Abstract
We have shown that electroacupuncture (EA) at P 5–6 (overlying median nerves) activates arcuate (ARC) neurons, which excite the ventrolateral periaqueductal gray (vlPAG) and inhibit cardiovascular sympathoexcitatory neurons in the rostral ventrolateral medulla (rVLM). To investigate whether the ARC inhibits rVLM activity directly or indirectly, we stimulated the splanchnic nerve to activate rVLM neurons. Micropipettes were inserted in the rVLM, vlPAG, and ARC for neural recording or injection. Microinjection of kainic acid (KA; 1 mM, 50 nl) in the ARC blocked EA inhibition of the splanchnic nerve stimulation-induced reflex increases in rVLM neuronal activity. Microinjection of d,l-homocysteic acid (4 nM, 50 nl) in the ARC, like EA, inhibited reflex increases in the rVLM neuronal discharge. The vlPAG neurons receive convergent input from the ARC, splanchnic nerve, P 5–6, and other acupoints. Microinjection of KA bilaterally into the rostral vlPAG partially reversed rVLM neuronal responses and cardiovascular inhibition during d,l-homocysteic acid stimulation of the ARC. On the other hand, injection of KA into the caudal vlPAG completely reversed these responses. We also observed that ARC neurons could be antidromically activated by stimulating the rVLM, and that ARC perikarya was labeled with retrograde tracer that had been microinjected into the rVLM. These neurons frequently contained β-endorphin and c-Fos, activated by EA stimulation. Therefore, the vlPAG, particularly, the caudal vlPAG, is required for ARC inhibition of rVLM neuronal activation and subsequent EA-related cardiovascular activation. Direct projections from the ARC to the rVLM, which serve as an important source of β-endorphin, appear also to exist.
Keywords: arcuate nucleus; ventrolateral periaqueductal gray; rostral ventrolateral medulla; d,l-homocysteic acid; kainic acid
cardiovascular disease is the greatest cause of death in middle-aged and elderly North Americans and Europeans. Although western science has developed a number of effective treatment strategies for this disease, treatment is not perfect and often is associated with side effects. As such, there has been increasing interest from the western countries in exploring alternative medicinal treatments and considering new therapies, such as acupuncture, for cardiovascular disease. According to the World Health Organization, acupuncture is effective in more than 40 medical conditions, including cardiac pain and hypertension (24). Experimental studies have shown that low-current, low-frequency electroacupuncture (EA) employed over deep nerves, like P 5–6 (pericardial meridian) overlying the median nerve, effectively inhibits cardiovascular sympathoexcitatory reflexes and the rostral ventrolateral medulla (rVLM) presympathetic neuronal responses. Conversely, electrical stimulation of acupoints in regions that do not overlie major somatic pathway or simple insertion of a needle without manual or electrical stimulation evokes little or no acupuncture-cardiovascular response (35). Thus there are clear point-specific cardiovascular responses during and after EA stimulation. The arcuate (ARC) nucleus, which serves as an important source of opioid peptides in the hypothalamus (9, 27), is thought to participate in the analgesic effect of acupuncture (33, 34). Additionally, previous investigation (13) has shown that surgical elimination or electrolytic lesions in the ARC abolish EA inhibition of the defensive pressor response, suggesting that the ARC nucleus may be essential for EA inhibition of cardiovascular function. These studies, however, have not distinguished between axons from other cells passing through the ARC region and the perikarya of ARC neurons. Somatic afferent stimulation during EA activates neurons in the ARC and ventrolateral periaqueductal gray (vlPAG) and inhibits activity in the rVLM (12–14, 18, 32). We have suggested that a long-loop pathway involving the ARC and vlPAG modulates cardiovascular sympathoexcitatory bulbospinal neurons in the rVLM during EA (30). In this regard, our recent work has shown that EA along the pericardial meridian located on both forelimbs at P 5–6 acupoints activates ARC neurons in the ventral hypothalamus, which, in turn, provide excitatory projections to vlPAG (18). In other experiments, we demonstrated that the vlPAG inhibits activity of cardiovascular sympathoexcitatory bulbospinal neurons in the rVLM, as well as increased sympathetic outflow in response to excitatory visceral reflexes. These data strongly suggest the existence of an ARC-vlPAG-rVLM neuronal pathway that serves as part of a long-loop pathway participating in EA inhibition of visceral excitatory cardiovascular reflexes (32). By inference, we have suggested that the ARC nucleus regulates rVLM activity. However, this possibility has not been proven unequivocally. Furthermore, these data do not distinguish between poly- (e.g., through vlPAG) and monosynaptic pathways that link the ARC to the rVLM in EA modulation of sympathetic outflow and blood pressure regulation. In this regard, previous anterograde tracing studies have suggested that putative opiocortin neurons in the ARC may influence a descending system, including the nuclei raphe and the adjacent reticularis gigantocellularis pars alpha located medial to the rVLM (27). However, there is no evidence demonstrating direct projections between the ARC and the rVLM that participate in modulation of cardiovascular function.
The aim of the present study, therefore, was to investigate the functional roles of direct and indirect projections from the ARC to the rVLM that participate in the long-loop EA inhibitory effect of reflex rVLM neuronal activation and ultimately cardiovascular sympathoexcitation. We hypothesized that direct and indirect pathways between these two nuclei exist and that both might serve important roles in regulation of blood pressure by EA. A preliminary study of this work has been published (19).
METHODS AND MATERIALS
Surgical Preparation
The experimental preparations and protocols for this study were reviewed and approved by the Animal Care and Use Committee of the University of California, Irvine, CA. The studies conformed to the American Physiological Society guidelines and principles for research involving animals. Adult cats of either sex (2.1–3.6 kg) were anesthetized by intramuscular injection of ketamine (40 mg/kg) followed by intravenous α-chloralose (50 mg/kg). Additional α-chloralose (5 mg/kg) was given to maintain an adequate depth of anesthesia, as assessed by the lack of a response to noxious toe pinch, a respiratory pattern that followed the ventilator (i.e., not overbreathing), and a stable blood pressure. The trachea was intubated, and respiration was maintained artificially (model 66, Harvard Apparatus, South Natick, MA). Gallamine triethiodide (4 mg/kg) was administered intravenously before neural activity was recorded to avoid muscle movement during stimulation of the somatic nerves. Arterial blood gases and pH were measured periodically in all animals with a blood-gas analyzer (model ABL3, Radiometer, Copenhagen, Denmark). Arterial Po2 and Pco2 were kept within normal limits (CO2 30–35 mmHg; Po2 >100 Torr) by enriching the inspired O2 supply and adjusting the ventilation rate or volume. Arterial pH was maintained between 7.35 and 7.43 and corrected, as necessary, by administering 8% sodium bicarbonate. Body temperature was monitored with a rectal probe connected to a thermistor (model 44TD, Yellow Springs Instrument, Yellow Springs, OH) and was maintained within the range of 36–38°C by a water heating pad and a heating lamp.
The left femoral vein was cannulated for administration of drugs and fluids. Systemic arterial blood pressure was monitored with a strain-gauge pressure transducer (model 1290, Hewlett-Packard, Waltham, MA) attached to a cannula inserted in the left femoral artery.
A laparotomy provided exposure of the gallbladder or isolation of the splanchnic nerve. The splanchnic nerve was placed on a bipolar stimulating electrode connected to an isolation unit and a stimulator (Grass, model S88). Hypoxy dental glue (Pentron, Wallington, CT) was used to isolate the electrodes and hold the intact nerve in place. The abdominal wall was closed with clips to maintain moisture in the abdominal cavity and to prevent heat loss. The neural axis of the cat was stabilized with a stereotaxic head frame (Kopf). A dorsal craniotomy was performed to expose the ARC, vlPAG, and rVLM for recording extracellular activity and for bilateral microinjection of agonists, antagonists, or vehicle controls.
During renal sympathetic nerve recordings, an incision was made in the left flank region of the cat, and the retroperitoneal renal sympathetic nerve was exposed. A dissecting microscope (Zeiss) was used to isolate a branch of the renal nerve from connective tissue. The nerve was covered with warm mineral oil and placed across one pole of the recording electrode. The other pole of the recording electrode was grounded with a saline presoaked cotton thread to the animal.
Stimulation, Recording, and Microinjection Methods
The splanchnic nerve was stimulated with a current of 0.4–0.6 mA, using a pulse width of 0.5 ms at 2 Hz with a Grass stimulator (model S88K), which was sufficient to induce a reflex increase in blood pressure. We also briefly stimulated the ARC, P 5–6, and several other acupoints to examine for evidence of convergent input in neurons located in the rostral (A 0–3) vlPAG. Electrical stimulation was applied bilaterally using pulses of 1–4 mA, 0.5-ms duration, and 2 Hz at the P 5–6, LI 4–11, LI 6–7, H 5–7, St 36–37, Sp 6–9, K 1-B 37, or G 37–39 acupoints (Table 1). We further evaluated the responses of these vlPAG neurons during 30 min of EA at P 5–6. Our laboratory has demonstrated previously that EA at P 5–6 stimulates the median nerve and modulates sympathoexcitatory cardiovascular responses (7, 15–17, 30, 31). Using the atlas of Bureš et al. (4) as a guide, glass pipettes with platinum recording wires were positioned perpendicularly to the cortex (0.7–1.0 mm lateral on either side of the midline, 0–3 mm and 9.5–11.5 mm rostral to the tentorium) and were lowered 22 and 28 mm from the dorsal surface of the midbrain and hypothalamus to access the vlPAG and ARC, respectively. Stainless steel tubes (guide tubes 0.8 mm and injection tubes 0.4 mm in diameter) for microinjection were inserted into the ARC and rostral vlPAG using the same coordinates as the recording electrodes. In another group of animals, guide tubes were positioned using a 77° angle from the dorsal surface, 5 mm anterior to the tentorium to approach the caudal vlPAG. The locations of both nuclei were identified preliminarily by microinjecting 50 nl of 4 nM d,l-homocysteic acid (DLH) to evoke small but reproducible decreases in blood pressure of 5–10 mmHg. DLH is a glutamate receptor agonist. Our laboratory and others (18, 22, 32) have used it to depolarize cell bodies in various regions of the brain, like the ARC, to demonstrate their actions on other distant nuclei, such as the vlPAG. Kainic acid (KA) also is a glutamate receptor agonist, which depolarizes neurons to an extent that causes depolarization blockade, thereby deactivating these cells for a short period of time. If KA is used in higher concentrations, it can cause cell death (10, 32). However, in the concentrations used in the present study, KA causes reversible inhibition of neurotransmission of cell bodies, but not axons of passage, hence making it a good agent to temporarily interrupt communication between nuclei (ARC to vlPAG). Recording electrodes in the medulla were positioned perpendicularly to the dorsal surface of the fourth ventricle of the medulla (2.5–4.2 mm lateral to the midline and 2.4–4.4 mm rostral to the obex) and were lowered 5–5.5 mm from the surface to reach the rVLM. The location of the rVLM was identified tentatively with stimulating electrodes (0.2–0.4 mA) to elicit small, 5–10 mmHg, increases in blood pressure. Single-unit extracellular activity in the ARC or rVLM was recorded with a single-barrel glass pipette containing 0.5 M sodium acetate and 2% Chicago sky blue (Sigma Chemical, St. Louis, MO). Evoked activity of ARC and rVLM neurons was recorded during stimulation of the splanchnic nerve and P 5–6 acupoints.
Table 1.
Locations and innervation of acupoints
| Acupoints | Meridians | Locations | Underlying Innervation |
|---|---|---|---|
| P 5–6 | Pericardium | Proximal to accessory carpel pad of forelimb, between ligaments of flexor carpi radialis and palmaris longus | Median nerve |
| LI 4–11 | Large intestine | LI 4: On dorsal aspect of front paw, between 1st and 2nd metacarpal bones; LI 11: On elbow between extensor carpi radialis longus and brevis | LI 4: Branch of median nerve; LI 11: Deep radial nerve |
| LI 6–7 | Large intestine | Radial side of dorsal surface on lower one-third of forelimb, between abductor pollicus longus, extensor pollicis brevis and longus | Superficial radial nerve |
| H 5–7 | Heart | Proximal to the ulnar end of wrist | Ulnar nerve |
| St 36–37 | Stomach | Anterolateral side of hindlimb, near anterior crest of tibia below knee, underneath tibialis anterior muscle | Deep peroneal nerve |
| Sp 6–9 | Spleen | Sp 6: Medial side of hindleg, above tip of medial malleolus, posterior to medial border of tibia; Sp 9: posterior and inferior to medial condyle of tibia | Tibial nerve |
| K 1-B 37 | Kidney and bladder | K1: Center of metatarsal pad of hindlimb; B 37: Lateral side digit 5, above claw | Branches of tibial nerve |
| St 2-G 2 | Stomach and gallbladder | Both on face. St 2: in depression of infraorbital foramen; G2: anterior to intertragic notch | Branches of trigeminal and facial nerves |
| G 37–39 | Gallbladder | Lateral side of hindleg, above tip of external malleolus, on anterior border of fibula | Superficial peroneal nerve |
See Ref. 26.
We used peristimulus time histograms to assess evoked responses to stimulation of the splanchnic and median nerves and to evaluate the influence of EA on ARC and rVLM neural activity. Action potentials were amplified with a preamplifier (Grass P511k, Grass Instrument, Quincy, MA) attached to a high-impedance probe (Grass P511k), filtered (0.3–10 kHz), and monitored with an oscilloscope (model TDS 360, Tektronix, Beaverton, OR). Potentials were analyzed both visually and with a SHMU 2006 (Shanghai Medical College of Fudan University, China) program to assess wave shape, spike height, and latency from the time of stimulation. Action potentials, blood pressure, and heart rate were digitized and analyzed offline with a Pentium IV computer.
We used collision testing to determine whether neurons from the ARC projected directly to the rVLM. To accomplish this, recording/stimulation electrodes were placed in the ARC and rVLM. The rVLM electrode was stimulated electrically (0.1–0.4 mA, 2 Hz, 0.5-ms pulses) to evoke a small, reproducible pressor response of 5–10 mmHg, which, along with anatomical evaluation after the experiment, confirmed its location. Neurograms were examined for evidence of collision of evoked antidromic spikes from the rVLM following either spontaneous or stimulus-induced orthodromic action potentials evoked by stimulating the median nerve or splanchnic nerve. Collision occurred within the critical interval (the latency plus the refractory period). Axonal conduction velocities of ARC nucleus neurons were estimated by dividing the distance between the recording electrodes in the ARC and stimulating electrodes in the rVLM by the antidromic latency. The criteria for antidromic response of the ARC neurons were a constant latency, an all or none evoked response, ability of the ARC recording to follow high-frequency stimulation of the rVLM, and a positive collision test (20, 32).
To further categorize the cardiovascular sympathetic neurons in the rVLM, responses of rVLM neurons were assessed by stimulating or unloading the baroreceptors following intravenous injection of 10 μg phenylephrine or 2.5 mg nitroglycerin, respectively.
A subgroup of rVLM neurons also was characterized by evaluating the relationship between rVLM discharge and renal sympathetic activity. To record renal nerve activity, a recording electrode was attached to a high-impedance probe (model P511k). The signal was amplified and processed through an audio amplifier. The amplified signal was monitored with an oscilloscope (model TDS 360, Tektronix, Beaverton, OR) and was processed with a Pentium IV computer for on- and offline analyses through an analog-to-digital converter four-channel data-acquisition system (SHMU). The electrical noise level in the neural recordings was determined by crushing the nerves at the end of the experiment. A window discriminator was set with a threshold just above the noise level so that only renal nerve discharge signals were counted. The relationship between neural activity, renal sympathetic nerve discharge, and blood pressure waves was assessed with time domain analysis using arterial pulse or spike-triggered averaging and with frequency domain analysis using coherence (1, 30, 32).
Microinjection of a Retrograde Tracer into rVLM
To anatomically examine for a direct projection between the ARC and rVLM that might be involved in EA-mediated inhibition, using stereotaxic positioning to guide placement of the tip of the injection pipette in the medulla in the region of the rVLM, we microinjected a retrograde tracer in eight rats (350–500 g). Of note, four of the eight rats were subjected to EA, while the other four rats received sham EA stimulation (see below for details). A mixture of ketamine-xylazine (80:12 mg/ml, Sigma) was used to induce (0.3–0.4 ml im) and maintain (0.1–0.2 ml im) anesthesia. Body temperature was monitored with a rectal probe and was maintained at 37°C. Heart rate and oxygen saturation were monitored using a pulse oximeter (Nonin Medical, Plymouth, MN). The rat was placed on a stereotaxic apparatus (David Kopf Instruments). A 1-in. incision was made to expose the skull. A burr hole (4 mm diameter) was made in the occipital bone, according to the following coordinates: 12.0–12.5 mm caudal from the bregma, 2.0–2.5 mm from the midline, and 8.5 mm deep from the dural surface (25). One hundred nanoliters of a retrogradely transported tracer, rhodamine-labeled fluorescent microspheres in suspension (0.04 μm, Molecular Probes, Eugene, OR), were injected into the rVLM through a glass micropipette. The wound was sutured shut. The microspheres were transported during the 10- to 12-day recovery and maintenance period.
Terminal procedures occurred 10–12 days after administration of the retrograde tracer. Rats were reanesthetized with the ketamine-xylazine, as described above. After tracheostomy and intubation, cannulation and monitoring for vital signs were similar to the procedures described above. Animals were stabilized for 4 h. EA or sham-operated controls for EA were conducted over a 30-min period, as described below. As noted in our laboratory's previous study (8), 90 min following termination of EA or the control procedure, rats were deeply anesthetized with a large dose of the ketamine-xylazine (0.5–0.7 ml im). Transcardial perfusion was performed using 500 ml of 0.9% saline solution followed by 500 ml of 4% paraformaldehyde. The hypothalamus and medulla oblongata were harvested and sliced into coronal sections with a cryostat microtome (Leica CM1850 Heidelberger Strasse, Nussloch, Germany). Sections were collected serially in cold cryoprotectant solution (6). The hypothalamus was sectioned (30 μm), and neurons were labeled with retrograde microsphere tracer and stained with β-endorphin and/or c-Fos visualized. Sections of the medulla oblongata (50 μm) were scanned to identify the sites of microinjection of the microsphere tracer.
Histochemistry and Immunohistochemistry
β-Endorphin immunohistochemical labeling.
After washing for 30 min (10 min × 3 times) in phosphate-buffered saline containing 0.3% Triton X-100 (PBST; pH = 7.4), hypothalamic sections were placed for 1 h in 1% normal donkey serum (Jackson Immunoresearch Laboratories, West Grove, PA). The sections were incubated with a primary rabbit polyclonal anti-β-endorphin antibody (1:400 dilutions, Chemicon International, Temecula, CA) at 4°C for 48 h. The tissues subsequently were rinsed three times (10 min for each rinse) in PBST and were incubated with fluorescein-conjugated donkey anti-rabbit antibody (1:100; Jackson Immunoresearch Laboratories) for 24 h at 4°C. Each section was mounted on a slide and was air dried. The slides were coverslipped using mounting medium (Vector Laboratories, Burlingame, CA). Immunohistochemical control studies were performed by omission of the primary or secondary antibodies and by preabsorption with excess (10 μg/ml) β-endorphin. No labeling was detected under these latter conditions.
Double-fluorescent immunohistochemical labeling for c-Fos + β-endorphin.
The staining procedures were similar to those used for single-fluorescent immunohistochemical labeling described above. Briefly, after treatment with PBST and 1% normal donkey serum, brain tissues were incubated with two primary antibodies, including a mouse monoclonal anti-c-Fos antibody (1:2,000 dilution, Santa Cruz Biotechnology) and a rabbit polyclonal anti-β-endorphin antibody (1:400, Chemicon International), for 48 h at 4°C. Sections then were incubated with fluorescein-conjugated donkey anti-rabbit antibody and coumarin-conjugated donkey anti-mouse antibody (all 1:100; Jackson Immunoresearch Laboratories) at 4°C for 24 h. The sections were mounted on slides and coverslipped with mounting medium (Vector Laboratories). In the immunohistochemical control studies, no stain was detected when the primary or secondary antibody was omitted.
Brain sections were scanned and examined with a standard fluorescent microscope (Nikon, E400, Melville, NY). Three epifluorescence filters (B-2A, G-2A, or UV-2A) equipped in a fluorescent microscope were used to identify single stains appearing as green (fluorescein), red (rhodamine), or blue (coumarin) in brain sections. Sections containing the ARC were identified according to their best matched standard stereotaxic plane, as shown in Paxinos and Watson's atlas for the rat (25).
After examination with a fluorescent microscope, selected sections were further evaluated with a laser scanning confocal microscope (Zeiss LSM 510, Meta System, Thornwood, NY) to confirm colocalization of two or three labels. This apparatus was equipped with Argon and HeNe lasers and allowed operation of multiple channels. Lasers of 488- and 543-nm wavelengths were used to excite fluorescein (green) and rhodamine (red), respectively. A 790-nm laser was applied for two-photon excitation of coumarin (blue). Digital fluorescent images were captured and analyzed with software (Zeiss LSM) provided with the confocal microscope. Each confocal section analyzed was limited to 0.5 μm thickness in the Z-plane. Images containing more than two colors in the same plane were merged to reveal the relationship between two labels (see Fig. 8). Single-, double-, and triple-labeled neurons were evaluated.
Experimental Protocols
ARC and vlPAG in rVLM inhibition by EA.
In this protocol, the splanchnic nerve was stimulated 30 times at a frequency of 2 Hz every 10 min. During EA, somatic stimulation was stopped transiently to evaluate the responses to splanchnic nerve stimulation, as we recorded information that allowed construction of the peristimulus time histogram. The evoked activity shown in bar histograms represents peak activity above the baseline firing rate.
ARC PROTOCOLS.
In eight time-control cats, the rVLM neural responses to repetitive splanchnic nerve stimulation were recorded every 10 min over a 100-min period. Medullary responses to 30 min of low-frequency EA also were evaluated in six other cats subjected to microinjection of the vehicle control immediately after EA. To determine the importance of the ARC in EA inhibition of the rVLM responses to splanchnic nerve stimulation in eight additional cats, we recorded poststimulus time histogram activity (30 stimuli, 2 Hz) before, during, and after 30 min of EA at P 5–6 acupoints, followed immediately by microinjection of 50 nl KA (1 mM) in the ARC.
VLPAG PROTOCOLS.
We recorded and later anatomically identified the locations of 34 neurons receiving convergent input from the ARC, splanchnic, and somatic afferents to determine their locations within the vlPAG. Their location served as an anatomical guide for microinjection of KA in the depolarization blockade protocol. In eight cats, rVLM activity was evaluated, while the splanchnic nerve was stimulated repetitively. As a surrogate for EA, after two-control splanchnic nerve stimulations, 50 nl of 4 nM DLH were injected in the ARC nucleus. To evaluate the role of the rostral vlPAG (A 0.1–3) in five animals and the caudal vlPAG (P 1-A 0) in four other animals, 50 nl KA (1 mM) were microinjected in the vlPAG, while 50 nl DLH (4 nM) were administered in the ARC to evoke rVLM responses. Four additional control animals were injected with normal saline (vehicle for KA) in the rostral and caudal vlPAG.
All rVLM neurons evaluated in this study were demonstrated to receive convergent input from the splanchnic, median, and baroreceptor nerves. A subgroup of six neurons was identified to be sympathoexcitatory by noting their close correlation with renal sympathetic nerve activity using spike-triggered averaging.
Collision testing was used in 14 cats to assess for evidence of direct projections from the ARC to the rVLM.
Hemodynamic protocol.
Pledgets of filter paper (1 cm2) soaked in a solution of bradykinin (BK; 10 μg/ml) applied to the gallbladder in five cats induced consistent reflex increases in blood pressure during repetitive gallbladder stimulation. Recovery periods of at least 10 min were provided between consecutive stimuli to prevent tachyphylaxis. In nine other animals, BK was applied 10 times to the gallbladder to induce repetitive increases in blood pressure over a period of 100 min. After the first two consecutive applications of BK, 50 nl DLH (4 nM) were microinjected in the ARC nucleus. KA (1 mM, 50 nl, in four cats) or saline (50 nl, in five other cats) was administered in the rostral or caudal vlPAG. Thus these protocols evaluated the magnitude of the gallbladder blood pressure reflex during excitation of the ARC following chemical inactivation of the cell bodies in the vlPAG.
C-Fos expression induced by EA.
Previously, our laboratory has shown a significant increase in c-Fos and in its colocalization with β-endorphin in the ARC of cats following EA stimulation at the P 5–6 acupoints, compared with sham-operated controls (9). In the present study, we sought to qualitatively identify c-Fos expression induced by EA in neurons containing both the retrograde tracer and β-endorphin. Four of eight rats were subjected to EA. Low-frequency EA (0.5-ms pulses, 2 Hz, 1–4 mA) at the P 5–6 acupoints was maintained for 30 min, as described in the above physiological protocols. The stimulation intensity was sufficient to produce moderate, repetitive paw flexion in each forelimb. In the other four rats, insertion of acupuncture needles into the P 5–6 acupoints without electrical stimulation served as sham controls for EA.
Verification of Injection and Recording Sites
Animals were euthanized with α-chloralose followed by intravenous saturated KCl at the end of each experiment. Recording sites were marked with 2% Chicago blue dye by either iontophoresis (5 min, 400 nA) or microinjection (0.1 μl). The hypothalamus, midbrain, and medulla were removed and fixed in 10% formalin for 4–7 days. Frozen serial 60 μm brain sections were cut with a freezing microtome (Leica CM 1850). Slices were stained with neural red and examined with a microscope (Nikon eclipse 6400) to identify recording, microinjection, and stimulation sites. These areas were reconstructed from the dye spots with New Bitmap Image plotted on coronal sections that were separated by 2 mm, with respect to the auditory line. Composite coronal caudal and rostral sections were composed from multiple tissue sections. Sections were scanned and then traced with Corel suite software. Nuclei were traced and superimposed with nuclear structures identified with the aid of Berman's atlas (2) for the medulla and the atlas of Bureš et al. (4) for the hypothalamus and midbrain of the cat.
Statistical Analysis
Values are presented as means ± SE. The assumption of normal data distribution was evaluated with the Kolmogorov-Smirnov test. Neural activity (spikes/30 stimuli) in response to splanchnic nerve stimulation before, during, and after EA, and after delivery of saline, DLH, or KA was assessed using a one-way repeated-measures analysis of variance, followed by the Holm-Sidak post hoc test. These tests represent a pairwise multiple-comparisons procedure. We utilized SigmaStat and SigmaPlot software (Jandel Scientific, San Rafael, CA) for statistical analysis and graphing. The 0.05 probability level was used to determine statistically significant differences.
RESULTS
Role of ARC in EA-rVLM Response
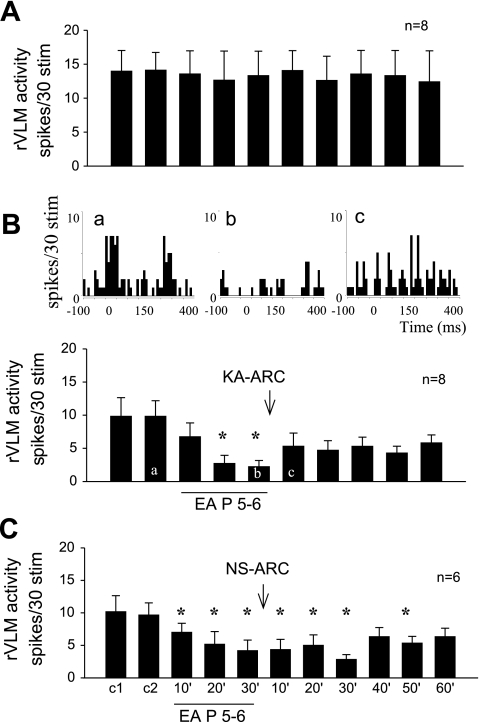
Evoked rVLM responses to stimulation of the splanchnic nerve 10 consecutive times in eight control cats were consistent, varying between 14 ± 3 and 12 ± 5 spikes/30 stimuli (Fig. 1A). Thirty minutes of low-frequency EA at P 5–6 (median nerve stimulation) reduced the rVLM response from 10 ± 2 to 2 ± 1 spikes/30 stimuli (P < 0.05). Bilateral microinjection of KA into the ARC nuclei in eight cats did not significantly alter baseline blood pressure or heart rate, but reversed the inhibitory influence of EA on the rVLM-evoked neuronal response from 2 ± 1 to 6 ± 3 spikes/30 stimuli (Fig. 1B). Microinjection of the saline control did not affect the EA-induced inhibition (from 10 ± 2 to 4 ± 2 spikes/30 stimuli), which lasted for more than 50 min (Fig. 1C).
Fig. 1.
Bilateral microinjection of kainic acid (KA) in arcuate (ARC) nucleus blocked electroacupuncture (EA) inhibition of rostral ventrolateral medulla (rVLM) neuronal response to splanchnic nerve stimulation. A: time control of rVLM response to stimulation of splanchnic nerve every 10 min. B, bottom: bilateral microinjection of KA into the ARC nucleus rapidly reversed EA inhibition of the rVLM neural response. *P < 0.05 compared with controls. Top: peristimulus time histograms demonstrate ventrolateral periaqueductal gray (vlPAG) neuronal responses during repeated 15-s (2-Hz) splanchnic nerve stimulations before (a) and after EA (b) at P 5–6 and following KA injection into the ARC (c). C: EA applied bilaterally at P 5–6 acupoints (located over median nerve) for 30 min inhibited splanchnic nerve stimulation-induced rVLM neural responses for more than 50 min. Microinjection of normal saline (NS) into the ARC did not influence the EA-related inhibition.
ARC-vlPAG-rVLM Pathway
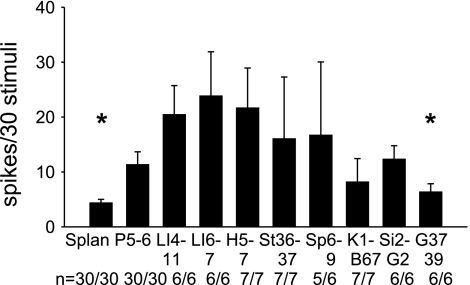
Convergent activity in 30 rVLM neurons was examined following injection of DLH into the cats' ARC nucleus and during separate electrical stimulation of the splanchnic or somatic nerves, located beneath acupoints. We found that the vlPAG neuronal responses to stimulation at P 5–6, LI 4–11, LI 6–7, H 5–7, St 36–37, SP 6–9, or St 2-G2 acupoints were greater than the responses to simulation of G 37–39 or splanchnic nerve. All neurons were located in the rostral portion of the vlPAG (A 0–3). Figure 2 shows responses to splanchnic and somatic nerve stimulation, plotted separately by responses to stimulation at each set of acupoints in the 30 neurons that were evaluated. Twenty-two of the vlPAG neurons also received input from the ARC nucleus. As we demonstrated in a previous study (32), EA at P 5–6 increases the vlPAG response to splanchnic nerve stimulation. Four other neurons located either dorsal, lateral, ventral (A 0.1–1), or more rostrally (A 7) in the vlPAG did not show any facilitation of the splanchnic nerve-evoked vlPAG response during EA. Stimulation of P 5–6 evoked somewhat larger responses than those occurring during splanchnic nerve or superficial peroneal nerve (G 37–39) stimulation. Combined with our previously published data, these results suggest that the A 0–3 region of the vlPAG plays a role in the EA cardiovascular response.
Fig. 2.
Convergent activity in the vlPAG between A 0.1–3. Bar histograms show evoked activity in vlPAG neurons during individual stimulation of the splanchnic nerve and nine sets of acupoints. Numbers below each bar indicate the number of responsive cells relative to number of cells studied. *Significantly different discharge rate compared with response during stimulation of P 5–6 (P < 0.05).
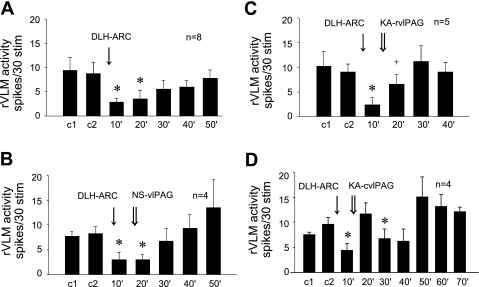
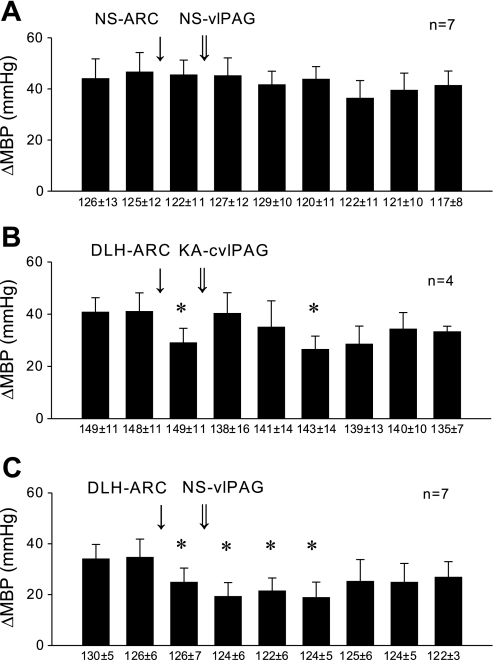
As a surrogate for EA, microinjection of DLH in the ARC of eight cats inhibited the rVLM-evoked responses from 9 ± 3 to 3 ± 1 spikes/30 stimuli, followed by recovery to 8 ± 2 spikes/30 stimuli after 50 min (Fig. 3A). Normal saline injected in the rostral or caudal vlPAG as a control did not alter the ARC DLH-related rVLM inhibition in four control animals (Fig. 3B). Conversely, bilateral microinjection of KA in the rostral vlPAG (A 0.1–3) partially reversed the ARC nucleus DLH-induced rVLM response (Fig. 3C), while inactivation of the caudal vlPAG (P 1-A 0) completely reversed the DLH-induced response (Fig. 3D).
Fig. 3.
Histograms displaying the effect of bilateral microinjection of d,l-homocysteic acid (DLH) and KA in ARC on the rVLM-evoked response to splanchnic nerve stimulation. A: microinjection of DLH in the ARC inhibited rVLM response for 20 min. B: microinjection of NS (vehicle) in the rostral (rvlPAG; n = 2) or caudal (cvlPAG; n = 2) vlPAG did not influence the inhibitory effect resulting from stimulation of the ARC with DLH. C: microinjection of KA in the rvlPAG partially reversed the inhibitory rVLM effect caused by microinjection of DLH in the ARC. D: microinjection of KA in the cvlPAG fully reversed the inhibitory rVLM effect of microinjection of DLH in the ARC *P < 0.05 compared with controls (c1, c2).
ARC-rVLM Projections
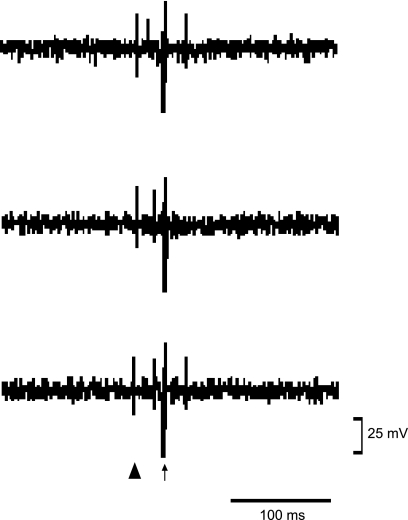
Stimulation of the rVLM in 6 of 14 cats induced antidromic responses in the ARC nucleus with a constant latency that averaged 17 ± 6 ms and was consistent during high-frequency stimulation at 200 Hz. Similar to our laboratory's previous work (18), we found that the spontaneous firing of neurons in the ARC was low. However, these neurons responded to stimulation of the splanchnic nerve and P 5–6 acupoints. Neural responses in the ARC to stimulation of the rVLM were “all or none” and had a threshold of 0.25 ± 0.03 mA. We observed collision of the antidromic and orthodromic potentials evoked by stimulation of P 5–6 in each animal (Fig. 4). The average distance between the rVLM and the ARC was 27 ± 2 mm, and the estimated axonal conduction velocity was calculated to be 2.7 ± 0.8 m/s. The mean refractory period was 3 ± 0.6 ms.
Fig. 4.
Tracing showing a single-unit recording of neural activity in ARC evaluating the response to antidromic stimulation of rVLM. Top: first spike is the orthodromic evoked potential induced during stimulation of P 5–6; second spike represents the response to antidromic stimulation of rVLM. Middle: the P 5–6-induced spike moved toward the rVLM antidromic stimulation and canceled the antidromic response. Bottom: the spike induced by stimulation of P 5–6 moved backward, and the antidromic response reappeared. ▴ and ↑ represent the stimulation artifacts of P 5–6 and rVLM, respectively.
Classification of rVLM Sympathetic Cardiovascular Neurons
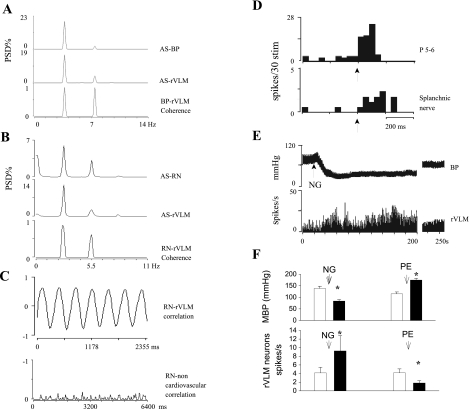
The rVLM neurons in cats evaluated in this study were found to function as cardiovascular sympathoexcitatory neurons, and, using the following criteria, a number were shown to be presympathetic in nature. First, 18 neurons subjected to frequency domain analysis exhibited a strong relationship to the cardiac cycle, demonstrating a strong average coherence of 0.85 ± 0.02 at 3.7 ± 0.2 Hz and a correlation of 0.8 ± 0.03 (Fig. 5A). Second, the subset of six neurons whose activity was recorded simultaneously with renal sympathetic nerve discharge uniformly showed a strong coherence and a high correlation (0.86) of discharge activities at a frequency of 3.3 Hz (Fig. 5, B and C). Third, 15 of the 18 neurons examined with respect to their response to baroreceptor input demonstrated increased activity (4 ± 1 to 9 ± 3 spikes/s) when blood pressure was lowered (138 ± 9 to 82 ± 6 mmHg) following intravenous nitroglycerin, and decreased activity (4 ± 1 to 2 ± 1 spikes/s) when blood pressure was raised (115 ± 8 to 180 ± 9 mmHg) with intravenous phenylephrine (Fig. 5, E and F).
Fig. 5.
Illustrations showing identification of cardiovascular sympathetic neurons in rVLM. A: autospectra (AS) of blood pressure (BP) and rVLM neuronal activity and the corresponding coherence function. Coherence was 0.87 at 3.79 Hz. B: AS of renal nerve (RN) and rVLM neuronal activity and the corresponding coherence of RN and rVLM neuronal firings. Coherence of 0.86 occurred at a frequency of 3.3 Hz. C, top: cross correlation of RN discharge and rVLM neural activity. Bottom: a neuron outside rVLM, showing no correlation with RN activity. D: histograms show the rVLM neuronal responses to stimulation of P 5–6 and splanchnic nerve. ↑, Stimulation of P 5–6 or splanchnic nerve. E: the change in BP and spontaneous discharge of an rVLM neuron after intravenous administration of nitroglycerin (NG). F: histograms show the change of BP and rVLM neuronal discharge after intravenous injection of NG and phenylephrine (PE) in 15 cats. MBP, mean BP; PSD, power spectral density. *, <0.05 compared with controls.
Roles of ARC and vlPAG in EA Inhibition of Visceral-Cardiovascular Reflexes
The reflex blood pressure responses in cats to repeated application of BK on the gallbladder were constant over time and were unaltered by microinjection of the vehicle, normal saline, in the ARC or the vlPAG (Fig. 6A). The blood pressure responses to application of BK on the gallbladder averaged 41 ± 6 mmHg. Like EA, DLH stimulation of the ARC nucleus significantly reduced the blood pressure responses to 29 ± 6 mmHg (P < 0.05). Chemical blockade of cell bodies in the caudal vlPAG (P 1-A 0) with KA completely reversed the DLH ARC-related inhibition (40 ± 8 mmHg, Fig. 6B), while microinjection of normal saline did not influence the DLH inhibitory response (Fig. 6C).
Fig. 6.
Histograms illustrating hemodynamic responses. A: repeated reflex BP responses were induced by application of bradykinin (BK) on gallbladder every 10 min. The responses were not altered by microinjection of NS into the rvlPAG or cvlPAG. B: inhibition of reflex BP response by bilateral injections of DLH in the ARC nucleus was blocked by bilateral injection of KA in the cvlPAG. C: inhibition of reflex BP response by injections of DLH in the ARC nucleus was not altered by bilateral injection of NS in the rvlPAG or cvlPAG. Numbers below the baseline indicate resting MBP, which were unchanged by the protocols. Δ, Change. *P < 0.05 compared with controls.
Location of Microinjection and Recording Sites
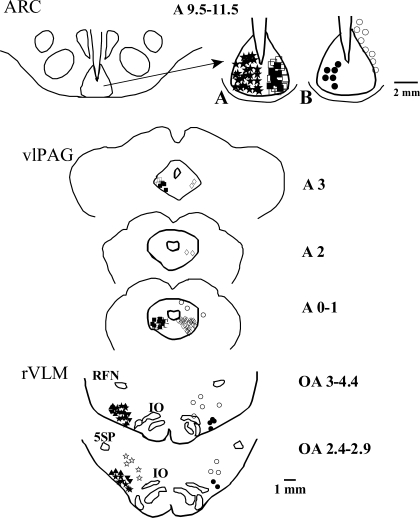
The 36 rVLM neurons of cats recorded in this study were located 2.4–4.4 mm rostral to obex, 2.8–3.8 mm lateral to the midline, and 0.1–1 mm from the ventral surface of the medulla, a region that was ventral and medial to the retrofacial nucleus and lateral to the inferior olive nucleus and pyramidal tract. By virtue of our selection process, all rVLM neurons received convergent input from the splanchnic and somatic nerves (P 5–6). Microinjection sites for all of the protocols localized in the ARC or vlPAG are shown in Fig. 7. Five rVLM neurons responding to somatic nerve stimulation during EA did not respond to splanchnic nerve stimulation. These neurons were not further studied. Six of the fourteen ARC neurons studied were activated antidromically from the rVLM. Eight additional neurons that were located dorsal or lateral to the ARC did not display antidromic responses during rVLM stimulation (Fig. 7B of ARC). Twenty-one rVLM neurons responding to splanchnic nerve stimulation were inhibited by DLH administered bilaterally in the ARC (Fig. 7, both panels of rVLM). Five other neurons located dorsal, lateral, or medial to the rVLM did not respond to microinjection of DLH in the ARC nucleus, demonstrating the medullary anatomical specificity of projections from this nucleus (Fig. 7, both panels of rVLM).
Fig. 7.
Composite map of brain sections showing locations of microinjection, stimulation, and recording sites in ARC, vlPAG, and rVLM. For simplicity, all recordings or microinjections are placed on one side. Top, left: location of the ARC in the ventral hypothalamus. Right: A: ★, DLH in ARC, n = 30; ▪, KA in ARC, n = 6; □, NS in ARC, n = 13. B: •, antidromic recordings in ARC, n = 6; ○, hypothalamic neurons unresponsive to antidromic stimulation of rVLM, n = 8. Middle: ▪, KA in rvlPAG (n = 5) and in cvlPAG (n = 8); □, NS in vlPAG, n = 16; ◊, recording of neuronal response to stimulation of the splanchnic nerve and somatic acupoints (n = 30); ○, recordings outside vlPAG at A 0.1–3 level (n = 3, another unresponsive neuron located more rostrally, A 7) is not shown in this figure. Bottom: ▴, rVLM neurons responding to EA, n = 12; ▾, rVLM evaluated for time control, n = 8; ★, rVLM neurons inhibited by DLH in ARC, n = 12; ⧫, DLH in ARC, KA in rvlPAG (n = 5) and cvlPAG (n = 4), and recording in rVLM; ⋆, medullary neurons outside rVLM unresponsive to DLH in ARC; n = 5; •, antidromic stimulation in rVLM, n = 6; ○, antidromic stimulation outside rVLM, n = 8. Retrofacial nucleus (RFN), nucleus of the inferior olive (IO), and alaminar spinal trigeminal nucleus (5SP) are indicated.
Retrograde Labeling and Immunohistochemical Staining
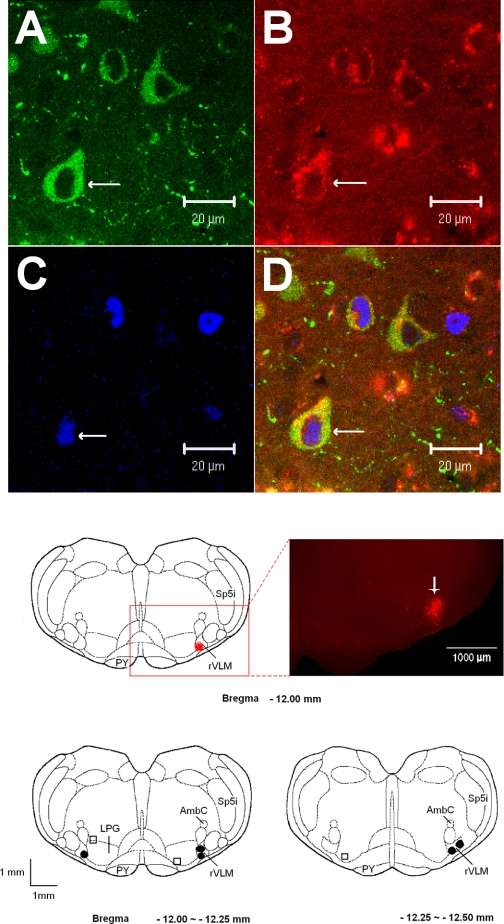
Following injection of microspheres into the rVLM in five rats subjected to EA (n = 3) or control stimulation (sham EA, n = 2), we observed that neurons containing the retrogradely transported microspheres were distributed rostral-caudally throughout the ARC and paraventricular nuclei. In the other three rats (one EA and two sham EA), injections were observed to be outside rVLM in adjacent regions (dorsal or medial to the rVLM; Fig. 8, bottom). Evaluation of the hypothalamus of the later group demonstrated no tracer cells in the ARC, whereas many cells containing the microsphere label were observed in the dorsomedial hypothalamic nucleus, dorsal hypothalamic, and posterior hypothalamic areas. Similar to our previous observations (9), we noted that β-endorphin was located in the ARC nucleus, but not in other hypothalamic nuclei of all five rats. We found some ARC neurons labeled with retrograde tracer that colocalized with β-endorphin. These double-labeled neurons represented 22% of the cells containing β-endorphin and 31% of neurons labeled with the microsphere tracer. Also, similar to our laboratory's previous findings in cats (9), we noted more Fos immunoreactivity located bilaterally in the ARC nucleus of EA-treated rats (n = 3) than the controls (n = 2). Moreover, colocalization of Fos nuclei with the retrograde tracer and/or β-endorphin was observed frequently in EA-treated rats, but only rarely in controls. Figure 8 demonstrates confocal images of neurons triple-labeled with c-Fos, microspheres, and β-endorphin in the ARC of a rat that had been subjected to EA.
Fig. 8.
A–D: confocal microscopic images displaying β-endorphin, retrogradely transported microsphere tracer injected 10 days earlier into rVLM and c-Fos, in the ARC nucleus (Bregma −1.72 mm) from a rat treated with EA. A–C: immunostaining of β-endorphin (green; A), retrograde microsphere tracer (red; B), and c-Fos (blue; C). D: merged images from A–C. Arrows in A–D indicate a neuron containing β-endorphin, microsphere c-Fos, and β-endorphin + microspheres + c-Fos, respectively. Middle: an original picture (right) represents the area indicated in the box on the left. This fluorescent microscopic image demonstrates the injection site of the retrograde microsphere tracer in the rVLM (bregma −12 mm) of a rat following EA. The bright red area indicated by the arrow shows an injected site of rhodamine-labeled fluorescent microsphere in the rVLM. Scale bar = 1,000 μm. Bottom: sections of rat medulla oblongata illustrating microinjection sites of the retrograde microsphere tracer. • and □ indicate injection sites inside and outside the rVLM, respectively. AmbC, ambig compact; LPG, lateral paragigantocellularis nucleus; PY, pyramidal tract; Sp5i, spinal 5 interpolar nucleus.
DISCUSSION
We demonstrated in the present study that KA-induced depolarization blockade of the ARC nucleus in the ventrolateral hypothalamus partially but rapidly reverses EA inhibition of cardiovascular visceral afferent-induced increases in rVLM neuronal activity. KA is known to induce depolarization blockade of neuronal function that is reversible under certain conditions, particularly those associated with the use of low concentrations of this agonist (10). Our studies show that microinjection of KA into the ARC does not significantly influence baseline blood pressure or heart rate. However, rVLM neuronal activity decreased transiently following KA, recovering to its original level within 10 min. The influence of KA blockade on EA-related changes in rVLM activity was observed after 10 min. These responses to KA are consistent with its known ability to initially depolarize neurons and then block their activity after a short delay (10). We found that microinjection of DLH in the ARC, like EA, inhibits rVLM neural responses to splanchnic nerve stimulation for more than 20 min, a response that recovers slowly, ∼50 min after injection of DLH. Collectively, these data suggest that the ARC nucleus serves as an important nucleus that processes information during prolonged inhibition by EA of cardiovascular sympathetic neurons in the rVLM.
Chemical blockade of cellular activity in the ARC partially reversed the EA-associated rVLM response, suggesting that either incomplete blockade of ARC neurons or the presence of other pathways not involving the ARC contribute to the EA-associated inhibitory response. These could include direct input from somatic afferents to the vlPAG, medullary raphe, or rVLM nuclei, since we have shown that EA can activate neurons in all three nuclei (23, 32). Either possibility could have occurred, but neither prevents the interpretation that during EA the ARC nucleus contributes to inhibition of rVLM activity.
As shown in Fig. 2, the responses of vlPAG neurons to stimulation of P 5–6, LI 4–11, LI 6–7, H 5–7, St 36–37, or St 2-G 2 acupoints are higher than the responses to activation of G 37–39 or splanchnic nerve stimulation. Point specificity is a complex issue. Our laboratory has previously demonstrated that this phenomenon is related to the degree of input to various regions that regulate sympathetic outflow, such as the rVLM (31). Thus, while a number of acupoints can have a small amount of input to the regions that comprise a long-loop pathway, including the vlPAG and the ARC nucleus, the influence of these points on this pathway, as well as the rVLM, appears to be related significantly to the extent of input.
Our previous studies have shown that the ARC provides excitatory projections to the vlPAG, and that chemical blockade of neural activity in the ARC decreases visceral afferent-related vlPAG activity, while similar blockade in the vlPAG attenuates EA-related cardiovascular modulation (18, 32). The present electrophysiological study extends these earlier observations by demonstrating that inhibition of rVLM neuronal response following vlPAG blockade also occurs during direct chemical stimulation of the ARC. Furthermore, similar to the effect of ARC KA blockade on sympathoexcitatory rVLM activity noted above, depolarization blockade of the ARC eliminates EA modulation of visceral-cardiovascular reflexes, while stimulation of the ARC in the absence of EA modulates these excitatory responses. Thus the ARC represents an important component of the long-loop pathway in EA-related inhibition of rVLM presynaptic neurons and excitatory cardiovascular reflex responses.
A significant remaining question is whether the ARC-rVLM inhibitory responses are poly- or monosynaptic. As noted above, our laboratory previously provided indirect evidence to suggest the presence of a polysynaptic pathway between the ventral hypothalamus and the medulla with one or more synapses in the vlPAG midbrain region (18, 32). The present study demonstrates that bilateral blockade of the rostral vlPAG partially reverses the ARC-related inhibitory rVLM response, while bilateral blockade of the caudal vlPAG completely reverses this response. Our laboratory's previous anatomical study likewise demonstrated the importance of the caudal region of the vlPAG in response to EA at P 5–6, since we observed relatively denser c-Fos labeling in this region compared with the rostral vlPAG (8). Thus the vlPAG, particularly the caudal portion of this nucleus, appears to be a necessary component of the ARC-rVLM long-loop modulatory pathway in the EA cardiovascular response.
Since the present study demonstrated that the vlPAG, especially the caudal vlPAG (A 0.6-P 1.5), is a required region for the inhibition on cardiovascular response (5), the importance of other pathways, including a direct connection between the ARC and the rVLM in the EA response, might be questioned. In this regard, early studies by Lovick (21) reported that, after injection of horseradish peroxidase in the nucleus paragigantocellularis lateralis (PGL) in the rVLM of cats, labeled cells were found in the dorsomedial, but not the ventromedial, hypothalamus. Lovick suggested the existence of direct projections from the dorsomedial hypothalamic areas to the PGL. Additionally, Li and Lovick (20) studying rats, documented antidromically stimulated neurons in the PGL in the medulla that project from perikarya in ventral hypothalamus. However, they did not observe direct projections from the ARC to the PGL. In the present study, we observed direct projections from the ARC to the rVLM. This was relatively specific, as we did not observe direct projections from the ARC to dorsal or medial regions adjacent to the rVLM. We recognize, however, that additional tracing studies would need to be carried out to carefully evaluate for the presence of connections between the ARC and other regions in the medulla.
Using collision techniques, we were able to detect the presence of direct projections between the ARC and the rVLM, although one might question the accuracy of antidromic stimulation to identify direct pathways between these two regions, since there could be current spread. However, these ARC neurons, which showed antidromic responses from rVLM stimulation, each received convergent afferent input from the splanchnic nerve and P 5–6 acupoints. It is also consistent with a previous study in which we detected β-endorphin-containing fibers but no cell bodies in the rVLM, even after colchicine treatment (8), leading us to speculate that opioid projections from the ARC, a rich source of β-endorphin-containing cells (3), may extend to the rVLM. In this regard, we recently confirmed the existence of two populations of ARC neurons containing excitatory (glutamate) and/or inhibitory neurotransmitters (including endorphins or enkephalins) that are activated by EA (9).
We have previously documented a role for inhibitory peptides, including opioids, with respect to the modulatory influence of EA on rVLM neuronal activity evoked by splanchnic stimulation. For example, our laboratory has shown that this EA-related inhibition is blocked by nonspecific opioid blockade with naloxone as well as with μ- and δ-opioid-receptor antagonism (7, 14, 29). We thus concluded that naloxone, as well as μ- and δ-opioid blockade, in this brain stem region modulates the EA-related cardioinhibitory response. Furthermore, opioid receptor blockade inhibits the prolonged inhibition by EA of sympathoexcitatory premotor sympathetic neurons in this region (29). The specific observation that μ-opioid-receptor blockade antagonizes the sympathatoexcitatory modulatory influence of EA strongly suggests that EA operates, in part, through a mechanism involving β-endorphin, since this neurotransmitter is one of the principal endogenous ligands from this receptor (11, 28).
More importantly, in the present investigation, we found that perikarya in the ARC of several rats were labeled with a retrogradely transported microsphere tracer following microinjection into the rVLM. The locations of injected tracer in the medulla of all five rats closely matched with the rVLM as defined by Paxinos and Watson's atlas for the rat (25). This site is located 2.0–2.6 mm lateral from the midline and 0.2–0.6 mm from ventral surface of the medulla. It is lateral to the paragigantocellularis nucleus, ventral to the Botzinger complex, and medial to the VII cranial nucleus at the high rostral level (25). Thus our tracing studies confirm the antidromic stimulation data by providing anatomical evidence for a direct neuronal projection from the ARC to the rVLM. Some of the retrogradely labeled neurons also contained β-endorphin and Fos induced by EA stimulation. The c-Fos, an immediate early gene, is a marker that identifies neurons activated by afferent input, for example, during EA (8, 9). Taken together, our previous and present findings suggest the existence for an opioid neuronal pathway projecting from the ARC to the rVLM that could underlie the opioid action of EA on rVLM activity. In this regard, we have shown previously that β-endorphin acting through μ-opioid receptors and enkephalin acting through δ-opioid receptors participate in EA modulation of sympathoexcitatory reflex responses (17). Furthermore, our anatomical data show that, while enkephalins are detected in the perikarya of EA-activated neurons in the rVLM, β-endorphin is detected in cell bodies of neither the rVLM nor vlPAG (9). Rather, β-endorphin is located only in cellular processes (axons) in the rVLM. Thus, while the vlPAG, especially the caudal vlPAG, is required for the EA inhibitory response, the ARC may directly supply endorphins to the rVLM.
Conclusion
Neurons in the ARC nucleus projecting to the rVLM potentially participate in EA inhibition of reflex cardiovascular sympathoexcitation. Our data indicate that the indirect, i.e., polysynaptic vlPAG projections from the ARC are required for EA regulation of sympathoexcitatory presympathetic rVLM activity and the cardiovascular excitatory reflex responses during EA, while a direct pathway between the ARC and rVLM might serve as a source of endorphins for EA cardiovascular modulation.
GRANTS
This work was supported by the National Heart, Lung, and Blood Institute, Bethesda, MD, Grants HL-72125, and HL-63313, and the Larry K. Dodge and Susan-Samueli Endowed Chairs (J. C. Longhurst).
Acknowledgments
The authors gratefully acknowledge Yinxiang Cao, Department of Physiology, Shanghai Medical College of Fudan University, China, for the development of a four-channel data-acquisition system and related software. We also thank Kin K. Hong, Ali R. Moazzami, Rainier Cabatbat, and Ryann Perry for technical assistance.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Barman S, Gebber G. Sequence of activation of ventrolateral and dorsal medullary sympathetic neurons. Am J Physiol Regul Integr Comp Physiol 245: R438–R447, 1983. [DOI] [PubMed] [Google Scholar]
- 2.Berman AL The Brainstem of the Cat: A Cytoarchitectonic Atlas With Stereotaxic Coordinates. Madison, WI: University of Wisconsin Press, 1968.
- 3.Bloom F, Battenberg E, Rossier J, Ling N, Guillemin R. Neurons containing B-endorphin in rat brain exist separately from those containing enkephalin: immunocytochemical studies. Proc Natl Acad Sci USA 75: 1591–1595, 1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bureš J, Petran M, Zachar J. Electrophysiological Methods in Biological Research. New York: Academic, 1967.
- 5.Carrive P, Bandler R. Viscerotopic organization of neurons subserving hypotensive reactions within the midbrain periaqueductal: a correlative functional and anatomical study. Brain Res 541: 206–216, 1991. [DOI] [PubMed] [Google Scholar]
- 6.Chan RKW, Sawchenko PE. Spatially and temporally differentiated patterns of c-Fos expression in brainstem catecholaminergic cell groups induced by cardiovascular challenges in the rat. J Comp Neurol 348: 433–460, 1994. [DOI] [PubMed] [Google Scholar]
- 7.Chao DM, Shen LL, Tjen-A-Looi S, Pitsillides KF, Li P, Longhurst JC. Naloxone reverses inhibitory effect of electroacupuncture on sympathetic cardiovascular reflex responses. Am J Physiol Heart Circ Physiol 276: H2127–H2134, 1999. [DOI] [PubMed] [Google Scholar]
- 8.Guo ZL, Moazzami A, Longhurst J. Electroacupuncture induces c-Fos expression in the rostral ventrolateral medulla and periaqueductal gray in cats: relation to opioid containing neurons. Brain Res 1030: 103–115, 2004. [DOI] [PubMed] [Google Scholar]
- 9.Guo Z, Longhurst J. Expression of c-Fos in arcuate nucleus induced by electroacupuncture: relations to neurons containing opioids and glutamate. Brain Res 1166: 65–76, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hade JS, Mifflin SW, Donta TS, Felder RB. Stimulation of parabrachial neurons elicits a sympathetically mediated pressor response in cats. Am J Physiol Heart Circ Physiol 255: H1349–H1358, 1988. [DOI] [PubMed] [Google Scholar]
- 11.Hawkins MF, Cubic B, Baumeister AA, Barton C. Microinjection of opioid antagonists into the substantia nigra reduces stress-induced eating in rats. Brain Res 584: 261–265, 1992. [DOI] [PubMed] [Google Scholar]
- 12.Huangfu DH, Huang Q, Li P. Afferent connections of the ventrolateral medulla in the rabbit-studied with HRP techniques. Chin J Physiol Sci 3: 86–95, 1987. [Google Scholar]
- 13.Huangfu DH, Li P. The role of nucleus arcuatus in the inhibitory effect of deep peroneal nerve inputs on defence reaction. Chin J Physiol Sci 3: 37–46, 1987. [Google Scholar]
- 14.Huangfu DH, Li P. The inhibitory effect of ARC-PAG-NRO system on the ventrolateral medullary neurons in the rabbit. Chin J Physiol Sci 4: 115–125, 1988. [Google Scholar]
- 15.Li P, Pitsillides KF, Rendig SV, Pan HL, Longhurst JC. Reversal of reflex-induced myocardial ischemia by median nerve stimulation: a feline model of electroacupuncture. Circulation 97: 1186–1194, 1998. [DOI] [PubMed] [Google Scholar]
- 16.Li P, Rowshan K,. Crisostomo M, Tjen-A-Looi SC, Longhurst JC. Effect of electroacupuncture on pressor reflex during gastric distention. Am J Physiol Regul Integr Comp Physiol 283: R1335–R1345, 2002. [DOI] [PubMed] [Google Scholar]
- 17.Li P, Tjen-A-Looi S, Longhurst JC. Rostral ventrolateral medullary opioid receptor subtypes in the inhibitory effect of electroacupuncture on reflex autonomic response in cats. Auton Neurosci 89: 38–47, 2001. [DOI] [PubMed] [Google Scholar]
- 18.Li P, Tjen-A-Looi SC, Longhurst JC. Excitatory projections from arcuate nucleus to ventrolateral periaqueductal gray in electroacupuncture inhibition of cardiovascular reflexes. Am J Physiol Heart Circ Physiol 290: H2535–H2542, 2006. [DOI] [PubMed] [Google Scholar]
- 19.Li P, Tjen-A-Looi SC, Longhurst JC. Role of arcuate nucleus and ventrolateral periaqueductal gray in electroacupuncture inhibition of sympathoexcitatory cardiovascular reflex response (Abstract). FASEB J 20: A734, 2006. [DOI] [PubMed] [Google Scholar]
- 20.Li P, Lovick TA. Excitatory projections from hypothalamic and midbrain defense regions to nucleus paragigantocellularis lateralis in the rat. Exp Neurol 89: 543–553, 1985. [DOI] [PubMed] [Google Scholar]
- 21.Lovick TA Projections from the diencephalon and mesencephalon to nucleus paragigantocellularis lateralis in the cat. Neuroscience 14: 853–861, 1985. [DOI] [PubMed] [Google Scholar]
- 22.Lovick T Differential control of cardiac and vasomotor activity by neurons in nucleus paragigantocellularis lateralis in the cat. J Physiol 389: 23–35, 1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Moazzami A, Tjen-A-Looi SC, Guo ZL, Longhurst JC. Serotoninergic projections from nucleus raphe pallidus to rostral ventrolateral medulla participate in acupuncture modulation of cardiovascular excitatory reflexes. FASEB J 582.23, 2007.
- 24.NIH Consensus Conference. Acupuncture. JAMA 280: 1518–1524, 1998. [PubMed] [Google Scholar]
- 25.Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. New York: Academic, 2005. [DOI] [PubMed]
- 26.Shi XM, Zhang MC. A Chinese-English Dictionary of Acupuncture and Moxibustion. Beijing, China: Huaxia Publishing, 1998.
- 27.Sim LJ, Joseph SA. Arcuate nucleus projections to brainstem regions which modulate nociception. J Chem Neuroanat 4: 97–109, 1991. [DOI] [PubMed] [Google Scholar]
- 28.Sun SY, Liu Z, Li P, Ingenito AJ. Central effects of opioid agonists and naloxone on blood pressure and heart rate in normotensive and hypertensive rats. Gen Pharmacol 27: 1187–1194, 1996. [DOI] [PubMed] [Google Scholar]
- 29.Tjen-A-Looi SC, Li P, Longhurst JC. Role of medullary GABA, opioids, and nociception in prolonged inhibition of cardiovascular sympathoexcitatory reflexes during electroacupuncture in cats. Am J Physiol Heart Circ Physiol 293: H3627–H3635, 2007. [DOI] [PubMed] [Google Scholar]
- 30.Tjen-A-Looi SC, Li P, Longhurst JC. Prolonged inhibition of rostral ventral lateral medullary premotor sympathetic neuron by electroacupuncture in cats. Auton Neurosci 106: 119–131, 2003. [DOI] [PubMed] [Google Scholar]
- 31.Tjen-A-Looi SC, Li P, Longhurst JC. Medullary substrate and differential cardiovascular response during stimulation of specific acupoints. Am J Physiol Regul Integr Comp Physiol 287: R852–R862, 2004. [DOI] [PubMed] [Google Scholar]
- 32.Tjen-A-Looi SC, Li P, Longhurst JC. Midbrain vIPAG inhibits rVLM cardiovascular sympathoexcitatory responses during acupuncture. Am J Physiol Heart Circ Physiol 290: H2543–H2553, 2006. [DOI] [PubMed] [Google Scholar]
- 33.Yin Q Hypothalamic arcuate nucleus and analgesia. Fudan Lectures Neurobiol 2–5: 33–46, 1986. [Google Scholar]
- 34.Yin Q Hypothalamic arcuate nucleus and the modulation of pain. CAPS News Communication 15: 198–201, 1996. [Google Scholar]
- 35.Zhou W, Fu LW, Tjen-A-Looi SC, Li P, Longhurst JC. Afferent mechanisms underlying stimulation modality-related modulation of acupuncture-related cardiovascular responses. J Appl Physiol 98: 872–880, 2005. [DOI] [PubMed] [Google Scholar]