Abstract
Full expression of reflex cutaneous vasodilation is dependent on cyclooxygenase- (COX) and nitric oxide synthase- (NOS) dependent mechanisms. Low-dose aspirin therapy is widely prescribed to inhibit COX-1 in platelets for atherothrombotic prevention. We hypothesized that chronic COX inhibition with daily low-dose aspirin therapy (81 mg) would attenuate reflex vasodilation in healthy human skin. Two microdialysis fibers were placed in forearm skin of seven middle-aged (57 ± 3 yr), normotensive, healthy humans with no preexisting cardiovascular disease, taking daily low-dose aspirin therapy (aspirin: 81 mg), and seven unmedicated, healthy, age-matched control (no aspirin, 55 ± 3 yr) subjects, with one site serving as a control (Ringer) and the other NOS inhibited (NOS inhibited: 10 mM NG-nitro-l-arginine methyl ester). Red cell flux was measured over each site by laser-Doppler flowmetry, as reflex vasodilation was induced by increasing core temperature (oral temperature) 1.0°C using a water-perfused suit. Cutaneous vascular conductance (CVC) was calculated (CVC = flux/mean arterial pressure) and normalized to maximal CVC (CVCmax; 28 mM sodium nitroprusside). CVCmax was not affected by either aspirin or NOS inhibition. The plateau in cutaneous vasodilation during heating (change in oral temperature = 1.0°C) was significantly attenuated in the aspirin group (aspirin: 25 ± 3% CVCmax vs. no aspirin: 50 ± 7% CVCmax, P < 0.001 between groups). NOS inhibition significantly attenuated %CVCmax in both groups (aspirin: 17 ± 2% CVCmax, no aspirin: 23 ± 3% CVCmax; P < 0.001 vs. control), but this attenuation was less in the no-aspirin treatment group (P < 0.001). This is the first observation that chronic low-dose aspirin therapy attenuates reflex cutaneous vasodilation through both COX- and NOS-dependent mechanisms.
Keywords: thermoregulation, prostaglandins, cyclooxygenase, nitric oxide
as core temperature rises, skin blood flow is first increased by withdrawal of adrenergic vasoconstrictor tone, and then, upon reaching a specific core temperature threshold, both sweating and active reflex cutaneous vasodilation occur (31). Reflex vasodilation is activated by cholinergic cotransmission (19) and is mediated by several putative vasodilator mechanisms, including the neurotransmitter vasoactive intestinal peptide (1), histamine receptor activation (38), and neurokinin-1 receptor activation (36). Furthermore, full expression of reflex cutaneous vasodilation is dependent on nitric oxide (NO) synthase- (NOS) (17, 33) and cyclooxygenase- (COX) mediated second-messenger mechanisms, which purportedly contribute independently to the rise in skin blood flow during hyperthermia (22).
Daily low-dose aspirin is the gold standard antiplatelet aggregation therapy for primary and secondary prevention of atherothrombotic disease (28). Aspirin is an irreversible inhibitor of both COX isoforms, resulting in decreased generation of both thromboxane A2 (TXA2), a potent vasoconstrictor and platelet aggregating agent, and prostacyclin (PGI2), a vasodilator and platelet inhibitor (28). In accordance with the imbalance theory (7), it has been suggested that the low-dose aspirin regimens inhibit the production of TXA2 via COX-1 in platelets for the life of the platelet (∼10 days) without inhibiting vascular COX-2 PGI2 production, thereby producing a favorable antiatherothombotic vascular profile (28). In human cutaneous microvasculature, direct in vivo measurements of arachidonic acid metabolisms suggest that low-dose aspirin therapy inhibits the production of PGI2 generation via COX-1 mechanisms (20, 35), potentially inhibiting cutaneous vasodilation. Taken together, these data suggest that low-dose aspirin therapy not only inhibits COX-1 in platelets, producing an antithrombotic effect, but may also alter COX-mediated vascular signaling.
Human aging in the absence of overt pathology is associated with an attenuated rise in skin blood flow during hyperthermia (10, 13–15) due to a reduction in NO- and non-NO-dependent vasodilation. Low-dose aspirin therapy is increasingly recommended in middle-aged and elderly populations for atherothrombotic disease prevention (29); however, the combined effects of primary human aging and inhibition of COX-dependent cutaneous vasodilatory pathways with low-dose aspirin may significantly impact reflex-mediated thermoregulatory mechanisms, including the potential to functionally lower the critical psychrometric limits for heat gain in individuals taking chronic aspirin therapy. Therefore, the purpose of this study was to examine the effect of chronic (>1 yr) low-dose aspirin therapy (81 mg daily) on reflex cutaneous vasodilation. We hypothesized that 1) daily low-dose aspirin therapy would attenuate reflex cutaneous vasodilation in healthy humans compared with age-matched control subjects; and 2) NOS inhibition would significantly decrease cutaneous vasodilation in both subject groups due to the independent roles of NOS- and COX-dependent pathways in reflex vasodilation (22).
METHODS
Subjects.
Experimental protocols were approved by the Institutional Review Board at The Pennsylvania State University and conformed to the guidelines set forth by the Declaration of Helsinki. Verbal and written consent were voluntarily obtained from all subjects before participation. Studies were performed on seven control subjects of similar age (55 ± 3 yr, 4 men, 3 women) and seven subject taking chronic low-dose aspirin therapy (aspirin group; 81 mg/day for >1 yr; 57 ± 3 yr; 3 men, 4 women). Maximal platelet inhibition with enteric-coated oral low-dose aspirin occurs within 7–14 days (27, 28), and recovery of platelet COX activity occurs at a rate of ∼10%/day (3, 5). Healthy subjects who were voluntarily taking low-dose aspirin as primary prevention of atherothrombotic disease were recruited for the study. Subjects taking aspirin for >1 mo were eligible to participate in the study; however, all of the subjects had been taking aspirin therapy for >1 yr. Data from a subset of the control group (n = 5) have been previously published (11, 12). Additional control subjects and subjects taking chronic low-dose aspirin therapy were recruited for this study.
Subjects underwent a complete medical screening, including a physician-supervised graded exercise test to evaluate the existence of underlying cardiovascular disease, blood chemistry, lipid profile evaluation (Quest Diagnostics Nichol Institute, Chantily, VA), resting electrocardiogram, and physical examination. All subjects were screened for the presence of cardiovascular, dermatological, and neurological disease. Subjects were taking aspirin as recommended by their personal physician, but none had a history or family history (first-degree relative) of atherothrombotic disease. Subjects were normally active, nondiabetic, nonsmokers, who were currently not taking medications, including vitamins, hormone replacement therapy, or oral contraceptives. All premenopausal women were studied on days 2–7 (follicular phase) of their menstrual cycle.
Instrumentation and measurements.
Protocols were performed in a thermoneutral laboratory with the subject in the semisupine position, with the experimental arm at heart level. Aspirin subjects maintained their normal aspirin regimen and took 81 mg (baby aspirin) the day before reporting to the laboratory for the experiment. To control for acute increases in aspirin plasma concentrations, subjects were instructed not to take aspirin the day of the study. In healthy subjects, low-dose, enteric-coated aspirin reaches peak plasma concentration within 3–4 h after ingestion and has a half-life of 15 min (2, 3, 5). Platelet function returns at ∼10%/day with the cessation of daily low-dose aspirin (28). Furthermore, 4 days of low-dose aspirin therapy effectively inhibit current induced vasodilation in human skin (32). On arrival at the laboratory, subjects were instrumented with two intradermal microdialysis fibers (MD 2000, Bioanalytical Systems) (10 mm, 20-kDa cutoff membrane) in the skin on the right ventral forearm. Microdialysis sites were at least 4.0 cm apart to ensure no cross-reactivity of pharmacological agents being delivered to the skin. Microdialysis fibers were placed at each site by first inserting a 25-gauge needle through unanesthetized skin using sterile technique. The entry and exit points were ∼2.5 cm apart. The microdialysis fibers were then threaded through the needle, and the needle was withdrawn, leaving the fibers in place. The microdialysis fibers were taped in place and perfused with lactated Ringer solution during the insertion trauma resolution period at a rate of 2.0 μl/min (Bee Hive controller and Baby Bee microinfusion pumps, Bioanalytical Systems) for 60–90 min.
To obtain an index of skin blood flow, cutaneous red blood cell flux was measured with an integrated laser-Doppler flowmeter probe placed in a local heater (MoorLAB, Temperature Monitor SH02, Moor Instruments, Devon, UK) on the skin directly above each microdialysis membrane. All laser-Doppler probes were calibrated using Brownian standard solution. Cutaneous vascular conductance (CVC) was calculated as flux divided by mean arterial pressure.
To control whole body temperature, subjects wore a water-perfused suit that covered the entire body, except head, hands, and experimental arm. Additionally, the subjects wore a water-impermeable outer garment over the water-perfused suit to minimize evaporative heat loss. The subject's electrocardiogram was monitored throughout the protocol, and blood pressure was measured via brachial auscultation every 5 min. Oral temperature (Tor) was continuously monitored during baseline and throughout whole body heating as an index of body core temperature with a thermistor placed in the sublingual sulcus. The subjects were instructed to keep the thermistor in the same location in the sublingual sulcus and not to open their mouths or speak during the protocol. Mean skin temperature was calculated as the unweighted average from six copper-constantan thermocouples placed on the chest, middle back, abdomen, upper arm, thigh, and calf. During the period of insertion trauma resolution and baseline measurement periods, thermoneutral water (34°C) was perfused through the suit to clamp body temperature. During whole body heating, 50°C water was perfused through the suit to raise subject's Tor by 1.0°C. Local skin temperature over each microdialysis site was separately maintained at 33°C (Moor Instruments SHO2, Devon, UK).
Experimental protocol.
Red cell flux over each microdialysis site was monitored as insertion trauma resolved over a 60- to 90-min period. Microdialysis sites were randomly assigned to receive either 1) 10.0 mM NG-nitro-l-arginine methyl ester (l-NAME) to inhibit NO production by NOS (15, 18, 25), or 2) lactated Ringer. l-NAME was mixed just before each experiment, dissolved in lactated Ringer solution, and sterilized using syringe microfilters (Acrodisc, Pall, Ann Arbor, MI).
Microdialysis sites were perfused continuously for at least 60 min before the start of the baseline and during the baseline and heating periods with assigned pharmacological agents at a rate of 2.0 μl/min. Baseline data were collected for 20 min before the start of whole body heating, after which whole body heating was initiated. At the end of the heating protocol, each microdialysis site was perfused with 28.0 mM sodium nitroprusside (Nitropress, Abbot Laboratories, Chicago, IL) at a rate of 4.0 μl/min to achieve maximal CVC (CVCmax) in combination with local heating of the skin to 43°C over each microdialysis site to ensure CVCmax (15, 24, 34).
Data acquisition and analysis.
Data were acquired using Labview or Windaq software, and National Instruments or Dataq data-acquisition systems (Austin, TX). The data were collected at 40 Hz, digitized, recorded, and stored on a personal computer for further analysis. CVC data were averaged over 3-min periods for baseline and every 0.1°C rise in Tor and are presented as a percentage of CVCmax (%CVCmax). Absolute CVCmax in each microdialysis site was calculated as the average of the stable plateau in laser-Doppler flux during 28 mM sodium nitroprusside infusion and local heating to 43°C divided by mean arterial pressure. Student's unpaired t-tests were used to determine significant differences between the groups for physical characteristics. Two-way repeated-measures ANOVA was conducted to detect 1) differences due to chronic low-dose therapy and pharmacological treatment on CVCmax; and 2) differences due to chronic aspirin therapy on the change (Δ) in %CVCmax between the control and the NOS-inhibited sites for every 0.1°C rise in Tor. A mixed-model three-way repeated-measures ANOVA was conducted to detect differences in %CVCmax between subject groups (chronic low-dose aspirin or age-matched control) at the pharmacological treatment sites within subject groups (control and l-NAME) over the rise in Tor (SAS, version 9.1). Tukey post hoc tests were performed when appropriate to determine where differences between groups and drug treatments occurred. The level of significance was set at α = 0.05. Values are presented as means ± SE.
RESULTS
Subject characteristics are presented in Table 1. Subject groups were well matched for body size, total cholesterol, high-density lipoprotein, low-density lipoprotein cholesterol, and blood pressure (all P > 0.05). Likewise, there was no difference in baseline Tor between the groups (P = 0.73).
Table 1.
Subject characteristics
| Age-Matched Control | Low-Dose Aspirin | |
|---|---|---|
| Sex (male, female) | 4, 3 | 3, 4 |
| Age, yr | 55±3 | 57±3 |
| BMI, kg/m2 | 24.0±1.0 | 26.4±1.6 |
| Total cholesterol, mg/dl | 183±11 | 195±9 |
| HDL, mg/dl | 60±6 | 65±5 |
| LDL, mg/dl | 107±8 | 112±9 |
| Fasting blood glucose, mg/dl | 91±3 | 95±5 |
| SBP, mmHg | 114±3 | 113±4 |
| DBP, mmHg | 77±1 | 74±3 |
| MAP, mmHg | 89±2 | 88±3 |
| Baseline Tor, °C | 36.3±0.1 | 36.3±0.1 |
Values are means ± SE. BMI, body mass index; HDL, high-density lipoprotein; LDL, low-density lipoprotein; SBP, systolic blood pressure; DBP, diastolic blood pressure; MAP mean arterial pressure; Tor, oral temperature.
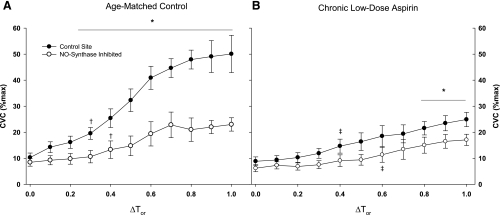
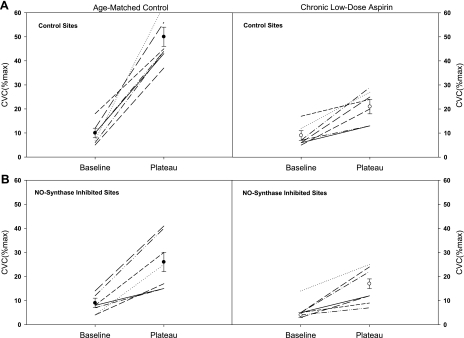
Group mean %CVCmax responses across the rise in body core temperature are presented in Fig. 1. In the nonaspirin group, control and NOS-inhibited sites were first significantly increased from baseline at ΔTor 0.3°C and 0.4°C, respectively (Fig. 1A). Furthermore, NOS inhibition significantly reduced %CVCmax compared with control sites at all ΔTor ≥ 0.3°C. In the aspirin group, control and NOS-inhibited sites were significantly increased from baseline at ΔTor 0.4°C and 0.6°C, respectively (Fig. 1B). NOS inhibition reduced %CVCmax compared with the control sites only when body core temperature was significantly elevated, ΔTor ≥ 0.8°C. The aspirin group had significantly attenuated %CVCmax at the control sites compared with the age-matched control group (P < 0.001), with increases in ΔTor ≥ 0.2°C. Individual and mean responses for baseline and the plateau in skin blood flow with an increase in Tor of 1.0°C are illustrated in Fig. 2. %CVCmax at the control sites were significantly attenuated in the aspirin group. NOS inhibition significantly decreased %CVCmax in the control subjects, but only minimally decreased %CVCmax in the chronic low-dose aspirin subjects.
Fig. 1.
Group mean ± SE cutaneous vascular conductance (CVC) as a percentage of maximal response during passive whole body heating. The control sites (•) and nitric oxide (NO) synthase-inhibited site (○) are shown in the age-matched control group (A) and chronic low-dose aspirin group (B). Percent maximal CVC (%CVCmax) was significantly attenuated in the chronic low-dose aspirin group. NO synthase inhibition significantly reduced %CVCmax in the age-matched control group with change in oral temperature (ΔTor) = 0.2°C, but only at significantly greater increases in Tor in the chronic aspirin therapy group: ΔTor = 0.8°C. *P < 0.05, significant difference vs. the control site within subject groups. †P < 0.05, beginning of significant difference from baseline within site in the age-matched control group. ‡P < 0.05, beginning of significant difference from baseline within site in the aspirin treatment group.
Fig. 2.
Individual and mean subject responses at baseline and at the plateau in %CVCmax. A: control sites; B: NO synthase inhibited sites. Left: data from the age-matched control subjects; right: chronic low-dose aspirin subject data. Subjects taking chronic low-dose aspirin therapy displayed a significantly attenuated increase in skin blood flow during hyperthermia in both the control sites and the NG-nitro-l-arginine methyl ester treated sites.
There were no significant effects of either chronic low-dose aspirin therapy (no aspirin: 1.7 ± 0.2 vs. aspirin: 1.9 ± 0.4 flux/mmHg; P = 0.72) or NOS inhibition (no aspirin: 1.8 ± 0.4 vs. aspirin: 1.5 ± 0.2 flux/mmHg; P = 0.22) on CVCmax.
DISCUSSION
The principal findings of this study include the important first observation that reflex cutaneous vasodilation is substantially attenuated in healthy humans voluntarily taking chronic low-dose aspirin for the primary prevention of atherothrombotic disease. Although baseline core temperature was similar between the groups, greater increases in core temperature were required to significantly increase skin blood flow from baseline in subjects taking low-dose aspirin. This decreased response is due in part to reduced NO-dependent vasodilation. While the precise mechanism(s) mediating this response remains unresolved, several possibilities exist. Because chronic low-dose aspirin (81 mg) inhibits COX-1 in platelets (28), these data may suggest that platelets may be activated during hyperthermia and release vasodilatory substances that increase skin blood flow. Alternatively, low-dose aspirin may have inhibited COX in platelets and in the cutaneous microvasculature, resulting in reduced cutaneous vasodilation. Finally, decreased blood viscosity resulting from platelet inhibition may decrease the shear stimulus on the cutaneous microvasculature during hyperthermia, resulting in the observed attenuated vasodilation.
In young, healthy humans, COX-dependent vasodilatory pathways contribute to reflex cutaneous vasodilation (22). To date, only non-isoform-specific COX inhibitors (ketorolac and aspirin) have been used to examine the contribution of COX-dependent mechanisms to reflex vasodilation, using localized administration of these inhibitors (intradermal microdialysis) to examine these mechanisms. Administration of these inhibitors at the concentrations used in previous studies is nonselective for COX isoform and location, i.e., platelet vs. vascular, making it difficult to delineate the precise COX isoform and cellular location synthesizing vasodilators during hyperthermia.
While aspirin is also a nonspecific COX inhibitor, the beneficial antithombotic effects of low-dose aspirin therapy are associated with its ability to alter the ratio of the production of TXA2 and PGI2 (21). Aspirin irreversibly acetylates platelet COX-1 in the presystemic (portal) circulation (30), thereby inhibiting platelet production of TXA2 for the life of the platelet (∼10 days). Thus it has been suggested that low-dose chronic (>5–6 days) aspirin therapy preferentially inhibits platelet COX-1-dependent TXA2 synthesis without affecting endothelial COX-dependent PGI2 synthesis. Much higher doses of aspirin (600 mg) are required to fully but acutely inhibit COX in the microvasculature (9, 27).
In contrast, low-dose enteric coated aspirin reaches peak plasma concentrations within 3–4 h after ingestion and has a half-life of 15 min (3, 27, 28). In the present study, subjects took their last dose of aspirin at least 16 h before the experiment to avoid potential acute inhibition of endothelial COX. Given the pharmacokinetics of enteric-coated, low-dose aspirin, it is unlikely that vascular COX was significantly inhibited. Taken together, these data support a role for platelet involvement in reflex vasodilation.
One interesting finding from the present study was that NO-dependent vasodilation was attenuated in subjects taking chronic low-dose aspirin (Fig. 2). It has been suggested that the NOS and COX vasodilatory pathways are independent of one another because the combined inhibition of both enzymes results in an additive reduction in total cutaneous vasodilation compared with NOS and COX inhibition alone (22). Our data would suggest that there may be cross talk between these two pathways, because chronic COX inhibition with aspirin resulted in a blunted relative NO contribution to reflex heating. There are several possible mechanisms for this finding, including 1) an aspirin-induced reduction cholinergic active vasodilator or vascular sensitivity mechanisms; 2) a decrease in platelet-released vasodilatory substances, which may induce vasodilation through NO-dependent mechanisms; and 3) a reduction in shear stress-mediated vasodilation.
One potential explanation for the severe attenuation in reflex vasodilation that we observed in subjects taking low-dose aspirin is that platelets may release vasodilatory substances that contribute to the increase in skin blood flow during hyperthermia. Platelets are known to release a variety of vasodilator substances, including serotonin and ADP (8, 16, 26). In vitro evidence suggests that vasodilation is attenuated after treatment with platelets from diabetic humans through impairments in the ATP/ADP pathway (26). In contrast to platelet inhibition with low-dose aspirin, specific inhibition with the platelet ADP receptor inhibitor clopidogrel does not alter sensory neurogenic vasodilation in human skin (32). Nonetheless, platelet-mediated vasodilation through serotonin and/or the ADP/ATP pathway during reflex heating remains a putative mechanism for the increase in skin that requires further exploration.
For a given rise in body core temperature, skin blood flow was significantly reduced with chronic low-dose aspirin therapy. This reduction in skin blood flow may be through lower endothelial shear stress. Although NO has not been found to cause cutaneous vasodilation after exposure to shear stress (37, 39), there is some indication that shear stress induces vasodilation through an interaction between NO and COX pathway (23). Also, platelet inhibition with aspirin therapy decreases blood viscosity, which further reduces the shear stimulus on the endothelium. These data suggest that platelet and vascular COX inhibition with aspirin may attenuate any shear-mediated cutaneous vasodilation during whole body heating.
Finally, COX-derived products can alter central hypothalamic thermoregulatory mechanisms, and we cannot discount the possibility that chronic systemic administration of low-dose aspirin may have altered central mechanisms. However, in the nonfebrile state, the administration of aspirin (50 mg/kg) (4) or other nonsteroidal anti-inflammatory drugs (800 mg ibuprofen) (6) has not been shown to alter the reflex vasodilatory responses as assessed by the thresholds for reflex vasodilation. In the present study, vasodilation was significantly attenuated in the aspirin therapy group, but baseline body temperature was similar between groups. Additional research assessing sweat output in conjunction with skin blood flow measurements is necessary to determine whether there is a decreased sensitivity of the sweating response, which would suggest that chronic low-dose aspirin may be altering central thermoregulatory mechanisms.
Limitations.
Subjects tested in this study were voluntarily taking 81 mg of aspirin daily for the primary prevention of atherothombotic events. The subjects were otherwise healthy, did not have a significant family history of cardiovascular disease (first-degree relative), and did not have any underlying cardiovascular risk/disease detectable with a maximal graded exercise test or blood chemistry analysis (Table 1). While we cannot exclude the possibility that these subjects may have had other unreported risk factors for vascular disease that lead them to engage in a low-dose aspirin regimen, it seems unlikely that underlying disease could explain the significantly attenuated skin blood flow responses observed in this study.
The 81-mg over-the-counter dose has become the standard therapy, proven as an effective antithrombotic agent in the prevention of myocardial infarction and stroke (28). In this study, we did not titrate the dose of aspirin based on body weight or blood volume: subjects were simply taking the over-the-counter dose based on availability and recommendation from their physician. It has been suggested that doses as low as 30 mg/day may be as effective as the 75-mg dose (29). It is possible that, by using the standard, commercially available 81-mg/dose, we observed significant COX inhibition in the vasculature and in the platelets not observed with lower doses. However, we did observe a significant attenuation in the skin blood flow response in otherwise healthy middle-aged subjects taking this commercially available dose of aspirin. Because primary human aging is also associated with attenuated reflex vasodilation, the combined effects of aging and chronic aspirin therapy may have significant thermoregulatory consequences.
In summary, we found that, in middle-aged men and women taking chronic low-dose aspirin, reflex cutaneous vasodilation is severely attenuated, due, in part, to reduced NO-dependent vasodilation. These data suggest that 1) platelets and/or platelet vessel wall signaling interactions may be involved in reflex vasodilation; 2) chronic low-dose aspirin may inhibit vascular COX, decreasing the synthesis of key vasodilators involved in reflex cutaneous vasodilation; and/or 3) alteration in blood viscosity due to aspirin therapy may decrease the shear stimulus on the cutaneous blood vessels, resulting in attenuated reflex vasodilation.
GRANTS
This research was supported by National Institutes of Health (NIH) Grant R01-AG-07004-17 (W. L. Kenney), American Heart Association Predoctoral Fellowship 0515392U (L. A. Holowatz), the American College of Sports Medicine Carl V. Gisolfi Memorial Student Research Grant (L. A. Holowatz), a Penn State Department of Kinesiology Dissertation Award, and NIH Grant M01-RR-10732 (General Clinical Research Center).
Acknowledgments
We are grateful for the intellectual, technical, and data collection assistance of Caitlin Thompson-Torgerson, Jane Pierzga, James Lang, David DeGroot, and John Jennings.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Bennett LA, Johnson JM, Stephens DP, Saad AR, Kellogg DL Jr. Evidence for a role for vasoactive intestinal peptide in active vasodilatation in the cutaneous vasculature of humans. J Physiol 552: 223–232, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bochner F, Williams DB, Morris PM, Siebert DM, Lloyd JV. Pharmacokinetics of low-dose oral modified release, soluble and intravenous aspirin in man, and effects on platelet function. Eur J Clin Pharmacol 35: 287–294, 1988. [DOI] [PubMed] [Google Scholar]
- 3.Bode-Boger SM, Boger RH, Schubert M, Frolich JC. Effects of very low dose and enteric-coated acetylsalicylic acid on prostacyclin and thromboxane formation and on bleeding time in healthy subjects. Eur J Clin Pharmacol 54: 707–714, 1998. [DOI] [PubMed] [Google Scholar]
- 4.Brooks-Asplund EM, Cannon JG, Kenney WL. Influence of hormone replacement therapy and aspirin on temperature regulation in postmenopausal women. Am J Physiol Regul Integr Comp Physiol 279: R839–R848, 2000. [DOI] [PubMed] [Google Scholar]
- 5.Cerletti C, Dell'Elba G, Manarini S, Pecce R, Di Castelnuovo A, Scorpiglione N, Feliziani V, de Gaetano G. Pharmacokinetic and pharmacodynamic differences between two low dosages of aspirin may affect therapeutic outcomes. Clin Pharmacokinet 42: 1059–1070, 2003. [DOI] [PubMed] [Google Scholar]
- 6.Charkoudian N, Johnson JM. Altered reflex control of cutaneous circulation by female sex steroids is independent of prostaglandins. Am J Physiol Heart Circ Physiol 276: H1634–H1640, 1999. [DOI] [PubMed] [Google Scholar]
- 7.Flavahan NA Balancing prostanoid activity in the human vascular system. Trends Pharmacol Sci 28: 106–110, 2007. [DOI] [PubMed] [Google Scholar]
- 8.Forstermann U, Mugge A, Bode SM, Frolich JC. Response of human coronary arteries to aggregating platelets: importance of endothelium-derived relaxing factor and prostanoids. Circ Res 63: 306–312, 1988. [DOI] [PubMed] [Google Scholar]
- 9.Heavey DJ, Barrow SE, Hickling NE, Ritter JM. Aspirin causes short-lived inhibition of bradykinin-stimulated prostacyclin production in man. Nature 318: 186–188, 1985. [DOI] [PubMed] [Google Scholar]
- 10.Holowatz LA, Houghton BL, Wong BJ, Wilkins BW, Harding AW, Kenney WL, Minson CT. Nitric oxide and attenuated reflex cutaneous vasodilation in aged skin. Am J Physiol Heart Circ Physiol 284: H1662–H1667, 2003. [DOI] [PubMed] [Google Scholar]
- 11.Holowatz LA, Kenney WL. Local ascorbate administration augments NO- and non-NO-dependent reflex cutaneous vasodilation in hypertensive humans. Am J Physiol Heart Circ Physiol 293: H1090–H1096, 2007. [DOI] [PubMed] [Google Scholar]
- 12.Holowatz LA, Kenney WL. Up-regulation of arginase activity contributes to attenuated reflex cutaneous vasodilatation in hypertensive humans. J Physiol 581: 863–872, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Holowatz LA, Thompson-Torgerson CS, Kenney WL. Altered mechanisms of vasodilation in aged human skin. Exerc Sport Sci Rev 35: 119–125, 2007. [DOI] [PubMed] [Google Scholar]
- 14.Holowatz LA, Thompson CS, Kenney WL. Acute ascorbate supplementation alone or combined with arginase inhibition augments reflex cutaneous vasodilation in aged human skin. Am J Physiol Heart Circ Physiol 291: H2965–H2970, 2006. [DOI] [PubMed] [Google Scholar]
- 15.Holowatz LA, Thompson CS, Minson CT, Kenney WL. Mechanisms of acetylcholine-mediated vasodilatation in young and aged human skin. J Physiol 563: 965–973, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kaul S, Padgett RC, Waack BJ, Brooks RM, Heistad DD. Effect of atherosclerosis on responses of the perfused rabbit carotid artery to human platelets. Arterioscler Thromb 12: 1206–1213, 1992. [DOI] [PubMed] [Google Scholar]
- 17.Kellogg DL Jr, Crandall CG, Liu Y, Charkoudian N, Johnson JM. Nitric oxide and cutaneous active vasodilation during heat stress in humans. J Appl Physiol 85: 824–829, 1998. [DOI] [PubMed] [Google Scholar]
- 18.Kellogg DL Jr, Liu Y, Kosiba IF, O'Donnell D. Role of nitric oxide in the vascular effects of local warming of the skin in humans. J Appl Physiol 86: 1185–1190, 1999. [DOI] [PubMed] [Google Scholar]
- 19.Kellogg DL Jr, Pergola PE, Piest KL, Kosiba WA, Crandall CG, Grossmann M, Johnson JM. Cutaneous active vasodilation in humans is mediated by cholinergic nerve cotransmission. Circ Res 77: 1222–1228, 1995. [DOI] [PubMed] [Google Scholar]
- 20.Kyrle PA, Eichler HG, Jager U, Lechner K. Inhibition of prostacyclin and thromboxane A2 generation by low-dose aspirin at the site of plug formation in man in vivo. Circulation 75: 1025–1029, 1987. [DOI] [PubMed] [Google Scholar]
- 21.Majerus PW Arachidonate metabolism in vascular disorders. J Clin Invest 72: 1521–1525, 1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McCord GR, Cracowski JL, Minson CT. Prostanoids contribute to cutaneous active vasodilation in humans. Am J Physiol Regul Integr Comp Physiol 291: R596–R602, 2006. [DOI] [PubMed] [Google Scholar]
- 23.Medow MS, Taneja I, Stewart JM. Cyclooxygenase and nitric oxide synthase dependence of cutaneous reactive hyperemia in humans. Am J Physiol Heart Circ Physiol 293: H425–H432, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Minson CT, Berry LT, Joyner MJ. Nitric oxide and neurally mediated regulation of skin blood flow during local heating. J Appl Physiol 91: 1619–1626, 2001. [DOI] [PubMed] [Google Scholar]
- 25.Minson CT, Holowatz LA, Wong BJ, Kenney WL, Wilkins BW. Decreased nitric oxide- and axon reflex-mediated cutaneous vasodilation with age during local heating. J Appl Physiol 93: 1644–1649, 2002. [DOI] [PubMed] [Google Scholar]
- 26.Oskarsson HJ, Hofmeyer TG. Platelets from patients with diabetes mellitus have impaired ability to mediate vasodilation. J Am Coll Cardiol 27: 1464–1470, 1996. [DOI] [PubMed] [Google Scholar]
- 27.Patrono C, Ciabattoni G, Patrignani P, Pugliese F, Filabozzi P, Catella F, Davi G, Forni L. Clinical pharmacology of platelet cyclooxygenase inhibition. Circulation 72: 1177–1184, 1985. [DOI] [PubMed] [Google Scholar]
- 28.Patrono C, Coller B, Dalen JE, FitzGerald GA, Fuster V, Gent M, Hirsh J, Roth G. Platelet-active drugs: the relationships among dose, effectiveness, and side effects. Chest 119: 39S–63S, 2001. [DOI] [PubMed] [Google Scholar]
- 29.Patrono C, Rocca B. Aspirin: promise and resistance in the new millennium. Arterioscler Thromb 28: s25–s32, 2008. [DOI] [PubMed] [Google Scholar]
- 30.Pedersen AK, FitzGerald GA. Dose-related kinetics of aspirin. Presystemic acetylation of platelet cyclooxygenase. N Engl J Med 311: 1206–1211, 1984. [DOI] [PubMed] [Google Scholar]
- 31.Roddie IC, Shepherd JT, Whelan RF. The contribution of constrictor and dilator nerves to the skin vasodilatation during body heating. J Physiol 136: 489–497, 1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rousseau P, Tartas M, Fromy B, Godon A, Custaud MA, Saumet JL, Abraham P. Platelet inhibition by low-dose aspirin but not by clopidogrel reduces the axon-reflex current-induced vasodilation in humans. Am J Physiol Regul Integr Comp Physiol 294: R1420–R1426, 2008. [DOI] [PubMed] [Google Scholar]
- 33.Shastry S, Dietz NM, Halliwill JR, Reed AS, Joyner MJ. Effects of nitric oxide synthase inhibition on cutaneous vasodilation during body heating in humans. J Appl Physiol 85: 830–834, 1998. [DOI] [PubMed] [Google Scholar]
- 34.Taylor WF, Johnson JM, O'Leary D, Park MK. Effect of high local temperature on reflex cutaneous vasodilation. J Appl Physiol 57: 191–196, 1984. [DOI] [PubMed] [Google Scholar]
- 35.Tuleja E, Mejza F, Cmiel A, Szczeklik A. Effects of cyclooxygenases inhibitors on vasoactive prostanoids and thrombin generation at the site of microvascular injury in healthy men. Arterioscler Thromb 23: 1111–1115, 2003. [DOI] [PubMed] [Google Scholar]
- 36.Wong BJ, Minson CT. Neurokinin-1 receptor desensitisation attenuates cutaneous active vasodilatation in humans. J Physiol 577: 1043–1051, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wong BJ, Wilkins BW, Holowatz LA, Minson CT. Nitric oxide synthase inhibition does not alter the reactive hyperemic response in the cutaneous circulation. J Appl Physiol 95: 504–510, 2003. [DOI] [PubMed] [Google Scholar]
- 38.Wong BJ, Wilkins BW, Minson CT. H1 but not H2 histamine receptor activation contributes to the rise in skin blood flow during whole body heating in humans. J Physiol 560: 941–948, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhao JL, Pergola PE, Roman LJ, Kellogg DL Jr. Bioactive nitric oxide concentration does not increase during reactive hyperemia in human skin. J Appl Physiol 96: 628–632, 2004. [DOI] [PubMed] [Google Scholar]