Abstract
Individuals with type 2 diabetes mellitus (T2DM) often exhibit microvascular dysfunction that may contribute to impaired thermoregulation, but potential mechanisms remain unclear. Our goals were to quantify skin blood flow responses and nitric oxide-mediated vasodilation during body heating in individuals with T2DM compared with nondiabetic control subjects of similar age. We measured skin blood flow (laser-Doppler flowmetry) in conjunction with intradermal microdialysis of NG-nitro-l-arginine methyl ester (l-NAME; nitric oxide synthase inhibitor) or vehicle during 45–60 min of whole body heating (WBH) in 10 individuals with T2DM and 14 control subjects. In six individuals from each group, we also measured forearm blood flow (FBF) by venous occlusion plethysmography on the contralateral forearm. FBF responses showed diminished absolute cutaneous vasodilation during WBH in the T2DM group (PANOVA < 0.01; peak FBF in control 13.1 ± 1.7 vs. T2DM 9.0 ± 1.6 ml·100 ml−1·min−1). However, the relative contribution of nitric oxide to the cutaneous vasodilator response (expressed as % of maximal cutaneous vascular conductance) was not different between groups (P > 0.05). We conclude that cutaneous vasodilator responses to WBH are decreased in individuals with T2DM, but the contribution of nitric oxide to this smaller vasodilation is similar between T2DM and control individuals. This decrease in cutaneous vasodilation is likely an important contributor to impaired thermoregulation in T2DM.
Keywords: metabolic diseases, microvasculature, heat stress, cutaneous circulation
microvascular complications of type 2 diabetes mellitus (T2DM) are well recognized as important contributors to the morbidity associated with the disease. Less well understood is how microvascular dysfunction in T2DM may affect thermoregulatory control mechanisms in the human cutaneous microcirculation. T2DM is associated with a higher incidence of heat-related illnesses such as heat stroke and heat exhaustion (15, 16), and individuals with T2DM have been shown to exhibit a decreased ability to tolerate elevated ambient temperatures (12, 13). However, the thermoregulatory mechanisms responsible for diabetic heat intolerance remain incompletely understood.
In humans, skin blood flow plays a primary role in the regulation of normal body temperature. As core temperature increases, the need for heat dissipation is met primarily by sympathetic active cutaneous vasodilation and sweating (2, 4, 14). In addition to whole body thermoregulation, skin blood flow also responds to local thermal stimuli. We recently observed (19, 22) a delayed threshold for the onset of active cutaneous vasodilation during whole body heating, as well as decreased vasodilation during local warming of the skin in subjects with T2DM compared with healthy control subjects. Because local warming causes vasodilation that is largely dependent on nitric oxide (NO) (8, 10), these latter findings lend support to the idea that NO-dependent vasodilation is decreased in patients with T2DM. Pharmacological evidence also suggests impaired NO-dependent vasodilator responsiveness in human skin. Studies involving local iontophoresis of acetylcholine and nitroprusside have shown impaired vasodilation to both endothelium-dependent and endothelium-independent NO stimuli in some groups of T2DM patients (1, 20).
In the present study, our goal was to further evaluate mechanisms of cutaneous vasodilation during whole body heating in T2DM by assessing the contribution of NO to active cutaneous vasodilation in individuals with T2DM compared with control subjects of similar age. We conducted whole body heating with water-perfused suits and used laser-Doppler measurement of skin blood flow in combination with intradermal microdialysis of NG-nitro-l-arginine methyl ester [l-NAME; nitric oxide synthase (NOS) inhibitor] or vehicle (Ringer solution). We tested the hypothesis that individuals with T2DM have a smaller contribution of NO to active cutaneous vasodilation during whole body heating compared with control subjects. Since data obtained from laser-Doppler measurements of skin blood flow are semiquantitative in nature, an additional goal of these studies was to obtain a more quantitative comparison of absolute levels of vasodilation between control and T2DM subjects; for this purpose, we measured forearm blood flow (FBF) by venous occlusion plethysmography.
METHODS
Subjects.
The study included 10 individuals with T2DM (3 female, 7 male) and 14 control subjects (7 female, 7 male) of similar age. All subjects were screened via telephone to rule out any comorbidities that could influence microvascular or thermoregulatory function. Subjects were excluded if they showed signs/symptoms of neuropathy or had a history of cardiovascular disease. In addition, any subjects taking thiazolidinediones or first-generation sulfonylureas (e.g., glyburide) were excluded from participation. Of the 10 women studied, 6 (3 control, 3 T2DM) were postmenopausal and none of these was taking hormone replacement therapy.
Subjects reported to the Mayo Clinical Research Unit (CRU) for a screening visit and again on the day of the study. Written informed consent was obtained before any procedures began. The protocol for this experiment was approved by the Institutional Review Board at the Mayo Clinic in Rochester, MN. Any T2DM subjects taking oral antidiabetic agents were instructed to temporarily withdraw their medication for 2 wk before the study visit. Subjects were requested to monitor their reflectance meter glucose values daily at home. If glucose values exceeded 300 mg/dl on more than one occasion during the 2-wk period, they were asked to resume their antidiabetic medications and were excluded from further participation.
Screening visit.
Study participants reported to the CRU at 7:00 AM for a screening visit. All subjects had a 12-lead ECG, and fasting blood samples were drawn and analyzed for glucose and hemoglobin A1c. Body composition was measured by dual-energy X-ray absorptiometry (DEXA). All individuals with T2DM, and all control subjects over 55 yr of age, participated in a treadmill exercise test conducted at the Mayo Cardiovascular Health Clinic with a standard Bruce protocol, to identify and exclude subjects with occult cardiovascular disease.
Study visit.
Subjects arrived at the CRU at 7:00 AM after an overnight fast. Blood glucose was measured in all T2DM subjects before any procedures began. Subjects changed into a two-piece water-perfused suit that covered their entire body except the head, hands, and feet. The left sleeve was rolled up to allow space for two microdialysis fibers (MD-2000; Bioanalytical Systems) that were placed intradermally on the ventral side of the left forearm, as previously described (10, 19). To help guide fiber placement, two 25-gauge needles were inserted into the dermal layer of the skin. Each needle had entry and exit points ∼2.5 cm apart. The microdialysis fibers were threaded through the lumen of each needle until the 1-cm microdialysis membrane was completely within the dermis. The needles were removed, and the fibers were taped in place.
Integrating Periflux laser-Doppler flowmetry (LDF) probes (Periflux System 5000; Perimed, Stockholm, Sweden) were placed above each microdialysis fiber to measure skin blood flow. The probes were held in place by adhesive and by specialized holders that are able to measure and control local skin temperature over an area of 12 cm2 (Perimed). A three-lead ECG was used for heart rate monitoring. Continuous beat-to-beat blood pressure measurements were obtained by Finometer on the left middle finger.
FBF was measured by venous occlusion plethysmography on the arm contralateral to the LDF measurements (n = 6 in each group). For these measurements, the right sleeve of the suit was rolled up to allow placement of the plethysmography strain gauge. Briefly, a pediatric blood pressure cuff was placed around the wrist and inflated to suprasystolic levels (220 mmHg) to arrest the circulation of the hand, and a venous occlusion cuff was placed on the upper arm and rapidly inflated to 60 mmHg for 7 of every 15 s, yielding one blood flow value every 15 s (3, 6). FBF is expressed as milliliters per 100 ml of tissue per minute.
Protocol.
After microdialysis fiber placement, subjects rested supine for 2 h to ensure that any hyperemia caused by the needle insertion had subsided. During this time, vehicle (Ringer solution) was perfused through both fibers at a rate of 4 μl/min with a microperfusion pump (Harvard 22 syringe pump; Harvard Apparatus, South Natick, MA). After the 2-h waiting period, and before the first drug administration, a 5-min baseline (pre-l-NAME baseline) was recorded at both sites. A control site (vehicle) and a treated site (l-NAME) were randomly assigned. l-NAME (10 mM) was perfused for 50 min at the treated site while the control site continued with vehicle microdialysis, both at a pump rate of 4 μl/min. To observe the effect of l-NAME on resting cutaneous blood flow, another 5-min baseline was recorded after 50 min of l-NAME microdialysis (l-NAME baseline). During this time, baseline FBF was measured on the contralateral arm for 3 min.
After baseline recordings were complete, we began the whole body heating protocol as previously described (22). Warm water was pumped through the tube-lined suit from a hot water bath set at 50°C. A temperature probe (model ON-402-PP, Omega Engineering, Stamford, CT) was placed in the mouth in the sublingual sulcus to measure internal temperature (Tsl). Subjects were encouraged to refrain from talking or opening their mouths to prevent any movement of the probe. Heating took place for 45–60 min until an ∼1°C increase in Tsl was observed. LDF was recorded continuously at vehicle and l-NAME sites. FBF was measured for 2-min periods at each of the following increments in Tsl: 0.3, 0.5, 0.7, and 1.0°C.
After whole body heating, cool water was temporarily perfused through the suits to return study participants to normal body temperature. At the end of each experiment, to cause maximum vasodilation, local temperature was increased to 43°C with specialized holders surrounding the laser-Doppler flow probes and sodium nitroprusside (SNP; 28 mM) was perfused through both fibers at a rate of 4 μl/min for 40 min.
Data analysis.
All data were recorded at 100 Hz with a Windaq data acquisition system (Dataq Instruments) and were subsequently analyzed off-line. LDF values were divided by mean arterial pressure to derive an index of cutaneous vascular conductance (CVC). CVC data are expressed as a percentage of maximal CVC (%maxCVC), calculated as the average of the last 2 min of CVC during SNP microdialysis. FBF was calculated with the slope (first derivative) of the volume changes during each venous occlusion period, using data analysis software in the Windaq program (Dataq Instruments). Forearm vascular conductance (FVC) was calculated as FBF/MAP × 100 (where MAP is mean arterial pressure) and expressed as milliliters per 100 ml per minute per 100 mmHg.
To analyze the effects of l-NAME on resting CVC, we calculated CVC during the last 2 min of the pre-l-NAME baseline and the l-NAME baseline. The NO contribution to baseline CVC was calculated as the difference between these two values. CVC responses during WBH were compared by using 120-s averages at four specific increases in Tsl: 0.3, 0.5, 0.7, and 1.0°C. FBF and FVC responses were calculated as 2-min averages during the same increments in internal temperature. Contributions of NO during whole body heating were calculated as the difference in CVC between vehicle site and l-NAME site.
Statistics.
Results are expressed as means ± SE. Comparisons between control and T2DM groups were made with unpaired Student's t-test for baseline values and two-way analysis of variance (ANOVA) for values during body heating. Statistical significance was accepted for P < 0.05.
RESULTS
Subject characteristics.
As shown in Table 1, individuals in the T2DM group were of similar age as the control group, but were somewhat heavier and had higher body mass index (BMI) values. Interestingly, %body fat was similar between groups. As expected, individuals with T2DM had higher fasting plasma glucose values and higher hemoglobin A1c levels.
Table 1.
Descriptive data for T2DM and control subjects
| Control | T2DM | |
|---|---|---|
| n | 14 (7 female, 7 male) | 10 (3 female, 7 male) |
| Age, yr | 55±3 | 57±3 |
| Height, cm | 170±2 | 173±2 |
| Weight, kg | 73±4 | 84±3† |
| BMI, kg/m2 | 25±1 | 28±1* |
| %Fat | 35±2 | 36±2 |
| Fasting glucose, mg/dl | 95±4 | 145±10* |
| Hemoglobin A1c, % | 5.4±0.1 | 6.7±0.3* |
Values are means ± SE for n subjects. T2DM, type 2 diabetes mellitus; BMI, body mass index.
P < 0.05 vs. control;
P = 0.05 vs. control.
Baseline CVC.
CVC was significantly higher in T2DM subjects compared with control subjects before l-NAME administration (T2DM 16 ± 2 vs. control 11 ± 1%maxCVC; P < 0.05) and was not different between groups during l-NAME (T2DM 10 ± 1 vs. control 8 ± 1%maxCVC; P > 0.05). The difference between groups in pre-l-NAME baseline CVC was also present when CVC was expressed as absolute values [T2DM 0.36 ± 0.04 vs. control 0.28 ± 0.02 arbitrary units (au)/mmHg; P < 0.05], suggesting that this difference was not an artifact of normalization to maximum CVC. The calculated contribution of NO to baseline CVC in the two groups demonstrated a strong trend to be greater in the T2DM group compared with control subjects (T2DM 4 ± 1 vs. control 1 ± 1%maxCVC; P = 0.06).
Baseline FBF and FVC.
Baseline FBF was 2.0 ± 0.3 ml·100 ml−1·min−1 in the control group and 1.6 ± 0.3 ml·100 ml−1·min−1 in the T2DM group (P > 0.05). FVC was also not different at baseline between groups (control 2.2 ± 0.3 vs. T2DM 1.7 ± 0.3 ml·100 ml−1·min−1·100 mmHg−1; P > 0.05).
Forearm vascular responses to whole body heating.
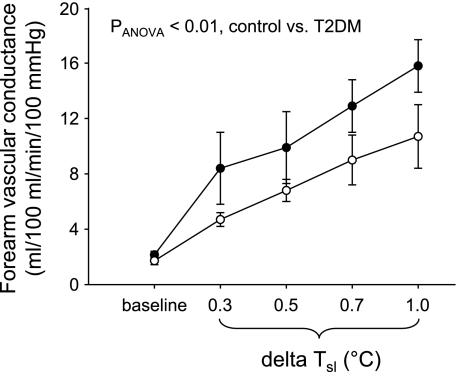
Figure 1 shows FVC during whole body heating in control and T2DM groups. As can be seen in Fig. 1, FVC was significantly lower during whole body heating in the T2DM group (PANOVA < 0.01). FBF values were also lower during heating in the T2DM group (PANOVA < 0.01). For example, FBF at the peak of heat stress (ΔTsl = 1.0°C) was 13.1 ± 1.7 ml·100 ml−1·min−1 in control subjects and 9.0 ± 1.6 ml·100 ml−1·min−1 in T2DM subjects. Similar results were obtained when FBF and FVC data were expressed as changes from baseline (Δ). ΔFBF and ΔFVC were both lower during body heating in the T2DM group (PANOVA < 0.02). For example, ΔFBF at the peak of heat stress (ΔTsl = 1.0°C) was 11.1 ± 2.2 ml·100 ml·min−1 in control and 7.3 ± 1.7 ml·100 ml−1·min−1 in T2DM.
Fig. 1.
Forearm vascular conductance at baseline and during whole body heating in control subjects (•) and individuals with type 2 diabetes mellitus (T2DM; ○) (n = 6 in each group). As shown, cutaneous vasodilator responses were significantly lower during body heating in the T2DM group (PANOVA < 0.01).
CVC responses to whole body heating.
Increases in body temperature resulted in progressive increases in %maxCVC at vehicle-treated LDF sites that were similar in control subjects and in individuals with T2DM at each of the following increments in Tsl: 0.3°C: control 28 ± 6 vs. T2DM 30 ± 7%maxCVC; 0.5°C: control 40 ± 6 vs. T2DM 35 ± 7%maxCVC; 0.7°C: control 45 ± 7 vs. T2DM 40 ± 7%maxCVC; 1.0°C: control 54 ± 6 vs. T2DM 50 ± 6%maxCVC (P > 0.05 for all). The corresponding absolute values for CVC were as follows: 0.3°C: control 0.63 ± 0.11 vs. T2DM 0.65 ± 0.22 au/mmHg; 0.5°C: control 0.94 ± 0.16 vs. T2DM 0.75 ± 0.23 au/mmHg; 0.7°C: control 1.08 ± 0.19 vs. T2DM 0.83 ± 0.23 au/mmHg; 1.0°C: control 1.31 ± 0.17 vs. T2DM 1.06 ± 0.22 au/mmHg (P > 0.05 for all).
Contribution of NO.
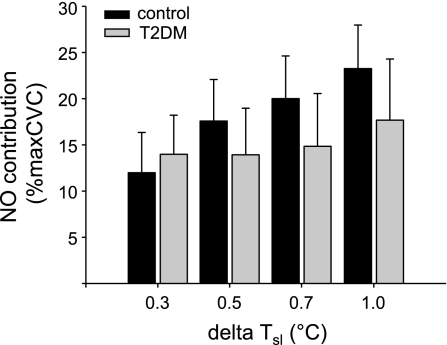
Figure 2 shows the calculated contribution of NO during body heating. This contribution of NO was quite variable during body heating within both groups and was not different between groups (P > 0.05).
Fig. 2.
Calculated contribution of nitric oxide (NO) to cutaneous vascular conductance (CVC) at increases in internal temperature (ΔTsl) of 0.3, 0.5, 0.7, and 1.0°C during body heating. Although overall the contribution of NO was similar between groups (P > 0.05), there was extensive variability in NO contribution within each group, as has been noted for this variable in previous studies in humans. %maxCVC, % maximal CVC.
Maximum CVC.
Absolute values for maximum CVC during SNP microdialysis were 2.56 ± 0.25 au/mmHg for control subjects and 2.24 ± 0.23 au/mmHg for T2DM subjects (P = 0.077). This represented a 12.5% smaller maximum CVC in the T2DM group.
DISCUSSION
The major new findings of the present study are that 1) the absolute cutaneous vasodilator responses to whole body heating, as assessed by venous occlusion plethysmography, were significantly lower during body heating in T2DM subjects compared with control subjects (Fig. 1); 2) within this smaller overall vasodilation, the relative contribution of NO to cutaneous vasodilation during whole body heating was similar between groups (Fig. 2); and 3) individuals with T2DM tended to show a greater contribution of NO to baseline levels of skin blood flow compared with healthy control subjects.
Laser-Doppler measurement of cutaneous microvascular perfusion in humans has many advantages: the measurement is continuous, noninvasive, and specific to the cutaneous microcirculation (5, 11). One of the limitations of LDF measurement is that it is semiquantitative in nature, and the absolute values recorded can depend on factors other than vasodilation, such as the extent of vascularity in a given site of measurement (5). To compare results among subjects, data are often normalized to a maximum vasodilator response, such as the nitroprusside-mediated vasodilation used in the present study (7–9). However, this may complicate interpretation when two groups are being compared who may have different maximum vasodilator capacities, as in the present study (19, 22). For these reasons, we decided to complement our LDF measurements with measurements of FBF by venous occlusion plethysmography. These FBF measurements are discontinuous and are not specific to the cutaneous circulation, but increases in FBF and vascular conductance during resting heating are confined to the skin circulation, and thus can be used to measure cutaneous vasodilation in a resting forearm (4, 5, 21). In the present study, this afforded us the valuable opportunity to demonstrate a quantitative difference in cutaneous vasodilator responses between our two groups. The FBF and vascular conductance data demonstrate significantly lower vasodilator responses in our T2DM group (see Fig. 1). This is consistent with our hypothesis that decreased skin blood flow during body heating is an important contributor to the potential for impaired thermoregulation in individuals with T2DM (22).
In this context, we were somewhat surprised to note a smaller difference in absolute values for maximum local CVC (during microdialysis of SNP) in our present study compared with those we have previously reported (19, 22). The difference seen in the present study was ∼12.5% (P = 0.077), compared with the 20–25% that we have seen previously (19, 22). It is not clear to us why this was the case, although interindividual variability in maximum vasodilation within both groups of subjects probably contributed. Nevertheless, the trend in the present study was consistent with our previous reports of lower maximum vasodilation in the T2DM population.
In human skin, NO has been shown to contribute ∼30% to the reflex (whole body) vasodilator response that is essential for heat dissipation during core hyperthermia (7, 18). In the present study, our goal was to evaluate whether the NO contribution to whole body cutaneous vasodilation was diminished in individuals with T2DM. A strength of the present study was that we were able to combine laser-Doppler and venous occlusion plethysmography measurements of skin blood flow. We found that overall vasodilator responses were less in the T2DM group, but that the contribution of NO was similar between groups. Average values for NO contribution were somewhat lower in the T2DM group (see Fig. 2).
The extensive interindividual variability in NO contribution noted in the present study could have led to an inability to detect a decrease in the NO contribution that may be physiologically significant in some individuals with T2DM. It was not feasible for us to increase our sample size enough to overcome this limitation (sample size analysis showed that 165 subjects/group would have been required for 80% power to detect a statistically significant difference, given the amount of variability). The interindividual variability in NO contribution to reflex cutaneous vasodilation has been noted in previous studies in healthy humans (17, 18) and remains a challenge to interpretation of the present data. In this context, we feel that an appropriate interpretation of our present FBF and CVC data, taken together, is that the NO contribution represents a similar percentage of a smaller overall vasodilation in the T2DM group.
In addition to measuring responses to body heating, a secondary goal of the present study was to evaluate the contribution of NO to skin blood flow during baseline (normothermic) conditions in our two groups. We found that the contribution of NO to resting skin blood flow, or “tonic” vasodilation, was greater in our T2DM group compared with the control subjects. Since NO-mediated vasodilation has generally been shown to be decreased in T2DM, it may seem paradoxical that the contribution of NO to baseline skin blood flow would be greater in our T2DM group. A possible explanation relates to the potential impairment of sympathetic vasoconstrictor control of skin blood flow in T2DM at baseline (22). Less sympathetically mediated vasoconstriction would increase baseline blood flow, and shear stress, which would then increase basal release of NO from the endothelium. Although not conclusive at the present time, this possibility could explain why both bretylium treatment (22) and NOS inhibition (19) abolished differences in baseline CVC between control and T2DM groups.
In a previous study (19), we reported that comparison of baseline CVC between vehicle-treated sites and l-NAME-treated sites showed a greater difference (NO contribution) in T2DM subjects compared with control subjects, but we did not measure CVC before and during NOS inhibition at the same site. We followed up in the present study by evaluating whether a comparison of baseline CVC before and after l-NAME administration at the same site would show similar results. Our present results are consistent with those from our previous study: baseline CVC values were greater in the T2DM group before, but not during, NOS inhibition, and the calculated contribution of NO to baseline CVC showed a strong trend to be greater in the T2DM group (P = 0.06).
With regard to clinical relevance for diabetes, it is important to note that the individuals in our T2DM group were relatively healthy. We excluded subjects with significant clinical neuropathy, with heart disease or history of heart disease, and/or with other comorbidities associated with T2DM. In doing so, our attempt was to isolate any specific influences of T2DM (as separate from other potentially confounding variables) on cutaneous microvascular control during whole body heating. However, this also limited our subject population to relatively healthy individuals with T2DM. Individuals with less well-controlled T2DM might have more significant impairments in cutaneous vasodilation and NO-mediated responses to whole body heating. Furthermore, the effects, if any, of ambient glucose concentrations and long-term glycemic control on thermoregulatory control of the skin circulation deserve further study.
In summary, we report that absolute skin blood flow responses to whole body heating (measured as FBF) were decreased in individuals with T2DM compared with control subjects of similar age. In the context of the smaller overall response, the relative contribution of NO was similar between groups. Our T2DM subjects also showed a trend for greater contribution of NO to resting (pre-heat stress) vascular tone compared with the control group. In combination with our previous studies (19, 22), our present data suggest that impaired vasodilation in the skin circulation is a major contributor to the potential for impaired thermoregulation during hyperthermia in individuals with T2DM (12, 15, 16).
GRANTS
This work was supported by National Institutes of Health Grants HL-73884 and CTSA-UL1-RR-024150 (to the Mayo Clinic).
Acknowledgments
We are grateful for the assistance of David Sletten, and we thank the subjects for their participation in these studies.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Caballero AE, Arora S, Saouaf R, Lim SC, Smakowski P, Park JY, King GL, LoGerfo FW, Horton ES, Veves A. Microvascular and macrovascular reactivity is reduced in subjects at risk for Type 2 diabetes. Diabetes 48: 1856–1862, 1999. [DOI] [PubMed] [Google Scholar]
- 2.Charkoudian N Skin blood flow in adult human thermoregulation: how it works, when it does not, and why. Mayo Clin Proc 78: 603–612, 2003. [DOI] [PubMed] [Google Scholar]
- 3.Charkoudian N, Joyner MJ, Sokolnicki LA, Johnson CP, Eisenach JH, Dietz NM, Curry TB, Wallin BG. Vascular adrenergic responsiveness is inversely related to tonic activity of sympathetic vasoconstrictor nerves in humans. J Physiol 572: 821–827, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Johnson JM, Proppe DW. Cardiovascular adjustments to heat stress. In: Handbook of Physiology. Environmental Physiology. Bethesda, MD: Am Physiol Soc, 1996, sect. 4, vol. 1, p. 215–244. [Google Scholar]
- 5.Johnson JM, Taylor WF, Shepherd AP, Park MK. Laser-Doppler measurement of skin blood flow: comparison with plethysmography. J Appl Physiol 56: 798–803, 1984. [DOI] [PubMed] [Google Scholar]
- 6.Joyner MJ, Dietz NM, Shepherd JT. From Belfast to Mayo and beyond: the use and future of plethysmography to study blood flow in human limbs. J Appl Physiol 91: 2431–2441, 2001. [DOI] [PubMed] [Google Scholar]
- 7.Kellogg DL, Crandall CG, Liu Y, Charkoudian N, Johnson JM. Nitric oxide and cutaneous active vasodilation during heat stress in humans. J Appl Physiol 85: 824–829, 1998. [DOI] [PubMed] [Google Scholar]
- 8.Kellogg DL, Liu YK, Kosiba IF, O'Donnell D. Role of nitric oxide in the vascular effects of local warming of the skin in humans. J Appl Physiol 86: 1185–1190, 1999. [DOI] [PubMed] [Google Scholar]
- 9.Minson CT, Berry LT, Joyner MJ. Nitric oxide and neurally mediated regulation of skin blood flow during local heating. J Appl Physiol 91: 1619–1626, 2001. [DOI] [PubMed] [Google Scholar]
- 10.Minson CT, Holowatz LA, Wong BJ, Kenney WL, Wilkins BW. Decreased nitric oxide- and axon reflex-mediated cutaneous vasodilation with age during local heating. J Appl Physiol 93: 1644–1649, 2002. [DOI] [PubMed] [Google Scholar]
- 11.Oberg PA Laser-Doppler flowmetry. Crit Rev Biomed Eng 18: 125–163, 1990. [PubMed] [Google Scholar]
- 12.Petrofsky JS, Besonis C, Rivera D. Circulatory responses to hydrotherapy and dry heat in individuals with type 2 diabetes. Int J Ther Rehabil 12: 491–497, 2005. [Google Scholar]
- 13.Petrofsky JS, Besonis C, Rivera D, Schwab E, Lee S. Impairment in orthostatic tolerance during heat exposure in individuals with Type I and Type II diabetes. Med Sci Monit 11: CR153–CR159, 2005. [PubMed] [Google Scholar]
- 14.Rowell LB Cardiovascular adjustments to thermal stress. In: Handbook of Physiology. The Cardiovascular System: Peripheral Circulation and Organ Blood Flow. Bethesda, MD: Am Physiol Soc, 1983, sect. 2, vol. III, p. 967–1023. [Google Scholar]
- 15.Schuman SH Patterns of urban heat-wave deaths and implications for prevention: data from New York and St. Louis during July, 1966. Environ Res 5: 59–75, 1972. [DOI] [PubMed] [Google Scholar]
- 16.Semenza JC, McCullough JE, Flanders WD, McGeehin MA, Lumpkin JR. Excess hospital admissions during the July 1995 heat wave in Chicago. Am J Prev Med 16: 269–277, 1999. [DOI] [PubMed] [Google Scholar]
- 17.Shastry S, Dietz NM, Halliwill JR, Reed AS, Joyner MJ. Effects of nitric oxide synthase inhibition on cutaneous vasodilation during body heating in humans. J Appl Physiol 85: 830–834, 1998. [DOI] [PubMed] [Google Scholar]
- 18.Shastry S, Minson CT, Wilson SA, Dietz NM, Joyner MJ. Effects of atropine and l-NAME on cutaneous blood flow during body heating in humans. J Appl Physiol 88: 467–472, 2000. [DOI] [PubMed] [Google Scholar]
- 19.Sokolnicki LA, Roberts SK, Wilkins BW, Basu A, Charkoudian N. Contribution of nitric oxide to cutaneous microvascular dilation in individuals with type 2 diabetes mellitus. Am J Physiol Endocrinol Metab 292: E314–E318, 2007. [DOI] [PubMed] [Google Scholar]
- 20.Veves A, Akbari CM, Primavera J, Donaghue VM, Zacharoulis D, Chrzan JS, DeGirolami U, LoGerfo FW, Freeman R. Endothelial dysfunction and the expression of endothelial nitric oxide synthetase in diabetic neuropathy, vascular disease, and foot ulceration. Diabetes 47: 457–463, 1998. [DOI] [PubMed] [Google Scholar]
- 21.Wenger CB, Bailey RB, Roberts MF, Nadel ER. Interaction of local and reflex thermal effects in control of forearm blood flow. J Appl Physiol 58: 251–257, 1985. [DOI] [PubMed] [Google Scholar]
- 22.Wick DE, Roberts SK, Basu A, Sandroni P, Fealey RD, Sletten D, Charkoudian N. Delayed threshold for active cutaneous vasodilation in patients with Type 2 diabetes mellitus. J Appl Physiol 100: 637–641, 2006. [DOI] [PubMed] [Google Scholar]