Abstract
Inflammation is a necessary albeit insufficient component of tumorigenesis in some cancers. Infectious agents directly implicated in tumorigenesis have been shown to induce inflammation. This process involves both the innate and adaptive components of the immune system which contribute to tumor angiogenesis, tumor tolerance and metastatic properties of neoplasms. Recently, heat-shock proteins have been identified as mediators of this inflammatory process and thus may provide a link between infection-mediated inflammation and subsequent cancer development. In this review, the role of heat-shock proteins in infection-induced inflammation and carcinogenesis will be discussed.
Introduction
Since the time of Rudolf Ludwig Karl Virchow, inflammation has been implicated as a necessary albeit insufficient component in tumorigenesis in some cancers [1,2]. Recent research has characterized several molecular mechanisms that demonstrate such a link. In addition, numerous infectious agents have been directly implicated as the source of this inflammatory pathway. Studies have shown that the innate and adaptive immune systems that respond to these infections may be directly responsible for tumor angiogenesis, tumor tolerance and in some cases metastatic mechanisms by providing the tumor with cytokines that promote these processes. One of the more recent discoveries has been the role of heat-shock proteins as mediators of this immune-mediated process via tumor peptide presentation [3]. In this review, we will discuss briefly the anti-cancer properties of heat-shock proteins and emphasize their critical faculties in infection-mediated inflammation-dependent tumorigenesis.
An estimated 10.9 million new cases of cancer occurred in 2002 worldwide. In 1990 investigators at the International Agency for Research on Cancer estimated that approximately 9% of cancers in the United States and 20% of cancers in developing countries could be attributed to infectious agents [4]. This geographic disparity may be due to the higher prevalence of cancer-related infectious agents in developing countries [5]. Cancers caused by such infections theoretically occur as a result of direct cell targeting with subsequent tumor suppressor gene inactivation, as in human papilloma virus (HPV), prolonged local inflammation by bacteria residing outside of tumor cells, such as H. pylori, or immune suppression by viral agents, such as human immunodeficiency virus [6-8]. Conversely, in the 1700s cancer patients who cleared bacterial infections occasionally experienced remission of their established malignancies [9]. In the late 1800s, Dr. William B. Coley of the New York Cancer Center noted the regression of sarcoma in patients who developed erysipelas [10]. Despite these isolated findings, the preponderance of evidence shows that infections contribute to carcinogenesis rather than counter it. A comprehensive explanation of this relationship has yet to be described.
Inflammation, tumor immunity and tumorigenesis
Inflammation is a localized protective response elicited by injury or destruction of tissues which serves to destroy, dilute or wall off both the injurious agent and the injured tissue. The inflammatory response to infections as well as other stimuli involves a myriad of defenses, including both the innate and adaptive arms of the immune system.
The innate immune system is comprised of myeloid cells such as macrophages and dendritic cells, and innate lymphocytes such as natural killer cells, all of which lack immunologic memory. This cellular component of the innate immune system can either kill engulfed microbes using toxins including superoxide anion, hydroxyl radical and nitric oxide or process antigens in a MHC-dependent manner. Extracellular antigens such as bacterial toxins are presented by MHC class II on antigen presenting cells (APCs) to CD4+ T cells whereas intracellular antigens such as viral antigens are presented by MHC class I to CD8+ T cells [11]. These APCs are stimulated by germline-encoded innate receptors such as Toll-like receptors (TLRs) to program adaptive immunity (both cellular and humoral immunity) via cytokines, co-stimulatory molecules in addition to present antigens to T cells [12].
The immune system therefore can function to modulate tumorigenic pathogen-induced chronic inflammatory responses or to identify and eliminate tumor cells. The latter process now known as immunologic tumor surveillance was first proposed by Burnet in 1957 [13]. When these events result in tumor clearance, it is known as elimination. If not cleared, a state of equilibrium between the tumor-suppressive immune system and tumor growth can occur. If tumor immunoediting progresses, the tumor grows or escapes [14,15]. Tumor immunologists in the past several decades have been focusing on the immune system to counter cancer. Increasing evidence is uncovering the paradoxical roles of the immune system to promote tumorigenesis.
The ancient Roman physician Galen (129 – 199 C.E.) was the first to posit the causal relationship between cancer and inflammation. In 1863, the "Father of Pathology," Rudolf Virchow perpetuated the notion that cancers must be due to prolonged irritation of various sorts. Similarly, Dr. C. Heitzman declared in 1883 that the "so-called small cellular infiltration [of Virchow] of the connective tissue was the 'pre-stage of cancer"' [16]. Since that time, the study of inflammation has become increasingly complicated, albeit more cohesive, in its associations with cancer [17]. Ultimately, chronic inflammation has been shown to contribute to tumorigenesis by causing DNA damage, promoting neoangiogenesis and compromising tumor immunosurveillance mechanisms.
Free radicals are thought to mediate tumorigenesis in the context of inflammation. Excess oxidative/nitrosative stress results in the generation of reactive oxygen species (ROS) such as hydroxyl radicals (OH·) and ultimately the accumulation of protein peroxidation, DNA damage and lipid peroxidation (LPO) (Figure 1) [18]. ROS and reactive nitrogen species (RNS) can damage both nuclear and mitochondrial DNA, RNA, lipids and proteins by nitration, oxidation and halogenation reactions, leading to an increased mutation load [19]. The LPO products [trans-4-hydroxy-2-nonenal (HNE), 4-hydroperoxy-2-nonenal (HPNE), and malondialdehyde (MDA)] can drift far from membranes and cause exocyclic adducts on DNA that are potentially promutagenic if not removed [20].
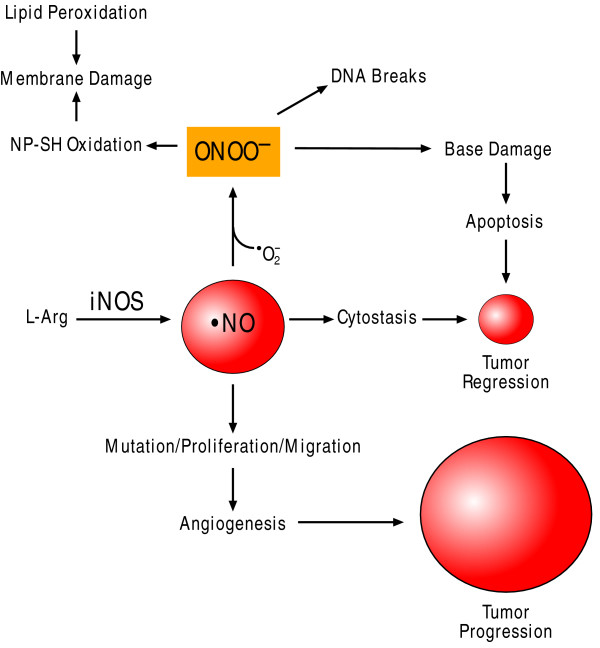
Figure 1.
Growth and inhibitory effects of free radicals on tumors. The unchecked production of hydroxyl radicals and other reactive oxygen species (ROS) leads to protein and lipid peroxidation as well as DNA damage which increase mutation load resulting in either tumor regression or tumor progression. In response to intracellular protozoa, classically-activated macrophages produce nitric oxide (NO) from arginine (L-arg) using the iNOS enzyme. H.pylori disinhibits iNOS in the gastric mucosa by attenuating the expression of HSP70 and HSP27. Tumor-associated macrophages (TAM) are not toxic to tumor cells because of their limited production of NO.
In human lung bronchial epithelial cells, the proinflammatory cytokine TNF-α has been shown to induce production of such ROS with a concomitant increase in 8-oxo-deoxyguanosine, a marker for oxidative DNA damage. The source of the ROS was shown to be spermine oxidase [21]. In vivo humans and experimental animals have been found to harbor carcinogenic N-nitrosamines formed by the deamination of DNA bases by N2O3 [22].
In the case of colon cancer, commensal intestinal flora can activate TLRs on the luminal surface of intestinal epithelial cells [23]. This interaction activates intracellular IKK-β and ultimately NF-κB, the key regulator of inflammation found in many solid tumors [24]. NF-κB is a homo- or hetero-dimeric transcription factor of the Rel family. NF-κB activates genes involved in cell proliferation (e.g., c-myc, cyclins), as well as cell survival (e.g., c-FLIP, c-IAP1, c-IAP2, XIAP, Bcl-XL, Bfl-1/A1 and p53) [25]. NF-κB contributes unevenly to the pro-apoptotic and anti-apoptotic pathways dependent upon its role in homeostasis or tumor development, respectively [26]. In a pro-inflammatory state, NF-κB contributes to the activation of COX-2, iNOS and matrix metalloproteinase (MMP-9). Furthermore, NF-κB is responsible for the expression of adhesion molecules and cell-surface metalloproteases, including MMP-9 and MMP-2, substances which degrade the extracellular matrix (ECM) to allow for metastases [27,28]. Downstream of NF-κB, increased expression of pro-inflammatory COX-2 has been demonstrated in colorectal adenomatous polyps and has been linked to the induction of tumorigenic DNA damage [18].
The tumor microenvironment features an important inflammatory cell component as well. Currently, it is believed that there are three types of activated macrophages. The classically activated macrophage which responds to intracellular pathogens is stimulated by IFN-γ, stimulates T-cells with IL-12, and produces nitric oxide (NO) from arginine using the iNOS2 enzyme (Figure 1). The so-called alternatively activated macrophages are stimulated by IL-4, fail to make NO, and inhibit T cell proliferation, but are able to produce IL-1-receptor antagonist and IL-10. The type 2-activated macrophages induce TH2-type humoral immune responses to antigen, such as IL-10 generation which results in IL-4 production by T cells, and leads to an anti-inflammatory milieu [29].
One key inflammatory component to tumor sustenance first discovered in the late 1970s is the infiltration of tumor-associated macrophages (TAM) which are attracted by monocyte chemotactic protein (MCP-1), RANTES and CCL5. TAMs accumulate in poorly vascularized and relatively hypoxic zones of tumor where hypoxia-inducible factors (HIF-1 and HIF-2) predominate and promote expression of pro-angiogenic VEGF, bFGF, and CXCL8 [30-34]. Like type 2-activated macrophages, TAMs release IL-10, PGE-2, TGF-β and other cytokines that inhibit antigen presentation and normal DC activity [35]. They are not cytotoxic for tumor cells because of their limited production of NO and proinflammatory cytokines and due to the production of IL-10 which dampens cytotoxic T-cell reactivity [36,37]. The Sea squirt-derived trabectidin has a selective cytotoxic effect on TAMs by binding to the minor groove of DNA and reducing IL-6 production, resulting in tumor growth suppression [38].
When functioning in concert, these processes may prevent adequate immunosurveillance. As proof of principle, Luo et al demonstrated that a legumain-based DNA vaccine induced a robust CD8+ T cell response against TAMs, dramatically reducing their presence in tumor tissues and decreasing proangiogenic TGF-β, TNF-α, MMP-9 and VEGF. Subsequently, tumor angiogenesis, tumor growth and metastases were suppressed [39].
Heat-shock proteins
First discovered accidentally in 1962 by Ritossa et al and isolated in 1974 by Tissieres et al, heat-shock proteins (HSPs) are a highly conserved group of protein products generated as a result of natural stressors, such as fever and active commensal gut microflora, or non-natural stressors, such as hyperthermia, NSAIDS, aspirin, nutrient withdrawal, ROS, proteasome inhibition, UV radiation and chemotherapy-induced DNA damage [40,41]. They promote cell survival by preventing mitochondrial outer membrane permeabilization, cytochrome c release, caspase activation and apoptosome assembly [42]. HSPs assist in general protein folding to prevent non-specific aggregation of misfolded or unfolded proteins which would otherwise be rendered nonfunctional. This folding process is facilitated by cofactors such as Hsp70/Hsp90 Organizing Protein (HOP) which associates with Hsp70 and Hsp90 to mediate the transfer of polypeptides from Hsp70 to Hsp90. Conversely, Hsp70 and Hsp90 may associate with the ubiquitin ligase CHIP and lead to proteasomal degradation of a misfolded protein.
Highly inducible HSPs such as HSP70 and HSP27 are transcriptionally controlled by heat shock transcription factor trimers, such as hsf1. For example, hsf1 represses transcription when bound to HSP70 during attenuation of the heat shock response as a negative feedback mechanism [43]. In a normal host, hsf1 enhances organismal survival and longevity. In cancer, however, hsf1 in particular has been found to be overexpressed and to contribute to invasion and metastasis by permitting increased cell proliferation and by decreasing cell death [44-47]. As expected, genetic deletion of hsf1 protects mice from experimental tumors [48].
HSP activation can directly affect both innate and adaptive immunity, although controversial studies and opinions exist in the field [49-51]. The innate immune responses induced by HSPs include cytokine and chemokine release by professional APCs and T-cells, maturation of DCs by upregulating the expression of costimulatory and antigen-presenting molecules such as B7-1, B7-2 and MHC-II molecules, induction of migration of DC to draining lymph nodes and activation of NK cells [52].
For example the HSP gp96 interacts with TLR2/4 resulting in the activation of NF-κB-driven reporter genes and mitogen- and stress-activated protein kinases. Gp96 also induces the degradation of IκBα in DCs while simultaneously stimulating both the innate and adaptive immune system [53]. Gp96-activated DCs release proinflammatory cytokines resulting in the induction of an inflammatory response by the innate component of the immune system [54]. Necrotic tumor cell-derived mammalian gp96 and hsp70 signal APCs via CD14, TLRs and CD91 (Figure 2) [55-58]. Tumor-derived Hsp70 can also activate NK cells having a high cell surface density of CD94 [59] by inducing NKG2D ligands on the surface of DCs [60]. This scenario may be particularly relevant in melanoma which overexpresses Hsp70.
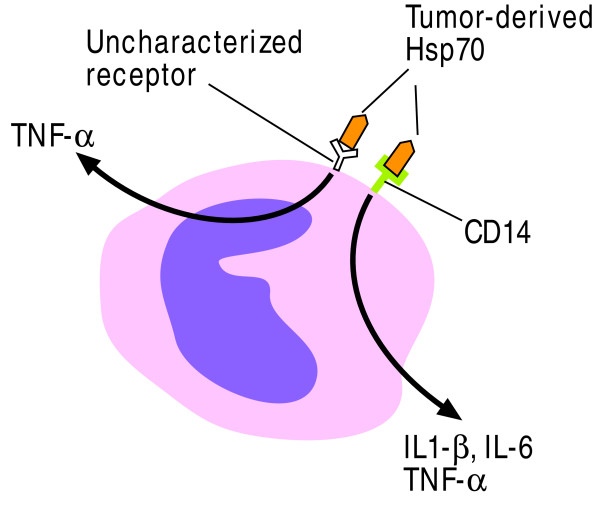
Figure 2.
Heat-shock protein signal cascade. Necrotic tumor-derived mammalian gp96 and HSP70 can signal antigen-presenting cells (APCs) via CD14, and other receptors such as TLRs and CD91 which remain to be fully determined.
The immunogenic potential of gp96-peptide complexes was first demonstrated by Srivastava et al [61,62]. When manipulated, tumor-derived gp96 vaccine induces T cell priming and tumor rejection [63-65]. When HSP-peptide complexes are procured by APCs, peptide is transferred from HSPs to MHC molecules for recognition by T cells [61]. Dai et al found that cell surface expression of gp96 leads to the priming and maintenance of both CD4+ and CD8+ T cell immunity against tumors and potentiates cross-presentation of intracellular antigens to MHC-I for activation of CD8+ T cells [66]. The interaction of gp96 with DCs leads to the preferential expansion of antigen-specific CD8+ T cells in vitro and in vivo in a TLR4-dependent manner [67]. These CD8+ T cells can then contribute to tumor immunosurveillance.
Furthermore, HSPs have been shown to induce T cell regulation of chronic inflammation [68]. HSPs can chaperone both steroid and non-steroid hormone receptors. Interestingly, steroids can interact with HSP-bound glucocorticoid receptors and increase the expression of IκBα, preventing the nuclear translocation of the pro-inflammatory molecule NF-κB [69,70].
Heat-shock proteins and tumorigenesis
The histologic evidence of chronic inflammation resulting from an infection is insufficient to explain a tumorigenic mechanism. This shortcoming can partially be reconciled by the identification of HSPs in and around tumors (Figure 3). Heat-shock proteins can be produced by tumors, microbes, and even inflammatory cells in the tumor microenvironment. Only recently have HSPs been implicated as biochemical elements of both anti-tumor immunity [3] and oncogenesis [48].
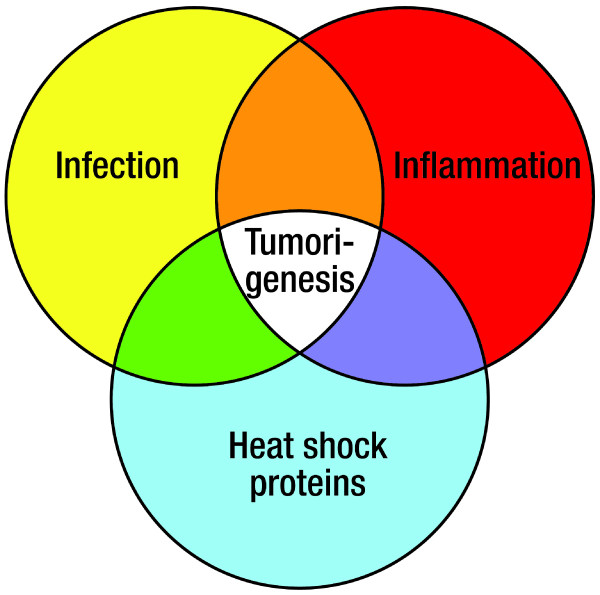
Figure 3.
Venn diagram demonstrating a model of the tumorigenic relationship between infection, chronic inflammation and microbial- or host-derived heat-shock proteins.
Unique HSPs activated in cancer cells have been well-documented and correlated with tumor cell proliferation, differentiation, invasion, metastasis and prognosis. Frequently, the tumor-derived HSPs are acetylated [71] and cannot be directly compared with native HSP or microbial HSP. Gp96 from tumor cells demonstrate greatly altered glycosylation patterns compared to host cell gp96, which may elucidate deficiencies in immune surveillance [72]. Tumor-derived Hsp90 can rescue wild type proteins as well as unstable mutant proteins implicated in carcinogenesis. Moreover, tumor-derived HSP90 is present entirely in multi-chaperone complexes with high ATPase activity, unlike non-tumor HSP90 [73,74]. For example, in chronic lymphocytic leukemia (CLL), ZAP-70+ lymphocytes express activated HSP90 which binds and stabilizes ZAP-70 with several HSP co-chaperones [75].
The HSP90 family consists of cytoplasmic HSP90β, inducible α-form, GRP94/gp96 and mitochondrial TRAP1/hsp75. HSP90 is a constitutively active, molecular chaperone that assists in folding of signature tumorigenic proteins such as HER-2/ErbB2, Akt, Raf-1, v-Src, and Bcr-Abl [76]. HSP90 is overexpressed in a wide variety of solid and hematologic malignancies and correlates with a poor prognosis [77]. The expression of endoplasmic reticulum regulator HSP70-member GRP78 (also known as BiP), glucose-regulated protein GRP94/gp96, or HSP90 has been associated significantly with vascular invasion and intrahepatic metastasis [78]. HSP90 may even promote invasion of metastases by chaperoning NF-kB-dependent MMP-2 [79].
Cultured cells and transgenic mice have been shown to exhibit cellular transformation and tumor formation when forced to over express intracellular HSP27 or HSP70 [80-83]. It has even been proposed that by interacting with mutant p53 and various oncogene products such as pp60-v-src, fes and fgr, these HSPs may alter cell cycle regulation and contribute to the anti-apoptotic mechanism of tumorigenesis [84].
HSP70 belongs to a family of inducible chaperone proteins frequently present on the plasma membrane of colon, lung, pancreas and breast cancer metastases [85]. This ATP-dependent chaperone can be induced by a variety of stimuli, including chemotherapy. HSP70 is a powerful anti-apoptotic protein that reduces caspase activation and suppresses mitochondrial damage and nuclear fragmentation [86]. HSP70 can even subvert apoptosis by blocking the translocation of Bax, which results in stabilization of the outer mitochondrial membrane [87]. HSP70 is also a potent activator of the human complement system in an antibody-independent fashion [88]. In defense, cancer cells block complement-mediated killing by expressing membrane complement regulatory proteins, such as CD46, CD55, CD35 and CD59 [89].
HSP27 of the inducible small HSP family has been shown to inhibit the mitochondrial release of SMAC (second mitochondrial-derived activator of caspase), the master regulator of apoptosis, to confer resistance of multiple myeloma cells to dexamethasone [90]. Conford et al have found a high correlation between the level of HSP27 expression and the Gleason score in prostate cancer [91]. HSP40, HSP60, and HSP70 expressions are up-regulated in response to the development of high grade intraepithelial neoplasia and cervical cancer [92]. These examples begin to unveil the complex relationship between HSPs and cancer formation.
Microbes, inflammation, heat-shock proteins and cancer
The parasitic origin of cancer was originally suggested by Paget in 1887 [93].
"I believe that microbe parasites, or substances produced by them, will some day be found in essential relation with cancer and cancerous disease."
In 1913, Dr. Johannes Fibiger, the pathological anatomist in Copenhagen, produced numerous cancers in the fore-stomach of rats by feeding them a nematode taken from the muscles of a cockroach [94]. Similarly, Bullock and Curtis produced hepatic sarcomas in rats by feeding them tapeworm eggs from cats [95]. And Schistosoma, a parasitic trematode or fluke discovered in 1851 by Theodor Bilharz, has been shown to cause chronic local inflammation which seems to increase the risk of developing squamous cell bladder cancer [96]. Over 200 million people in tropical and subtropical countries are believed infected by any of six species of schistosomes. In Egypt alone, 27% of the 2500 new cancer patients each year have bladder cancers attributed to schistosomiasis [97].
Adult schistosome trematodes are found in the venous plexus around the urinary bladder. Any eggs released can then traverse the bladder wall and cause hematuria. Immune responses during the early stages of schistosomiasis infection are directed against antigens of schistosomula, and demonstrate a TH1 profile. With the onset of egg laying, TH1 responses are replaced by vigorous TH2 responses directed against egg antigens. The result is a tissue granuloma surrounding eggs characterized by an infiltrate of TH2 cells, eosinophils, macrophages and fibroblasts within a dense collagen-rich matrix. Schistosome-induced macrophages and neutrophils are important sources of endogenous oxygen or hydroxyl radicals, which are also implicated in the formation of carcinogenic N-nitrosamines [98]. These inflammatory cells may induce genotoxic effects, such as mutations, sister chromatid exchanges and DNA strand breaks [99-101]. They may also participate in the activation of procarcinogens, such as aromatic amines and polycyclic aromatic hydrocarbons, generating carcinogenic metabolites [102]. An increased number of inflammatory cells in the urinary bladder of schistosomal patients may enhance the carcinogenic potential of these agents by increasing their rate of activation. Furthermore, in patients with S. haematobium and bladder cancer, TAMs attracted to the bladder can produce TNF alpha, a key component of inflammation which is upregulated by HSP60 and HSP90.
HSP can be produced by a wide variety of parasitic organisms as detailed by Maresca et al [103]. HSP86, HSP70, HSP60, HSP58, HSP27 have all been detected in S. mansoni. In superficial transitional cell bladder cancer, the loss of surface expression of tumor-derived HSP60 and HSP90 was correlated with a poor prognosis, possibly explained by the inability of T cells and NK cells to recognize these tumor cells [104]. In the future these findings may champion parasite-derived HSPs as potential carcinogens.
Bacteria have also been implicated as a cause of cancer. In 1893, Bizzozero discovered a spirochete in the stomach of dogs. This finding has since been verified by numerous scientists including Salomon in 1896 and Krienitz in 1906 who related a similar finding to gastric cancer in a human patient [105]. More importantly, in 1983 the microbe now known as Helicobacter pylori was identified as a trigger of gastric cancer and gastric lymphoma [106-108]. H. pylori is associated with infiltration by neutrophils and mononuclear cells in gastric mucosa, likely attracted by granulocyte macrophage colony-stimulating factor and RANTES. Subsequently macrophages and monocytes respond to the presence of H. pylori via TLR2 resulting in NF-κB activation and the release of early proinflammatory cytokines, such as IL-1β. Macrophage-derived migration inhibitory factor (MIF) is a potent cytokine produced by H. pylori that overrides tumor suppressor p53 activity by suppressing its transcriptional activity. The result is increased DNA damage by inflammatory cells [109]. Furthermore, H. pylori infection disinhibits iNOS (Figure 1) in the presence of lipopolysaccharide by significantly attenuating the expression of HSP70 and HSP27 [110]. As expected, increased iNOS expression and subsequent oxidative damage has been found in gastric mucosa chronically infected with H. pylori [111].
Corresponding increases in various cytokines including IL-1β, IL-6, IL-8, and TNF-α have also been identified [112]. Investigators have shown that H. pylori must directly contact the host cell in order to up-regulate IL-8 [113]. NF-κB-dependent expression of IL-8 has been correlated with increased vascularity in human gastric carcinomas [114]. Takenaka et al have demonstrated how H. pylori-derived HSP60 can activate NF-κB and mitogen-activated protein kinase (MAPK) and induce IL-8 production and secretion through TLR-2 and TLR-4 pathways in KATO III human gastric epithelial cells [115,116]. HSP62, a member of the HSP60 chaperonin family and homologue of the H. pylori HSP known as GroEL, has been shown to participate in the extracellular assembly of H. pylori -derived urease, a known virulence factor [117]. These mechanisms provide insight into the relationship between H. pylori infection, inflammation, HSPs and tumorigenesis.
Chlamydial HSP60 has also been recognized as a potential extracellular stimulus of oncogenesis in that it is found in pre-neoplastic lesions and can bind TLRs, inducing a cascade of signaling which leads to neoangiogenesis, macrophage activation and anti-apoptosis mediated by complexing with Bax and Bak [118]. However, there is limited evidence which can implicate microbial or host HSPs as directly carcinogenic.
Parasites and bacteria are not the only culprits. In 1911, Dr. Peyton Rous of the Rockefeller Institute first demonstrated the RNA retrovirus causally associated with sarcomas in chickens for which he received the Nobel Prize in 1966 [119]. Since then several human cancers have been attributed to viral infections although the exact mechanism has not been elucidated in every case.
In 1963, Blumberg discovered the Hepatitis B virus (HBV), which is now known to cause hepatocellular carcinoma (HCC) in humans. Cell surface expression of viral HBsAg and HBcAg in association with MHC class I molecules activates CD8+ cytotoxic T lymphocytes which can then produce IFN-gamma. Hepatic GRP94/gp96, an endoplasmic reticulum-associated member of the HSP90 family, has been observed in association with HBV DNA and core antigen protein in biopsies of HCC [120,121]. Hepatic gp96 expression has been correlated with the degree of tumor differentiation and tumor size [120]. The exact role of gp96 in this case has not been determined. Interestingly, expression of the SMAC-inhibitor HSP27 has been shown to correlate with prognosis, disease-free and overall survival in patients with HBV-associated HCC [122].
The Epstein-Barr virus (EBV) is highly prevalent in humans (≥ 90% worldwide are carriers). In 1964, Epstein described EBV in association with endemic Burkitt's lymphoma in Central Africa, a highly aggressive but potentially curable form of non-Hodgkin lymphoma, as well as nasopharyngeal carcinoma. EBV is able to bind CD21 on B cells, a critical event to the induction of HSPs and the transformation of some B cells enabling them to become independent of the usual regulatory factors, including T cells. Cheung et al described in detail the coordinate induction of HSP70 and HSP90 at mRNA and protein levels upon EBV infection in vitro. Induction of HSPs and transformation of B cells were dependent on EBV-induced trans-membrane Ca2+ currents, but not on EBV gene products. Blockade of HSP induction prevented transformation [123]. This evidence has been essential for deciphering the role of HSPs in tumorigenesis.
Conclusion
For over two millennia scientists have speculated the etiology of cancer. In some instances such as tobacco use, there is a preponderance of evidence demonstrating a direct carcinogenic link with tobacco use. The roles of chronic infections and chronic inflammation have been repeatedly investigated as tumorigens for over a century with only a handful of confirmed associations relative to the diversity of human neoplasms and pathogens. Nevertheless, the worldwide population burden of infectious organisms makes understanding their role in human disease of paramount importance to cancer prevention strategies. Molecular studies have been able to dissect the pathophysiology of carcinogenesis on many levels. The direct and indirect involvement of microbial or host heat-shock proteins in the malignant transformation of a chronically infected host has been shown to be integral. This review attempts to assemble the evidence implicating heat-shock proteins in the neoplastic process.
Ever since the crucial role of heat shock proteins in cancer pathophysiology was established, efforts to inhibit their carcinogenic capacity have taken many forms. Heat-shock protein vaccines using conjugated tumor peptides [124,125] and direct HSP90 inhibitors such as 17-(Allylamino)-17-demethoxygeldanamycin (17-AAG) [76] have been investigated in clinical trials. Currently these interventions have not proven efficacy clinically, although they seem promising in vitro and in early phase trials. It remains to be seen whether or not manipulation of one HSP at a time will lead to meaningful tumor responses and/or survival benefit.
Competing interests
The authors declare that they have no competing interests.
Authors' contributions
All authors, MG and ZL, participated in drafting and editing the manuscript. MG and ZL read and approved the final manuscript. The cited work from the laboratory of ZL was supported by grants from NIH, DHHS, USA.
Authors' information
The authors provided specialized, multidisciplinary clinical care for hematology and oncology patients at the University of Connecticut Neag Comprehensive Cancer Center – John Dempsey Hospital. ZL is currently an Associate Professor in the Department of Immunology at the Univesity of Connecticut and a clinical scholar of the Leukemia and Lymphoma Society, USA. MG has completed fellowship training at the University of Connecticut and is currently in private practice in Maryland.
Acknowledgments
Acknowledgements
Graphic design by Linda Tenukas, Biomedical Communications, University of Connecticut is gratefully acknowledged.
Contributor Information
Mark G Goldstein, Email: mark.goldstein@comcast.net.
Zihai Li, Email: zli@up.uchc.edu.
References
- Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002;420:860–867. doi: 10.1038/nature01322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantovani A, Allavena P, Sica A, Balkwill F. Cancer-related inflammation. Nature. 2008;454:436–444. doi: 10.1038/nature07205. [DOI] [PubMed] [Google Scholar]
- Srivastava P. Roles of heat-shock proteins in innate and adaptive immunity. Nat Rev Immunol. 2002;2:185–194. doi: 10.1038/nri749. [DOI] [PubMed] [Google Scholar]
- Pisani P, Parkin DM, Munoz N, Ferlay J. Cancer and infection: estimates of the attributable fraction in 1990. Cancer Epidemiol Biomarkers Prev. 1997;6:387–400. [PubMed] [Google Scholar]
- Yu S. Primary prevention of hepatocellular carcinoma. J Gastroenterol Hepatol. 1995;10:674–682. doi: 10.1111/j.1440-1746.1995.tb01370.x. [DOI] [PubMed] [Google Scholar]
- Lazo PA. The molecular genetics of cervical carcinoma. Br J Cancer. 1999;80:2008–2018. doi: 10.1038/sj.bjc.6690635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feigal EG. AIDS-associated malignancies: research perspectives. Biochim Biophys Acta. 1999;1423:C1–9. doi: 10.1016/s0304-419x(98)00033-x. [DOI] [PubMed] [Google Scholar]
- Isaacson PG. Gastric MALT lymphoma: from concept to cure. Ann Oncol. 1999;10:637–645. doi: 10.1023/A:1008396618983. [DOI] [PubMed] [Google Scholar]
- Starnes CO. Coley's toxins in perspective. Nature. 1992;357:11–12. doi: 10.1038/357011a0. [DOI] [PubMed] [Google Scholar]
- Richardson MA, Ramirez T, Russell NC, Moye LA. Coley toxins immunotherapy: a retrospective review. Altern Ther Health Med. 1999;5:42–47. [PubMed] [Google Scholar]
- Cresswell P. Antigen processing and presentation. Immunol Rev. 2005;207:5–7. doi: 10.1111/j.0105-2896.2005.00320.x. [DOI] [PubMed] [Google Scholar]
- Medzhitov R, Janeway CA., Jr Decoding the patterns of self and nonself by the innate immune system. Science. 2002;296:298–300. doi: 10.1126/science.1068883. [DOI] [PubMed] [Google Scholar]
- Burnet M. Cancer: a biological approach. III. Viruses associated with neoplastic conditions. IV. Practical applications. Br Med J. 1957;1:841–847. doi: 10.1136/bmj.1.5023.841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swann JB, Smyth MJ. Immune surveillance of tumors. J Clin Invest. 2007;117:1137–1146. doi: 10.1172/JCI31405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn GP, Old LJ, Schreiber RD. The three Es of cancer immunoediting. Annu Rev Immunol. 2004;22:329–360. doi: 10.1146/annurev.immunol.22.012703.104803. [DOI] [PubMed] [Google Scholar]
- Jones M. Carcinoma on the Floor of the Pelvis: Two Discoveries in Cancerous Disease. Transactions of the American Microscopial Sciety. 1898;20:165–176. doi: 10.2307/3221255. [DOI] [Google Scholar]
- Balkwill F, Mantovani A. Inflammation and cancer: back to Virchow? Lancet. 2001;357:539–545. doi: 10.1016/S0140-6736(00)04046-0. [DOI] [PubMed] [Google Scholar]
- Bartsch H, Nair J. Chronic inflammation and oxidative stress in the genesis and perpetuation of cancer: role of lipid peroxidation, DNA damage, and repair. Langenbecks Arch Surg. 2006;391:499–510. doi: 10.1007/s00423-006-0073-1. [DOI] [PubMed] [Google Scholar]
- Ohshima H, Tatemichi M, Sawa T. Chemical basis of inflammation-induced carcinogenesis. Arch Biochem Biophys. 2003;417:3–11. doi: 10.1016/S0003-9861(03)00283-2. [DOI] [PubMed] [Google Scholar]
- Esterbauer H, Schaur RJ, Zollner H. Chemistry and biochemistry of 4-hydroxynonenal, malonaldehyde and related aldehydes. Free Radic Biol Med. 1991;11:81–128. doi: 10.1016/0891-5849(91)90192-6. [DOI] [PubMed] [Google Scholar]
- Babbar N, Casero RA., Jr Tumor necrosis factor-alpha increases reactive oxygen species by inducing spermine oxidase in human lung epithelial cells: a potential mechanism for inflammation-induced carcinogenesis. Cancer Res. 2006;66:11125–11130. doi: 10.1158/0008-5472.CAN-06-3174. [DOI] [PubMed] [Google Scholar]
- Ohshima H, Bartsch H. Chronic infections and inflammatory processes as cancer risk factors: possible role of nitric oxide in carcinogenesis. Mutat Res. 1994;305:253–264. doi: 10.1016/0027-5107(94)90245-3. [DOI] [PubMed] [Google Scholar]
- Rakoff-Nahoum S, Paglino J, Eslami-Varzaneh F, Edberg S, Medzhitov R. Recognition of commensal microflora by toll-like receptors is required for intestinal homeostasis. Cell. 2004;118:229–241. doi: 10.1016/j.cell.2004.07.002. [DOI] [PubMed] [Google Scholar]
- Pikarsky E, Porat RM, Stein I, Abramovitch R, Amit S, Kasem S, Gutkovich-Pyest E, Urieli-Shoval S, Galun E, Ben-Neriah Y. NF-kappaB functions as a tumour promoter in inflammation-associated cancer. Nature. 2004;431:461–466. doi: 10.1038/nature02924. [DOI] [PubMed] [Google Scholar]
- Li Q, Verma IM. NF-kappaB regulation in the immune system. Nat Rev Immunol. 2002;2:725–734. doi: 10.1038/nri910. [DOI] [PubMed] [Google Scholar]
- Karin M, Lin A. NF-kappaB at the crossroads of life and death. Nat Immunol. 2002;3:221–227. doi: 10.1038/ni0302-221. [DOI] [PubMed] [Google Scholar]
- Baldwin AS., Jr The NF-kappa B and I kappa B proteins: new discoveries and insights. Annu Rev Immunol. 1996;14:649–683. doi: 10.1146/annurev.immunol.14.1.649. [DOI] [PubMed] [Google Scholar]
- Baldwin AS. Control of oncogenesis and cancer therapy resistance by the transcription factor NF-kappaB. J Clin Invest. 2001;107:241–246. doi: 10.1172/JCI11991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosser DM. The many faces of macrophage activation. J Leukoc Biol. 2003;73:209–212. doi: 10.1189/jlb.0602325. [DOI] [PubMed] [Google Scholar]
- Talks KL, Turley H, Gatter KC, Maxwell PH, Pugh CW, Ratcliffe PJ, Harris AL. The expression and distribution of the hypoxia-inducible factors HIF-1alpha and HIF-2alpha in normal human tissues, cancers, and tumor-associated macrophages. Am J Pathol. 2000;157:411–421. doi: 10.1016/s0002-9440(10)64554-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goede V, Brogelli L, Ziche M, Augustin HG. Induction of inflammatory angiogenesis by monocyte chemoattractant protein-1. Int J Cancer. 1999;82:765–770. doi: 10.1002/(SICI)1097-0215(19990827)82:5<765::AID-IJC23>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- Luboshits G, Shina S, Kaplan O, Engelberg S, Nass D, Lifshitz-Mercer B, Chaitchik S, Keydar I, Ben-Baruch A. Elevated expression of the CC chemokine regulated on activation, normal T cell expressed and secreted (RANTES) in advanced breast carcinoma. Cancer Res. 1999;59:4681–4687. [PubMed] [Google Scholar]
- Ueno T, Toi M, Saji H, Muta M, Bando H, Kuroi K, Koike M, Inadera H, Matsushima K. Significance of macrophage chemoattractant protein-1 in macrophage recruitment, angiogenesis, and survival in human breast cancer. Clin Cancer Res. 2000;6:3282–3289. [PubMed] [Google Scholar]
- Robinson SC, Scott KA, Wilson JL, Thompson RG, Proudfoot AE, Balkwill FR. A chemokine receptor antagonist inhibits experimental breast tumor growth. Cancer Res. 2003;63:8360–8365. [PubMed] [Google Scholar]
- Mantovani A, Sozzani S, Locati M, Allavena P, Sica A. Macrophage polarization: tumor-associated macrophages as a paradigm for polarized M2 mononuclear phagocytes. Trends Immunol. 2002;23:549–555. doi: 10.1016/S1471-4906(02)02302-5. [DOI] [PubMed] [Google Scholar]
- Mills CD, Kincaid K, Alt JM, Heilman MJ, Hill AM. M-1/M-2 macrophages and the Th1/Th2 paradigm. J Immunol. 2000;164:6166–6173. doi: 10.4049/jimmunol.1701141. [DOI] [PubMed] [Google Scholar]
- Lamagna C, Aurrand-Lions M, Imhof BA. Dual role of macrophages in tumor growth and angiogenesis. J Leukoc Biol. 2006;80:705–713. doi: 10.1189/jlb.1105656. [DOI] [PubMed] [Google Scholar]
- Allavena P, Signorelli M, Chieppa M, Erba E, Bianchi G, Marchesi F, Olimpio CO, Bonardi C, Garbi A, Lissoni A, et al. Anti-inflammatory properties of the novel antitumor agent yondelis (trabectedin): inhibition of macrophage differentiation and cytokine production. Cancer Res. 2005;65:2964–2971. doi: 10.1158/0008-5472.CAN-04-4037. [DOI] [PubMed] [Google Scholar]
- Luo Y, Zhou H, Krueger J, Kaplan C, Lee SH, Dolman C, Markowitz D, Wu W, Liu C, Reisfeld RA, Xiang R. Targeting tumor-associated macrophages as a novel strategy against breast cancer. J Clin Invest. 2006;116:2132–2141. doi: 10.1172/JCI27648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritossa F. A new puffing pattern induced by temperature shock and DNP in Drosophila. Experientia. 1962;18:571–572. doi: 10.1007/BF02172188. [DOI] [Google Scholar]
- Tissieres A, Mitchell HK, Tracy UM. Protein synthesis in salivary glands of Drosophila melanogaster: relation to chromosome puffs. J Mol Biol. 1974;84:389–398. doi: 10.1016/0022-2836(74)90447-1. [DOI] [PubMed] [Google Scholar]
- Beere HM. Death versus survival: functional interaction between the apoptotic and stress-inducible heat shock protein pathways. J Clin Invest. 2005;115:2633–2639. doi: 10.1172/JCI26471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi Y, Mosser DD, Morimoto RI. Molecular chaperones as HSF1-specific transcriptional repressors. Genes Dev. 1998;12:654–666. doi: 10.1101/gad.12.5.654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu C. Heat shock transcription factors: structure and regulation. Annu Rev Cell Dev Biol. 1995;11:441–469. doi: 10.1146/annurev.cb.11.110195.002301. [DOI] [PubMed] [Google Scholar]
- McMillan DR, Xiao X, Shao L, Graves K, Benjamin IJ. Targeted disruption of heat shock transcription factor 1 abolishes thermotolerance and protection against heat-inducible apoptosis. J Biol Chem. 1998;273:7523–7528. doi: 10.1074/jbc.273.13.7523. [DOI] [PubMed] [Google Scholar]
- Hoang AT, Huang J, Rudra-Ganguly N, Zheng J, Powell WC, Rabindran SK, Wu C, Roy-Burman P. A novel association between the human heat shock transcription factor 1 (HSF1) and prostate adenocarcinoma. Am J Pathol. 2000;156:857–864. doi: 10.1016/S0002-9440(10)64954-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang JH, Yao MZ, Zhang ZL, Zhang YH, Wang YG, Liu XY. HSF1 blockade-induced tumor thermotolerance abolishment is mediated by JNK-dependent caspase-3 activation. Biochem Biophys Res Commun. 2004;321:736–745. doi: 10.1016/j.bbrc.2004.07.018. [DOI] [PubMed] [Google Scholar]
- Dai C, Whitesell L, Rogers AB, Lindquist S. Heat shock factor 1 is a powerful multifaceted modifier of carcinogenesis. Cell. 2007;130:1005–1018. doi: 10.1016/j.cell.2007.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srivastava P. Interaction of heat shock proteins with peptides and antigen presenting cells: chaperoning of the innate and adaptive immune responses. Annu Rev Immunol. 2002;20:395–425. doi: 10.1146/annurev.immunol.20.100301.064801. [DOI] [PubMed] [Google Scholar]
- Quintana FJ, Cohen IR. Heat shock proteins as endogenous adjuvants in sterile and septic inflammation. J Immunol. 2005;175:2777–2782. doi: 10.4049/jimmunol.175.5.2777. [DOI] [PubMed] [Google Scholar]
- Nicchitta CV. Re-evaluating the role of heat-shock protein-peptide interactions in tumour immunity. Nat Rev Immunol. 2003;3:427–432. doi: 10.1038/nri1089. [DOI] [PubMed] [Google Scholar]
- Lewis JJ. Therapeutic cancer vaccines: using unique antigens. Proc Natl Acad Sci USA. 2004;101:14653–14656. doi: 10.1073/pnas.0404839101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vabulas RM, Braedel S, Hilf N, Singh-Jasuja H, Herter S, Ahmad-Nejad P, Kirschning CJ, Da Costa C, Rammensee HG, Wagner H, Schild H. The endoplasmic reticulum-resident heat shock protein Gp96 activates dendritic cells via the Toll-like receptor 2/4 pathway. J Biol Chem. 2002;277:20847–20853. doi: 10.1074/jbc.M200425200. [DOI] [PubMed] [Google Scholar]
- Singh-Jasuja H, Scherer HU, Hilf N, Arnold-Schild D, Rammensee HG, Toes RE, Schild H. The heat shock protein gp96 induces maturation of dendritic cells and down-regulation of its receptor. Eur J Immunol. 2000;30:2211–2215. doi: 10.1002/1521-4141(2000)30:8<2211::AID-IMMU2211>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- Chen W, Syldath U, Bellmann K, Burkart V, Kolb H. Human 60-kDa heat-shock protein: a danger signal to the innate immune system. J Immunol. 1999;162:3212–3219. [PubMed] [Google Scholar]
- Todryk S, Melcher AA, Hardwick N, Linardakis E, Bateman A, Colombo MP, Stoppacciaro A, Vile RG. Heat shock protein 70 induced during tumor cell killing induces Th1 cytokines and targets immature dendritic cell precursors to enhance antigen uptake. J Immunol. 1999;163:1398–1408. [PubMed] [Google Scholar]
- Basu S, Binder RJ, Suto R, Anderson KM, Srivastava PK. Necrotic but not apoptotic cell death releases heat shock proteins, which deliver a partial maturation signal to dendritic cells and activate the NF-kappa B pathway. Int Immunol. 2000;12:1539–1546. doi: 10.1093/intimm/12.11.1539. [DOI] [PubMed] [Google Scholar]
- Somersan S, Larsson M, Fonteneau JF, Basu S, Srivastava P, Bhardwaj N. Primary tumor tissue lysates are enriched in heat shock proteins and induce the maturation of human dendritic cells. J Immunol. 2001;167:4844–4852. doi: 10.4049/jimmunol.167.9.4844. [DOI] [PubMed] [Google Scholar]
- Gross C, Schmidt-Wolf IG, Nagaraj S, Gastpar R, Ellwart J, Kunz-Schughart LA, Multhoff G. Heat shock protein 70-reactivity is associated with increased cell surface density of CD94/CD56 on primary natural killer cells. Cell Stress Chaperones. 2003;8:348–360. doi: 10.1379/1466-1268(2003)008<0348:HSPRIA>2.0.CO;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiao Y, Liu B, Li Z. Activation of NK cells by extracellular heat shock protein 70 through induction of NKG2D ligands on dendritic cells. Cancer Immun. 2008;8:12. [PMC free article] [PubMed] [Google Scholar]
- Srivastava PK, Udono H, Blachere NE, Li Z. Heat shock proteins transfer peptides during antigen processing and CTL priming. Immunogenetics. 1994;39:93–98. doi: 10.1007/BF00188611. [DOI] [PubMed] [Google Scholar]
- Srivastava P. Heat shock proteins come of age: primitive functions acquire new roles in an adaptive world. Immunity. 1998;8:657–665. doi: 10.1016/S1074-7613(00)80570-1. [DOI] [PubMed] [Google Scholar]
- Tamura Y, Peng P, Liu K, Daou M, Srivastava PK. Immunotherapy of tumors with autologous tumor-derived heat shock protein preparations. Science. 1997;278:117–120. doi: 10.1126/science.278.5335.117. [DOI] [PubMed] [Google Scholar]
- Udono H, Srivastava PK. Heat shock protein 70-associated peptides elicit specific cancer immunity. J Exp Med. 1993;178:1391–1396. doi: 10.1084/jem.178.4.1391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng H, Dai J, Stoilova D, Li Z. Cell surface targeting of heat shock protein gp96 induces dendritic cell maturation and antitumor immunity. J Immunol. 2001;167:6731–6735. doi: 10.4049/jimmunol.167.12.6731. [DOI] [PubMed] [Google Scholar]
- Dai J, Liu B, Caudill M, Zheng H, Qiao Y, Podack ER, Li Z. Cell surface expression of heat shock protein gp96 enhances cross-presentation of cellular antigens and the generation of tumor-specific T cell memory. Cancer Immunity. 2003;3:1–5. [PubMed] [Google Scholar]
- Ramirez SR, Singh-Jasuja H, Warger T, Braedel-Ruoff S, Hilf N, Wiemann K, Rammensee HG, Schild H. Glycoprotein 96-activated dendritic cells induce a CD8-biased T cell response. Cell Stress Chaperones. 2005;10:221–229. doi: 10.1379/CSC-117R.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Eden W, Zee R van der, Prakken B. Heat-shock proteins induce T-cell regulation of chronic inflammation. Nat Rev Immunol. 2005;5:318–330. doi: 10.1038/nri1593. [DOI] [PubMed] [Google Scholar]
- Franchimont D, Kino T, Galon J, Meduri GU, Chrousos G. Glucocorticoids and inflammation revisited: the state of the art. NIH clinical staff conference. Neuroimmunomodulation. 2002;10:247–260. doi: 10.1159/000069969. [DOI] [PubMed] [Google Scholar]
- Heck S, Bender K, Kullmann M, Gottlicher M, Herrlich P, Cato AC. I kappaB alpha-independent downregulation of NF-kappaB activity by glucocorticoid receptor. EMBO J. 1997;16:4698–4707. doi: 10.1093/emboj/16.15.4698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, Rao R, Shen J, Tang Y, Fiskus W, Nechtman J, Atadja P, Bhalla K. Role of acetylation and extracellular location of heat shock protein 90alpha in tumor cell invasion. Cancer Res. 2008;68:4833–4842. doi: 10.1158/0008-5472.CAN-08-0644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suriano R, Ghosh SK, Ashok BT, Mittelman A, Chen Y, Banerjee A, Tiwari RK. Differences in glycosylation patterns of heat shock protein, gp96: implications for prostate cancer prevention. Cancer Res. 2005;65:6466–6475. doi: 10.1158/0008-5472.CAN-04-4639. [DOI] [PubMed] [Google Scholar]
- Kamal A, Thao L, Sensintaffar J, Zhang L, Boehm MF, Fritz LC, Burrows FJ. A high-affinity conformation of Hsp90 confers tumour selectivity on Hsp90 inhibitors. Nature. 2003;425:407–410. doi: 10.1038/nature01913. [DOI] [PubMed] [Google Scholar]
- Prodromou C, Pearl LH. Structure and functional relationships of Hsp90. Curr Cancer Drug Targets. 2003;3:301–323. doi: 10.2174/1568009033481877. [DOI] [PubMed] [Google Scholar]
- Castro JE, Prada CE, Loria O, Kamal A, Chen L, Burrows FJ, Kipps TJ. ZAP-70 is a novel conditional heat shock protein 90 (Hsp90) client: inhibition of Hsp90 leads to ZAP-70 degradation, apoptosis, and impaired signaling in chronic lymphocytic leukemia. Blood. 2005;106:2506–2512. doi: 10.1182/blood-2005-03-1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitesell L, Lindquist SL. HSP90 and the chaperoning of cancer. Nat Rev Cancer. 2005;5:761–772. doi: 10.1038/nrc1716. [DOI] [PubMed] [Google Scholar]
- Yano M, Naito Z, Tanaka S, Asano G. Expression and roles of heat shock proteins in human breast cancer. Jpn J Cancer Res. 1996;87:908–915. doi: 10.1111/j.1349-7006.1996.tb02119.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim SO, Park SG, Yoo JH, Park YM, Kim HJ, Jang KT, Cho JW, Yoo BC, Jung GH, Park CK. Expression of heat shock proteins (HSP27, HSP60, HSP70, HSP90, GRP78, GRP94) in hepatitis B virus-related hepatocellular carcinomas and dysplastic nodules. World J Gastroenterol. 2005;11:2072–2079. doi: 10.3748/wjg.v11.i14.2072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eustace BK, Sakurai T, Stewart JK, Yimlamai D, Unger C, Zehetmeier C, Lain B, Torella C, Henning SW, Beste G, et al. Functional proteomic screens reveal an essential extracellular role for hsp90 alpha in cancer cell invasiveness. Nat Cell Biol. 2004;6:507–514. doi: 10.1038/ncb1131. [DOI] [PubMed] [Google Scholar]
- Jaattela M. Over-expression of hsp70 confers tumorigenicity to mouse fibrosarcoma cells. Int J Cancer. 1995;60:689–693. doi: 10.1002/ijc.2910600520. [DOI] [PubMed] [Google Scholar]
- Garrido C, Fromentin A, Bonnotte B, Favre N, Moutet M, Arrigo AP, Mehlen P, Solary E. Heat shock protein 27 enhances the tumorigenicity of immunogenic rat colon carcinoma cell clones. Cancer Res. 1998;58:5495–5499. [PubMed] [Google Scholar]
- Volloch VZ, Sherman MY. Oncogenic potential of Hsp72. Oncogene. 1999;18:3648–3651. doi: 10.1038/sj.onc.1202525. [DOI] [PubMed] [Google Scholar]
- Seo JS, Park YM, Kim JI, Shim EH, Kim CW, Jang JJ, Kim SH, Lee WH. T cell lymphoma in transgenic mice expressing the human Hsp70 gene. Biochem Biophys Res Commun. 1996;218:582–587. doi: 10.1006/bbrc.1996.0103. [DOI] [PubMed] [Google Scholar]
- Jolly C, Morimoto RI. Role of the heat shock response and molecular chaperones in oncogenesis and cell death. J Natl Cancer Inst. 2000;92:1564–1572. doi: 10.1093/jnci/92.19.1564. [DOI] [PubMed] [Google Scholar]
- Schmitt E, Gehrmann M, Brunet M, Multhoff G, Garrido C. Intracellular and extracellular functions of heat shock proteins: repercussions in cancer therapy. J Leukoc Biol. 2007;81:15–27. doi: 10.1189/jlb.0306167. [DOI] [PubMed] [Google Scholar]
- Buzzard KA, Giaccia AJ, Killender M, Anderson RL. Heat shock protein 72 modulates pathways of stress-induced apoptosis. J Biol Chem. 1998;273:17147–17153. doi: 10.1074/jbc.273.27.17147. [DOI] [PubMed] [Google Scholar]
- Stankiewicz AR, Lachapelle G, Foo CP, Radicioni SM, Mosser DD. Hsp70 inhibits heat-induced apoptosis upstream of mitochondria by preventing Bax translocation. J Biol Chem. 2005;280:38729–38739. doi: 10.1074/jbc.M509497200. [DOI] [PubMed] [Google Scholar]
- Prohaszka Z, Singh M, Nagy K, Kiss E, Lakos G, Duba J, Fust G. Heat shock protein 70 is a potent activator of the human complement system. Cell Stress Chaperones. 2002;7:17–22. doi: 10.1379/1466-1268(2002)007<0017:HSPIAP>2.0.CO;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fishelson Z, Donin N, Zell S, Schultz S, Kirschfink M. Obstacles to cancer immunotherapy: expression of membrane complement regulatory proteins (mCRPs) in tumors. Mol Immunol. 2003;40:109–123. doi: 10.1016/S0161-5890(03)00112-3. [DOI] [PubMed] [Google Scholar]
- Chauhan D, Li G, Hideshima T, Podar K, Mitsiades C, Mitsiades N, Catley L, Tai YT, Hayashi T, Shringarpure R, et al. Hsp27 inhibits release of mitochondrial protein Smac in multiple myeloma cells and confers dexamethasone resistance. Blood. 2003;102:3379–3386. doi: 10.1182/blood-2003-05-1417. [DOI] [PubMed] [Google Scholar]
- Cornford PA, Dodson AR, Parsons KF, Desmond AD, Woolfenden A, Fordham M, Neoptolemos JP, Ke Y, Foster CS. Heat shock protein expression independently predicts clinical outcome in prostate cancer. Cancer Res. 2000;60:7099–7105. [PubMed] [Google Scholar]
- Castle PE, Ashfaq R, Ansari F, Muller CY. Immunohistochemical evaluation of heat shock proteins in normal and preinvasive lesions of the cervix. Cancer Lett. 2005;229:245–252. doi: 10.1016/j.canlet.2005.06.045. [DOI] [PubMed] [Google Scholar]
- Paget J. The Morton lecture on cancer and cancerous diseases, delivered at the Royal College of Surgeons of England, on Friday, November 11, 1887. London: Longmans, Green & Co; 1887. [Google Scholar]
- Smith EF. Some Newer Aspects of Cancer Research. Science. 1925;61:595–601. doi: 10.1126/science.61.1589.595. [DOI] [PubMed] [Google Scholar]
- Bullock FDCM. The Experimental Production of Sarcoma of the Liver of Rats. Proc N Y Path Soc. 1920;20:149–175. [Google Scholar]
- Ross AG, Bartley PB, Sleigh AC, Olds GR, Li Y, Williams GM, McManus DP. Schistosomiasis. N Engl J Med. 2002;346:1212–1220. doi: 10.1056/NEJMra012396. [DOI] [PubMed] [Google Scholar]
- Elsebai I. Parasites in the etiology of cancer–bilharziasis and bladder cancer. CA Cancer J Clin. 1977;27:100–106. doi: 10.3322/canjclin.27.2.100. [DOI] [PubMed] [Google Scholar]
- Marletta MA. Mammalian synthesis of nitrite, nitrate, nitric oxide, and N-nitrosating agents. Chem Res Toxicol. 1988;1:249–257. doi: 10.1021/tx00005a001. [DOI] [PubMed] [Google Scholar]
- Shacter E, Beecham EJ, Covey JM, Kohn KW, Potter M. Activated neutrophils induce prolonged DNA damage in neighboring cells. Carcinogenesis. 1988;9:2297–2304. doi: 10.1093/carcin/9.12.2297. [DOI] [PubMed] [Google Scholar]
- Weitberg AB. Effect of combinations of antioxidants on phagocyte-induced sister-chromatid exchanges. Mutat Res. 1989;224:1–4. doi: 10.1016/0165-1218(89)90002-5. [DOI] [PubMed] [Google Scholar]
- Weitzman SA, Stossel TP. Mutation caused by human phagocytes. Science. 1981;212:546–547. doi: 10.1126/science.6259738. [DOI] [PubMed] [Google Scholar]
- O'Brien PJ. Radical formation during the peroxidase catalyzed metabolism of carcinogens and xenobiotics: the reactivity of these radicals with GSH, DNA, and unsaturated lipid. Free Radic Biol Med. 1988;4:169–183. doi: 10.1016/0891-5849(88)90025-1. [DOI] [PubMed] [Google Scholar]
- Maresca B, Carratu L. The biology of the heat shock response in parasites. Parasitol Today. 1992;8:260–266. doi: 10.1016/0169-4758(92)90137-Q. [DOI] [PubMed] [Google Scholar]
- Lebret T, Watson RW, Molinie V, O'Neill A, Gabriel C, Fitzpatrick JM, Botto H. Heat shock proteins HSP27, HSP60, HSP70, and HSP90: expression in bladder carcinoma. Cancer. 2003;98:970–977. doi: 10.1002/cncr.11594. [DOI] [PubMed] [Google Scholar]
- Kasai KKR. The stomach Spirochete occurring in Mammals. J Parasitol. 1919;6:1–11. doi: 10.2307/3271009. [DOI] [Google Scholar]
- Marshall BJ, Warren JR. Unidentified curved bacilli in the stomach of patients with gastritis and peptic ulceration. Lancet. 1984;1:1311–1315. doi: 10.1016/S0140-6736(84)91816-6. [DOI] [PubMed] [Google Scholar]
- Parsonnet J, Hansen S, Rodriguez L, Gelb AB, Warnke RA, Jellum E, Orentreich N, Vogelman JH, Friedman GD. Helicobacter pylori infection and gastric lymphoma. N Engl J Med. 1994;330:1267–1271. doi: 10.1056/NEJM199405053301803. [DOI] [PubMed] [Google Scholar]
- Correa P. Helicobacter pylori and gastric carcinogenesis. Am J Surg Pathol. 1995;19:S37–43. [PubMed] [Google Scholar]
- Hudson JD, Shoaibi MA, Maestro R, Carnero A, Hannon GJ, Beach DH. A proinflammatory cytokine inhibits p53 tumor suppressor activity. J Exp Med. 1999;190:1375–1382. doi: 10.1084/jem.190.10.1375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeo M, Park HK, Kim DK, Cho SW, Kim YS, Cho SY, Paik YK, Hahm KB. Restoration of heat shock protein70 suppresses gastric mucosal inducible nitric oxide synthase expression induced by Helicobacter pylori. Proteomics. 2004;4:3335–3342. doi: 10.1002/pmic.200400951. [DOI] [PubMed] [Google Scholar]
- Iacopini F, Consolazio A, Bosco D, Marcheggiano A, Bella A, Pica R, Paoluzi OA, Crispino P, Rivera M, Mottolese M, et al. Oxidative damage of the gastric mucosa in Helicobacter pylori positive chronic atrophic and nonatrophic gastritis, before and after eradication. Helicobacter. 2003;8:503–512. doi: 10.1046/j.1523-5378.2003.00172.x. [DOI] [PubMed] [Google Scholar]
- Yamaoka Y, Kita M, Kodama T, Sawai N, Kashima K, Imanishi J. Induction of various cytokines and development of severe mucosal inflammation by cagA gene positive Helicobacter pylori strains. Gut. 1997;41:442–451. doi: 10.1136/gut.41.4.442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crabtree JE, Farmery SM, Lindley IJ, Figura N, Peichl P, Tompkins DS. CagA/cytotoxic strains of Helicobacter pylori and interleukin-8 in gastric epithelial cell lines. J Clin Pathol. 1994;47:945–950. doi: 10.1136/jcp.47.10.945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitadai Y, Haruma K, Sumii K, Yamamoto S, Ue T, Yokozaki H, Yasui W, Ohmoto Y, Kajiyama G, Fidler IJ, Tahara E. Expression of interleukin-8 correlates with vascularity in human gastric carcinomas. Am J Pathol. 1998;152:93–100. [PMC free article] [PubMed] [Google Scholar]
- Takenaka R, Yokota K, Ayada K, Mizuno M, Zhao Y, Fujinami Y, Lin SN, Toyokawa T, Okada H, Shiratori Y, Oguma K. Helicobacter pylori heat-shock protein 60 induces inflammatory responses through the Toll-like receptor-triggered pathway in cultured human gastric epithelial cells. Microbiology. 2004;150:3913–3922. doi: 10.1099/mic.0.27527-0. [DOI] [PubMed] [Google Scholar]
- Zhao Y, Yokota K, Ayada K, Yamamoto Y, Okada T, Shen L, Oguma K. Helicobacter pylori heat-shock protein 60 induces interleukin-8 via a Toll-like receptor (TLR)2 and mitogen-activated protein (MAP) kinase pathway in human monocytes. J Med Microbiol. 2007;56:154–164. doi: 10.1099/jmm.0.46882-0. [DOI] [PubMed] [Google Scholar]
- Evans DJ, Jr, Evans DG, Engstrand L, Graham DY. Urease-associated heat shock protein of Helicobacter pylori. Infect Immun. 1992;60:2125–2127. doi: 10.1128/iai.60.5.2125-2127.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirchhoff SR, Gupta S, Knowlton AA. Cytosolic heat shock protein 60, apoptosis, and myocardial injury. Circulation. 2002;105:2899–2904. doi: 10.1161/01.CIR.0000019403.35847.23. [DOI] [PubMed] [Google Scholar]
- Rous P. Landmark article (JAMA 1911;56:198). Transmission of a malignant new growth by means of a cell-free filtrate. By Peyton Rous. JAMA. 1983;250:1445–1449. doi: 10.1001/jama.250.11.1445. [DOI] [PubMed] [Google Scholar]
- Yao DF, Wu XH, Su XQ, Yao M, Wu W, Qiu LW, Zou L, Meng XY. Abnormal expression of HSP gp96 associated with HBV replication in human hepatocellular carcinoma. Hepatobiliary Pancreat Dis Int. 2006;5:381–386. [PubMed] [Google Scholar]
- Meng SD, Gao T, Gao GF, Tien P. HBV-specific peptide associated with heat-shock protein gp96. Lancet. 2001;357:528–529. doi: 10.1016/S0140-6736(00)04050-2. [DOI] [PubMed] [Google Scholar]
- Harimoto N, Shimada M, Aishima S, Kitagawa D, Itoh S, Tsujita E, Maehara S, Taketomi A, Tanaka S, Shirabe K, Maehara Y. The role of heat shock protein 27 expression in hepatocellular carcinoma in Japan: special reference to the difference between hepatitis B and C. Liver Int. 2004;24:316–321. doi: 10.1111/j.1478-3231.2004.0927.x. [DOI] [PubMed] [Google Scholar]
- Cheung RK, Dosch HM. The growth transformation of human B cells involves superinduction of hsp70 and hsp90. Virology. 1993;193:700–708. doi: 10.1006/viro.1993.1178. [DOI] [PubMed] [Google Scholar]
- Testori A, Richards J, Whitman E, Mann GB, Lutzky J, Camacho L, Parmiani G, Tosti G, Kirkwood JM, Hoos A, et al. Phase III comparison of vitespen, an autologous tumor-derived heat shock protein gp96 peptide complex vaccine, with physician's choice of treatment for stage IV melanoma: the C-100-21 Study Group. J Clin Oncol. 2008;26:955–962. doi: 10.1200/JCO.2007.11.9941. [DOI] [PubMed] [Google Scholar]
- Wood C, Srivastava P, Bukowski R, Lacombe L, Gorelov AI, Gorelov S, Mulders P, Zielinski H, Hoos A, Teofilovici F, et al. An adjuvant autologous therapeutic vaccine (HSPPC-96; vitespen) versus observation alone for patients at high risk of recurrence after nephrectomy for renal cell carcinoma: a multicentre, open-label, randomised phase III trial. Lancet. 2008;372:145–154. doi: 10.1016/S0140-6736(08)60697-2. [DOI] [PubMed] [Google Scholar]