Abstract
Bone marrow cells were isolated from the humeri of C57BL/6 mice after a 13-day flight on the space shuttle Space Transportation System (STS)-118 to determine how spaceflight affects differentiation of cells in the granulocytic lineage. We used flow cytometry to assess the expression of molecules that define the maturation/activation state of cells in the granulocytic lineage on three bone marrow cell subpopulations. These molecules included Ly6C, CD11b, CD31 (platelet endothelial cell adhesion molecule-1), Ly6G (Gr-1), F4/80, CD44, and c-Fos. The three subpopulations were small agranular cells [region (R)1], larger granular cells (R2), which were mostly neutrophils, and very large, very granular cells (R3), which had properties of macrophages. Although there were no composite phenotypic differences between total bone marrow cells isolated from spaceflight and ground-control mice, there were subpopulation differences in Ly6C (R1 and R3), CD11b (R2), CD31 (R1, R2, and R3), Ly6G (R3), F4/80 (R3), CD44high (R3), and c-Fos (R1, R2, and R3). In particular, the elevation of CD11b in the R2 subpopulation suggests neutrophil activation in response to landing. In addition, decreases in Ly6C, c-Fos, CD44high, and Ly6G and an increase in F4/80 suggest that the cells in the bone marrow R3 subpopulation of spaceflight mice were more differentiated compared with ground-control mice. The presence of more differentiated cells may not pose an immediate risk to immune resistance. However, the reduction in less differentiated cells may forebode future consequences for macrophage production and host defenses. This is of particular importance to considerations of future long-term spaceflights.
Keywords: phenotypic markers, granulocytic lineage, differentiation
bone marrow is a complex microenvironment that is necessary for the generation of red and white blood cells (hematopoiesis) in adult animals (1, 58, 61). This organ can be disrupted by numerous factors including stress, bone changes, alterations in circadian rhythm, and irradiation (4, 35, 44, 45), all of which occur during spaceflight. Therefore, it is not surprising that spaceflight decreased the number of bone marrow-derived colony-forming units [CFU-monocyte (M) or CFU-granulocyte/monocyte (GM)] during some spaceflights (26, 49, 50, 57). Spaceflight also has an impact on monocytes, cells that have recently emerged from the bone marrow. The number of monocytes in circulation declined (53), and some lacked the expression of insulin-like growth factor (IGF) receptors (34). A mouse model used to simulate some of the physiological changes associated with spaceflight, antiorthostatic suspension (56), also diminished the number of macrophage progenitor cells (CFU-M) in the bone marrow (6–8, 19, 20, 49). This strengthens the hypothesis that physiological changes that occur in response to spaceflight affect bone marrow cells.
Although many spaceflights have been done with rats, only Space Transportation System (STS)-108 addressed the impact of spaceflight on immune parameters in adult mice. Indeed, there was a decrease in the number and percentage of blast cells and an increase in the number of CD34+ cells in the bone marrow in flight animals assessed after the landing of STS-108 (41). However, there were no differences in the numbers of mature granulocytes or monocytes in the bone marrow of those same animals (41).
Several countries are preparing for space travel in the future (29, 40). Given the importance of bone marrow to the maintenance of host red and white blood cell populations, additional information is needed on the changes that occur in bone marrow cells in response to spaceflight. The invasive nature of the collection of human bone marrow specimens precludes extensive study of human bone marrow. Therefore, rodent models are essential to revealing clues about how spaceflight impacts this critical system. To this end, we had access to normal mouse bone marrow that was part of the Commercial Biomedical Test Module-2 (CBTM-2) payload experiment. Past spaceflight rodent studies indicated that there are effects on early blast cells (CFU-GM) in bone marrow (49, 50). Therefore, we assessed bone marrow cells for the expression of differentiation and activation molecules to determine whether spaceflight affects specific subpopulations of cells in the granulocytic lineage, e.g., macrophages and neutrophils.
MATERIALS AND METHODS
Antibodies used for phenotyping.
Fluorescein isothiocyanate (FITC)-conjugated anti-Ly6C (Clone AL-21), FITC-anti-IgM (Clone RA-22), phycoerythrin (PE)-conjugated anti-CD31 (Clone MEC13.3), PE-anti-IgG2a (Clone R35-95), PE-anti-CD44 (Clone IM7), and PE-anti-IgG2b (Clone A95-1) were purchased from BD Pharmingen. PE-anti-CD11b (Clone M1-70), PE-anti-IgG2b (Clone eB149/0H5), PE-anti-Ly6G (Clone RB6–8C5), PE-anti-IgG2b (Clone eB149/0H5), allophycocyanin (APC)-conjugated anti-F4/80 (Clone BM8), and APC-anti-IgG2a (Clone eBR2a) were purchased from eBioscience. PE-anti-c-Fos (Clone 4) and PE-anti-IgG2b (clone not categorized) were purchased from Santa Cruz Biotechnology.
Mice.
Female C57BL/6 mice (n = 28) were 9 wk old at the beginning of the experiment. The mice were obtained from Charles River Laboratories (Wilmington, MA) and were housed at the National Aeronautics and Space Administration (NASA) Space Life Sciences Laboratory Facility (SLSL) at Kennedy Space Center. The Institutional Animal Care and Use Committees of NASA, Kansas State University, the University of Colorado, and Loma Linda University approved all procedures.
All mice were acclimated to the food bar diet (39, 63) before their selection for the Flight or Ground-control group (16, 48). Mice were housed in animal enclosure module (AEM) housing for Ground-control (n = 12 mice) and Flight (n = 12 mice) groups and were given the same food bar diet. The Flight group was flown on the Space Shuttle Endeavour (STS-118), which flew for 13 days. Ground-control mice were exposed to environmental settings (temperature, relative humidity, and atmospheric CO2 levels) similar to the spaceflight animals with telemetry from the shuttle on a 48-h time delay. Additional mice were housed in standard vivarium conditions (control; n = 4 mice). Mice had access to food and water ad libitum. Lighting was on a 12:12-h light-dark cycle.
Sample collection.
Muscle strength testing and nuclear magnetic resonance imaging were performed before euthanasia with 100% CO2. Mice were euthanized within 3–4 h of landing. The humeri were recovered and cleaned of all nonosseous tissue for the control, Flight, or Ground-control mice in three consecutive sampling days. Because of the experimental design of the primary experiments of the CBTM-2 payload, only humeri were available for this secondary experiment. This was an acceptable alternative to obtaining bone marrow from hindlimb bones because in response to skeletal unloading the CFU-M colony formation was similar between bone marrow isolated from humeri and bone marrow isolated from hindlimbs (femora and tibiae) (6, 7). The marrow cavity was flushed with a sterile PBS solution. Red blood cells were lysed with ACK lysing buffer reagent (0.15 M NH4Cl, 10 mM KHCO3, and 0.1 mM Na2EDTA). Cells were centrifuged (300 g, 5 min) and washed two times with PBS to remove serum components. Cells were counted on a hemacytometer and suspended in 2% formalin in PBS at a concentration of 4 × 106 cells/ml. These cells were shipped at 4°C overnight to Kansas State University for further analyses.
Bone marrow-derived cell labeling and fluorescence-activated cell sorting analysis.
After arrival at Kansas State University, bone marrow cells were recounted and cell concentrations were adjusted to 1 × 107 cells/ml. Phenotypic analysis of bone marrow-derived cells was performed by fluorescence-activated cell sorting (FACS) on two successive days. Bone marrow cells were blocked with PBS-goat serum (50:50; 50 μl) at 4°C for 0.5 h. FITC-anti-Ly6C or anti-IgM (0.5 μg), PE-anti-CD11b or anti-IgG2b (0.1 μg), APC-anti CD31 or anti-IgG2a (0.5 μg), PE-anti Ly6G or anti-IgG2b (0.1 μg), APC-anti-F4/80 or anti-IgG2a (1.4 μg), PE-anti-CD44 or anti-IgG2b (1 μg), and PE-anti-c-Fos or anti-IgG2b (3.8 μg) were added to the cell suspensions and incubated at 4°C for 1 h. In some instances, multiplexing of antibodies with compatible flurochromes was done (e.g., c-Fos and CD31 or Ly6C and CD31). The cells were then washed twice in Hanks’ balanced salt solution and resuspended in 1% formalin. A FACSCalibur flow cytometer (Becton Dickinson, Rockville, MD) was used for the analyses. A minimum of 20,000 events were collected for each sample. Sample gating was done so that ≥95% of the isotype control was in the lower left quadrant (e.g., Figs. 3–5) or was in the left gate on histograms (e.g., Fig. 2). Since the Flight and Ground-control bone marrow cell surface antigens were analyzed on different days (the day after shipping), the day-to-day differences in cell staining were normalized by using control cells that were prepared at the SLSL at Cape Canaveral and were stained at the same time as Flight or Ground samples (2 mice per labeling day, Controlflight and Controlground). Ground-control data were normalized to Flight data with the calculation normalized Ground data = (Controlflight/Controlground) × Ground-control sample value.
Statistical analysis.
Data were evaluated by factorial ANOVA with Tukey pairwise multiple comparison test and χ2-test (Statmost, Detaxiom Software, Los Angeles, CA). P values of <0.05 were selected to indicate significance. Data are presented as means ± SE for 12 mice per treatment group.
RESULTS
Bone marrow cell numbers and subpopulations.
Flight and Ground-control mice had body masses of 18.1 ± 0.2 g before flight. At landing, all mice appeared well groomed, with a normal coat appearance, but they appeared disoriented. Video documentation obtained in space on flight days 5 and 6 indicated that the mice adapted well to the space environment. They exhibited normal eating, grooming behavior, and active movement throughout the AEM, including running and “flying.” Veterinary examination at landing found the mice to be in good health despite weight loss. Ground-control mice weighed 18.7 ± 0.2 g (+3.3%) and Flight mice weighed 16.5 ± 0.3 g (−8.8%). We isolated an average of 6.9 ± 0.5 × 106 bone marrow cells per mouse from 12 Flight mice and similar numbers of bone marrow cells from 12 AEM-housed Ground-control mice (6.5 ± 0.3 × 106). There were no significant differences in gross bone morphology between treatment groups or changes in the bone marrow cell numbers between Flight and Ground-control mice.
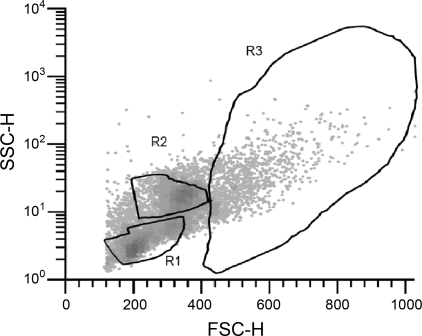
Bone marrow cells were identified by size (forward scatter) and granularity (side scatter) (Fig. 1). Region (R)1 identified small, agranular cells. R2 identified cells that were larger and more granular. As shown by the expression of Ly6G (Gr-1) and CD11b, this region contains many granulocytes (neutrophils) (Table 1). The largest, most granular cells were identified in R3. These cells had the highest level of staining for macrophages based on the macrophage phenotypic expression of F4/80 and CD11b, which are expressed by monocytes and macrophages in various tissues throughout the mouse (23) (Table 1).
Fig. 1.
Subpopulations of bone marrow cells separated into region (R)1, R2, and R3 subpopulations based on granularity [y-axis, side scatter (SSC)] and size [x-axis, forward scatter (FSC)].
Table 1.
Effect of spaceflight on bone marrow-derived cell phenotypic markers
| Cell Marker | Subpopulation | Spaceflight | Ground Control |
|---|---|---|---|
| Ly6C | TBM | 35±2† | 21±3 |
| R1 | 14±1† | 9±1 | |
| R2 | 33±3 | 37±4 | |
| R3 | 63±3† | 72±3 | |
| CD11b | TBM | 11±1* | 14±1 |
| R1 | 1±0 | 1±0 | |
| R2 | 50±3† | 4±0 | |
| R3 | 40±3 | 43±3 | |
| CD31 | TBM | 3±2* | 5±1 |
| R1 | 3±0† | 14±2 | |
| R2 | 2±1† | 10±2 | |
| R3 | 1±2* | 10±3 | |
| Ly6G | TBM | 28±2 | 31±2 |
| R1 | 0±0 | 0±0 | |
| R2 | 60±3 | 66±6 | |
| R3 | 26±1† | 67±4 | |
| F4/80 | TBM | 7±1† | 3±0 |
| R1 | 1±0* | 2±1 | |
| R2 | 6±1 | 4±2 | |
| R3 | 24±3† | 8±1 | |
| cFos | TBM | 0±2* | 0±5 |
| R1 | 2±0† | 5±1 | |
| R2 | 4±1† | 13±2 | |
| R3 | 15±3† | 45±3 | |
| CD44low | TBM | 17±1 | 16±1 |
| R1 | 57±1 | 59±1 | |
| R2 | 0±1 | 0±0 | |
| R3 | 0±1 | 0±1 | |
| CD44high | TBM | 62±2* | 69±2 |
| R1 | 29±2 | 28±1 | |
| R2 | 92±1* | 95±1 | |
| R3 | 64±4† | 83±2 |
Values are % means ± SE for 12 mice/group. TBM, total bone marrow; R1, region 1; R2, region 2; R3, region 3. t-Test:
P < 0.05,
P < 0.01.
We compared AEM-housed Ground-control mice and the mice that were flown in space. R1 contained 38 ± 1% of the bone marrow cells from Flight or Ground-control mice. R2 contained 38 ± 1% and 36 ± 1% of the bone marrow cells from Flight and Ground-control mice, respectively. R3 contained 11 ± 0% of the total bone marrow cell population for both treatment groups. Therefore, spaceflight did not radically disrupt the distribution of bone marrow cell subpopulations.
Total bone marrow phenotypic assessment.
We analyzed the level of Ly6C expression on the total bone marrow population. Ly6C expression is related to differentiation and identifies cells at an intermediate stage within the myeloid lineage, but it is absent from cells of the erythroid lineage (30). Its expression is completely lost by cells on final maturation into mature macrophages (17). Overall, 35 ± 2% of bone marrow cells from Flight animals were positive compared with 21 ± 3% (P < 0.01) positive for bone marrow cells from Ground-control mice. The high levels of Ly6C are consistent with the presence of high numbers of cells in the granulocytic lineage in bone marrow developing for host innate immune responses. Bone marrow cells were 3 ± 2% and 5 ± 1% (P < 0.05) positive for CD31 (macrophages early in differentiation; Refs. 17, 18, 62) for Flight and Ground-control mice, respectively.
When we examined bone marrow cell expression of CD11b, a β-integrin that participates in strong adhesion and trafficking (27), we saw that the overall expression was low in both mouse treatment groups compared with the expression in the R2 or R3 subpopulations. Bone marrow cells were 11 ± 1% and 14 ± 1% (P < 0.05) positive from Flight animals and Ground-control animals, respectively. These data are consistent with the low-level expression of CD11b in the whole bone marrow (33).
Ly6G is expressed predominantly on neutrophils. However, it can also be expressed on differentiating premonocytes as well as other cells (3, 21, 31). There were no significant differences in Ly6G expression between the two treatment groups (Table 1). Overall, expression of F4/80 (a macrophage-specific marker; Refs. 11, 25) was higher on bone marrow cells from Flight animals compared with Ground-control animals (7 ± 1% vs. 3 ± 0%, P < 0.01).
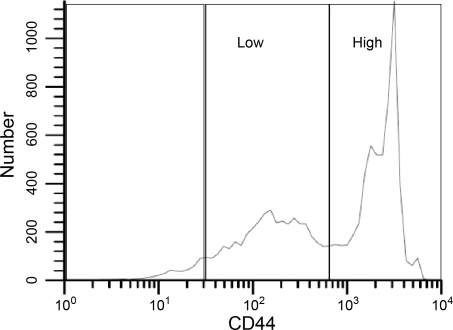
CD44 is another cell surface protein involved in cell-cell interactions, migration, cell adhesion, and lymphocyte activation (28). Some isoforms bind hyaluronic acid (32), which may be important in organizing and regulating the function of bone marrow cells (36, 46). Most bone marrow cells, especially granulocytes, express CD44 (55). Therefore, CD44 was also examined. In the total bone marrow, there were two subpopulations of CD44+ cells (Fig. 2). Therefore, for our analyses, we assessed the “CD44high” and “CD44low” populations separately. There were no differences in treatment groups in the percentage of cells that were CD44low. For CD44high cells, Flight and Ground-control bone marrow cells were 62 ± 2% and 69 ± 2% (P < 0.05), respectively.
Fig. 2.
Bone marrow-derived cells identified by low and high expression of CD44 surface antigen.
R1 subpopulation phenotypic assessment.
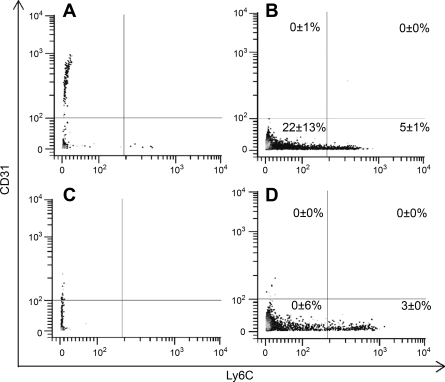
The assessment of Ly6C and CD31 in the R1 population showed that overall there was a significant increase in expression of Ly6C from 9 ± 1% in Ground-control samples to 14 ± 1% (P < 0.01) for Flight samples. The same trend was seen with Ly6C+CD31− cells (Fig. 3) from Ground-control and Flight samples (3% and 5%, respectively, P < 0.01). However, there were no differences in Ly6C+CD31+ or Ly6C−CD31+ cell percentages in the R1 subpopulation. Therefore, since Ly6C may discern differentiation (18), or may be important in the trafficking of CD8+ T lymphocytes (24), spaceflight may have an effect on the small agranular cells in the R1 subpopulation.
Fig. 3.
Ly6C and CD31 surface expression by bone marrow-derived cells in the R1 subpopulation. A: Flight group cells stained with isotype antibody. B: Flight cells stained with α-Ly6C and α-CD31 antibodies. C: Ground-control group cells stained with isotype antibody. D: Ground-control cells stained with anti-Ly6C and anti-CD31. Percentages are averages ± SE for 12 mice per treatment group.
There also was a decrease in CD31 from 14 ± 2% on Ground-control mouse bone marrow cells to 3 ± 0% on Flight mouse bone marrow cells (P < 0.01) in the R1 subpopulation. Because CD31 is involved in the transmigration of cells into tissue (10), these data also support the hypothesis that R1 cell trafficking may have been affected by spaceflight.
There was ≤2% expression of F4/80 and Ly6G in the R1 population. For c-Fos, bone marrow cells from Flight mice had slightly lower expression (2 ± 0% vs. 5 ± 1%; P < 0.01) than bone marrow cells from Ground-control mice. CD44low expression was 59 ± 1% and 57 ± 1% for Ground-control and Flight mouse bone marrow, respectively. CD44high expression (28 ± 1% and 29 ± 2% for Ground-control and Flight bone marrow, respectively) was significantly lower than CD44low expression in the R1 subpopulation. These data are consistent with observations that CD44 expression is higher on granulocytic cells (55). However, there was no statistically significant spaceflight effect on the expression of CD44 in the R1 subpopulation.
R2 subpopulation phenotypic assessment.
There were no significant differences in the expression of Ly6C, Ly6G, or F4/80 on bone marrow cells in the R2 subpopulation between Flight and Ground-control animals. There was a significant increase in the expression of CD11b on bone marrow cells from Flight mice (50 ± 3%) compared with Ground-control mice (4 ± 0%; P < 0.01). This reversely correlated with the expression of CD31 and c-Fos protein on bone marrow cells. Flight mice had 2 ± 1% and 4 ± 1% CD31- and c-fos-expressing cells compared with 10 ± 2% (P < 0.01) and 13 ± 2% (P < 0.01) expression by bone marrow from Ground-control mice. Interestingly, there was expression of CD44high by the R2 bone marrow cell subpopulation but no CD44low expression. Flight mice had 92 ± 1% CD44high-expressing cells compared with 95 ± 1% (P < 0.05) expression by bone marrow from Ground-control mice.
R3 subpopulation phenotypic assessment.
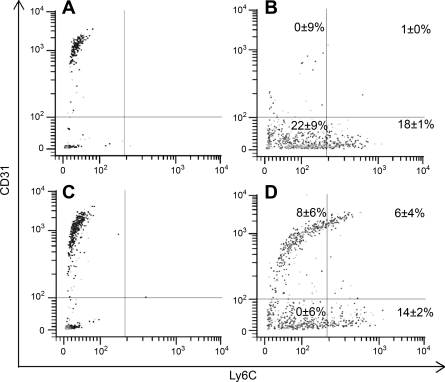
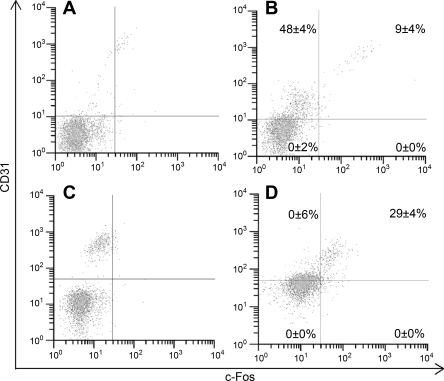
There was little difference in the R3 bone marrow cell expression of CD11b between Flight and Ground-control mice. Flight mice had 40 ± 3% CD11b-expressing cells compared with 43 ± 3% expression by bone marrow cells from Ground-control mice. There was a small but significant difference in the cell expression of Ly6C and CD31 between Flight and Ground-control mice. Flight mice had 63 ± 3% Ly6C-expressing cells compared with 72 ± 3% expression by bone marrow from Ground-control mice (P < 0.01). Flight mouse bone marrow was 1 ± 2% positive for CD31, whereas bone marrow from Ground-control mice was 10 ± 1% positive (P < 0.05). These data were consistent when these two phenotypes were looked at in combination. There was a decrease in the Ly6C−CD31+ subpopulation (Fig. 4). There also was a decrease in the Ly6C+CD31+ subpopulation in cells from Flight mice compared with cells from Ground-control mice (Fig. 4). The evaluation of c-Fos in the R3 subpopulation showed that there was a significant decrease in its expression (15 ± 3%) in Flight mice compared with Ground-control mice (45 ± 3%, P < 0.01). The same trend was seen with c-Fos+CD31+ cells from Flight and Ground-control mice (9 ± 4% and 29 ± 4%, respectively, P < 0.01). Bone marrow cells from flight that had the c-Fos−CD31+ phenotype were 48 ± 4% positive. However, c-Fos−CD31+ cells were 0 ± 6% (P < 0.01) for Ground-control mice (Fig. 5). No differences in c-Fos+CD31− cell percentages were seen in the R3 subpopulation (Fig. 5). Ground-control mouse bone marrow cells were 8 ± 1% positive for F4/80 compared with 24 ± 3% positive cells in the Flight samples (P < 0.05). This inversely correlated with the expression of Ly6G and CD44high, which were 67 ± 4% and 83 ± 2% positive, respectively, in Ground-control mouse bone marrow cells compared with 26 ± 1% and 64 ± 4% positive cells in Flight samples (P < 0.05).
Fig. 4.
Ly6C and CD31 surface expression by bone marrow-derived cells in the R3 subpopulation. A: Flight cells stained with isotype antibody. B: Flight cells stained with anti-Ly6C and anti-CD31 antibodies. C: Ground-control cells stained with isotype antibody. D: Ground-control cells stained with anti-Ly6C and anti-CD31. Percentages are averages ± SE for 12 mice per treatment group.
Fig. 5.
c-Fos and CD31 surface expression by bone marrow-derived cells in the R3 subpopulation. A: Flight cells stained with isotype antibody. B: Flight cells stained with anti-c-Fos and anti-CD31 antibodies. C: Ground-control cells stained with isotype antibody. D: Ground-control cells stained with anti-c-Fos and anti-CD31. Percentages are averages ± SE of 12 mice per treatment group.
DISCUSSION
Bone marrow cells from the CBTM-2 payload were analyzed in this study as a complete bone marrow population and as cell subpopulations distinguished by size and granularity. Ly6C was the only surface molecule significantly elevated on whole bone marrow cells from Flight mice compared with Ground-control mice. This contrasts with the observations of Pecaut et al. (41), who did not see changes in Ly6A/E in their analysis of mouse marrow after a 12-day flight on STS-108. This difference was probably because Ly6A/E is expressed on different cell populations than Ly6C, which was targeted in this study because of its higher specificity for cells in the granulocytic lineage than the other Ly6 family members (9, 42). This hypothesis is supported by the observations from STS-108 (41) and STS-118 (this report) in which there were no differences in total numbers of bone marrow cells isolated from Flight and Ground-control mice yet there were differences between bone marrow subpopulations (this report). It is possible that we might have observed a different outcome had we examined bone marrow from the femur instead of the humeri. However, experience with skeletally unloaded mice and antiorthostatic suspension suggests that the changes in bone (47) and bone marrow (6, 7) are systemic in response to unloading. Therefore, we do not think this is a serious concern.
Although Ly6C expression was elevated on whole bone marrow isolated from Flight mice compared with Ground-control mice, whole bone marrow composite phenotype expression demonstrated no significant difference between Flight and Ground-control mouse bone marrow (P > 0.2 by χ2-test). This is not surprising because bone marrow contains a heterogeneous population of cells that share phenotypes. Therefore, we analyzed subpopulations of cells that were distinguished on the basis of their size and granularity. Three subpopulations of cells were identified and assessed for the expression of Ly6C, CD11b, CD31, Ly6G, F4/80, CD44, and c-Fos.
Cell surface antigen expression differences were present within spaceflight subpopulations. For example, Ly6C expression was elevated by spaceflight in the small agranular R1 subpopulation but was lower in the R3 subpopulation. The R1 population contained the fewest numbers of macrophages and neutrophils based on the expression of F4/80, CD11b, and Ly6G. Given that Ly6C is expressed on cells at less mature stages within the myeloid lineage (30), these data suggest that the impact of spaceflight was on less mature cells compared with more mature cells in the granulocytic lineage. This hypothesis is supported by the expression of CD31 as well. CD31 expression distinguishes macrophages early in differentiation (17, 18, 62). c-Fos is also expressed during early myeloid cell development (38, 60). Therefore, c-Fos expression is normally expected concurrently with CD31 expression at initial macrophage differentiation stages under normal conditions. As differentiation proceeds, c-Fos expression ceases as CD31 levels begin to decline. The depression in the c-Fos+CD31+ population and the increase in the c-Fos−CD31+ population in R3 bone marrow cells from Flight mice suggest that the STS-118 spaceflight increased macrophage development, resulting in the presence of more differentiated cells than in the Ground-control mice.
CD11b was significantly elevated on bone marrow cells in the R2 population after spaceflight. CD11b is involved in trafficking and strong adhesion of macrophages and granulocytes in trafficking. Therefore, the elevation of this marker on the R2 subpopulation but not the R1 and R3 subpopulations may reflect the activation of the enriched neutrophil population in the R2 group in response to landing. Unfortunately, the experimental design of this study precluded the collection of blood for differential analysis. Therefore, we cannot make a direct comparison between what was occurring in the bone marrow and the peripheral blood cell populations. However, neutrophilia has been seen as a consequence of landing stress in past rodent flights (2, 13, 14, 26). Consequently, the specific impact on the R2 subpopulation would be consistent with those observations.
The greater decrease in expression of CD44high and Ly6G in the R3 bone marrow subpopulation from Flight mice but not the R1 and R2 subpopulations was also notable. In fact, there was a reverse correlation between the expression level of CD44high and Ly6G, which are strongly expressed on polymorphonuclear neutrophils (PMNs), and the increased expression of F4/80 in R3. Because F4/80 identifies mature macrophages, these data suggest that spaceflight caused increases in the percentage of mature macrophages in the bone marrow or decreased the percentage of PMNs. The stress of landing could induce the mobilization of bone marrow PMNs into circulation and cause such an impact (2, 13, 14, 26). Although this is an attractive hypothesis that would be consistent with data from other flights, there were no differences in bone marrow cell numbers between Flight mice and Ground-control mice to account for margination. Therefore, other factors may also be affecting the distribution of macrophages in the R3 subpopulation. The loss of cell numbers and a decrease in the percentage of monocytes/macrophages in the spleen of these flight mice (Baquai FP, Gridley DS, Slater JM, Luo-Owen X, Stodiek LS, Ferguson V, Chapes SK, Pecaut MJ, unpublished observations) would be consistent with increased trafficking of those cells to the bone marrow. Alternatively, the decrease in Ly6C, c-Fos, and/or Ly6G and increase in F4/80 might suggest that the cells in the R3 subpopulation were more differentiated in the Flight mice compared with the Ground-control mice. This hypothesis is consistent with the diminished expression of IGF-I receptor (IGF-1R) on monocytes during spaceflight (34), since the expression of IGF-1R is reduced as macrophages differentiate (5).
The shift in cellular phenotypes is a complex process. For this reason, we compared the composite phenotypes of each of the three subpopulations of Flight bone marrow cells and Ground-control bone marrow cells with both parametric (factorial ANOVA) and nonparametric (χ2-test) statistics. R1, R2, and R3 comparisons between Flight and Ground-control AEM-housed mice suggest that there are significant differences (P < 0.01) between cells in the two treatment groups for all three subpopulations with either type of analysis. The mice were analyzed after landing. Therefore, it is not possible to separate the landing effects from the flight effects. As shown by behavioral observations made at days 5–6 during flight (normal grooming, eating, drinking, and movement) and the veterinary examination after flight, the animals were healthy. The weight loss most likely occurred in the first 3 days of habituation to the change in gravity during the spaceflight (37, 51, 59). Nevertheless, the loss in body weight and the significant thymic atrophy (Baqai et al., submitted for publication) that were seen in these mice suggest that the mice were subjected to chronic stress beyond what was endured during landing. Therefore, it is not unreasonable to suggest that there are significant changes in bone marrow phenotype in response to the stress of the spaceflight experience. Bone marrow cells are plastic in their response to host factors (52). Furthermore, not only are changes in bone marrow cell distribution seen in rats and mice in response to spaceflight (41, 50), bone marrow cell distribution is responsive to other stressors such as reactive oxygen species (43), ionizing radiation (15), ovariectomy (22), and glucocorticoid administration (54). It is also not clear whether these differences translate into changes in immune competence (12), but the data are consistent with observations that the number of CFU-M and CFU-GM are decreased during spaceflight (26, 49, 50). The shift toward more differentiated cells in the R2 and R3 subpopulations would reduce the number of early CFU-GM available to respond to colony-stimulating factor to differentiate into the appropriate cell lineage. If humans aspire to interplanetary space travel, appropriate concern and planning are needed to manage the possible consequences of a long-term spaceflight on hematopoiesis.
GRANTS
This project has been supported in part by NASA Grant NAG2-1274, by the NASA space grant consortium, National Institutes of Health Grants AI-55052, AI-052206, RR-16475, and RR-17686, the Kansas Agriculture Experiment Station, the Terry C. Johnson Center for Basic Cancer Research, and the Department of Radiation Medicine LLURM Molecular Radiation Biology Laboratories, Loma Linda University. This is Kansas Agriculture Experiment Station Publication 09-023-J.
Acknowledgments
We thank Amgen for sponsoring this flight investigation and generously providing the tissues required to conduct this study. In particular, we thank the principal investigators at Amgen, H. Q. Han and David Lacey. We thank Alison Fedrow and Betsey Potts for their assistance in this project and Tammy Koopman for her assistance with flow cytometry. We also thank Ramona Bober and the rest of the support staff at the NASA SLSL at Kennedy Space Center. We also thank the students and technicians from the University of Colorado at Boulder and Clemson University for their help with tissue collection.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Abramson S, Miller G, Phillips R. The identification in adult bone marrow of pluripotent and restricted stem cells of the myeloid and lymphoid systems. J Exp Med 145: 1567–1579, 1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Allebban Z, Ichiki A, Gibson L, Jones J, Congdon C, Lange R. Effects of spaceflight on the number of rat peripheral blood leukocytes and lymphocyte subsets. J Leukoc Biol 55: 209–213, 1994. [DOI] [PubMed] [Google Scholar]
- 3.Ammon C, Meyer SP, Schwarzfischer L, Krause SW, Andreesen R, Kreutz M. Comparative analysis of integrin expression on monocyte-derived macrophages and monocyte-derived dendritic cells. Immunology 100: 364–369, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Amsel S, Maniatis A, Tavassoli M, Crosby W. The significance of intramedullary cancellous bone formation in the repair of bone marrow tissue. Anat Rec 164: 101–112, 1969. [DOI] [PubMed] [Google Scholar]
- 5.Arkins S, Rebeiz N, Brunke-Reese DL, Minshall C, Kelley KW. The colony-stimulating factors induce expression of insulin-like growth factor-I messenger ribonucleic acid during hematopoiesis. Endocrinology 136: 1153–1160, 1995. [DOI] [PubMed] [Google Scholar]
- 6.Armstrong JA, Balch S, Chapes SK. Interleukin-2 therapy reverses some immunosuppressive effects of skeletal unloading. J Appl Physiol 77: 584–589, 1994. [DOI] [PubMed] [Google Scholar]
- 7.Armstrong JA, Kirby-Dobbels K, Chapes SK. The effects of rM-CSF and rIL-6 therapy on immunosuppressed antiorthostatically suspended mice. J Appl Physiol 78: 968–975, 1995. [DOI] [PubMed] [Google Scholar]
- 8.Armstrong JW, Nelson KA, Simske SJ, Luttges MW, Iandolo JJ, Chapes SK. Skeletal unloading causes organ-specific changes in immune cell responses. J Appl Physiol 75: 2734–2739, 1993. [DOI] [PubMed] [Google Scholar]
- 9.Barclay AN, Birkeland ML, Brown MH, Beyers AD, Davis SJ, Somoza C, Williams AF. The Leukocyte Antigen Facts Book. San Diego, CA: Academic, 1993, p. 424.
- 10.Bogen SA, Baldwin HS, Watkins SC, Albelda SM, Abbas AK. Association of murine CD31 with transmigrating lymphocytes following antigenic stimulation. Am J Pathol 141: 843–854, 1992. [PMC free article] [PubMed] [Google Scholar]
- 11.Caminschi I, Lucas KM, O'Keeffe MA, Hochrein H, Laabi Y, Kontgen F, Lew AM, Shortman K, Wright MD. Molecular cloning of F4/80-like-receptor, a seven-span membrane protein expressed differentially by dendritic cell and monocyte-macrophage subpopulations. J Immunol 167: 3570–3576, 2001. [DOI] [PubMed] [Google Scholar]
- 12.Chapes SK, Ganta RR. Mouse infection models for space flight immunology. Adv Space Biol Med 10: 81–104, 2005. [DOI] [PubMed] [Google Scholar]
- 13.Chapes SK, Simske SJ, Forsman AD, Bateman TA, Zimmerman RJ. Effects of space flight and IGF-1 on immune function. Adv Space Res 23: 1955–1964, 1999. [DOI] [PubMed] [Google Scholar]
- 14.Chapes SK, Simske SJ, Sonnenfeld G, Miller ES, Zimmerman RJ. Effects of spaceflight and PEG-IL-2 on rat physiological and immunological responses. J Appl Physiol 86: 2065–2076, 1999. [DOI] [PubMed] [Google Scholar]
- 15.Coates PJ, Rundle JK, Lorimore SA, Wright EG. Indirect macrophage responses to ionizing radiation: implications for genotype-dependent bystander signaling. Cancer Res 68: 450–456, 2008. [DOI] [PubMed] [Google Scholar]
- 16.Dalton P, Gould M, Girten B, Stodieck LS, Bateman TA. Preventing annoyance from odors in spaceflight: a method for evaluating the sensory impact of rodent housing. J Appl Physiol 95: 2113–2121, 2003. [DOI] [PubMed] [Google Scholar]
- 17.de Bruijn MF, Slieker WA, van der Loo JC, Voerman JS, van Ewijk W, Leenen PJ. Distinct mouse bone marrow macrophage precursors identified by differential expression of ER-MP12 and ER-MP20 antigens. Eur J Immunol 24: 2279–2284, 1994. [DOI] [PubMed] [Google Scholar]
- 18.de Bruijn MF, van Vianen W, Ploemacher RE, Bakker-Woudenberg IA, Campbell PA, van Ewijk W, Leenen PJ. Bone marrow cellular composition in Listeria monocytogenes infected mice detected using ER-MP12 and ER-MP20 antibodies: a flow cytometric alternative to differential counting. J Immunol Methods 217: 27–39, 1998. [DOI] [PubMed] [Google Scholar]
- 19.Dunn C, Johnson P, Lange R. Hematopoiesis in antiorthostatic, hypokinesic rats. Physiologist 26: S133–S134, 1983. [Google Scholar]
- 20.Dunn C, Johnson P, Lange R, Perez L, Nessel R. Regulation of hematopoiesis in rats exposed to antiorthostatic, hypokinetic/hypodynamia. I. Model description. Aviat Space Environ Med 56: 419–426, 1985. [PubMed] [Google Scholar]
- 21.Ferret-Bernard S, Sai P, Bach JM. In vitro induction of inhibitory macrophage differentiation by granulocyte-macrophage colony-stimulating factor, stem cell factor and interferon-gamma from lineage phenotypes-negative c-kit-positive murine hematopoietic progenitor cells. Immunol Lett 91: 221–227, 2004. [DOI] [PubMed] [Google Scholar]
- 22.Garcia-Perez MA, Noguera I, Hermenegildo C, Martinez-Romero A, Tarin JJ, Cano A. Alterations in the phenotype and function of immune cells in ovariectomy-induced osteopenic mice. Hum Reprod 21: 880–887, 2006. [DOI] [PubMed] [Google Scholar]
- 23.Gordon S, Crocker PR, Morris L, Lee SH, Perry VH, Hume DA. Localization and function of tissue macrophages. Ciba Found Symp 118: 54–67, 1986. [DOI] [PubMed] [Google Scholar]
- 24.Hanninen A, Jaakkola I, Salmi M, Simell O, Jalkanen S. Ly-6C regulates endothelial adhesion and homing of CD8+ T cells by activating integrin-dependent adhesion pathways. Proc Natl Acad Sci USA 94: 6898–6903, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hume DA, Ross IL, Himes SR, Sasmono RT, Wells CA, Ravasi T. The mononuclear phagocyte system revisited. J Leukoc Biol 72: 621–627, 2002. [PubMed] [Google Scholar]
- 26.Ichiki A, Gibson L, Jago T, Strickland K, Johnson D, Lange R, Allebban Z. Effects of spaceflight on rat peripheral blood leukocytes and bone marrow progenitor cells. J Leukoc Biol 60: 37–43, 1996. [DOI] [PubMed] [Google Scholar]
- 27.Jones D, Anderson D, Burr B, Rudloff H, Smith C, Krater S, Schmalstieg F. Quantitation of intracellular Mac-1 (CD11b/CD18) pools in human neutrophils. J Leukoc Biol 44: 535–544, 1988. [DOI] [PubMed] [Google Scholar]
- 28.Lau L, Jamieson B, Somasundaram T, Ahmed R. Cytotoxic T-cell memory without antigen. Nature 369: 648–652, 1994. [DOI] [PubMed] [Google Scholar]
- 29.Lawler A Remaking NASA: how much space for science? Science 303: 610–612, 2004. [DOI] [PubMed] [Google Scholar]
- 30.Leenen PJ, de Bruijn MF, Voerman JS, Campbell PA, Van Ewijk W. Markers of mouse macrophage development detected by monoclonal antibodies. J Immunol Methods 174: 5–19, 1994. [DOI] [PubMed] [Google Scholar]
- 31.Leenen PJ, Slieker WA, Melis M, Van Ewijk W. Murine macrophage precursor characterization. I. Production, phenotype and differentiation of macrophage precursor hybrids. Eur J Immunol 20: 15–25, 1990. [DOI] [PubMed] [Google Scholar]
- 32.Lesley J, He Q, Miyake K, Hamann A, Hyman R, Kincade PW. Requirements for hyaluronic acid binding by CD44: a role for the cytoplasmic domain and activation by antibody. J Exp Med 175: 257–266, 1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.McKnight AJ, Gordon S. Membrane molecules as differentiation antigens of murine macrophages. Adv Immunol 68: 271–314, 1998. [DOI] [PubMed] [Google Scholar]
- 34.Meehan R, Neale L, Kraus E, Stuart C, Smith M, Cintron N, Sams C. Alteration in human mononuclear leucocytes following space flight. Immunology 76: 491–497, 1992. [PMC free article] [PubMed] [Google Scholar]
- 35.Mendez-Ferrer S, Lucas D, Battista M, Frenette PS. Haematopoietic stem cell release is regulated by circadian oscillations. Nature 452: 442–447, 2008. [DOI] [PubMed] [Google Scholar]
- 36.Minguell JJ Is hyaluronic acid the “organizer” of the extracellular matrix in marrow stroma? Exp Hematol 21: 7–8, 1993. [PubMed] [Google Scholar]
- 37.Morey E Spaceflight and bone turnover: correlation with a new rat model. Bioscience 29: 168–172, 1979. [Google Scholar]
- 38.Muller R, Curran T, Muller D, Guilbert L. Induction of c-fos during myelomonocytic differentiation and macrophage proliferation. Nature 314: 546–548, 1985. [DOI] [PubMed] [Google Scholar]
- 39.Munoz KA, Tischler ME. The effect of a space food bar diet on body and muscle mass in normal and hind-limb suspended rats. Aviat Space Environ Med 62: 875–878, 1991. [PubMed] [Google Scholar]
- 40.Normile D, Bagla P. Space exploration. Asian powers shoot for the moon with orbiting research missions. Science 317: 1163, 2007. [DOI] [PubMed] [Google Scholar]
- 41.Pecaut MJ, Nelson GA, Peters LL, Kostenuik PJ, Bateman TA, Morony S, Stodieck LS, Lacey DL, Simske SJ, Gridley DS. Effects of spaceflight on immunity in the C57BL/6 mouse. I. Immune population distributions. J Appl Physiol 94: 2085–2094, 2003. [DOI] [PubMed] [Google Scholar]
- 42.Pflugh DL, Maher SE, Bothwell ALM. Ly-6 superfamily members Ly-6A/E, Ly-6C, and Ly-6I recognize two potential ligands expressed by B lymphocytes. J Immunol 169: 5130–5136, 2002. [DOI] [PubMed] [Google Scholar]
- 43.Pyatt DW, Stillman WS, Irons RD. Reactive oxygen species mediate stem cell factor synergy with granulocyte/macrophage colony-stimulating factor in a subpopulation of primitive murine hematopoietic progenitor cells. Mol Pharmacol 49: 1097–1103, 1996. [PubMed] [Google Scholar]
- 44.Shibata Y, Dempsey W, Morahan P, Volkman A. Selectively eliminated blood monocytes and splenic suppressor macrophages in mice depleted of bone marrow by strontium 89. J Leukoc Biol 38: 659–669, 1985. [DOI] [PubMed] [Google Scholar]
- 45.Shibata Y, Volkman A. Restoration of prostaglandin releasing macrophage populations in lethally irradiated mice with spleen cells from bone marrow-depleted donors. J Leukoc Biol 49: 397–406, 1991. [DOI] [PubMed] [Google Scholar]
- 46.Siczkowski M, Andrew T, Amos S, Gordon MY. Hyaluronic acid regulates the function and distribution of sulfated glycosaminoglycans in bone marrow stromal cultures. Exp Hematol 21: 126–130, 1993. [PubMed] [Google Scholar]
- 47.Simske SJ, Greenberg A, Luttges MW. Effects of suspension-induced osteopenia on the mechanical behaviour of mouse long bones. J Mater Sci Mater Med 2: 43–50, 1991. [DOI] [PubMed] [Google Scholar]
- 48.Smith M, Johnson P, LeBlanc A. Animal enclosure module inflight test. In: Results of the Life Sciences DSOs Conducted Aboard the Space Shuttle 1981–1986, edited by Bungo MW, Bagian TM, Bowman MW, and Iovetan BM. Houston, TX: NASA, 1987, p. 75–77.
- 49.Sonnenfeld G, Mandel A, Konstantinova I, Berry W, Taylor G, Lesnyak A, Fuchs B, Rakhmilevich A. Spaceflight alters immune cell function and distribution. J Appl Physiol 73: 191S–195S, 1992. [DOI] [PubMed] [Google Scholar]
- 50.Sonnenfeld G, Mandel A, Konstantinova I, Taylor G, Berry W, Wellhausen S, Lesnyak A, Fuchs B. Effects of spaceflight on levels and activity of immune cells. Aviat Space Environ Med 61: 648–653, 1990. [PubMed] [Google Scholar]
- 51.Steffen J, Fell R, Musacchia X. Physiological responses during whole body suspension of adult rats. Physiologist 30: 94–95, 1987. [PubMed] [Google Scholar]
- 52.Stout RD, Jiang C, Matta B, Tietzel I, Watkins SK, Suttles J. Macrophages sequentially change their functional phenotype in response to changes in microenvironmental influences. J Immunol 175: 342–349, 2005. [DOI] [PubMed] [Google Scholar]
- 53.Taylor G, Neale L, Dardano J. Immunological analyses of U.S. Space Shuttle crew members. Aviat Space Environ Med 57: 213–217, 1986. [PubMed] [Google Scholar]
- 54.Trottier MD, Newsted MM, King LE, Fraker PJ. Natural glucocorticoids induce expansion of all developmental stages of murine bone marrow granulocytes without inhibiting function. Proc Natl Acad Sci USA 105: 2028–2033, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Trowbridge IS, Lesley J, Schulte R, Hyman R, Trotter J. Biochemical characterization and cellular distribution of a polymorphic, murine cell-surface glycoprotein expressed on lymphoid tissues. Immunogenetics 15: 299–312, 1982. [DOI] [PubMed] [Google Scholar]
- 56.Tsujimoto H, Ono S, Mochizuki H, Aosasa S, Majima T, Ueno C, Matsumoto A. Role of macrophage inflammatory protein 2 in acute lung injury in murine peritonitis. J Surg Res 103: 61–67, 2002. [DOI] [PubMed] [Google Scholar]
- 57.Vacek A, Bartonickov A, Rotkovsk D, Michurina T, Damaratskaya E, Serova L. The effects of weightlessness and increased gravity on hemopoietic stem cells of rats and mice. Physiologist 26: S131–S132, 1983. [Google Scholar]
- 58.van Furth R, Diesselhoff-den Dulk M. The kinetics of promonocytes and monocytes in the bone marrow. J Exp Med 132: 813–828, 1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wade CE, Ortiz RM, Baer LA. Increases in body mass of rats during spaceflight: models and measurements. Aviat Space Environ Med 71: 1126–1130, 2000. [PubMed] [Google Scholar]
- 60.Wang ZQ, Ovitt C, Grigoriadis A, Mohle-Steinlein U, Ruther U, Wagner E. Bone and haematopoietic defects in mice lacking c-fos. Nature 360: 741–745, 1992. [DOI] [PubMed] [Google Scholar]
- 61.Warren M, Vogel S. Bone marrow-derived macrophages: development and regulation at differentiation markers by colony-stimulating factor and interferons. J Immunol 134: 982–989, 1985. [PubMed] [Google Scholar]
- 62.Watt SM, Williamson J, Genevier H, Fawcett J, Simmons DL, Hatzfeld A, Nesbitt SA, Coombe DR. The heparin binding PECAM-1 adhesion molecule is expressed by CD34+ hematopoietic precursor cells with early myeloid and B-lymphoid cell phenotypes. Blood 82: 2649–2663, 1993. [PubMed] [Google Scholar]
- 63.Zerath E, Holy X, Andre C, Renault S. Effects of space food bar feeding on bone mass and metabolism in normal and unloaded rats. Nutr Res 22: 1309–1318, 2002. [DOI] [PubMed] [Google Scholar]