Abstract
In experiments on small bundles of intact fibers from a rat fast muscle, in vitro, we examined the decline in force in repeated tetanic contractions; the aim was to characterize the effect of shortening and of temperature on the initial phase of muscle fatigue. Short tetanic contractions were elicited at a control repetition rate of 1/60 s, and fatigue was induced by raising the rate to 1/5 s for 2–3 min, both in isometric mode (no shortening) and in shortening mode, in which each tetanic contraction included a ramp shortening at a standard velocity. In experiments at 20°C (n = 12), the force decline during a fatigue run was 25% in the isometric mode but was significantly higher (35%) in the shortening mode. In experiments at different temperatures (10–30°C, n = 11), the tetanic frequency and duration were adjusted as appropriate, and for shortening mode, the velocity was adjusted for maximum power output. In isometric mode, fatigue of force was significantly less at 30°C (∼20%) than at 10°C (∼30%); the power output (force × velocity) was >10× higher at 30°C than at 10°C, and power decline during a fatigue run was less at 30°C (∼20–30%) than at 10°C (∼50%). The finding that the extent of fatigue is increased with shortening contractions and is lower at higher temperatures is consistent with the view that force depression by inorganic phosphate, which accumulates within fibers during activity, may be a primary cause of initial muscle fatigue.
Keywords: exercise, temperature effects, muscle power, Fenn effect
deterioration of muscular performance during prolonged exercise is commonly referred to as fatigue, and in view of its obvious relevance to our daily physical activity, many studies have been carried out to elucidate the underlying mechanisms. The original experiments of Merton (28) on in situ human muscle showed that a main underlying cause of decline in force (fatigue) is indeed within muscle, namely, accumulation of metabolic products of activity. However, subsequent studies have shown that the etiology of fatigue of muscular performance can be rather complex and may involve impairment of various nervous mechanisms,; referred to as central fatigue (41), it seems that there is no one mechanism responsible and that in vivo muscle fatigue can be additionally caused by different neural mechanisms, specific to the task being performed (19, 29, 33).
The present study addresses an aspect of peripheral muscle fatigue. In that regard, experimental studies on directly stimulated muscle fibers in vitro have demonstrated that maximal isometric force declines (fatigue) when standard tetanic contractions are repeated at a certain high rate and recovers when subsequently “rested”: as reviewed in detail by Allen et al. (1), even peripheral muscle fatigue can be complex and may involve several causative factors. In general, however, such experiments indicated an initial force decline followed by a later more pronounced decline (31, 42). Whereas the late phase of fatigue, referred to as activation fatigue (18), was shown to be associated with impaired excitation-contraction coupling processes, the early force decline (myofibrillar fatigue) is thought to be largely due to accumulation of products of ATP hydrolysis such as inorganic phosphate (Pi) affecting the cross-bridge cycle (for recent reviews, see 1, 21). Indeed, experiments on skinned muscle fibers, where the intracellular chemical milieu can be controlled, have demonstrated that increased Pi depresses maximally Ca-activated force (5, 22) because of its effect on the force generation step in the cross-bridge/acto-myosin ATPase cycle (see 36).
Against the general background knowledge briefly summarized above (also see discussion), we have carried out experiments to determine what may be a predominant cause of early (myofibrillar) fatigue in intact (rat) muscle fibers; the rationale behind these experiments was the following. First, when an actively contracting muscle is allowed to shorten, the force it exerts is reduced but its rates of energy liberation and ATP hydrolysis are raised; the converse effects are obtained when an active muscle is stretched (10, 17, 20, 24). Hence, if a major contributing factor to early fatigue is the accumulation of Pi, a product released from actomyosin ATP hydrolysis, then the extent of muscle fatigue would be greater with shortening contractions doing external work than with isometric contractions. Second, maximal active force in mammalian muscle increases ∼2-fold (37) and the power output (force × shortening velocity) increases >10-fold (35) in warming from low (∼10°C) to physiological (>30°C) temperatures: however, in skinned fiber experiments, the sensitivity of active force to Pi is less at higher temperatures (7). Thus, if Pi-induced force depression is a major cause, the extent of early fatigue would be less at the physiological temperatures. This study therefore examined fatigue of isometric force (and power) following repeated short contractions without shortening (isometric mode) and with shortening (shortening mode) and at 10, 20, and 30°C; the trends indicated by our findings are consistent with the notion that the early muscle fatigue may be due to force depression by Pi accumulated during muscle activity.
METHODS
The basic experimental techniques are similar to those outlined in Roots et al. (38). In accordance with the UK Home Office (Schedule 1) humane killing procedure of animals, adult male rats (∼250 g) were killed with an overdose of anesthetic, pentobarbital sodium (Euthatal, Rhone, Merieux) administered intraperitoneally; furthermore, the study was reviewed and approved by the University of Bristol Ethical Review Committee for animal use and care, as required by the UK Animals (Scientific Procedures) Act 1986. The flexor hallucis brevis (FHB) muscle, which is known to contain predominantly (∼90%) fast type 2 fibers, was removed from the foot of the dead animal and small bundles of ∼5 muscle fibers were dissected from a muscle under dissecting microscope fitted with dark-field illumination (see 8, for details). Fiber bundles were mounted horizontally, using aluminum T clips attached to the tendons, between a force transducer (AE 801 element; AME, Horten, Norway) and servomotor in a stainless steel chamber (∼2 ml), the force transducer's natural resonance frequency was >5 kHz and the servomotor could apply ramp length steps of 20% optimal fiber length (L0) at various rates. The chamber was perfused (0.5 ml/min) with a constant through-flow of physiological saline solution containing (in mM) 109 NaCl, 5 KCl, 1 MgCl2, 1 NaH2PO4, 4 CaCl2, 10 pyruvate, 24 NaHCO3, and 200 mg/l bovine fetal serum; the solution was continuously bubbled with 95% O2-5% CO2. A Peltier device fitted beneath the chamber controlled the temperature and a thermocouple inside the chamber monitored the solution temperature changes. Fiber length was initially set to L0 to give maximum tetanic tension at 20°C, and sarcomere length measured by He-Ne laser diffraction was typically ∼2.5 μm.
Experimental protocols.
Based on some preliminary experiments, the experimental protocol adopted to examine fatigue was basically an adaptation of that of Westerblad et al. (45). Using a standard stimulation frequency and duration (see below), tetanic contractions were first elicited at a nonfatiguing repetition rate of 1/60 s as a control condition; to induce fatigue, the rate was increased to 1/5 s, when the tension declined rapidly at first, phase 1 of fatigue (see 31), and then reached a steady level (phase 2). After 20–30 contractions, the rate was returned to 1/60 s for the fibers to recover; a recovery to within 90–95% P0 (control tension) was considered acceptable before another procedure was initiated. Repetition rates higher than 1/5 s and durations longer than 2–3 min were avoided to prevent adverse effects of more prolonged (activation) fatigue, or phase 3 in the study of Lannergren et al. (31).
In one series of experiments on 12 preparations, fatigue of tension was compared between isometric and shortening modes; these experiments were done at 20°C and used tetanic contractions at a stimulation frequency of ∼70 Hz and duration of ∼1 s. For shortening mode, a ramp shortening of 20% L0 at ∼0.5 L0/s was introduced to each tetanic contraction, applied half-way along the isometric tension plateau (as seen in Fig. 2), so that isometric tension could still be measured before the ramp; other shortening velocities (0.4–1.0 L0/s) and durations were also used in some experiments, and they gave qualitatively similar data but they are not reported here. From such recordings throughout each experiment, measurements of isometric force before fatigue (P0 control) and during a fatiguing run were made to determine the time course and also the extent of fatigue at the 20th contraction. This procedure allowed examination, with and without shortening, of the time course of isometric tension fatigue, which was not readily possible with other protocols (e.g., 9, 15) in which isometric tension was recorded after a sustained isometric contraction of a certain duration or repeated isotonic contractions to cause fatigue.
In a second series of experiments on 11 preparations, recordings were made at 10, 20, and/or 30°C. Tetanic stimulation frequencies of ∼40, ∼70, and ∼250 Hz were used to obtain fused contractions at 10, 20, and 30°C. Since contractions are much faster at the higher temperatures, in examining fatigue using the same repetition rate, the duration of tetanic contractions was made correspondingly longer at lower temperatures (>2× longer at 10°C than at 30°C) to compensate for the lower contraction speed (∼ATPase rate); the durations of tetanic stimulation were ∼1.0, ∼0.7, and 0.4–0.5 s for 10, 20, and 30°C, respectively. As determined in a series of preliminary force-velocity analyses and from Roots and Ranatunga (39), shortening velocities were set to give the maximum power output at each temperature; they were ∼0.5, ∼1, and ∼2.5 L0/s for 10, 20, and 30°C, respectively; a shortening ramp was set to occur approximately halfway along the isometric tension plateau as in the first series.
The tension responses recorded in the shortening mode were used to estimate the mechanical power output, as velocity × force during shortening, at the different temperatures; as in our previous study (39), the P2 tension at the point of intersection between two lines across the P2 transition (as shown in Fig. 2B) was taken as the active force during steady shortening; thus, taking P2 tension in kilonewtons per square meter and the shortening velocity in L0 per second, the power output was calculated in units of watts per liter, or kilowatts per cubic meter.
Data recording and analysis.
Tension and length change signals were recorded onto computer via a CED 1401 Plus interface and Signal 3 software (Cambridge Electronic Design, Cambridge, UK). A two-channel chart recorder (Multitrace2, Lectromed) was also used to provide a record of the entire experimental time course. After each experiment tension and time course, measurements were made using the Signal software and analyzed further with FigP (Biosoft, Durham, NC), Excel (Microsoft), and Prism (GraphPad) software. Comparison of the extent of fatigue was made using Student's t-test on the pooled data for the 20th contraction (1st series) or the 30th contraction (2nd series); P < 0.05 was considered significant.
RESULTS
The data presented in this study are from experiments on 23 FHB fiber bundles; their mean (±SE) fiber length (L0) and width were 2.18 ± 0.044 mm and 203 ± 15 μm, respectively. The control isometric tension (P0) was 163 ± 13.7 kN/m2 at 20°C, which is in the range reported in two of our previous studies (32, 38).
Fatigue in isometric mode and shortening mode.
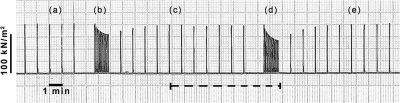
Figure 1 shows a chart recording from a typical experiment. During fatigue run in isometric mode (Fig. 1, b), the tension declines toward a plateau level of ∼0.8P0 and recovers to prefatigue level when returned to 1/60 s (Fig. 1, c). Within the duration of recording shown by the dashed horizontal line, each tetanic contraction was initially isometric but contained a subsequent ramp shortening phase. During fatigue run in this shortening mode (Fig. 1, d), the decline of isometric tension (i.e., tension before the ramp) is more pronounced (∼35%) than in isometric mode (∼20%) but returns to its original prefatigue level at 1/60 s (Fig. 1, e).
Fig. 1.
Fatigue protocol, with and without shortening. A chart recorder trace showing typical fatigue runs and recovery in an experiment at 20°C from one fiber bundle, with isometric and ramp shortening contractions. In isometric mode, contractions at 1/60 s (a) do not cause tension to fall; when changed to 1/5 s contraction rate (b) isometric tension falls toward a steady level. After ∼1.5 min, contraction rate is returned to 1/60 s and isometric tension gradually recovers to within 95% of its original value (c). Once recovered, a shortening ramp is introduced to each tetanic contraction; no fatigue occurs at 1/60 s but when the rate is increased to 1/5 s (d) isometric tension declines. The horizontal dashed line indicates the period of shortening mode, where ramp shortening was introduced to each contraction (see Fig. 2). After the second period of fatigue, contraction rate was returned to 1/60 s and tension recovered once more (e). Note that the extent of tension decline during the fatigue run is greater with shortening contractions than with isometric contractions (compare d with b).
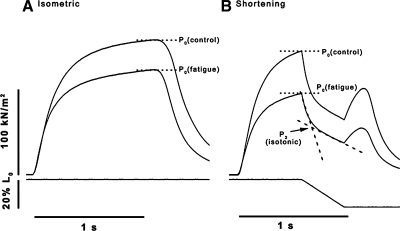
Figure 2 shows, at an expanded time scale, superimposed 1st and 20th contractions during a fatigue run, in isometric mode (Fig. 2A) and in shortening mode (Fig. 2B). It is noteworthy from Fig. 2A that the time course of tension relaxation is similar between control and fatigued contractions; measurements made on such experimental records (n > 12) showed that half-time of tension relaxation in the 20th (fatigued) contraction remained within ±10% of the control. The form of tension change during a ramp shortening (Fig. 2B) is similar to that described in our previous detailed study (38); a rapid fall in tension at the onset of the ramp is followed by a gradual transition phase (P2) and a slower decline in tension afterward until the end of the ramp. The P2 tension at the point of intersection between two lines across the P2 transition (as shown) was taken as the active force during shortening at this velocity.
Fig. 2.
Sample records of contractions before (control) and after fatigue. Tension responses (top traces) and length changes (bottom traces) from a preparation taken at time 0, the 1st contraction (P0 control), and at the 20th contraction (P0 fatigue) during a fatigue run at 1/5 s, at 20°C. A: 2 superimposed tension traces of isometric contractions (without ramp shortening). B: contractions with a ramp shortening of 20% optimal fiber length (L0), 0.5 L0/s, applied during tetanic tension plateau. Note that tension fatigue is greater in B, in the shortening mode. The tension response to a ramp shortening (B) is similar to that characterized in a previous study [Roots et al. (38)]; an initial rapid drop in tension is followed by a gradual transition phase (P2); P2 tension was estimated from the point of intersection between the lines fitted, as shown, and taken as tension during shortening for calculating power.
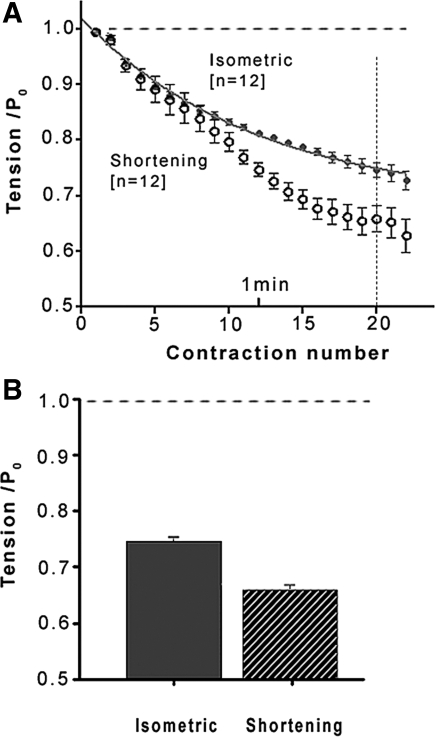
Figure 3A shows pooled data for the time course fatigue; data show that in both isometric mode and shortening mode, the tension falls steadily during a fatigue run, leveling off toward an approximate steady level. Taking the 20th contraction, Fig. 3B summarizes the difference in the extent of fatigue between the two conditions. Thus, at 20°C, the tension fatigue is ∼25% in isometric mode and it is higher, ∼35%, when during part of each contraction the fibers were shortening and doing external work; the difference is significant (paired t-test, P < 0.001).
Fig. 3.
Fatigue in isometric mode and shortening mode. A: pooled mean (±SE) tension data during a fatigue run, solid symbols in isometric mode and open symbols in shortening mode, from 12 fiber bundles. Tensions are normalized to control P0 (horizontal dashed line). A single-exponential curve fitted to the isometric mode data indicates that fatigue would reach a steady value of 0.71 ± 0.015 P0; it is 0.49 ± 0.06 P0 in shortening mode (curve not shown). B: mean (±SE) tension of the 20th contraction during a fatigue run in isometric mode (filled column) and in shortening mode (striped); the tensions were 0.75 ± 0.012 P0 in isometric and 0.65 ± 0.025 P0 with shortening; the difference is significant (paired t-test, P < 0.01).
Fatigue at different temperatures.
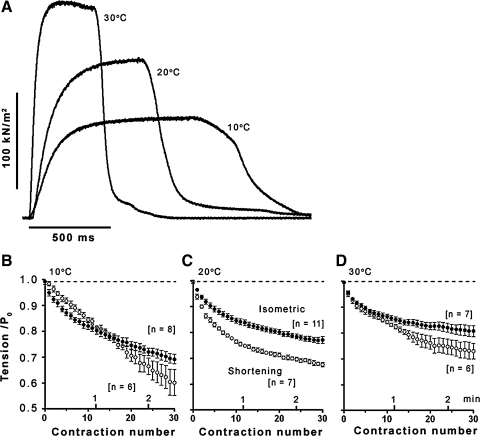
In experiments on 11 fiber bundles, we examined fatigue at different temperatures using essentially the same protocol as above. The mean (±SE) tetanic tensions recorded in these experiments were 107 ± 7.1, 170 ± 21.1, and 214 ± 29.5 kN/m2 for 10, 20, and 30°C, respectively; the data show a twofold increase of force in warming from 10 to 30°C as found in our previous studies (see references in 8). Isometric tetanic contractions from one fiber bundle in Fig. 4A show the marked increase of tension on warming; they also show that the tension rise and relaxation are much faster at the higher temperatures. Hence, in examining fatigue using the same repetition rate, the duration of tetanic contractions was made correspondingly longer at lower temperatures (see methods) to compensate for the lower contraction speed (∼ATPase rate).
Fig. 4.
Fatigue at different temperatures. A: sample traces from one preparation illustrating the tetanic contractions used in fatigue experiments: note that contractions are faster and the tension is increased at high temperatures, as in previous studies [Coupland and Ranatunga (8)]. Tetanic durations (and frequency) were accordingly adjusted as shown (see methods). B–D: pooled data (11 preparations; n for each temperature is given) for tension decline during a fatigue run (at 1/5 s) at 10 (B), 20 (C), and 30°C (D); presentation is similar to Fig. 3A. Filled symbols, data in isometric mode: fatigue decreases from B (10°C) to D (30°C). A single-exponential curve fitted to each data set (as in Fig. 3A) gave steady fatigued tension levels of 0.63 ± 0.03 P0 for 10°C, 0.75 ± 0.01 P0 for 20°C, and 0.81 ± 0.01 P0 for 30°C; their time constants of approach to steady level were ∼100, ∼55, and ∼40 s, respectively. Open symbols, data in shortening mode; at each temperature, fatigue is higher in shortening mode, but the time course for 10°C shows complexity (see text).
Figure 4, B–D, shows the pooled data for the time course of fatigue at the three different temperatures. In isometric mode (filled symbols), tension declines toward a steady level but the tension decline is temperature sensitive, being greater at the lower temperatures. Fatigue was also examined in the shortening mode (open symbols), using velocities appropriate for maximal power output at each temperature (see methods). At 20 and 30°C, the general pattern of tension fatigue in the shortening mode, with a rapid phase of decline that approaches a steady level, appears similar to isometric. Within the time scale employed, the data at 10°C show some complexity; the tension continues to decline with no distinct phases visible. In general, however, the tension decline by 20–30 contractions is larger in shortening mode than in isometric mode, at all temperatures.
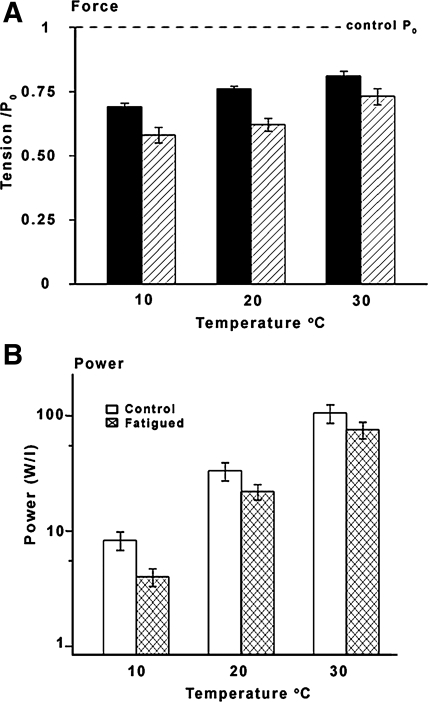
Figure 5A illustrates, in the form of histograms, a summary data for the extent of fatigue in the 30th contraction at the three different temperatures. At 10°C, the extent of fatigue is ∼30% in isometric mode (filled columns) and ∼40% in shortening mode (hatched columns); corresponding values for the extent of fatigue at 30°C, ∼20% and ∼25% respectively, are significantly smaller (see legend of Fig. 5A for details). Figure 5B shows the power output (force during shortening × velocity, see methods) at the different temperatures, determined from tension responses in the shortening mode. Although the absolute power recorded in control conditions (before fatigue) in the present experiments (open columns) is less than in our previous studies that did not investigate fatigue, a >10-fold increase from 10°C to 30°C is similar to the previous results (35, 39). When examined for the 30th contraction, the power is significantly decreased (paired t-test) during fatigue (cross-hatched columns); also, power decline during fatigue is significantly higher, ∼50%, at 10°C than at higher temperatures (∼25–30% at 20–30°C).
Fig. 5.
Summary data for fatigue at different temperature. A: mean (±SE) data for isometric tension of the 30th contraction (as a ratio of control P0; dashed line) at different temperatures (n as in Fig. 4) in isometric mode (filled columns) and in shortening mode (hatched columns). Extent of fatigue is less at higher temperatures and at each temperature shortening mode causes more fatigue than isometric. Statistically significant differences (t-test; P < 0.05) are found for fatigue data in isometric mode between 10 and 20°C and 10 and 30°C and between isometric vs. shortening data at 20 and 10°C. B: mechanical power, calculated as P2 tension (see Fig. 2) in kN/m2 multiplied by shortening velocity in L0/s, is plotted in units of watts/liter (W/l) on a logarithmic ordinate. The histograms show the mean (±SE; n = 6, 7) absolute power for prefatigue control (open columns) and for 30th fatiguing contraction (cross-hatched columns) at different temperatures. Note that the power output shows >10-fold increase from 10°C (∼8 W/l) to 30°C (∼105 W/l) and the differences between temperatures are significant (P < 0.05); also, the power at each temperature is significantly reduced (paired t-test, P < 0.05) after fatigue. The fatigue of power is greater at 10°C (∼50%) than at 20 and 30°C (70–75%).
DISCUSSION
Findings from the present study.
This study examined fatigue of isometric force in intact mammalian (fast) muscle fibers, throughout fatigue runs both in isometric mode (no shortening) and in shortening mode (with ramp shortening during part of each contraction) (see Fig. 1, Fig. 2); corresponding data were also obtained over a range of temperatures (10–30°C). Two basic findings are 1) that fatigue of force was greater in shortening mode than in isometric mode (Fig. 1, Fig. 3), and 2) that the extent of fatigue decreased with increase of temperature from 10°C to more physiological 30°C (Fig. 4); similar trends were seen in the mechanical power output calculated from the shortening contractions (see Fig. 5).
Causes of peripheral muscle fatigue in general.
Although the ATP level in intact muscle fibers during activity would be effectively maintained by creatine phosphokinase reaction (see 11, 46), accumulation of Pi, H+, and ADP, etc., and also impairment of excitation and/or excitation-contraction coupling (E-C coupling) can play some role in fatigue (see 1, 21, 26). We used repetition of short contractions to induce fatigue and examined only the early phase of fatigue. Allen et al. (2) noted increased Ca2+ transients, and Edman and Lou (18) found tension to be caffeine insensitive during this initial tension fatigue in intact muscle fibers and identified it as myofibrillar fatigue. Moreover, the tension relaxation is largely unaffected (Fig. 2 and text) during fatigue in our experiments; a marked slowing of tension relaxation (2- to 4-fold) during fatigue has been reported before but with different protocols, for example, in rat muscle with a long (∼15 s) tetanic contraction (15) and in human muscle in vivo (25) that used ischemia for fatigue. Thus it is reasonable to assume that the force fatigue in our experiments arises from effects on the cross-bridge cycle of accumulation of metabolic products rather than from direct or indirect effects on E-C coupling.
Although ADP potentiates force and depresses speed of shortening (see references in Ref. 6), the likely contributors to myofibrillar fatigue would be increased Pi and H+, both of which depress active force in skinned fibers (5, 22). Such skinned fiber experiments were initially limited to low temperatures, but the recent careful studies of Fitts and colleagues (14, 27) do show that both increased Pi and H+ depress active force (and power) at the more physiological temperatures of ∼30°C (see also 26). Moreover, increase of H+ (acidosis) and Pi has been recorded during fatigue in human muscle (see 3). In experiments on intact mammalian muscle fibers, on the other hand, acidosis has been shown to have little depressive effect on the active force at physiological temperatures (34, 44), and, on the basis of additional experimentation such as fatigue in creatine kinase-deficient mouse muscle (see 12, 13), Westerblad et al. (43) indeed concluded that increased Pi rather than low pH is the major cause of muscle fatigue; nevertheless, acidosis may play a role in high intensity fatigue as indicated by the experiments of Chin et al. (4).
Increase of fatigue with shortening.
There have been previous studies examining muscle fatigue using shortening contractions (9, 15); although not at the detailed level carried out here (e.g., Fig. 3), the results from those studies also showed greater fatigue with shortening than with isometric contractions. It is well known that the energy production (and the acto-myosin ATPase rate) in muscle is increased with shortening (and decreased with lengthening), a cardinal principle of muscle contraction commonly referred to as the Fenn effect (see introduction and references in 46). If indeed the cross-bridge/ATPase cycle proceeds more readily during steady muscle shortening, then the increased fatigue in the shortening mode (Figs. 3 and 4) would be expected: everything else being similar, Pi accumulation within muscle fibers during a fatigue run with shortening would be greater than in the isometric mode.
Fatigue at different temperatures.
Studies on mammalian muscle contraction in vitro and muscle performance in vivo at different temperatures have indicated that muscle fatigue is also temperature sensitive; interestingly, optimal muscle performance (or less fatigue) was obtained at 25–30°C than at higher or lower temperatures (see 40 and references therein). In experiments on human muscle under ischemic conditions, the extent of fatigue was found to be greater, and its onset faster, at 37°C than at 22°C (16); a faster onset of fatigue at 37°C than at 22°C was also found in mouse muscle experiments (30). Although a faster onset of fatigue at higher temperatures is seen in our data (see Fig. 4 legend), the extent of fatigue is less at higher than at lower temperatures; this may be due to the specific experimental protocols we adopted to examine the cause(s) of initial fatigue (see below).
In a previous study on skinned mammalian fast (rabbit psoas) muscle fibers, we showed that, in warming from 10 to 30°C, the maximally Ca-activated isometric force increased ∼2-fold, as found for tetanic force in intact fibers, but the depressive effect of Pi on active force was decreased (7); similar observations on the temperature sensitivity of the Pi effects on force have been reported in other studies (see 14). We adopted an experimental protocol that was aimed at relating such effects to fatigue in intact fibers (see methods); our finding that fatigue is less marked at high (30°C) than at low temperature (10°C) would be consistent with force depression by Pi being a main cause of fatigue.
It is noteworthy that high levels of Pi have been actually measured during similar level of muscle fatigue in other studies (25–30 mM in human muscle in Ref. 3, ∼10 mM in fish muscle in Ref. 11). A rough calculation may be made of the level of Pi accumulation during a fatigue run in our experiments. Taking an actin-activated active site (cross bridge) concentration (c) of 0.15 mM and an acto-myosin ATPase rate (r) of ∼5 s−1 in isometric muscle fiber (at ∼12°C) (23), a series of n (= 30) tetanic contractions of ∼1 s duration (d) in a fatigue run would liberate (c×r×d×n) or ∼22 mM Pi around myofibrils. Pi removal in intact fibers is known to be slow (see 11); if tension recovery after a fatigue run reflects this process, it takes ∼6–7 min (Fig. 1) and the Pi removal rate is ∼0.0028 s−1 [1/(6 × 60 s)]. On that basis, Pi removed during 4 s × 29 rest intervals in a fatigue run would be ∼0.32 (i.e., 4 s × 29 × 0.0028 s−1) of Pi produced. Hence, for the 30th contraction the Pi level would have risen to 22 mM × (1 − 0.32) or ∼15 mM. Although ATPase rate would be higher at higher temperatures, the contraction duration was shortened, and, hence, it was assumed that similar levels of Pi (10–20 mM) accumulated during the fatigue runs. In skinned fiber experiments, the isometric tension depression with 25 mM added Pi was ∼50% at 10°C and <20% at 30°C (Fig. 2 in Ref. 7). Given the experimental uncertainties involved in both studies, tension fatigue of ∼30% at 10°C and ∼20% at 30°C, obtained here in the isometric mode (Fig. 5), seem broadly consistent; Pi accumulation can be assumed to be higher during more dynamic contractions (shortening mode) and fatigue of force would be greater. Hence, to a first approximation, the decreased Pi sensitivity of active force can be considered a key contributor to the decrease in the extent of fatigue at higher temperature, as in Fig. 5A.
Effect of fatigue on power.
Our normal body movements during exercise are reliant on muscle power generation, a function of velocity and force, both of which (and also the curvature of force vs. velocity relation) are temperature sensitive (see references in 46). In the present study, shortening velocities were specifically chosen to obtain maximal power output at each temperature, and a fatigue run was also conducted. Although the absolute power values are lower in the present results, muscle power increases approximately exponentially with temperature and a >10-fold increase is seen from 10 to 30°C (Fig. 5B, a semilog plot). de Haan et al. (15) found a greater fall in power compared with that of isometric tension in fatigued intact muscles at 26°C, as indicated in our data. Our data also show that the extent of power reduction following fatigue was high at 10°C and lower at higher temperatures (see Fig. 5 and results), consistent with the finding of Debold et al. (14) that peak power was reduced to a greater extent at 15°C than at 30°C with 30 mM Pi. Since ADP decreases speed of shortening, its effect on power decline during fatigue also cannot be excluded (see 6); further experiments would be worthwhile.
In summary, the trends indicated by our experiments on intact muscle fibers suggest that the extent of low-intensity, reversible fatigue is temperature sensitive so that it is less pronounced at the physiological temperatures than at low temperatures; a decreased sensitivity of force to inorganic phosphate, which accumulates during activity and depresses force, can be the primary cause of such temperature-sensitivity differences in early fatigue. Other effects such as changes during activity in pH, ADP, phosphorylation, etc., may not be fully excluded, particularly, for higher intensity fatigue (see 21, 26).
GRANTS
We thank The Wellcome Trust for financial support of our research.
Acknowledgments
We thank Barbara Girard and Robert Wood for assistance in some experiments.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Allen DG, Lamb GD, Westerblad H. Skeletal muscle fatigue: cellular mechanisms. Physiol Rev 88: 287–332, 2008. [DOI] [PubMed] [Google Scholar]
- 2.Allen DG, Lee JA, Westerblad H. Intracellular calcium and tension during fatigue in isolated single muscle fibres from Xenopus laevis. J Physiol 415: 433–458, 1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cady EB, Jones DA, Lynn J, Newham DJ. Changes in force and intracellular metabolites during fatigue of human skeletal muscle. J Physiol 418: 311–325, 1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chin ER, Allen DG. The contribution of pH-dependent mechanisms to fatigue at different intensities in mammalian single muscle fibres. J Physiol 512: 831–840, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cooke R, Franks K, Luiciani GB, Pate E. The inhibition of rabbit skeletal muscle contraction by hydrogen ions and phosphate. J Physiol 398: 77–97, 1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Coupland ME, Pinniger GJ, Ranatunga KW. Endothermic force generation, temperature-jump experiments and effects of increased [MgADP] in rabbit psoas muscle fibres. J Physiol 567: 471–492, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Coupland ME, Puchert E, Ranatunga KW. Temperature dependence of active tension in mammalian (rabbit psoas) muscle fibres: effect of inorganic phosphate. J Physiol 536: 879–891, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Coupland ME, Ranatunga KW. Force generation induced by rapid temperature jumps in intact mammalian (rat) skeletal muscle fibres. J Physiol 548: 439–449, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cummins ME, Rabinder SS, Curtin NA. Fatigue of isolated mouse muscle due to isometric tetani with high power output. Q J Exp Physiol 74: 951–95, 1989. [DOI] [PubMed] [Google Scholar]
- 10.Curtin NA, Davies RE. Chemical and mechanical changes during stretching of activated frog skeletal muscle. Cold Spring Harb Symp Quant Biol 37: 619–626, 1973. [Google Scholar]
- 11.Curtin NA, Kushmerick MJ, Wiseman RW, Woledge RC. Recovery after contraction of white muscle fibres from the dogfish Scyliorhinus canicula. J Exp Biol 200: 1061–1071, 1997. [DOI] [PubMed] [Google Scholar]
- 12.Dahltedt AJ, Katz A, Wieringa B, Westerblad H. Is creatine kinase responsible for fatigue? Studies of isolated skeletal muscle deficient in creatine kinase. FASEB J 14: 982–990, 2000. [DOI] [PubMed] [Google Scholar]
- 13.Dahltedt AJ, Westerblad H. Inhibition of creatine kinase reduces the rate of fatigue-induced decrease in tetanic [Ca2+]i in mouse skeletal muscle. J Physiol 533: 639–649, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Debold EP, Dave H, Fitts RH. Fibre type and temperature dependence of inorganic phosphate: implications for fatigue. Am J Physiol Cell Physiol 287: C673–C681, 2004. [DOI] [PubMed] [Google Scholar]
- 15.De Haan A, Jones DA, Sargeant AJ. Changes in velocity of shortening, power output and relaxation rate during fatigue of rat medial gastrocnemius muscle. Pflügers Arch 413: 422–428, 1989. [DOI] [PubMed] [Google Scholar]
- 16.De Ruiter CJ, De Haan A. Temperature effect on the force-velocity relation ship of the fresh and fatigued human adductor pollicis muscle. Pflügers Arch 440: 163–170, 2000. [DOI] [PubMed] [Google Scholar]
- 17.Edman KAP, Elzinga G, Noble MI. Enhancement of mechanical performance by stretch during tetanic contractions of vertebrate skeletal muscle fibres. J Physiol 281: 139–155, 1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Edman KAP, Lou F. Myofibrillar fatigue versus failure of activation during repetitive stimulation of frog muscle fibres. J Physiol 457: 655–673, 1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Enoka RM, Duchateau J. Muscle fatigue: what, why and how it influences muscle function. J Physiol 586: 11–23, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fenn WO The relationship between the work performed and the energy liberated in muscular contraction. J Physiol 58: 373–395, 1924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fitts RH The cross-bridge cycle and skeletal muscle fatigue. J Appl Physiol 104: 551–558, 2008. [DOI] [PubMed] [Google Scholar]
- 22.Godt RE, Nosek TM. Changes of intracellular milieu with fatigue or hypoxia depress contraction of skinned rabbit skeletal and cardiac muscle. J Physiol 412: 155–180, 1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.He ZH, Chillingworth RK, Brune M, Corrie JET, Webb MR, Ferenczi MA. The efficiency of contraction in rabbit skeletal muscle fibres, determined from the rate of release of inorganic phosphate. J Physiol 517: 839–854, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hill AV The heat of shortening and the dynamic constants of muscle. Proc R Soc Lond B 126: 136–195, 1938. [Google Scholar]
- 25.Jones DA, De Ruiter CJ, De Haan A. Change in contractile properties of human muscle in relationship to the loss of power and slowing of relaxation seen with fatigue. J Physiol 576: 913–922, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Karatzaferi C, Franks-Skiba K, Cooke R. Inhibition of shortening velocity of skinned skeletal muscle fibers in conditions that mimic fatigue. Am J Physiol Regul Integr Comp Physiol 294: R948–R955, 2008. [DOI] [PubMed] [Google Scholar]
- 27.Knuth ST, Dave H, Peters JR, Fitts RH. Low cell pH depresses peak power in rat skeletal muscle fibres at both 30°C and 15°C: implications for muscle fatigue. J Physiol 575: 887–899, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Merton PA Voluntary strength and fatigue. J Physiol 123: 553–564, 1954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Millet GY, Martin V, Lattier G, Ballay Y. Mechanisms contributing to knee extensor strength loss after prolonged running exercise. J Appl Physiol 94: 193–198, 2003. [DOI] [PubMed] [Google Scholar]
- 30.Moopanar TR, Allen DG. Reactive oxygen species reduce myofibrillar Ca2+ sensitivity in fatiguing mouse skeletal muscle at 37°C. J Physiol 564: 189–199, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lannergren J, Westerblad H. Force decline due to fatigue and intracellular acidification in isolated fibres from mouse skeletal muscle. J Physiol 434: 307–322, 1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pinniger GJ, Ranatunga KW, Offer GW. Crossbridge and non-crossbridge contributions to tension in lengthening muscle: force-induced reversal of the power stroke. J Physiol 573: 627–643, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Racinais S, Girard O, Micallef JP, Perrey S. Failed excitability of spinal motorneurons induced by prolonged running exercise. J Neurophysiol 97: 596–603, 2007. [DOI] [PubMed] [Google Scholar]
- 34.Ranatunga KW Effects of acidosis on tension development in mammalian muscle. Muscle Nerve 10: 439–444, 1987. [DOI] [PubMed] [Google Scholar]
- 35.Ranatunga KW Temperature dependence of mechanical power output in mammalian (rat) skeletal muscle. Exp Physiol 83: 371–376, 1998. [DOI] [PubMed] [Google Scholar]
- 36.Ranatunga KW Effects of inorganic phosphate on endothermic force generation in muscle. Proc R Soc Lond B 266: 1381–1385, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ranatunga KW, Wylie SR. Temperature dependent transitions in isometric contractions of rat muscle. J Physiol 339: 87–95, 1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Roots H, Offer GW, Ranatunga KW. Comparison of the tension responses to ramp shortening and lengthening in intact mammalian muscle fibres: crossbridge and non-crossbridge contributions. J Muscle Res Cell Motil 28: 123–139, 2007. [DOI] [PubMed] [Google Scholar]
- 39.Roots H, Ranatunga KW. An analysis of the temperature dependence of force, during steady shortening at different velocities, in (mammalian) fast muscle fibres. J Muscle Res Cell Motil 29: 9–24, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Segal SS, Faulkner JA, White TP. Skeletal muscle fatigue in vitro is temperature dependent. J Appl Physiol 61: 660–665, 1986. [DOI] [PubMed] [Google Scholar]
- 41.Taylor JL, Gandevia SC. A comparison of central aspects of fatigue in submaximal and maximal voluntary contractions. J Appl Physiol 104: 542–550, 2008. [DOI] [PubMed] [Google Scholar]
- 42.Vawda F, Ranatunga KW, Geeves MA. Effects of hydrostatic pressure on fatiguing frog muscle fibres. J Muscle Res Cell Motil 17: 631–636, 1996. [DOI] [PubMed] [Google Scholar]
- 43.Westerblad H, Allen DG, Lännergren J. Muscle fatigue: lactic acid or inorganic phosphate the major cause? News Physiol Sci 17: 17–21, 2002. [DOI] [PubMed] [Google Scholar]
- 44.Westerblad H, Bruton JD, Lännergren J. The effect of intracellular pH on contractile function of intact, single fibres of mouse muscles declines with increase in temperature. J Physiol 500: 193–204, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Westerblad H, Dahlstedt AJ, Lännergren J. Mechanisms underlying reduced maximum shortening velocity during fatigue of intact, single fibres of mouse muscle. J Physiol 510: 269–277, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Woledge RC, Curtin NA, Homsher E. Energetic Aspects of Muscle Contraction. London: Academic, 1985. [PubMed]