Abstract
A synthetic amphiphilic block copolymer Pluronic P85 (P85) was shown to be among the most potent inhibitors of Pgp efflux system in the blood–brain barrier (BBB) and capable of enhancing delivery of Pgp substrates to the brain. The purpose of this work is to evaluate the effects of P85 on amino acid transport in BBB. Primary bovine brain micro-vessel endothelial cells (BBMEC) grown on membrane inserts were used as an in vitro BBB model. Expression of amino acid transporters, like large neutral amino acid transporter 1, cationic amino acid transporter 1, and small neutral amino acid transporter 1, were confirmed by reverse transcriptase polymerase chain reaction. Effects of P85 on amino acid transporters were examined using their substrates: 3H-phenylalanine, 3H-lysine, and 3H-methylaminoisobutyric acid, respectively. BBMEC permeability studies were carried out in apical (AP) to basolateral (BL) and BL to AP directions. P85 added at the AP side had little, if any, effect on AP to BL (“blood to brain”) transport for all examined amino acids in BBMEC monolayers. However, 0.1% P85 added at the BL side significantly increased the BL to AP transport of these substrates. Furthermore, the effective concentrations of P85 were also shown to induce plasma membrane depolarization and increase intracellular sodium concentration in BBMEC, which can contribute to the effects of the copolymer on the energy-dependent transport systems. All together, despite profound effects on transport system(s) at the brain side of cell monolayers, P85 had no effect on AP to BL transport of amino acids in brain microvessel endothelial cell model.
Keywords: blood-brain barrier, amino acid transporter, Pluronic P85
Introduction
The blood–brain barrier (BBB) is a primary interface between the central nervous system and peripheral circulation. It consists of brain microvessel endothelial cells (BMVEC), astrocytes, and pericytes. BMVEC form tight junctions, have low pinocytic activity, and express efflux proteins, notably, P-glycoprotein (Pgp), which all restrict transport of toxic substances to the brain (Pardridge 1998). At the same time, BBB facilitates brain transport of nutrients and hormones essential for normal metabolism and growth. The protective function of the BBB hinders delivery of diagnostic and therapeutic agents to the brain. It is a major obstacle to diagnosis and therapy of brain tumors and neurodegenerative disorders, such as Alzheimer’s and Parkinson’s diseases, stroke, amyotrophic lateral sclerosis, multiple sclerosis, HIV-1-associated dementia, and lysosomal storage diseases. Furthermore, treatment of depression, severe pain, fungal infections, obesity, and other disorders also requires efficient drug transport across BBB.
A synthetic amphiphilic block copolymer Pluronic P85 (P85) was shown to be among the most potent inhibitors of the Pgp efflux system in BBB and capable of enhancing delivery of Pgp substrates to the brain (Batrakova et al. 1999). It is a block copolymer of ethylene oxide (EO) and propylene oxide (PO) arranged in a basic A–B–A structure: EO26–PO40–EO26. It acts through a unique “double punch” mechanism, concurrently inhibiting Pgp ATPase activity in the cell membrane and depleting adenosine triphosphate (ATP) in BMVEC (Batrakova et al. 2001a). A formulation containing Pluronic and doxorubicin, SP1049C, was successfully evaluated in phase II clinical trial to treat esophageal cancer (Armstrong et al. 2006). Similar compositions capable of inhibiting Pgp can also be used to enhance drug delivery across BBB (Batrakova et al. 1999, 2001b). However, other transport systems need to be evaluated along with Pgp to assess selectivity of Pluronic effects in BBB. An earlier study suggested that P85 slightly inhibits monocarboxylate transporter 1 and has no effect on glucose transporter 1 (Batrakova et al. 2004c).
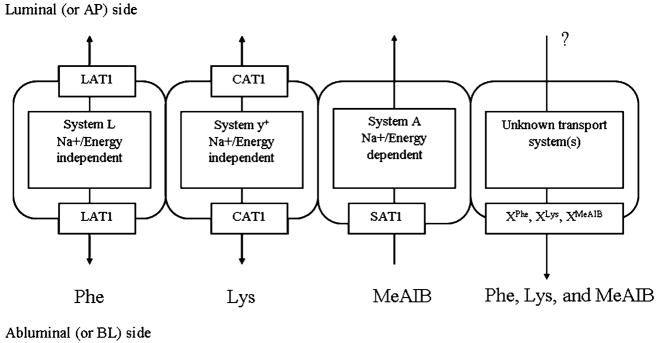
The present work evaluates transport of amino acids essential for brain function and metabolism (Oldendorf 1971; Molina et al. 1997; Araujo et al. 2001). The amino acid transport systems in BBB include large neutral amino acid transporter 1 (LAT1), cationic amino acid transporter 1 (CAT1), and small neutral amino acid transporter 1 (SAT1; Stoll et al. 1993; Matsuo et al. 2000; Sugawara et al. 2000; Umeki et al. 2002). Their localization, directionality, and energy requirements in BBB are summarized in Fig. 1. More recently, in addition to these amino acid transporters, several other transporters were uncovered in BBB, which have various localization, specificity, and energy requirements (Hawkins et al. 2006; Ohtsuki and Terasaki 2007; Omidi et al. 2008). In this work, effects of P85 on amino acid transport to the brain are assessed using monolayers of primary bovine brain micro-vessel endothelial cells (BBMEC) as an in vitro model of the BBB. L-Phenylalanine (Phe), L-lysine (Lys), and methylaminoisobutyric acid (MeAIB) are used as respective substrates for LAT1, CAT1, and SAT1. The solute transport across BBMEC monolayers is characterized in two directions: apical (AP) to basolateral (BL) and BL to AP. The block copolymer is added at each side to elucidate its effects on luminal (blood facing) and abluminal (brain facing) transport systems.
Fig. 1.
Amino acid transporters in BBB: localization, directionality, and energy requirements
Results demonstrate that P85 has little, if any, effect on AP to BL transport of Phe, Lys, or MeAIB, suggesting that the copolymer does not affect luminal transport systems in in vitro BBB. However, it increases BL to AP transport of each of these substrates. This may be due to the interaction of the copolymer with abluminal transport system(s) in brain capillary cells (Fig. 1). All together, this study demonstrates selectivity of the block copolymer with respect to different transport systems in BBB and reinforces the need to carefully evaluate lack of adverse effects on amino acid transport to the brain in animal models.
Materials and methods
Materials
P85 was kindly provided by BASF Corp. (Parispany, NJ, USA). Molecular mass of PO block was approximately 2,500, and EO content was approximately 50% by weight. P85 and radioactively labeled substrates for amino acid transporters were dissolved in assay buffer containing 150 mM NaCl, 25 mM NaHCO3, 10 mM glucose, 10 mM 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid, 3 mM KCl, 1.2 mM MgSO4, 1.4 mM CaCl2, and 0.4 mM K2HPO4, pH=7.4. 3H-Phe (specific activity 90 Ci/mmol, 1 mCi/ml, 100 nM), 3H-Lys (specific activity 58 Ci/mmol, 1 mCi/ml, and 100 nM), and 3H-MeAIB (specific activity 80 Ci/mmol, 1 mCi/ml, and 100 nM) were obtained from the American Radiolabeled Chemicals, Inc. (St Louis, MO, USA). All cell culture media ingredients were obtained from GIBCO Life Technologies, Inc. (Grand Island, NY, USA) and Sigma, Inc. (St Louis, MO, USA).
Cells isolation and culture
BBMEC were isolated from fresh cow brains using a combination of enzymatic digestion and density centrifugation as described earlier (Miller et al. 1992). For accumulation studies, the cells were seeded at a density of 50,000 cells/cm2 in 24-well plates precoated with collagen (BD Biosciences, Bedford, MA, USA) and fibronectin (Sigma), and cultured in minimum essential medium: F12 culture medium supplemented with 10% (w/w) horse serum, heparin sulfate (100 ug/ml), amphotericin B (2.5 μg/ml), and gentamicin (50 μg/ml). Medium was changed every other day; confluent BBMEC monolayers were obtained typically by day 14. For transport experiments, cells were seeded in the 12-well trans-well inserts (Corning Incorporated Life Sciences, Corning, NY, USA) precoated with collagen and fibronectin and used for flux studies when the cells were confluent (typically, in 14 days).
Reverse transcriptase polymerase chain reaction
Total RNA was extracted from approximately 1×107 BBMEC using the TRIzol kit (Invitrogen, Grand Island, NY, USA) as described elsewhere (Boado and Pardridge 1991). Approximately 1×107 cells were used for RNA extraction. The quality of RNA was verified by 1% agarose gel stained with ethidium bromide. Two micrograms of total RNA was reverse transcribed using an M-MLV Reverse Transcriptase kit (Promega Corp. Madison, WI, USA) according to the manufacturer’s protocol. The bovine LAT1, CAT1, SAT1, and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) genes were amplified by reverse transcriptase polymerase chain reactions (RT-PCRs) using PCR Core Systems (Promega Corp.). Primers are shown in Table 1. The products were analyzed by electrophoresis in 2% agarose gel stained with ethidium bromide. The gel images were captured with GEL DOC 2000 (Bio-Rad, Hercules, CA, USA).
Table 1.
Summary of primer sequences for LAT1 CAT1, SAT1 and GADPH
| Gene | bp | Forward primer sequence | Reverse primer sequence | GeneBank accession |
|---|---|---|---|---|
| LAT1 | 266 | 5′-tacttccttggggtctggtg | 5′-gtatctgcggacatccacct | AF174615 |
| CAT1 | 346 | 5′-gagctctggccttcatcac | 5′-ttctccgtgagctgccagtt | NM_013111 |
| SAT1 | 350 | 5′-ttctagcccccagactcaga | 5′-gtgagttcacagcgctcaag | AY039236 |
| GADPH | 427 | 5′-tgaccccttcattgaccttc | 5′-ggtcataagtccctccacga | BTU85042 |
3H-Phe, 3H-Lys, and 3H-MeAIB permeability studies
BBMEC permeability studies were carried out as described earlier (Batrakova et al. 2001b). Briefly, confluent monolayers of BBMEC (cultured in 12-well transwell inserts; trans-endothelium electrical resistances above 120 Ω·cm2) grown on polycarbonate membranes were placed in the membrane transport system (AMIE system, Bethlehern, PA, USA). First, cell monolayers were pretreated for 30 min at 37°C with a substrate-free assay buffer or P85 solutions (0.01–1 wt.%). Second, 3H-Phe, 3H-Lys, or 3H-MeAIB in an assay buffer with/without P85 were added to AP or BL sides of the monolayers. Appearance of substrates in the receiver chamber was monitored by measuring its radioactivity at various time intervals (0–120 min). All transport experiments were conducted in triplicate. The permeability coefficients were calculated as follows (Nerurkar et al. 1997):
where Pe is the effective permeability coefficient (cm/s), A is a cross-sectional area of the cell monolayers (cm2), ΔMR/Δt is a linear rate of appearance of mass in the receiver chamber (μmol/min), CD(0) is the initial concentration of amino acid in the donor chamber, CR is the concentration of amino acids in the receiver chamber at each time point, and Δt is the time interval. Using 14C-mannitol as a paracellular marker, we determined that an increase in paracellular permeability of the BBMEC monolayers at the highest doses of 1% P85 did not exceed 30%. No permeability changes were observed with lower doses of the copolymer (data were not shown in this paper).
Membrane potential
BBMEC monolayers were grown in 24-well plates precoated with collagen. Cells were collected by trypsinization, and concentration was adjusted to one million per milliliter. Cells were treated either with an assay buffer (control) or different concentrations of P85 (0.0001–5%) for 30 min. After that, 1 μM bis-(1,3-dibutylbarbituric acid) trimethine oxonol [DiBAC; Batrakova et al. 2001a (3); Invitrogen, Molecular Probes, Carlsbad, CA, USA] was added, and cells were incubated for additional 10 min. After that, cells were washed with cold phosphate-buffered saline and P85, and fluorescence intensity was determined by fluorescence-activated cell sorting with excitation 488 nm and emission 530 nm.
Sodium concentration measurement
BBMEC monolayers were grown for 2 weeks in chambered coverglasses for confocal microscopy precoated with collagen. Two-micromolar sodium green (Invitrogen) was added to the media, and cells were incubated for 20 min for equilibration. After that, 0.1% P85 was added, and images were taken every 15 min on a Zeiss LSM using a 488-nm laser for excitation.
Statistical analysis
All statistical tests were performed by the two-tailed Student’s t-test with Microsoft Excel. A minimum p value of 0.05 was estimated as the significance level for the all tests. All experiments were completed in triplicates, and the results were presented as means±SEM.
Results
Expression of amino acid transporters in BBMEC
Expression of LAT1, CAT1, and SAT1 in BBMEC cultured was confirmed by RT-PCR (Fig. 2).
Fig. 2.
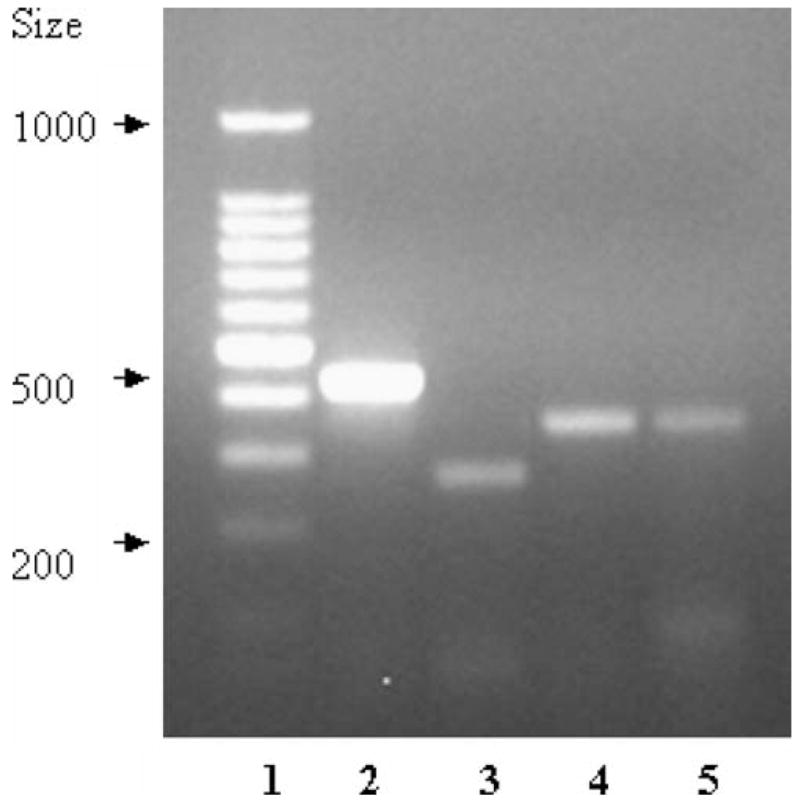
RT-PCR analysis of expression of amino acid transporters in BBMEC. The lanes correspond to standard markers (lane 1), GADPH (lane 2), bovine LAT1 complementary DNA (lane 3), bovine CAT1 (lane 4), and bovine SAT1 (lane 5)
Effect of P85 on transport of Phe across BBMEC monolayers
Effects of P85 on 3H-Phe transport across BBMEC monolayers were examined using cells grown on 12-well trans-well inserts; see Fig. 3 (transport kinetics) and Table 2 (permeability coefficients). A single isolation of BBMEC provides from one to six plates allowing four groups per plate using triplicates. Each plate usually includes a control assay buffer and treatment groups. Therefore, we used multiple isolations and overlapping treatment groups in the analysis. Since there was some variability in the transport kinetics for different BBMEC isolations, the permeability coefficients are analyzed below for same isolations.
Fig. 3.
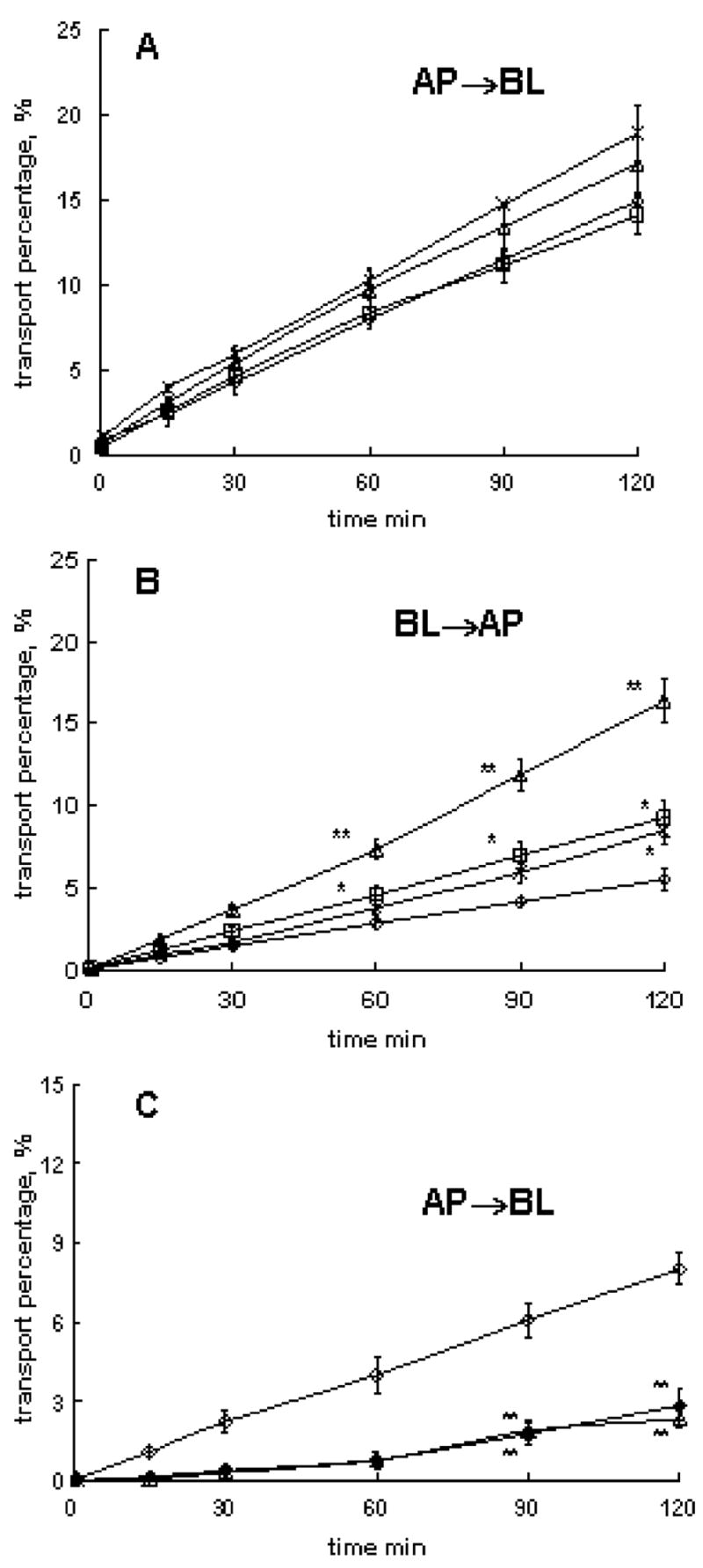
Effects of P85 on 3H-Phe transport in different directions across BBMEC monolayers: a AP to BL transport of 3H-Phe with/without P85 at the AP side (isolation 1), b BL to AP transport of 3H-Phe with/without P85 at BL side (isolation 2), c AP to BL transport of 3H-Phe with/without P85 or 2-mM unlabeled Phe at BL side (isolation 3). The treatment groups included: a, b assay buffer controls (open diamonds), 0.01 wt.% P85 (crosses), 0.1 wt.% P85 (open triangles), and 1 wt.% P85 (open squares) and c assay buffer (open diamond), 0.1% P85 (open triangle), and 2-mM unlabeled Phe in assay buffer (filled diamond). BBMEC monolayers were grown on 12-well transwell inserts. Statistical comparisons were made pair-wise between (1) each P85 and (2) assay buffer groups: p<0.05 (*) and p<0.01 (**), n=3
Table 2.
Permeability coefficients of 3H-Phe across BBMEC monolayers (×10−5 cm/s)
| # isolation | Transport direction | Assay buffer control | P85 treatment groups
|
|||
|---|---|---|---|---|---|---|
| Application side | 0.01% P85 | 0.1% P85 | 1%P85 | |||
| 1 | AP to BL | 30.2±0.1 | AP | 37.4±3.2 | 34.9±3.1 | 28.8±2.3 |
| 2 | BL to AP | 11.6±1.4 | BL | 17.9±1.8* | 34.5±2.8** | 19.5±2.3* |
| 3 | AP to BL | 16.8±1.5 | BL | – | 7.0±0.8* | – |
| 3 | AP to BL | 7.3±0.1a | BL | – | – | – |
Differences between the assay buffer control and P85 treatment groups were considered significant
In these groups, 2-mM unlabeled Phe was added at the BL side of BBMEC monolayers, while 3 H-Phe was applied on the AP side
p<0.05;
p<0.01, n=3
P85 added at the AP side had little, if any, effects on AP to BL flux of 3H-Phe (Fig. 3a and Table 2, isolation 1). However, the copolymer added at the BL side significantly increased BL to AP transport of the substrate (Fig. 3b and Table 2, isolation 2). The magnitude of this effect varied, but it was reproduced for different isolations. The maximal permeability increases were observed with 0.1% P85. At the same time, 0.1% P85 added at the BL side significantly decreased AP to BL transport of 3H-Phe (Fig. 3c and Table 2, isolation 3). Notably, 0.1% P85 had no effect on paracellular transport of 14C-mannitol in either direction (data not shown). It is unlikely that P85 affected activity of LAT1 since it had no effect on the 3H-Phe transport at the AP side of BBMEC monolayers. Therefore, we suggest possible effects of P85 on a transport system other than LAT1, located at the BL side of BBMEC monolayers, XPhe (Fig. 1). It can be an influx (blood to brain) transporter, which is inhibited by P85 at the BL side. Alternatively, it can be an efflux (brain to blood) transporter, which is activated by P85. The influx transporter XPhe is more likely because addition of 2 mM unlabeled Phe at the BL side resulted in a decrease of AP to BL transport of 3H-Phe (Fig. 3c). All together, P85 has little, if any, effect on LAT1 at the AP side of the monolayers and affects some transport system XPhe at the BL side.
Effect of P85 on transport of Lys across BBMEC monolayers
A similar approach was used to evaluate effects of P85 on the transport of a cationic amino acid across BBMEC monolayers. The copolymer added at the AP side did not affect 3H-Lys flux in AP to BL direction (Fig. 4a and Table 3, isolations 4, 5, and 6). In contrast, P85 added at the BL side considerably increased the BL to AP flux of the substrate (Fig. 4b and Table 3, isolations 4 and 8). Furthermore, AP to BL flux of 3H-Lys was considerably decreased by adding P85 at the BL side (Fig. 4c and Table 3, isolations 6, 7, and 9). Like in the previous case, this could be either due to inhibition of an influx transporter or activation of an efflux transporter at the BL side. The former, i.e., influx transporter, XLys, is more likely, because addition of excess of unlabeled Lys (10 mM) to the BL side significantly inhibited AP to BL flux of 3H-Lys (Fig. 4c and Table 3, isolation 9). Interestingly, unlabeled Lys at the BL side also inhibited BL to AP transport of 3H-Lys with or without P85 (Fig. 4c and Table 3, isolation 8), which suggests that Lys excess inhibits all transport systems present (influx and efflux). All together, we conclude that P85 does not affect Lys AP to BL transport system(s) at the AP side. However, it appears to inhibit a Lys transport system, XLys at the BL side (Fig. 1).
Fig. 4.
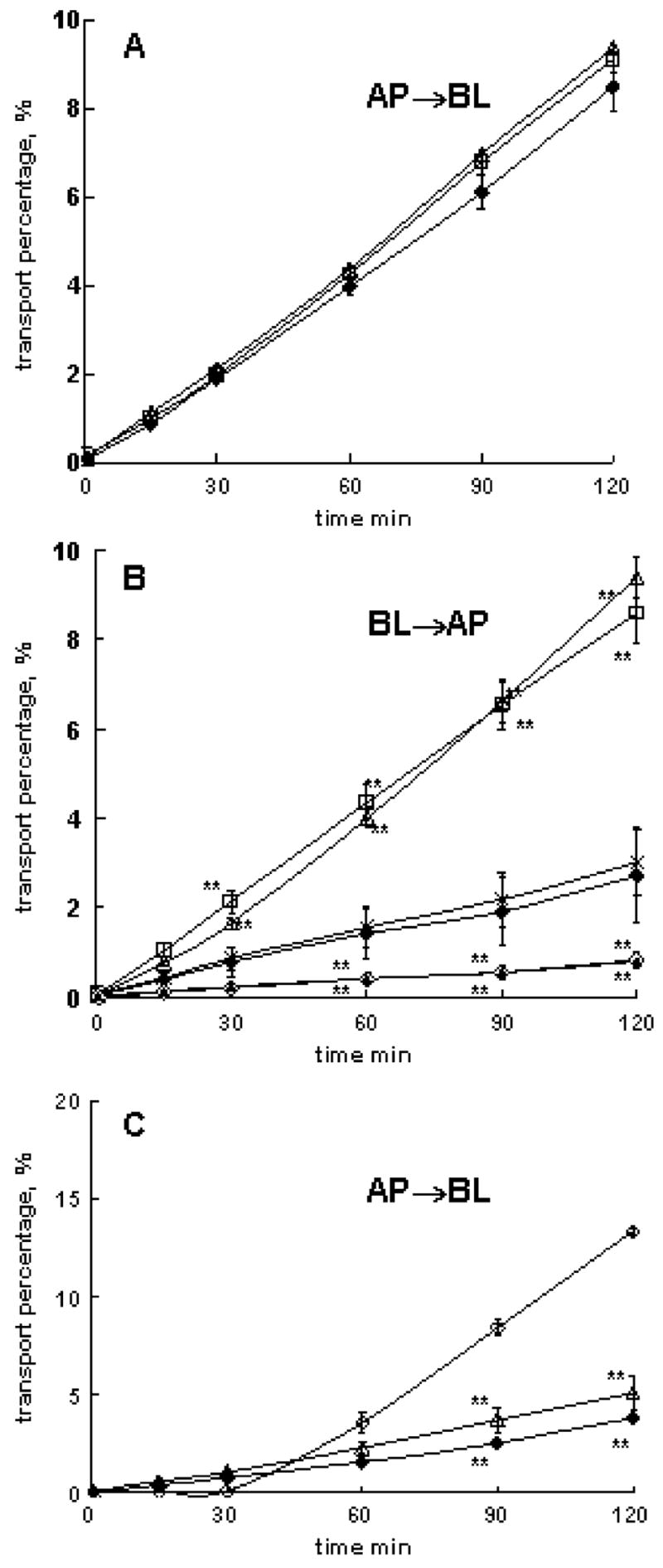
Effects of P85 on 3H-Lys transport in different directions across BBMEC monolayers: a AP to BL transport with/without P85 at the AP side (isolation 5), b BL to AP transport with/without P85 and 10-mM unlabeled Lys at BL side (isolation 8), c AP to BL transport with/without P85 or 10-mM unlabeled Lys at BL side (isolation 9). Assay buffer (open diamonds), 0.01 wt.% P85 (crosses), 0.1 wt.% P85 (open triangles), 1 wt.% P85 (open squares), assay buffer with 10 mM unlabeled lysine at BL side (filled diamonds). Statistical comparisons were made pair-wise between (1) each P85 or unlabeled Lys groups and (2) assay buffer groups that did not contain unlabeled Lys added: p<0.05 (*) and p<0.01 (**), n=3
Table 3.
Permeability coefficients of 3H-Lys across BBMEC monolayers (×10−5 cm/s)
| # isolation | Transport direction | Assay buffer Control | P85 treatment groups
|
|||
|---|---|---|---|---|---|---|
| Application side | 0.01% P85 | 0.1% P85 | 1%P85 | |||
| 4 | AP to BL | 8.7±1.8 | AP | – | 11.8±1.3 | – |
| 4 | BL to AP | 12.2±1.7 | BL | – | 15.5±1.1* | – |
| 5 | AP to BL | 18.0±1.3 | AP | – | 19.8±0.2 | 18.3±1.4 |
| 6 | AP to BL | 11.6±0.1a | AP | – | 11.8±0.7 | – |
| 6 | AP to BL | 11.6±0.1a | BL | – | 5.1±0.4** | – |
| 7 | AP to BL | 7.9±0.12 | BL | 2.8±0.2** | 4.7±0.5* | 3.4±0.2** |
| 8 | BL to AP | 5.5±2.5 | BL | 6.2±1.5 | 20.2±1.1** | 18.3±1.3** |
| 8 | BL to AP | 1.7±0.2b, ** | BL | – | 1.6±0.1b, ** | – |
| 9 | AP to BL | 29.6±0.9 | BL | – | 10.8±1.9** | – |
| 9 | AP to BL | 7.9±0.4b, ** | BL | – | – | – |
The differences between the assay buffer control and treatment groups were considered significant
Same data
In these groups, 10-mM unlabeled Lys was added at the BL side of BBMEC monolayers along with 3 H-Lys, and the comparisons were made with the BL to AP assay buffer group, in which unlabeled Lys was not added. In all other cases, the comparisons were made between P85 treatment and respective assay buffer control groups
p<0.05;
p<0.01, n=3
Effect of P85 on transport of MeAIB across BBMEC monolayers
Small nonessential neutral amino acid transporters are uniquely expressed at the abluminal side of the BBB (Sanchez del Pino et al. 1992; Hawkins et al. 2006). Consistent with that, the AP to BL flux of 3H-MeAIB was not affected by P85 added at the AP side (Fig. 5a and Table 4, isolations 10 and 11). In contrast, at the BL side, P85 increased the BL to AP flux of 3H-MeAIB (Fig. 5b and Table 4, isolations 10 and 11). The magnitude of this effect varied for different BBMEC isolations and depended on P85 concentration. Furthermore, like in the previous cases, P85 added at the BL side significantly decreased AP to BL flux of 3H-MeAIB (Fig. 5c and Table 4, isolation 12). In contrast, unlabeled MeAIB applied from the BL side significantly increased AP to BL transport of the labeled substrate (Fig. 5c and Table 4, isolation 12), presumably due to inhibition of BL to AP efflux mediated by SAT1. Therefore, P85 and unlabeled MeAIB added at the BL had opposite effects on AP to BL transport of 3H-MeAIB. This reinforces that P85 affected a different transport system at the BL side, XMeAIB, rather than SAT1 (Fig. 1). This transport system most likely has opposite directionality (AP to BL) compared to SAT1 (system A).
Fig. 5.
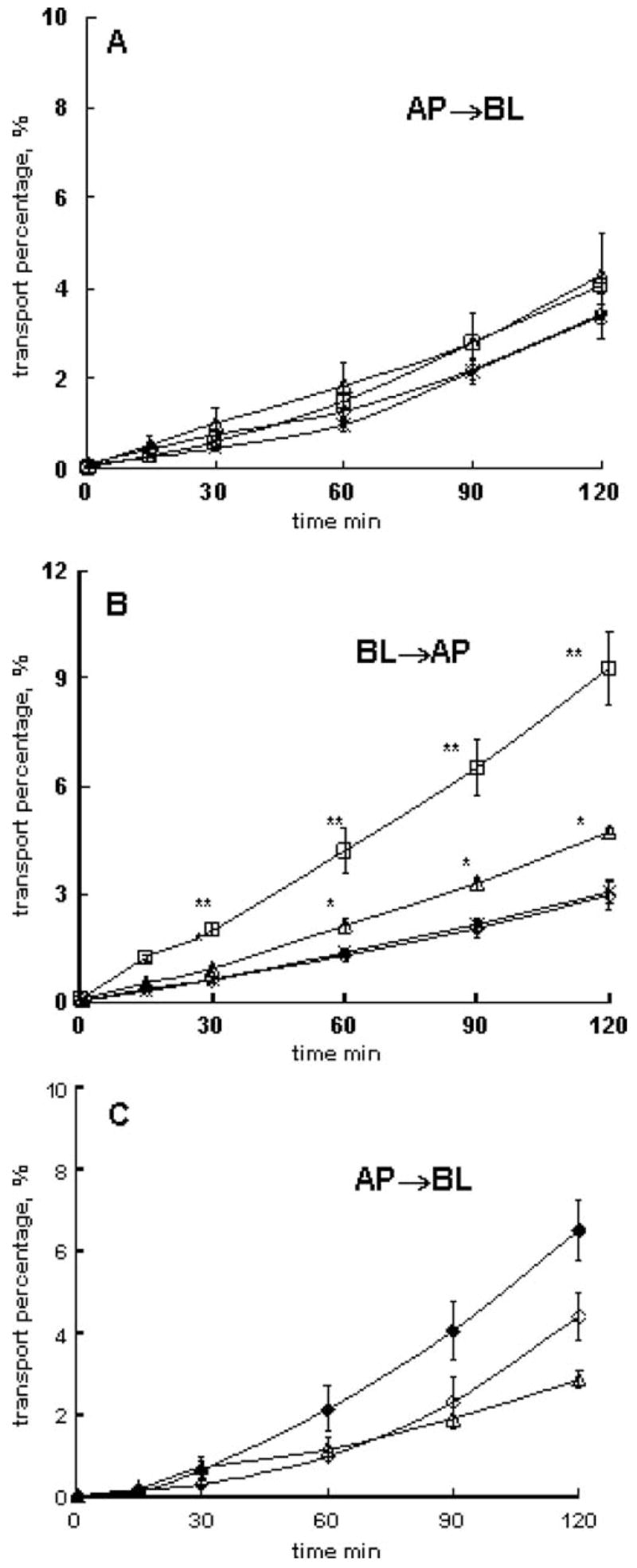
Effect of P85 on 3H-MeAIB transport in different directions across BBMEC monolayers: a AP to BL transport with/without P85 at the AP side (isolation 11), b BL to AP transport with/without P85 at BL side (isolation 11), c AP to BL transport with/without P85 or 50-mM unlabeled MeAIB at BL side (isolation 12). Assay buffer (open diamonds), 0.1 wt.% P85 (open triangles), assay buffer with 50-mM unlabeled MeAIB at BL side (filled diamonds). BBMEC monolayers were grown on 12-well transwell inserts. Statistical comparisons were made pair-wise between (1) each P85 and (2) assay buffer groups: p<0.05 (*) and p<0.01 (**), n=3
Table 4.
Permeability coefficients of 3H-MeAIB across BBMEC monolayers (×10−5 cm/s)
| # isolation | Transport direction | Assay buffer control | P85 treatment groups
|
|||
|---|---|---|---|---|---|---|
| Application side | 0.01% P85 | 0.1% P85 | 1%P85 | |||
| 10 | AP to BL | 7.2±0.7 | AP | – | 6.9±0.4 | – |
| 10 | BL to AP | 7.6±0.3 | BL | – | 16.6±0.5** | – |
| 11 | AP to BL | 6. 8±0.6 | AP | 6.0±1.0 | 8.7±1.7 | 8.7±1.5 |
| 11 | BL to AP | 6.2±0.9 | BL | 6.4±0.6 | 9.9±0.2* | 19.3±2.2** |
| 12 | AP to BL | 9.0±1.8 | BL | – | 5.9±0.4 | – |
| 12 | AP to BL | 14.0±1.9a | BL | – | – | – |
Differences between the assay buffer control and P85 treatment groups were considered significant
In these groups, 50-mM unlabeled MeAIB was added at the BL side of BBMEC monolayers, while 3 H-MeAIB was applied on the AP side
p<0.05;
p<0.01, n=3
Substrate interactions at the BL side of BBMEC monolayers
To evaluate possible interactions between Phe, Lys, and MeAIB, the BL to AP permeability of each substrate was examined with or without excess of another substrate added at the BL side of the BBMEC monolayers (Table 5). A saturating concentration (2 mM) was chosen for each unlabeled substrate. The results clearly demonstrate that none of the selected substrates significantly affects transport of another substrate with or without P85. At the same time, addition of P85 at the BL side of the cell monolayers resulted in significant increases of the permeability of each studied substrate. All together, this suggests that each substrate is carried by a different transport system inhibited by P85. Alternatively, there could be only one transporter located at the BL side with broad substrate specificity that is affected by P85. In this case, however, such transporter should have different binding sites for each of the substrates to ensure that they do not compete with each other.
Table 5.
Substrate interactions at the BL side of BBMEC monolayers (same isolation for all groups)
| 3H-probe | BL P85 concentration | BL to AP permeability coefficients with our without second substrate added (×10−5 cm/s)
|
|||
|---|---|---|---|---|---|
| None | Phe | Lys | MeAIB | ||
| 3H-Phe | 0 | 32.2±0.8 | – | 33.9±0.5 | 29.0±4.8 |
| 0.1% | 39.5±1.9 | – | 40.7±3.1 | 40.2±4.7 | |
| 3H-Lys | 0 | 18.4±2.0 | 19.7±3.1 | – | 18.5±4.4 |
| 0.1% | 33.4±0.5 | 29.8±0.7 | – | 34.3±3.4 | |
| 3H-MeAIB | 0 | 11.6±1.5 | 14.2±1.0 | 10.0±1.5 | – |
| 0.1% | 15.3±1.6 | 13.2±5.6 | 14.9±3.6 | – | |
Effect of P85 on membrane potential in BBMEC
Previous work demonstrated that P85 induces ATP depletion in BBMEC monolayers (Batrakova et al. 2001a, 2003a). This may result in a decrease of membrane potential, which is supported by ATP-dependent systems. Therefore, we evaluated effects of various doses of P85 on the plasma membrane potential in BBMEC. The membrane potential in live cells was measured using a bis-oxanol probe DiBAC (Batrakova et al. 2001a; Pratap et al. 1990; Bortner et al. 2001). As presented in Fig. 6, at low concentrations of P85 (0.0001% to 0.1%), the fluorescence intensity of the probe increases, which is indicative of the membrane depolarization. Interestingly, at high concentrations of P85 (1% and 5%), the fluorescence decreases. These concentrations are well above the critical micelle concentration (CMC) of P85 (approx. 0.0 3%; Kozlov et al. 2000). Therefore, it is likely that the observed decrease in the fluorescence intensity is due to solubilization (entrapment) of the DiBAC (Batrakova et al. 2001a) in the micelles that can considerably decrease the uptake of solutes in cells (Batrakova et al. 1998). Notably, the portion of the dye entrapped in the micelles increases as the concentration of the copolymer increases, which explains the observed concentration dependence of the fluorescence above the CMC (Miller et al. 1997). All together, the data suggest that 0.0001% to 0.1% P85 induces depolarization of the plasma membrane.
Fig. 6.
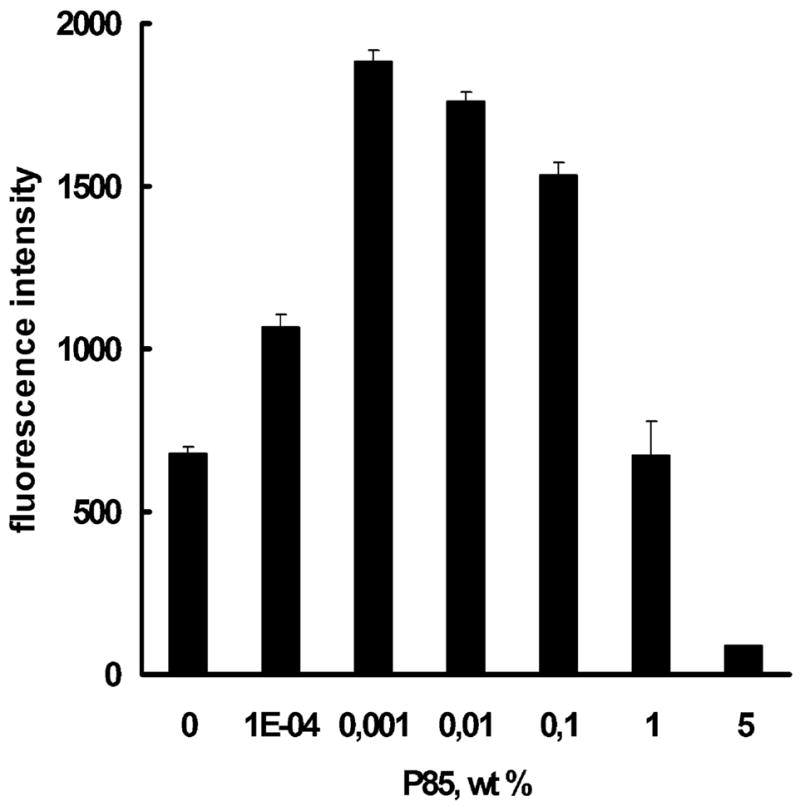
Effect of P85 on plasma membrane potential in BBMEC as measured by mean fluorescence of DiBAC (Batrakova et al. 2001a) (3). Data are mean+SEM (n=5)
Effect of P85 on intracellular sodium concentration
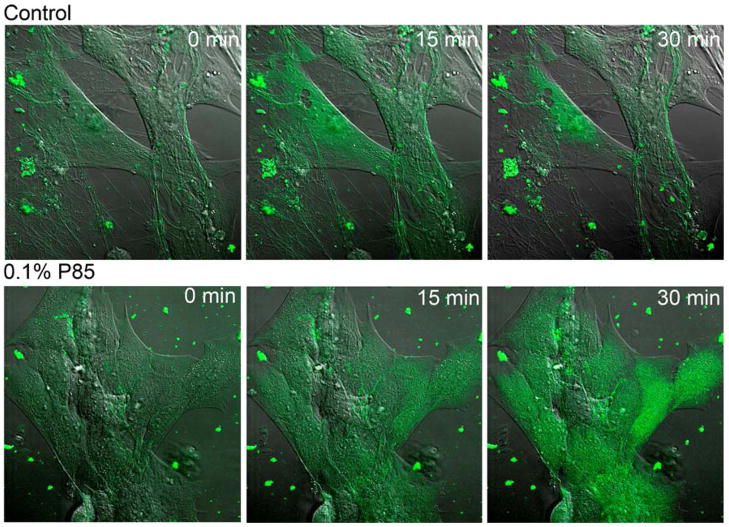
Sodium gradient is necessary for the proper function of selected amino acid transport systems in BMVEC (Ohtsuki and Terasaki 2007). Therefore, we determined whether exposure to P85 can result in an increase of the intacellular sodium concentration in BBMEC. For this purpose, a fluorescent probe, sodium green was used (Minta and Tsien 1989). As seen in Fig. 7, exposure of BBMEC to 0.1% P85 resulted in an increase in fluorescence of the cells as early as after 15 min of exposure. This was indicative of the increase in the intracellular sodium concentration in BBMEC monolayers.
Fig. 7.
Confocal microscopy images of BBMEC with 0.1% P85 and without P85 (control) at different time points of exposure. Increased fluorescence of the dye indicates increased intracellular sodium concentration. ×63 magnification
Discussion
All together, the results suggest that P85 has little, if any, effect on AP to BL transport of amino acids in our in vitro BBB model. When added at the AP side of the BBMEC monolayers, the copolymer had nearly no effect on AP to BL permeability of Phe, Lys, or MeAIB. In contrast, exposure of BBMEC monolayers to P85 added at the BL side considerably increased the BL to AP transport and decreased the AP to BL transport of each of these substrates. The doses of P85 that were used in these studies are comparable to its doses that induce inhibition of Pgp in vitro (0.01% to 1%; Batrakova et al. 1999). Furthermore, these doses are attainable to the doses that realize in vivo. Specifically, during the first 20 h after intravenous injection of 100 μl 1% P85 solution in mice, the copolymer concentration in plasma exceeds 0.001% (Batrakova et al. 2004b). The same dose of P85 was shown to increase brain delivery of a Pgp substrate, digoxin, in a mouse model (Batrakova et al. 1999, 2001b).
The changes in the flux of the amino acids observed with the P85 added at the BL side of BBMEC monolayers are unexpected and intriguing. Nonspecific increases in cell membrane permeability or disruption of tight junctions by P85 are unlikely based on the previous evidence that the copolymer does not alter membrane transport or paracellular flux of mannitol and other solutes (Batrakova et al. 1999, 2001b). Furthermore, such nonspecific effects would not explain the directionality differences in the copolymer’s activities observed in this study. First, the copolymer added at the AP side practically did not increase the AP to BL permeability of Phe, Lys, or MeAIB. Second, the copolymer added at the BL side decreased AP to BL permeability of these substrates. Finally, there were no changes in paracellular permeability of mannitol in either direction in the presence of P85. Therefore, P85 most likely affected some specific transport system(s) for Phe, Lys, and MeAIB located at the abluminal side (BL) of the brain endothelial cells. Since there was no interaction between all these substrates at the BL side with or without the copolymer, there are probably three different abluminal transport systems for each substrate affected by P85. There is, however, a possibility of existence of a single abluminal system, which has different binding sites for each of the three substrates.
These transport systems affected by P85 appear to be distinct from the well-known transporters of these substrates. For example, since P85 had no effect on LAT1 and CAT1 at the luminal side, the effects of P85 at the abluminal side appear to involve different transport system(s), but not LAT1 or CAT1, which are also expressed at this side. Some preliminary conclusions about the directionality of these new systems, XPhe and XLys, can also be made. Specifically, they are likely to have AP to BL directionality (influx) and are inhibited by the copolymer. Several new sodium-dependent transporters were described in the BBB. For example, large neutral amino acids are pumped out of the brain by several sodium-dependent efflux transporters located at the abluminal side of the BBB (Hawkins et al. 2005, 2006). The transport of Lys and other cationic amino acids was also reported to be mediated by a sodium-independent, voltage-sensitive transporter y+, with a prevalence of activity on the abluminal membrane (O’Kane et al. 2006). At this point, the data do not allow relating the effects of P85 to the activity of any of these abluminal transporters. Notably, we propose that P85 inhibits the influx transporters in the BBB. In contrast, at least in the case of Phe, the known sodium-dependent abluminal transporters appear to be efflux (Hawkins et al. 2005, 2006).
The analysis is even more complex in the case of small neutral amino acid transport. For example, small nonessential neutral amino acid transporters (e.g., system A) are sodium-dependent and expressed only at the abluminal side (Hawkins et al. 2006). However, in our model, the MeAIB flux was almost equal in both directions in the absence of P85. This may be explained by leakiness of the BBMEC monolayers with respect to MeAIB. However, such conclusion would contradict a significant increase in the AP to BL permeability of the BBMEC monolayers with excess of unlabeled 50 mM MeAIB added at the BL side (Table 4). Furthermore, a significant increase in BL to AP transport of MeAIB with P85 added at the BL but not at the AP side is very unusual. Since 0.1% P85 was shown to decrease membrane potential and increase sodium concentration in the cells, one would expect that it should also inhibit system A, but not activate it. All together, the data suggest that the copolymer affects a different MeAIB transport system, XMeAIB, with opposite directionality compared to system A. Specifically, we believe that this system transports MeAIB in AP to BL direction and is inhibited with P85 added at the abluminal side. Future studies should confirm or refute this suggestion.
Finally, we would like to discuss a possible relation of P85 effects to changes in intracellular ATP levels, membrane potential, and sodium concentration. Our previous work has shown that P85 rapidly (within 15 min) decreased ATP levels in BBMEC monolayers (Batrakova et al. 2001a). Notably, the ATP depletion was observed with P85 added at either AP or BL side of the cells. The contribution of ATP depletion to inhibition of an energy-dependent transport system in BBB was clearly demonstrated in the case of Pgp (Batrakova et al. 2001a). However, ATP depletion alone was not sufficient for inhibition of Pgp, since the transporter was only inhibited when P85 was added at the AP side but not when it was added at the BL side. Pgp is uniquely expressed at the AP side of BBMEC, and the direct contact between the Pgp expressing membrane and P85 was essential for inhibition. Most likely, P85 binds with the membrane through its hydrophobic PPO chain and induces structural changes of the membrane in the vicinity of Pgp. This leads to an inhibition of ATPase activity of Pgp and overall decrease of Vmax/Km of this transporter with respect to its substrates (Batrakova et al. 2004a). The ATPase inhibition and ATP depletion combined result in profound inhibition of the Pgp efflux system (Batrakova et al. 2001a). Notably, P85 shows some selectivity with respect to Pgp compared to other ATP-dependent drug efflux pumps. In particular, comparison of P85 effects on Pgp with its effects on multidrug-resistant pumps MRP1 and MRP2 suggests that the copolymer is much more potent in inhibiting Pgp than MRP1 and MRP2 (Batrakova et al. 2003b). This was despite the fact that P85 induced ATP depletion in the MRP1- and MRP2-overexpressing cells as well as in Pgp-expressing cells. The possible reason of apparent selectivity of P85 with respect to Pgp was that the copolymer was much less effective in inhibiting the MRP1 and MRP2 ATPase activity (Batrakova et al. 2004a). As a result, in the presence of P85, MRP1 and MRP2 were able to function at much lower concentrations of ATP than Pgp. Similar factors, i.e., differential response of transporters to the P85-induced changes in the membrane structure, may explain why some transporters in this study may be affected while others may not.
In this work, we demonstrate for the first time that P85 can also induce plasma membrane depolarization and increase intracellular sodium concentration in BBMEC. Interestingly, however, the membrane depolarization does not appear to be directly related to the ATP depletion. This follows from the fact that considerable depolarization was observed at the P85 concentration as low as 0.001%, while ATP depletion is usually observed at 0.01% and above (Batrakova et al. 2001a). There is a more clear correlation with the changes in the membrane’s microviscosity induced by P85 (“membrane fluidization”; Batrakova et al. 2001a), which is observed at lower concentrations of the copolymer. However, ATP depletion is a likely reason for increase in the sodium intracellular concentration, since sodium gradient is sustained by ATP-dependent mechanisms. The decrease of the sodium gradient in BBMEC may contribute to inhibition of sodium-dependent amino acid transporters. Importantly, no sodium dependency of amino acid transport has been detected at the luminal side of the BMVEC, which appear to have only facilitative carriers (Hawkins et al. 2006). This is consistent with the observed absence of the P85 effects on the amino acid transport at the AP side of the BBMEC. However, when P85 was added at the BL side, this resulted in changes of both AP to BL (decrease) and BL to AP (increase) permeabilities of Phe, Lys, and MeAIB. It is possible that similar to the inhibition of Pgp, concurrent restriction of energy and binding of the copolymer with the membrane carrying a transporter is necessary for inhibition of this transporter. If this is the case, it may be important for the use of P85 in vivo, when it is injected intravenously and is unlikely to be rapidly accumulated at the abluminal side of the BBB in the concentrations sufficient for affecting the amino acid transport. However, the effects of the copolymer observed at the amino acid transport at the BL side of the BBMEC monolayers are not understood at this time and require further careful investigation. Such future studies may be of considerable interest if, for example, new abluminal amino acid transporters are discovered.
Acknowledgments
This study was supported by the National Institutes of Health grant NS36229. We are grateful to Dr. Alexander Russell Hyde at University of Dundee for giving helpful suggestions.
Abbreviations
- AIB
aminoisobutyric acid
- BBB
blood–brain barrier
- BMVEC
brain microvessel endothelial cells
- BBMEC
bovine brain microvessel endothelial cells
- 3H-Phe
3H-phenylalanine
- 3H-Lys
3H-lysine
- 3H-MeAIB
3H-methylaminoisobutyric acid
- Phe
phenylalanine
- Lys
lysine
- MeAIB
methylaminoisobutyric acid
- LAT
large neutral amino acid transporter
- CAT
cationic amino acid transporter
- SAT
small neutral amino acid transporter
- Pgp
P-glycoprotein
- AP
apical side
- BL
basolateral side
Contributor Information
Xiaobin Zhang, Department of Pharmaceutical Sciences and Center for Drug Delivery and Nanomedicine, College of Pharmacy, University of Nebraska Medical Center, Omaha, NE 68198, USA.
Daria Y. Alakhova, Department of Pharmaceutical Sciences and Center for Drug Delivery and Nanomedicine, College of Pharmacy, University of Nebraska Medical Center, Omaha, NE 68198, USA
Elena V. Batrakova, Department of Pharmaceutical Sciences and Center for Drug Delivery and Nanomedicine, College of Pharmacy, University of Nebraska Medical Center, Omaha, NE 68198, USA
Shu Li, Department of Pharmaceutical Sciences and Center for Drug Delivery and Nanomedicine, College of Pharmacy, University of Nebraska Medical Center, Omaha, NE 68198, USA.
Zhihui Yang, Department of Pharmaceutical Sciences and Center for Drug Delivery and Nanomedicine, College of Pharmacy, University of Nebraska Medical Center, Omaha, NE 68198, USA.
Yili Li, Department of Pharmaceutical Sciences and Center for Drug Delivery and Nanomedicine, College of Pharmacy, University of Nebraska Medical Center, Omaha, NE 68198, USA.
Alexander V. Kabanov, Department of Pharmaceutical Sciences and Center for Drug Delivery and Nanomedicine, College of Pharmacy, University of Nebraska Medical Center, Omaha, NE 68198, USA Center for Drug Delivery and Nanomedicine, Durham Research Center, Room 1036, 985830 Nebraska Medical Center, Omaha, NE 68198-5830, USA, e-mail: akabanov@unmc.edu.
References
- Araujo P, Wassermann GF, Tallini K, Furlanetto V, Vargas CR, Wannmacher CM, et al. Reduction of large neutral amino acid levels in plasma and brain of hyperleucinemic rats. Neurochem Int. 2001;38:529–537. doi: 10.1016/S0197-0186(00)00100-5. [DOI] [PubMed] [Google Scholar]
- Armstrong A, Brewer J, Newman C, Alakhov V, Pietrzynski G, Campbell S, Corrie P, Ranson M, Valle JW. SP1049C as first-line therapy in advanced (inoperable or metastatic) adenocarcinoma of the oesophagus: a phase II window study. 2006 ASCO Annual Meeting Proceedings Part I; 2006. p. 4080. [Google Scholar]
- Batrakova EV, Han HY, Alakhov V, Miller DW, Kabanov AV. Effects of Pluronic block copolymers on drug absorption in Caco-2 cell monolayers. Pharm Res. 1998;15:850–855. doi: 10.1023/A:1011964213024. [DOI] [PubMed] [Google Scholar]
- Batrakova EV, Li S, Miller DW, Kabanov AV. Pluronic P85 increases permeability of a broad spectrum of drugs in polarized BBMEC and Caco-2 cell monolayers. Pharm Res. 1999;16:1366–1372. doi: 10.1023/A:1018990706838. [DOI] [PubMed] [Google Scholar]
- Batrakova EV, Li S, Vinogradov SV, Alakhov VY, Miller DW, Kabanov AV. Mechanism of Pluronic effect on P-glycoprotein efflux system in blood–brain barrier: contributions of energy depletion and membrane fluidization. J Pharmacol Exp Ther. 2001a;299:483–493. [PubMed] [Google Scholar]
- Batrakova EV, Miller DW, Li S, Alakhov VY, Kabanov AV, Elmquist WF. Pluronic P85 enhances the delivery of digoxin to the brain: in vitro and in vivo studies. J Pharmacol Exp Ther. 2001b;296:551–557. [PubMed] [Google Scholar]
- Batrakova EV, Li S, Alakhov VY, Miller DW, Kabanov AV. Optimal structure requirements for Pluronic block copolymers in modifying P-glycoprotein drug efflux transporter activity in bovine brain microvessel endothelial cells. J Pharmacol Exp Ther. 2003a;304:845–854. doi: 10.1124/jpet.102.043307. [DOI] [PubMed] [Google Scholar]
- Batrakova EV, Li S, Alakhov VY, Elmquist WF, Miller DW, Kabanov AV. Sensitization of cells overexpressing multidrug-resistant proteins by Pluronic P85. Pharm Res. 2003b;20:1581–1590. doi: 10.1023/A:1026179132599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batrakova EV, Li S, Li Y, Alakhov VY, Kabanov AV. Effect of Pluronic P85 on ATPase activity of drug efflux transporters. Pharm Res. 2004a;21:2226–2233. doi: 10.1007/s11095-004-7675-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batrakova EV, Li S, Li Y, Alakhov VY, Elmquist WF, Kabanov AV. Distribution kinetics of a micelle-forming block copolymer Pluronic P85. J Control Release. 2004b;100:389–397. doi: 10.1016/j.jconrel.2004.09.002. [DOI] [PubMed] [Google Scholar]
- Batrakova EV, Zhang Y, Li Y, Li S, Vinogradov SV, Persidsky Y, et al. Effects of Pluronic P85 on GLUT1 and MCT1 transporters in the blood–brain barrier. Pharm Res. 2004c;21:1993–2000. doi: 10.1023/B:PHAM.0000048189.79606.6e. [DOI] [PubMed] [Google Scholar]
- Boado RJ, Pardridge MM. A one-step procedure for isolation of poly(A)+ mRNA from isolated brain capillaries and endothelial cells in culture. J Neurochem. 1991;57:2136–2139. doi: 10.1111/j.1471-4159.1991.tb06433.x. [DOI] [PubMed] [Google Scholar]
- Bortner CD, Gomez-Angelats M, Cidlowski JA. Plasma membrane depolarization without repolarization is an early molecular event in anti-Fas-induced apoptosis. J Biol Chem. 2001;276:4304–4314. doi: 10.1074/jbc.M005171200. [DOI] [PubMed] [Google Scholar]
- Hawkins RA, Mokashi A, Simpson IA. An active transport system in the blood–brain barrier may reduce levodopa availability. Exp Neurol. 2005;195:267–271. doi: 10.1016/j.expneurol.2005.04.008. [DOI] [PubMed] [Google Scholar]
- Hawkins RA, O’Kane RL, Simpson IA, Vina JR. Structure of the blood–brain barrier and its role in the transport of amino acids. J Nutr. 2006;136:218–226. doi: 10.1093/jn/136.1.218S. [DOI] [PubMed] [Google Scholar]
- Kozlov MY, Melik-Nubarov NS, Batrakova EV, Kabanov AV. Relationship between Pluronic block copolymer structure, critical micellization concentration and partitioning coefficients of low molecular mass solutes. Macromolecules. 2000;33:3305–3313. doi: 10.1021/ma991634x. [DOI] [Google Scholar]
- Matsuo H, Tsukada S, Nakata T, Chairoungdua A, Kim DK, Cha SH, et al. Expression of a system L neutral amino acid transporter at the blood–brain barrier. Neuroreport. 2000;11:3507–3511. doi: 10.1097/00001756-200011090-00021. [DOI] [PubMed] [Google Scholar]
- Miller D, Audus K, Borchardt R. Application of cultured bovine brain endothelial cells of the brain microvasiculature in the study of the blood–brain barrier. J Tissue Cult Methods. 1992;14:217–224. doi: 10.1007/BF01409014. [DOI] [Google Scholar]
- Miller DW, Batrakova EV, Waltner TO, Alakhov V, Kabanov AV. Interactions of Pluronic block copolymers with brain microvessel endothelial cells: evidence of two potential pathways for drug absorption. Bioconjug Chem. 1997;8:649–657. doi: 10.1021/bc970118d. [DOI] [PubMed] [Google Scholar]
- Minta A, Tsien RY. Fluorescent indicators for cytosolic sodium. J Biol Chem. 1989;264:19449–19457. [PubMed] [Google Scholar]
- Molina JA, Jimenez-Jimenez FJ, Gomez P, Vargas C, Navarro JA, Orti-Pareja M, et al. Decreased cerebrospinal fluid levels of neutral and basic amino acids in patients with Parkinson’s disease. J Neurol Sci. 1997;150:123–127. doi: 10.1016/S0022-510X(97)00069-5. [DOI] [PubMed] [Google Scholar]
- Nerurkar MM, Ho NF, Burton PS, Vidmar TJ, Borchardt RT. Mechanistic roles of neutral surfactants on concurrent polarized and passive membrane transport of a model peptide in Caco-2 cells. J Pharm Sci. 1997;86:813–821. doi: 10.1021/js960483y. [DOI] [PubMed] [Google Scholar]
- O’Kane RL, Vina JR, Simpson I, Zaragoza R, Mokashi A, Hawkins RA. Cationic amino acid transport across the blood–brain barrier is mediated exclusively by system y+ Am J Physiol Endocrinol Metab. 2006;291:E412–E419. doi: 10.1152/ajpendo.00007.2006. [DOI] [PubMed] [Google Scholar]
- Ohtsuki S, Terasaki T. Contribution of carrier-mediated transport systems to the blood–brain barrier as a supporting and protecting interface for the brain; importance for CNS drug discovery and development. Pharm Res. 2007;24:1745–1758. doi: 10.1007/s11095-007-9374-5. [DOI] [PubMed] [Google Scholar]
- Oldendorf WH. Brain uptake of radiolabeled amino acids, amines, and hexoses after arterial injection. Am J Physiol. 1971;221:1629–1639. doi: 10.1152/ajplegacy.1971.221.6.1629. [DOI] [PubMed] [Google Scholar]
- Omidi Y, Barar J, Ahmadian S, Heidari HR, Gumbleton M. Characterization and astrocytic modulation of system L transporters in brain microvasculature endothelial cells. Cell Biochem Funct. 2008 doi: 10.1002/cbf.1455. [DOI] [PubMed] [Google Scholar]
- Pardridge WM. Introduction to the blood–brain barrier: methodology, biology, and pathology. Cambridge Univ. Press; New York: 1998. [Google Scholar]
- Pratap PR, Novak TS, Freedman JC. Two mechanisms by which fluorescent oxonols indicate membrane potential in human red blood cells. Biophys J. 1990;57:835–849. doi: 10.1016/S0006-3495(90)82603-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez del Pino MM, Hawkins RA, Peterson DR. Neutral amino acid transport by the blood–brain barrier. Membrane vesicle studies. J Biol Chem. 1992;267:25951–25957. [PubMed] [Google Scholar]
- Stoll J, Wadhwani KC, Smith QR. Identification of the cationic amino acid transporter (System y+) of the rat blood–brain barrier. J Neurochem. 1993;60:1956–1959. doi: 10.1111/j.1471-4159.1993. tb13428.x. [DOI] [PubMed] [Google Scholar]
- Sugawara M, Nakanishi T, Fei YJ, Huang W, Ganapathy ME, Leibach FH, et al. Cloning of an amino acid transporter with functional characteristics and tissue expression pattern identical to that of system A. J Biol Chem. 2000;275:16473–16477. doi: 10.1074/jbc.C000205200. [DOI] [PubMed] [Google Scholar]
- Umeki N, Fukasawa Y, Ohtsuki S, Hori S, Watanabe Y, Kohno Y, et al. mRNA expression and amino acid transport characteristics of cultured human brain microvascular endothelial cells (hBME) Drug Metab Pharmacokinet. 2002;17:367–373. doi: 10.2133/dmpk.17.367. [DOI] [PubMed] [Google Scholar]