Abstract
Substantial evidence suggests that the behavioral and reinforcing effects of cocaine can be mediated by the central dopaminergic systems. Repeated injections of cocaine produce an increase in locomotor activity and the expression of tyrosine hydroxylase (TH) in the main dopaminergic areas. Protoberberine alkaloids affect neuronal functions. Coptidis rhizoma (CR) and its main compound, berberine (BER) reduced the dopamine content in the central nervous system. In order to investigate the effects of CR or BER on the repeated cocaine-induced neuronal and behavioral alterations, we examined the influence of CR or BER on the repeated cocaine-induced locomotor activity and the expression of TH in the brain by using immunohistochemistry. Male SD rats were given repeated injections of saline or cocaine hydrochloride (15 mg/kg, i.p. for 10 consecutive days) followed by one challenge injection on the 4th day after the last daily injection. Cocaine challenge (15 mg/kg, i.p) produced a larger increase in locomotor activity and expression of TH in the central dopaminergic areas. Pretreatment with CR (50, 100, 200 and 400 mg/kg, p.o.) and BER (200 mg/kg, p.o.) 30 min before the daily injections of cocaine significantly inhibited the cocaine-induced locomotor activity as well as TH expression in the central dopaminergic areas. Our data demonstrate that the inhibitory effects of CR and BER on the repeated cocaine-induced locomotor activity were closely associated with the reduction of dopamine biosynthesis and post-synaptic neuronal activity. These results suggest that CR and BER may be effective for inhibiting the behavioral effects of cocaine by possibly modulating the central dopaminergic system.
Keywords: cocaine, berberine, Coptidis rhizome, locomotor activity, tyrosine hydroxylase, ventral tegmental area.
Introduction
Repeated exposure to psychostimulants induces sensitization to their behavioral stimulant effects. Behavioral sensitization is the enhanced motor-stimulant response that occurs with repeated exposure to such psychostimulants as cocaine or amphetamine (1,2).
Cocaine is a potent and widely abused psychostimulant that exerts behavioral and neuropharmacologic effects; these effects may be mediated by the central dopaminergic systems. Especially, the mesolimbic system from the ventral tegmental area (VTA) to the nucleus accumbens (NAc), one of the main central dopaminergic systems, has been implicated in the processes of drug addiction and this includes behavioral sensitization (3,4). For example, many studies reveal that the reinforcing effects of cocaine are related to the blockade of the dopamine (DA) reuptake systems (5) and the consequent increases in binding of DA to its post-synaptic receptors (6). The inhibition of DA re-uptake has been demonstrated in several brain nuclei such as the NAc, the striatum, the VTA and the medial prefrontal cortex (3,7–9). DA receptor antagonists also block the cocaine-induced increases of locomotor activity and stereotypy, the decrease of food intake (10) and they block the cocaine-induced reinstatement of drug seeking behavior (11,12). In addition, several studies have shown that repeated exposure to cocaine produced the expression of tyrosine hydroxylase (TH) enzyme activity for DA biosynthesis in mesolimbic DA pathways (13,14).
Coptidis rhizoma (Coptis chinensis FRANCH, CR) has been widely used as a Korean traditional medicine for hundreds of years. CR exerts therapeutic effects on disorders such as anxiety, depression and substance abuse in human and animal studies (15,16) and CR and its main compound, berberine (BER) produced a variety of biological effects on the central nervous system (17–20). In particular, protoberberine alkaloids such as BER, palmatine and coptisine inhibited DA biosynthesis (21,22). BER reduces the dopamine content in PC12 cells (18,23).
However, the effects of CR or BER on cocaine-induced neurochemical and behavioral alterations have not been investigated. Therefore, in the present study, we determined to examine whether CR or BER could affect the repeated cocaine-induced locomotor activity. The expressions of TH in the VTA were also examined by using immunohistochemical methods in order to determine a possible mechanism underlining the suppressive effects of CR and BER on cocaine-induced behavioral sensitization in rats.
Methods
Subject
Male Sprague–Dawley rats weighing 260–270 g each were obtained from Samtaco Animal Corp. (Seoul, Korea). The experiment began at least 7 days after their arrival. Rats were maintained on ad libitum food and water and they were maintained on a 12 h light-dark cycle (lights on at 7:00) at an ambient temperature of 22–24°C with a controlled relative humidity of 55%. All experiments were approved by the Kyung Hee University Institutional Animal Care and Use Committee.
Preparation of the Drugs and the Methanol Extracts of Coptidis Rhizoma
CR was purchased from an oriental drug store (Jungdo Inc. Seoul, Korea). The voucher specimens (no. KH-CR01) are deposited at the herbarium located in the College of Oriental Medicine, Kyung Hee University. CR (100 g) was cut into small pieces and extracted three times in a reflux condenser for 24 h each time using 85% methanol. The solutions were combined, filtered through Whatman no. 1 filter paper and concentrated using a rotary vacuum evaporator, this was followed by lyophilization. The yield was 12.8% (w/w). CR consists of the major active component, berberine (4–7%) and also of small amount of columbamine, coptisine, groenlandicine, berberastine and thalifendine. Cocaine hydrochloride (Macharlan, Smiss Limit, UK) and berberine (Sigma, St Louis MO, USA) were obtained from the standard commercial suppliers. Cocaine hydrochloride was dissolved in 0.9% saline and CR and berberine were dissolved in distilled water.
Experimental Design
The experiment consisted of three phases: a 10 day developmental phase, a 3 day withdrawal phase and a 1 day challenging phase. The experiment was designed to investigate the effect of CR and BER on the behavioral sensitization produced by repeated injection of cocaine. The rats were divided into eight groups. The two groups were pre-treated with saline [0.9% NaCl, i.p. saline-treated rats (SAL) group; n = 4] or cocaine [15 mg/kg, i.p. cocaine-induced rats (COC) group; n = 7] one daily for 10 consecutive days, after which time the rats were challenged with the same dose of saline or cocaine, 72 h after the last treatment, respectively. The acute cocaine treated group (15 mg/kg, i.p. CON group; n = 5) received saline for 10 days, after which time the rats were challenged with cocaine, 72 h after the last treatment. The other experimental groups were pre-treated with CR 50 mg/kg (CR50 + COC group, p.o. n = 5), CR 100 mg/kg (CR100 + COC group, p.o. n = 4), CR 200 mg/kg (CR200 + COC group, p.o. n = 8), CR 400 mg/kg (CR400 + COC group, p.o. n = 5) and berberine 200 mg/kg (BER200 + COC group, p.o. n = 5), respectively, 30 min before the injections of cocaine during a 10 day development phase. We did a pilot dose–response experiment with using BER 50, 100 and 200 mg/kg and we found that BER 200 mg/kg produced a maximal effect. Also, the dosage (200 mg/kg) chosen in this study is a relatively standard one that other workers have reported on in a previous animal study (24). Their locomotor activity was measured for 1 h after every injection of cocaine or saline.
Measurement of Locomotor Activity
The rats were individually housed prior to behavioral testing. Locomotor activity was measured in a rectangular container (40 × 40 × 45 cm3) that was equipped with a video camera above the center of the floor as described previously (25). The walls and floor were made of clear plastic and they were painted black. Locomotor activity was monitored by a videotracking system using the S-MART program (PanLab, Barcelona, Spain). Rats were allowed to adapt themselves for 1 h in the container and the distance they traveled was recorded every 10 min during a 1 h baseline and during a 1 h after treatment. The measure of locomotor activity was indicated by cm.
Immunohistochemistry for Tyrosine Hydroxylase
One hour after the last behavioral testing, rats were deeply anesthetized with sodium pentobarbital (80 mg/kg, i.p.) then perfused through the ascending aorta with normal saline (0.9%) and this was followed by 800 ml of 4% paraformaldehyde in 0.1 M phosphate-buffered saline (PBS). The brains were removed, post-fixed overnight, cryoprotected in 20% sucrose, and cut by a cryostat as 30 μm coronal sections. The sections were obtained according to the rat atlas of Paxinos and Watson (26) and stored in PBS solution for immunocytochemical processing. Sections were immunostained for TH protein by the avidin-biotin-peroxidase method. Sections were rinsed three times for 5 min each in PBS, then incubated for 72 h at 4°C with a primary polyclonal antiserum (sheep anti-TH; Chemicon, Temecula, CA, USA) at a titer of 1: 2000 in PBS containing 0.3% Triton-X100 (PBST). The sections were washed for 5 min in PBST and then incubated for 120 min in PBST containing biotinylated goat anti-sheep IgG antibody at a 1: 200 dilution (Vector Laboratories, Burlingame, CA, USA). Following a 90 min incubation in the Elite standard vecta stain avidin–biotin complex (ABC) re-agent (Vector Laboratories, Burlingame, CA, USA), the sections were again washed three times for 5 min each time in PBS, then incubated in a medium containing 0.05% 3′-diaminobenzidine tetrahydrochloride (DAB: Sigma, St Louis, MO, USA) with 0.01% H2O2 for 1 min to reveal the immunoreactivity. Finally, the tissue was rinsed in PBS; this was followed by a brief rinse in dH2O and the tissues mounted individually onto slides. After allowing the slides to air dry, they were cover-slipped. The tegmental cells were counted at ×400 magnifications using a microscope rectangle grid that measured 50 × 50 microns, according to the rat atlas (26). The cells within the tegmental areas were counted on each of three sections per animal.
Statistical Analysis
Experimental results were expressed as means ± SE The behavioral data were analyzed by one-way ANOVA using the SPSS program (Version 8.0). Statistical differences among groups were further analyzed using Tukey's post hoc test. The immunohistologic data were calculated and analyzed by one-way ANOVA followed by the Tukey's post hoc technique. P values < 0.05 were considered to be significant.
Results
Cocaine-induced Locomotor Activity Higher in the Chronic Group
When the rats that were given repeated cocaine treatments were then challenged with systemic administration of cocaine, their behavioral responses were significantly increased compared with those of the saline-induced rats (SAL group) or acute cocaine-induced rats (CON group). The locomotor activity across time for 1 h after saline and cocaine challenges is shown in Fig. 1. The behavioral response to cocaine challenge in the repeated COC group was significantly higher than that in the saline-treated group (P < 0.001) and acute cocaine-treated group (P < 0.001). ANOVAs (8 × 11, treatment × time) performed on the activity scores following the drug injections indicated a significant effect of a group difference [F (7, 35) = 79.506, P < 0.001], effect of day [F (10, 350) = 1.602, P < 0.05], but not group × day interaction [F (70, 350) = 0.913, P = 0.672]. Tukey's post hoc comparisons indicated that the behavioral response to cocaine challenge in the repeated cocaine-treated group was significantly higher than that in the acute cocaine treated group (P < 0.001).
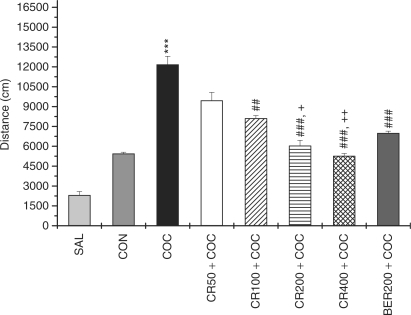
Figure 1.
Effect of CR and BER on the locomotor activity in cocaine- or saline-pre-treated rats. Rats were pre-treated with saline (SAL group) or cocaine (15 mg/kg, i.p. COC group) one daily for 10 consecutive days, after which time the rats were challenged with the same dose of saline or cocaine, 72 h after the last treatment. The acute cocaine treated group (15 mg/kg, i.p. CON group) received saline for 10 days, after which time the rats were challenged with cocaine, 72 h after the last treatment. The other experimental groups were pre-treated with CR 50 mg/kg (CR50 + COC group), CR 100 mg/kg (CR100 + COC group), CR 200 mg/kg (CR200 + COC group), CR 400 mg/kg (CR400 + COC group) and berberine 200 mg/kg (BER200 + COC group), 30 min before the injections of cocaine during a 10 days development phase. Significance with Tukey's test following a repeated ANOVA is indicated as ***P < 0.001 versus SAL group and CON group, or ##P < 0.01, ###P < 0.001 versus COC group and +P < 0.05, ++P < 0.01 versus CR50 + COC group. The vertical lines indicate SE.
CR and BER Significantly Blocked Chronic Cocaine-induced Locomotor Activity
Administration of CR or BER before the cocaine injection significantly blocked the effects of cocaine on locomotor activity during the 60 min testing period compared with the COC group, as is seen in Fig. 1. CR50, CR100, CR200, CR400 mg/kg and BER 200 mg/kg administrated 30 min before the cocaine injection decreased the cocaine-induced locomotor activity to 9463.6 ± 606.7 (P = 0.154), 8115.8 ± 201.4 (P < 0.05) 6052.1 ± 399.2 (P < 0.001), 5273.8 ± 186.6 (P < 0.001) and 7000.3 ± 142.9 (P < 0.001), respectively, when compared with the COC group's locomotor activity numbers of 12183 ± 618.1 [F (7,35) = 15.404, P < 0.001]. In a pilot study it was shown that the only CR (100 mg/kg) or BER (100 mg/kg)-treated groups did not show any change in locomotor activity when compared with the saline-treated group (P = 0.924, P = 0.902).
CR and BER inhibited cocaine-induced TH-like immunoreactivity
The SAL group received saline instead of a test substance here because the previous studies revealed that TH-like immunoreactivity was increased due to the handling and injection stress in a variety of brain areas. Following the systemic injections of cocaine, a massive amount of TH was present in the ventral tegmental area (Figs 2 and 3).
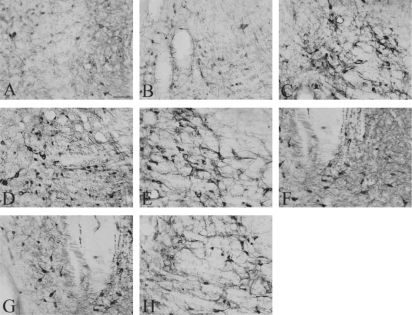
Figure 2.
Representative photographs showing the TH expression in the ventral tegmental area of SAL (A), CON (B), COC (C), CR50 + COC (D), CR100 + COC (E), CR200 + COC (F), CR400 + COC (G) and BER200 + COC (H) groups. The scale bar represents 25 μm.
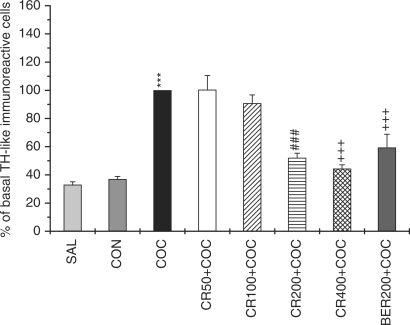
Figure 3.
Expression of TH in the ventral tegmental area after systemic injections of saline or cocaine with Coptidis rhizoma and Berberine. The results are presented as the mean ± SE. The total number of TH-like immunoreactive neurons within a (50 × 50 μm) grid over the areas at × 400 magnification. Significance with Tukey's test following a one-way ANOVA is indicated as ***P < 0.001 versus SAL group and CON group, or ###P < 0.001 and +++P < 0.001 versus COC group. The cells within the tegmental cell area were counted on each of three sections per animal.
Measures of one-way ANOVAs on the numbers of TH-like immunoreactive cells revealed a significant difference among the groups [F (7,141) = 32.930, P < 0.001). Post hoc comparisons revealed the COC group showed more increase in the TH expression than that of the SAL group (P < 0.001) and CON group (P < 0.001) (Fig. 2). CR50, CR100, CR200, CR400 mg/kg and BER 200 mg/kg administration 30 min before the cocaine injection decreased the numbers of TH-like immunoreactive cells to 100.39 ± 10.11 (P = 1.000), 90.83 ± 5.90 (P = 0.897), 52.07 ± 3.32 (P < 0.001), 44.39 ± 2.77 (P < 0.001) and 59.41 ± 9.39 (P < 0.001), respectively, when compared with the COC group's TH-like immunoreactive cells of 100 ± 0.
Discussion
Systemic challenge with cocaine successfully produced a significant increase in locomotor activity and TH immunoreactivity in the ventral tegmental area, which is the major projection area of the central dopamine system. The results of present study demonstrated that repeated daily injections of cocaine produced a higher increase in locomotor activity to a subsequent systemic challenge with cocaine. Current results also clearly showed that a pre-treatment with CR and BER significantly suppressed the chronic cocaine-induced sensitization of locomotor activity and reduced the chronic cocaine-induced increases of TH expression in the VTA in the central DA pathways. These findings suggest that a pre-treatment with CR and BER reduces the development of locomotor activity in response to cocaine by modulating dopamine synthesis in the VTA. It is well known that the mesolimbic system from the VTA to the NAc mediates the behavioral and reinforcing effects of cocaine (27). In the present study, repeated injections of cocaine produced increases in locomotor activity and increases in the TH-like immunoreactivity in the central DA systems. These results agree with the other previous evidence (12,28).
The cocaine-treated group showed a significant increase in locomotor activity, when compared with the saline-treated group. However, there was no significant difference in locomotor activity between the CR and berberine only-treated groups. The CR or BER injections did not produce any significant changes in locomotor activity, indicating that CR or BER did not produce any significant increase in behavioral activity. Meanwhile, the CR and BER administered 30 min prior to the cocaine injection inhibited chronic cocaine-induced sensitization of locomotor activity when compared with the chronic cocaine-treated group. These results suggest that CR and BER inhibit the chronic cocaine-induced psychologic dependence, as determined by the behavioral sensitization paradigm.
Current results demonstrated that pre-treatment of CR 200 mg/kg and CR 400 mg/kg produced a significantly higher inhibitory effect on activity than BER 200 mg/kg. Since it is known that CR contains plamatine and coptisine as well as BER and previous reports have shown that palamatine and cotisine reduced behavioral activity and DA biosynthesis (18,29,30), perhaps other alkaloids may affect cocaine-induced neuronal and behavioral alterations. Future analyses are needed to investigate neurochemical and behavioral effects of these components on cocaine-induced sensitization.
We found that a pre-treatment of CR and BER significantly inhibited development of the cocaine-induced behavioral sensitization and tegmental TH to subsequent cocaine challenge. The present results also suggest that pre-treatment of CR and BER inhibition of the repeated cocaine-induced hyperactivity is closely related with the inhibition of the activated dopaminergic systems that is produced by cocaine. Therefore, the inhibitory effect of CR and BER on behavioral activity reflects the blockade of dopaminergic biosynthesis or transmission. This suggestion is strongly strengthened by previous studies showing that treatment of BER suppressed DA biosynthesis in the brain (18,31,32).
Even though several studies have indicated that protoberberberins may have pharmacologic effects on the central DA system (18,23,30,31), this is the first demonstration of BER action on cocaine-induced behavioral and neurochemical activity. Little is currently known about the action of BER on the central DA system at cellular or molecular levels. Other kinds of protoberberines, the tetrahydroprotoberberines (THPBs), were previously shown to display the highest affinity for the D1- and D2-like receptors (30,33–36). Our preliminary data suggest that BER may bind to DA-receptor sites (unpublished observations). In addition to the clinical study of CR or BER on cocaine addiction, further pharmacologic actions of BER on the central dopaminergic systems should be investigated in the future.
Taken together, CR has been empirically prescribed as a traditional medicine for the treatment of substance abuse, but its major therapeutic actions have not been well known. However, our results demonstrated that CR and BER have central effects and thus contribute to the restoration of a normal biochemical balance in the dopaminergic system. Therefore, our results might provide a mechanistic rationale for herbal treatments including CR for treatment of drug abuse.
In summary, the present results demonstrated that CR and BER inhibit chronic cocaine-induced sensitization and they may modulate the cocaine-induced DA transmission at both the pre- and post-synaptic levels. These results suggest that CR and BER may be effective for inhibiting the behavioral effects of cocaine by modulating the central dopaminergic system.
Acknowledgments
This study was supported by a grant of the SRC program of KOSEF (R11-2005-014) and the Korea Research Foundation (KRF-2006-311-H00001), Korea.
References
- 1.Richtard NM, Woods SC, Berger SP, Strakowski SM. D3 dopamine receptor, behavioral sensitization and psychosis. Neurosci Biobehav Rev. 2001;25:427–43. doi: 10.1016/s0149-7634(01)00023-9. Review. [DOI] [PubMed] [Google Scholar]
- 2.Heimer DS, Bahovic-Vuksanovic DS, Shoaib M, Shippenberg TS. Development of behavioral sensitization to cocaine: Influence of kappa opioid receptor agonists. J Pharmacol Exp Ther. 1995;275:150–63. [PubMed] [Google Scholar]
- 3.Bradberry CW, Roth RH. Cocaine increases extracellular dopamine in rat nucleus accumbens and ventral tegmental area as shown by in vivo microdialysis. Neurosci Lett. 1989;103:97–102. doi: 10.1016/0304-3940(89)90492-8. [DOI] [PubMed] [Google Scholar]
- 4.Steketee JD. Neurotransmitter systems of the medial prefrontal cortex: potential role in sensitization to psychostimulants. Brain Res Brain Res Rev. 2003;41:203–28. doi: 10.1016/s0165-0173(02)00233-3. Review. [DOI] [PubMed] [Google Scholar]
- 5.Ritz MC, Cone EJ, Kuhar MJ. Cocaine inhibition of ligand binding at dopamine, norepinephrineand serotonin transporters: a structure-activity study. Life Sci. 1990;46:635–45. doi: 10.1016/0024-3205(90)90132-b. [DOI] [PubMed] [Google Scholar]
- 6.Caine SB, Negus SS, Mello NK, Patel S, Bristow L, Kulagowski J, et al. Role of dopamine D2-like receptors in cocaine self-administration: studies with D2 receptor mutant mice and novel D2 receptor antagonists. J Neurosci. 2002;22:2977–88. doi: 10.1523/JNEUROSCI.22-07-02977.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Church WH, Justice JB, Jr, Byrd LD. Extracellular dopamine in rat striatum following uptake inhibition by cocaine, nomifensine and benztropine. Eur J Pharmacol. 1987;139:345–8. doi: 10.1016/0014-2999(87)90592-9. [DOI] [PubMed] [Google Scholar]
- 8.Izenwasser S, Werling LL, Cox BM. Comparison of the effects of cocaine and other inhibitors of dopamine uptake in rat striatum, nucleus accumbens, olfactory tubercle and medial prefrontal cortex. Brain Res. 1990;520:303–9. doi: 10.1016/0006-8993(90)91719-w. [DOI] [PubMed] [Google Scholar]
- 9.Kalivas PW, Duffy P. Effect of acute and daily cocaine treatment on extracellular dopamine in the nucleus accumbens. Synapse. 1990;5:48–58. doi: 10.1002/syn.890050104. [DOI] [PubMed] [Google Scholar]
- 10.Spealman RD. Antagonism of behavioral effects of cocaine by selective dopamine receptor blockers. Psychopharmacology (Berl) 1990;101:142–5. doi: 10.1007/BF02253732. [DOI] [PubMed] [Google Scholar]
- 11.Crombag HS, Grimm JW, Shaham Y. Effect of dopamine receptor antagonists on renewal of cocaine seeking by reexposure to drug-associated contextual cues. Neuropsychopharmacology. 2002;27:1006–15. doi: 10.1016/S0893-133X(02)00356-1. [DOI] [PubMed] [Google Scholar]
- 12.Weissenborn R, Deroche V, Koob GF, Weiss F. Effects of dopamine agonists and antagonists on cocaine-induced operant responding for a cocaine-associated stimulus. Psychopharmacology (Berl) 1966;126:311–22. doi: 10.1007/BF02247382. [DOI] [PubMed] [Google Scholar]
- 13.Vrana SL, Vrana KE, Koves TR, Smith JE, Dworkin SI. Chronic cocaine administration increases CNS tyrosine hydroxylase enzyme activity and mRNA levels and tryptophan hydroxylase enzyme activity levels. J Neurochem. 1993;61:2262–8. doi: 10.1111/j.1471-4159.1993.tb07468.x. [DOI] [PubMed] [Google Scholar]
- 14.Beitner-Johnson D, Nestler EJ. Morphine and cocaine exert common chronic actions on tyrosine hydroxylase in dopaminergic brain reward regions. J Neurochem. 1991;57:344–7. doi: 10.1111/j.1471-4159.1991.tb02133.x. [DOI] [PubMed] [Google Scholar]
- 15.Lee B, Hahm DH, Lee HJ, Shim I. Effect of Coptidis Rhizoma on repeated cocaine-induced behavioral sensitization and Fos-like immunoreactivity in the brain of the rats. Korean Journal of Biological and Physiological Psychology. 2002;13:57–69. [Google Scholar]
- 16.Lee HJ, Son DH, Lee SK, Lee J, Jun CD, Jeon BH, et al. Extreact of Coptidis Rhizoma induced cytochrome-c dependent apoptosis in immortalized and malignant human oral keratinocytes. Phytother Res. 2006;20:773–9. doi: 10.1002/ptr.1956. [DOI] [PubMed] [Google Scholar]
- 17.Hwang YS, Shin CY, Huh Y, Ryu JH. Hwangryun-Hae-Dok-tang (Huanglian-Jie-Du-Tang) extract and its constituents reduce ischemia-reperfusion brain injury and neutrophil infiltration in rats. Life Sci. 2002;71:2105–17. doi: 10.1016/s0024-3205(02)01920-3. [DOI] [PubMed] [Google Scholar]
- 18.Shin JS, Kim EI, Kai M, Lee MK. Inhibition of dopamine biosynthesis by protoberberine alkaloids in PC12 cells. Neurochem Res. 2002;25:363–8. doi: 10.1023/a:1007541020736. [DOI] [PubMed] [Google Scholar]
- 19.Wang X, Xing D, Wang W, Lei F, Su H, Du L. The uptake and transport behavior of berberine in Coptidis Rhizoma extract through rat primary cultured cortical neurons. Neurosci Lett. 2005;379:132–7. doi: 10.1016/j.neulet.2004.12.050. [DOI] [PubMed] [Google Scholar]
- 20.Yamahara J. Behavioral pharmacology of berberine-type alkaloids. Central depressive action of Coptidis rhizoma and its constituents. Nippon Yakurigaku Zasshi. 1976;72:899–908. doi: 10.1254/fpj.72.899. [DOI] [PubMed] [Google Scholar]
- 21.Clement-Cormier YC, Meyerson LR, Phillips H, Davis VE. Dopamine receptor topography. Characterization of antagonist requirements of striatal dopamine-sensitive adenylate cyclase using protoberberine alkaloids. Biochem Pharmacol. 1979;28:3123–9. doi: 10.1016/0006-2952(79)90622-1. [DOI] [PubMed] [Google Scholar]
- 22.Harsing LG, Lonart G, Vizi SE. Berbanes; search for novel alpha-2 adrenoceptor antagonists. Pol J Pharmacol Pharm. 1988;40:697–708. [PubMed] [Google Scholar]
- 23.Lee MK, Kim HS. Inhibitory effects of protoberberine alkaloids from the roots of Coptis japonica on catecholamine biosynthesis in PC12 cells. Planta Med. 1996;62:31–4. doi: 10.1055/s-2006-957791. [DOI] [PubMed] [Google Scholar]
- 24.Xu X, Malave A. Protective effect of berberine on cyclophosphamide-induced haemorrhagic cystitis in rats. Pharmacol Toxicol. 2001;88:232–7. doi: 10.1034/j.1600-0773.2001.d01-109.x. [DOI] [PubMed] [Google Scholar]
- 25.Chae Y, Yang CH, Kwon YK, Kim MR, Pyun KH, Hahm DH, et al. Acupunture attenuates repeated nicotine-induced behavioral sensitization and c-Fos expression in the nucleus accumbens and striatum of the rat. Neurosci Lett. 2004;358:87–90. doi: 10.1016/j.neulet.2003.12.121. [DOI] [PubMed] [Google Scholar]
- 26.Paxinos G, Watson C. The Rat Brain in Stereotaxic Co-ordinates. New York: Academic Press; 1986. [Google Scholar]
- 27.Pecins-Thompson M, Peris J. Behavioral and neurochemical changes caused by repeated ethanol and cocaine administration. Phychopharmacology(Berl) 1993;110:443–50. doi: 10.1007/BF02244651. [DOI] [PubMed] [Google Scholar]
- 28.Uslaner J, Badiani A, Norton CS, Day HE, Watson SJ, Akil H, et al. Amphetamine and cocaine induced different patterns of c-fos mRNA expression in the striatum and subthalamic nucleus depending on environmental context. Eur J Neurosci. 2001;13:1977–83. doi: 10.1046/j.0953-816x.2001.01574.x. [DOI] [PubMed] [Google Scholar]
- 29.Hsieh MT, Su SH, Tsai HY, Peng WH, Hsieh CC, Chen CF. Effects of palmatine on motor activity and the concentration of central monoamines and its metabolites in rats. Jpn J Pharmacol. 1993;61:1–5. doi: 10.1254/jjp.61.1. [DOI] [PubMed] [Google Scholar]
- 30.Xu SX, Yu LP, Han YR, Chen Y, Jin GZ. Effects of tetrahydroprotoberberines on dopamine receptor subtypes in brain. Zhongguo Yao Li Xue Bao. 1989;10:104–10. [PubMed] [Google Scholar]
- 31.Sethi ML. Inhibition of reverse transcriptase activity by protoberberine alkaloids and structure-activty relationship. J Pharm Sci. 1983;72:538–41. doi: 10.1002/jps.2600720515. [DOI] [PubMed] [Google Scholar]
- 32.Tang W, Eisenbrand G. Coptids in Chinese Drugs of Plant Origin. Berlin, Heidelberg, New York: Springer; 1992. pp. 362–71. [Google Scholar]
- 33.Wang LM, Zhang XX, Jin GZ. Effect of tetrahydroprotoberberines on dopamine D2 receptors in ventral tegmental area of rat. Zhongguo Yao Li Xue Bao. 1997;18:143–6. [PubMed] [Google Scholar]
- 34.Xuan B, Li DX, Wang M. Protective effects of tetrahydro-protoberberines on experimental myocardial infarction in rats. Acta Pharmacol Sin. 1992;13:167–71. [PubMed] [Google Scholar]
- 35.Jin GZ, Sun BC. Neuropharmacological effects of (-)-stepholidine and its analogues on brain dopaminergic system. Adv Exp Med Biol. 1995;363:27–8. doi: 10.1007/978-1-4615-1857-0_5. [DOI] [PubMed] [Google Scholar]
- 36.Yamahara J. Behavioral pharmacology of berberine type alkaloids (α). Central depressant effect of tetrahydroberberine (THB) and its related compound. Folia Pharmcol Japo. 1976;72:909–27. [PubMed] [Google Scholar]