Abstract
Lys-48-linked polyubiquitination regulates a variety of cellular processes by targeting ubiquitinated proteins to the proteasome for degradation. Although polyubiquitination had been presumed to occur by transferring ubiquitin molecules, one at a time, from an E2 active site to a substrate, we recently showed that the endoplasmic reticulum-associated RING finger ubiquitin ligase gp78 can mediate the preassembly of Lys-48-linked polyubiquitin chains on the catalytic cysteine of its cognate E2 Ube2g2 and subsequent transfer to a substrate. Active site-linked polyubiquitin chains are detected in cells on Ube2g2 and its yeast homolog Ubc7p, but how these chains are assembled is unclear. Here, we show that gp78 forms an oligomer via 2 oligomerization sites, one of which is a hydrophobic segment located in the gp78 cytosolic domain. We further demonstrate that a gp78 oligomer can simultaneously associate with multiple Ube2g2 molecules. This interaction is mediated by a novel Ube2g2 surface distinct from the predicted RING binding site. Our data suggest that a large gp78–Ube2g2 heterooligomer brings multiple Ube2g2 molecules into close proximity, allowing ubiquitin moieties to be transferred between neighboring Ube2g2s to form active site-linked polyubiquitin chains.
Keywords: crystallography, endoplasmic reticulum-associated protein degradation, gp78, polyubiquitin chain
Covalent attachment of the 76-residue ubiquitin to polypeptides regulates the stability, localization, or activity of the modified proteins (1–3). This modification requires concerted actions of 3 types of enzymes: an activating enzyme (E1) that forms a thioester linkage (designated herein as “∼”) between its catalytic cysteine and the carboxyl group of the Gly-76 in ubiquitin; a conjugating enzyme (E2) that receives ubiquitin from an E1; and an ubiquitin ligase (E3) that catalyzes the transfer of ubiquitin from the E2 active-site cysteine to a substrate (4). To date, 2 E1s and dozens of E2 enzymes have been identified in mammals, whereas the number of E3s is in the range of several hundreds (5–8). Many E3 enzymes contain a RING finger domain that interacts transiently with a cognate E2 to mediate polyubiquitination reactions (4, 9–12).
Ubiquitination often occurs in the form of a polymer whereby the C-terminal Gly-76 in an ubiquitin moiety is linked to a lysine residue in another ubiquitin molecule. It had been presumed that polyubiquitin chains are formed by conjugating ubiquitin moieties, one at a time, first to a lysine residue in a substrate, and then to a lysine in the previously-attached ubiquitin molecule. This appears to be the case for some E2s (13–15). However, we and several other groups recently found that ubiquitin chains can also be preassembled on the catalytic cysteine of the E2 enzyme Ube2g2 and its yeast homologue Ubc7p both in vitro and in vivo (16–19). These ubiquitin-conjugating enzymes are associated with endoplasmic reticulum (ER) membranes to modify misfolded polypeptides that have been exported from the ER lumen in a process termed ER-associated protein degradation (ERAD) (20–22). Intriguingly, polyubiquitin chains preassembled on the active site of Ube2g2 can be subsequently transferred en bloc to a substrate (16), which offers a novel mechanism of polyubiquitination.
In this article, we study the mechanism of Ube2g2 active site-associated polyubiquitination mediated by the ER-associated RING finger ligase gp78. Our biochemical and structural analyses reveal a mode of E3–E2 interaction that occurs within a large gp78–Ube2g2 heterooligomeric complex. This interaction brings multiple Ube2g2s into close proximity to form active site-linked polyubiquitin chains. Given that Ube2g2 is a major E2 involved in ERAD, our findings provide important insights into how misfolded ER proteins are polyubiquitinated for proteasomal degradation.
Results
gp78 Contains 2 Oligomerization Sites, One of Which Is Located Within the gp78 Cytosolic Domain.
We demonstrated that gp78 can mediate the assembly of polyubiquitin chains on the active site of Ube2g2 and subsequent transfer to a substrate. We also showed that gp78 forms an oligomer that might be critical for E2 active site-associated polyubiquitination (16). gp78 is a multispanning membrane protein anchored to the ER membrane with its catalytic domain facing the cytosol (23, 24). To identify the domains responsible for gp78 oligomerization, we expressed Flag-tagged, full-length gp78 (Flag-gp78) together with GFP-fusion proteins comprising either the N-terminal transmembrane segments (gp78N-GFP) or the C-terminal cytosolic domain (gp78C-GFP) of gp78 in cells. Immunoprecipitation experiments showed that both gp78N-GFP and gp78C-GFP coprecipitated with Flag-gp78, unlike the control GFP protein (Fig. 1 A and B), suggesting that gp78 contains at least 2 oligomerization sites, one in the transmembrane region and the other in its cytosolic domain.
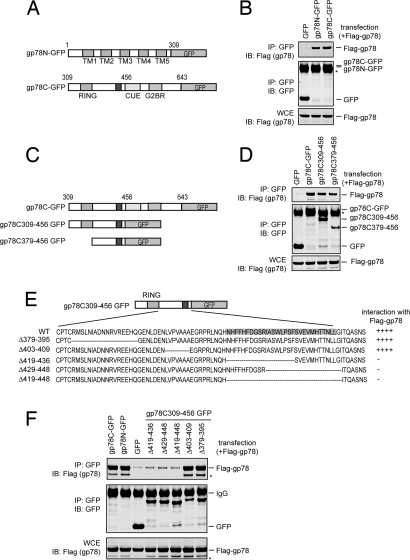
Fig. 1.
Oligomerization of gp78. (A) Schematic representation of the gp78 variants tested in B. (B) Both the cytosolic domain and the transmembrane segments of gp78 can interact with full-length gp78. Detergent extracts of 293T cells transfected with the indicated plasmids were subjected to immunoprecipitation (IP) followed by immunoblotting (IB) with the indicated antibodies. Note that the expressed gp78 proteins comigrate with IgG (*). WCE, whole cell extract. (C–F) Mapping the region in the gp78 cytosolic domain that is necessary for its self-association. (C) Schematic representation of the gp78 variants tested in D. (D) As in B, except that plasmids expressing the indicated gp78 variants were analyzed. (E) Schematic representation of the gp78 variants used in F. (F) As in B, except that plasmids expressing the indicated gp78 variants were analyzed. * indicates a gp78 degradation product.
We focused our study on gp78C because the transmembrane segments of gp78 are dispensable for the ligase activity (16, 23). Using a set of gp78C deletion mutants (Fig. 1C), we mapped the region required for gp78C oligomerization to a segment of 77 amino acids downstream of the RING domain (Fig. 1D). Further deletion analyses identified the element responsible for gp78C oligomerization as a hydrophobic segment containing amino acids 419–448 of gp78, which is predicted to form a loop (Fig. 1 E and F).
A Monomeric gp78C Mutant Retains Ubiquitin Ligation Activity but Fails to Promote Ube2g2 Active Site-Associated Polyubiquitination.
To investigate the role of E3 oligomerization in Ube2g2 active site-associated polyubiquitination, we wanted to generate a monomeric gp78 mutant. To this end, we made a loop-deleted gp78C mutant lacking residues 411–448 (gp78CΔL). Wild-type gp78C and gp78CΔL were purified as both GST- and maltose-binding protein (MBP)-tagged proteins. Removal of the tag from gp78C caused the protein to become insoluble, whereas cleavage of the tag from gp78CΔL yielded soluble material (Fig. 2A). Gel filtration and analytical ultracentrifugation analyses showed that gp78CΔL was a monomer regardless of whether or not it contained the MBP tag (Fig. S1a). In contrast, MBP-gp78C formed large-size oligomers (>670 kDa). As expected, GST-gp78C formed oligomers even larger than MBP-gp78C, presumably caused by the dimerization of GST (Fig. S1b).
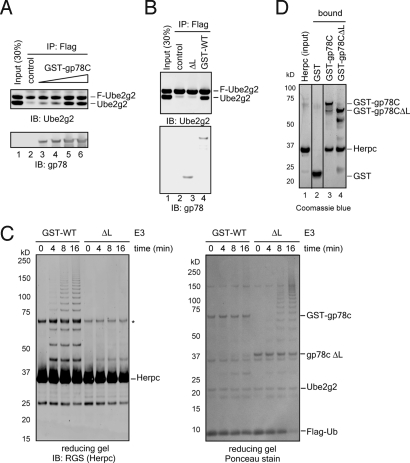
Fig. 2.
Oligomerization of gp78C is required for Ube2g2 active site-linked polyubiquitination, but is dispensable for ubiquitin ligation activity. (A) Purified recombinant gp78C variants. (B) Polyubiquitinating activity of gp78CΔL. Polyubiquitination reaction conducted with E1, Ube2g2, Flag-Ub, and the indicated E3 ligases was analyzed under both nonreducing (−DTT) and reducing (+DTT) conditions by immunoblotting. Arrows indicate the mobility shift for Ube2g2 active site-linked ubiquitin chains upon treatment with DTT. * indicates Ube2g2∼Ub thioester. Note that the ubiquitin chains synthesized by gp78CΔL (ΔL) exhibit a pattern different from that generated by GST-gp78C (GST-WT) on a nonreducing gel. (C) gp78CΔL cannot synthesize ubiquitin chains on the Ube2g2 active site. Ubiquitin chains synthesized in the absence (no E3) or presence of the indicated gp78C variants were analyzed by immunoblotting. E2∼Ub(n), E2-linked ubiquitin chains. Note that the Flag antibody has a higher affinity for polyubiquitin chains than for monoubiquitin. (D) Purified recombinant MBP-gp78C variants. (E) Polyubiquitinating activity of MBP-gp78CΔL. Polyubiquitination reactions conducted with the indicated MBP-tagged gp78C variants were analyzed under both nonreducing (−DTT) and reducing (+DTT) conditions. Arrows indicate the mobility shift for Ube2g2 active site-linked ubiquitin chains upon treatment with DTT. (F) Ubiquitin chains synthesized by MBP-gp78CΔL (MBP-ΔL) are not linked to Ube2g2.
Because the gp78 N-terminal transmembrane domains also contain a dimerization site, we assumed that the oligomeric state of GST-gp78C might more closely resemble that of full-length gp78. Moreover, at least for gp78CΔL, the GST tag had little effect on its ligase activity. In contrast, the MBP tag significantly reduced its activity, as judged by a single-round ubiquitin ligation assay (Fig. S2). We therefore chose GST-gp78C as a reference when studying the activity of the untagged monomeric gp78CΔL mutant. As demonstrated, GST-gp78C stimulated the formation of Ube2g2 active site-linked ubiquitin chains that could be converted to free chains by the reducing agent DTT (Fig. 2B, lanes 13–16 versus lanes 9–12) (16). Accordingly, these chains could be recognized by Ube2g2 antibody (Fig. 2C, lanes 2 and 5) or coprecipitated with Ube2g2 under a nonreducing condition (Fig. S3). By contrast, gp78CΔL was capable of promoting the synthesis of polyubiquitin chains, but the chains were not linked to the Ube2g2 active site as their mobility on SDS/PAGE gel was not altered by DTT (Fig. 2B, lanes 1–8). Consistently, ubiquitin chains formed in the presence of gp78CΔL could not be recognized by the Ube2g2 antibody (Fig. 2C, lanes 3 and 6), nor did they coprecipitate with Ube2g2 (Fig. S3). The polyubiquitin ladder generated by gp78CΔL appeared to be unanchored chains, as judged by the size increment of ≈9 kDa, which corresponded to the size of Flag-ubiquitin. Consistent with this interpretation, these chains could be rapidly disassembled by isopeptidase T, a deubiquitinating enzyme that only disassembles unanchored chains (25, 26). Together, these results indicate that the monomeric gp78C mutant cannot promote Ube2g2 active site-associated polyubiquitination although it retains ligase activity. This conclusion was further validated by using MBP-tagged gp78C and a corresponding loop deleted gp78C mutant (Fig. 2 D–F).
We next used an established single-round ubiquitin turnover assay to further examine the ligase activity of gp78CΔL. To this end, Ube2g2 precharged with Flag-UbK48R (donor) was incubated with Ube2g2 precharged with untagged wild-type ubiquitin (acceptor). The UbK48R mutant lacks Lys-48 and therefore can only serve as donor because ubiquitin chains synthesized by gp78C are linked exclusively via Lys-48 (16). As demonstrated (16), wild-type gp78C could catalyze the transfer of Flag-UbK48R from an E2 active site to a second Ube2g2∼ubiquitin thioester to form diubiquitin-linked Ube2g2 (Fig. 3A, lanes 6–8 and 14–16). In contrast, the monomeric gp78CΔL mutant failed to form diubiquitin-linked Ube2g2 (Fig. 3A, lanes 9–12). Instead, it produced a small amount of unanchored diubiquitin. This was caused by the transfer of Flag-UbK48R to free ubiquitin present in the reaction. The formation of such a diubiquitin species has been used as an indicator of ligase activity for some RING E3s, and the amount of diubiquitin generated depends on the concentration of the ubiquitin acceptor (13, 27). Indeed, when the Ube2g2∼Flag-UbK48R thioester was incubated with excess ubiquitin, gp78CΔL could generate diubiquitin more efficiently than wild-type gp78C (Fig. 3B, lanes 9–12 versus 13–16). These data confirm that oligomerization of gp78C is required for charging the Ube2g2 active site with polyubiquitin. Our results also indicate that having ubiquitin ligase activity is not sufficient to stimulate E2 active site-associated polyubiquitination.
Fig. 3.
gp78CΔL stimulates the formation of free diubiquitin but is defective in building Ube2g2-linked diubiquitin. (A) As indicated by the experimental scheme, the transfer of Flag-UbK48R from Ube2g2 to an Ube2g2∼ubiquitin thioester was monitored by immunoblotting with Ube2g2 (E2) and Flag antibodies. * indicates a nonspecific band. (B) As indicated by the experimental scheme, the transfer of Flag-UbK48R from Ube2g2 to free ubiquitin was monitored by immunoblotting with Ube2g2 and Flag antibodies.
The Monomeric gp78C Mutant Does Not Promote Ube2g2 Self-Association and Is Defective in Substrate Polyubiquitination.
Because the transfer of ubiquitin between Ube2g2∼Ub thioesters requires multiple Ube2g2 molecules in close proximity, we tested whether a gp78C oligomer could simultaneously bind multiple Ube2g2s. We incubated Flag-Ube2g2 with untagged Ube2g2 in the presence or absence of gp78C, and then immunoprecipitated Flag–Ube2g2 by Flag antibody. Immunoblotting showed that GST-gp78C promoted the coprecipitation of untagged Ube2g2 with Flag–Ube2g2 (Fig. 4A, lanes 3–6). In contrast, gp78CΔL failed to do so, although it was capable of binding to Ube2g2 (Fig. 4B, lane 3). Thus, a gp78 oligomer can recruit multiple Ube2g2 molecules to form a large E3–E2 heterooligomer.
Fig. 4.
The monomeric gp78C mutant cannot promote Ube2g2 self-assembly and is defective in polyubiquitination of Herpc. (A) gp78C promotes Ube2g2 self-assembly. Equal amount of Flag-tagged (F-Ube2g2) and untagged Ube2g2 were mixed in the presence of either GST (control) or increasing amounts of GST-gp78C (lanes 3–6). Flag-Ube2g2 and its associated proteins were immunoprecipitated by anti-Flag agarose beads and analyzed by immunoblotting. Lane 1 shows input (30% of Ube2g2 used in the binding experiment). (B) gp78CΔL does not facilitate Ube2g2 self-assembly. As in A, except that both GST-gp78C (GST-WT) and gp78CΔL (ΔL) were tested at a single concentration. (C) gp78CΔL is defective in Herpc polyubiquitination. Polyubiquitination reactions conducted with E1, Ube2g2, Ub, Herpc, and the indicated gp78 variants were analyzed under reducing condition by immunoblotting and Ponceau staining. * indicates a nonspecific band. (D) gp78CΔL can interact with Herpc. The indicated GST-fusion proteins were immobilized on glutathione beads and incubated with purified Herpc (lanes 2–4). Bound proteins were eluted and analyzed by SDS/PAGE and Coomassie blue staining. Lane 1 shows Herpc input (30%).
To determine the role of gp78 oligomerization in substrate ubiquitination, we tested the ability of gp78CΔL to polyubiquitinate Herpc, an in vitro model substrate of gp78C (16). We showed that wild-type gp78C forms Lys-48-linked polyubiquitin chains on Lys-61 in Herpc (16). Interestingly, under the same condition, gp78CΔL ubiquitinated Herpc at a significantly reduced rate, although it efficiently generated ubiquitin chains that appeared unanchored (Fig. 4C). The defect in substrate polyubiquitination was not caused by lack of substrate association because in a GST pull-down experiment gp78CΔL associated with Herpc similarly to wild-type gp78C (Fig. 4D). We therefore conclude that oligomerization of gp78 is also required for efficient polyubiquitination of some gp78 substrates.
Structure of Ube2g2 Bound with a gp78 G2BR Peptide.
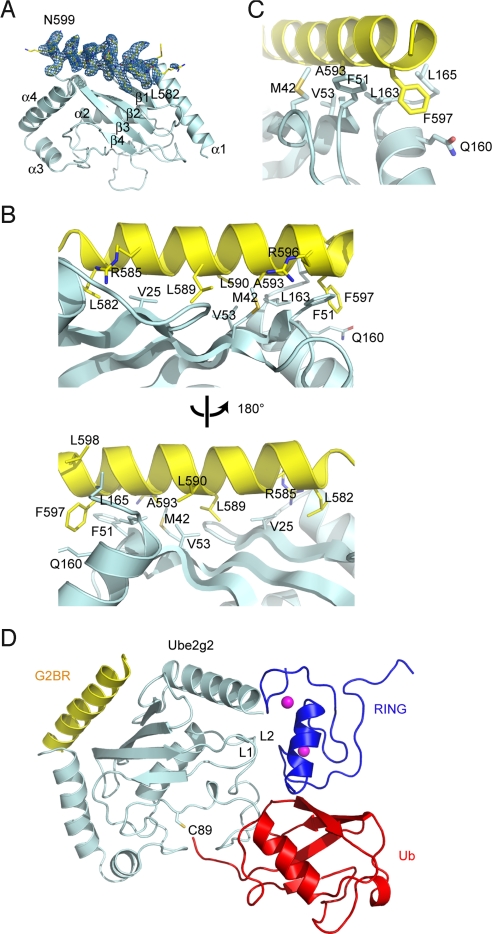
RING finger E3s usually interact with cognate E2s via the RING domain. However, gp78 was reported to contain a second E2 binding site named G2BR (24). To understand how gp78 interacts with Ube2g2 to assemble a functional E3–E2 heterooligomer, we determined the crystal structure of Ube2g2 bound to a 28-residue peptide corresponding to the G2BR domain of gp78. Crystals in space group P43212 were formed by using an Ube2g2–G2BR complex preassembled on a size exclusion column. Iterative model building and refinement produced a model comprising 334 residues of 2 Ube2g2 monomers and 21 residues of the G2BR peptide per asymmetric unit with Rfree = 25.6% and R = 21.6% to dmin = 2.76 Å (Table S1). The quality of the electron density map was excellent except for Ube2g2 residues 96–107 and G2BR residues 580–581 and 600.
The 2 Ube2g2 monomers in an asymmetric unit are essentially identical, with a rmsd of 0.82 Å over 165 Cα atoms. However, only 1 Ube2g2 interacts with a G2BR peptide, which is likely a result of crystal packing as the binding site on the second Ube2g2 is involved in crystal contact formation. The structure of Ube2g2 in our complex superimposed well onto that of Ube2g2 solved in isolation with a rmsd of 0.97 Å out of 165 Cα atoms (28). Ube2g2 forms a compact domain comprising an antiparallel β-sheet with 4 strands (β1–β4) and 4 α-helices (α1–α4; Fig. 5A). In the Ube2g2–G2BR structure, 21 consecutive residues of the G2BR peptide (from Arg-580 to Lys-600) form a helix, resting on an Ube2g2 surface formed by strands β1–β3 and the terminal portion of the helix α4 (Fig. 5A).
Fig. 5.
The structure of Ube2g2 bound to the gp78 G2BR domain. (A) Overall structure showing the Ube2g2–G2BR complex. Contoured at 3.5 σ around G2BR is a 2Fo − Fc σA-weighted annealed omit map omitting G2BR. (B) The contacts between G2BR and Ube2g2. (C) A close-up view on the most critical contacts around A593 and F597 of G2BR. (D) The geometry of G2BR binding compared with RING binding and the active site. RING domain (blue) of the c-Cbl–UbcH7 complex (Protein Data Bank ID code 1FBV), and Ub (red) of the Mms2-Ubc13∼Ub covalent complex (Protein Data Bank ID code 2GMI) are docked onto the Ube2g2–G2BR complex based on E2 structural alignments. Overlay matrices were determined by DALI.
The interface between Ube2g2 and G2BR buries a total surface area of 1,474 Å2. The interaction is formed predominantly by side chains of hydrophobic residues. Specifically, 8 residues in G2BR (Leu-582, Arg-585, Leu-589, Leu-590, Ala-593, Arg-596, Phe-597, Leu-598) form a combined 21 contacts (defined by pairs of carbon atoms that are <3.9 Å apart) with Ube2g2 (Fig. 5B). Among them, residues Ala-593 and Phe-597 make particularly extensive interactions as they together create 9 specific contacts: Ala-593 inserts into a pocket formed by Met-42, Val-53, and Leu-163, whereas Phe-597 snuggly fills up a depression formed by Phe-51, Gln-160, Leu-163, and Leu-165 of Ube2g2 (Fig. 5C). On the Ube2g2 side, Leu-163 and Leu-165 together make 7 specific contacts with G2BR by using their δ carbons, and Met-42 and Val-25 each forms 3 contacts with G2BR. In addition, 2 intermolecular hydrogen bonds were observed: NE and NH2 of G2BR Arg-594 each form a H-bond with O of Ube2g2 Leu-163. Phe-51 of Ube2g2 also interacts with Arg-596 in G2BR through hydrophobic contacts to its CG and C atoms, but the charged head group of Arg-596 forms no salt bridges and its CG atom also has relatively weak electron density, suggesting that this interaction does not contribute much to the affinity between Ube2g2 and gp78 (see below).
To understand the relative geometry of G2BR and RING binding to Ube2g2, we aligned the structure of Ube2g2–G2BR with that of UbcH7 in complex with a RING ligase by using the respective E2 as a template. The result reveals that the G2BR binding site is located opposite to the predicted RING binding surface on Ube2g2 (Fig. 5D). Moreover, both the G2BR and RING binding sites are well removed from the Ube2g2 catalytic center.
G2BR–Ube2g2 Interaction Is Required for Ube2g2-Dependent Polyubiquitination.
To understand the role of G2BR–Ube2g2 interaction in regulating Ube2g2 active site-associated polyubiquitination, we generated Ube2g2 variants that contained either single or multiple amino acid substitutions on the newly-identified G2BR binding surface. These mutants exhibited similar elution profiles to wild-type Ube2g2 on a size exclusion column, suggesting that they were properly folded. Substituting Met-42 and Phe-51 with Ala had little effect on the Ube2g2–gp78 interaction (Fig. 6A), whereas the L165A, L163A double mutant was severely defective. Moreover, a quadruple mutant combining all 4 mutations was completely inactive and did not bind G2BR (Fig. 6A). As a result, the Ube2g2 quadruple mutant could not be recruited to a gp78C–Ube2g2 heterooligomer (Fig. 6B). These results indicate that Leu-163 and Leu-165 in the carboxyl terminus of Ube2g2 are more critical for G2BR binding, whereas Met-42 and Phe-51 may play a minor role.
Fig. 6.
Ube2g2–G2BR interaction is required for active site-linked polyubiquitination and substrate ubiquitination. (A) Interaction of Ube2g2 with gp78C is mediated by the G2BR domain. GST-tagged gp78C immobilized on glutathione beads was incubated with the indicated Ube2g2 variants. The bound proteins were analyzed by immunoblotting. (B) The Ube2g2–G2BR binding is required for Ube2g2 self-interaction. As in Fig. 4A except that the indicated untagged Ube2g2 variants were incubated with Flag–Ube2g2 in the absence (−) or presence (+) of GST-gp78C. (C and D) Surface plasmon resonance analyses of GST–gp78C–Ube2g2 interaction. (C) Representative sensorgrams for the binding of Ube2g2 to GST-gp78C. (D) The relative binding of wild-type Ube2g2 (E2 WT) and the quadruple mutant (E2 quad.) to GST-gp78C. (E) Active site-linked polyubiquitination by Ube2g2 variants. The reactions were analyzed under a nonreducing condition by immunoblotting. (F) Polyubiquitination of Herpc by Ube2g2 variants. As in E, except that the reaction also contains Herpc and that the reaction was analyzed under reducing condition.
The interaction between gp78C and Ube2g2 was further examined by surface plasmon resonance experiments. Injection of Ube2g2 onto a carboxymethyl dextran-coated gold surface immobilized with GST-gp78C resulted in a concentration-dependent response (Fig. 6C). Thermodynamic evaluation of the data showed that Ube2g2 bound GST-gp78C with a KD of ≈5 nM, whereas the quadruple Ube2g2 mutant barely interacted with GST-gp78C (Fig. 6D). Interestingly, a RING deleted gp78C mutant bound Ube2g2 with an affinity similar to wild-type gp78C, as judged by pull-down experiments (Fig. S4), suggesting that the stable interaction of gp78 with Ube2g2 is largely mediated by the G2BR domain.
As anticipated, in vitro ubiquitination experiments showed that the L165A L163A double and the quadruple mutants were defective in assembling Ube2g2 active site-linked polyubiquitin chains (Fig. 6E) and could not efficiently polyubiquitinate Herpc (Fig. 6F). By contrast, the M42A and F51A mutants were able to assemble Ube2g2 active site-linked polyubiquitin chains and they had normal polyubiquitinating activities toward Herpc. Thus, the high-affinity Ube2g2–G2BR interaction is required for both Ube2g2 active site-linked polyubiquitination and substrate polyubiquitination. We therefore conclude that the G2BR domain is crucial for assembling a stable E3–E2 heterooligomer to fulfill the polyubiquitination function of gp78.
Discussion
In this article, we show that gp78 contains at least 2 oligomerization sites, which lead to the formation of large-size gp78 oligomers. We further demonstrate that a gp78 oligomer can bring multiple Ube2g2∼ubiquitin thioesters into close proximity, which presumably makes Ube2g2-linked ubiquitin a preferred acceptor for chain extension and thus promote E2 active site-linked polyubiquitination.
It has been well established that RING finger E3s bind cognate E2s via the RING domain (4). This interaction, albeit weak, is essential for promoting the transfer of ubiquitin from E2s to substrates. RING binding requires 3 residues located at the tips of the L1 and L2 loops, which are generally conserved among E2s including Ube2g2 (29). Thus, we assume that gp78 may also have a weak interaction with Ube2g2 via its RING domain. In this context, it is intriguing that a recent study identified a second E2 binding domain named G2BR in gp78 (24). The G2BR domain dramatically enhances the affinity of gp78 to Ube2g2. By contrast, removal of the RING domain does not have any significant affect on the gp78C–Ube2g2 interaction. These features distinguish Ube2g2 from other E2s that only transiently interact with E3 ligases via the RING domain (29), and thus suggest that Ube2g2 may operate by a distinct mechanism. Indeed, the residues critical for G2BR binding (L163 and L165) are largely conserved among the Ube2g2 family members, but are absent from other E2s. Moreover, unlike some E3s, gp78 does not undergo ubiquitination by Ube2g2.
The generally weak E2–E3 interactions via the RING binding site may be critical for efficient polyubiquitination of many substrates. E2s presumably need to dissociate from an E3-substrate complex before they can be recharged for the next round of the transfer reaction because the RING binding site on E2s overlaps with the E1 interaction surface (30). A transient E2–E3 interaction is therefore favored as it allows >1 E2∼ubiquitin thioester to efficiently cycle on and off from a relatively stable E3–substrate complex and thus enhance processivity, which is commonly defined in the ubiquitin field as the number of ubiquitin molecules transferred in a single round of substrate–E3 interaction. However, dynamic E2–E3 interactions may not be suitable for certain substrates, particularly for those with a high dissociation constant for E3 enzymes, because it is conceivable that these substrates may be released from a ligase before a polyubiquitin chain is fully built. The fact that diverse ERAD substrates are modified by a relatively small number of ER-associated ubiquitin ligases suggests that substrate recognition must be promiscuous and transient in nature. ERAD substrates may therefore require a special mechanism to promote polyubiquitination. We propose that its processivity may be achieved via oligomerization and enhanced E2–E3 interaction (RING and G2BR binding regions). These features allow a gp78 oligomer to simultaneously bind multiple Ube2g2∼ubiquitin thioesters and thus reduce the reliance on recycle of E2s for chain extension. Moreover, the formation of such large-size E3–E2 heterooligomers favors assembly of ubiquitin chains on the Ube2g2 active site. The transfer of preassembled ubiquitin chains from Ube2g2 to a substrate may help to overcome the detrimental effect of substrate dissociation on polyubiquitination.
Interestingly, it was recently suggested that UbcH5c may also need to be assembled into an ordered oligomeric complex to processively polyubiquitinate substrates. In this case, an ordered UbcH5c oligomer may be formed by using a so-called “back-side ubiquitin binding surface,” which is also present in the SUMO E2 Ubc9 (31, 32). The noncovalent E2–ubiquitin interaction would allow 1 UbcH5c to interact with an ubiquitin molecule conjugated to the active site of a second UbcH5c (31). Structural comparison suggests that the back-side ubiquitin binding surface, if existing in Ube2g2, overlaps with the G2BR binding site on Ube2g2 (Fig. S5). Thus, the mechanism by which G2BR assembles Ube2g2 oligomers must be distinct from that used by UbcH5c. Nonetheless, an ordered E2∼ubiquitin thioester assembly may provide a common mechanism that may enhance the processivity of polyubiquitination by allowing multiple E2∼ubiquitin thioesters to act simultaneously in a reaction.
Materials and Methods
Crystallization, Data Collection, and Processing.
The G2BR peptide corresponding to gp78 residues 574–601 (NH2-SADERQRMLVQRKDELLQQARKRFLNKS-COOH) was synthesized by Elim Biopharmaceuticals. Lyophilized peptide was dissolved at 1 mg/mL in protein buffer (100 mM NaCl, 20 mM Hepes, pH 7.0) supplemented with 10% DMSO. G2BR (2.2 mg) was mixed with 8 mg of Ube2g2, loaded onto a HiLoad 16/60 Superdex 75 gel filtration column. The eluted peak containing both Ube2g2 and G2BR was concentrated to 8.4 mg/mL.
Initial screens of crystallization condition were carried out by using the 96-well format Protein Complex Suite (Qiagen) on a Phoenix robot (Art Robbins Instruments) identified condition G7 (0.1 M Tris, pH 7.5, 3 M Na Formate) as the only hit. Crystals of Ube2g2/G2BR complex were grown by using hanging drop vapor diffusion at 20 °C. A total of 1.5 μL of the complex was mixed with equal volume of mother liquor consisting of 2.8 M Na Formate, 100 mM Tris (pH 7.7), 5 mM DTT and equilibrated against 1 mL of mother liquor. Crystals were directly frozen out of mother liquor into liquid nitrogen. A native dataset was collected at SSRL 11-1 and recorded on a MarMosaic 325 CCD detector. Integration, scaling, and merging of the diffraction data were performed by HKL2000 (33).
Structure Determination and Refinement.
The structure was solved by molecular replacement using Phaser (34) with the human Ube2g2 structure (Protein Data Bank ID code 2cyx) as a search model. Data in the resolution range 50 to 2.76 Å were used in both rotation and translation calculations, which gave an obvious solution with significant contrast, resulting in 2 Ube2g2 molecules in the asymmetric unit with a Matthews coefficient (VM) of 4.47 Å3·Da−1 and a solvent content of 72.5%. The G2BR helix was built by using COOT (35) and its register was guided by some of its well-resolved side-chain electron density. Because of additional electron density, 2 residues of the cloning artifact sequence were added at the N terminus of Ube2g2. The resulting model was refined by using CNS 1.2 (36). The refinement consisted of alternating rounds of torsion angle molecular dynamics simulated annealing, individual restrained thermal factor refinement, and model building in COOT.
Additional methods are available in SI Text.
Supplementary Material
Acknowledgments.
We thank N. Soetandyo (National Institute of Diabetes and Digestive and Kidney Diseases) for technical assistance and M. Krause and M. Gellert (National Institute of Diabetes and Digestive and Kidney Diseases) for critical reading of the manuscript. This work was supported by the intramural research program of the National Institute of Diabetes and Digestive and Kidney Diseases, National Institutes of Health.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
Data deposition: The atomic coordinates have been deposited in the Protein Data Bank, www.pdb.org (PDB ID code 3FSH).
This article contains supporting information online at www.pnas.org/cgi/content/full/0808564106/DCSupplemental.
References
- 1.Weissman AM. Themes and variations on ubiquitylation. Nat Rev Mol Cell Biol. 2001;2:169–178. doi: 10.1038/35056563. [DOI] [PubMed] [Google Scholar]
- 2.Welchman RL, Gordon C, Mayer RJ. Ubiquitin and ubiquitin-like proteins as multifunctional signals. Nat Rev Mol Cell Biol. 2005;6:599–609. doi: 10.1038/nrm1700. [DOI] [PubMed] [Google Scholar]
- 3.Mukhopadhyay D, Riezman H. Proteasome-independent functions of ubiquitin in endocytosis and signaling. Science. 2007;315:201–205. doi: 10.1126/science.1127085. [DOI] [PubMed] [Google Scholar]
- 4.Pickart CM. Mechanisms underlying ubiquitination. Annu Rev Biochem. 2001;70:503–533. doi: 10.1146/annurev.biochem.70.1.503. [DOI] [PubMed] [Google Scholar]
- 5.Jin J, Li X, Gygi SP, Harper JW. Dual E1 activation systems for ubiquitin differentially regulate E2 enzyme charging. Nature. 2007;447:1135–1138. doi: 10.1038/nature05902. [DOI] [PubMed] [Google Scholar]
- 6.Chiu YH, Sun Q, Chen ZJ. E1–L2 activates both ubiquitin and FAT10. Mol Cell. 2007;27:1014–1023. doi: 10.1016/j.molcel.2007.08.020. [DOI] [PubMed] [Google Scholar]
- 7.Pelzer C, et al. UBE1L2, a novel E1 enzyme specific for ubiquitin. J Biol Chem. 2007;282:23010–23014. doi: 10.1074/jbc.C700111200. [DOI] [PubMed] [Google Scholar]
- 8.Kerscher O, Felberbaum R, Hochstrasser M. Modification of proteins by ubiquitin and ubiquitin-like proteins. Annu Rev Cell Dev Biol. 2006;22:159–180. doi: 10.1146/annurev.cellbio.22.010605.093503. [DOI] [PubMed] [Google Scholar]
- 9.Lorick KL, et al. RING fingers mediate ubiquitin-conjugating enzyme (E2)-dependent ubiquitination. Proc Natl Acad Sci USA. 1999;96:11364–11369. doi: 10.1073/pnas.96.20.11364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Xie Y, Varshavsky A. The E2–E3 interaction in the N-end rule pathway: The RING-H2 finger of E3 is required for the synthesis of multiubiquitin chain. EMBO J. 1999;18:6832–6844. doi: 10.1093/emboj/18.23.6832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Joazeiro CA, Weissman AM. RING finger proteins: Mediators of ubiquitin ligase activity. Cell. 2000;102:549–552. doi: 10.1016/s0092-8674(00)00077-5. [DOI] [PubMed] [Google Scholar]
- 12.Deffenbaugh AE, et al. Release of ubiquitin-charged Cdc34-S-Ub from the RING domain is essential for ubiquitination of the SCF(Cdc4)-bound substrate Sic1. Cell. 2003;114:611–622. doi: 10.1016/s0092-8674(03)00641-x. [DOI] [PubMed] [Google Scholar]
- 13.Petroski MD, Deshaies RJ. Mechanism of lysine 48-linked ubiquitin-chain synthesis by the cullin–RING ubiquitin–ligase complex SCF–Cdc34. Cell. 2005;123:1107–1120. doi: 10.1016/j.cell.2005.09.033. [DOI] [PubMed] [Google Scholar]
- 14.Rodrigo-Brenni MC, Morgan DO. Sequential E2s drive polyubiquitin chain assembly on APC targets. Cell. 2007;130:127–139. doi: 10.1016/j.cell.2007.05.027. [DOI] [PubMed] [Google Scholar]
- 15.Jin L, Williamson A, Banerjee S, Philipp I, Rape M. Mechanism of ubiquitin-chain formation by the human anaphase-promoting complex. Cell. 2008;133:653–665. doi: 10.1016/j.cell.2008.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li W, Tu D, Brunger AT, Ye Y. A ubiquitin ligase transfers preformed polyubiquitin chains from a conjugating enzyme to a substrate. Nature. 2007;446:333–337. doi: 10.1038/nature05542. [DOI] [PubMed] [Google Scholar]
- 17.Ravid T, Hochstrasser M. Autoregulation of an E2 enzyme by ubiquitin-chain assembly on its catalytic residue. Nat Cell Biol. 2007;9:422–427. doi: 10.1038/ncb1558. [DOI] [PubMed] [Google Scholar]
- 18.Cao J, et al. Ufd1 is a cofactor of gp78 and plays a key role in cholesterol metabolism by regulating the stability of HMG-CoA reductase. Cell Metab. 2007;6:115–128. doi: 10.1016/j.cmet.2007.07.002. [DOI] [PubMed] [Google Scholar]
- 19.Bazirgan OA, Hampton RY. Cue1p is an activator of Ubc7p E2 activity in vitro and in vivo. J Biol Chem. 2008;283:12797–12810. doi: 10.1074/jbc.M801122200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hampton RY. ER-associated degradation in protein quality control and cellular regulation. Curr Opin Cell Biol. 2002;14:476–482. doi: 10.1016/s0955-0674(02)00358-7. [DOI] [PubMed] [Google Scholar]
- 21.Meusser B, Hirsch C, Jarosch E, Sommer T. ERAD: The long road to destruction. Nat Cell Biol. 2005;7:766–772. doi: 10.1038/ncb0805-766. [DOI] [PubMed] [Google Scholar]
- 22.Ye Y. The role of the ubiquitin-proteasome system in ER quality control. Essays Biochem. 2005;41:99–112. doi: 10.1042/EB0410099. [DOI] [PubMed] [Google Scholar]
- 23.Fang S, et al. The tumor autocrine motility factor receptor, gp78, is a ubiquitin protein ligase implicated in degradation from the endoplasmic reticulum. Proc Natl Acad Sci USA. 2001;98:14422–14427. doi: 10.1073/pnas.251401598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chen B, et al. The activity of a human endoplasmic reticulum-associated degradation E3, gp78, requires its Cue domain, RING finger, and an E2-binding site. Proc Natl Acad Sci USA. 2006;103:341–346. doi: 10.1073/pnas.0506618103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Amerik A, Swaminathan S, Krantz BA, Wilkinson KD, Hochstrasser M. In vivo disassembly of free polyubiquitin chains by yeast Ubp14 modulates rates of protein degradation by the proteasome. EMBO J. 1997;16:4826–4838. doi: 10.1093/emboj/16.16.4826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Reyes-Turcu FE, et al. The ubiquitin binding domain ZnF UBP recognizes the C-terminal diglycine motif of unanchored ubiquitin. Cell. 2006;124:1197–1208. doi: 10.1016/j.cell.2006.02.038. [DOI] [PubMed] [Google Scholar]
- 27.Ozkan E, Yu H, Deisenhofer J. Mechanistic insight into the allosteric activation of a ubiquitin-conjugating enzyme by RING-type ubiquitin ligases. Proc Natl Acad Sci USA. 2005;102:18890–18895. doi: 10.1073/pnas.0509418102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Arai R, et al. Structure of human ubiquitin-conjugating enzyme E2 G2 (UBE2G2/UBC7) Acta Crystallogr F. 2006;62:330–334. doi: 10.1107/S1744309106009006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zheng N, Wang P, Jeffrey PD, Pavletich NP. Structure of a c-Cbl–UbcH7 complex: RING domain function in ubiquitin-protein ligases. Cell. 2000;102:533–539. doi: 10.1016/s0092-8674(00)00057-x. [DOI] [PubMed] [Google Scholar]
- 30.Eletr ZM, Huang DT, Duda DM, Schulman BA, Kuhlman B. E2-conjugating enzymes must disengage from their E1 enzymes before E3-dependent ubiquitin and ubiquitin-like transfer. Nat Struct Mol Biol. 2005;12:933–934. doi: 10.1038/nsmb984. [DOI] [PubMed] [Google Scholar]
- 31.Brzovic PS, Lissounov A, Christensen DE, Hoyt DW, Klevit RE. A UbcH5/ubiquitin noncovalent complex is required for processive BRCA1-directed ubiquitination. Mol Cell. 2006;21:873–880. doi: 10.1016/j.molcel.2006.02.008. [DOI] [PubMed] [Google Scholar]
- 32.Knipscheer P, van Dijk WJ, Olsen JV, Mann M, Sixma TK. Noncovalent interaction between Ubc9 and SUMO promotes SUMO chain formation. EMBO J. 2007;26:2797–2807. doi: 10.1038/sj.emboj.7601711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Otwinowski Z, et al. Crystal structure of trp repressor/operator complex at atomic resolution. Nature. 1988;335:321–329. doi: 10.1038/335321a0. [DOI] [PubMed] [Google Scholar]
- 34.McCoy AJ, Grosse-Kunstleve RW, Storoni LC, Read RJ. Likelihood-enhanced fast translation functions. Acta Crystallogr D. 2005;61:458–464. doi: 10.1107/S0907444905001617. [DOI] [PubMed] [Google Scholar]
- 35.Emsley P, Cowtan K. COOT: Model-building tools for molecular graphics. Acta Crystallogr D. 2004;60:2126–2132. doi: 10.1107/S0907444904019158. [DOI] [PubMed] [Google Scholar]
- 36.Brunger AT. Version 1.2 of the Crystallography and NMR system. Nat Protoc. 2007;2:2728–2733. doi: 10.1038/nprot.2007.406. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.