Abstract
Childhood diseases are often accompanied by chronic inflammation, which is thought to negatively impact growth. Interleukin-6 (IL-6) is typically cited as an indicator of inflammation and is linked to impaired growth. This study was designed to isolate and identify potential effects of chronic IL-6 exposure on skeletal muscle growth during development. A second aim was to determine if endurance exercise, thought to antagonize chronic inflammation, would interact with any effects of IL-6. The muscles of one leg of rapidly growing rats were exposed to IL-6 or vehicle for 14 days. Subgroups of IL-6-infused rats were provided access to running wheels. Local IL-6 infusion resulted in ∼13% muscle growth deficit (myofibrillar protein levels). Exercise (>4,000 m/day) prevented this deficit. IL-6 infusion increased mRNA for suppressor of cytokine signaling-3 (SOCS3) and tumor necrosis factor-α (TNF-α), and this was not prevented by exercise. IL-6 infusion increased the mRNAs for atrogin, insulin-like growth factor-I (IGF-I), and IGF binding protein-4 (IGFBP4), and these effects were mitigated by exercise. Exercise stimulated an increase in total RNA (∼19%) only in the IL-6-infused muscle, suggesting that a compensatory increase in translational capacity was required to maintain muscle growth. This study indicates that IL-6 exposure during periods of rapid growth in young animals can retard growth possibly via interactions with key growth factors. Relatively high volumes of endurance-type exercise do not exacerbate the negative effects of IL-6 and in fact were found to be beneficial in protecting muscle growth.
Keywords: cytokine, inflammation, running, protection
a number of childhood disease states are accompanied by chronic inflammation (23, 29, 41, 51, 61, 83). In addition to overt disease states, conditions such as childhood obesity and metabolic syndrome are also marked by abnormal inflammatory mediators (59, 77). Interleukin-6 (IL-6) is often reported to be elevated in these conditions and is an indicator of chronic inflammation (72). However, there is evidence that IL-6 can serve in either a pro- or anti-inflammatory role (e.g., 62, 72). An anti-inflammatory role for IL-6 in disease states would explain the frequent correlation between systemic IL-6 levels and severity of the disease. In contrast to a potentially palliative role for IL-6, there is evidence to suggest that the growth defect associated with some childhood diseases involving chronic inflammation may be mediated by increased circulating IL-6, most likely via the depression of plasma IGF-I levels (20, 52). This conclusion is supported by the known potential for interactions between proinflammatory cytokines and growth factors such as growth hormone (GH) and insulin-like growth factor-I (IGF-I) (20, 52, 79, 80). In particular, recent findings that cytokine and growth factor signaling share common intracellular pathways and negative-feedback mechanisms [e.g., suppressor of cytokine signaling (SOCS) family] suggest a mechanism for interaction and possibly interference (6, 33, 34, 53, 66, 67, 71, 78, 82).
In addition to observational studies, several attempts at intervention indicate that elevated levels of IL-6 per se may negatively impact growth. For example, transgenic mice that overexpress IL-6 have decreased growth that can be mitigated by IL-6-neutralizing antibodies (20, 21). Similarly, in animal models of inflammatory bowel disease (IBD), treatment with IL-6-neutralizing antibodies restores growth (7, 69). Recently, the therapeutic use of an antibody that prevents formation of the IL-6-IL-6 receptor (IL-6R) complex has also shown great promise for the treatment of systemic juvenile idiopathic arthritis (JIA) (84).
We previously reported that chronic local infusion of nonsystemic doses of IL-6 into targeted skeletal muscles of adult rats led to significant muscle atrophy, as evidenced by decreased total and myofibrillar protein content (39). In the context of the broader literature, we interpret this finding as an indication that IL-6 per se has direct effects on skeletal muscle that result in catabolism. Recently, van Hall et al. (77a) reported that acute systemic infusion of IL-6 into healthy human subjects, at levels that stimulate an intense exercise response, caused a decrease in muscle protein synthesis and therefore a small increase in net muscle protein breakdown (77a). These authors speculated that this effect was due to an IL-6-induced decrease in circulating amino acid pools rather than a direct effect of IL-6 on muscle. Alternatively, there is substantial evidence that IL-6 can activate AMP kinase (AMPK) in a number of tissues, including skeletal muscle cells (5, 28, 68). In skeletal muscle, Bolster et al. (12) reported that AMPK activity suppresses protein synthesis in the rat by downregulating mammalian target of rapamycin (mTOR) signaling. A number of subsequent studies have verified that AMPK can downregulate protein synthesis via its effects on the mTOR pathway (24, 48). This mechanism can explain the observation that IL-6 decreases the activity of p70 S6 kinase (S6K) (28), a substrate of mTOR and a critical regulator of translation (42, 46).
The relationship between IL-6 and skeletal muscle in particular presents a paradox. Muscle cells are known to produce IL-6 in culture and in vivo (9, 35). In muscle, IL-6 has the potential to stimulate myoblast or satellite cell proliferation (13, 64), and promote angiogenesis, all features indicative of anabolic processes (19). However, a number of studies have also established that chronic IL-6 exposure can have catabolic effects on skeletal muscle (31, 39, 65). It is most likely that some of the seemingly paradoxical effects of IL-6 exposure may be related to temporal factors. For example, chronic exposure, as seen during inflammation, may have very different effects from acute increases, such as those seen with exercise. However, one concern would be the overlay of exercise-induced IL-6 on a background of chronic inflammation.
Although increases in systemic levels of IL-6 often correlate with manifestations of a given disease state, there have been few in vivo studies that have assessed the direct impact of chronic elevations of IL-6 on specific tissues in the absence of substantial systemic effects. As noted above, evidence from studies that counter the effects of IL-6 also indicate that this cytokine can exert catabolic effects. However, these systemic studies cannot speak to the question of mechanisms by which IL-6 may affect specific tissues. The present work was designed to measure the effects of elevated IL-6 on skeletal muscle in the absence of a more generalized, systemic elevation of this cytokine. Specifically, we focused on the imposition of a local elevation of IL-6 on skeletal muscle during a period of rapid developmental growth, such as would occur in a growing child who suffers from an inflammatory disease. A second aim of this work was to measure the interactive effects of chronic IL-6 elevation and endurance-mode exercise on muscle growth.
For this study, our working hypotheses were that chronic IL-6 exposure would negatively impact muscle growth and that exercise had the potential to exacerbate this effect. To test these hypotheses, chronic IL-6 exposure, localized to one skeletal muscle, was imposed in rapidly growing rats. Changes in muscle growth and molecular markers of muscle anabolic (e.g., IGF-I system, myostatin, etc.) and catabolic (e.g., atrogin) regulators were measured. In addition, molecular markers of potential cytokine-growth factor interactions (SOCS) were evaluated.
METHODS
Animals.
The protocols use in this study were approved by the University of California-Irvine Institutional Animal Care and Use Committee.
Four-week-old Female Sprague-Dawley rats were purchased from Taconic Farms. Rats were initially housed in groups of four with access to noninstrumented mouse running wheels to allow for familiarization with running. Approximately 1 wk later, rats were single-housed with access to a full-sized rat running wheel, and the running distance of each rat was determined for 3 days. Following this screening period, rats whose running distances deviated from the mean by 1 SD were removed from the potential study population to avoid introducing a bias by including animals with widely varying running behaviors.
The pump implantation surgery was considered to be the start of the study. At this time, the rats were ∼5.5 wk of age, and body mass was 118 ± 1 g ( n = 36).
Rats were randomly assigned to one of three groups: sham group rats received unilateral implantation of a miniosmotic pump and catheter filled with vehicle. IL-6C group rats received unilateral implantation of pumps and catheters filled with IL-6 (recombinant rat IL-6, Sigma no. I0406). R-IL-6 group rats also received unilateral implantation of pumps and catheters filled with IL-6. For the duration of the study, sham group and IL-6C rats were single-housed in standard vivarium cages with access to food and water ad libitum. R-IL-6 rats were housed in standard size vivarium cages fitted with Nalgene rat running wheels instrumented to record revolutions and were allowed access to food and water ad libitum.
Local infusion.
The local infusion protocol used in this study has been described in previous publications (4, 36, 39). Infusion was accomplished via a catheter (0.006-in. I.D. Teflon, Cole-Parmer) attached to a miniosmotic pump (Alzet model 2002, Alza). The miniosmotic pumps were filled under aseptic conditions following the manufacturer's instructions. For catheter implantation, the rats were anesthetized with ketamine/acepromazine, 80/2 mg/kg, and incisions were made in the skin overlying the gastrocnemius muscle (∼1.5-cm incision) and on the back (∼0.5-cm incision).
For these small animals, the catheter placement was modified relative to the previous studies (4, 36, 39). In this instance the fenestrated catheter tip was placed adjacent to the target muscle as opposed to being inserted under the muscle fascia. The catheter was passed through the biceps femoris muscle, then placed between the lateral and medial heads of the gastrocnemius well distal to the location of nerves and blood vessels. The catheter fenestrations were oriented toward the medial head of the gastrocnemius (MG). The distal end of the catheter was closed off and secured to the plantaris tendon with sutures (4-0 Ethicon). The proximal end of the catheter was tunneled under the skin to the back incision. The proximal end of the catheter was then mated with the osmotic pump, which had been primed by preincubation in sterile saline at 37°C. The pump was then placed under the skin via the back incision, and both incisions were closed. At the termination of the infusion protocols, the osmotic pumps were removed and any remaining infusate was aspirated via a syringe to verify pump function.
Previous work has demonstrated that the local infusion of vehicle per se does not result in change in any measured variables (4, 17; and Adams, unpublished observations). In the present study, the sham-infused rats experienced the same procedures as the IL-6-infused animals with the exception that IL-6 was excluded from the muscle infusate to reconfirm that vehicle infusion had no effect on muscle mass.
IL-6 dose.
In children, conditions such as congenital heart disease, chronic renal failure, insulin-dependent diabetes, sickle cell disease, Crohns' disease, and JIA are associated with circulating IL-6 levels reported to range from 10 to 71 ng/l (7, 23, 29, 41, 51, 55, 61, 83). In the present study, the dose of IL-6 delivered to the target muscle was designed to simulate a modest level of systemic IL-6 elevation (22 ng/l). This dose would be similar to that seen chronically in systemic JIA (51, 55, 61) but also similar to the plasma IL-6 levels transiently attained following strenuous exercise (58, 76).
The dose of IL-6 selected for this study the same as that used in a previous study in adult rats (39), e.g., 0.0014 pg·mg muscle−1·h−1. Assuming a blood volume of ∼7 ml in ∼100-g rats (22), an equivalent systemic dose of IL-6 would be 0.154 ng. The objective of the present study was to deliver a dose reflecting a somatic dose of 0.154 ng to just the target muscle, hence 0.28 pg/muscle, assuming a starting muscle mass of 200 mg for an ∼100-g female. In the present study, the dose of 0.28 pg, if it were delivered to the systemic circulation, would equal 0.04 pg/ml or 40 pg/l. It is important to note that, with regard to the whole body, the IL-6 dose used is vanishingly small (compare 40 pg to 22 ng from Ref. 76) and many times lower than would be used for sepsis-related studies (54, 77b). Vehicle for this study consisted of 0.9% saline.
Tissue collection and analysis.
Fourteen days after the implantation surgeries, the rats were killed using Pentosol euthanasia solution. The rats were killed within 2 h of the end of the dark cycle when most running activity occurs. After the induction of deep anesthesia, but before the cessation of breathing, blood was collected from the left ventricle via the diaphragm using a heparinized syringe. The ventricles were removed and weighed. The soleus, plantaris, MG, lateral gastrocnemius (LG), and tibalis anterior (TA) muscles of both the infused and contralateral leg were dissected free of connective tissue, weighed, snap-frozen, and stored at −80°C for later analysis.
Outcome variables.
The data reported in this study include measures of muscle growth and anabolic potential. Molecular indicators of the anabolic state of the muscle were selected based on an extensive history of work by our group and others that have established their relevance in the context of the regulation of muscle size in rodents and humans (e.g., 1, 10).
Muscle protein analysis.
Muscle protein was determined from whole muscle homogenates using the biuret method (32). Total muscle protein was calculated from the product of the concentration and the wet weight of the muscle sample recorded at time of death. Total myofibrillar protein was determined as previously described using a modification of the method of Solaro et al. (36, 73).
DNA determination.
DNA concentration was measured in whole muscle homogenates using a fluorometric assay for the DNA binding fluorochrome bisbenzimide H-33258 (Calbiochem, San Diego, CA). Calf thymus DNA was used as a standard (49).
Total RNA isolation.
Total RNA was extracted from preweighed frozen muscle samples using the TRI Reagent (Molecular Research Center, Cincinnati, OH) according to the company's protocol, which is based on the method described by Chomczynski and Sacchi (18). Extracted RNA was precipitated from the aqueous phase with isopropanol and, after washing with ethanol, dried and suspended in a known volume of nuclease-free water. The RNA concentration was determined by optical density at 260 nm (using an OD260 unit equivalent to 40 μg/ml). The muscle total RNA concentration is calculated based on total RNA yield and the weight of the extracted muscle piece. The RNA samples were stored frozen at −80°C to be used subsequently in determining the specific mRNA expression using relative RT-PCR procedures.
Reverse transcription.
One microgram of total RNA was reverse transcribed for each muscle sample using the SuperScript II RT from Gibco BRL and a mix of oligo(dT) (100 ng/reaction) and random primers (200 ng/reaction) in a 20-μl total reaction volume at 45°C for 50 min, according to the provided protocol. At the end of the RT reaction, the tubes were heated at 90°C for 5 min to stop the reaction and then were stored at −80°C until used in the PCR reactions for specific mRNA analyses.
PCR.
As described previously (36, 81), relative RT-PCR method using 18S as internal standard (Ambion, Austin, TX) was applied to study the expression of specific mRNAs of interest, including IGF-I. We have previously published the sequence for the IGF-I primers (37). The primer sequences for SOCS-2, SOCS-3, atrogin-1, MURF-1, and myostatin have also been published previously (27, 39). The primers used for IL-6 and its receptor and TNF-α and its receptor are provided in Table 1. Primers were purchased from Life Technology and Gibco. All primers were tested for their compatibility with the alternate 18S primers. It was verified that 18S was not different between groups.
Table 1.
Primers used for IL-6 and its receptor and TNF-α and its receptor
| Target mRNA | PCR Primer Sequence, 5′→3′ | Product Size, bp | GenBank Accession No. |
|---|---|---|---|
| IL-6 | Fwd: CAGAAATACAAAGAAATGATGGATGCT | 242 | NM_012589 |
| Rev: ATTGGAAGTTGGGGTAGGAAGGAC | |||
| IL-6 receptor | Fwd: AGGTGGCCCAGCATCAATGTGTCA | 228 | NM_017020.2 |
| Rev: TTCCGTACTGATCCTCGTGGTTGTCATAGT | |||
| TNF-α | Fwd: GGGGGCCTCCAGAACTCC | 218 | NM_012675.2 |
| Rev: TGGGCTACGGGCTTGTCAC | |||
| TNF-α receptor | Fwd: ACTGTGCGGCAGGCATGTTTACCC | 215 | NM_130426 |
| Rev: GAAGCCAGGGCCACACTTGCTCA |
Fwd, forward; Rev, reverse.
In each PCR reaction, 18S ribosomal RNA was coamplified with the target cDNA (mRNA) to serve as an internal standard and to allow correction for differences in starting amounts of total RNA. The 18S primers were mixed with competimers at an optimized ratio that could range from 1:4 to 1:10 to bring down the 18S signal, which allows its linear amplification to the same range as the coamplified target mRNA (10, Ambion, Relative RT-PCR kit protocol).
For each specific target mRNA, the reverse transcription and PCR reactions were carried under identical conditions using the same reagents premix for all the samples to be compared in the study. To validate the consistency of the analysis procedures, at least one representative from each group was included in each RT-PCR run.
Amplifications were carried out in a Stratagene Robocycler with an initial denaturing step of 3 min at 96°C, followed by 25 cycles of 1 min at 96°C, 1 min at 55°C (55–60°C depending on primers), 1 min at 72°C, and a final step of 3 min at 72°C. PCR products were separated on a 2–2.5% agarose gel by electrophoresis and stained with ethidium bromide, and signal quantification was conducted by laser-scanning densitometry, as reported previously (81). In this approach, each specific mRNA signal is normalized to its corresponding 18S. For each primer set, PCR conditions (cDNA dilutions, 18S competimer/primer mix, MgCl2 concentration, and annealing temperature) were set to optimal conditions, so that both the target mRNA and 18S product yields are in the linear range of the semilog plot when the yield is expressed as a function of the number of cycles (11). Selected PCR results (SOCS3, atrogin, and 18S) were confirmed using real-time PCR.
Immunoblot.
As reported previously (38), the total amount insulin (Y1146)/IGF-I (Y1131) receptor and SOCS3 protein was examined by immunoblotting using antibodies from Cell Signaling Technology.
Plasma cytokine measurements: blood sampling and analysis.
Aliquoted plasma was stored at −80°C until assayed. Secreted cytokine levels in rat plasma were measured using commercially available ELISA kits manufactured by R&D Systems (Minneapolis, MN). Cytokines measured were IL-1β, with an intra-assay coefficient of variation (CV) of 4.1–5.7%, interassay CV 3.9–8.8% and sensitivity ≤ 5.0 pg/ml; TNF-α, with an intra-assay CV of 2.1–5.1%, interassay CV 8.8–9.7%, and sensitivity ≤ 5.0 pg/ml; and IL-6, with an intra-assay CV 4.5–8.8%, interassay CV 7.0–10.0%, and sensitivity of 22 pg/ml.
Muscle myosin heavy chain protein determination.
Skeletal muscle myosin heavy chain (MHC) proteins were separated on acrylamide gels (10% T, 2.5% C) using a standard SDS-PAGE technique. Briefly, 2.5 μg of total denatured protein was loaded per lane. The gels were run at constant current (30 mA) for ∼2.5 h at 22°C. At the completion of electrophoresis, the gels were stained with Brilliant Blue G 250 (Sigma Chemical), destained, and then scanned using a Molecular Dynamics Laser Scanning Personal Densitometer (Sunnyvale, CA). The MHC bands were identified based on their molecular weight and comparisons with purified protein. The intensity of the bands of interest was calculated via volume integration, which is based on the sum of pixel density within a rectangle containing the entire band with local background correction (ImageQuant Software, Molecular Dynamics).
Statistical analysis.
All values are reported as means and standard error of the mean (SE). Between-muscle and between-group analysis was conducted using a one-way ANOVA and Student-Neuman-Keuls (SNK) posttest (PRISM-Graphpad). For statistical purposes, all comparisons included the contralateral and treatment muscles from each animal, including the sham group. Pearson's correlation analysis was used to assess the relationship between IGF-I and Col-3 mRNA using the Prism software package. For all statistical tests, the 0.05 level of confidence was accepted for statistical significance.
RESULTS
Muscle effects: sham group.
As previously reported by us and others, no differences were detected in any of the measured variables between the vehicle-infused and contralateral muscles of the sham-group rats (4, 17, 36, 39). Data from the contralateral sham-group muscles have been omitted from the graphical representations but were included in the statistical analyses.
Somatic effects of local muscle IL-6 infusion and exercise.
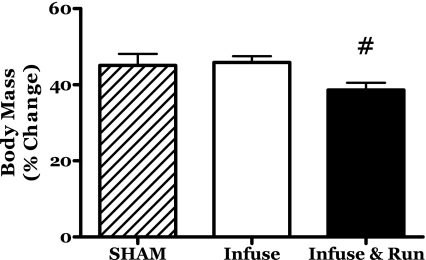
The young female rats in this study increased in body mass by ∼45% in the sham and IL-6C groups (Fig. 1). The rats in the R-IL-6 group exhibited 39% body mass increase. Lesser body weight gains are commonly observed in female rats provided unlimited access to running wheels (e.g., 8, 57).
Fig. 1.
Changes in body mass during 14 days of localized muscle infusion of vehicle (sham), IL-6, or IL-6 plus concurrent voluntary running. #P < 0.05 vs. sham and IL-6.
Relative to body mass, the mass of the left ventricle from the IL-6C rats was not different from that of the sham group but was 6.8% lower (P < 0.05) than that of the R-IL-6 rats (sham, 2.56 ± 0.03; IL-6C, 2.50 ± 0.03; R-IL-6, 2.67 ± 0.06 mg/g body).
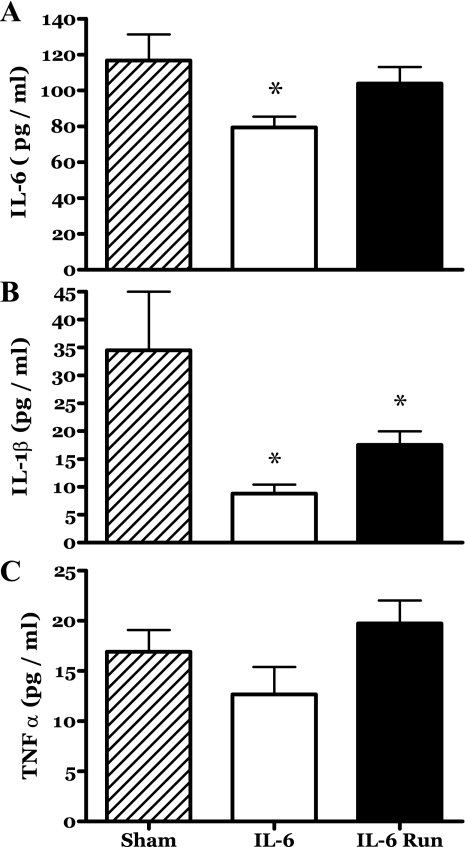
Circulating (plasma) levels of proinflammatory cytokines associated with IL-6 were determined at death. Plasma IL-6 levels of the sham group rats were similar to those recently reported by others (e.g., 16, 47). The plasma IL-6 levels of the IL-6C rats were significantly depressed (−32%) relative to the sham group (Fig. 2A). Exercise appeared to counter this decrease in circulating IL-6 such that the levels seen in the R-IL-6 group were not statistically different from those of the sham group rats (Fig. 2A). In pilot studies we found that 14 days of voluntary running had no effect on circulating IL-6 levels (data not shown).
Fig. 2.
Blood plasma concentrations of selected cytokines during 14 days of localized muscle infusion of vehicle (sham), IL-6, or IL-6 plus concurrent voluntary running. *P < 0.05 vs. sham.
Plasma IL-1β levels were also depressed in the IL-6C rats as well as the R-IL-6 animals (Fig. 2B). The plasma levels of TNF-α appeared to follow a similar pattern to those of IL-6 (Fig. 2C). However, no statistically significant changes were detected.
Run distances.
The rats provided with running wheels (R-IL-6 group) averaged 4,462 ± 682 m/day over the course of the study. The final 6 days of the study, this average was 5,561 ± 565 m.
Muscle DNA.
No differences were detected in the concentration of DNA present in the MG muscles from any group (not shown).
Muscle: exercise effects.
Voluntary running resulted in a number of responses that were independent of the IL-6 treatment, i.e., they occurred in both the infused and contralateral muscles exclusively in the R-IL-6 group.
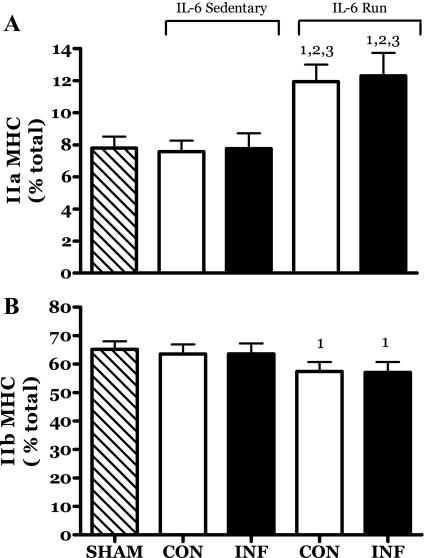
As might be expected, there was a reduction in the proportion of type IIb MHC protein and an increase in the proportion of type IIa in the MG muscle (Fig. 3). This is a classical phenotypic shift observed in many training studies (3). IL-6 infusion had no impact on this process.
Fig. 3.
Effects of 14 days of localized muscle infusion of vehicle (sham), IL-6, or IL-6 plus concurrent voluntary running on myosin heavy chain (MHC) type IIa (A) and type IIb (B) protein proportions in rat medial gastrocnemius (MG) muscles. CON, contralateral muscle; INF, IL-6-infused muscle. 1 P < 0.05 vs. sham; 2 P < 0.05 vs. sedentary IL-6 contralateral. 3 P < 0.05 vs. sedentary IL-6 infused.
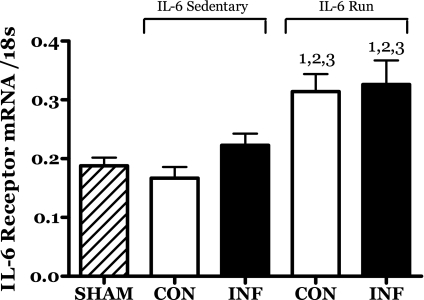
The expression and/or accumulation of the mRNA for the IL-6 receptor subunit was significantly increased in both the IL-6-infused and contralateral muscles of the running group (Fig. 4) but not in the sham or IL-6C groups.
Fig. 4.
Effects of 14 days of localized muscle infusion of vehicle (sham), IL-6, or IL-6 plus concurrent voluntary running on IL-6 receptor mRNA levels in rat MG muscles. 1 P < 0.05 vs. sham. 2 P < 0.05 vs. sedentary IL-6 contralateral. 3 P < 0.05 vs. sedentary IL-6 infused.
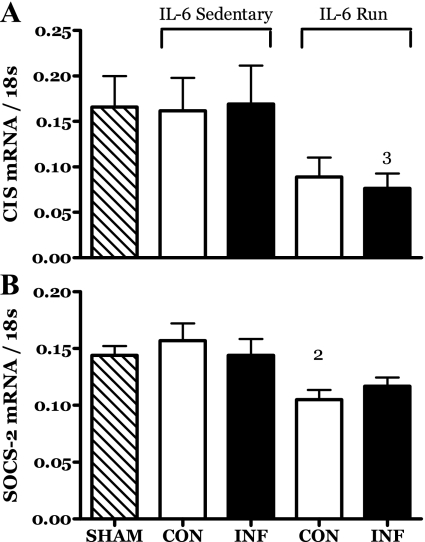
The mRNA levels of two members of the SOCS family appeared to be depressed in the muscles of running rats, apparently independent of IL-6 infusion status. The mRNA for cytokine-inducible SH2 protein (CIS) was significantly decreased in the IL-6-infused muscles of runners while that of the contralateral muscle was not different from the infused but failed to achieve significance at the 0.05 level relative to other groups (Fig. 5A). The mRNA for SOCS2 also appeared to be decreased in response to exercise in the contralateral muscle (Fig. 5B).
Fig. 5.
Effects of 14 days of localized muscle infusion of vehicle (sham), IL-6, or IL-6 plus concurrent voluntary running on mRNA levels for CIS (A) and suppressor of cytokine signaling 2 (SOCS2) (B) in rat MG muscles. 2 P < 0.05 vs. sedentary IL-6 contralateral. 3 P < 0.05 vs. sedentary IL-6 infused.
Muscle: IL-6 effects sensitive to exercise.
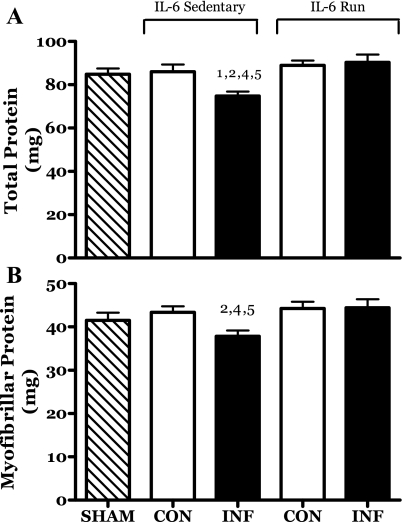
A number of IL-6-induced changes in skeletal muscle appeared to be, to some extent, countered by exercise. The IL-6-infused muscles of sedentary rats demonstrated a significant retardation in growth. This is evidenced in the 13% lower content of both total and myofibrillar protein relative to the contralateral muscles (Fig. 6). No deficit was seen in this parameter in muscles from either the sham or R-IL-6 groups. Similarly, the MG muscle mass of the IL-6C infused muscles was significantly less than that of the contralateral muscle (2.0 ± 0.04 vs. 2.2 ± 0.04 mg/g body, P = 0.03). There were no significant differences in MG mass in the sham or R-IL-6 groups. In addition, the mass of the soleus, plantaris, and LG muscles of the IL-6C rats did not differ between legs (data not shown).
Fig. 6.
Effects of 14 days of localized muscle infusion of vehicle (sham), IL-6, or IL-6 plus concurrent voluntary running on MG muscle total (A) and myofibrillar (B) protein content. 1 P < 0.05 vs. sham. 2 P < 0.05 vs. sedentary IL-6 contralateral. 4 P < 0.05 vs. run contralateral muscle. 5 P < 0.05 vs. IL-6-infused run muscle.
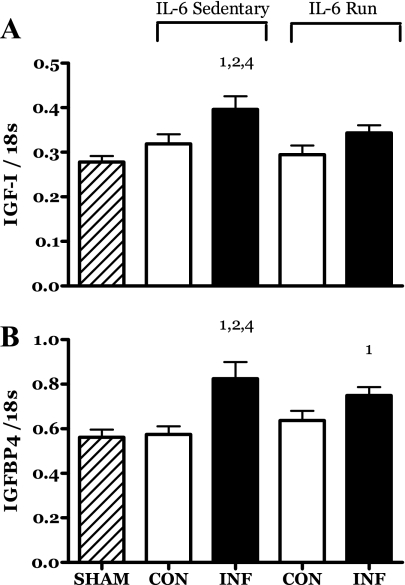
IL-6 infusion resulted in an increase in the levels of mRNA for IGF-I and IGF binding protein -4 (IGFBP4) (Fig. 7, A and B). This response was partially (IGFBP4) or completely (IGF-I) attenuated by the running exercise (Fig. 7, A and B).
Fig. 7.
Effects of 14 days of localized muscle infusion of vehicle (sham), IL-6, or IL-6 plus concurrent voluntary running on the level of IGF-I (A) and IGF binding protein 4 (IGFBP4) (B) mRNA present in rat MG muscle. 1 P < 0.05 vs. sham. 2 P < 0.05 vs. sedentary IL-6 contralateral. 4 P < 0.05 vs. run contralateral muscle.
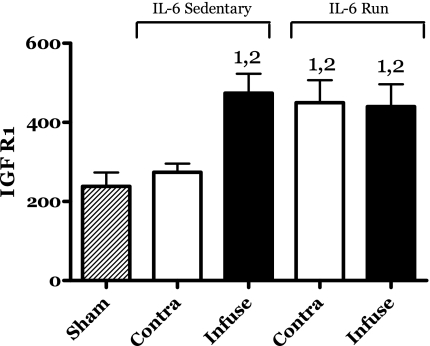
IL-6 infusion resulted in an increase in the protein levels of the IGF-I/insulin receptor (IGFR1) (Fig. 8). Running exercise also increased the levels of this protein. However, no additional increases in IGFR1 protein were observed in the IL-6-infused muscles of the running group (Fig. 8).
Fig. 8.
Effects of 14 days of localized muscle infusion of vehicle (sham), IL-6, or IL-6 plus concurrent voluntary running on the IGF-I/insulin receptor (IGFR1) protein level present in rat MG muscle. 1 P < 0.05 vs. sham. 2 P < 0.05 vs. sedentary IL-6 contralateral.
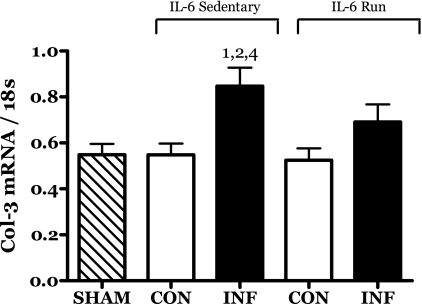
The abundance of the mRNA for procollagen III (Col-3) was significantly increased in the IL-6C infused muscles (Fig. 9). The increase in Col-3 mRNA was blunted by running exercise in the R-IL-6 rats.
Fig. 9.
Effects of 14 days of localized muscle infusion of vehicle (sham), IL-6, or IL-6 plus concurrent voluntary running on the level of procollagen III (Col-3) mRNA present in rat MG muscle. 1 P < 0.05 vs. sham. 2 P < 0.05 vs. sedentary IL-6 contralateral. 4 P < 0.05 vs. run contralateral muscle.
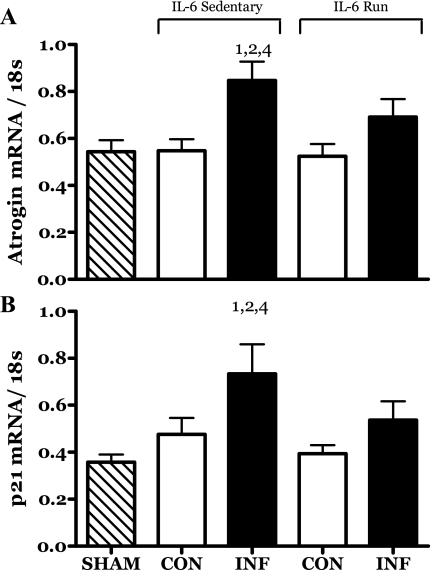
The mRNA levels of both atrogin and cyclin-dependent kinase inhibitor p21 (p21) were significantly increased by IL-6 infusion (Fig. 10, A and B). This response was partially blunted by running exercise such that any changes were no longer significant.
Fig. 10.
Effects of 14 days of localized muscle infusion of vehicle (sham), IL-6, or IL-6 plus concurrent voluntary running on the level of atrogin (A) and p21 (B) mRNA present in rat MG muscle. 1 P < 0.05 vs. sham. 2 P < 0.05 vs. sedentary IL-6 contralateral. 4 P < 0.05 vs. run contralateral muscle.
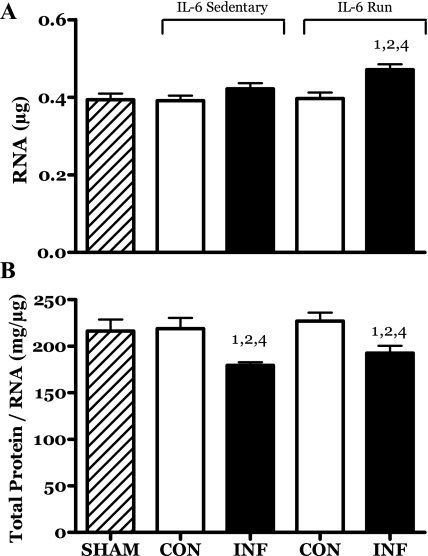
The total RNA content of the R-IL-6 infused (but not contralateral) muscles was significantly increased. This result suggests a positive interactive effect of these two treatments on the protein translational capacity of these muscles (Fig. 11A).
Fig. 11.
Effects of 14 days of localized muscle infusion of vehicle (sham), IL-6, or IL-6 plus concurrent voluntary running on the amount of total RNA (A) and the ratio of total protein to total RNA (B) present in rat MG muscle. 1 P < 0.05 vs. sham. 2 P < 0.05 vs. sedentary IL-6 contralateral. 4 P < 0.05 vs. run contralateral muscle.
Muscle: IL-6 effects not sensitive to exercise.
IL-6 infusion resulted in a similar decrease in the ratio of total protein (mg) to total RNA (μg) in the muscles of both the IL-6C and IL-6R rats (Fig. 11B).
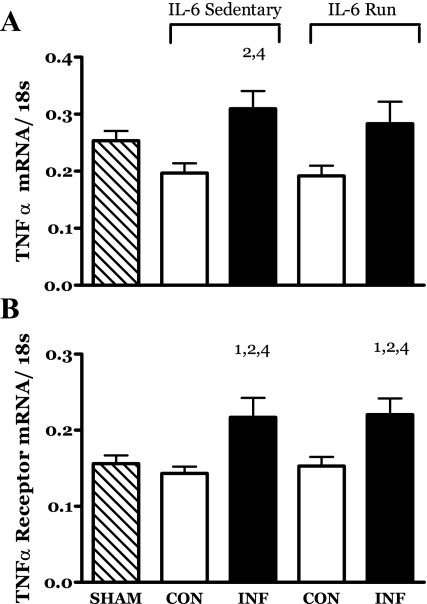
IL-6 infusion resulted in an increase in the mRNA for TNF-α in the muscles of the IL-6C rats (Fig. 12A). The TNF-α mRNA levels in the muscles of the R-IL-6 rats appeared to follow a similar pattern. However, the increase in TNF-α mRNA in the R-IL-6 infused muscles was not significant at the 0.05 level.
Fig. 12.
Effects of 14 days of localized muscle infusion of vehicle (sham), IL-6, or IL-6 plus concurrent voluntary running on the level of TNF-α (A) and TNF-α receptor (B) present in rat MG muscle. 1 P < 0.05 vs. sham. 2 P < 0.05 vs. sedentary IL-6 contralateral. 4 P < 0.05 vs. run contralateral muscle.
The mRNA for the TNF-α receptor was significantly increased in the IL-6-infused muscles of both the IL-6C and R-IL-6 groups (Fig. 12B).
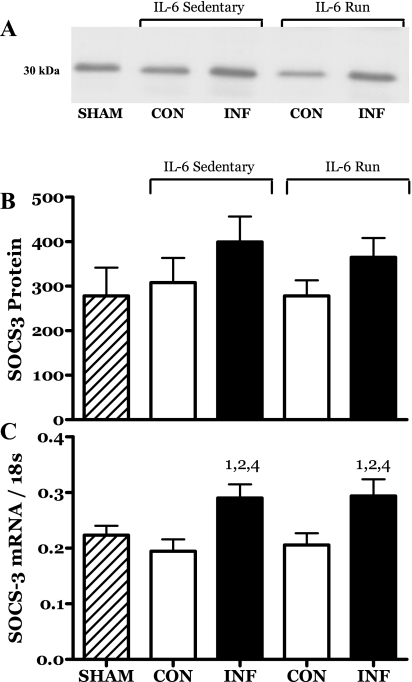
The levels of mRNA for SOCS3 were also significantly increased in both groups of IL-6-infused muscles (Fig. 13). However, there were no significant differences detected in SOCS3 protein levels (Fig. 13).
Fig. 13.
Effects of 14 days of localized muscle infusion of vehicle (sham), IL-6, or IL-6 plus concurrent voluntary running on the level of SOCS3 mRNA and protein present in rat MG muscle. Representative immunoblot for SOCS3 protein (A), SOCS3 protein in densitometer units (not significant) (B), and SOCS3 mRNA (C). 1 P < 0.05 vs. sham. 2 P < 0.05 vs. sedentary IL-6 contralateral. 4 P < 0.05 vs. run contralateral muscle.
Muscle: no effects.
None of the treatment or control muscles demonstrated statistically significant differences in the mRNA levels for IL-6, myogenin, cyclin D1, IGFBP5, or myostatin (data not shown).
DISCUSSION
A number of chronic diseases in children are associated with systemic inflammation as evidenced by increased levels of proinflammatory cytokines. The primary rationale for the present study was the need to distinguish the direct effects of IL-6, during developmental growth in skeletal muscle, from more complex, indirect, and generalized effects that might result from delivery of the IL-6 to the whole body. The present study was designed to determine if chronic IL-6 exposure would interfere with the growth of a single targeted muscle in rapidly growing rats and whether endurance-mode exercise would have an interactive effect on this process.
IL-6-induced muscle growth retardation.
In the present study we found that localized IL-6 infusion resulted in an apparent decrement in muscle growth. In particular, the IL-6-infused muscles of the sedentary IL-6 group (IL-6C) had 13% lower myofibrillar protein content than the contralateral muscles (Fig. 6B). This magnitude of myofibrillar protein deficit is similar to that seen when the effects of IGF-I were blocked by local infusion of an inhibitor of one component of IGF-I intracellular signaling in a previous study (36).
In contrast to our original hypothesis, voluntary running appeared to protect muscles exposed to chronic elevations in IL-6 from the growth-inhibiting effect of IL-6 exposure but did not promote a hypertrophy response (e.g., R-IL-6 vs. sham) (Fig. 6B). One possible explanation for this result might be that the increased contractile activity simply served to flush the infused IL-6 from the local muscle environment. However, it was observed that the mRNA for SOCS3 was elevated equally in both the IL-6C and R-IL-6 infused muscles (Fig. 13C). In the context of IL-6, SOCS3 is a known target of IL-6 signaling, serving as part of a negative-feedback mechanism (6, 75). While increased SOCS3 expression is not exclusive to IL-6 signaling, in the present study it was found only in the IL-6-infused muscles. We therefore interpret this result as a validation of the efficacy of IL-6 delivery protocol used in this study.
Localized exposure to chronically elevated IL-6 did not appear to affect the developmental program of skeletal muscles with regard to phenotype as assessed by MHC protein expression. The proportions of the various MHC isoforms were essentially the same in the IL-6-infused contralateral (IL-6C) and sham group muscles (Fig. 3 and data not shown). Similarly, IL-6 infusion did not effect the classical training-induced alteration in MHC profile of the R-IL-6 rats (Fig. 3).
The pool of total RNA present in muscle consists primarily (>85%) of ribosomal RNA (rRNA). As such, substantial increases in this parameter are thought to reflect an increase in potential translational capacity (i.e., more ribosomes) (40). IL-6 infusion per se had no effect on the amount of RNA in muscle (Fig. 11). Neither did running change this value. However, the combination of IL-6 infusion and running resulted in an increase in RNA (Fig. 11). The fact that the level of RNA in the growth-impaired IL-6C infused muscles was unchanged, while it increased in the IL-6-infused muscles of the runners, suggests that one of the impacts of IL-6 infusion may be a decrease in translational efficiency (i.e., protein per unit RNA). This conclusion is supported by the significant decrease in the protein-to-RNA ratio seen in both the IL-6C and IL-6R muscles (Fig. 11B). This interpretation would suggest that running stimulated a compensatory increase in rRNA, enabling these muscles to overcome the IL-6-induced defect and maintain protein production and therefore growth. However, this compensatory response did not appear to alter the decline in translational efficiency in that the protein-to-RNA ratios were the same in both the sedentary and running groups (Fig. 11A).
IL-6 independent exercise effects.
A number of changes in muscle appeared to be independent of the IL-6 treatment. Perhaps most interesting was the training-induced increase in the level of mRNA for the IL-6 receptor (IL-6R). The magnitude of this increase was similar in both the infused and contralateral muscles of the runners (Fig. 4). It could be speculated that this response reflects some relationship with the well-established role of acute, exercise-induced increases in IL-6 expression (26). In support of this, Keller et al. (45) reported that endurance training increased IL-6R mRNA expression in human muscle.
Exercise training appeared to decrease the level of mRNA for SOCS2 and CIS, two members of the suppressor of cytokine signaling (SOCS) family that are very similar in structure (75). SOCS2 and CIS are thought to play a role in negative feedback to GH signaling, thereby limiting the anabolic, growth-related effects of that growth factor (34, 66). However, there are reports that, in some tissues, SOCS2 can inhibit IGF-I-regulated growth as well (e.g., 56).
We have previously reported that resistance exercise can depress SOCS2 mRNA in rat skeletal muscle (38). In that context, we assumed that this was associated with responses that would promote muscle hypertrophy. However, endurance-mode exercise training, such as voluntary running in the present study, is not generally associated with muscle hypertrophy (63). It is possible that the changes in SOCS2 and CIS observed in the present study may reflect negative feedback associated with running exercise-induced increases in GH possibly functioning as a regulatory element in the control of glucose homeostasis as opposed to a growth-related role (14, 44, 85).
Exercise-independent IL-6 effects.
IL-6 infusion stimulated an increase in the production and/or accumulation of the mRNA for TNF-α and its receptor in the gastrocnemius muscles of rats (Fig. 12A). The finding that the mRNA for TNF-α and its receptor was increased by IL-6 was unexpected. Studies imposing systemic increases in IL-6 generally report that TNF-α levels are suppressed. For example, Starkie et al. (74) reported that exogenous IL-6 repressed endotoxin-induced increases in plasma TNF-α in humans. While other studies have indicated that IL-6 can increase circulating TNF-α (43), this has not been a common finding (62). When attempting to interpret the present findings, it is important to note that the treatment was localized to muscle and that the changes in TNF-related mRNA were observed in the targeted muscle. If, in fact, the increased TNF-α was realized as protein, it had no systemic manifestation (Fig. 2) and thus may not be at odds with previous reports.
The levels of mRNA for SOCS3 were significantly increased in both groups of IL-6-infused muscles (Fig. 13C). As noted above, one known role for SOCS3 is negative feedback to IL-6 receptor signaling via the inhibition of Janus kinase (Jak) activity (6, 75). The induction of SOCS3 mRNA is thought to underlie the growth impairment observed in transgenic mice overexpressing IL-6 (53). One possible mechanism for IL-6-induced growth impairment may be the known reliance of GH, IGF-I, and insulin on JAK signaling cascades. This commonality in signaling intermediates renders these growth factors sensitive to inhibition by increased levels of SOCS3 (33, 67, 71, 78, 82). In addition to the canonical Jak/STAT pathway associated with SOCS, there is a growing body of evidence that indicates that SOCS proteins, and in particular SOCS3, interact with and inhibit receptor-mediated activation of insulin receptor substrate (IRS) (50). IRS is a key initial mediator of insulin and IGF-I signaling, both of which can promote anabolism in skeletal muscle. Taken together, the results from the literature suggest that one mechanism by which IL-6 might promote catabolic effects in skeletal muscle may be via SOCS3-mediated inhibition of signaling pathways associated with anabolic stimuli. In the context of an immune response, the rationale for this mechanism is clear. One of the primary responses to physiological insults such as infection is a mobilization of resources such as amino acids from skeletal muscle. IL-6-stimulated SOCS feedback may serve to coordinately suppress competing anabolic processes to prevent a futile cycle of protein synthesis at a time when amino acid mobilization is required.
In the present study, exercise did not alter the IL-6-induced increase in SOCS3 mRNA (Fig. 13C). This would suggest that the “protective” effects of exercise on growth do not directly involve downregulation of IL-6-related signaling.
Exercise-IL-6 interactions.
Most notably, voluntary running exercise normalized muscle growth (Fig. 6). A number of additional IL-6-induced changes in skeletal muscle appeared to be, to some extent, countered by exercise.
As we observed previously in adult rats (39), local IL-6 exposure stimulated an increase in the mRNA for IGF-I in the muscles of growing rats. However, this effect was eliminated by the running protocol. We speculate that the increase in IGF-I mRNA may represent a compensatory response, possibly to decreased efficacy of IGF-I intracellular signaling, essentially resulting in resistance to IGF-I. In support of this hypothesis, there was a significant increase in IGF-I/insulin receptor protein in the IL-6-infused vs. contralateral muscles (Fig. 8). It is possible that exercise in some way normalized IGF-I signaling, obviating the stimulus for this compensatory response. In support of this, running induced similar increases in IGFR1 in both the contralateral and IL-6-infused muscles, suggesting that the effects of running obviated the stimulus for a compensatory response to IL-6-induced interference with IGF-I signaling (Fig. 8).
Increased procollagen III mRNA (Fig. 9) is often seen in parallel with increased levels of IGF-I (2, 60). However, in each case this was a function of increased mechanical loading. In the present study, the changes observed in procollagen III mRNA levels appear to directly mirror the IGF-I response to these treatments and were significantly correlated (r = 0.40, P = 0.007). Taken together with the increased IGF-I mRNA seen in this and our previous IL-6 infusion study, this result suggests that the compensatory IGF-I response may be ineffective in conserving muscle contractile protein but effective in stimulating increased collagen mRNA.
Atrogin is a muscle-specific ubiquitin E3 ligase that has been associated with muscle protein turnover (30). In the present study, atrogin mRNA was increased in the growth-retarded IL-6-infused muscles (Fig. 10A), possibly indicating an increase in the protein degradation machinery of these muscles. Exercise appeared to partially blunt this effect of IL-6 such that an increase in atrogin mRNA could not be detected statistically.
Similar to atrogin, the expression of cyclin-dependent kinase inhibitor p21 was increased by IL-6 infusion, and this effect was partially blunted by running (Fig. 10B). Increased p21 expression is generally associated with the inhibition of cell proliferation. It is possible that, in the present context, increased p21 mRNA may be related to a downregulation of the ongoing satellite cell proliferation associated with growth (70). In this context it is important to note that the concentrations of DNA were similar in all of the muscles from this study. While this is a rather gross measure, it may indicate that the myonuclear complement of the constituent muscle fibers of each muscle was appropriate for the myofiber size. This would allow for the speculation that one of the growth defects in the IL-6 muscles was a reduction in cell proliferation, thereby limiting myofiber growth due to myonuclear domain limitations.
Potential study limitations.
A potential limitation to the present study is the partial failure of the assumption that the localized infusion of IL-6 would have no systemic effects. Analysis of the circulating levels of IL-6 and IL-1β indicated that there were decreases in the circulating levels of these proteins in the IL-6-infused rats. This is somewhat surprising in that the dose of IL-6 delivered to the muscle was extremely low. For example, if the entire output of IL-6 was delivered directly into the circulation, as opposed to the muscle interstitial space, it would represent a concentration of 0.04 pg/ml. By comparison, the recent work by van Hall et al. (77a) achieved sustained plasma IL-6 concentrations of 140 pg/ml. Since the sham group animals were handled in every respect the same as the two IL-6 treatment groups, it is clear that these results are related to the IL-6 treatment. We do not, however, feel that this substantially compromised the findings of this study. IL-6 infusion had no apparent effects on the somatic growth of the rats, or the growth of the heart or of the contralateral and adjacent ipsilateral skeletal muscles.
Summary.
Studies have found that acute elevation of IL-6 results in a negative protein balance in skeletal muscle (77a), while chronic IL-6 exposure results in a growth deficit that can be countered by IL-6 neutralization (7, 20, 21, 84, 69) sometimes in the absence of changes in the state of inflammation (69).
The primary finding of the present study, that IL-6 delivered directly to a skeletal muscle inhibited the growth of that muscle, contributes to this growing body of evidence that indicates that chronic elevations in IL-6 per se may be mediating the catabolic effects of some disease states.
The implications of this research are that IL-6 elevation may be a promising and important candidate for therapy aimed at protecting growth in children with chronic inflammation. Further, these results indicate that, with regard to the growth of skeletal muscle, appropriate volumes of exercise do no harm and may provide a protective effect.
GRANTS
This work was supported by National Institutes of Health Grant P01-HD-048721 (Project 1, G. R. Adams).
Acknowledgments
We thank Phuc Tran, Alvin Yu, Jasleen Saini, Marinelle Camilon, Tiffany Yu, Julianne Lynn, Phillip Bucur, Sandy Liu, and Nkiruka Ojukwv for technical support.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Adams GR, Haddad F, Baldwin KM. Time course of changes in markers of myogenesis in overloaded rat skeletal muscles. J Appl Physiol 87: 1705–1712, 1999. [DOI] [PubMed] [Google Scholar]
- 2.Adams GR, Haddad F, Bodell PW, Tran PD, Baldwin KM. Combined isometric, concentric and eccentric resistance exercise prevents unloading induced muscle atrophy in rats. J Appl Physiol 103: 1644–1654, 2007. [DOI] [PubMed] [Google Scholar]
- 3.Adams GR, Hather BM, Baldwin KM, Dudley GA. Skeletal muscle myosin heavy chain composition and resistance training. J Appl Physiol 74: 911–915, 1993. [DOI] [PubMed] [Google Scholar]
- 4.Adams GR, McCue SA. Localized infusion of IGF-I results in skeletal muscle hypertrophy in rats. J Appl Physiol 84: 1716–1722, 1998. [DOI] [PubMed] [Google Scholar]
- 5.Al-Khalili L, Bouzakri K, Glund S, Lonnqvist F, Koistinen HA, Krook A. Signaling specificity of interleukin-6 action on glucose and lipid metabolism in skeletal muscle. Mol Endocrinol 20: 3364–3375, 2006. [DOI] [PubMed] [Google Scholar]
- 6.Aringer M, Cheng A, Nelson JW, Chen M, Sudarshan C, Zhou YJ, O'Shea JJ. Janus kinases and their role in growth and disease. Life Sci 64: 2173–2186, 1999. [DOI] [PubMed] [Google Scholar]
- 7.Ballinger A Fundamental mechanisms of growth failure in inflammatory bowel disease. Horm Res, 58 Suppl 1: 7–10, 2002. [DOI] [PubMed] [Google Scholar]
- 8.Banu MJ, Orhii PB, Mejia W, McCarter RJ, Mosekilde L, Thomsen JS, Kalu DN. Analysis of the effects of growth hormone, voluntary exercise, and food restriction on diaphyseal bone in female F344 rats. Bone 25: 469–480, 1999. [DOI] [PubMed] [Google Scholar]
- 9.Bartoccioni E, Michaelis D, Hohlfeld R. Constitutive and cytokine-induced production of interleukin-6 by human myoblasts. Immunol Lett 42: 135–138, 1994. [DOI] [PubMed] [Google Scholar]
- 10.Bickel CS, Slade J, Mahoney E, Haddad F, Dudley GA, Adams GR. Time course of molecular responses of human skeletal muscle to acute bouts of resistance exercise. J Appl Physiol 98: 482–488, 2005. [DOI] [PubMed] [Google Scholar]
- 11.Bickel CS, Slade JM, Haddad F, Adams GR, Dudley GA. Acute molecular responses of skeletal muscle to resistance exercise in able-bodied and spinal cord-injured subjects. J Appl Physiol 94: 2255–2262, 2003. [DOI] [PubMed] [Google Scholar]
- 12.Bolster DR, Crozier SJ, Kimball SR, Jefferson LS. AMP-activated protein kinase suppresses protein synthesis in rat skeletal muscle through down-regulated mammalian target of rapamycin (mTOR) signaling. J Biol Chem 277: 23977–23980, 2002. [DOI] [PubMed] [Google Scholar]
- 13.Cantini M, Massimino ML, Rapizzi E, Rossini K, Catani C, Libera LD, Carraro U. Human satellite cell proliferation in vitro is regulated by autocrine secretion of IL-6 stimulated by a soluble factor(s) released by activated monocytes. Biochem Biophys Res Commun 216: 49–53, 1995. [DOI] [PubMed] [Google Scholar]
- 14.Cappon JP, Ipp E, Brasel JA, Cooper DM. Acute effects of high fat and high glucose meals on the growth hormone response to exercise. J Clin Endocrinol Metab 76: 1418–1422, 1993. [DOI] [PubMed] [Google Scholar]
- 16.Carrizo GJ, Wu R, Cui X, Dwivedi AJ, Simms HH, Wang P. Adrenomedullin and adrenomedullin-binding protein-1 downregulate inflammatory cytokines and attenuate tissue injury after gut ischemia-reperfusion. Surgery 141: 245–253, 2007. [DOI] [PubMed] [Google Scholar]
- 17.Chakravarthy MV, Davis BS, Booth FW. IGF-I restores satellite cell proliferative potential in immobilized old skeletal muscle. J Appl Physiol 89: 1365–1379, 2000. [DOI] [PubMed] [Google Scholar]
- 18.Chomczynski P, Sacchi N. Single step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem 162: 156–159, 1987. [DOI] [PubMed] [Google Scholar]
- 19.Cohen T, Nahari D, Cerem LW, Neufeld G, Levi BZ. Interleukin 6 induces the expression of vascular endothelial growth factor. J Biol Chem 271: 736–741, 1996. [DOI] [PubMed] [Google Scholar]
- 20.DeBenedetti F, Alonzi T, Moretta A, Lazzaro D, Costa P, Poli V, Martini A, Ciliberto G, Fattori E. Interleukin 6 causes growth impairment in transgenic mice through a decrease in insulin-like growth factor-1. A model for stunted growth in children with chronic inflammation. J Clin Invest 99: 643–650, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.DeBenedetti F, Meazza C, Oliveri M, Pignatti P, Vivarelli M, Alonzi T, Fattori E, Garrone S, Barreca A, Martini A. Effect of IL-6 on IGF binding protein-3: a study in IL-6 transgenic mice and in patients with systemic juvenile idiopathic arthritis. Endocrinology 142: 4818–4826, 2001. [DOI] [PubMed] [Google Scholar]
- 22.Diehl KH, Hull R, Morton D, Pfister R, Rabemampianina Y, Smith D, Vidal JM, van de Vorstenbosch C. A good practice guide to the administration of substances and removal of blood, including routes and volumes. J Appl Toxicol 21: 15–23, 2001. [DOI] [PubMed] [Google Scholar]
- 23.Dogan Y, Akarsu S, Ustundag B, Yilmaz E, Gurgoze MK. Serum IL-1beta, IL-2, and IL-6 in insulin-dependent diabetic children. Mediators Inflamm 2006(1): 59206, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dubbelhuis PF, Meijer JA. Hepatic amino acid-dependent signaling is under the control of AMP-dependent protein kinase. FEBS Lett 521: 39–42, 2002. [DOI] [PubMed] [Google Scholar]
- 25.Eckel LA, Houpt TA, Geary N. Spontaneous meal patterns in female rats with and without access to running wheels. Physiol Behav 70: 397–405, 2000. [DOI] [PubMed] [Google Scholar]
- 26.Febbraio MA, Hiscock N, Sacchetti M, Fischer CP, Pedersen BK. Interleukin-6 is a novel factor mediating glucose homeostasis during skeletal muscle contraction. Diabetes 53: 1643–1648, 2004. [DOI] [PubMed] [Google Scholar]
- 27.Garma T, Kobayashi C, Haddad F, Adams GR, Bodell PW, Baldwin KM. Similar acute molecular responses to equivalent volumes of isometric, lengthening, or shortening mode resistance exercise. J Appl Physiol 102: 135–143, 2007. [DOI] [PubMed] [Google Scholar]
- 28.Glund S, Deshmukh A, Long YC, Moller T, Koistinen HA, Caidahl K, Zierath JR, Krook A. Interleukin-6 directly increases glucose metabolism in resting human skeletal muscle. Diabetes 56: 1630–1637, 2007. [DOI] [PubMed] [Google Scholar]
- 29.Goldstein SL, Leung JC, Silverstein DM. Pro- and anti-inflammatory cytokines in chronic pediatric dialysis patients: effect of aspirin. Clin J Am Soc Nephrol 1: 979–986, 2006. [DOI] [PubMed] [Google Scholar]
- 30.Gomes MD, Lecker SH, Jagoe RT, Navon A, Goldberg AL. Atrogin-1, a muscle-specific F-box protein highly expressed during muscle atrophy. Proc Natl Acad Sci USA 98: 14440–14445, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Goodman MN Interleukin-6 induces skeletal muscle protein breakdown in rats. Proc Soc Exp Biol Med. 205: 182–185, 1994. [DOI] [PubMed] [Google Scholar]
- 32.Gornall AG, Bardawill CJ, David MM. Determination of serum proteins by means of the biuret reaction. J Biol Chem 177: 751–766, 1949. [PubMed] [Google Scholar]
- 33.Greenhalgh CJ, Alexander WS. Suppressors of cytokine signalling and regulation of growth hormone action. Growth Horm IGF Res 14: 200–206, 2004. [DOI] [PubMed] [Google Scholar]
- 34.Greenhalgh CJ, Rico-Bautista E, Lorentzon M, Thaus AL, Morgan PO, Willson TA, Zervoudakis P, Metcalf D, Street I, Nicola NA, Nash AD, Fabri LJ, Norstedt G, Ohlsson C, Flores-Morales A, Alexander WS, Hilton DJ. SOCS2 negatively regulates growth hormone action in vitro and in vivo. J Clin Invest 115: 397–406, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hacham M, Cristal N, White RM, Segal S, Apte RN. Complementary organ expression of IL-1 vs. IL-6 and CSF-1 activities in normal and LPS-injected mice. Cytokine 8: 21–31, 1996. [DOI] [PubMed] [Google Scholar]
- 36.Haddad F, Adams GR. Inhibition of MAP/ERK kinase prevents IGF-I induced hypertrophy in rat muscles. J Appl Physiol 96: 203–210, 2004. [DOI] [PubMed] [Google Scholar]
- 37.Haddad F, Adams GR. Acute cellular and molecular responses to resistance exercise. J Appl Physiol 93: 394–403, 2002. [DOI] [PubMed] [Google Scholar]
- 38.Haddad F, Adams GR. Aging sensitive cellular and molecular mechanisms associated with skeletal muscle hypertrophy. J Appl Physiol 100: 1188–1203, 2006. [DOI] [PubMed] [Google Scholar]
- 39.Haddad F, Zaldivar FP, Cooper DM, Adams GR. IL-6 induced skeletal muscle atrophy. J Appl Physiol 98: 911–917, 2005. [DOI] [PubMed] [Google Scholar]
- 40.Hannan KM, Hannan RD, Rothblum LI. Transcription by RNA polymerase I. Front Biosci 3: 376–398, 1998. [DOI] [PubMed] [Google Scholar]
- 41.Hibbert JM, Hsu LL, Bhathena SJ, Irune I, Sarfo B, Creary MS, Gee BE, Mohamed AI, Buchanan ID, Al-Mahmoud A, Stiles JK. Proinflammatory cytokines and the hypermetabolism of children with sickle cell disease. Exp Biol Med 230: 68–74, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Holz MK, Ballif BA, Gygi SP, Blenis J. mTOR and S6K1 mediate assembly of the translation preinitiation complex through dynamic protein interchange and ordered phosphorylation events. Cell 123: 569–580, 2005. [DOI] [PubMed] [Google Scholar]
- 43.Huber SA, Sakkinen P, Conze D, Hardin N, Tracy R. Interleukin-6 exacerbates early atherosclerosis in mice. Arterioscler Thromb Vasc Biol 19: 2364–2367, 1999. [DOI] [PubMed] [Google Scholar]
- 44.Jørgensen JO, LMøller Krag M, Billestrup N, Christiansen JS. Effects of growth hormone on glucose and fat metabolism in human subjects. Endocrinol Metab Clin North Am 36: 75–87, 2007. [DOI] [PubMed] [Google Scholar]
- 45.Keller C, Steensberg A, Hansen AK, Fischer CP, Plomgaard P, Pedersen BK. Effect of exercise, training, and glycogen availability on IL-6 receptor expression in human skeletal muscle. J Appl Physiol 99: 2075–2078, 2005. [DOI] [PubMed] [Google Scholar]
- 46.Kimball SR Interaction between the AMP-activated protein kinase and mTOR signaling pathways. Med Sci Sports Exerc 38: 1958–1964, 2006. [DOI] [PubMed] [Google Scholar]
- 47.Koçtürk S, Kayatekin BM, Resmi H, Açikgöz O, Kaynak C, Ozer E. The apoptotic response to strenuous exercise of the gastrocnemius and soleus muscle fibers in rats. Eur J Appl Physiol 102: 515–524, 2008. [DOI] [PubMed] [Google Scholar]
- 48.Krause U, Bertrand L, Hue L. Control of p70 ribosomal protein S6 kinase and acetyl-CoA carboxylase by AMP-activated protein kinase and protein phosphatases in isolated hepatocytes. Eur J Biochem 269: 3751–3759, 2002. [DOI] [PubMed] [Google Scholar]
- 49.Labarca C, Paigen K. A simple, rapid, and sensitive DNA assay procedure. Anal Biochem 102: 344–352, 1980. [DOI] [PubMed] [Google Scholar]
- 50.Lebrun P, Van Obberghen E. SOCS proteins causing trouble in insulin action. Acta Physiol 192: 29–36, 2008. [DOI] [PubMed] [Google Scholar]
- 51.Levälampi T, Honkanen V, Lahdenne P, Nieminen R, Hakala M, Moilanen E. Effects of infliximab on cytokines, myeloperoxidase, and soluble adhesion molecules in patients with juvenile idiopathic arthritis. Scand J Rheumatol 36: 189–193, 2007. [DOI] [PubMed] [Google Scholar]
- 52.Lieskovska J, Guo D, Derman E. IL-6-overexpression brings about growth impairment potentially through a GH receptor defect. Growth Horm IGF Res 12: 388–398, 2002. [DOI] [PubMed] [Google Scholar]
- 53.Lieskovska J, Guo D, Derman E. Growth impairment in IL-6-overexpressing transgenic mice is associated with induction of SOCS3 mRNA. Growth Horm IGF Res 13: 26–35, 2003. [DOI] [PubMed] [Google Scholar]
- 54.Ling PR, Schwartz JH, Bistrian BR. Mechanisms of host wasting induced by administration of cytokines in rats. Am J Physiol Endocrinol Metab 272: E333–E339, 1997. [DOI] [PubMed] [Google Scholar]
- 55.Lotito AP, Campa A, Silva CA, Kiss MH, Mello SB. Interleukin 18 as a marker of disease activity and severity in patients with juvenile idiopathic arthritis. J Rheumatol 34: 823–830, 2007. [PubMed] [Google Scholar]
- 56.Michaylira CZ, Simmons JG, Ramocki NM, Scull BP, McNaughton KK, Fuller CR, Lund PK. Suppressor of cytokine signaling-2 limits intestinal growth and enterotrophic actions of IGF-I in vivo. Am J Physiol Gastrointest Liver Physiol 291: G472–G481, 2006. [DOI] [PubMed] [Google Scholar]
- 57.Natali AJ, Wilson LA, Peckham M, Turner DL, Harrison SM, White E. Different regional effects of voluntary exercise on the mechanical and electrical properties of rat ventricular myocytes. J Physiol 541: 863–875, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Nemet D, Rose-Gottron CM, Mills PJ, Cooper DM. Effect of water polo practice on cytokines, growth mediators, and leukocytes in girls. Med Sci Sports Exerc 35: 356–363, 2003. [DOI] [PubMed] [Google Scholar]
- 59.Nemet D, Wang P, Funahashi T, Matsuzawa Y, Tanaka S, Engelman L, Cooper DM. Adipocytokines, body composition, and fitness in children. Pediatr Res 53: 148–152, 2003. [DOI] [PubMed] [Google Scholar]
- 60.Olesen JL, Heinemeier KM, Haddad F, Langberg H, Flyvbjerg A, Kjaer M, Baldwin KM. Expression of insulin-like growth factor I, insulin-like growth factor binding proteins, and collagen mRNA in mechanically loaded plantaris tendon. J Appl Physiol 101: 183–188, 2006. [DOI] [PubMed] [Google Scholar]
- 61.Ou LS, See LC, Wu CJ, Kao CC, Lin YL, Huang JL. Association between serum inflammatory cytokines and disease activity in juvenile idiopathic arthritis. Clin Rheumatol 21: 52–56, 2002. [DOI] [PubMed] [Google Scholar]
- 62.Pedersen BK IL-6 signalling in exercise and disease. Biochem Soc Trans 35: 1295–1297, 2007. [DOI] [PubMed] [Google Scholar]
- 63.Putman CT, Xu X, Gillies E, MacLean IM, Bell GJ. Effects of strength, endurance and combined training on myosin heavy chain content and fibre-type distribution in humans. Eur J Appl Physiol 92: 376–384, 2004. [DOI] [PubMed] [Google Scholar]
- 64.Quinn LS, Haugk KL, Grabstein KH. Interleukin-15: A novel anabolic cytokine for skeletal muscle. Endocrinology 136: 3669–3672, 1995. [DOI] [PubMed] [Google Scholar]
- 65.Raj DS, Shah H, Shah VO, Ferrando A, Bankhurst A, Wolfe R, Zager PG. Markers of inflammation, proteolysis, and apoptosis in ESRD. Am J Kidney Dis 42: 1212–1220, 2003. [DOI] [PubMed] [Google Scholar]
- 66.Ram PA, Waxman DJ. SOCS/CIS protein inhibition of growth hormone-stimulated STAT5 signaling by multiple mechanisms. J Biol Chem 274: 35553–35551, 1999. [DOI] [PubMed] [Google Scholar]
- 67.Ridderstråle M, Amstrup J, Hilton DJ, Billestrup N, Tornqvist H. SOCS-3 is involved in the downregulation of the acute insulin-like effects of growth hormone in rat adipocytes by inhibition of Jak2/IRS-1 signaling. Horm Metab Res 35: 167–177, 2003. [DOI] [PubMed] [Google Scholar]
- 68.Ruderman NB, Keller C, Richard AM, Saha AK, Luo Z, Xiang X, Giralt M, Ritov VB, Menshikova EV, Kelley DE, Hidalgo J, Pedersen BK, Kelly M. Interleukin-6 regulation of AMP-activated protein kinase. Potential role in the systemic response to exercise and prevention of the metabolic syndrome. Diabetes 55, Suppl 2: S48–S54, 2006. [DOI] [PubMed] [Google Scholar]
- 69.Sawczenko A, Azooz O, Paraszczuk J, Idestrom M, Croft NM, Savage MO, Ballinger AB, Sanderson IR. Intestinal inflammation-induced growth retardation acts through IL-6 in rats and depends on the −174 IL-6 G/C polymorphism in children. Proc Natl Acad Sci USA 102: 13260–13265, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Schultz E, McCormick KM. Skeletal muscle satellite cells. Rev Physiol Biochem Pharmacol 123: 213–257, 1994. [DOI] [PubMed] [Google Scholar]
- 71.Shi H, Tzameli I, Bjorbaek C, Flier JS. Suppressor of cytokine signaling 3 is a physiological regulator of adipocyte insulin signaling. J Biol Chem 279: 34733–34740, 2004. [DOI] [PubMed] [Google Scholar]
- 72.Sjöholm A, Nyström T. Inflammation and the etiology of type 2 diabetes. Diabetes Metab Res Rev 22: 4–10, 2006. [DOI] [PubMed] [Google Scholar]
- 73.Solaro J, Pang DC, Briggs FN. The purification of cardiac myofibrils with Triton X-100. Biochim Biophys Acta 245: 259–262, 1971. [DOI] [PubMed] [Google Scholar]
- 74.Starkie RL, Ostrowski SR, Jauffred S, Febbraio MA, Pedersen BK. Exercise and IL-6 infusion inhibit endotoxin-induced TNF-a production in humans. FASEB J 17: 884–886, 2003. [DOI] [PubMed] [Google Scholar]
- 75.Starr R, Hilton DJ. Negative regulation of the JAK/STAT pathway. Bioessays 21: 47–52, 1999. [DOI] [PubMed] [Google Scholar]
- 76.Steensberg A, Febbraio MA, Osada T, Schjerling P, van Hall G, Saltin B, Pedersen BK. Interleukin-6 production in contracting human skeletal muscle is influenced by pre-exercise muscle glycogen content. J Physiol 537: 633–639, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Trayhurn P, Wood IS. Adipokines: inflammation and the pleiotropic role of white adipose tissue. Br J Nutr 92: 347–355, 2004. [DOI] [PubMed] [Google Scholar]
- 77a.van Hall G, Steensberg A, Fischer C, Keller C, Møller K, Moseley P, Pedersen BK. Interleukin-6 markedly decreases skeletal muscle protein turnover and increases nonmuscle amino acid utilization in healthy individuals. J Clin Endocrinol Metab 93: 2851–2858, 2008. [DOI] [PubMed] [Google Scholar]
- 77b.vanderMeer MJ, Sweep CG, Rijnkels CE, Pesman GJ, Tilders FJ, Kloppenborg PW, Hermus AR. Acute stimulation of the hypothalamic-pituitary-adrenal axis by IL-1B, TNFa and IL-6: A dose response study. J Endocrinol Invest 19: 175–182, 1996. [DOI] [PubMed] [Google Scholar]
- 78.Velloso LA, Carvalho CR, Rojas FA, Folli F, Saad MJ. Insulin signalling in heart involves insulin receptor substrates-1 and -2, activation of phosphatidylinositol 3-kinase and the JAK 2-growth related pathway. Cardiovasc Res 40: 96–102, 1998. [DOI] [PubMed] [Google Scholar]
- 79.Wada Y, Sato M, Niimi M, Tamaki M, Ishida T, Takahara J. Inhibitory effects of interleukin-1 on growth hormone secretion in conscious male rats. Endocrinology 136: 3936–3941, 1995. [DOI] [PubMed] [Google Scholar]
- 80.Wolf M, Bohm S, Brand M, Kreymann G. Proinflammatory cytokines interleukin 1 beta and tumor necrosis factor alpha inhibit growth hormone stimulation of insulin-like growth factor I synthesis and growth hormone receptor mRNA levels in cultured rat liver cells. Eur J Endocrinol 135: 729–737, 1996. [DOI] [PubMed] [Google Scholar]
- 81.Wright C, Haddad F, Qin A, Baldwin KM. Analysis of myosin heavy chain mRNA expression by RT-PCR. J Appl Physiol 83: 1389–1396, 1997. [DOI] [PubMed] [Google Scholar]
- 82.Yadav A, Kalita A, Dhillon S, Banerjee K. JAK/STAT3 pathway is involved in survival of neurons in response to insulin-like growth factor and negatively regulated by suppressor of cytokine signaling-3. J Biol Chem 280: 31830–31840, 2005. [DOI] [PubMed] [Google Scholar]
- 83.Yilmaz E, Ustundag B, Sen Y, Akarsu S, Kurt AN, Dogan Y. The levels of ghrelin, TNF-alpha, and IL-6 in children with cyanotic and acyanotic congenital heart disease. Mediators Inflamm 2007: 32403, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Yokota S, Miyamae T, Imagawa T, Iwata N, Katakura S, Mori M, Woo P, Nishimoto N, Yoshizaki K, Kishimoto T. Therapeutic efficacy of humanized recombinant anti-interleukin-6 receptor antibody in children with systemic-onset juvenile idiopathic arthritis. Arthritis Rheum 52: 818–825, 2005. [DOI] [PubMed] [Google Scholar]
- 85.Zoladz JA, Duda K, Konturek SJ, Sliwowski Z, Pawlik T, Majerczak J. Effect of different muscle shortening velocities during prolonged incremental cycling exercise on the plasma growth hormone, insulin, glucose, glucagon, cortisol, leptin and lactate concentrations. J Physiol Pharmacol 53: 409–422, 2002. [PubMed] [Google Scholar]