Abstract
Moutan Cortex, a widely used traditional Chinese medicine for the treatment of various diseases, is the root bark of Paeonia suffruticosa Andrews (Paeoniaceae). Most of the pharmacological investigations of Moutan Cortex have been addressed to its central nervous system activities, anti-oxidative and sedative actions. Otherwise, there are few reports about the active compounds with anti-inflammatory activity of Moutan Cortex. The aim of the present study was to screen and identify bioactive compounds with anti-inflammatory effect from Moutan Cortex. With the aid of preparative high performance liquid chromatography (HPLC) technique, ethyl acetate and ethanol extract of Moutan Cortex were isolated into twenty-two fractions. Bioactivities of these fractions were evaluated by measuring expression of tumor necrosis factor-α (TNF-α) in rat synoviocytes subjected to interleukin-1β (IL-1β). Eight compounds were isolated from six active fractions and identified by HPLC/MSn. Purified compounds, paeoniflorin, paeonol and pentagalloylglucose resulted in dose-dependent inhibition of TNF-α synthesis and IL-6 production in synoviocytes treated with proinflammatory mediator. These results suggested that paeonol, paeoniflorin, glycosides and pentagalloylglucose contribute to the anti-inflammatory effect of Moutan Cortex.
Keywords: moutan cortex, proinflammatory mediator, screening trials, synoviocytes, tumor necrosis factor (TNF), IL-6
Introduction
Moutan Cortex, the root bark of Paeonia suffruticosa Andrews (Paeoniaceae), has been used extensively as a traditional Chinese medicine (TCM) for treating various diseases in China and in other eastern Asian countries. Moutan Cortex is used to treat a plethora of disease classes such as atherosclerosis, infection and inflammation, among many others. A variety of compounds, such as paeonoside, paeonolide, apiopaeonoside, paeoniflorin, oxypaeoniflorin, benzoyloxypaeoniflorin, benzoylpaeoniflorin, paeonol and sugars, etc. have been identified and determined in Moutan Cortex (1). Many clinical and experimental studies have reported that crude extracts of Moutan Cortex have therapeutic effects on ischemic heart diseases (2,3). Further, recent pharmacological studies revealed that Moutan Cortex can inhibit the production of reactive oxygen species (ROS) (4) and some paeonol glycosides exhibited radical scavenging effects (5). Matsuda H et al. (6) reported the isolation of five paeonol glycosides and their radical scavenging effects.
The synoviocytes cells are important part of joint tissue and can produce pro-inflammatory mediators that are key players in pathophysiology of arthritis. The occurrence of inflammatory reactions in the synovial membrane has been demonstrated as the initial motive of temporomandibular disorders (TMD) (7), similar to the pathologic changes of osteoarthritis (OA) and rheumatoid arthritis (RA) (8,9). It has been reported that extracts from the roots of Paeonia lactiflora Pall, another medicinal herb with analogous chemical composition of Moutan Cortex, can possess anti-inflammatory effects on arthritis rats by modulating the pro-inflammatory mediators production and change the ultrastructure of synoviocytes (10). However, the bioactive compounds of Moutan Cortex, which are potential to confer inflammatory mediator in cellular models, have not yet been evaluated.
Many in vitro cell models including tumor cell lines and macrophages have been used to screen herbal extracts for anti-inflammatory activity. An in vitro model, which can appropriately represent the joint tissue and allow monitoring of physiological markers in response to treatment agents, has been established in previous report (11). The aim of the present study was to screen and identify active compounds from Moutan Cortex with anti-inflammatory action. Fractions from the ethyl-acetate and ethanol extracts of Moutan Cortex were obtained by preparative high performance liquid chromatography (HPLC) and assayed for their abilities of reducing expression of tumor necrosis factor-α (TNF-α). Eight compounds in six fractions with significant activity were identified. Dose-dependent effects of three purified compounds, paeoniflorin, paeonol, and pentagalloylglucose have been studied.
Methods
Plant Materials and Reagents
Moutan Cortex was purchased from Hangzhou Traditional Chinese Medicine Company (Hangzhou, China). The crude sample was authenticated morphologically by Assistant Prof. Heqing of Zhejiang University. A voucher specimen has been deposited in the herbaria (Department of Chinese Medicine Sciences & Engineering, Zhejiang University, China), under the acquisition number of DP-1L.
Ethylacetate (EtOAc), methanol and ethanol were of analytical grade from Hangzhou Reagent Company (Hangzhou, China). Acetonitrile for HPLC analysis was of HPLC grade from E. Merck (Darmstadt, Germany). Water for HPLC analysis was purified by a Milli-Q academic water purification system (Milford, MA, USA). For cell culture, DMEM medium and collagenase II were purchased from Gibco (CA, USA); trypsin was obtained from Sigma (St. Louis, MO USA); fetal calf serum was purchased from HyClone (Logan, USA).
Extraction and Isolation
Air-dried plant material (250 g) were crushed and macerated consecutively with the mixture of ethylacetate (EtOAc) and ethanol (v:v 1:1) and 70% ethanol solution for 1 day. The extract obtained was filtered and the solvent removed in vacuum and two fractions were obtained: extract A with lower polarity and extract B with higher polarity. The yield ratio was 8.6% and 16.4%, respectively.
The extract A was transferred into a silica gel column and chromatographed using a step gradient solvent system starting with petroleum ether, chloroform and ending with methanol. The elution of a mixture of chloroform and methanol (10:1) were collected and dried in vacuum to get extract C. The extract B was subjected to a silica gel column using a step gradient solvent system with methanol aqueous solutions (5%, 70% and 100%) and the elution of 70% methanol was collected to obtain extract D.
Further separations of extract C and extract D were performed on a Hanbang RP-18 semi-preparative column (5 μm, 250 × 10 mm; Hanbon Sci. & Tech, China) and Agilent 1100 series preparative HPLC system (Waldbronn, Germany) equipped with a binary high-pressure mixing pump and fraction collector. The flow rate was 3 mL/min and the detection wavelength of the diode array detector was set at 230 nm. For extract C, the following gradient profile with water (solvent A) and acetonitrile (solvent B, E. Merck) was used: solvent B, 0–40 min, 20–80%; 40–50 min, 80–95%. Nine different fractions were collected according to the time schedule as shown in Table 1. Extract D was separated in the same manner with a gradation: solvent B, 0–8 min, 10–19%; 8–50 min, 19–35%; 50–65 min, 35–95%; 65–70 min, 95%. Thirteen fractions were obtained.
Table 1.
Collection procedure of fractions by preparative-HPLC; the number in the table represents the started time of collection for each fraction
| No. | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| extract C | 4.0 | 7.6 | 9.7 | 14.2 | 18.4 | 21.8 | 26.5 | 30.3 | 35.5 | / | / | / | / |
| extract D | 4.0 | 7.7 | 10.7 | 15.8 | 18.8 | 23.2 | 27.4 | 33.1 | 40.2 | 44.4 | 48.5 | 58.0 | 67.0 |
Identification of Active Compounds
Preliminary phytochemical investigations of fractions were carried out by HPLC/Msn. The chromatographic separation was performed on a Lichrospher C18 column (5 μm, 4.6 × 250 mm, Hanbang Science & Technology) with an Agilent 1100 HPLC system (Waldbroonn, Germany) equipped with a quaternary pump, vacuum degasser, autosampler, diode-array detector, column heater-cooler and ChemStation system. A LCQ DECA XPplus mass spectrometer (Thermo Finnigan, San Jose, CA, USA) equipped with an ESI interface and an ion trap mass analyzer was used to carry out the MS and MSn. The column temperature was set at 30°C. Detection was at 230 nm and spectrograms were recorded between 200 and 400 nm at the apex of each peak.
Pure chemicals of paeoniflorin and paeonol were purchased from the National Institute for the Control of Pharmaceutical and Biological Products (Beijing, China). Compounds in the samples were identified by comparison of the retention time and spectrum for each peak with the corresponding standard. By comparison of molecular weight, UV absorbance and mass fragments information with those of published data, a number of compounds can be tentatively identified.
Cultured Synoviocytes and In Vitro Screening Model
Synoviocytes were isolated from the double condyle of rat temporomandibular joint and cultured according to a described procedure with minor modifications (12). The use of animals was in accordance with Guideline for the Care and Use of Laboratory Animals of Zhejiang University. Briefly, the double condyles of 3 months old Sprague–Dawley rats (obtained from the Zhejiang Center of Laboratory Animals, China) were excised, the synovium of bilaminar zone (BZ) were separated, washed extensively with phosphate buffered saline (PBS), then minced and digested three times by 0.125% trypsin and 0.05% collagenase II at 37°C for 30 min. Synoviocytes were seeded into a cover plate, incubated in DMEM (Gibco, Grand Island, NY, USA) supplemented with l-glutamine, streptomycin, penicillin and 10% fetal calf serum at 37°C in a humidified atmosphere containing 5% CO2. A pre-treatment of fractions on synoviocytes was performed to evaluate cell viability through trypan blue dye and morphological observation.
To evaluate anti-inflammatory effects of different fractions, synoviocytes were seeded onto 24-well plates at 1 × 105 cells/well. Synoviocytes were next incubated with control media alone or with fraction (10−5 g mL−1) 1 h before reincubation with 10 ng mL−1 interleukin-1β (IL-1β) or control media alone for 1 h. Supernatant medium samples were taken for the detection of TNF-α concentration by enzyme-linked immunosorbent assay (ELISA) (Biosource International, Camarillo, CA, USA). A commercial rat-specific IL-6 ELISA kit (Quantikine, R&D Systems) was used to quantify IL-6. The effectiveness of tested fractions was determined by comparing TNF-α concentration in treated group to the untreated cells.
Statistics
Results were expressed as mean ± S.E.M. Statistical analysis between groups was carried out by one-way analysis of variance and the Newman–Keuls Student t-test. The level of significance was chosen as P < 0.05.
Results
Anti-Inflammatory Effects of Fractions
The fractions obtained were combinations of a few compounds with similar chemical properties. Evaluation of cell viability indicated that incubation of synoviocytes with fractions of Moutan Cortex for 1 day at concentration of 10 g mL−1 does not reduce the percent of viable cells. There was no significant difference in cell number between treated and untreated control cells (P > 0.05).
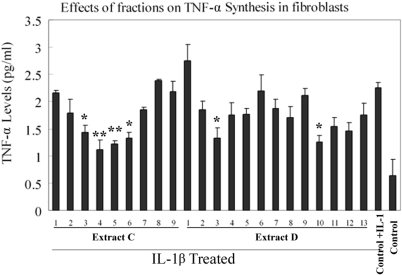
The anti-inflammatory activities of totally 22 fractions were assayed in IL-1β stimulated synoviocytes. It has been found that the level of TNF-α in synoviocytes incubated with pro-inflammatory mediators was significantly increased in comparison with the untreated control. It was observed that four adjacent fractions from extract C, i.e. fraction C-3, C-4, C-5, C-6, exhibited similar efficacy on suppressing the release of TNF-α. Additionally, two fractions in extract D, D-3 and D-10, significantly affect production of TNF-α (Fig. 1).
Figure 1.
Inhibition of tumor necrosis factor-α (TNF-α) synthesis by fractions of Moutan Cortex. Synoviocytes were incubated with control medium alone or (10 μg mL−1 of fraction for 1 h) before the addition of control medium alone or 10 ng mL−1 interleukin-1β (IL-1β). The cells were reincubated for (24 h at 37°C, 5% carbon dioxide) and the secreted TNF-α concentration were measured with ELISA. Compared with control group (*P < 0.05, **P < 0.01, n = 3).
Identification of Compounds in Active Fractions
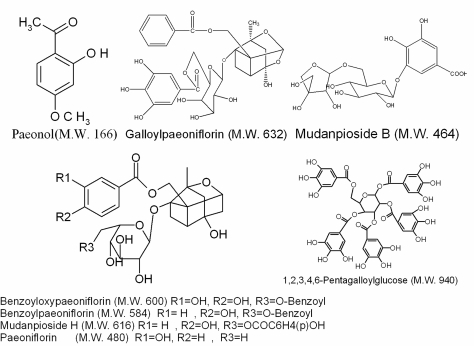
To illustrate chemical composition of those bioactive fractions, the major constituents were identified by HPLC/MSn. Five paeonol glycosides including paeonol, paeoniflorin and its derivatives in extract C and two other active compounds, mudanoside B and 1,2,3,4,6-pentagalloylglucose in extract D were identified. Major constituents of active fractions were further isolated by preparative HPLC and purified. Some purified compounds, such as paeonol and paeoniflorin were identified by comparing MS/MSn data with standard samples. Other compounds, including pentagalloylglucose, galloylpaeoniflorin, benzoylpaeoniflorin, benzoyl- oxypaeoniflorin, mudanpioside B and mudanpioside H were identified by comparing MSn and NMR data with previously reported data. Major compounds of those fractions are listed in Table 2 and their structures are shown in Fig. 2.
Table 2.
HPLC-DAD-MSn data of identified compounds in active fractions
| Fraction | M.W. | MSn | UV (nm) | Compounds |
|---|---|---|---|---|
| C-3 | 480 | 449 [M-H-CH2O] 327 [M-H-CH2O-(Benzoic acid)] 165[M-H-CH2O-(Benzoic acid)-Glucose] | 195, 235 | Paeoniflorin |
| C-4 | 616 | 585 [M-H-CH2O 447[M-H-CH2O-(p-hydroxybenzoic acid)] | 215, 270 | Mudanpioside H |
| C-4 | 632 | 491 [M-H-C6H5COOH] 313 [M-H- C6H5COOH- gallic acid] | 230 | Galloylpaeoniflorin |
| C-5 | 600 | 569 [M-H-CH2O] 477 [M-H-CH2O-(Benzoic acid)] | 230, 260 | Benzoyloxypaeoniflorin |
| C-5 | 584 | 461 [M-H-(Benzoicacid)]− 431 [M-H-(Benzoicacid) –CH2O]− 165[M-H-(Benzoicacid)-CH2O-(Benzoicacid)-Glucose]− 137[M-H-(Benzoicacid)-CH2O-(Benzoicacid)-Glucose-co]− | 230 | Benzoylpaeoniflorin |
| C-6 | 166 | 149 [M+H-H2O]+ 121 [M+H-H2O-CO]+ | 230, 280 | Paeonol |
| D-3 | 464 | 301 [M-H- C6H10O5] | 215, 275 | Mudanoside B |
| D-10 | 940 | 769 [M-H-gallic acid]− 617 [M-H-gallicacid-galloy]− 313 [M-H-gallicacid-galloy-2galloy]− | 212, 280 | 1,2,3,4,6-Pentagalloylglucose |
Figure 2.
Structures of active compounds.
Dose-Dependent Effect of Purified Compounds on TNF-α Level
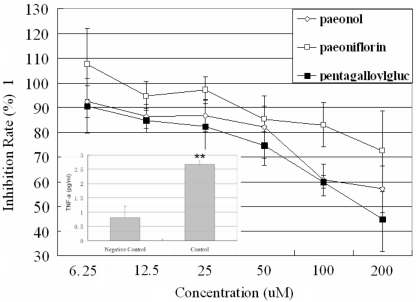
Anti-inflammatory effects of three major components, paeonol, paeoniflorin and pentagalloylglucose at concentrations ranging from 6.1 to 200 μM were tested on the above cell model. As shown in Fig. 3, in comparison of the negative control (untreated synoviocytes), TNF-α level in control group (IL-1 treated synoviocytes) significantly increased (P < 0.01). Paeonol, paeoniflorin and pentagalloylglucose can significantly suppress the increase of TNF-α level in dose-dependent manner compared with the control group. The ratio of TNF-α concentration in treated well to untreated well was used to present the anti-inflammatory effect of those compounds. Paeonol decreased the level of TNF-α with maximum inhibition (57%) at a concentration of 200 μM, whilst pentagalloylglucose achieved better anti-inflammatory effect (45%) at the same concentration. Paeoniflorin exhibited a maximum inhibition (72%).
Figure 3.
Dose-dependent inhibition of TNF-α synthesis induced by paeonol (^), paeoniflorin (□), and pentagalloylglucose (▪) on synoviocytes. Bars represent standard deviations, n = 4. The percentage of inhibition rate was calculated as follows: inhibition rate [(%) = 100 × (TNF-α level in treated well/TNF-α level in the control well)]. TNF-α level in negative control (untreated synoviocytes) and control (IL-1 treated synoviocytes) was expressed as bar graph. Comparing with control group, *P < 0.05, **P < 0.01.
Dose-Dependent Effect of Purified Compounds on IL-6 Level
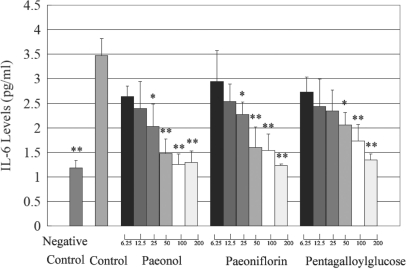
IL-6 is one of the most potent cytokines that promote inflammatory events through expansion and activation of T cells, differentiation of B cells and induction of the acute phase response (13). Several cytokines including TNF-α, IL-1 can stimulate the synthesis of IL-6 (14,15). To examine anti-inflammatory effects of purified compounds, effects of paeonol, paeoniflorin and pentagalloylglucose on the production of IL-6 in IL-1β stimulated synoviocytes were studied. As shown in Fig. 4, paeonol, paeoniflorin and pentagalloylglucose significantly decreased IL-6 level in dose-dependent manner.
Figure 4.
Dose-dependent inhibition of IL-6 level induced by paeonol, paeoniflorin and pentagalloylglucose in the range of (6.25–200 μM) on IL-1 stimulated synoviocytes. TNF-α level in negative control (untreated synoviocytes) and control (IL-1 treated synoviocytes) was also expressed. Bars represent standard deviations (n = 6). Comparing with control group, *P < 0.05, **P < 0.01.
In summary, the present data illustrated that the purified compounds were bioactive compounds of Moutan Cortex with anti-inflammatory activity.
Discussion
In the study on anti-anaphylactic activity of paeonol by Kim et al. (16), paeonol exhibited significant regulation on the release of TNF-α. Although anti-inflammatory effect of paeonol on synoviocytes has not been reported, a recent study reveals that paeonol dose-dependently inhibited TNF-α and IL-1β formation in the rat paw exudates after carrageenan injection (17). Chun et al. (18) also reported anti-inflammatory activity of the methanol extract of Moutan Cortex in RAW264.7 cells after treatement with, lipopolyssacharide (LPS) and suggested that the anti-inflammatory effects were induced through the inhibition of iNOS and COX-2 expression by suppressing the phosphorylation of I-κBα and the activation of NF-κB. Therefore, the possible mechanisms by which paeonol exerts its anti-inflammatory effect may be associated with decreased production of pro-inflammatory cytokines, NO and increased production of IL-10. Total glucosides of paeony (TGP) has been proven to be an anti-inflammatory extract by modulating phosphorylation of MAPKs from synoviocytes (10). Paeoniflorin and its derivatives such as benzoylpaeoniflorin and galloylpaeoniflorin are major components of TGP. Thus, it suggests that the possible mechanism for anti-inflammatory effect of paeoniflorin may be attributed to the activation of the MARKs cascades and other transcription factors. Pentagalloylglucose is a newly found component with anti-endotoxin effect both in endotoxemic mice and rats. The reduction of the endotoxin levels in rats tightly associated with the TNF-α level (19). Therefore, pentagalloylglucose might suppress TNF-α release in a dose dependent manner.
In recent years, it has been proven that extracts or compounds from many herbs may provide a safe and effective adjunctive therapeutic approach for the treatment of osteoarthritis and RA (20). Tohda et al. (21) has compared anti-inflammatory activities of six different species of Curcuma herbs in adjuvant arthritis model mice and found a most effective specie. Thus, it can be expected to find active compounds from herbs for the therapy of temporomandibular syndrome. Traditionally, temporomandibular disorder (TMD) is treated with conservative therapy. Some alternative and complementary therapy for TMD patients has also been introduced, including intra-articular injection of superoxide dismutase (SOD) (22) and sodium hyaluronate (SH) (23). It has been reported that extracts of Moutan Cortex and paeonol can increase SOD activity in ischemic brain tissue (24) and cultured synoviocytes (25). The chemical and biological investigations carried out in this work lead to the detection of several active compounds in Moutan Cortex as inhibitor of pro-inflammatory mediator. Though the exact mechanism of action responded for the anti-inflammatory effects of those compounds still need further investigation, the result of present study offers a new way for the treatment of TMD.
To our knowledge, bioavailabilities of some identified active compounds are not good. However, Liu et al. (26) suggested that poor bioavailability of paeoniflorin can be significantly increased by other chemicals such as sinomenine and verapamil. It also reported that absorption of paeoniflorin after oral administration of the two herbal formulations was significantly greater than that after oral administration alone (27). Therefore, bioavailability of the isolated compounds and their interaction on pharmacokinetics should be further evaluated in our future study to help design rational dosage regimens. It can be expected that new agents can be developed from extracts and active compounds with anti-inflammatory effect of Moutan Cortex for patients with temporomandibular dysfunction who fail to respond to conservative treatment.
Acknowledgments
This project was financially supported by a grant from the National Natural Science Foundation of China (No. 30471900).
References
- 1.Chen G, Zhang L, Zhu Y. Determination of glycosides and sugars in Moutan Cortex by capillary electrophoresis with electrochemical detection. J Pharm Biomed Anal. 2006;41:129–34. doi: 10.1016/j.jpba.2005.11.001. [DOI] [PubMed] [Google Scholar]
- 2.Ma Y, Liu J, Sun W. Effect of Moutan cortex on dynamics of blood stream in ischemic dog model. Shanxi Medicine. 1984;13:212–4. [Google Scholar]
- 3.Li Y, Zhang W, Huang L, Shi J. Pharmacological of cortex moutan and core. Zhongguo Zhong Yao Za Zhi. 1997;22:214–6. [PubMed] [Google Scholar]
- 4.Rho S, Chung HS, Kang M, Lee E, Cho C, Kim H, et al. Inhibition of production of reactive oxygen species and gene expression profile by treatment of ethanol extract of moutan cortex radicis in oxidative stressed PC12 cells. Biol Pharm Bull. 2005;28:661–6. doi: 10.1248/bpb.28.661. [DOI] [PubMed] [Google Scholar]
- 5.Yoshikawa M, Uchida E, Kawaguchi A, Kitagawa I, Yamahara J. Galloyl-oxypaeoniflorin, suffruticosides A, B, C, and D, five new antioxidative glycosides, and suffruticoside E, A paeonol glycoside, from Chinese moutan cortex. Chem Pharm Bull (Tokyo) 1992;40:2248–50. doi: 10.1248/cpb.40.2248. [DOI] [PubMed] [Google Scholar]
- 6.Matsuda H, Ohta T, Kawaguchi A, Yoshikawa M. Bioactive constituents of chinese natural medicines. VI. Moutan cortex. (2): structures and radical scavenging effects of suffruticosides A, B, C, D, and E and galloyl-oxypaeoniflorin. Chem Pharm Bull (Tokyo) 2001;49:69–72. doi: 10.1248/cpb.49.69. [DOI] [PubMed] [Google Scholar]
- 7.Gynther GW, Holmlund AB, Reinholt FP. Synovitis in internal derangement of the temporomandibular joint: correlation between arthroscopic and histologic findings. J Oral Maxillofac Surg. 1994;52:913–7. doi: 10.1016/s0278-2391(10)80066-7. [DOI] [PubMed] [Google Scholar]
- 8.Gynther GW, Dijkgraaf LC, Reinholt FP, Holmlund AB, Liem RS, de Bont LG. Synovial inflammation in arthroscopically obtained biopsy specimens from the temporomandibular joint: a review of the literature and a proposed histologic grading system. J Oral Maxillofac Surg. 1998;56:1281–6. doi: 10.1016/s0278-2391(98)90609-7. [DOI] [PubMed] [Google Scholar]
- 9.Dijkgraaf LC, Liem RS, van der Weele LT, de Bont LG. Correlation between arthroscopically observed changes and synovial light microscopic findings in osteoarthritic temporomandibular joints. Int J Oral Maxillofac Surg. 1999;28:83–9. [PubMed] [Google Scholar]
- 10.Zheng YQ, Wei W. Total glucosides of paeony suppresses adjuvant arthritis in rats and intervenes cytokine-signaling between different types of synoviocytes. Int Immunopharmacol. 2005;5:1560–73. doi: 10.1016/j.intimp.2005.03.010. [DOI] [PubMed] [Google Scholar]
- 11.Frondoza CG, Sohrabi A, Polotsky A, Phan PV, Hungerford DS, Lindmark L. An in vitro screening assay for inhibitors of proinflammatory mediators in herbal extracts using human synoviocyte cultures. In Vitro Cell Dev Biol Anim. 2004;40:95–101. doi: 10.1290/1543-706x(2004)040<0095:aivsaf>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 12.Nagai H, Miyamoto Y, Nakata A, Hatakeyama S, Iwanami Y, Fukuda M. Isolation and characterization of synovial cells from the human temporomandibular joint. J Oral Pathol Med. 2006;35:104–10. doi: 10.1111/j.1600-0714.2006.00369.x. [DOI] [PubMed] [Google Scholar]
- 13.Kamimura D, Ishihara K, Hirano T. IL-6 signal transduction and its physiological roles: the signal orchestration model. Rev Physiol Biochem Pharmacol. 2003;149:1–38. doi: 10.1007/s10254-003-0012-2. [DOI] [PubMed] [Google Scholar]
- 14.Okuda Y, Bardin CW, Hodgskin LR, Morris PL. Interleukins-1 alpha and -1 beta regulate interleukin-6 expression in Leydig and Sertoli cells. Recent Prog Horm Res. 1995;50:367–72. doi: 10.1016/b978-0-12-571150-0.50022-6. [DOI] [PubMed] [Google Scholar]
- 15.Stephan JP, Syed V, Jegou B. Regulation of Sertoli cell IL-1 and IL-6 production in vitro. Mol Cell Endocrinol. 1997;134:109–18. doi: 10.1016/s0303-7207(97)00172-x. [DOI] [PubMed] [Google Scholar]
- 16.Kim SH, Kim SA, Park MK, Kim SH, Park YD, Na HJ, et al. Paeonol inhibits anaphylactic reaction by regulating histamine and TNF-alpha. Int Immunopharmacol. 2004;4:279–87. doi: 10.1016/j.intimp.2003.12.013. [DOI] [PubMed] [Google Scholar]
- 17.Chou TC. Anti-inflammatory and analgesic effects of paeonol in carrageenan-evoked thermal hyperalgesia. Br J Pharmacol. 2003;139:1146–52. doi: 10.1038/sj.bjp.0705360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chun SC, Jee SY, Lee SG, Park SJ, Lee JR, Kim SC. Anti-Inflammatory Activity of the Methanol Extract of Moutan Cortex in LPS-Activated Raw264.7 Cells. Evid Based Complement Alterna Med. 2006 doi: 10.1093/ecam/nel093. doi:10.1093/ecam/nel093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Genfa L, Jiang Z, Hong Z, Yimin Z, Liangxi W, Guo W, et al. The screening and isolation of an effective anti-endotoxin monomer from Radix Paeoniae Rubra using affinity biosensor technology. Int Immunopharmacol. 2005;5:1007–17. doi: 10.1016/j.intimp.2005.01.013. [DOI] [PubMed] [Google Scholar]
- 20.Ahmed S, Anuntiyo J, Malemud CJ, Haqqi TM. Biological basis for the use of botanicals in osteoarthritis and rheumatoid arthritis: a review. Evid Based Complement Alternat Med. 2005;2:301–8. doi: 10.1093/ecam/neh117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tohda C, Nakayama N, Hatanaka F, Komatsu K. Comparison of Anti-inflammatory Activities of Six Curcuma Rhizomes: A Possible Curcuminoid-independent Pathway Mediated by Curcuma phaeocaulis Extract. Evid Based Complement Alternat Med. 2006;3:255–60. doi: 10.1093/ecam/nel008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lin Y, Pape HD, Friedrich R. Use of superoxide dismutase (SOD) in patients with temporomandibular joint dysfunction—a preliminary study. Int J Oral Maxillofac Surg. 1994;23:428–9. doi: 10.1016/s0901-5027(05)80038-4. [DOI] [PubMed] [Google Scholar]
- 23.Alpaslan GH, Alpaslan C. Efficacy of temporomandibular joint arthrocentesis with and without injection of sodium hyaluronate in treatment of internal derangements. J Oral Maxillofac Surg. 2001;59:613–8. doi: 10.1053/joms.2001.23368. [DOI] [PubMed] [Google Scholar]
- 24.Zhang G, Yu Z, Zhao H. Protective effect of paeonol on repeated cerebral ischemia in rats. Zhong Yao Cai. 1997;20:626–8. [PubMed] [Google Scholar]
- 25.Lee SE, Hwang HJ, Ha JS, Jeong HS, Kim JH. Screening of medicinal plant extracts for antioxidant activity. Life Sci. 2003;73:167–79. doi: 10.1016/s0024-3205(03)00259-5. [DOI] [PubMed] [Google Scholar]
- 26.Liu ZQ, Jiang ZH, Liu L, Hu M. Mechanisms responsible for poor oral bioavailability of paeoniflorin: Role of intestinal disposition and interactions with sinomenine. Pharm Res. 2006;23:2768–80. doi: 10.1007/s11095-006-9100-8. [DOI] [PubMed] [Google Scholar]
- 27.Yang XG, Peng B, Zhang GH, Wei LL, Nie SF, Pan WS. Studies of the pharmacokinetics of paeoniflorin in two Jing-Zhi-Guan-Xin formulations after oral administration to beagle dogs. J Pharm Biomed Anal. 2006;41:320–4. doi: 10.1016/j.jpba.2005.11.004. [DOI] [PubMed] [Google Scholar]