Abstract
Abrupt neurotoxic destruction of >70% of the pre-Bötzinger complex (preBötzC) in awake goats results in respiratory and cardiac failure (Wenninger JM, Pan LG, Klum L, Leekley T, Bastastic J, Hodges MR, Feroah TR, Davis S, Forster HV. J Appl Physiol 97: 1629–1636, 2004). However, in reduced preparations, rhythmic respiratory activity has been found in other areas of the brain stem (Huang Q, St. John WM. J Appl Physiol 64: 1405–1411, 1988; Janczewski WA, Feldman JL. J Physiol 570: 407–420, 2006; Lieske SP, Thoby-Brisson M, Telgkamo P, Ramierz JM. Nature Neurosci 3: 600–607, 2000; St. John WM, Bledsoe TA. J Appl Physiol 59: 684–690, 1985); thus we hypothesized that, when the preBötzC is destroyed incrementally over weeks, time-dependent plasticity within the respiratory network will result in a respiratory rhythm capable of maintaining normal blood gases. Microtubules were bilaterally implanted into the presumed preBötzC of seven goats. After recovery from surgery, studies were completed to establish baseline values for respiratory parameters. At weekly intervals, increasing volumes (in order 0.5, 1, 5, and 10 μl) of ibotenic acid (IA; 50 mM) were then injected into the preBötzC. All IA injections resulted in an acute tachypnea and dysrhythmia featuring augmented breaths, apneas, and increased breath-to-breath variation in breathing. In studies at night, apneas were nearly all central and occurred in the awake state. Breath-to-breath variation in breathing was greater (P < 0.05) during wakefulness than during non-rapid eye movement sleep. However, one week after the final IA injection, the breathing pattern, breath-to-breath variation, and arterial blood gases and pH were unchanged from baseline, but there was a 20% decrease in respiratory frequency (f) and CO2 sensitivity (P < 0.05), as well as a 40% decrease in the ventilatory response to hypoxia (P < 0.001). In subsequent histological analysis of the presumed preBötzC region of lesioned goats, it was determined that there was a 90 and 92% reduction from control goats in total and neurokinin-1 receptor neurons, respectively. Therefore, it was concluded that 1) the dysrhythmic effects on breathing are state dependent; and 2) after incremental, near total destruction of the presumed preBötzC region, time-dependent plasticity within the respiratory network provides a rhythm capable of sustaining normal arterial blood gases.
Keywords: plasticity, respiratory rhythmogenesis
the site(s) within the brain stem and the mechanisms of respiratory rhythm and pattern generation remain controversial. For several decades, one major theory, as proposed by Lumsden in the 1920s (20, 21), has been that respiratory rhythm and pattern generation are mediated by a pontine-medullary circuit. Subsequently, this view has been confirmed and extended by the studies by St. John et al. (41–45), which demonstrated that a presumable respiratory rhythm is sustained in the mylohyoid branch of the trigeminal nerve, after the phrenic rhythm is abolished by chemical lesions in the medulla in decerebrate cats (15, 42). Thus it has been hypothesized that, in isolation, the medulla is only capable of generating the gasping rhythm, and that the rhythm originating in the rostral pons from the trigeminal nerve “may underlie the neurogenesis of eupnea” (42).
Another major theory is that respiratory rhythm and pattern are generated uniquely by pacemaker or a network of rhythmogenic neurons in the pre-Bötzinger complex (preBötzC) in the medulla. A landmark in vitro study supporting this view by Smith et al. (35) found that serial transections of a neonatal rat medulla eliminate respiratory rhythm when the preBötzC is abolished. Subsequently, some (19, 23, 48, 49), but not all (40, 52), investigations found that the preBötzC is critical for respiratory rhythm in reduced preparations. As a result, these data have led to different opinions concerning the role of the preBötzC in the control of breathing.
Recent findings in awake goats (51) and rats (47) appear consistent with the concept of a unique role of the preBötzC in the generation of the eupneic breathing rhythm and pattern. For example, Wenninger et al. (51) found in adult, awake goats that abrupt neurotoxin-induced lesions, which destroyed 70% of the preBötzC, resulted in a severe disruption of respiratory rhythm and pattern. Ultimately, these goats died of cardiac and respiratory failure or had to be euthanized due to severe hypoventilation, arterial hypoxia, and hypercapnia (51). More recently, Tan et al. (47) used a novel approach to rapidly silence somatostatin-expressing neurons in the preBötzC of awake rats, which also “induced a persistent apnea without any respiratory movements to rescue their breathing.” However, it has also been found that ventilatory support was required for ∼20 h in order for cats to recover from injections of a neurotoxin into the ventrolateral solitary nucleus (2). In addition, apneas and irregular breathing occurred in awake goats after neurotoxic lesions in the facial, vestibular, and raphe nuclei (7, 14). Accordingly, it is not clear whether the apneas or hypoventilation caused by abrupt lesions within the preBötzC establish that the preBötzC has a unique role in the generation of eupnea.
To gain insight into the concept that eupnea is generated uniquely by the preBötzC, the present study aimed to destroy the preBötzC in a stepwise, incremental manner over several weeks. The rationale for our hypothesis was based on the evidence that multiple areas within the brain stem appear capable of generating rhythmic respiratory activity (15, 17, 19, 42) and also the evidence that the respiratory network has the capacity for plasticity (2, 6, 8, 10, 27, 40–42). Accordingly, we hypothesized that, when the preBötzC was destroyed in a stepwise, incremental manner over several weeks, time-dependent plasticity within the respiratory network would result in a respiratory rhythm and pattern capable of sustaining normal arterial blood gases during both awake and asleep states.
METHODS
Data were obtained on 13 adult female goats, weighing 43.1 ± 2.91 kg. Only female goats were studied. No differences have been found between female and castrated male goats in changes in breathing after lesions within the preBötzC (50, 51). Seven goats were implanted with microtubules (MTs) within the preBötzC, one goat was implanted with a MT outside the preBötzC, and five goats were not implanted with any medullary MTs and were utilized for histological control purposes only. Goats were housed and studied in an environmental chamber with a fixed ambient temperature and photoperiod. All goats were allowed free access to hay and water, except for periods of study. The goats were trained to stand comfortably in a stanchion during periods of study. All aspects of the study were reviewed and approved by the Medical College of Wisconsin Animal Care Committee before the studies were initiated.
Surgical procedures.
Before surgery, the goats were anesthetized with a combination of ketamine and xylazine (15 mg/kg and 1.25 mg/kg, respectively) and intubated. During surgery, they were mechanically ventilated with 1.5% halothane in 100% O2. All surgeries were performed under sterile conditions. The initial surgery on eight goats involved the following: 1) implanting electrodes into the diaphragm (DIA), abdominal (ABD), and three different upper airway (UAW) muscles to monitor respiratory muscle activity; 2) implanting electrodes for electroencephalogram (EEG) and electrooculogram (EOG) recordings; and 3) elevating a 5-cm segment of each carotid artery subcutaneously for eventual insertion of a catheter. Ceftifur sodium (Naxcel) (8 mg/kg) was administered daily (intramuscularly) postoperatively to minimize infection.
After at least 2 wk of recovery, seven goats underwent a second surgery to bilaterally implant 70-mm-long ( 1.27 mm outer diameter, 0.84 mm inner diameter) stainless steel MTs into the preBötzC. In an eighth goat, a MT was implanted into a nonrespiratory medullary area, located near the dorsal surface and lateral to the nucleus of the solitary tract. To implant the MTs, an occipital craniotomy was created, and the dura mater was excised to expose the posterior cerebellum and the dorsal view of the caudal medulla. The MTs were implanted into the preBötzC based on previously delineated coordinates (50) (2–3 mm rostral to obex, 4–5 mm lateral to the midline, and 4–6 mm from the dorsal surface). The MTs were chronically fixed using stainless steel screws and dental acrylic.
The goats were continuously monitored at least 24 h after brain surgery, or until the goat reached stable conditions. Buprenorphrine hydrochloride (Buprenex, 0.005 mg/kg) was administered for pain, as needed. Following the brain surgery, the goats were placed on a regimen of medication to minimize infection (chloremphenicol, 20 mg/kg iv, 3 times/day for 3 days) and swelling (dexamethansome, intravenously, 3 times/day for 7 days, starting with 0.4 mg/kg and decreasing 0.05 mg·kg−1·day−1). Every day thereafter until death, the goats were medicated with ceftifur sodium and gentamyacin (Gentamax 100; 6 mg/kg im, once per day).
Previous studies have demonstrated that injections of the neurotoxin ibotenic acid (IA) into the preBötzC area can cause obstructive apnea (51), most presumably due to damage of nucleus ambiguus (NA), a nucleus known to contain motoneurons of the UAW muscles. Thus, in the present study, when the goats exhibited signs of UAW obstruction, a third surgery was performed to create a tracheostomy (n = 6). In five goats, the tracheostomy was performed 5 days after the injection of 5 μl of IA into the preBötzC. In the sixth goat, the tracheostomy was performed 2 wk after the implantation of the MT into the preBötzC area. A 5-cm midline incision was made longitudinally caudal to the cricoid cartilage, and then three to four cartilage rings were cut at the midline. The skin was sutured to the cartilage rings to prevent closure. After the tracheostomy, the goats were aerosolized everyday with 10% gentamyacin in sterile water to help prevent infection.
Experimental procedures and protocol.
Studies commenced after a minimum 2-wk recovery period from the brain surgery. Before the present study, this same group of animals was used to study respiratory chemosensitivity in the preBötzC utilizing a microdialysis-induced focal acidosis, as well as to study the effects of μ-opioid receptor agonist microinjections in the preBötzC. For the present study before any IA injections were made, the goats were initially studied for several days to establish normal eupneic breathing rhythm and pattern while awake, as well as to assess ventilatory sensitivity to CO2 and hypoxia. Then, to determine the goats’ normal physiological variation of the ventilatory variables (see Physiologic variables below), seven goats were monitored during room air breathing conditions for 5 h during the day, and six of the seven were also monitored for 5 h at night. Thereafter, the design for all goats consisted of several injections of IA (50 mM) to destroy the preBötzC area in a stepwise manner. The IA injections were made at weekly intervals, starting with 0.5 μl, and progressing to 1.0, 5.0, and 10 μl. After a 15-min control period, a unilateral injection of IA was made into the preBötzC through the MT during eupneic breathing conditions. Physiological variables were monitored for 1 h, and then an identical 0.5 μl, 1-μl, or 5-μl injection was made into the contralateral MT. However, due to previous findings that a bilateral injection of 10 μl of IA caused cardiac and respiratory failure (51), the injection of 10 μl into the left and right preBötzC was separated by 1 wk. Blood gases were sampled every 0.5 h. This exact schedule was also followed in the goat that had a MT implanted in the medulla outside of the preBötzC. Throughout these studies during the day, the goats were loosely tied in a stanchion so that they could not resume sternal recumbancy, which is the normal sleep posture for goats.
After each 5-h IA study outlined above, the goats were permitted to eat and drink freely for 5 h. However, in an attempt to consolidate sleep, the goats were not allowed to assume sternal recumbancy. Then, to determine whether IA had any effects on physiological variables during non-rapid eye movement (NREM) or rapid eye movement (REM) sleep, the goats (6 of 7) were allowed to assume sternal recumbancy, and, 10 h after the IA injections, they were monitored for another 5 h. This time frame corresponded to the normal sleep period for goats (9 PM to 2 AM). In addition, the goats were also studied during the same nighttime hours 5 days after each IA injection (1–2 days before the next larger volume of IA).
On several days between each injection, all physiological variables were monitored during eupneic, hypercapnic, and/or hypoxic breathing conditions. After a 15-min eupneic control period, CO2 sensitivity was assessed by sequentially increasing inspired CO2 to 3, 5, and 7% for 5-min intervals. Arterial blood was sampled over the last 2 min of each level of CO2. Hypoxia sensitivity was assessed after a 15-min control period by decreasing inspired O2 to 10.8% for 30 min, followed by a 15-min room air recovery. Arterial blood was sampled during minutes 6 and 26 of hypoxia and over the last 2 min of the recovery period.
Physiological variables.
During all studies, inspiratory flow (V̇i) was measured with a pneumotach by attaching a breathing valve to either a custom-fitted mask secured to the goat's muzzle (airway-intact goats), or an endotracheal tube (tracheostomized goats). During daytime studies, expired air was collected in a spirometer, allowing for determination of expired volume and the concentration of CO2 and O2 in expired air needed for metabolic rate calculations. V̇i, blood pressure (mean arterial blood pressure), heart rate, EMG activity, and during nighttime studies, EEG and EOG were continuously recorded on both a Grass recorder and Codas software. Arterial blood was sampled in duplicate for blood-gas and pH measurements. Rectal temperature was monitored at regular intervals.
Histological studies.
After completion of the experimental protocol, all goats, as well as the histological control goats, were euthanized (Beuthanasia), and the brains were perfused with physiological buffer solution and fixed with 4% paraformaldehyde. The medullas were excised and placed in a 4% paraformaldehyde solution for 24–48 h and then placed in a 30% sucrose solution. The medullas were frozen and then serial sectioned (25 μm) in the transverse plane (n = 12) or the sagittal plane (n = 1), and the sections were adhered to chrome alum-coated slides. For the transverse sections, one series was stained with hematoxylin and eosin for identification of living and dead neurons. Dead neurons have a strikingly different morphology (round) and color (pale pink) from live neurons. A second series in six goats (5 transverse and 1 sagittal) was used for Nissl staining to further document the total number of living neurons. A third series was used for neurokinin-1 (NK1) receptor immunoreactivity by complexing anti-NK1 receptor (primary antibody) with biotinylated anti-mouse (secondary antibody). After the antibody-antigen complex was incubated, it was localized by avidin (Vector ABC Elite) and developed with diaminobenzene. Neurons were counted at a distance from 2.0–4.6 mm rostral to obex. Living and dead neurons and NK1-positive neurons were counted in NA and the presumed preBötzC region (1.5 × 1.5 mm area just ventral to NA). Finally, neurons were also counted in a 1.5 × 1.5 mm region ventral and lateral to the presumed preBötzC.
Data analysis.
Pulmonary ventilation (V̇i; l/min), f (breaths/min), tidal volume (Vt; liters), expiratory (Te; s) and inspiratory times (Ti; s), respiratory muscle activity (mV), and, in addition for night studies, the EEG and EOG activity were analyzed on a breath-by-breath basis. The EEG and EOG activity was utilized to visually distinguish between states of consciousness. Wakefulness was defined as a low-voltage, high-frequency EEG, with absent EOG activity. NREM sleep was characterized by a low-frequency (<2 Hz), high-voltage EEG (2–3 times greater than that found in wakefulness), with absent EOG. REM sleep was defined as having a low-voltage, high-frequency EEG, similar to wakefulness, but concurrent with frequent and distinct bursting activity (>30 μV) from the EOG.
For the day studies, the breath-by-breath values were averaged into 5-min bins. All bins were divided by the average of the bins in the control period, and then the responses to each condition (CO2, hypoxia and post-IA injection) were expressed and statistically analyzed on a percentage of control basis. The breath-to-breath coefficient of variation (CV) (SD/mean) was calculated for each 5-min bin. For each 5-h study, the number of augmented breaths (Vt 2.5 times greater than average Vt) and apneas (Te 2.5 times greater than average Te) were tabulated. Either a two-way ANOVA for repeated measures followed by the Bonferroni post hoc test, or a paired t-test was utilized to establish statistically significant (P ≤ 0.05) changes in measured variables.
For the night studies, each breath was categorized as awake, NREM sleep, or REM sleep. The breaths were then further categorized as “quiet,” regular breaths, or those that were “irregular/disrupted,” characterized by any respiratory muscle activity that was inappropriate for normal, eupneic breathing. These disruptions included swallowing, coughing, mastication, and eructation, as well as any abnormal patterns that could not be identified as a specific behavior. The rationale for this second categorization was as stated by Bolser et al. (4): “the presence of elements that are normally silent in close proximity to spontaneously active respiratory neurons raises the possibility that some interventions that are intended to study the neurogenesis of breathing might instead be influencing components of the holarchical system that are only conditionally active” (2); thus it was essential to make a delineation between the two types of breaths (quiet vs. disrupted). For each of the multiple categories of breaths, there were a limited number that were continuous; thus, rather than bin the data at specific time intervals as in the day studies, the data were binned and averaged for each 100 sequential breaths for each category. The CV of the breath-by-breath data was thus calculated on a 100-breath basis. Augmented breaths and apneas were tabulated as in the daytime studies. Statistically significant changes were established as outlined above.
RESULTS
Histological verification of the preBötzC.
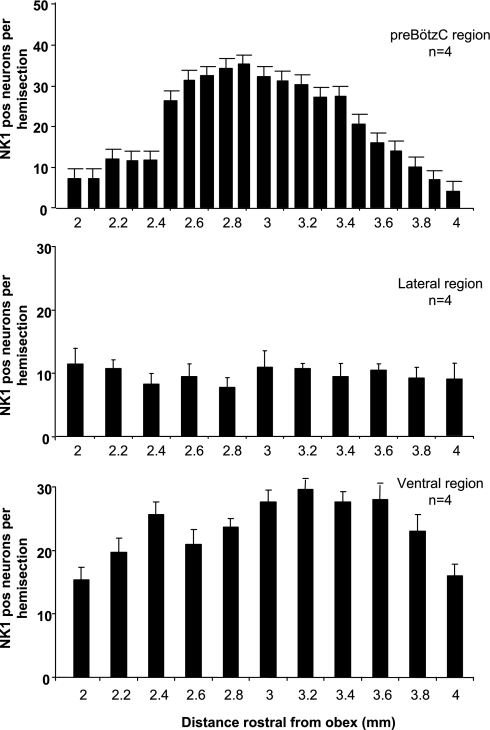
As shown in Fig. 1, A–C, ventral to NA and 4–5 mm lateral to the midline of the medulla in goats, there are numerous NK1-positive neurons. In agreement with previous data (50), we presently found, in four control goats, a distinct increase (P < 0.001) in the number of NK1-positive neurons in a 1.5 × 1.5-mm region just ventral to NA, 2.5 and 3.5 mm rostral to obex (Fig. 2A). We presume this region represents the preBötzC for this species. Between 2.5 and 3.5 mm rostral to obex, there was no distinct increase (P > 0.10) in NK1-positive neurons in the regions lateral (Fig. 2B) or ventral to the presumed preBötzC (Fig. 2C).
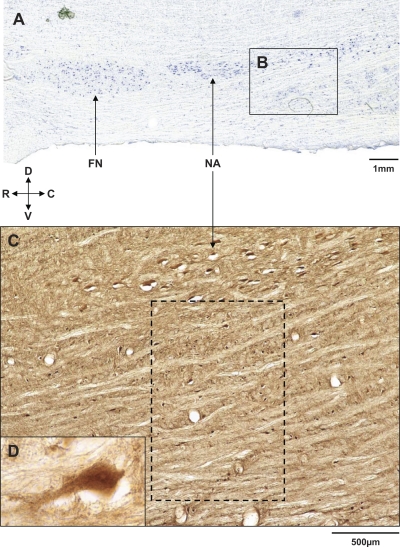
Fig. 1.
Location of the presumed pre-Bötzinger complex (preBötzC) in goats. A: medullary parasagittal section of an unoperated control goat that was Nissl stained to illustrate the ventrolateral respiratory column, including nucleus ambiguus (NA) and the facial nucleus (FN) (×1). Inset B extends from ∼2.0 to 4.6 mm rostral to obex, and we presume the portion 2.5–3.5 mm rostral to obex and ventral to NA is the preBötzC. C: parasagittal section (×4) from the same goat 4.7 mm lateral to the midline and 2.0–4.6 mm rostral to obex (comparable to inset B); it is immunostained for neurokinin-1 (NK1) receptor (NK1R) expressing neurons. The dashed box extends from 2.5 to 3.5 mm rostral to obex, and we presume it is the preBötzC area. Inset D (×40) is a neuron from within the dashed box in C. D, dorsal; V, ventral; R, rostral; C, caudal.
Fig. 2.
Total number of NK1R-positive neurons in the presumed preBötzC, and the areas just lateral and ventral to the preBötzC. Panels are counts of NK1R-positive neurons in 1.5 × 1.5 mm areas 2–4 mm rostral to obex. Top: number of neurons just ventral to NA. Note the marked increase (P < 0.001) in NK1R-positive neurons between 2.5 and 3.5 mm rostral to obex. We presume this region is the preBötzC in goats. There was no significant (P > 0.10) rostral-caudal change in NK1-positive neurons in the areas just lateral (middle) or ventral (bottom) to the presumed preBötzC.
Tachypnea and dysrhythmia during first 5 h after the injections of IA.
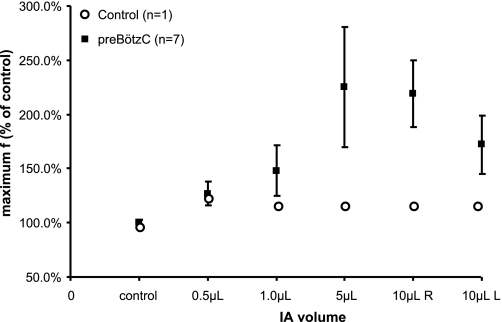
There was a range of responses among the goats in the magnitude and duration of the tachypnea after each injection of IA. Nevertheless, regardless of the volume of IA injected, f was significantly increased (P < 0.05, n = 7) after each injection (Fig. 3). Frequency increased in a dose-dependent manner after the 0.5-, 1-, and 5-μl bilateral injections, increasing maximally by 26 (P = 0.002), 48 (P < 0.001), and 159% (P < 0.001) above control, respectively. In each case, f increased significantly after the contralateral (second) injection was made and remained elevated for the entire duration of the 5-h study. Breathing f was also significantly increased after the 10-μl unilateral injections on the left (120%) and right sides (72%), but the maximal tachypnea was less severe than after the 5-μl bilateral injections, nor was the tachypnea sustained over the 5 h after the injections. This attenuated tachypnea may indicate the preceding IA injections had already destroyed the majority of the rhythmogenic neurons in the presumed preBötzC region. There was no time-dependent increase in f during a 5-h control study on each of the seven goats (Fig. 3, P > 0.05), and, in a control goat, f did not change when IA was injected unilaterally into a medullary area dorsal and lateral to the preBötzC (Fig. 3).
Fig. 3.
Injection of ibotenic acid (IA) into the preBötzC stimulates breathing frequency (f). ▪, Average (± SE, n = 7) percent increase in f from the preinjection period for the 5 h following no IA injection (control) and for 5 h after bilateral injections of 0.5, 1.0, and 5.0 μl of IA and for 5 h after 10 μl unilateral (right R, and left L) injections. At all volume injections of IA, f was significantly (P < 0.05) greater than during the 5-h control study. ○, IA had no effect on f when injected into the medulla of a goat with microtubules implanted outside the preBötzC.
Some, but not all, other measured physiological variables were also affected after the IA injections. V̇i was not significantly elevated after the 0.5 μl (P = 0.235) and 1-μl (P = 0.998) injections, but was increased within 5–40 min after the 5-μl (P = 0.002), 10-μl left, and 10-μl right injections (P < 0.001). After the 1-, 5-, and 10-μl right injections, Vt was significantly decreased by 24, 41, and 23% below control (P < 0.001), respectively, but was unaffected after the 0.5 μl (P = 0.532) or the 10-μl left injection (P = 0.678). Ti was also significantly decreased by 25–50% after every injection except the 0.5 μl (P < 0.05), as was Te (P < 0.05) except after the 0.5 μl and 1-μl volumes. Mean arterial blood pressure was significantly increased only after the 1- (P = 0.003) and 5-μl injections (P < 0.001). There were no significant changes in heart rate, O2 consumption, pH, arterial Pco2 (PaCO2), or arterial Po2 (PaO2) (P > 0.05) during the 5 h after any injection of IA.
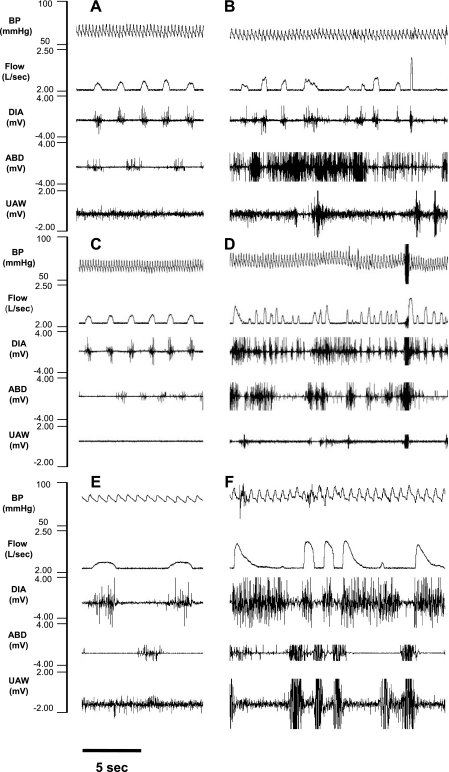
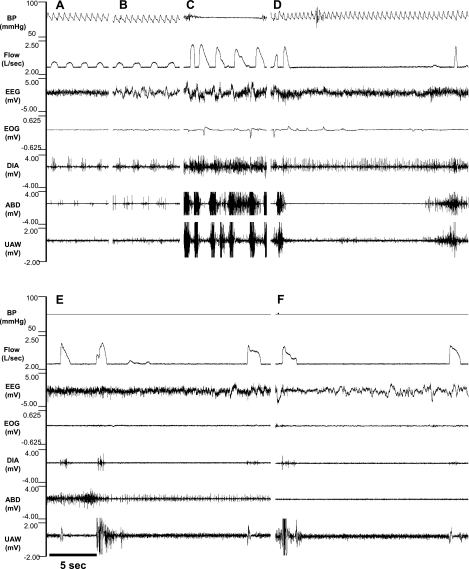
As illustrated for one goat in Fig. 4, A, C, and E, before each injection, DIA contractions were in phase with Vi, and ABD contractions were out of phase with Vi. However, during the 5 h after the IA injections, there were periods of irregular/disrupted breathing that were not identical after each injection. The 500-nl bilateral injection caused the least severe effects; the DIA continued to contract appropriately in phase with Vi, but there were periods in which there was strong tonic ABD activity throughout expiration and inspiration (Fig. 4B). After the 1-μl bilateral injection, both the tachypnea and disruptions became more severe, including periods of prolonged Te, as well as large Vt breaths that occurred with the simultaneous contraction of the DIA, ABD, and UAW muscles (Fig. 4D). The final 10-μl unilateral injection caused unusual markedly augmented breaths separated by large, gasp-like breaths that occurred when the DIA, ABD, and UAW muscles contracted simultaneously (Fig. 4F). We emphasize that, as shown in Fig. 4, C and E, there was a regular eupneic breathing pattern before each injection, indicating that the goat had recovered from the irregular breathing during the initial 5 h after the injection of IA 1 wk earlier.
Fig. 4.
Representation of disrupted breathing and respiratory muscle patterns after IA injections into the preBötzC of 1 awake goat. A, C, and E: tracings taken from the control period before the injection of 0.5 μl (B), 1.0 μl (D), and 10 μl (F) of IA. Control panels A, C, and E show that the diaphragm (DIA) contracted in phase with inspiratory flow (V̇i) and a smaller abdominal (ABD) muscle contraction occurred out-of-phase with Vi, which is the normal pattern for goats. Note in B, D, and F the increased f after IA injections. B also shows irregular breathing after 0.5 μl IA injection with prominent ABD activity throughout inspiration and expiration. D and F both show irregular breathing and simultaneous contraction of the DIA and ABD muscle activity. Note in F the gasp-like Vi followed by prolonged apneas. It is noteworthy that, as shown in C and E, there was a regular breathing and muscle activation pattern before each injection, indicating that the goat had fully recovered from the irregular patterns observed 1 wk earlier after the previous IA injection. BP, blood pressure; UAW, upper airway.
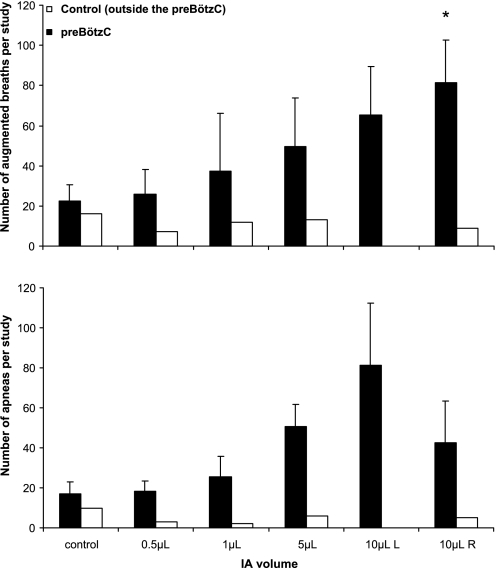
These qualitative examples of the dysrhythmia were substantiated by the significant increase in the breath-to-breath CV of Te (P < 0.01, data not shown), and by the increased number of augmented breaths (P < 0.01, Fig. 5A) and apneas (P = 0.06, Fig. 5B). In all goats after the first 10-μl injection of IA, and in six of seven goats after the 5-μl injection of IA, the number of augmented breaths and apneas was greater than during the 5-h control study. It should be noted that the apneas were primarily central (vs. obstructive), and the number increased as the volume of injected IA increased; there were 33 ± 6.3, 50 ± 9.8, and 65 ± 10.2% more central apneas after the 1-, 5-, and 10-μl injections, respectively, than during the control study.
Fig. 5.
Total number of augmented breaths (top) and apneas (bottom) over the 5 h of the control study and the first 5 h after each injection of IA into the preBötzC. Solid bars, means (± SE) of 7 goats that had injections into the preBötzC; open bars, data from the goat that had injections outside the preBötzC. Augmented breaths and apneas were computed as a tidal volume (Vt) 2.5 times greater than the average Vt and expiratory time (Te) 2.5 greater than average Te, respectively. Analysis of variance indicated significant (P < 0.05) and nearly significant (P = 0.061) differences in augmented breaths and apneas, respectively, between the six 5-h studies. *Post hoc analysis indicated only augmented breaths after the last IA injection differed significantly from control. However, in all goats after the first 10-μl injection and in 6 of 7 goats after the 5-μl injections, the number of augmented breaths and apneas was greater than during the control study. Note that there were no changes in the number of augmented breaths and apneas when IA was injected outside the preBötzC (open bars).
Physiological functions 9–14 h after IA injections (normal sleep period).
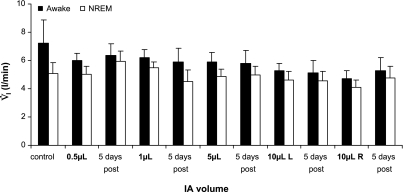
During the studies at night before any injections were made, the goats were awake 66% of the time, which was significantly (P < 0.05) greater than the 33% of time in NREM sleep and 1% of time in REM sleep. There was no significant change in this sleep pattern with any injection of IA. Because REM sleep was not observed in all goats, or after all studies, breathing variables during REM sleep will not be reported herein. In contrast to the first 5 h after the IA injections, the goats were not tachypneic 10–15 h after the injections. During quiet, regular breathing on each night, V̇i (Fig. 6), f, and Vt were significantly reduced during NREM sleep compared with the awake state by an average of 12.5 ± 0.06, 7.2 ± 0.10, and 5.8 ± 0.001%, respectively. The Ti and Te were 4.8 ± 0.01 and 6.8 ± 0.02% greater (P < 0.05) during NREM sleep than while awake, respectively. V̇i, f, Vt, and Ti during wakefulness and NREM sleep did not change significantly (P > 0.10) over the course of the 11 studies at night, and the differences between the awake and NREM sleep states were not altered by the IA injections. In agreement with previous findings (22), arterial blood gases did not differ between awake and NREM sleep states on the control night, and there were no changes as the volume of IA injected was increased (Table 1).
Fig. 6.
V̇i during quiet, regular breathing was significantly (P < 0.01) less each night during non-rapid eye movement (NREM) sleep than during the awake state. There was no significant (P > 0.010) change in V̇i during either state over the 11 studies at night.
Table 1.
Average values for arterial blood gases and pH and breathing variables before any (pre-IA) and 1 wk after all IA injections (post-IA)
| PaCO2, Torr | PaO2, Torr | pHa | V̇i, l/s | f, breaths/min | Vt, liters | Ti, s | Te, s | |
|---|---|---|---|---|---|---|---|---|
| Daytime | ||||||||
| Pre-IA | 41.0±0.5 | 100.8±2.9 | 7.430±0.01 | 6.83±0.55 | 22.8±2.4 | 0.338±0.03 | 1.15±0.14 | 1.96±0.27 |
| Post-IA | 40.6±0.6 | 97.9±2.5 | 7.444±0.01 | 5.1±0.58 | 18.1±1.84* | 0.308±0.02 | 1.36±0.17 | 2.46±0.23* |
| Nighttime | ||||||||
| Pre-IA awake | 41.6±0.4 | 97.6±1.8 | 7.424±0.006 | 7.25±1.63 | 20.4±2.1 | 0.420±.14 | 1.38±0.28 | 2.00±0.24 |
| Post-IA awake | 41.1±0.9 | 95.7±6.9 | 7.411±0.015 | 5.30±0.91 | 16.6±1.5 | 0.323±0.02 | 1.31±0.07 | 2.50±0.32 |
| Pre-IA NREM | 40.6±0.7 | 98.0±2.8 | 7.425±0.004 | 5.08±0.76 | 19.9±2.3 | 0.271±0.04 | 1.13±0.07 | 2.07±0.32 |
| Post-IA NREM | 39.3±1.2 | 101.0±5.1 | 7.422±0.003 | 4.75±0.84 | 15.8±1.64 | 0.304±0.03 | 1.36±0.08 | 2.59±0.28 |
Values are average ± SE. IA, ibotenic acid; NREM, non-rapid eye movement; PaCO2, arterial Pco2; PaO2, arterial Po2; pHa, arterial pH; V̇i, inspired pulmonary ventilation; f, breathing frequency; Vt, tidal volume; Ti and Te, inspiratory and expiratory time, respectively. One week after the final injection, f was significantly decreased (P < 0.05) and Te was significantly increased (
P < 0.05), but all other values were not changed from the pre-IA values.
Ten to fifteen hours after the injections of IA, there were periods of quiet, regular breathing (Fig. 7) interspersed with periods of disrupted/irregular breathing patterns. For example, in one goat after the first 10-μl IA injection, the regular pattern (Fig. 7, A and B) was interspersed with periods when V̇i increased to ∼2.5 greater than normal, which was coincident with a strong, simultaneous contraction of the ABD and UAW muscle and tonic DIA activity (Fig. 7C). Often, these large breaths during wakefulness (Fig. 7D) were followed by prolonged apneas, which were as long as 20 s in duration. During the 5 h of this night study, there were a total of 57 apneas, with 56 during wakefulness and 1 during NREM, and all but 1 was central in nature. In contrast, in this animal's preinjection control study, no central apneas were observed, and five nights after the final injection, there were only six central apneas awake and two central apneas during sleep.
Fig. 7.
There were prolonged central apneas primarily during the awake state in goats 10–15 h after injections of IA into the preBötzC. A–D: recordings from one goat; E and F: recordings from a second goat. All recordings are for periods 10–15 h after injections of 10 μl of IA. A and B: regular breathing during awake and NREM sleep states, respectively. C and D: gasp-like breaths coincident with strong contraction of the ABD and an UAW muscle. Also note the prolonged central apnea in D. Similarly, in another goat, there are also gasp-like breaths followed by prolonged central apneas (E and F). EEG, electroencephalogram; EOG, electrooculogram.
In a second goat after a 10-μl injection, there were periods of a gasp-like breathing pattern during wakefulness, characterized by a sharp inspiratory rise time and steep decrementing slope (Fig. 7, E and F). These breaths were immediately followed by a central apnea that lasted up to 20 s. During this 5-h night study, there were a total of 51 apneas, all of which were central, and all occurred during wakefulness. These apneas contrast with the absence of apneas in this goat's control study. There were only 20 central apneas while awake and 3 during NREM sleep 5 days after the final IA injection.
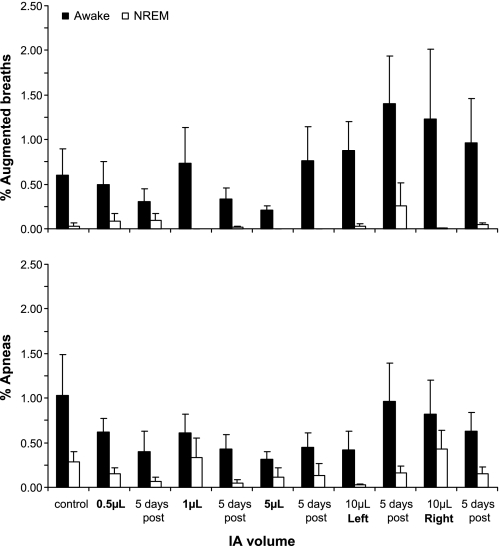
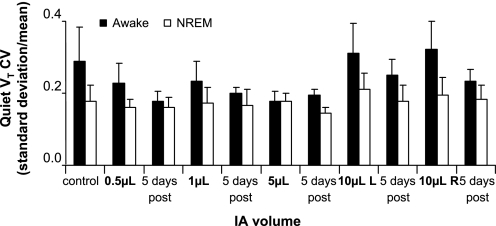
On every night of sleep studies, a prominent finding was that the number of apneas and augmented breaths were greater (P < 0.05) during wakefulness than during NREM sleep (Fig. 8). A second prominent finding was that, as shown in Figs. 7, E and F, the apneas were primarily central at the higher volumes of IA injections, whereas, in contrast on the control night, a majority of the apneas were obstructive. The volume of IA that elicited the greatest number of apneas and augmented breaths was not consistent among the goats. Note that 5 days after the final IA injection, the number of apneas in both the awake and NREM sleep states had returned to control levels. The finding that the disruptions in breathing pattern were primarily during the awake state was further substantiated quantitatively by the finding that the breath-to-breath CV for V̇i, Ti, and Vt (Fig. 9) were 32 ± 0.8 (P = 0.02), 26 ± 0.3 (P = 0.01), and 29 ± 0.9% (P = 0.03) greater, respectively, during quiet, regular wakefulness compared with quiet NREM sleep. There was no statistically significant change in the CVs (P > 0.05) over the several studies at night, but there was a trend of increased variability after each of the 10-μl injections, which then recovered to at or below preinjection control values 5 days after the injections (Fig. 9). A final indication of the state-dependent variability was that, during NREM sleep, 87% of the breaths were quiet, regular breaths, whereas, in the awake state, only 64% were quiet, regular breaths.
Fig. 8.
During all studies at night, the occurrence of augmented breaths and apneas was significantly (P < 0.001) greater in the awake state than during NREM sleep. The raw number of these events was greatest during the awake state, but presenting the raw number would be somewhat misleading, as the total awake time and total number of awake breaths were three to four times greater for the awake state than for NREM sleep. Thus the differences were normalized by determining the percentage of total breaths for each state that were augmented or apneic. There was considerable variation among the goats in the volume of injected IA that caused these events; thus there was no statistically significant change in these events across the 11 night studies. Values are means ± SE of 6 goats.
Fig. 9.
During quiet, regular breathing, the coefficient of variation (CV) for Vt was on 10 of 11 nights greater in the awake state than during NREM sleep. Values are means ± SE of 6 goats. Note the trend of an increased CV on the night that 10 μl were injected, followed 5 days later by a decrease, particularly in the awake state.
Chronic changes after the IA injections.
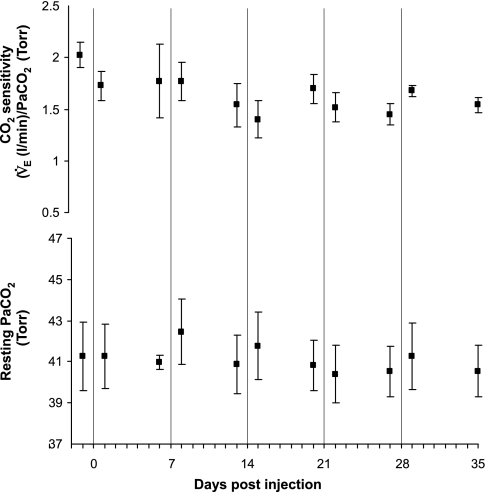
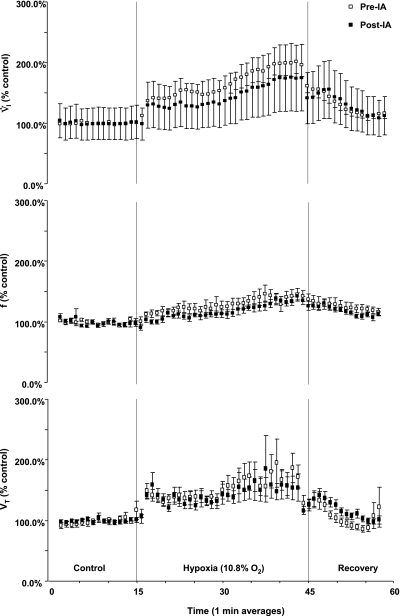
The day immediately following each IA injection study, all measured respiratory variables were unchanged from the baseline values measured the previous day before the injections of IA. Moreover, 1 wk after the final 10-μl injection, during awake and NREM sleep, V̇i, Vt, Ti, arterial pH, PaO2, PaCO2 (Table 1, Fig. 10), and the CV of all respiratory variables were unchanged from the control studies before the initial injection of IA (P > 0.05). The f, however, was significantly decreased, and Te was significantly increased (P < 0.03) (Table 1). Compared with studies before the lesions were created, CO2 sensitivity (change in minute ventilation/change in PaCO2) was significantly decreased from 2.025 ± 0.12 to 1.566 ± 0.13 (P < 0.05) (Fig. 10), and the peak ventilatory response to hypoxia was decreased from 244 ± 28 to 204 ± 60% of the prehypoxia control values (P < 0.001) (Fig. 11) 1 wk after the final injection. In the goat with MTs implanted outside the preBötzC area, CO2 and hypoxic sensitivity were not altered after the same schedule of IA injections.
Fig. 10.
Bilateral injection of IA into the preBötzC attenuated CO2 sensitivity [change in (Δ) minute ventilation (V̇e)/Δarterial Pco2 (PaCO2)], but did not alter PaCO2 while breathing room air. Values are means ± SE of data obtained on 7 awake goats before any injections (control) and then 1 and 6 days after each injection. The vertical lines denote the day in order of 0.5-, 1.0-, 5.0-μl bilateral and 10-μl unilateral injections of IA into the preBötzC.
Fig. 11.
IA injections decreased the ventilatory response to hypoxia (10.8% O2). V̇i (P < 0.001), but not f or Vt (P > 0.05), was reduced after the series of IA injections were completed. The data (means ± SE) were averaged in 1-min intervals and expressed as a percentage of control. The dashed lines delineate room air control, hypoxia, and room air recovery periods.
Lesion size and medullary damage.
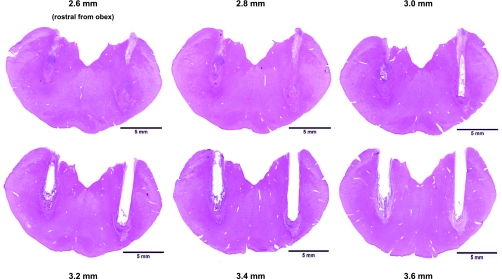
As shown in Fig. 12, the tissue damage caused by the MT and the IA extended over the range of 2.5–3.5 mm rostral to obex. We quantitated the extent of the lesion in the region of the implanted MT by counting, at 50-μm intervals, the number of living neurons identified by either the Nissl or the hematoxylin and eosin stains. There was excellent correlation between the counts of the two stains. For the presumed preBötzC region, NA, and the region ventral to presumed preBötzC, the hematoxylin and eosin vs. Nissl counts for living neurons in the lesioned goats was 51 ± 19 vs. 46 ± 19, 12 ± 4 vs. 13 ± 7, and 89 ± 41 vs. 82 ± 40, respectively. We found a range of only 0–16 dead neurons in the presumed preBötzC region of the seven lesioned goats, and believe the small number of dead neurons reflect the scavenging of dead neurons that occurred over the month following the first IA injection.
Fig. 12.
The preBötzC was nearly totally destroyed after the IA injections. These transverse sections from the medulla of one goat (no. 4 in Tables 2 and 3) stained with hematoxylin & eosin demonstrate the neuronal destruction caused by the implanted microtubule (area devoid of tissue) and by the injections of IA (disrupted tissue) 2.6–3.6 mm rostral to obex, which encompasses the presumed preBötzC area in goats.
Due to small differences in goat medullary morphology, not all of the MTs in goats were placed exactly in the same region, giving rise to the differences in living neurons remaining at the completion of the study. For example, in seven goats, the total number of living neurons remaining within the presumed preBötzC region ranged from 2 to 116 (Table 2). Similarly, in the region 0.4 mm caudal and 1.0 mm rostral to the presumed preBötzC region, the total number of living neurons ranged from 1 to 103 (Table 2). However, on average, for the preBötzC region, and the regions caudal and rostral to the preBötzC, there were only 10, 11, and 18% of the total living neurons remaining, compared with the control, unlesioned goats.
Table 2.
Total number of living neurons for each individual animal within the NA, presumed preBötzC, and area ventral to the preBötzC, from 2.0 to 4.6 mm rostral from obex, 1 wk after the final 10-μl IA injection
| Goat No. | 2.0–2.4 mm Rostral From Obex |
2.5–3.5 mm Rostral From Obex | 3.6–4.6 mm Rostral From Obex | ||||||
|---|---|---|---|---|---|---|---|---|---|
| NA | Caudal preBötzC | Ventral area | NA | Presumed preBötzC | Ventral area | NA | Rostral preBötzC | Ventral area | |
| 1 | 11 | 22 | 129 | 30 | 87 | 138 | 80 | 103 | 143 |
| 2* | 10 | 2 | 46 | 16 | 4 | 49 | 23 | 1 | 25 |
| 3 | 0 | 0 | 29 | 17 | 61 | 88 | 17 | 12 | 53 |
| 4 | 9 | 10 | 19 | 11 | 2 | 18 | 0 | 19 | 14 |
| 5 | 0 | 0 | 32 | 12 | 26 | 37 | 19 | 32 | 58 |
| 6 | 0 | 0 | 33 | 0 | 22 | 7 | 7 | 64 | 54 |
| 7 | 16 | 56 | 156 | 9 | 116 | 243 | 31 | 83 | 251 |
| Control | 38±1.0 | 133±2.4 | 272±10.5 | 62±1.0 | 428±1.1 | 347±1.1 | 79±2.5 | 255±3.2 | 430±4.3 |
On average, between 2.5 and 3.5 mm rostral to obex, there were only 22, 10, and 24% of the total neurons remaining within the nucleus ambiguus (NA), presumed pre-Bötzinger complex (preBötzC) region, and region ventral to the preBötzC, respectively, compared with control, unoperated goats.
Not used in sleep studies.
There was also variation between the goats in the number of living neurons in NA and the region ventral to the presumed preBötzC. For example, in one goat only 7 living neurons remained in NA between 2.5 and 3.5 mm rostral to obex, while the group average for living neurons was 14 (Table 2), or 22% of normal. Similarly, for the region ventral to the presumed preBötzC, the range of living neurons was 7–243 (Table 2), while the group average was 83 neurons, or 24% of normal.
For the seven goats, there was a range of 0–84 NK1-positive neurons remaining within the presumed preBötzC (Table 3). In four of the goats, there were less than 10 NK1-positive neurons remaining in the region (Table 3). On average, for the preBötzC, and the regions caudal and rostral to the preBötzC, there were only 8, 11, and 12% of the total NK1-positive remaining, compared with the control, unlesioned animals.
Table 3.
Total number of neurokinin-1-positive neurons for each individual animal within, caudal, and rostral to the presumed preBötzC, 1 wk after the final 10 μl IA injection
| Goat No. | 2.0–2.4 mm Rostral From Obex: Caudal preBötzC | 2.5–3.5 mm Rostral From Obex: Presumed preBötzC | 3.6–4.6 mm Rostral From Obex: Rostral preBötzC |
|---|---|---|---|
| 1 | 7 | 37 | 48 |
| 2* | 0 | 2 | 0 |
| 3 | 0 | 25 | 0 |
| 4 | 6 | 0 | 9 |
| 5 | 0 | 6 | 0 |
| 6 | 0 | 6 | 25 |
| 7 | 25 | 84 | 32 |
| Control | 49±1.1 | 302±1.3 | 142±2.2 |
On average, between 2.5 and 3.5 mm rostral to obex, there were only 11, 8, and 12% of the total neurokinin-1-positive neurons remaining in the caudal, presumed, and rostral portions of the preBötzC, respectively, compared with control, unoperated goats.
Not used in sleep studies.
DISCUSSION
Major conclusions.
The first prominent finding of this study is that all goats had a normal eupneic breathing pattern and normal arterial blood gases during both awake and NREM sleep states after an average >90% destruction of total neurons, and 92% destruction of NK1-positive neurons within the presumed preBötzC region. These finding are in marked contrast to previous findings of Wenninger et al. (51), who found that, after bilateral destruction of approximately only 70% of the preBötzC, three of three goats with intact airways died, and four of four tracheostomized goats had to be euthanized due to severe hypoventilation. The second prominent finding is that the acute disruptions of the normal respiratory rhythm and pattern from the IA injections are greater during the awake state than during NREM sleep; however, in both states, a normal eupneic rhythm and pattern returned over days after the IA injections.
Anatomical and functional definition of the preBötzC.
Several studies have attempted to define the boundaries of the preBötzC (12, 13, 32, 33, 46, 48). It has been shown in adult rats that there is a distinct increase in NK1-positive neurons within the ventral respiratory group (VRG), in an region just ventral to NA (12, 13, 46, 48). Subsequently, it was found that somatostatin-positive neurons in the region of the VRG supposedly define the boundaries of the preBötzC (46, 47), while others have found that, in neonatal rats, there is a core of rhythmogenic neurons 0.5 mm caudal to the facial nucleus essential for respiratory rhythmogenesis (32, 33). From these studies, the general consensus is that the exact boundary of the preBötC or boundary of the medullary neurons responsible for the eupneic respiratory rhythm in adult mammals is not precisely established, nor is there a single anatomic marker that accurately defines the preBötC. However, it has been generally accepted that the preBötzC includes a region within the VRG that has a high density of NK1- and somatostatin-expressing neurons.
Previously published data (50) and data in Fig. 2 of the present study substantiate that, in goats, there is a distinct increase in NK1-positive neurons between 2.5 and 3.5 mm rostral to obex, which, as in rat, is just ventral to NA. Accordingly, the MTs were implanted from the dorsal medullary surface to the ventral border of NA and the dorsal border of the presumed preBötzC between 2.5 and 3.5 mm rostral to obex (Fig. 12).
Several laboratories found that microinjection of a glutamate receptor agonist along the rostral-to-caudal direction of the VRG in anesthetized rats or cats elicits a tachypneic and dysrhythmic phrenic nerve response only when injected into the preBötzC (23, 37, 49). This laboratory has also previously reported (51) and currently corroborated, that injections into the preBötzC region of the neurotoxin IA (a glutamate receptor agonist that binds irreversibly and preferentially to N-methyl-d-aspartate receptors; Ref. 26), induces a significant tachypnea (Fig. 3) and dysrhythmia (Figs. 4 and 7). Injection of IA into a nonrespiratory medullary area does not induce these changes; thus these findings support the conclusion that the MTs were placed correctly within the preBötzC.
Acute apnea/hypoventilation and irregular respiratory rhythm in awake and sleeping goats after injection of IA into the preBötzC.
This laboratory has previously demonstrated that phasic DIA activity is completely abolished within 2 h after a bilateral 10-μl injection of IA into the preBötzC of adult awake goats, and a profound hypoventilation, generated by phasic expiratory muscle activity, results in severe arterial hypercapnia and hypoxemia (51). These goats either died or had to be euthanized, as there was no evidence of recovery within 5 h after the injections. Histological analysis indicated that 70% of neurons in the presumed preBötzC region were destroyed in these goats. More recently, Tan et al. (47) showed that abrupt silencing of somatostatin-expressing neurons in the preBötzC of rats also “induced a persistent apnea without any respiratory movements to rescue their breathing.”
The present study has shown that, after a series of smaller volume injections of IA, the 10-μl IA injection into the preBötzC also resulted in periods of abnormal breathing patterns and apneas over the first 5 h after the injections (Fig. 4) and also when the goats were monitored 10–15 h after the injections (Fig. 7). However, unlike the earlier study (51), the present study did not find any profound and prolonged hypoventilation and severe arterial hypercapnia and hypoxemia. Most striking was that none of the goats died or had to be euthanized within 5 h after the injections. The difference in responses to 10-μl injections in the previous and present study likely reflects that the lower volume IA injections in the present study initiated plasticity within the respiratory control network, which was critical to the goat's survival after the 10 μl in the present study. Twenty-four hours to 1 wk after the IA injections, the goats were healthy, with normal arterial pH and blood gases, normal rhythmic DIA and ABD muscle activity, and a normal eupneic breathing pattern during both awake (Fig. 4, C and E) and NREM states. Furthermore, 1 wk after the completion of the entire IA injection series, the only significant changes from the pre-IA injections were a decreased f and increased Te. This recovery occurred, despite an average of only 45 ± 15.3 living neurons remaining in the presumed preBötzC region, or 10% of the normal (Table 2), whereas 30% of normal remained in the earlier study. Moreover, nearly 80% of the neurons in NA 2.5–3.5 mm rostral to obex were also destroyed (Table 2). Finally, in the region ventral to the presumed preBötzC, there were, on average, only 83 living neurons, or 25% of normal (Table 2).
The greater variability of breathing during wakefulness than NREM sleep is consistent with the previous findings that 7–14 days after injection of saporin conjugated to substance P (SAP-SP) into the preBötzC of goats resulted in abnormal breathing only during wakefulness and not sleep (50). It is noteworthy that the greater variability while awake was not due to more frequent disturbances of breathing by nonrespiratory behaviors, as these were excluded from the regular breathing category. Equally noteworthy is that nearly all of the apneas occurred in the awake state, and a large majority of the apneas were central, indicating dysfunction of rhythm-generating mechanisms. The difference in apnea frequency between wakefulness and NREM sleep cannot be due to a relatively less excitatory input to the rhythm generator, such as serotinergic, adrenergic, and cholinergic inputs, because these decrease during NREM sleep compared with wakefulness (1). Thus it seems that findings of this present study reflect the possibility that preBötzC has a state-dependent role in respiratory rhythmogenesis, or as has been postulated by others (34, 36), the site and/or mechanism of rhythm generation is state dependent.
Implications of rhythmogenic plasticity within the respiratory network.
The normal breathing after the gradual, stepwise destruction of the preBötzC in contrast to the sustained apnea after abrupt destruction of the preBötzC indicates that time-dependent plasticity occurs within the respiratory network after gradual destruction of this region. It seems unlikely that this plasticity occurred solely within the presumed preBötzC region, because, in four goats, only 2, 4, 22, and 26 living neurons were found in this region. A second possibility is that the preBötzC is not accurately defined by the region of a high density in NK1-positive neurons, 2.5–3.5 mm rostral to obex. In other words, the rhythm-generating neurons may not be restricted to this region. Indeed, if neurons expressing NK1 receptors are rhythmogenic, then the region ventral to the presumed preBötzC might be rhythmogenic, as this region has a relatively high number of neurons expressing NK1 receptors. However, in two goats, only 7 and 31 living neurons remained in the entire region 2.4–4.6 mm rostral to obex. Moreover, in the one goat with only 2 living neurons in the presumed preBötzC region, only 11 living neurons were found in NA and only 18 were found in the region ventral to the presumed preBötzC. Despite these examples of near total destruction of the presumed preBötzC region, as well as the large or complete destruction in the surrounding tissue, it remains conceivable that plasticity occurred in the few living neurons within and surrounding the presumed preBötzC (19, 32).
Another possible explanation of plasticity could be that a reconfiguration occurred elsewhere within the respiratory network (19, 32). It could be that rhythmogenic neurons within the parafacial respiratory group (pFRG), which have been hypothesized to generate an expiratory rhythm in juvenile rats (6, 16, 17), are reconfigured to generate an inspiratory rhythm. However, there are no known data on adult mammals documenting the type of preinspiratory neurons in the pFRG that are rhythmogenic as in neonatal rats. On the other hand, since, in adult rats, neurons within the Bötzinger complex that are expiratory during normal oxygenation are reconfigured to a preinspiratory pattern during hypoxia (9), it is conceivable that similar reconfiguration occurs in the Bötzinger complex or pFRG after destruction of the preBötzC.
There is evidence that neurons within the pontine respiratory group (PRG) have a role in respiratory timing, and there are projections from the PRG to the DIA (3, 5, 15, 20, 21, 41–45). Moreover, in decerebrate cats, a presumed respiratory rhythm is sustained in the mylohyoid branch of the trigeminal nerve after phrenic rhythm is abolished by chemical lesions in the medulla (42). It has also been shown that lesions in the PRG result in disruptions in respiratory rhythm and pattern that are more profound during NREM sleep than in the awake state (5). These and other findings led to the hypothesis that a major role of PRG neurons was in stabilizing and preventing large fluctuations in breathing during sleep (1, 5). Thus it is plausible that, while the preBötzC has a critical role in generating and maintaining a stable inspiratory rhythm during wakefulness, the PRG is responsible for maintaining stable breathing during sleep, and, since the PRG was intact in our goats, the breathing pattern remained most stable during NREM sleep.
Moreover, after the destruction of the preBötzC, the PRG may have assumed the dominant role in respiratory rhythmogenesis during both awake and sleep states.
The preBötzC and sleep-disordered breathing.
In two major aspects, the present data are in contrast to the findings of McKay et al. (24, 25). First, they found that lesioning the preBötzC in rats with SAP-SP resulted in progressive respiratory disturbances, first in REM sleep, and then days later in NREM sleep and the awake state (25). Second, they found no recovery in their disturbances, whereas we observed recovery within 24 h after the major disruption. They concluded that “sleep disordered breathing results from loss of preBötzC neurons and could underlie death during sleep” (24). The data from the present study do not support this conclusion, as the majority of the disrupted breathing was observed during wakefulness, and not during sleep. The differences between this study and that of McKay cannot be related to the choice of neurotoxin, because Wenninger et al. (50) using SAP-SP lesions also found disturbances only while awake and not during NREM sleep in goats with intact airways. A possible explanation is that the goat may have more effective compensatory mechanisms than the rat to maintain homeostasis during sleep. The fact that rats, but not goat, are nocturnal could also contribute to the difference between species. Finally, this species difference in effect of arousal state on breathing variability emphasizes the limitations in extrapolating from data on nonhuman mammals to humans.
Lesioning the preBötzC attenuates CO2 and hypoxia sensitivity.
The preBötzC has also been implicated in both CO2/H+ and O2 chemosensitivity. Indeed, evidence from our laboratory has shown that a microdialysis-induced focal acidosis of the preBötzC increased V̇i, solely as a function of increased f, by 10% (18). Moreover, it has already been shown that the ventilatory response to elevated inspired CO2 is significantly decreased in awake rats when the preBötzC is lesioned with SAP-SP (11). Furthermore, Nattie and Li (28, 29) and Hodges et al. (14) have shown that destroying NK1-positive neurons at others chemosensitive sites, specifically the retrotrapezoid nucleus/parapyramidal region and the medullary raphe, also decreases the ventilatory response to elevated inspired CO2 in awake rats and goats. In the present study, CO2 sensitivity and the ventilatory response to hypoxia were also significantly attenuated after the IA injection in the preBötzC. Thus it is conceivable that the destruction of NK1 neurons, as well as other potential chemosensitive and rhythmogenic neurons in the preBötzC, is responsible for the significant decrease in the response to both hypercapnia and hypoxia. In other words, the data from this study appear to support previous data that there are both CO2-H+ (18, 38) and hypoxic (39) chemoreceptors within the preBötzC. Moreover, the present data also are consistent with the concept of a distributed and not specialized areas of CO2-H+ chemoreceptors within the brain (30). Finally, because there was a normal respiratory pattern in response to these breathing conditions, albeit diminished, it appears that the redundant chemosensitive sites within the respiratory network were able to reconfigure to a new respiratory rhythm generator.
Conclusions.
In support of previous studies (2, 6, 8, 10, 27, 40–42), data herein demonstrate the remarkable plasticity of the respiratory control system; there was time-dependent normalization of respiratory rhythm and pattern after near total destruction of a site (preBötzC), which, when abruptly destroyed in the awake state, results in highly irregular breathing and/or prolonged/terminal apneas. The neural substrate of the plasticity likely incorporates areas beyond the presumed preBötzC area; in two lesioned goats, there were only 7 and 31 living neurons within and surrounding the preBötzC region. However, an alternative conclusion would be that only a few neurons in the preBötzC region are required to mediate the complex functions of rhythm and pattern generation across multiple states and conditions. The data herein suggest the site and/or mechanism of rhythm and pattern generation are state dependent; 10–15 h after lesioning the preBötzC, prolonged central apneas were nearly exclusively in the awake state. Accordingly, either the rhythm-generating mechanisms within the preBötzC region are state dependent, or another brain stem site has a major role in generating the rhythm during NREM sleep. Finally, in contrast to the recent focus on the preBötzC, which has been based largely on data from reduced preparations, the data from this study during physiological wakefulness and NREM sleep emphasize that regions in the brain stem other than the preBötzC conceivably have or are capable of a major role in respiratory rhythm and pattern generation.
GRANTS
The authors’ work was supported by National Heart, Lung, and Blood Institute Grant HL-25739 and the Department of Veterans Affairs.
Acknowledgments
We are grateful to Dr. Don McCrimmon for sharing expertise and knowledge, which greatly aided in the successful completion of this study.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Bellingham MC, Ireland MF. Contribution of cholinergic systems to state-dependent modulation of respiratory control. Respir Physiol Neurobiol 131: 135–144, 2002. [DOI] [PubMed] [Google Scholar]
- 2.Berger AJ, Cooney KA. Ventilatory effects of kainic acid injections of the ventrolateral solitary nucleus. J Appl Physiol 52: 131–140, 1982. [DOI] [PubMed] [Google Scholar]
- 3.Bertrand F, Hugelin A. Respiratory synchronizing function of nucleus parabrachialis medialis: pneumotaxic mechanisms. J Neurophysiol 34: 189–207, 1971. [DOI] [PubMed] [Google Scholar]
- 4.Bolser DC, Poliacek I, Jakus J, Fuller DD, Davenport PW. Neurogenesis of cough, other airway defensive behaviors and breathing: a holarchical system? Respir Physiol Neurobiol 152: 255–265, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Caille D, Vibert JF, Hugelin A. Apneusis and apnea after parabrachial or Kolliker-Fuse lesion; influence of wakefulness. Respir Physiol 45: 79–91, 1981. [DOI] [PubMed] [Google Scholar]
- 6.Feldman JL, Mitchell GS, Nattie EE. Breathing: rhythmicity, plasticity, chemosensitivity. Annu Rev Neurosci 26: 239–266, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Feroah TR, Forster HV, Fuentes CG, Martino P, Hodges M, Wenninger J, Pan L, Rice T. Perturbations in three medullary nuclei enhance fractionated breathing in awake goats. J Appl Physiol 94: 1508–1518, 2003. [DOI] [PubMed] [Google Scholar]
- 8.Forster HV Plasticity in the control of breathing following sensory denervation. J Appl Physiol 94: 784–794, 2003. [DOI] [PubMed] [Google Scholar]
- 9.Fortuna MG, West GH, Stornetta RL, Guyenet PG. Botzinger expiratory-augumenting neurons and the parafacial respiratory. J Neurosci 28: 2506–2515, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gautier H, Bertrand F. Respiratory effects of pneumotaxic center lesions and subsequent vagotomy in chronic cats. Respir Physiol 23: 71–85, 1975. [DOI] [PubMed] [Google Scholar]
- 11.Gray PA, Janczewski WA, Mellen N, McCrimmon DR, Feldman JL,. Normal breathing requires preBotzinger complex neurokinin-1 receptor-expressing neurons. Nat Neurosci 4: 927–930, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gray PA, Rekling JC, Bocchiaro CM, Feldman JL. Modulation of respiratory frequency by peptidergic input to rhythmogenic neurons in the preBotzinger complex. Science 286: 1566–1568, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Guyenet PG, Sevigny CP, Weston MC, Stornetta RL. Neurokinin-1 receptor-expressing cells of the ventral respiratory group are functionally heterogeneous and predominantly glutamatergic. J Neurosci 22: 3806–3816, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hodges MR, Opansky C, Qian B, Davis S, Bonis J, Bastasic J, Leekley T, Pan LG, Forster HV. Transient attenuation of CO2 sensitivity after neurotoxic lesions in the medullary raphe area of awake goats. J Appl Physiol 97: 2236–2247, 2004. [DOI] [PubMed] [Google Scholar]
- 15.Huang Q, St. John WM. Respiratory neural activities after caudal to rostral ablation of medullary regions. J Appl Physiol 64: 1405–1411, 1988. [DOI] [PubMed] [Google Scholar]
- 16.Janczewski WA, Onimaru H, Homma I, Feldman J. Opioid-resistant respiratory pathway from the preinspiratory neurones to abdominal muscles: in vivo and in vitro study in the newborn rat. J Physiol 545: 1017–1026, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Janczewski WA, Feldman JL. Distinct rhythm generators for inspiration and expiration in the juvenile rat. J Physiol 570: 407–420, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Krause KL, Forster HV, Davis SE, Kiner T, Bonis JM, Pan LG, Qian B. Focal acidosis in the pre-Botzinger complex area of awake goats induces a mild tachypnea. J Appl Physiol. In press. [DOI] [PMC free article] [PubMed]
- 19.Lieske SP, Thoby-Brisson M, Telgkamo P, Ramierz JM. Reconfiguration of the neural network controlling multiple breathing patterns: eupnea, sighs, and gasps. Nat Neurosci 3: 600–607, 2000. [DOI] [PubMed] [Google Scholar]
- 20.Lumsden T Observations on the respiratory center. J Physiol Lond 57: 153–160, 1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lumsden T The regulation of respiration. J Physiol Lond 58: 81–91, 1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Martino PF, Forster HV, Feroah T, Wenninger JM, Hodges MR, Pan LG. Do neurotoxic lesions in rostral medullary nuclei induce/accentuate hypoventilation during NREM sleep? Respir Physiol Neurobiol 138: 59–75, 2003. [DOI] [PubMed] [Google Scholar]
- 23.McCrimmon DR, Monnier A, Hayashi F, Zuperku E. Pattern formation and rhythm generation in the ventral respiratory group. Clin Exp Pharmacol Physiol 27: 126–131, 2000. [DOI] [PubMed] [Google Scholar]
- 24.McKay LC, Janczewski WA, Feldman JL. Sleep-disordered breathing after targeted ablation of preBotzinger complex neurons. Nat Neurosci 8: 1142–1144, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McKay LC, Feldman JL. Unilateral ablation of pre-Bötzinger complex disrupts breathing during sleep but not wakefulness. Am J Respir Crit Care Med 178: 89–95, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Michelot D, Melendez-Howell LM. Amanita muscaria: chemistry, biology, toxicology, and ethnomycology. Mycol Res 107: 131–146, 2003. [DOI] [PubMed] [Google Scholar]
- 27.Mitchell GS, Johnson SM,. Neuroplasticity in respiratory motor control. J Appl Physiol 94: 358–374, 2003. [DOI] [PubMed] [Google Scholar]
- 28.Nattie EE, Li A. Neurokinin-1 receptor-expressing neurons in the ventral medulla are essential for normal central and peripheral chemoreception in the conscious rat. J Appl Physiol 101: 1596–1606, 2006. [DOI] [PubMed] [Google Scholar]
- 29.Nattie EE, Li A. Substance P-saporin lesion of neurons with NK1 receptors in one chemoreceptor site in rats decreases ventilation and chemosensitivity. J Physiol 544: 603–616, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nattie EE, Li A. Central chemoreception is a complex system function that involves multiple brain stem sites J Appl Physiol. In press. [DOI] [PMC free article] [PubMed]
- 31.Ramirez JM, Zuperku EJ, Alheid GF, Lieske SP, Ptak K, McCrimmon DR. Respiratory rhythm generation: converging concepts from in vitro and in vivo approaches? Respir Physiol Neurobiol 131: 43–56, 2002. [DOI] [PubMed] [Google Scholar]
- 32.Ruangkittisakul AS, Schwarzacher S, Seccia L, Ma Y, Bobocea N, Poon BY, Funk GD, Ballanyi K. Generation of eupnea, and sigh by a spatiochemically organized inspiratory network. J Neurosci 28: 2447–2458, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ruangkittisakul AS, Schwarzacher S, Seccia L, Poon BY, Ma Y, Funk GD, Ballanyi K. High sensitivity to neuromodulator-activated signaling pathways at physiological [K+] of confocally imaged respiratory center neurons in on-line-calibrated newborn rat brainstem slices. J Neurosci 26: 11870–11880, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rybak IA, Shevtsova NA, St-John WM, Paton JF, Pierrefiche O. Endogenous rhythm generation in the pre-Bötzinger complex and ionic currents: modelling and in vitro studies. Eur J Neurosci 18: 239–257, 2003. [DOI] [PubMed] [Google Scholar]
- 35.Smith JC, Ellenberger HH, Ballanyi K, Richter DW, Feldman JL. Pre-Bötzinger complex: a brainstem region that may generate respiratory rhythm in mammals. Science 254: 726–729, 1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Smith JC, Butera RJ, Koshiya N, Del Negro C, Wilson CG, Johnson SM. Respiratory rhythm generation in neonatal and adult mammals: the hybrid pacemaker-network model. Respir Physiol 122: 131–147, 2000. [DOI] [PubMed] [Google Scholar]
- 37.Solomon IC, Edelman NH, Neubauer JA. Patterns of phrenic motor output evoked by chemical stimulation of neurons located in the pre-Bötzinger complex in vivo. J Neurophysiol 81: 1150–1161, 1999. [DOI] [PubMed] [Google Scholar]
- 38.Solomon IC, Edelman NH, O'Neal MH. CO2/H+.chemoreception in the cat pre-Bötzinger complex in vivo. J Appl Physiol 88: 1996–2007, 2000. [DOI] [PubMed] [Google Scholar]
- 39.Solomon IC, Edelman NH, Neubauer JA. Pre-Bötzinger Complex functions as a central hypoxia chemosensor for respiration in vivo. J Neurophysiol 83: 2854–2868, 2000. [DOI] [PubMed] [Google Scholar]
- 40.St Jacques R, St. John WM. Transient, reversible apnoea following ablation of the pre-Bötzinger complex in rats. J Physiol 520: 303–314, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.St. John WM, Glasser RL, King RA. Rhythmic respiration in awake, vagotomized cats with chronic pneumotaxic center lesions. Respir Physiol 15: 233–244, 1972. [DOI] [PubMed] [Google Scholar]
- 42.St. John WM, Bledsoe TA. Genesis of rhythmic respiratory activity in pons independent of medulla. J Appl Physiol 59: 684–690, 1985. [DOI] [PubMed] [Google Scholar]
- 43.St John WM, Rybak IA, Paton JE. Potential switch from eupnea to fictive gasping after blockade of glycine transmission and potassium channels. Am J Physiol Regul Integr Comp Physiol 283: R721–R731, 2002. [DOI] [PubMed] [Google Scholar]
- 44.St-John WM, Paton JF. Role of pontile mechanisms in the neurogenesis of eupnea. Respir Physiol Neurobiol 143: 321–332, 2004. [DOI] [PubMed] [Google Scholar]
- 45.St-John WM, Paton JF. Role of pontile mechanisms in the neurogenesis of eupnea. Respir Physiol Neurobiol 143: 321–332, 2004. [DOI] [PubMed] [Google Scholar]
- 46.Stornetta RL, Rosin DL, Wang H, Sevigny CP, Weston MC, Guyenet PG. A group of glutamatergic interneurons expressing high levels of both neurokinin-1 receptors and somatostatin identifies the region of the pre-Bötzinger complex. J Comp Neurol 455: 499–512, 2003. [DOI] [PubMed] [Google Scholar]
- 47.Tan W, Janczewski WA, Yang P, Shao XM, Callaway EM, Feldman JL. Silencing preBotzinger complex somatostatin-expressing neurons induces persistent aprea in awake rat. Nat Neurosci 11: 538–540, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wang H, Stornetta RL, Rosin DL, Guyenet PG. Neurokinin-1 receptor-immunoreactive neurons of the ventral respiratory group in the rat. J Comp Neurol 434: 128–146, 2001. [DOI] [PubMed] [Google Scholar]
- 49.Wang H, Germanson TP, Guyenet PG. Depressor and tachypneic responses to chemical stimulation of the ventral respiratory group are reduced by ablation of neurokinin-1 receptor-expressing neurons. J Neurosci 22: 3755–3764, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wenninger JM, Pan LG, Klum L, Leekley T, Bastastic Hodges MR J, Feroah T, Davis S, Forster HV. Small reduction of neurokinin-1 receptor-expressing neurons in the pre-Bötzinger complex area induces abnormal breathing periods in awake goats. J Appl Physiol 97: 1620–1628, 2004. [DOI] [PubMed] [Google Scholar]
- 51.Wenninger JM, Pan LG, Klum L, Leekley T, Bastastic J, Hodges MR, Feroah TR, Davis S, Forster HV. Large lesions in the pre-Bötzinger complex area eliminate eupneic respiratory rhythm in awake goats. J Appl Physiol 97: 1629–1636, 2004. [DOI] [PubMed] [Google Scholar]
- 52.Wu M, Haxhiu MA, Johnson SM. Hypercapnic and hypoxic responses require intact neural transmission from the pre-Bötzinger Complex. Respir Physiol Neurobiol 146: 33–46, 2004. [DOI] [PubMed] [Google Scholar]