Abstract
Tendon is mainly composed of collagen and an aqueous matrix of proteoglycans that are regulated by enzymes called matrix metalloproteinases (MMPs) and tissue inhibitors of metalloproteinases (TIMPs). Although it is known that resistance exercise (RE) and sex influence tendon metabolism and mechanical properties, it is uncertain what structural and regulatory components contribute to these responses. We measured the mRNA expression of tendon's main fibrillar collagens (type I and type III) and the main proteoglycans (decorin, biglycan, fibromodulin, and versican) and the regulatory enzymes MMP-2, MMP-9, MMP-3, and TIMP-1 at rest and after RE. Patellar tendon biopsy samples were taken from six individuals (3 men and 3 women) before and 4 h after a bout of RE and from a another six individuals (3 men and 3 women) before and 24 h after RE. Resting mRNA expression was used for sex comparisons (6 men and 6 women). Collagen type I, collagen type III, and MMP-2 were downregulated (P < 0.05) 4 h after RE but were unchanged (P > 0.05) 24 h after RE. All other genes remained unchanged (P > 0.05) after RE. Women had higher resting mRNA expression (P < 0.05) of collagen type III and a trend (P = 0.08) toward lower resting expression of MMP-3 than men. All other genes were not influenced (P > 0.05) by sex. Acute RE appears to stimulate a change in collagen type I, collagen type III, and MMP-2 gene regulation in the human patellar tendon. Sex influences the structural and regulatory mRNA expression of tendon.
Keywords: collagen, proteoglycan, matrix metalloproteinases
the tendon is a metabolically active structure that transmits force from muscle to bone for mechanical movement. The extracellular matrix (ECM) of tendon mainly consists of collagen and elastin fibers surrounded by an aqueous matrix of proteoglycans, glycosaminoglycans (GAGs), and glycoproteins (20). Collagen, specifically collagen type I, is the most abundant protein in tendon, forming 60–80% of tendon dry weight (20). Collagen type III is found in significantly less abundance than collagen type I, but increased expression is an indicator of tendon injury (9, 11, 47). Collagen turnover, an important component of a healthy ECM, is regulated by a group of enzymes called matrix metalloproteinases (MMPs) and tissue inhibitors of metalloproteinases (TIMPs) (37).
Physical activity stimulates mechanical and structural alterations in tendon (22, 25, 26, 28, 44), which can subsequently influence muscle performance (6). Cross-sectional studies demonstrate that tendon size is greater in individuals who habitually exercise than in untrained individuals (21, 49), and it has been shown that prolonged resistance training can induce tendon hypertrophy (22). Increased tendon size is a potential result of elevated collagen synthesis, which has been demonstrated to increase after an acute bout of aerobic exercise (29, 39). On the contrary, some prolonged resistance training studies show that training can induce tendon adaptations, such as increased tendon stiffness, without changes in tendon size (25, 26, 28, 44). Therefore, components of the ECM may be adapting independent of tendon hypertrophy to cause these mechanical alterations. The role of each ECM component in these training adaptations has yet to be elucidated (36).
Mechanical properties and metabolism in tendon have been shown to be different in men and women (27, 35, 38, 58). The elastic modulus of tendon is significantly decreased in women compared with men, possibly leading to an inefficient matrix for force transfer between muscle and bone (27). In addition, cross-sectional studies reveal that tendons of habitually trained women are the same size as those of untrained women, whereas men's tendons are hypothesized to hypertrophy with training (58). Tendon collagen synthesis at rest and after an acute bout of exercise is significantly lower in women than in men (38). It is uncertain whether other structural and regulatory components of tendon are influenced by sex to cause these mechanical and metabolic differences.
Given the limited amount of information regarding tendon adaptations to exercise in humans, the main objective of this investigation was to identify changes in mRNA expression of some of the main structural and regulatory components of tendon after an acute bout of resistance exercise (RE). A secondary objective of this investigation was to identify sex differences in mRNA expression of the structural and regulatory genes of tendon at rest. The structural components of tendon, including the main fibrillar collagens, collagen type I and collagen type III, and the main proteoglycans, decorin, biglycan, fibromodulin, and versican, were studied. The regulatory components, including the enzymes MMP-2, MMP-9, MMP-3, and TIMP-1, were studied as well.
MATERIALS AND METHODS
Subjects and Experimental Design
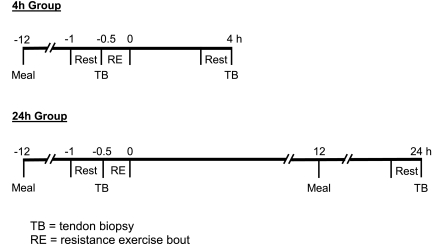
Twelve recreationally active individuals (6 men and 6 women) were included in this investigation. For the analysis of mRNA expression after RE, subjects were divided into two groups of six, with equal numbers of men and women in each group: a 4-h group (27 ± 1 yr old, 173 ± 1 cm height, 74.8 ± 4.1 kg body wt) and a 24-h group (23 ± 1 yr old, 167 ± 1 cm height, 62.6 ± 4.8 kg body wt). Sex comparisons of mRNA expression were made using the resting (preexercise) measurements in the six men (26 ± 1 yr old, 174 ± 3 cm height, 77.2 ± 2.8 kg body wt) and six women (24 ± 1 yr old, 166 ± 3 cm height, 60.2 ± 4.2 kg body wt). After approval by the Institutional Review Board at Ball State University, all the procedures, risks, and benefits associated with the study were explained to the subjects before they signed a consent form, which adhered to the guidelines of the Institutional Review Board. The experimental design is presented in Fig. 1 and described in detail below.
Fig. 1.
Experimental protocol. The first biopsy in each group was used for the resting sex comparisons.
Dietary and Activity Control
The evening meals were supplied before the rest (preexercise) and 24-h postexercise tendon biopsies in liquid form (Ensure Plus, Ross, Columbus, OH; 53% carbohydrate, 15% protein, and 32% fat) and provided 50% of the subject's estimated caloric need [1.5 times the subject's predicted resting metabolic rate (12)]. This level of dietary control standardized the composition, amount, and timing (i.e., duration of fast) of the final meal consumed before the morning tendon biopsies. In addition, subjects were asked to refrain from physical activity or exercise training 3 days before the start of the study and for the duration of the study. Subjects in the 4-h group rested quietly in the laboratory during the 4-h postexercise period; subjects in the 24-h group were discharged and returned on the following morning for the postexercise biopsy.
RE Protocol
Each subject completed a bout of unilateral knee extension exercise, with the right leg on a knee extension weight stack device (Cybex Eagle, Medway, MA). The workload was set to ∼70% of the subject's concentric one repetition maximum, which was determined ≥1 wk before the resting tendon biopsy procedure. After 5 min of light cycling (∼50 W) and 2 sets of 5 repetitions with light loads, each subject completed 3 sets of 10 repetitions, with 2 min of rest between sets (43).
Tendon Biopsy
After 30 min of supine rest, a patellar tendon biopsy was taken before exercise (left leg) and 4 (4-h group) or 24 (24-h group) h after the RE bout (right leg). All biopsies were taken from the central portion of the patellar tendon by the same investigator in a consistent fashion for all subjects. Tendon tissue was obtained after local anesthesia (1.0 ml of 1% lidocaine HCl) using a Bard Magnum biopsy instrument and a 14-gauge needle (models MG1522 and MN1410, CR Bard, Covington, GA) (19, 39, 41). The tissue was immediately inspected, nontendon tissue was removed under ×10–20 magnification, and tendon tissue was placed in a preweighed RNase- and DNase-free tube containing 400 μl of RNAlater (Ambion, Austin, TX). Each tube was reweighed to obtain the tissue weight (5.04 ± 0.26 mg), incubated at 4°C for 24 h, and then stored at −20°C until mRNA analysis (described below).
Tendon RNA Analysis
Disruption and homogenization of tissue.
Each tendon sample was removed from RNAlater and powdered/ground into small pieces in a liquid nitrogen-filled mortar that was kept on ice. Before use, the mortar and pestle were treated with RNaseZap (Ambion), autoclaved, and stored at −20°C. The liquid nitrogen-tissue suspension was transferred to a liquid nitrogen-cooled six-well culture plate that was kept on ice. The liquid nitrogen was allowed to evaporate from the well, and with care taken to prevent thawing of the sample, 600 μl of lysis buffer (RNeasy Mini Kit, Qiagen, Valencia, CA) were added to the well. The solution was pipetted up and down several times for collection of the ground tissue into the tip, and then the solution was loaded onto a QIAshredder spin column (Qiagen). The solution was homogenized by passage through the QIAshredder spin column homogenizer during a 3-min spin at 15,000 g. The supernatant was carefully transferred to a new tube, leaving a well-formed pellet on the bottom of the spin column collection tube.
Total RNA extraction and RNA quality check.
RNA was extracted from the supernatant using the RNeasy Mini Kit according to the manufacturer's protocol (Qiagen). Elimination of genomic DNA contamination was done on-column by DNase digestion following the protocol provided by the manufacturer with the RNeasy Mini Kit (Qiagen).
One microliter of each total RNA extract [1:1 (vol/vol) with water] was analyzed using the RNA 6000 Pico LabChip kit on a bioanalzyer (model 2100, Agilent Technologies, Palo Alto, CA). This system reported detailed information about quantity and integrity of the RNA samples. Each RNA sample was electrophoretically separated into two peaks of 18S and 28S rRNA. Data were displayed as a gel-like image and an electropherogram (16, 43, 53). Sample analyses were performed as described by the manufacturer. On average, the yield of total RNA from tendon tissue was 8.07 ± 1.16 ng RNA/mg tendon wet wt.
Reverse transcription.
Oligo(dT)-primed first-strand cDNA was synthesized using SuperScript II RT (Invitrogen, Carlsbad, CA). This system was optimized for sensitive RT-PCR on small amounts of RNA. A first reaction mix of 12 μl for each sample, consisting of 8 μl of RNA extract (2.4 ng), 1 μl of 10 mM dNTP, 1 μl of oligo(dT)12–18 (0.5 μg/μl), and 2.0 μl of nuclease-free water, was incubated at 65°C for 5 min and then placed on ice for 1 min. A second reaction mix of 7 μl, consisting of 4 μl of 5× first-strand buffer, 2 μl of 0.1 M DTT, and 1 μl of RNaseOUT recombinant RNase inhibitor, was added to the first reaction mix and incubated at 42°C for 2 min. Finally, 1 μl (50 U) of SuperScript II RT was added to each tube (giving a total volume of 20 μl), incubated at 42°C for 50 min and then at 70°C for 15 min to terminate the reaction, and chilled to −4°C. The resulting cDNA samples were diluted with water to a final volume of 40 μl. All thermal incubations and chilling were done in a Peltier thermal cycler with dual-block DNA engine (MJ Research, Waltham, MA) to provide temperature homogeneity and identical temperature ramping for all samples.
Real-time PCR.
Quantification of mRNA transcription was performed in a 72-well centrifugal real-time cycler (Rotor-Gene 3000, Corbett Research, Mortlake, NSW, Australia). All primers for each gene of interest (GOI) were mRNA specific and designed for gene expression real-time PCR analysis (Vector NTI Advance 9 software, Invitrogen) using SYBR Green chemistry (Table 1). GAPDH was chosen as the housekeeping gene (HKG) for the internal control on the basis of previous validation (53). The PCR mix consisted of 12.5 μl of SYBR Green JumpStart Taq Ready Mix (Sigma-Aldrich, St. Louis, MO), 0.5 μl each of 10 μM forward and reverse primers, 2.5 μl of cDNA, and nuclease-free water to a final volume of 25 μl. Each tendon sample was prepared in duplicate for all assays (coefficient of variation = 0.58 ± 0.03%).
Table 1.
Primer pair and amplicon information for genes of interest and housekeeping gene (GAPDH)
| GenBank No. | Amplicon Size, bp | Annealing Temperature, °C | mRNA Region, bp | |
|---|---|---|---|---|
| Collagen type I | NM_000088 | 112 | 60 | 207–318 |
| 5′-GGCCAAGACGAAGACATCCCACCAA-3′ (sense) | ||||
| 5′-TGCCGTTGTCGCAGACGCAGAT-3′ (antisense) | ||||
| Collagen type III | NM_000090 | 124 | 60 | 1400–1523 |
| 5′-GAGGTGGTGCAGGTGAGCCTGGTAA-3′ (sense) | ||||
| 5′-GATCCATCCTTGCCATCTTCGCCTT-3′ (antisense) | ||||
| Decorin | NM_009120 | 116 | 60 | 954–1069 |
| 5′-CACCAATCCGCTGAAGAGCTCAGGAA-3′ (sense) | ||||
| 5′-GAGGAAGACCTTGAGGAATGCTGGTGATA-3′ (antisense) | ||||
| Biglycan | NM_001711 | 103 | 60 | 741–843 |
| 5′-GAACTGCATCGAGATGGGCGGGAA-3′ (sense) | ||||
| 5′-CTTGGCCTCTGAGATGCGCAGGTA-3′ (antisense) | ||||
| Fibromodulin | NM_002023 | 108 | 60 | 891–998 |
| 5′-GCTAGACCTCTCCTACAACCAGCTGCAGAA-3′ (sense) | ||||
| 5′-AAGCTGCTGATGGAGAACTCATTGATCCTA-3′ (antisense) | ||||
| Versican (V1) | NM_004385 | 112 | 60 | 1209–1320 |
| 5′-TGTGGAGGTGGTCTACTTGGGGTGAGAA-3′ (sense) | ||||
| 5′-CACTCATTCGACGTTTAAAGCAGTAGGC-3′ (antisense) | ||||
| MMP-2 | NM_004530 | 103 | 60 | 2097–2199 |
| 5′-TGGATCCTGGCTTCCCCAAGCTCAT-3′ (sense) | ||||
| 5′-AAGAAGTAGCTGTGACCGCCGCCCT-3′ (antisense) | ||||
| MMP-9 | NM_004994 | 94 | 60 | 1176–1269 |
| 5′-GCTTCTGCCCGGACCAAGGATACAGTT-3′ (sense) | ||||
| 5′-GCCTCCGGCACTGAGGAATGATCTAA-3′ (antisense) | ||||
| MMP-3 | NM_002422 | 89 | 59 | 671–759 |
| 5′-ATGGACAAAGGATACAACAGGGACCAA-3′ (sense) | ||||
| 5′-TGTTGGCTGAGTGAAAGAGACCCAGG-3′ (antisense) | ||||
| TIMP-1 | NM_003254 | 131 | 60 | 410–540 |
| 5′-AAGCCTTAGGGGATGCCGCTGACAT-3′ (sense) | ||||
| 5′-GAGTCCATCCTGCAGTTTTCCAGCAAT-3′ (antisense) | ||||
| GAPDH | NM_002046 | 114 | 59 | 10–123 |
| 5′-CCTCCTGTTCGACAGTCAGCCGCAT-3′ (sense) | ||||
| 5′-GCGCCCAATACGACCAAATCCGTT-3′ (sense) |
MMP, matrix metalloproteinase; TIMP, tissue inhibitor of metalloproteinase.
The PCR parameters were as follows: initial denaturing at 95°C for 2 min to activate the Taq polymerase followed by 45 cycles of 20 s at 95°C, 20 s at 59°C or 60°C (Table 1), and 20 s at 72°C with fluorescence acquisition. A melt curve analysis was generated by the Rotor-Gene software after the end of the final cycle for each sample by continuous monitoring of SYBR Green fluorescence throughout the temperature ramp from 72°C to 99°C in 1°C increments and a 5-s hold at each increment. A single melt peak observed for each sample was used to validate the presence of a single product.
The amplification data were analyzed in two ways. 1) mRNA expression of each GOI at 4 and 24 h after exercise was compared with levels at rest using the 2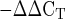 relative quantification method (31, 43, 59). 2) Sex differences in mRNA expression at rest (before exercise) were evaluated for each GOI using the 2
relative quantification method (31, 43, 59). 2) Sex differences in mRNA expression at rest (before exercise) were evaluated for each GOI using the 2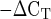 relative quantification method (31, 43, 52, 57). These methods are based on the assumption that the difference in threshold cycles (ΔCT) between the GOI and the HKG is proportional to the relative expression of the GOI (31, 52).
relative quantification method (31, 43, 52, 57). These methods are based on the assumption that the difference in threshold cycles (ΔCT) between the GOI and the HKG is proportional to the relative expression of the GOI (31, 52).
For each real-time PCR assay, a dilution curve was generated using gene-specific primers to evaluate reaction efficiencies. As template for the curve, cDNA was reverse transcribed from 200 ng of human skeletal muscle RNA (Ambion), a twofold dilution series was prepared, and each dilution was assayed in duplicate. The real-time PCR amplification calculated by the Rotor-Gene software was specific and highly efficient, generating an average reaction efficiency of 1.06 ± 0.01, an R2 of 0.99 ± 0.002, and a slope of 3.20 ± 0.02. A single melt peak was observed for each GOI.
MMP-9 mRNA expression was not quantifiable in any of the subjects at rest or after exercise. Although MMP-9 expression was detectable in most of the samples, amplification occurred at high CT values (∼36) and was not reproducible in duplicate. The average CT values for GAPDH at rest and 4 and 24 h after exercise were 21.64 ± 0.18, 21.44 ± 0.26, and 21.94 ± 0.16, respectively.
Statistical Analysis
A paired t-test was applied to determine whether acute RE influences mRNA expression of each GOI by comparing mRNA levels at rest with mRNA levels 4 and 24 h after exercise. A two-sample t-test was applied to determine whether sex influences mRNA expression of each GOI at rest. Significance was set at an α-level of 0.05 for all comparisons. Values are means ± SE.
RESULTS
mRNA Expression After RE
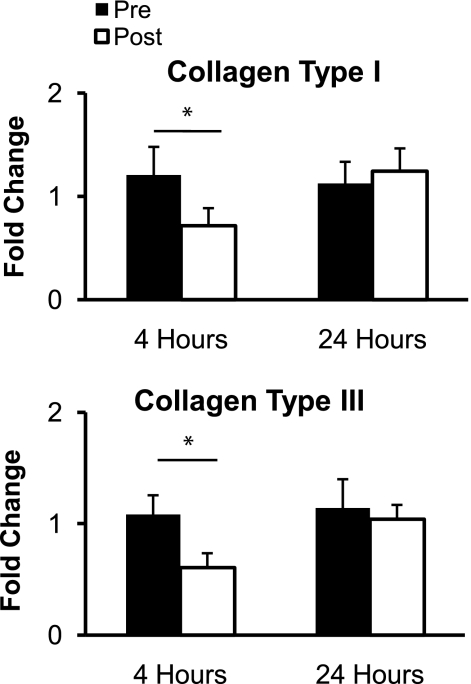
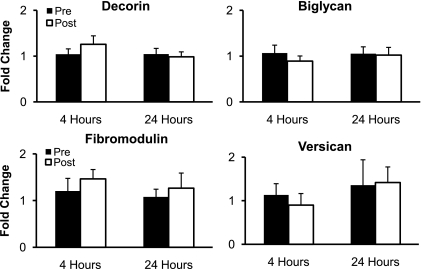
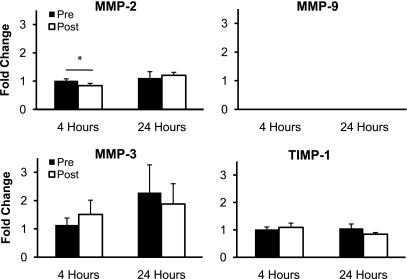
The mRNA expression of collagen type I and collagen type III significantly decreased (P < 0.05) 4 h after RE but returned to resting levels 24 h after RE (P > 0.05; Fig. 2). The mRNA expression of the proteoglycans decorin, biglycan, fibromodulin, and versican did not significantly change (P > 0.05) from resting levels at 4 or 24 h after RE (Fig. 3). The mRNA expression of the regulatory enzyme MMP-2 significantly decreased (P < 0.05) 4 h after RE and returned to resting levels 24 h after exercise (P > 0.05; Fig. 4). The mRNA expression of MMP-3 and TIMP-1 did not significantly change (P > 0.05) from resting levels at 4 or 24 h after RE (Fig. 4). MMP-9 mRNA expression was not quantifiable at rest or after RE (Fig. 4).
Fig. 2.
Mean mRNA expression of collagen type I and collagen type III at rest (Pre) and 4 and 24 h after exercise (Post). Expression levels for each gene were normalized to GAPDH using the 2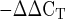 method. *P < 0.05.
method. *P < 0.05.
Fig. 3.
Mean mRNA expression of the proteoglycans decorin, biglycan, fibromodulin, and versican at rest and 4 and 24 h after exercise. Expression levels were normalized to GAPDH using the 2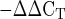 method.
method.
Fig. 4.
Mean mRNA expression of the regulatory genes matrix metalloproteinase (MMP)-2, MMP-9, MMP-3, and tissue inhibitor of metalloproteinase-1 (TIMP-1) at rest and 4 and 24 h after exercise. Expression levels were normalized to GAPDH using the 2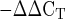 method. *P < 0.05.
method. *P < 0.05.
mRNA Expression at Rest: Men vs. Women
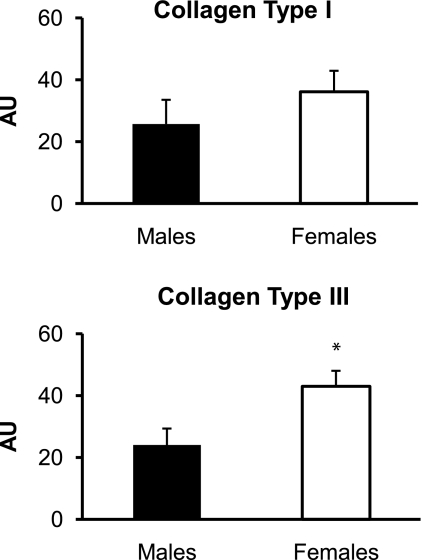
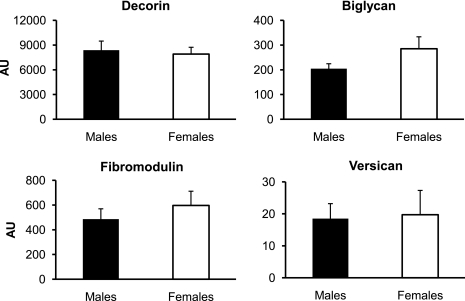
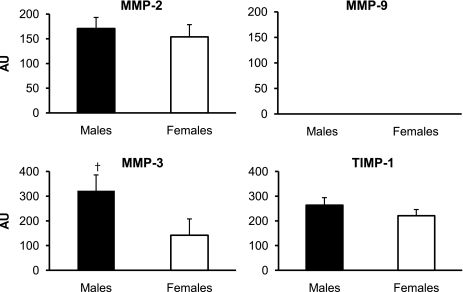
The mRNA expression of collagen type III at rest was significantly higher (P < 0.05) in the women than in the men, whereas collagen type I mRNA expression was similar (P > 0.05) between sexes (Fig. 5). The mRNA expression of the proteoglycans decorin, biglycan, fibromodulin, and versican at rest was not significantly different (P > 0.05) between the women and the men (Fig. 6). There was a trend toward higher (P = 0.08) MMP-3 mRNA expression at rest in the men than in the women, whereas the mRNA expression of MMP-2 and TIMP-1 at rest was not significantly different (P > 0.05; Fig. 7). MMP-9 mRNA expression was not quantifiable at rest in the men or the women (Fig. 7).
Fig. 5.
Mean mRNA expression of collagen type I and collagen type III in men and women at rest. Expression levels for each gene of interest were normalized to GAPDH using the 2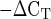 method. AU, arbitrary units (2
method. AU, arbitrary units (2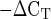 × 103). *P < 0.05 vs. males.
× 103). *P < 0.05 vs. males.
Fig. 6.
Mean mRNA expression of the proteoglycans decorin, biglycan, fibromodulin, and versican in men and women at rest. Expression levels for each gene of interest were normalized to GAPDH using the 2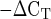 method.
method.
Fig. 7.
Mean mRNA expression of the regulatory genes MMP-2, MMP-9, MMP-3, and TIMP-1 in men and women at rest. Expression levels for each gene were normalized to GAPDH using the 2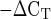 method. †P = 0.08 vs. females.
method. †P = 0.08 vs. females.
DISCUSSION
To our knowledge, this is the first investigation of the mRNA expression of structural and regulatory genes in tendon of men and women after an acute bout of RE. Acute RE elicited a regulatory response in gene expression in the patellar tendon, as indicated by the downregulation of collagen type I, collagen type III, and MMP-2 mRNA expression. However, the proteoglycans decorin, biglycan, fibromodulin, and versican were refractory to the high-intensity mechanical loading of acute RE. In addition, sex influenced gene regulation of the structural (collagen type III) and regulatory (MMP-3) components of tendon.
Exercise Response
As a first approximation, it seems reasonable to suggest that the downregulation of collagen type I and collagen type III mRNA expression 4 h after the RE bout infers that protein synthesis of the two main fibrillar collagens of tendon is reduced in the first several hours after RE. This idea is based on the assumption that collagen mRNA expression is a reflection of collagen protein synthesis, although it must be kept in mind that there are multiple regulatory steps between collagen gene transcription and incorporation of protein into the collagen fibril (30, 42). In support of this assumption, changes in collagen mRNA expression have been shown to mirror changes in collagen protein synthesis (33, 50). The response to RE in the present investigation is in contrast to the work of Miller et al. (39), who reported that collagen protein synthesis in the human patellar tendon was significantly stimulated 6 h after an acute bout of aerobic leg-kicking exercise. By incorporating stable isotopically labeled amino acids into collagen protein, Miller et al. directly measured collagen synthesis. Although both investigations used a knee extension exercise, the loading stimulus placed on the patellar tendon as a result of aerobic leg kicking (2,100 total contractions over 60 min) was different from that attributable to the bout of RE (30 total contractions over 2 min). However, the return to baseline (i.e., the increase from 4 to 24 h) in collagen type I and collagen type III mRNA expression in the present investigation corresponds with the previously reported increase and peak in collagen protein synthesis from 6 to 24 h after aerobic exercise (39). Therefore, it is possible that the regulation of collagen turnover and, ultimately, the extent and time course of collagen turnover are dependent on the mode, duration, and intensity of the exercise bout. It is unlikely that the different nutritional status of the subjects between the present study (fasted) and the study of Miller et al. (fed) played a role in the responses to acute exercise, inasmuch as it has been shown that feeding does not appear to impact collagen turnover (5, 40). In addition, collagen turnover appears to be influenced by other factors, such as multiple bouts of activity and disuse (7, 14). For example, after 4 days of eccentric, concentric, or isometric training, collagen type I and collagen type III mRNA expression significantly increased in a rat tendon model (14). These studies indicate that collagen turnover is dynamic and can be influenced variably by different manipulations.
Previous in vitro (23, 48) and in vivo (60) animal studies demonstrate that mechanical loading induces changes in proteoglycan expression in tendon. The main functions of tendon proteoglycans are regulation of fibrillogenesis and maintenance of tendon structure (61). It appears that the RE bout used in the present investigation was not sufficient to induce alterations in proteoglycan gene regulation. Independent of proteoglycan abundance, posttranslational modifications of the proteoglycans, such as alterations to the GAG chains that bind to the core protein (61), may influence tendon mechanical properties. Alterations in GAG content have been observed in prolonged run-trained animal tendons (55, 60), in aged tendons (55), and in human tendinopathy (10, 46).
MMPs are the primary enzymes responsible for the ECM breakdown in tendon necessary for protein turnover (56). Although there are 23 known types of MMPs in humans (56), we chose the MMPs that have been indicated to play a significant role in ECM maintenance of tendon. The gelatinases MMP-2 and MMP-9 are involved in the ECM turnover induced by tissue injury (1, 34, 45) and exercise (24). MMP-2 also functions as an interstitial collagenase (2). MMP-3 functions to degrade a range of ECM components, as well as activate other MMPs (37), and is induced by mechanical loading (3, 54). The lack of overall response of MMPs in the present investigation suggests that tendon tissue breakdown was not induced by the mechanical loading of RE. In addition, MMPs are inhibited by TIMP-1 (56), and the finding that TIMP-1 mRNA expression was not influenced by the exercise bout corresponds with the overall MMP response. Furthermore, although the reduction of MMP-2 mRNA at 4 h was modest, the return to resting levels 24 h after RE paralleled the changes of collagen type I and collagen type III mRNA. The lack of quantifiable MMP-9 mRNA expression in tendon at rest and after exercise was somewhat surprising, because MMP-9 has been shown to be responsive to an exercise stimulus in human tendon (24) and muscle (51). However, the exercise bout in these studies consisted of 1 h of aerobic running or cycling, and, as previously discussed, different exercise stimuli may induce different cellular responses.
Sex Differences
Although it is known that risk for connective tissue injuries is increased in women compared with men, the cause for this trend is uncertain (4, 13, 35). Tendon has been used as a model to explore sex differences in mechanical and metabolic properties of connective tissue (27, 35, 38, 58). In the present study, we sought to better understand these sex differences by comparing gene expression in the structural and regulatory components of tendon between men and women at rest. It is of particular interest that resting collagen type III mRNA expression in tendon was greater in women than in men. Studies of tendinopathy and tendon rupture in humans show that increased collagen type III mRNA expression (15) and protein content (9, 11, 47) are indicators of injured tendon tissue. In addition, studies show that even healthy tendon in individuals who do not display outward symptoms of tendinopathy can exhibit signs of a pathological phenotype on a microscopic level (18, 47), including increased collagen type III expression (47). Collagen type III forms smaller and less organized fibrils than collagen type I, which can cause tendon to be more susceptible to rupture (47). It should be noted that none of the women included in the present study had a history of patellar tendinopathy.
A trend toward decreased MMP-3 mRNA expression in women at rest also has implications concerning tendon pathology. Expression of MMP-3 mRNA (8, 15, 17, 32) and protein (8, 45) is downregulated in injured and ruptured tendons; therefore, MMP-3 is thought to be a crucial enzyme for maintenance of a healthy ECM in tendon (45). Expression of lower levels of MMP-3 mRNA at rest in women than in men may indicate impaired ECM maintenance, leading to increased injury susceptibility.
Expression of the other GOIs (collagen type I, decorin, biglycan, fibromodulin, versican, MMP-2, MMP-9, and TIMP-1) was not different between men and women at rest. We conclude that these targets are not key components leading to sex differences in metabolic and biomechanical properties of tendon.
Conclusions
The present investigation provides novel insight into the influence of acute exercise on the molecular responses of the patellar tendon. Gene regulation for structural and regulatory components of tendon was altered, signifying that a cellular response was triggered by acute RE. The present investigation also suggests that the increased risk for connective tissue injury in women compared with men may be related to structural and regulatory expression differences in tendon.
GRANTS
This work was supported by National Institute on Aging Grant R01 AG-020532 (T. A. Trappe).
Acknowledgments
The authors thank the subjects for their participation.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Agren MS Gelatinase activity during wound healing. Br J Dermatol 131: 634–640, 1994. [DOI] [PubMed] [Google Scholar]
- 2.Aimes RT, Quigley JP. Matrix metalloproteinase-2 is an interstitial collagenase. Inhibitor-free enzyme catalyzes the cleavage of collagen fibrils and soluble native type I collagen generating the specific 3/4- and 1/4-length fragments. J Biol Chem 270: 5872–5876, 1995. [DOI] [PubMed] [Google Scholar]
- 3.Archambault JM, Elfervig-Wall MK, Tsuzaki M, Herzog W, Banes AJ. Rabbit tendon cells produce MMP-3 in response to fluid flow without significant calcium transients. J Biomech 35: 303–309, 2002. [DOI] [PubMed] [Google Scholar]
- 4.Arendt E, Dick R. Knee injury patterns among men and women in collegiate basketball and soccer. NCAA data and review of literature. Am J Sports Med 23: 694–701, 1995. [DOI] [PubMed] [Google Scholar]
- 5.Babraj JA, Cuthbertson DJ, Smith K, Langberg H, Miller B, Krogsgaard MR, Kjaer M, Rennie MJ. Collagen synthesis in human musculoskeletal tissues and skin. Am J Physiol Endocrinol Metab 289: E864–E869, 2005. [DOI] [PubMed] [Google Scholar]
- 6.Bojsen-Moller J, Magnusson SP, Rasmussen LR, Kjaer M, Aagaard P. Muscle performance during maximal isometric and dynamic contractions is influenced by the stiffness of the tendinous structures. J Appl Physiol 99: 986–994, 2005. [DOI] [PubMed] [Google Scholar]
- 7.de Boer MD, Selby A, Atherton P, Smith K, Seynnes OR, Maganaris CN, Maffulli N, Movin T, Narici MV, Rennie MJ. The temporal responses of protein synthesis, gene expression and cell signalling in human quadriceps muscle and patellar tendon to disuse. J Physiol 585: 241–251, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.de Mos M, van El B, DeGroot J, Jahr H, van Schie HT, van Arkel ER, Tol H, Heijboer R, van Osch GJ, Verhaar JA. Achilles tendinosis: changes in biochemical composition and collagen turnover rate. Am J Sports Med 35: 1549–1556, 2007. [DOI] [PubMed] [Google Scholar]
- 9.Eriksen HA, Pajala A, Leppilahti J, Risteli J. Increased content of type III collagen at the rupture site of human Achilles tendon. J Orthop Res 20: 1352–1357, 2002. [DOI] [PubMed] [Google Scholar]
- 10.Fu SC, Chan KM, Rolf CG. Increased deposition of sulfated glycosaminoglycans in human patellar tendinopathy. Clin J Sport Med 17: 129–134, 2007. [DOI] [PubMed] [Google Scholar]
- 11.Goncalves-Neto J, Witzel SS, Teodoro WR, Carvalho-Junior AE, Fernandes TD, Yoshinari HH. Changes in collagen matrix composition in human posterior tibial tendon dysfunction. Joint Bone Spine 69: 189–194, 2002. [DOI] [PubMed] [Google Scholar]
- 12.Harris JA, Benedict FG. A Biometric Study of Basal Metabolism in Man. Washington, DC: Carnegie Institution, 1919.
- 13.Hart DA, Archambault JM, Kydd A, Reno C, Frank CB, Herzog W. Gender and neurogenic variables in tendon biology and repetitive motion disorders. Clin Orthop Relat Res: 44–56, 1998. [PubMed]
- 14.Heinemeier KM, Olesen JL, Haddad F, Langberg H, Kjaer M, Baldwin KM, Schjerling P. Expression of collagen and related growth factors in rat tendon and skeletal muscle in response to specific contraction types. J Physiol 582: 1303–1316, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ireland D, Harrall R, Curry V, Holloway G, Hackney R, Hazleman B, Riley G. Multiple changes in gene expression in chronic human Achilles tendinopathy. Matrix Biol 20: 159–169, 2001. [DOI] [PubMed] [Google Scholar]
- 16.Jemiolo B, Trappe S. Single muscle fiber gene expression in human skeletal muscle: validation of internal control with exercise. Biochem Biophys Res Commun 320: 1043–1050, 2004. [DOI] [PubMed] [Google Scholar]
- 17.Jones GC, Corps AN, Pennington CJ, Clark IM, Edwards DR, Bradley MM, Hazleman BL, Riley GP. Expression profiling of metalloproteinases and tissue inhibitors of metalloproteinases in normal and degenerate human Achilles tendon. Arthritis Rheum 54: 832–842, 2006. [DOI] [PubMed] [Google Scholar]
- 18.Kannus P, Jozsa L. Histopathological changes preceding spontaneous rupture of a tendon. A controlled study of 891 patients. J Bone Joint Surg Am 73: 1507–1525, 1991. [PubMed] [Google Scholar]
- 19.Kartus J, Movin T, Papadogiannakis N, Christensen LR, Lindahl S, Karlsson J. A radiographic and histologic evaluation of the patellar tendon after harvesting its central third. Am J Sports Med 28: 218–226, 2000. [DOI] [PubMed] [Google Scholar]
- 20.Kjaer M Role of extracellular matrix in adaptation of tendon and skeletal muscle to mechanical loading. Physiol Rev 84: 649–698, 2004. [DOI] [PubMed] [Google Scholar]
- 21.Kongsgaard M, Aagaard P, Kjaer M, Magnusson SP. Structural Achilles tendon properties in athletes subjected to different exercise modes and in Achilles tendon rupture patients. J Appl Physiol 99: 1965–1971, 2005. [DOI] [PubMed] [Google Scholar]
- 22.Kongsgaard M, Reitelseder S, Pedersen TG, Holm L, Aagaard P, Kjaer M, Magnusson SP. Region specific patellar tendon hypertrophy in humans following resistance training. Acta Physiol (Oxf) 191: 111–121, 2007. [DOI] [PubMed] [Google Scholar]
- 23.Koob TJ, Clark PE, Hernandez DJ, Thurmond FA, Vogel KG. Compression loading in vitro regulates proteoglycan synthesis by tendon fibrocartilage. Arch Biochem Biophys 298: 303–312, 1992. [DOI] [PubMed] [Google Scholar]
- 24.Koskinen SO, Heinemeier KM, Olesen JL, Langberg H, Kjaer M. Physical exercise can influence local levels of matrix metalloproteinases and their inhibitors in tendon-related connective tissue. J Appl Physiol 96: 861–864, 2004. [DOI] [PubMed] [Google Scholar]
- 25.Kubo K, Kanehisa H, Fukunaga T. Effects of different duration isometric contractions on tendon elasticity in human quadriceps muscles. J Physiol 536: 649–655, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kubo K, Kanehisa H, Fukunaga T. Effects of resistance and stretching training programmes on the viscoelastic properties of human tendon structures in vivo. J Physiol 538: 219–226, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kubo K, Kanehisa H, Fukunaga T. Gender differences in the viscoelastic properties of tendon structures. Eur J Appl Physiol 88: 520–526, 2003. [DOI] [PubMed] [Google Scholar]
- 28.Kubo K, Kanehisa H, Ito M, Fukunaga T. Effects of isometric training on the elasticity of human tendon structures in vivo. J Appl Physiol 91: 26–32, 2001. [DOI] [PubMed] [Google Scholar]
- 29.Langberg H, Skovgaard D, Petersen LJ, Bulow J, Kjaer M. Type I collagen synthesis and degradation in peritendinous tissue after exercise determined by microdialysis in humans. J Physiol 521: 299–306, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Laurent GJ Dynamic state of collagen: pathways of collagen degradation in vivo and their possible role in regulation of collagen mass. Am J Physiol Cell Physiol 252: C1–C9, 1987. [DOI] [PubMed] [Google Scholar]
-
31.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2
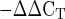 method. Methods
25: 402–408, 2001. [DOI] [PubMed] [Google Scholar]
method. Methods
25: 402–408, 2001. [DOI] [PubMed] [Google Scholar] - 32.Lo IK, Marchuk LL, Hollinshead R, Hart DA, Frank CB. Matrix metalloproteinase and tissue inhibitor of matrix metalloproteinase mRNA levels are specifically altered in torn rotator cuff tendons. Am J Sports Med 32: 1223–1229, 2004. [DOI] [PubMed] [Google Scholar]
- 33.LuValle P, Ninomiya Y, Rosenblum ND, Olsen BR. The type X collagen gene. Intron sequences split the 5′-untranslated region and separate the coding regions for the non-collagenous amino-terminal and triple-helical domains. J Biol Chem 263: 18378–18385, 1988. [PubMed] [Google Scholar]
- 34.Madlener M Differential expression of matrix metalloproteinases and their physiological inhibitors in acute murine skin wounds. Arch Dermatol Res 290 Suppl: S24–S29, 1998. [DOI] [PubMed] [Google Scholar]
- 35.Magnusson SP, Hansen M, Langberg H, Miller B, Haraldsson B, Westh EK, Koskinen S, Aagaard P, Kjaer M. The adaptability of tendon to loading differs in men and women. Int J Exp Pathol 88: 237–240, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Magnusson SP, Hansen P, Kjaer M. Tendon properties in relation to muscular activity and physical training. Scand J Med Sci Sports 13: 211–223, 2003. [DOI] [PubMed] [Google Scholar]
- 37.Magra M, Maffulli N. Molecular events in tendinopathy: a role for metalloproteases. Foot Ankle Clin 10: 267–277, 2005. [DOI] [PubMed] [Google Scholar]
- 38.Miller BF, Hansen M, Olesen JL, Schwarz P, Babraj JA, Smith K, Rennie MJ, Kjaer M. Tendon collagen synthesis at rest and after exercise in women. J Appl Physiol 102: 541–546, 2007. [DOI] [PubMed] [Google Scholar]
- 39.Miller BF, Olesen JL, Hansen M, Dossing S, Crameri RM, Welling RJ, Langberg H, Flyvbjerg A, Kjaer M, Babraj JA, Smith K, Rennie MJ. Coordinated collagen and muscle protein synthesis in human patella tendon and quadriceps muscle after exercise. J Physiol 567: 1021–1033, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mittendorfer B, Andersen JL, Plomgaard P, Saltin B, Babraj JA, Smith K, Rennie MJ. Protein synthesis rates in human muscles: neither anatomical location nor fibre-type composition are major determinants. J Physiol 563: 203–211, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Movin T Tendon tissue sampling. Scand J Med Sci Sports 10: 368–371, 2000. [DOI] [PubMed] [Google Scholar]
- 42.Parsons M, Kessler E, Laurent GJ, Brown RA, Bishop JE. Mechanical load enhances procollagen processing in dermal fibroblasts by regulating levels of procollagen C-proteinase. Exp Cell Res 252: 319–331, 1999. [DOI] [PubMed] [Google Scholar]
- 43.Raue U, Slivka D, Jemiolo B, Hollon C, Trappe S. Myogenic gene expression at rest and after a bout of resistance exercise in young (18–30 yr) and old (80–89 yr) women. J Appl Physiol 101: 53–59, 2006. [DOI] [PubMed] [Google Scholar]
- 44.Reeves ND, Maganaris CN, Narici MV. Effect of strength training on human patella tendon mechanical properties of older individuals. J Physiol 548: 971–981, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Riley GP, Curry V, DeGroot J, van El B, Verzijl N, Hazleman BL, Bank RA. Matrix metalloproteinase activities and their relationship with collagen remodelling in tendon pathology. Matrix Biol 21: 185–195, 2002. [DOI] [PubMed] [Google Scholar]
- 46.Riley GP, Harrall RL, Constant CR, Chard MD, Cawston TE, Hazleman BL. Glycosaminoglycans of human rotator cuff tendons: changes with age and in chronic rotator cuff tendinitis. Ann Rheum Dis 53: 367–376, 1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Riley GP, Harrall RL, Constant CR, Chard MD, Cawston TE, Hazleman BL. Tendon degeneration and chronic shoulder pain: changes in the collagen composition of the human rotator cuff tendons in rotator cuff tendinitis. Ann Rheum Dis 53: 359–366, 1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Robbins JR, Evanko SP, Vogel KG. Mechanical loading and TGF-β regulate proteoglycan synthesis in tendon. Arch Biochem Biophys 342: 203–211, 1997. [DOI] [PubMed] [Google Scholar]
- 49.Rosager S, Aagaard P, Dyhre-Poulsen P, Neergaard K, Kjaer M, Magnusson SP. Load-displacement properties of the human triceps surae aponeurosis and tendon in runners and non-runners. Scand J Med Sci Sports 12: 90–98, 2002. [DOI] [PubMed] [Google Scholar]
- 50.Rowe DW, Moen RC, Davidson JM, Byers PH, Bornstein P, Palmiter RD. Correlation of procollagen mRNA levels in normal and transformed chick embryo fibroblasts with different rates of procollagen synthesis. Biochemistry 17: 1581–1590, 1978. [DOI] [PubMed] [Google Scholar]
- 51.Rullman E, Rundqvist H, Wagsater D, Fischer H, Eriksson P, Sundberg CJ, Jansson E, Gustafsson T. A single bout of exercise activates matrix metalloproteinase in human skeletal muscle. J Appl Physiol 102: 2346–2351, 2007. [DOI] [PubMed] [Google Scholar]
- 52.Schmittgen TD, Zakrajsek BA. Effect of experimental treatment on housekeeping gene expression: validation by real-time, quantitative RT-PCR. J Biochem Biophys Methods 46: 69–81, 2000. [DOI] [PubMed] [Google Scholar]
- 53.Trappe TA, Carroll CC, Jemiolo B, Trappe SW, Dossing S, Kjaer M, Magnusson SP. Cyclooxygenase mRNA expression in human patellar tendon at rest and after exercise. Am J Physiol Regul Integr Comp Physiol 294: R192–R199, 2008. [DOI] [PubMed] [Google Scholar]
- 54.Tsuzaki M, Bynum D, Almekinders L, Yang X, Faber J, Banes AJ. ATP modulates load-inducible IL-1β, COX 2, and MMP-3 gene expression in human tendon cells. J Cell Biochem 89: 556–562, 2003. [DOI] [PubMed] [Google Scholar]
- 55.Vailas AC, Pedrini VA, Pedrini-Mille A, Holloszy JO. Patellar tendon matrix changes associated with aging and voluntary exercise. J Appl Physiol 58: 1572–1576, 1985. [DOI] [PubMed] [Google Scholar]
- 56.Visse R, Nagase H. Matrix metalloproteinases and tissue inhibitors of metalloproteinases: structure, function, and biochemistry. Circ Res 92: 827–839, 2003. [DOI] [PubMed] [Google Scholar]
- 57.Weinheimer EM, Jemiolo B, Carroll CC, Harber MP, Haus JM, Burd NA, Lemoine JK, Trappe SW, Trappe TA. Resistance exercise and cyclooxygenase (COX) expression in human skeletal muscle: implications for COX-inhibiting drugs and protein synthesis. Am J Physiol Regul Integr Comp Physiol 292: R2241–R2248, 2007. [DOI] [PubMed] [Google Scholar]
- 58.Westh E, Kongsgaard M, Bojsen-Moller J, Aagaard P, Hansen M, Kjaer M, Magnusson SP. Effect of habitual exercise on the structural and mechanical properties of human tendon, in vivo, in men and women. Scand J Med Sci Sports 18: 23–30, 2008. [DOI] [PubMed] [Google Scholar]
- 59.Yang Y, Creer A, Jemiolo B, Trappe S. Time course of myogenic and metabolic gene expression in response to acute exercise in human skeletal muscle. J Appl Physiol 98: 1745–1752, 2005. [DOI] [PubMed] [Google Scholar]
- 60.Yoon HJ, Brooks R, Hwan Kim Y, Terada M, Halper J. Proteoglycans in chicken gastrocnemius tendons change with exercise. Arch Biochem Biophys 412: 279–286, 2003. [DOI] [PubMed] [Google Scholar]
- 61.Yoon JH, Halper J. Tendon proteoglycans: biochemistry and function. J Musculoskelet Neuronal Interact 5: 22–34, 2005. [PubMed] [Google Scholar]