronic changes in rMT in response to the five interventions were analyzed by comparing rMT before an intervention, respectively, within sessions 1, 5, and 10 with a group (5) by session (3) ANOVA. Chronic changes in recruitment curves in response to the five interventions were analyzed by comparing normalized MEP amplitudes before an intervention, respectively, within sessions 1, 5, and 10 with a group (5) by session (3) by stimulation intensity (8) ANOVA. In case of a significant F value, we used a Tukey's post hoc contrast to identify the means that were different at P < 0.05. Pearson product moment correlation coefficients were computed between rMT and MVC and the changes in MVC between sessions 1 and 10 in the right FDI. In addition, correlation coefficients were computed between percent changes in MVC and Mmax-normalized MEP size measured at the highest stimulation intensity (i.e., 1.6 of rMT) from session 1 to session 10 in Vol, Vol+Sham, Vol+rTMS, and rTMS_only groups.
RESULTS
Muscle Strength
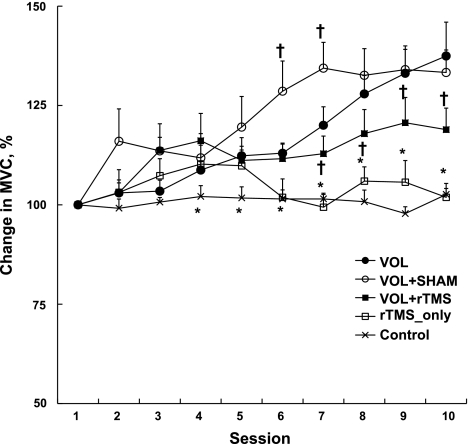
ANOVA revealed a significant group by session interaction in MVC of the right FDI (F = 4.7, P = 0.001). The FDI force at baseline was 32.0 (±2.9), 35.2 (±2.5), 35.2 (±3.4), 35.5 (±4.2), and 33.8 (±4.1) N in Vol, Vol+Sham, Vol+rTMS, rTMS_only, and control groups, respectively. Figure 1 shows the percent changes in MVC. From first to last session, MVC increased 37.5% (±8.5) in the Vol group and a similar amount, 33.3% (±16.0) in the Vol+Sham group. These improvements were significantly more than the 18.9% (±15.3) in the Vol+rTMS group. The 1.9% (±9.9) change in the rTMS_only group and the 2.6% (±4.2) in the control group were not significant and were less than Vol, Vol+Sham, and Vol+rTMS groups (all P < 0.05). The data suggest that rTMS interacted with exertion of voluntary strength because it interfered with improvements in voluntary strength, but it did not reduce voluntary strength when it was applied by itself without strength training.
Fig. 1.
Chronic effects: % change in maximal voluntary force (maximal voluntary contraction, MVC)of the right first dorsal interosseus (FDI) after 500 MVCs at 70–80% intensity over 10 sessions. Vol, voluntary training; Vol+Sham, voluntary training combined with sham transcranial magnetic stimulation (TMS) at 1 Hz; Vol+rTMS, voluntary training combined with repetitive TMS (rTMS) at 1 Hz; rTMS_only, rTMS at 1 Hz only. Values are means ± SE. *rTMS_only and control significantly different from Vol, Vol+Sham, and Vol+rTMS, P < 0.05; †significantly different from all other groups, P < 0.05.
On the basis of the before and after session measurement of MVC, we were able to determine whether any of the interventions produced changes in voluntary force acutely, within each session. A group by session ANOVA of the acute, within-session percent changes in MVC revealed a significant interaction (F = 7.4, P = 0.001). There were no acute changes in MVC in Vol, Vol+Sham, and rTMS_only groups, but the Vol+rTMS group revealed small but significant reductions in MVC of −3.2% (±1.0), −4.1% (±0.8), −5.2% (±1.0), −5.5% (±1.1), −6.5% (±1.2), −5.5% (±1.1), and −6.2% (±1.2) in sessions 4–10, respectively.
The right ADM force at baseline was 11.9 (±1.3), 15.4 (±1.8), 13.5 (±1.9), 15.4 (±1.8), and 14.5 (±1.8) N in Vol, Vol+Sham, Vol+rTMS, rTMS_only, and control groups, respectively. There were no significant changes in the MVC of the ADMs in any group (group main effect F = 0.7, P = 0.611; time main effect F = 1.0, P = 0.374; group by time interaction F = 0.8, P = 0.625). There was no significant group by session interaction (F = 0.6, P = 0.678), group (F = 1.1, P = 0.333), and session (F = 0.5, P = 0.727) main effect in twitch-evoked force elicited in response to a supramaximal stimulus of the ulnar nerve in the right FDI. There also were no changes in twitch-evoked forces in the ADM control muscle.
TMS Data
We refer to “acute effects” as effects that occurred within sessions 1, 5, and 10 and to “chronic effects” as effects that occurred between sessions 1–5, 1–10, and 5–10. The group by session ANOVA revealed that none of the interventions affected Mmax between sessions 1, 5, and 10 (group main effect F = 0.2, P = 0.893; session main effect F = 1.5, P = 0.231; group by session interaction F = 0.9, P = 0.470). None of the interventions produced significant acute (i.e., within sessions 1, 5, and 10) changes in rMT in the right FDI (range of P values from 0.111 to 0.996) or in the right ADM (range of P values from 0.111 to 0.631). The mean of the rMT in the right FDI was 48.5 (±2.4) before (pooled across intervention groups and sessions 1, 5, 10) and 47.9 (±2.6) after intervention.
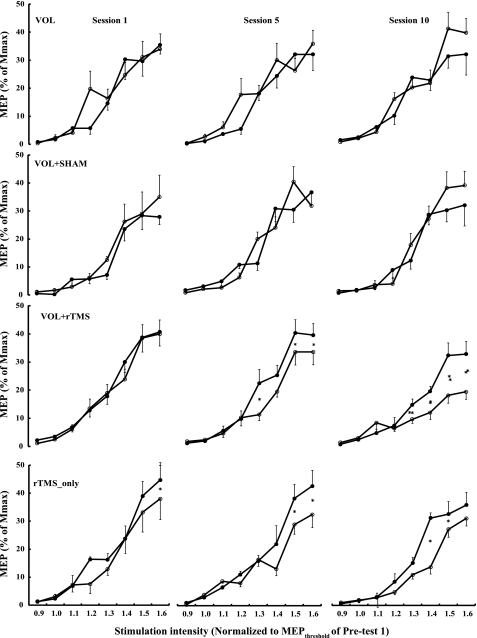
Figure 2 shows the acute effects of the interventions on TMS recruitment curves in the right FDI, and Table 1 summarizes the statistical analyses. The relevant aspects of these analyses were the time main effect (pre, post, 2 levels) and the time by stimulation intensity (8 levels) interaction. A significant time effect signifies a global reduction in MEP size within a session, and a significant interaction term would indicate a change in slope of the recruitment curve within a given session. The Vol and Vol+Sham interventions did not affect MEP size and recruitment slope acutely within sessions 1, 5, and 10. In contrast, the Vol+rTMS intervention reduced MEP size 20% within session 5 (time main effect, P = 0.003) and 32% in session 10 (time main effect, P = 0.037). In addition, this intervention also significantly modified the slope recruitment curve in session 5 (P = 0.001) and session 10 (P = 0.003), especially at the high stimulation intensities. The rTMS_only intervention also reduced the slope of recruitment curve MEP size in session 5 (interaction, P = 0.015) and session 10 (interaction, P = 0.027), with a significant 25% reduction in overall MEP size (time effect, P = 0.012). Again, the interaction was caused by the reductions in MEP size at the high stimulation intensities, as indicated in Fig. 2. There were no changes in the control group. There were also no changes in MEP size of the right ADM after the interventions applied to the right FDI (data not shown).
Fig. 2.
Acute effects of 4 interventions on the size of motor-evoked potentials (MEPs) in session 1, session 5, and session 10. The interventions were voluntary strength training of the right FDI (Vol), voluntary strength training combined with sham rTMS at 1 Hz (Vol+Sham), voluntary strength training combined with 1 Hz rTMS (Vol+rTMS), and rTMS only without strength training (rTMS_only). •, Data collected before an intervention; ○, data collected immediately after the completion of an intervention within a given session. x-Axis: TMS stimulation intensity normalized to the individual MEP threshold determined before a given intervention in session 1. y-Axis: MEP amplitude (means ± SE) normalized to individual maximum action potential amplitude (Mmax) established before a given intervention in each session. There were no changes in the control group, and these data are not shown. *P < 0.05 based on Tukey's post hoc contrast. These data compare the acute effects of a particular intervention within a session.
Table 1.
Acute effects
| Intervention | Session | Time Main Effect |
Time by Stimulation Intensity Interaction | ||
|---|---|---|---|---|---|
| F value | P value | F value | P value | ||
| Vol | 1 | 0.46 | 0.518 | 1.72 | 0.121 |
| 5 | 2.21 | 0.180 | 1.41 | 0.124 | |
| 10 | 0.57 | 0.474 | 2.01 | 0.072 | |
| Vol+Sham | 1 | 0.49 | 0.503 | 0.35 | 0.925 |
| 5 | 0.05 | 0.825 | 1.66 | 0.097 | |
| 10 | 0.84 | 0.389 | 1.87 | 0.090 | |
| Vol+rTMS | 1 | 3.16 | 0.119 | 2.74 | 0.061 |
| 5 | 18.96 | 0.003 | 4.12 | 0.001 | |
| 10 | 6.62 | 0.037 | 3.59 | 0.003 | |
| rTMS_only | 1 | 2.63 | 0.149 | 1.47 | 0.199 |
| 5 | 3.21 | 0.116 | 2.84 | 0.015 | |
| 10 | 11.32 | 0.012 | 2.51 | 0.027 | |
| Control | 1 | 0.13 | 0.726 | 1.89 | 0.091 |
| 5 | 1.23 | 0.305 | 1.02 | 0.430 | |
| 10 | 1.06 | 0.338 | 1.64 | 0.146 | |
Summary of ANOVAs comparing transcranial magnetic stimulation (TMS) recruitment curves immediately before and after an intervention within sessions 1, 5, and 10, respectively.Time main effect refers to the immediate effect of an intervention on motor-evoked potential (MEP) size collapsed across 8 stimulation intensities within sessions 1, 5, and 10, respectively. Time by stimulation intensity interaction refers to the comparison of MEP amplitudes measured at 8 intensities immediately before and after an intervention. Vol, voluntary contraction; Sham, sham TMS; rTMS, repetitive TMS. Significant P values are in bold.
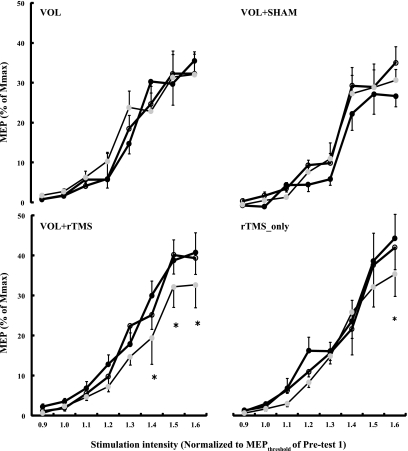
We compared the chronic effects of five interventions on MEP size with a group by session by intensity ANOVA, using in this analysis only the preintervention recruitment curves from sessions 1, 5, and 10. The most relevant aspect of this ANOVA was the group by session two-way interaction (F = 4.7, P = 0.001) and the group by session by intensity three-way interaction (F = 1.5, P = 0.026), the latter indicating that the five interventions modulated the recruitment curves differently across sessions 1, 5, and 10. Figure 3 shows that the Vol and Vol+Sham interventions did not chronically affect MEP size. In contrast, the magnitude of MEP decreased significantly in Vol+rTMS and rTMS_only groups compared with the Vol, Vol+Sham, and control groups. Specifically, the overall MEP size (i.e., pooled across stimulation intensities) remained similar in Vol (sessions 1 vs. 10: 15.5 ± 2.6% vs. 16.4 ± 3.1% of Mmax) and Vol+Sham (sessions 1 vs. 10: 12.4 ± 3.8% vs. 14.6 ± 2.1% of Mmax) groups. However, in the Vol+rTMS group the MEP amplitudes decreased from 19.1% (±3.0) in session 1 to 18.2% (±2.2) in session 5 and further decreased (P < 0.05) to 14.1% (±2.9) of Mmax in session 10, with a overall 26% decrease from session 1 to 10 (P < 0.05). In the rTMS_only group, the MEP amplitudes decreased from 18.1% (±3.4) in session 1 to 17.4% (±2.2) in session 5 and further decreased to 15.9% (±2.4) of Mmax in session 10, with an overall 12% reduction from session 1 to session 10 (P < 0.05). In both of these interventions, therefore, the majority of reductions in MEP occurred in the latter half of the interventions. Figure 3 identifies the specific stimulation intensities at which significant between-session reductions occurred. There were no significant chronic changes in MEP amplitude in the right ADM (data not shown).
Fig. 3.
Chronic effects of 4 interventions on size of MEPs in session 1 (black circles), session 5 (white circles), and session 10 (gray circles). Interventions were voluntary strength training of the right FDI (Vol), voluntary strength training combined with sham rTMS at 1 Hz (Vol+Sham), voluntary strength training combined with 1 Hz rTMS (Vol+rTMS), and rTMS only without strength training (rTMS_only). x-Axis, TMS stimulation intensity normalized to the individual MEP threshold determined before a given intervention in session 1. y-Axis, MEP amplitude (mean ± SE) normalized to individual Mmax established before a given intervention in each session. There were no changes in the control group, and these data are not shown. *P < 0.05 based on Tukey's post hoc contrast relative to sessions 1 and 5. These data compare the chronic effects of a particular intervention across sessions 1, 5, and 10.
Correlation Analyses
There was no significant relationship between rMT and MVC in the data pooled across all subjects at session 1 (r = 0.02, P = 0.834), session 5 (r = 0.01, P = 0.887), and session 10 (r = 0.00, P = 0.911). The correlation between the percent changes in MVC and the changes in the size of the MEP measured at the highest stimulation intensity, computed from data between sessions 1 and 10, were low in Vol, rTMS+Vol, and Vol+Sham groups (range r = 0.01–0.57). There also was no significant relationship between changes in rMT and changes in the size of the Mmax-normalized MEP measured at 1.6 rMT (data not shown).
DISCUSSION
The main finding of the present study was that low-frequency rTMS of M1 diminished exercise-induced gains in MVC. The diminishment in strength gains was limited to Vol+rTMS, whereas rTMS_only did not significantly affect strength gains, suggesting an interaction between voluntary command and the effects of rTMS on M1. These adaptations were not accompanied by changes in twitch forces and were spatially confined to the trained right FDI, because changes in voluntary force and corticospinal excitability were not significant in the untrained right ADM.
The strength training paradigm of the right FDI produced comparable gains in MVC reported previously in the FDI and other small finger muscles without inducing changes in a control muscle, the right ADM (33, 34, 59). The absence of strength gains in the ADM suggests a spatial specificity of the observed training effects in MVC. In addition, the absence of change in MVC in the nonexercising control group, visiting the laboratory as many times as the intervention groups (27), suggests that there was no significant contribution from the testing protocol per se to strength gains observed in the intervention groups.
There is a strong consensus that functional modifications in specific structures of the central nervous system mediate some of the adaptations produced by high-intensity exercise (1, 9, 16, 27, 30, 49, 59). However, evidence remains elusive as to whether the site of adaptations is supraspinal, spinal, or both. A group of animal and human studies suggests that prolonged exercise training with high-intensity voluntary contractions vis-à-vis skill training produces little or no adaptation in the corticospinal neurons and M1 (9, 30, 44). However, resistance training of the tibialis anterior was associated with a 32% increase in MEP amplitude produced by TMS during low-level contractions without changes in M-wave amplitude, indicating a role for spinal, corticospinal neurons, possibly M1 (25). In addition, several other studies showed that adaptations to acute and chronic voluntary and electrical stimulation-evoked muscle contractions, without a skill component, increased volitional drive from supraspinal centers (4, 13, 15, 24, 31, 32) without concomitant changes in H reflex, measured at rest or during mild voluntary contraction (1, 10, 13, 15, 23, 24, 35, 38, 50, 55). However, the interpretation of these studies must be viewed carefully because they do not provide direct evidence for M1's involvement in strength gains and some studies did find increases in H reflex after resistance training (1, 4, 15, 32). In addition, several of these studies reported an increase in the amplitude of the V wave after strength training without changes in H-reflex amplitude at rest and during mild voluntary contractions, implying a role for supraspinal structures in training-induced increases in voluntary force. M1 is arguably the primary supraspinal structure involved in controlling voluntary force (3). We must exercise caution in interpreting the V-wave and H-reflex studies. It is possible that the V waves, recorded during MVCs, and the H reflexes, recorded at rest and during mild contractions, measure the excitability of different motor units. In addition, a change in V-wave amplitude with training could be due to changes in the properties of the Ia afferent: motoneuronal synapse, intrinsic motoneuron properties, and changes in motoneuron firing rate (that could be due to spinal or supraspinal effects), making it difficult to pinpoint the site of adaptation.
The present study expands on these findings by using a new approach, and the data point to a possible role of M1 in adaptations to chronic exercise involving a simple skill. Our data qualitatively agree with previous human and animal studies showing that voluntary strength training can increase MVC without concurrent increase in corticospinal excitability (Figs. 2 and 3) (9, 30, 44). Indeed, corticospinal excitability may remain unchanged because of the low level of task complexity and the limited peripheral feedback normally present in the motor tasks used in such training programs. However, here we addressed the role of M1 in mediating voluntary strength gains with a different approach and asked the question, “Does chronic interference with M1 by rTMS affect strength gains produced by exercise training?” We used this approach because low-frequency rTMS is known to cause widespread inhibition of cortical circuits including M1 excitability (45). While several studies reported that low-rate rTMS does not interfere with simple motor skills such as paced fist clenching (43), finger tapping (11, 58), and finger acceleration during pinching (40), a few studies did report impairments in motor output, including reaction time and finger tracking performance (7, 51). In addition, prior motor training also reduced the response to a subsequent facilitatory rTMS protocol, suggesting that rTMS influences functionally important circuits (53). Because none of these studies administered rTMS chronically (over several days or weeks), the possibility exists that long-term, low-rate rTMS of M1 would reduce its excitability to a level that would interfere with the command needed to generate voluntary force, implicating a role for M1 in mediating increase in voluntary force. Consistent with this suggestion and the results of some (7, 51) but not all (11, 43, 58) studies, we also observed acute (within session) small (∼3–6%) but significant reductions in MVC.
Indeed, an important difference between previous rTMS studies and the present work is that in the previous studies motor practice and rTMS were limited just to one or, at most, a few sessions (7, 11, 43, 45, 51, 58). We found that chronic low-rate, 1-Hz rTMS diminished gains in voluntary force production as healthy individuals exercised their right FDI over 10 sessions with a total of 500 voluntary contractions at 70–80% of MVC. Figure 1 shows that rTMS's diminishing effects on strength gains appeared around the sixth session. We applied rTMS during the 1-min-long rest periods instead of the 5-s-long muscle contractions. Such an experimental arrangement allowed us to control the timing of delivery of the rTMS trains and avoid fluctuations in force that the rTMS pulses would have caused. Such an approach relies on rTMS to exert its inhibitory effects on the involved cortical structures after the rTMS trains are delivered. It is well established that 1-Hz rTMS, consisting of just 150 pulses, i.e., one-half of the number of pulses used in the present study (56), can have aftereffects lasting 2–10 min and reduce corticospinal excitability by 25% or more (45). Therefore, the effect from rTMS that ultimately interfered with motor output was the result of an interaction between the lingering aftereffects of rTMS and the neural command for muscle contraction. That corticospinal excitability was reduced in Vol+rTMS but not in Vol is evidence that such an interaction occurred. Such an interaction was also demonstrated in a different experimental context in which theta-burst stimulation given after a voluntary contraction inhibited MEPs (21). Still, the precise mechanism of how rTMS interfered with motor performance is unclear. One possibility is that chronic 1-Hz rTMS activated cortical inhibitory interneurons (11, 17, 18, 36, 37, 40, 46, 56, 57) and reduced cortical and corticospinal excitability (Figs. 2 and 3) and the responsiveness of M1 to stimulation arising from central drive. Another possibility is that rTMS reduced synaptic efficacy in cortical and corticospinal neurons so that for a given excitatory input from central drive there was less postsynaptic activity akin to long-term depression. Third, rTMS is known to reduce sensitivity in M1 to somatosensory activity (57), so that rTMS may have interfered with the afferent input from the contracting FDI. Hence, the site of rTMS becomes less responsive to activity in motor areas, including M1, involved in execution of the FDI contraction (36).
We used TMS-induced activation of the right FDI to determine the optimal scalp position for rTMS so that the right FDI M1 was definitively in the center of the stimulation field. To test whether rTMS at this site actually interfered with M1, we studied rTMS-induced changes in single-pulse TMS measures of corticospinal excitability, i.e., rMT and MEP size, before and after the rTMS procedure. Consistent with previous reports (11, 17, 18, 36, 37, 40, 46, 56, 57), 1-Hz rTMS resulted in an acute reduction of corticospinal excitability (Fig. 2). Specifically, our results agree with Togue et al.'s data that 150–600 rTMS stimuli delivered at 1 Hz and an intensity of 95% of rMT reduced MEP size by 25% for ∼10 min after rTMS (56). In addition, the initial acute reduction in MEP size in session 1 accumulated to produce a chronic reduction over the 10 sessions, a new finding. We found an overall reduction in MEP size of 26% in the Vol+rTMS group and 12% in the rTMS_only group after 3,000 pulses of 1-Hz rTMS at 120% of rMT between session 1 and session 10 (Figs. 2 and 3). These data are proof that rTMS interfered with M1 acutely and chronically. We are not aware of any studies that have used 1-Hz rTMS chronically for 10 sessions and quantified its effects on MEP size. The rTMS effects were likely to be confined to M1 because previous studies found no changes in various measures of spinal excitability in healthy adults (29, 56). Overall the present data qualitatively complement the findings of Bäumer et al. (6), although they stimulated the premotor area and not M1, showing that 1-Hz rTMS produced cumulative changes in M1 when repeated within 24 h.
The present study cannot determine the specific features of M1 or corticospinal function that can contribute to strength training-induced modulation of voluntary force. Imaging studies suggest that changes in voluntary force could be mediated by the recruitment of cortical areas that are not directly involved in the control of the same movement, resulting in an increase in the area of motor cortex activated (14). rTMS could have interfered with this expansion and affected strength gains. There is evidence for intracortical synaptic reorganization and an increase in cortical excitability following repetitive motor tasks (5) and this reorganization being linked to short-term and long-term potentiation (2). Increased M1 output after resistance training can lead to motor unit synchronization that can increase voluntary force (Ref. 52, but see Ref. 34), and chronic rTMS can affect cortical oscillatory behavior producing such synchronization (12). Still, we cannot tell whether any of these mechanisms played a role under the present experimental conditions.
There are several limitations to the present study. We did not take TMS measurements during muscle contraction, to reduce subjects' exposure to TMS, and this limits the interpretation of the data. This study still leaves unresolved the precise location of adaptation in the nervous system. We did not test for any spinal effects. Considering the inconsistent results from TMS (9, 25, 30) and peripheral nerve stimulation studies (1, 4, 13, 15, 23, 24, 32, 35, 38), a role for segmental effects cannot be dismissed. We intentionally designed the study to consist of only 10 sessions so that the nature of adaptation would primarily be neuronal (9); it is still possible that a portion of the strength gains was due to muscle hypertrophy. We tried to check this by measuring twitch force. We, as others (9), found no changes in the twitch-evoked forces, suggesting that indeed some central factor was involved in the adaptations to the exercise program. It is still possible that intramuscular changes also contributed to strength improvements in the absence of twitch changes through force-frequency effects. The twitch force measurements must be interpreted with caution because ulnar nerve stimulation activates the dorsal as well as the antagonist palmar interossei, which can bias the data (cf. Refs. 9, 54). In addition, tetanic stimulation of the ulnar nerve could have been more sensitive to detect spatial specificity than the twitch measurements. Although we prevented the movement of the index finger, rTMS of the FDI cortical area still produced sporadic twitches in the FDI. Tendon and skin receptors respond to these induced twitches and could have contributed to the rTMS effects. Finally, we did not administer rTMS to a cortical area other than M1 of the FDI area to determine the motor cortical specificity of rTMS's effects on strength gains and also used only one frequency and one intensity. Considering that rTMS of occipital and dorsolateral prefrontal cortex did not affect motor consolidation in a previous study (41), we excluded such control experiments.
In summary, we found that voluntary strength training increased MVC without significant changes in corticospinal excitability measured with TMS. Real but not sham 1-Hz rTMS of the hand area M1 interfered with strength gains, and the diminishment in strength gains was moderately but significantly associated with the reductions in M1 excitability. None of the interventions affected Mmax amplitude, twitch force, or the voluntary force in the spatially separate right ADM. Although contributions from spinal sources cannot be excluded, the data suggest that M1 may play a role in mediating neural adaptations to strength training.
GRANTS
This work was supported in part by National Institute of Neurological Disorders and Stroke Grant NS-049783 and by a Research Development Grant from East Carolina University's Division of Research and Graduate Studies.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Aagaard P, Simonsen EB, Andersen JL, Magnusson P, Dyhre-Poulsen P. Neural adaptation to resistance training: changes in evoked V-wave and H-reflex responses. J Appl Physiol 92: 2309–2318, 2002. [DOI] [PubMed] [Google Scholar]
- 2.Asanuma H, Pavlides C. Neurobiological basis of motor learning in mammals. Neuroreport 8: i–vi, 1997. [PubMed] [Google Scholar]
- 3.Ashe J Force and the motor cortex. Behav Brain Res 87: 255–269, 1997. [DOI] [PubMed] [Google Scholar]
- 4.Boerio D, Jubeau M, Zory R, Maffiuletti NA. Central and peripheral fatigue after electrostimulation-induced resistance exercise. Med Sci Sports Exerc 37: 973–978, 2005. [PubMed] [Google Scholar]
- 5.Buonomano DV, Merzenich MM. Cortical plasticity: from synapses to maps. Annu Rev Neurosci 21: 149–186, 1998. [DOI] [PubMed] [Google Scholar]
- 6.Bäumer T, Lange R, Liepert J, Weiller C, Siebner HR, Rothwell JC, Munchau A. Repeated premotor rTMS leads to cumulative plastic changes of motor cortex excitability in humans. Neuroimage 20: 550–560, 2003. [DOI] [PubMed] [Google Scholar]
- 7.Carey JR, Fregni F, Pascual-Leone A. rTMS combined with motor learning training in healthy subjects. Restor Neurol Neurosci 24: 191–199, 2006. [PubMed] [Google Scholar]
- 8.Carroll TJ, Riek S, Carson RG. Reliability of the input-output properties of the cortico-spinal pathway obtained from transcranial magnetic and electrical stimulation. J Neurosci Methods 112: 193–202, 2001. [DOI] [PubMed] [Google Scholar]
- 9.Carroll TJ, Riek S, Carson RG. The sites of neural adaptation induced by resistance training in humans. J Physiol 544: 641–652, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Casabona A, Polizzi MC, Perciavalle V. Differences in H-reflex between athletes trained for explosive contractions and non-trained subjects. Eur J Appl Physiol Occup Physiol 61: 26–32, 1990. [DOI] [PubMed] [Google Scholar]
- 11.Chen R, Classen J, Gerloff C, Celnik P, Wassermann EM, Hallett M, Cohen LG. Depression of motor cortex excitability by low-frequency transcranial magnetic stimulation. Neurology 48: 1398–1403, 1997. [DOI] [PubMed] [Google Scholar]
- 12.Chen WH, Mima T, Siebner HR, Oga T, Hara H, Satow T, Begum T, Nagamine T, Shibasaki H. Low-frequency rTMS over lateral premotor cortex induces lasting changes in regional activation and functional coupling of cortical motor areas. Clin Neurophysiol 114: 1628–1637, 2003. [DOI] [PubMed] [Google Scholar]
- 13.Del Balso C, Cafarelli E. Adaptations in the activation of human skeletal muscle induced by short-term isometric resistance training. J Appl Physiol 103: 402–411, 2007. [DOI] [PubMed] [Google Scholar]
- 14.Dettmers C, Connelly A, Stephan KM, Turner R, Friston KJ, Frackowiak RS, Gadian DG. Quantitative comparison of functional magnetic resonance imaging with positron emission tomography using a force-related paradigm. Neuroimage 4: 201–209, 1996. [DOI] [PubMed] [Google Scholar]
- 15.Duclay J, Martin A, Robbe A, Pousson M. Spinal reflex plasticity during maximal dynamic contractions after eccentric training. Med Sci Sports Exerc 40: 722–734, 2008. [DOI] [PubMed] [Google Scholar]
- 16.Enoka RM Neural adaptations with chronic physical activity. J Biomech 30: 447–455, 1997. [DOI] [PubMed] [Google Scholar]
- 17.Fierro B, Piazza A, Brighina F, La Bua V, Buffa D, Oliveri M. Modulation of intracortical inhibition induced by low- and high-frequency repetitive transcranial magnetic stimulation. Exp Brain Res 138: 452–457, 2001. [DOI] [PubMed] [Google Scholar]
- 18.Fitzgerald PB, Brown TL, Daskalakis ZJ, Chen R, Kulkarni J. Intensity-dependent effects of 1 Hz rTMS on human corticospinal excitability. Clin Neurophysiol 113: 1136–1141, 2002. [DOI] [PubMed] [Google Scholar]
- 19.Gabriel DA, Kamen G, Frost G. Neural adaptations to resistive exercise: mechanisms and recommendations for training practices. Sports Med 36: 133–149, 2006. [DOI] [PubMed] [Google Scholar]
- 20.Gandevia SC Spinal and supraspinal factors in human muscle fatigue. Physiol Rev 81: 1725–1789, 2001. [DOI] [PubMed] [Google Scholar]
- 21.Gentner R, Wankerl K, Reinsberger C, Zeller D, Classen J. Depression of human corticospinal excitability induced by magnetic theta-burst stimulation: evidence of rapid polarity-reversing metaplasticity. Cereb Cortex 18: 2046–2053, 2008. [DOI] [PubMed] [Google Scholar]
- 22.Goldspink DF The influence of immobilization and stretch on protein turnover of rat skeletal muscle. J Physiol 264: 267–282, 1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gondin J, Duclay J, Martin A. Neural drive preservation after detraining following neuromuscular electrical stimulation training. Neurosci Lett 409: 210–214, 2006. [DOI] [PubMed] [Google Scholar]
- 24.Gondin J, Duclay J, Martin A. Soleus- and gastrocnemii-evoked V-wave responses increase after neuromuscular electrical stimulation training. J Neurophysiol 95: 3328–3335, 2006. [DOI] [PubMed] [Google Scholar]
- 25.Griffin L, Cafarelli E. Transcranial magnetic stimulation during resistance training of the tibialis anterior muscle. J Electromyogr Kinesiol 17: 446–452, 2007. [DOI] [PubMed] [Google Scholar]
- 26.Hallett M Transcranial magnetic stimulation: a primer. Neuron 55: 187–199, 2007. [DOI] [PubMed] [Google Scholar]
- 27.Herbert RD, Dean C, Gandevia SC. Effects of real and imagined training on voluntary muscle activation during maximal isometric contractions. Acta Physiol Scand 163: 361–368, 1998. [DOI] [PubMed] [Google Scholar]
- 28.Herbert RD, Gandevia SC. Muscle activation in unilateral and bilateral efforts assessed by motor nerve and cortical stimulation. J Appl Physiol 80: 1351–1356, 1996. [DOI] [PubMed] [Google Scholar]
- 29.Huang YZ, Edwards MJ, Bhatia KP, Rothwell JC. One-Hz repetitive transcranial magnetic stimulation of the premotor cortex alters reciprocal inhibition in DYT1 dystonia. Mov Disord 19: 54–59, 2004. [DOI] [PubMed] [Google Scholar]
- 30.Jensen JL, Marstrand PC, Nielsen JB. Motor skill training and strength training are associated with different plastic changes in the central nervous system. J Appl Physiol 99: 1558–1568, 2005. [DOI] [PubMed] [Google Scholar]
- 31.Jubeau M, Zory R, Gondin J, Martin A, Maffiuletti NA. Effect of electrostimulation training-detraining on neuromuscular fatigue mechanisms. Neurosci Lett 424: 41–46, 2007. [DOI] [PubMed] [Google Scholar]
- 32.Jubeau M, Zory R, Gondin J, Martin A, Maffiuletti NA. Late neural adaptations to electrostimulation resistance training of the plantar flexor muscles. Eur J Appl Physiol 98: 202–211, 2006. [DOI] [PubMed] [Google Scholar]
- 33.Keen DA, Yue GH, Enoka RM. Training-related enhancement in the control of motor output in elderly humans. J Appl Physiol 77: 2648–2658, 1994. [DOI] [PubMed] [Google Scholar]
- 34.Kidgell DJ, Sale MV, Semmler JG. Motor unit synchronization measured by cross-correlation is not influenced by short-term strength training of a hand muscle. Exp Brain Res 175: 745–753, 2006. [DOI] [PubMed] [Google Scholar]
- 35.Lagerquist O, Zehr EP, Docherty D. Increased spinal reflex excitability is not associated with neural plasticity underlying the cross-education effect. J Appl Physiol 100: 83–90, 2006. [DOI] [PubMed] [Google Scholar]
- 36.Lee L, Siebner HR, Rowe JB, Rizzo V, Rothwell JC, Frackowiak RS, Friston KJ. Acute remapping within the motor system induced by low-frequency repetitive transcranial magnetic stimulation. J Neurosci 23: 5308–5318, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Maeda F, Keenan JP, Tormos JM, Topka H, Pascual-Leone A. Modulation of corticospinal excitability by repetitive transcranial magnetic stimulation. Clin Neurophysiol 111: 800–805, 2000. [DOI] [PubMed] [Google Scholar]
- 38.Maffiuletti NA, Pensini M, Scaglioni G, Ferri A, Ballay Y, Martin A. Effect of electromyostimulation training on soleus and gastrocnemii H- and T-reflex properties. Eur J Appl Physiol 90: 601–607, 2003. [DOI] [PubMed] [Google Scholar]
- 39.Muellbacher W, Ziemann U, Boroojerdi B, Cohen L, Hallett M. Role of the human motor cortex in rapid motor learning. Exp Brain Res 136: 431–438, 2001. [DOI] [PubMed] [Google Scholar]
- 40.Muellbacher W, Ziemann U, Boroojerdi B, Hallett M. Effects of low-frequency transcranial magnetic stimulation on motor excitability and basic motor behavior. Clin Neurophysiol 111: 1002–1007, 2000. [DOI] [PubMed] [Google Scholar]
- 41.Muellbacher W, Ziemann U, Wissel J, Dang N, Kofler M, Facchini S, Boroojerdi B, Poewe W, Hallett M. Early consolidation in human primary motor cortex. Nature 415: 640–644, 2002. [DOI] [PubMed] [Google Scholar]
- 42.Oldfield RC The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia 9: 97–113, 1971. [DOI] [PubMed] [Google Scholar]
- 43.Pascual-Leone A, Tormos JM, Keenan J, Tarazona F, Canete C, Catala MD. Study and modulation of human cortical excitability with transcranial magnetic stimulation. J Clin Neurophysiol 15: 333–343, 1998. [DOI] [PubMed] [Google Scholar]
- 44.Remple MS, Bruneau RM, VandenBerg PM, Goertzen C, Kleim JA. Sensitivity of cortical movement representations to motor experience: evidence that skill learning but not strength training induces cortical reorganization. Behav Brain Res 123: 133–141, 2001. [DOI] [PubMed] [Google Scholar]
- 45.Ridding MC, Rothwell JC. Is there a future for therapeutic use of transcranial magnetic stimulation? Nat Rev Neurosci 8: 559–567, 2007. [DOI] [PubMed] [Google Scholar]
- 46.Romero JR, Anschel D, Sparing R, Gangitano M, Pascual-Leone A. Subthreshold low frequency repetitive transcranial magnetic stimulation selectively decreases facilitation in the motor cortex. Clin Neurophysiol 113: 101–107, 2002. [DOI] [PubMed] [Google Scholar]
- 47.Rooney KJ, Herbert RD, Balnave RJ. Fatigue contributes to the strength training stimulus. Med Sci Sports Exerc 26: 1160–1164, 1994. [PubMed] [Google Scholar]
- 48.Rossini PM, Barker AT, Berardelli A, Caramia MD, Caruso G, Cracco RQ, Dimitrijevic MR, Hallett M, Katayama Y, Lucking CH. Non-invasive electrical and magnetic stimulation of the brain, spinal cord and roots: basic principles and procedures for routine clinical application. Report of an IFCN committee. Electroencephalogr Clin Neurophysiol 91: 79–92, 1994. [DOI] [PubMed] [Google Scholar]
- 49.Sale DG Neural adaptation to resistance training. Med Sci Sports Exerc 20: S135–S145, 1988. [DOI] [PubMed] [Google Scholar]
- 50.Scaglioni G, Ferri A, Minetti AE, Martin A, Van Hoecke J, Capodaglio P, Sartorio A, Narici MV. Plantar flexor activation capacity and H reflex in older adults: adaptations to strength training. J Appl Physiol 92: 2292–2302, 2002. [DOI] [PubMed] [Google Scholar]
- 51.Schlaghecken F, Munchau A, Bloem BR, Rothwell J, Eimer M. Slow frequency repetitive transcranial magnetic stimulation affects reaction times, but not priming effects, in a masked prime task. Clin Neurophysiol 114: 1272–1277, 2003. [DOI] [PubMed] [Google Scholar]
- 52.Semmler JG, Nordstrom MA. Motor unit discharge and force tremor in skill- and strength-trained individuals. Exp Brain Res 119: 27–38, 1998. [DOI] [PubMed] [Google Scholar]
- 53.Stefan K, Wycislo M, Gentner R, Schramm A, Naumann M, Reiners K, Classen J. Temporary occlusion of associative motor cortical plasticity by prior dynamic motor training. Cereb Cortex 16: 376–385, 2006. [DOI] [PubMed] [Google Scholar]
- 54.Szubski C, Burtscher M, Loscher WN. Neuromuscular fatigue during sustained contractions performed in short-term hypoxia. Med Sci Sports Exerc 39: 948–954, 2007. [DOI] [PubMed] [Google Scholar]
- 55.Tinazzi M, Zanette G. Modulation of ipsilateral motor cortex in man during unimanual finger movements of different complexities. Neurosci Lett 244: 121–124, 1998. [DOI] [PubMed] [Google Scholar]
- 56.Touge T, Gerschlager W, Brown P, Rothwell JC. Are the after-effects of low-frequency rTMS on motor cortex excitability due to changes in the efficacy of cortical synapses? Clin Neurophysiol 112: 2138–2145, 2001. [DOI] [PubMed] [Google Scholar]
- 57.Tsuji T, Rothwell JC. Long lasting effects of rTMS and associated peripheral sensory input on MEPs, SEPs and transcortical reflex excitability in humans. J Physiol 540: 367–376, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wassermann EM, Grafman J, Berry C, Hollnagel C, Wild K, Clark K, Hallett M. Use and safety of a new repetitive transcranial magnetic stimulator. Electroencephalogr Clin Neurophysiol 101: 412–417, 1996. [PubMed] [Google Scholar]
- 59.Yue G, Cole KJ. Strength increases from the motor program: comparison of training with maximal voluntary and imagined muscle contractions. J Neurophysiol 67: 1114–1123, 1992. [DOI] [PubMed] [Google Scholar]