Abstract
We explored the neurochemical mechanism of electroacupuncture's (EA) protective effect on brain function in focal cerebral ischemia rats, using cerebral ischemia/reperfusion rats established by the middle cerebral artery occlusion (MCAO) method. Adult male Sprague–Dawley rats were randomly divided into four groups: Sham, Sham+EA, MCAO and MCAO+EA. The rats in Sham+EA and MCAO+EA were accepted EA treatment at ‘GV26’ and ‘GV20’ acupoints for 30 min. Electric stimulation was produced by a G-6805 generator and neurological deficit scores were recorded. Mitochondria respiratory function and the activities of respiratory enzymes were measured by a computer-aided Clark oxygen electrode system. Results showed that EA treatment might reduce the neurological deficit score, and significantly improve respiratory control ratio (RCR), the index of mitochondrial respiratory function, and increase the activities of succinic dehydrogenase, NADH dehydrogenase and cytochrome C oxidase in the MCAO rats. Results suggest that EA might markedly decrease the neurological deficit score, promote the activities of respiratory enzymes and reduce the generation of reactive oxygen species (ROS), resulting in improvement of respiratory chain function and anti-oxidative capability of brain tissues in the infarct penumbra zone. This be a mechanism of EA's anti-injury effect on brain function in MCAO rats.
Keywords: cytochrome C oxidase, electroacupuncture, mitochondria, NADH dehydrogenase, respiratory control ratio, stroke, succinic dehydrogenase
Introduction
Stroke is a major cause of death and disability all over the world. Statistical data show that 85% of strokes are caused by embolism of a cerebral blood vessel (1). Many animal models have been adopted to study ischemic stroke. Clinical statistical data have shown that the middle cerebral artery (MCA) is the most commonly part occluded, making the MCAO model an ideal one for cerebral ischemia/reperfusion. Improved Longa is the most common method to operate on the MCAO model that is used not only to research brain ischemic injury, but also reperfusion damage (2,3).
Clinically, acupuncture has shown significant therapeutic benefits for stroke patients (4–6), and the mechanism of these benefits has been under more and more scrutiny. Previous experimental data from our laboratory provided some evidence that electroacupuncture (EA) might attenuate the activities of superoxide dismutase (SOD) and glutathione peroxidase (GSH-Px), decrease the content of malondialdehyde (MDA) and ameliorate mitochondrial DNA (mtDNA) in the infarcted area of the brain in the penumbra zone of MCAO rats. These data suggest that EA might alleviate oxidative stress and thus protect cerebral function in MCAO rats (7).
Mitochondria are essential organelles of energy metabolism and play a key role in processes such as oxidative phosphorylation, aerobic metabolism of glucose and fat, calcium signaling, apoptosis and generation of reactive oxygen species (ROS) (8–10). The respiratory chain engenders an electron leak and generated ROS while transferring the electron and under pathological conditions such as ischemia or hypoxia, the electron leak increases and therefore leads to an increase of ROS (11), resulting in mitochondrial dysfunction. Mitochondrial enzymes including succinic dehydrogenase, NADH dehydrogenase and Cytochrome C oxidase are the vital enzymes in the respiratory chain, their activities are closely connected with mitochondrial respiratory function, energy metabolism and the production of ROS (12) and can easily be attacked by ROS (13). Some data have shown that when the brain cortex of mice is exposed to chronic hypoxia, the activities of cytochrome oxidase and NADH-CoQ reductase decreased and oxygen consumption reduced (14,15). According to these results, we suppose that mitochondrial oxidative stress is a chief cause of brain cell damage after cerebral ischemia/reperfusion and that EA might improve the activities of antioxidant enzymes and the function of mitochondria to alleviate brain injury induced by ischemia. To gather more evidence to support our hypothesis we designed these experiments, and were able to further explore the mechanism of EA protection of brain function in MCAO rats.
Methods
Rats
Male Sprague–Dawley rats (weight 200–220 g) purchased from Animal Resources Center, Shanghai Medical College of Fudan University, were divided randomly into four groups by using computerized random number allocation: Sham (n = 12), Sham+EA (n = 12), MCAO (n = 12) and MCAO+EA (n = 12). Half of every group was used to estimate activities of mitochondrial respiratory enzymes, and the other half was assigned to analysis of mitochondrial respiratory function. All experimental procedures involving the use of animals were conducted in accordance with NIH Guidelines and approved by the Animal Use and Care Committee for Fudan University.
MCAO Operation
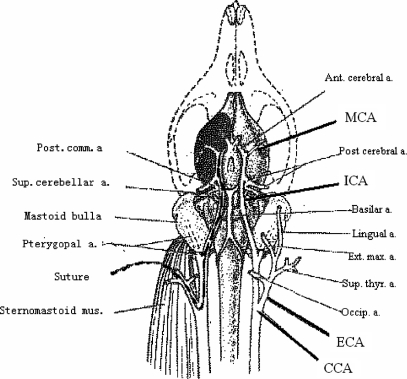
The operation of MCAO was carried out according to previously described methods (7). The rats were anesthetized by 7% chloral hydrate (1 ml 200 g−1 ip) and placed in dorsal recumbency. A longitudinal incision of 1.5 cm in length was made in the midline of the ventral cervical skin. The right common carotid artery (CCA), right internal carotid artery (ICA) and right external carotid artery (ECA) were exposed and carefully isolated. A nylon monofilament (40 mm in length and 0.24 mm in diameter), its tip rounded by flame-heating, was inserted from the lumen of the ECA to that of the right ICA to occlude the origin of the right middle cerebral artery (MCA) (Fig. 1). The right MCA was occluded for 90 min and after that cerebral blood flow (CBF) was restored by withdrawal of the nylon thread. Twenty-four hours after reperfusion, rats were decapitated. Animal body temperature was maintained at 37 ± 1°C during and after operation. The sham group underwent the same surgical procedure without inserting the nylon thread.
Figure 1.
Schematic diagram illustrates extra-intercranial vascular relation and the transient focal cerebral ischemia model induced by middle cerebral artery occlusion in rat. A 4-0 nylon suture was inserted from the internal carotid artery to the origin of middle cerebral artery to occlude blood flow there.
Acupuncture Treatment
Two stainless steel needles (φ = 0.5 mm) inserted at two head acupoints of ‘GV 26’ (Renzhong, located at the midline just 1mm below the top of nose) inserted 1 mm and ‘GV 20’ (Baihui, located at the midmost point of parietal bone) inserted 4 mm. After the operation, the Sham+EA and MCAO+EA rats received EA treatment immediately for 60 min with a 15 min intermission. Electric stimulation was generated by an EA apparatus (Model G-6805) and the stimulation parameter were disperse-dense waves of 5/20 Hz (28.5 ms/15 ms pulse duration) of frequency and current density of 2–4 mA.
Index of Neurological Deficits Test
Researchers and another assistant who was not involved in this experiment scored the neurological deficits at 24 h after the reperfusion using a modified Zea-Longa's scoring system (16), grade 0: no observable deficit, grade 1: when rats were suspended by the tail, the left forelimb extended incompletely, grade 2: left forelimb dysfunction, grade 3: left forelimb flexed tightly close to the chest, grade 4: unidirectional (left) circling spontaneously, grade 5: plus decreased resistance to lateral push, grade 6: unidirectional circling around a fixed point and grade 7: lying on one side without strength to support its weight.
Isolation of Brain Mitochondria
Brain mitochondria of the perifocal penumbra zone was isolated by differential centrifugation, according to the method of Mizuno et al. (17). All procedures were carried out on ice except centrifugation, which was done at 4°C. Rats were weighed, decapitated, and the brains immediately removed, minced into fine pieces and rinsed in isolation buffer (250 mM sucrose, 5 mM Tris-HCl and 1 mM EDTA, pH 7.4 at 20°C) by glass homogenizers until the supernatant appeared clear. The homogenates were centrifuged at 1300 g for 15 min. The supernatant was saved and spun at 10 000 g for 10 min. The resulting pellet was rinsed once by resuspension in isolation buffer and spinning at 10 000 g for 10 min. The mitochondrial pellet was resuspended in reaction buffer (0.3 M sucrose, 0.01 M KCl, 0.0002 M EDTA, 0.004 M MgCl2, 0.2% BSA, 0.01 M K3PO4 buffer, 0.01 M Tris-HCl, PH 7.4) stored at −20°C. Mitochondrial protein concentration was determined with the Bradford assay using BSA as a standard (18).
Measurement of Mitochondrial Respiratory Function and the Activities of Respiratory Enzymes
Function of Mitochondrial respiration and activities of respiratory enzymes were measured by a computer-aided Clark oxygen electrode system. The hardware of the system was constituted with computer (HP pavilion dv 5220), ADC-DAC board, a Clark type oxygen electrode, a closed chamber with constant temperature and electromagnetic stirrer. The software (Huan LJ 0201) was exploited by Shanghai Medical College, Fudan University. The procedure of operation was following: after preparing electrode system, oxygen electrode was balanced in reaction chamber with reaction buffer (300 mM mannitol, 10 mM KCl, 0.2 mM EDTA, 4 mM MgCl2, 0.2%BSA, 0.01 M K3PO4 buffer and 0.01 M Tris-HCl, pH 7.4 at 20°C) and succinic acid. After stabilization, mitochondria (3 mg ml−1 protein) and following ADP (final concentration for 0.25 mM) was added respectively through a small portal. The final volume was 2.8 ml and the assay temperature was 30°C. State β and state χ was registered and RCR was calculated. While estimating respiratory enzymes, K3PO4 buffer replaced the reaction buffer, and the content of mitochondrial protein was 1 mg ml−1. The reactive substrates of succinic dehydrogenase, NADH dehydrogenase and cytochrome C oxidase were respectively: succinic acid, cytochrome C; NADH, cytochrome C; TMPD, Vit C and cytochrome C, and each reaction rate was examined. The results were calculated automatically by software according to correlated original data.
Statistics
Statistical analysis of neurological deficits scores was managed by non-parametric Mann–Whitney U rank sum test, the other comparisons of the four groups were made using analysis of one-way ANOVA followed by post-hoc protected least-significant difference test. The criterion for statistical difference was P < 0.05.
Results
Reduction of the Neurological Deficit Score
Behavioral tests may reflect the neurological deficit in rat brain function. In the experiment we observed that the neurological deficit was aggravated transitorily with recovered blood flow. The situation was stable till 24 h after reperfusion. Neurological deficits scored at 24 h after reperfusion are shown in Table 1. The difference of neurological deficit scores between MCAO and MCAO+EA was significant (P < 0.05).
Table 1.
Scores of neurological deficit in rats
| Score of neurological deficit in each rat | |||||||
|---|---|---|---|---|---|---|---|
| I | II | III | IV | V | VI | Median | |
| MCAO | 4 | 5 | 5 | 6 | 5 | 6 | 5 |
| MCAO+EA | 3 | 4 | 3 | 4 | 4 | 4 | 4* |
*P < 0.05 compared with MCAO group.
Nonparametric Mann-Whitney U rank sum test was used to analyze statistical significance.
Improvement of Mitochondrial Respiratory Function
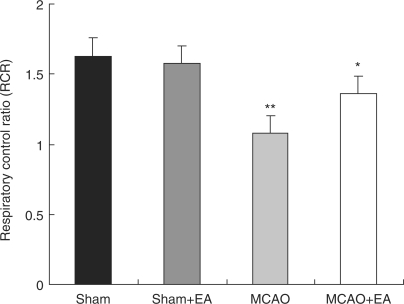
Fresh isolated brain mitochondria (8.4 mg) in 2.8 ml of the reactive medium were energized with 5 mmol l−1 succinate and 0.25 mmol l−1 ADP. It is well known that State 3 reflects the oxygen uptake capacity of the mitochondria after injecting ADP, depending on excessive oxidizable substrates. State 4 reflects mitochondrial dissipation rate after ADP is exhausted. RCR (state 3/state 4) reflects coupling extent of oxidation and phosphorylation, which is well correlated with mitochondrial functional state. Our results showed that RCR in the penumbra zone decreased about 35% compared with the sham rats after ischemia/reperfusion. RCR increased about 17% after EA treatment in the penumbra area (Fig. 2).
Figure 2.
EA treatment on mitochondrial respiratory function in the penumbra zone after ischemia/reperfusion. One-way ANOVA followed by post-hoc protected least-significant difference test was used to analyze statistical significance. **P < 0.01 compared with Sham group; *P < 0.01 compared with MCAO alone.
Respiratory control ratio (RCR) was lower in the MCAO than in the Sham (**P < 0.01). RCR was obviously higher in the MCAO+EA than in the MCAO (*P < 0.01), however it was lower than in the Sham and Sham+EA.
Enhancement of Mitochondrial Respiratory Enzymatic Activities
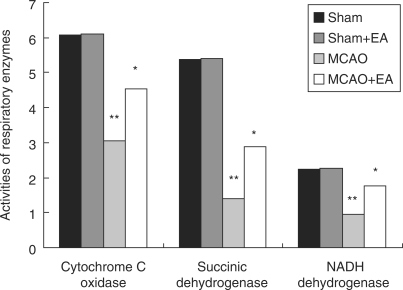
Mitochondrial pellets were frozen and thawed three times after storing at −20°C for 24 h and were used to examine the activities of mitochondrial respiratory enzymes. Results showed that activities of succinic dehydrogenase, NADH dehydrogenase and cytochrome C oxidase decreased by about 74%, 58% and 50%, and they increased about by 27%, 36% and 25% after EA treatment (Fig. 3).
Figure 3.
Effect of EA treatment on mitochondrial respiratory enzymes in the penumbra zone after ischemia/reperfusion; the unit of each numerical value is nmol O2 min mg−1 protein. *P < 0.01 compared with MCAO group; **P < 0.01 compared with Sham group.
The activities of succinic dehydrogenase, NADH dehydrogenase and cytochrome C oxidase were lower in the MCAO than in the Sham (**P < 0.01). EA significantly enhanced enzymatic activities in the MCAO rats’ cerebral mitochondria (*P < 0.01).
Discussion
Since Longa et al. (19) brought forward the criterion of neurological deficit score for animal experiments of cerebral ischemia, the neurological deficit score has improved increasingly and is now broadly applied to estimate the whole curative effect of diversified treatments on all kinds of animals (20,21). Clinically, neurological deficit score and infarct lesion volume were regarded as the important indices for estimation of stroke prognosis. In a previous study, we observed higher neurological deficit scores the bigger the infarct volume in the MCAO rats and there was a positive linear correlation between them (r = 0.863, Spearman statistical method) (7). In this experiment, we adopted the neurological deficit as an index for screening out successful MCAO models.
Mitochondria are the control center of cell survival or death. Under physiological conditions, mitochondrial electron transfer chain may generate ATP and provide body energy. Mitochondrial energy metabolism is extremely sensitive to impairment by reactive oxygen and nitrogen species (ROS/RNS), and oxidative stress limited metabolic recovery and promoted the intrinsic pathway of apoptosis (22). Oxidative stress might be the primary mediator of neurological injury and an important mechanism of cell apoptosis following cerebral ischemia/reperfusion. Several lines of evidence have indicated that mitochondria might participate in oxidative stress damage (23). Super oxide is a normal byproduct of mitochondrial respiration and accounted for 0.1% of O2 consumed by mitochondria. Under some circumstances such as ischemia/reperfusion (I/R), ROS increased via electron leak, leading to oxidative damage of proteins, lipids, RNA and DNA and subsequently cell apoptosis. Ischemic regions could be divided into the core and penumbra zones according to the property of neuron damage. The histological penumbra zone represented the area of reversible damage in the ischemic regions; The penumbra zone was the theoretical basis of the reperfusion time window, and it could be saved (24). While the supply of blood to the penumbra area renewed, reperfusion induced the mechanism of neuron damage (25), suggesting the pathological changes in the penumbra zone close to the infarct core deteriorated. The penumbra zone became an emphasis of research but still the mechanism of cell survival in the penumbra zone is unclear.
Oxygen consumption was measured by single Clark-type oxygen electrodes in most traditional studies (26,27). Rehncrona et al. (28) reported that after 30 min of ischemia there was a decrease in RCR and that mitochondrial dysfunction persisted during recirculation in rats with incomplete ischemia. The reason for persistent inhibition of mitochondrial function during recirculation was, however, unsettled. In this article we introduced a computer-aided Clark electrode system to examine brain mitochondrial oxygen consumption in MCAO rats. The measurement end of the polyethylene electrode was covered by pellicle and did not directly contact reaction buffer so voltage could not affect the reaction buffer containing mitochondria, thus maintaining environmental stability. In addition, stability of the electrode was long-lasting because only oxygen could go through the polyethylene pellicle while other substances that might affect it were blocked. Computer tracked and computed experimental data automatically, which improved precision, objectivity, convenience and efficiency of the whole experiment. Our results showed that I/R significantly decreased mitochondrial RCR in the penumbra zone, which was possibly the direct result of ROS damage. EA could improve the function of mitochondrial respiration in the penumbra zone, and thereby increase the energy supply.
The respiratory chain located in the mitochondrial inner membrane contains four enzyme systems (complex-, α, β, χ), co-enzyme Q and cytochrome C, which catalyzes oxidation of biologic substrates and coupled with ATPase composes molecular structure to transform biologic energy. The activities of succinic dehydrogenase, NADH dehydrogenase and complex IV (cytochrome C oxidase) directly affect the ratio of mitochondrial electron transport and oxidative phosphorylation. Succinic dehydrogenase, NADH dehydrogenase and cytochrome C oxidase are symbolic enzymes of respiratory chain, participating in energy metabolism and generation of ROS. These respiratory enzymes as important proteins in the mitochondria are vulnerable to the attack of ROS. It has been proved that H2O2 markedly damages genes of cytochrome C oxidase, encoded by mtDNA, which ultimately reduces activity of cytochrome C oxidase (29). We observed that the activities of succinic dehydrogenase, NADH dehydrogenase and cytochrome C oxidase in the penumbra zone were lower following I/R,which was possibly the direct or indirect result of ROS damage. EA improved the activities of succinic dehydrogenase, NADH dehydrogenase and cytochrome C oxidase in the penumbra zone and reduced ROS generation by ameliorating respiration chain function, but whether the effect of EA on those enzymes is direct or indirect still requires further investigation.
Certain clinical studies have revealed that acupuncture was an effective therapy for ischemic stroke (30–32). Kim (31) reported that electrical stimulation at GV26 and CV24 was beneficial for blood pressure, heart rate and CBF in ischemic stroke patients. Li (32) observed beneficial effects of acupuncture on aphasia due to ischemic stroke at an early stage, and found the WAB AQ index evaluation was better in the acupuncture group 15 days and 30 days after treatment than in the control group, suggesting that acupuncture has synergistic action with standard medication and rehabilitation. Lewith et al. (33) have systematically researched and reviewed the literature looking at how acupuncture affects brain activation as measured by functional magnetic resonance imaging and positron emission tomography, which show that specific and largely predictable areas of brain activation and deactivation occur when considering the traditional Chinese functions attributable to certain specific acupuncture points.
These results provided verification for effectiveness of acupuncture or EA on several clinical conditions including pain relief, and nausea. These clinical observations validated by animal experiments (7,34) and our present study, which sheds some light on the scientific mechanism of acupuncture therapeutics, provide conclusive evidence for the clinical application of acupuncture. However, recent evidence (35) shows that nitric oxide plays an important role in mediating the cardiovascular responses to EA stimulation through the gracile nucleus-thalamic pathway. Other substances, including serotonin, catecholamines, inorganic chemicals and amino acids such as glutamate and α-aminobutyric acid (GABA), have been proposed as mediators of the cardiovascular effects of acupuncture, but at present their role is still poorly understood. Therefore, further evidence of the neurobiological mechanisms of acupuncture should be the aim of research in the future.
In conclusion, our present results suggest that EA might decrease the neurological deficit score markedly, promote the activities of respiratory enzymes and reduce the generation of ROS. This results in improvement of the respiratory chain function and the anti-oxidative capability of brain tissues in the infarct penumbra zone, which may be one way that EA can protect the brain function of MCAO rats from injury.
Acknowledgements
The project was supported by the Science Fund of Jiangsu Science and Technology Committee (No. KJS03049).
References
- 1.Richard Green A, Tomas Odergren, Tim Ashwood. Animal models of stroke: do they have value for discovering neuroprotective agents? Trends Pharmacol Sci. 2003;24:402–8. doi: 10.1016/S0165-6147(03)00192-5. [DOI] [PubMed] [Google Scholar]
- 2.Kudo M, Aoyama A, Ichimori S, Fukunaga N. An animal model of cerebral infarction? Homologous blood clot emboli in rats. Stroke. 1982;13:505–8. doi: 10.1161/01.str.13.4.505. [DOI] [PubMed] [Google Scholar]
- 3.koiZumi J, Yoshida Y, Nakazawa T. Experimental studies of ischemic brain edema a new experimental model of cerebral embolism in rats in which recirculation can be introduced in the ischemia area. Stroke. 1986;8:1–8. [Google Scholar]
- 4.Ulett GA, Han SHan JS. Electroacupuncture mechanisms and clinical application. Biol Psychiatry. 1998;44:129–38. doi: 10.1016/s0006-3223(97)00394-6. [DOI] [PubMed] [Google Scholar]
- 5.Liu R. Clinical experience in acupuncture treatment of apoplexy. J Tradit Chin Med. 2005;25:190–2. [PubMed] [Google Scholar]
- 6.Hopwood V, Lewith GT. Does acupuncture help stroke patients become more independent? J Altern Complement Med. 2005;11:175–7. doi: 10.1089/acm.2005.11.175. [DOI] [PubMed] [Google Scholar]
- 7.Wang CX, Zhong RL, Chen BY. Protective effect of electroacupuncture on cerebral function via ameliorating oxidative stress in MCAO rats. Neurosci Bull. 2005;21:153–7. [Google Scholar]
- 8.Andreyev AY, Kushnareva YE, Starkov AA. Mitochondrial metabolism of reactive oxygen species. Biochemistry (Mosc) 2005;70:200–14. doi: 10.1007/s10541-005-0102-7. [DOI] [PubMed] [Google Scholar]
- 9.Wallace DC. Mitochondrial DNA sequence variation in human evolution and disease. Proc Natl Acad Sci USA. 1994;91:8739–46. doi: 10.1073/pnas.91.19.8739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dyall SD, Brown MT, Johnson PJ. Ancient invasions: from endosymbionts to organelles. Science. 2004;304:253–7. doi: 10.1126/science.1094884. [DOI] [PubMed] [Google Scholar]
- 11.Young TA, Cunningham CC, Bailey SM. Reactive oxygen species production by the mitochondrial respiratory chain in isolated rat hepatocytes and liver mitochondria studies using myxothiazol. Arch Biochem Biophys. 2002;405:65–72. doi: 10.1016/s0003-9861(02)00338-7. [DOI] [PubMed] [Google Scholar]
- 12.Ludwig B, Bender E, Arnold S, Hüttemann M, Lee I, Kadenbach B. Cytochrome C oxidase and the regulation of oxidative phosphorylation. Chembiochem. 2001;2:392–403. doi: 10.1002/1439-7633(20010601)2:6<392::AID-CBIC392>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 13.Yang ZP, Dettbarn WD. Lipid peroxidation and changes in cytochrome c oxidase and xanthine oxidase activity in organophosphorus anticholinesterase induced myopathy. J Physiol. 1998;92:157–61. doi: 10.1016/s0928-4257(98)80002-8. [DOI] [PubMed] [Google Scholar]
- 14.Chavez JC, Pichiule P, Boero J, Arregui A. Reduced mitochondrial respiration in mouse cerebral cortex during chronic hypoxia. Neurosci Lett. 1995;193:169–72. doi: 10.1016/0304-3940(95)11692-p. [DOI] [PubMed] [Google Scholar]
- 15.La Manna JC, Kutina-Nelson KL, Hritz MA, Huang Z, Wong-Riley MTT. Decreased rat brain cytochrome oxidase activity after prolonged hypoxia. Brain Res. 1996;720:1–6. doi: 10.1016/0006-8993(95)01495-0. [DOI] [PubMed] [Google Scholar]
- 16.Zausinger S, Hungerhuber E, Baethmann A. Neurological impairment in rats after transient middle cerebral artery occlusion: a comparative study under various treatment paradigms. Brain Res. 2000;863:94–105. doi: 10.1016/s0006-8993(00)02100-4. [DOI] [PubMed] [Google Scholar]
- 17.Mizuno Y, Saitoh T, Sone N. Inhibition of mitochondrial NADH-ubiquinone oxidoreductase activity by 1-methyl-4-phenylpyridinium ion. Biochem Biophys Res Commun. 1987;143:294–9. doi: 10.1016/0006-291x(87)90664-4. [DOI] [PubMed] [Google Scholar]
- 18.Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Analytical Biochem. 1976;72:248–54. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 19.Longa EZ, Weinstein PR, Carlson S, Cummins R. Reversible middle cerebral artery occlusion without craniectomy in rats. Stroke. 1989;20:84–91. doi: 10.1161/01.str.20.1.84. [DOI] [PubMed] [Google Scholar]
- 20.Ahmed R, Zuberi BF, Afsar S. Stroke scale score and early prediction of outcome after stroke. J Coll Physician Surg Pak. 2004;14:267–9. [PubMed] [Google Scholar]
- 21.Suzuki M, Sasamata M, Miyata K. Neuroprotective effects of YM872 coadministered with t-PA in a rat embolic stroke model. Brain Res. 2003;959:169–72. doi: 10.1016/s0006-8993(02)03759-9. [DOI] [PubMed] [Google Scholar]
- 22.Dugan LL, Kim-Han JS. Astrocyte mitochondria in vitro models of ischemia. J Bioenerg Biomembr. 2004;36:317–21. doi: 10.1023/B:JOBB.0000041761.61554.44. [DOI] [PubMed] [Google Scholar]
- 23.Murin R, Drgova A, Kaplán P, Dobrota D, Lehotský J. Ischemia/Reperfusion-induced oxidative stress causes structural changes of brain membrane proteins and lipids. Gen Physiol Biophys. 2001;20:431–8. [PubMed] [Google Scholar]
- 24.Schaller B, Graf R. Cerebral ischemia and reperfusion the pathophysiologic concept as a basis for clinical therapy. J Cereb Blood Flow Metab. 2004;24:351–71. doi: 10.1097/00004647-200404000-00001. [DOI] [PubMed] [Google Scholar]
- 25.Caraceni P, Domenicali M, Vendemiale G, Grattagliano I, Pertosa A, Nardo B. The reduced tolerance of rat fatty liver to ischemia reperfusion is associated with mitochondrial oxidative injury. J Surg Re. 2005;124:160–8. doi: 10.1016/j.jss.2004.10.007. [DOI] [PubMed] [Google Scholar]
- 26.Chance B, Williams GR. The respiratory chain and oxidative phosphorylation. Adv Enzymol. 1956;17:65–134. doi: 10.1002/9780470122624.ch2. [DOI] [PubMed] [Google Scholar]
- 27.Clark JB, Nicklas WJ. The metabolism of rat brain mitochondria. J Biol Chem. 1970;245:4724–31. [PubMed] [Google Scholar]
- 28.Rehncrona S, Mela L, Siesjo BK. Recovery of brain mitochondrial function in the rat after complete and incomplete cerebral ischemia. Stroke. 1979;10:437–46. doi: 10.1161/01.str.10.4.437. [DOI] [PubMed] [Google Scholar]
- 29.Dobson AW, Grishko V, LeDoux SP, Kelley MR, Wilson GL, Gillespie MN. Enhanced mtDNA repair capacity protects pulmonary artery endothelial cells from oxidant-mediated death. Am J Physiol Lung Cell Mol Physiol. 2002;283:L205–10. doi: 10.1152/ajplung.00443.2001. [DOI] [PubMed] [Google Scholar]
- 30.Quanming Si, Gencheng Wu, Xiaoding Cao. Effects of electroacupuncture on acute cerebral infarction. Acupuncture & Electro-Therapeutics Res Int J. 1998;23:81–92. doi: 10.3727/036012998816356562. [DOI] [PubMed] [Google Scholar]
- 31.Kim YS, Jun HJ, Chae YY, Park HJ, Kim BH, Chang IM, et al. The practice of Korean medicine: an overview of clinical trials in acupuncture. Evid Based Complement Alternat Med. 2005;2:325–52. doi: 10.1093/ecam/neh102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li JA. Clinical observation on acupuncture for treatment of aphasia due to ischemic stroke at the early stage. Zhongguo Zhen Jiu. 2005;25:760–2. [PubMed] [Google Scholar]
- 33.Lewith GT, White PJ. Jeremie Pariente. Investigating acupuncture using brain imaging techniques: the current state of play. Evid Based Complement Alternat Med. 2005;2:315–9. doi: 10.1093/ecam/neh110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhao P, Zhi N, Chen G, Cheng JS. Electro-acupuncture attenuates nitric oxide release from rat striatum after transient middle cerebral artery occlusion. Acupuncture & Electro-Therapeutics Res Int J. 2000;25:101–7. doi: 10.3727/036012900816356163. [DOI] [PubMed] [Google Scholar]
- 35.Ma SX. Neurobiology of acupuncture: toward CAM. Evid Based Complement Alternat Med. 2004;1:41–7. doi: 10.1093/ecam/neh017. [DOI] [PMC free article] [PubMed] [Google Scholar]